Abstract
Background and aims
Diabetes is associated with accelerated arterial intimal thickening that contributes to the increased cardiovascular disease seen in this population. In healthy arteries, intimal thickening is inhibited by elevated levels of the cyclin-dependent kinase inhibitor, p27Kip1, and intimal thickening is promoted by activation of the mammalian Target of Rapamycin to promote degradation of p27Kip1 protein. Recently, we reported that two microRNAs, miR-221 and -222, which promote intimal thickening via down-regulation of mRNA encoding p27Kip1, are elevated in the arteries of diabetic patients. To determine if these miRNAs are critical to the increased intimal thickening under diabetic conditions, we examined the regulation of p27Kip1 in a mouse model of diabetes.
Methods
Comparisons of p27Kip1 signaling in NONcNZO10 mice fed a diabetogenic versus control diet were performed using immunochemistry and real-time PCR.
Results
Vascular smooth muscle cells and arteries of diabetic mice exhibited decreased levels of p27Kip1 that derived from destabilization of p27Kip1 mRNA in an extracellular signal response kinase-1/2 (ERK-1/2) dependent manner. The activity of ERK-1/2 is increased in the arteries of diabetic mice and promotes an increase in miR-221 and -222. Inhibition of miR-221 and -222 restores normal levels of p27Kip1 mRNA and protein in the arteries of diabetic mice and reduces intimal thickening following wire injury.
Conclusions
These data suggest diabetes is accompanied by increases in arterial miR-221 and -222 expression that promotes intimal thickening. Inhibition of the increased miR-221 and -222 may be efficacious in the prevention of the cardiovascular complications of diabetes.
Keywords: Diabetes Mellitus, Intimal thickening, miR-221, miR-222, p27Kip1
1. Introduction
Diabetes mellitus is accompanied by an increase in intimal thickening, a central component of cardiovascular diseases, including atherosclerosis and restenosis following percutaneous transluminal coronary angioplasty1–4. Intimal thickening consists of the proliferation and migration of vascular smooth muscle cells (VSMCs) in the intimal layer of an artery. Intimal thickening results from activation of multiple signal transduction pathways in response to mitogenic stimulation of VSMCs. Activation of one of these, the mammalian Target of Rapamycin (mTOR) pathway, promotes the degradation of the cyclin-dependent kinase inhibitor, p27Kip1. Elevated p27Kip1 protein, as seen in healthy arteries and quiescent VSMCs, blocks intimal thickening through inhibition of VSMC proliferation and migration that occur in response to mitogenic stimulation5–8. Inhibition of mTOR with the macrolide antibiotic rapamycin or its analogs is highly effective at preventing VSMC proliferation and migration9–11. In diabetic patients, the use of mTOR inhibitors is less effective at preventing in-stent restenosis12.
Recently, we reported that two microRNAs that promote intimal thickening, miR-221 and -222, are elevated in the arteries of patients with diabetes13, 14. miR-221 and -222 accelerate intimal thickening by promoting the degradation of mRNA encoding p27Kip1, in VSMCs15, 16. Loss of p27Kip1 expression is associated with increased VSMC proliferation and migration and resistance to rapamycin9, 11, 17–19. Whether this increase in miR-221 and -222 in response to diabetes promotes an increase in intimal thickening and resistance to rapamycin remains to be seen.
We and others have also reported that changes in insulin signaling associated with diabetes result in an increase in the activation of the ERK-1/2 pathway17, 20. Prolonged activation of this pathway promotes increased expression of miR-221 and -22221. Thus, the presence of diabetes may lead to a switch in the regulation of p27Kip1 from mTOR regulation at the protein level to ERK-1/2 regulation at the mRNA level. We hypothesize that this loss of p27Kip1 through increased miR-221 and -222 promotes the increase in intimal thickening seen in diabetic patients.
Here we report that, in a mouse model of type 2 diabetes, neointimal hyperplasia following femoral wire injury is increased in a miR-221 and -222 dependent manner. This is derived from the down-regulation of p27Kip1 in an mTOR independent manner. The dysregulation of mTOR and p27Kip1 occurs in response to increased activation of the ERK-1/2 pathway that results in an increase in miR-221 and -222 that destabilizes p27Kip1 mRNA. These data provide a molecular basis for the increased neointimal hyperplasia in diabetic subjects.
2. Materials and methods
2.1. Mouse model of type 2 diabetes
The NONcNZO10/LtJ mouse, a recombinant cogenic mouse model of type 2 diabetes, develops an obesity associated form of diabetes when fed a moderate fat (MF) diet of 11% fat (LabDiet, 5K20) for 12 weeks22–24. NONcNZO10 mice weaned onto 11% fat diet and aged 12 weeks to induce diabetes served as our diabetic model (DM). Our non-diabetic control mice (ND) were NONcNZO10/LtJ mice maintained on a 4% fat diet (LabDiet, 5K54). The diabetic state of all mice was confirmed using fed blood glucose measurement with the DM group having greater than 350 mg/dL. All procedures were performed with approval of the Institutional Animal Care and Use Committee.
2.2. Cell culture
Murine VSMCs were isolated from the abdominal aortae of DM (DM-VSMCs) and ND (ND-VMSCs) mice as previously described17. Greater than 90% purity of the VSMC cultures was confirmed by positive staining for α-smooth muscle actin. VSMCs were sub-cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Invitrogen, Inc. Carlsbad, CA) supplemented with 20% fetal bovine serum (FBS, Invitrogen, Inc.) at 37°C in a humidified 5% CO2 atmosphere with the media being replaced every ~48 h. All tissue culture experiments were conducted using a minimum of 3 independent isolates from ND and DM mice.
2.3. Cell proliferation
The percent of proliferating VSMCs was seeded at ~50% confluence and incubated in DMEM supplemented with 0.5% FBS for 24 h, followed by incubation in DMEM supplemented with 20% FBS for 24 h. During the final 5 h, treatment medium was supplemented with 10 µM BrdU. Following fixation and permeabilization, cells were treated with 300 µg/mL DNAse in phosphate buffered saline for 1 h at 37°C. Cells were stained with an anti-BrdU antibody conjugated to fluorescein isothiocyanate (FITC, BD Pharmingen) at 1:50 dilution and 4',6-diamidino-2-phenylindole (DAPI). The percentage of total cells (DAPI) staining positive for BrdU was calculated for four high power fields and a minimum of 100 cells for each condition.
2.4. Cell migration
Cell migration was measured as previously described.10 VSMCs at less than 50% confluence were pretreated with rapamycin in normal growth media for up to 48 h prior to seeding onto cell inserts. VSMCs (2 × 105) were seeded into the cell inserts and incubated for 4 h over DMEM containing chemoattractant (PDGF, 10 ng/mL) or 0.1% bovine serum albumin (BSA). For comparison of migration rates, data are expressed as the mean of four high power fields of triplicate samples normalized to the number of cells migrating toward media without chemoattractant.
2.5. Real-time PCR
Total RNA was isolated from VSMCs and arterial tissues using the miRNeasy Plus Mini kit (Qiagen, Inc., Valencia, CA). For measurement of mRNA, 10 ng of total RNA from each sample was analyzed to determine the relative amounts of mRNA encoding p27Kip1 and hypoxanthine phosphoribosyltransferase 1 (hprt) using QuantiTect SYBR Green one-step RT-PCR (Qiagen) and QuantiTect primer assays (Supplemental Table 1). For miRNA measurement, the Qiagen miRScript II RT Kit was used to prepare cDNA, which was then used as a template in the miScript SYBR Green PCR Kit reaction according to manufacturer’s instructions. The expression of mRNAs and miRNAs was normalized to the expression of hprt and U6, respectively, for each sample, and the fold difference between samples was calculated using the 2−ΔΔCt method. For measurement of the RNA half-life, VSMCs were treated with actinomycin D (5 ng/mL), stimulated with insulin (10 nM). Prior to measuring p27Kip1 half-life, VSMCs were treated with either the ERK-1/2 pathway inhibitor, PD98059 (20 µM), siRNA targeting ERK-2, control oligonucleotide (Ambion), or vehicle. The levels of p27Kip1 and hprt were determined as described above and the half-life was calculated as t1/2 = ln(2)/k where k is the first order rate of decay constant.
2.6. Protein measurements
Phosphorylation of ERK-1/2 at threonine 202 and tyrosine 204 (p-ERK-1/2) and total ERK-1/2 and p27Kip1 were measured using DuoSet IC ELISA kits (R&D Systems, Inc) according to manufacturer’s instructions. Western blots were prepared as previously described11 and probed with antibodies purchased from BD Biosciences (p27Kip1), Cell Signaling Technology (Danvers, MA, β-actin, p-Akt (Ser473), total Akt,), and Bethyl Laboratories (Montgomery, TX, 4E-BP1). All primary antibodies were used at a 1:1000 dilution except for the β-actin which was used at 1:2000. Blots were incubated with primary antibody overnight at 4°C with the exceptio n of p27Kip1, which was incubated for 1 h at 25°C. Blots were incubated with secondary a ntibodies (Vector Laboratories, Inc., Burlingame, CA) for 1 h at 25°C. Phosphorylation of insulin receptor substrate-1 (IRS-1) at serine 307 residue and p70S6kinase at tyrosine 421 and serine 424 residues was measured using the AKT pathway 7-Plex Panel (ThermoFisher, Inc.) and the Bio-Plex 200 Suspension Array System (Bio-Rad, Inc.) according the manufacturer’s instructions.
2.7. Mouse femoral wire injury model
The mouse femoral artery wire injury model of Roque et al. was used, with minor modifications25. Mice were injured via three passes of a 0.009” diameter angioplasty guidewire. Antisense to miR-221 and -222 (2’OMe-miR-222) was administered locally via a perivascular 30% Pluronic F-127 gel containing 1% HiPerfect transfection reagent (Qiagen) and 90 µg of either control oligonucleotide or 2’OMe-miR-222. Inhibition of the ERK-1/2 pathway was achieved via daily intraperitoneal injections of U0126 (7.5 mg/kg/d, Tocris Bioscience). U0126 was used in these studies based on previous studies demonstrating its efficacy in similar in vivo models26. Femoral arteries were harvested at 14 days post-injury. Tissues were fixed in formalin and stained with Mason’s Trichrome stain. The area of the intima, media, and lumen of each artery was measured using Adobe Photoshop.
2.8. Statistics
All data are expressed as the mean ± standard deviation with the exception of Figure 5 B and C where the data are reported as mean ± standard error of the mean for clarity of the figure. For the mRNA half-life studies, ANCOVA was used to test for statistical differences with time treated as a covariate. Student’s t-test was used to compare the means of two samples. Statistical analysis between multiple groups was performed using one way ANOVA, and Tukey’s HSD test was used to compare the individual mean values. A p-value < 0.05 was considered significant.
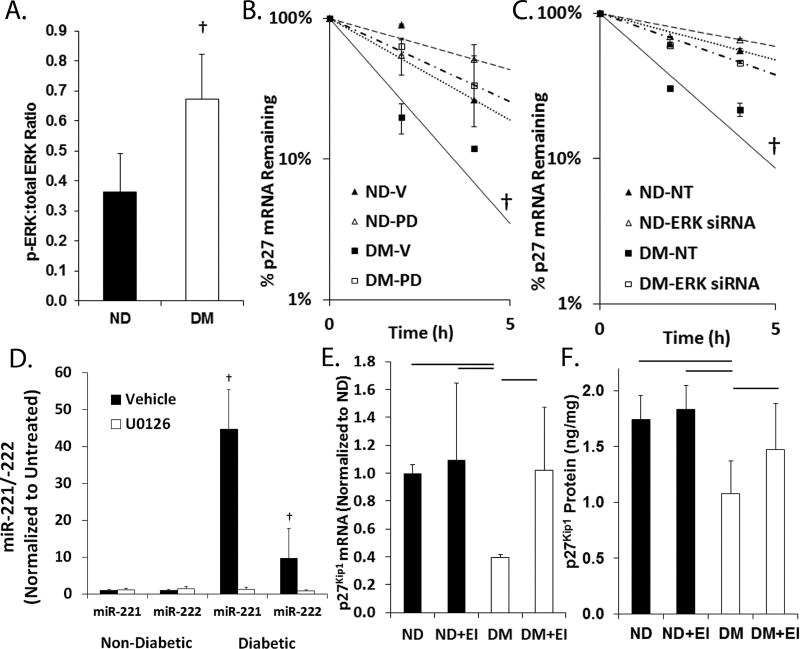
Figure 5. Increased ERK-1/2 activity promotes increased miR-221 and -222 as well as p27Kip1 mRNA destabilization.
(A) Ratio of phosphorylated to total ERK-1/2 in the aortae of DM (n=3) and ND (n=4) mice as measured by ELISA. Bar p < 0.05. (B) Half-life of p27Kip1 mRNA in ND-VSMCs and DM-VSMCs treated with vehicle (Veh) and PD98059 (PD). Data are presented as mean of triplicate samples ± standard error of the mean. † p < 0.05. (C) Half-life of p27Kip1 mRNA in ND-VSMCs and DM-VSMCs transfected with non-targeting control oligonucleotides (NT) or siRNA targeting ERK 1/2 (ERK siRNA). Data are presented as mean of triplicate samples ± standard error of the mean. † p < 0.05. (D) Relative levels of miR-221 and -222 in serum stimulated ND-VSMCs and DM-VSMCs treated with vehicle or U0126 (50 µmol/L) overnight. Data are normalized to the vehicle treated samples. Bar p < 0.05. (E) Relative p27Kip1 mRNA levels in the aortae of ND mice treated with vehicle (ND, n=3) or U0126 (ND+EI, n=5) and DM mice treated with vehicle (DM, n=4) or U0126 (DM+EI, n=4). Data are normalized to ND. Bar p < 0.05. (F) Relative p27Kip1 protein levels in the aortae of ND mice treated with vehicle (ND, n=4) or U0126 (ND+EI, n=4) and DM mice treated with vehicle (DM, n=4) or U0126 (DM+EI, n=6). Bar p < 0.05.
3. Results
3.1. Diabetes induces an increase in miR-221 and -222 that reduces p27Kip1 protein levels and increases intimal hyperplasia
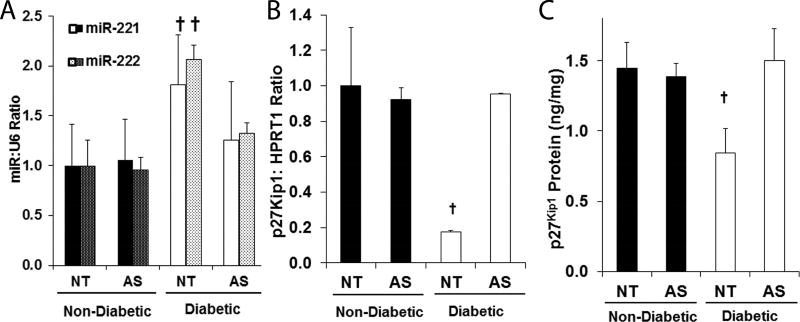
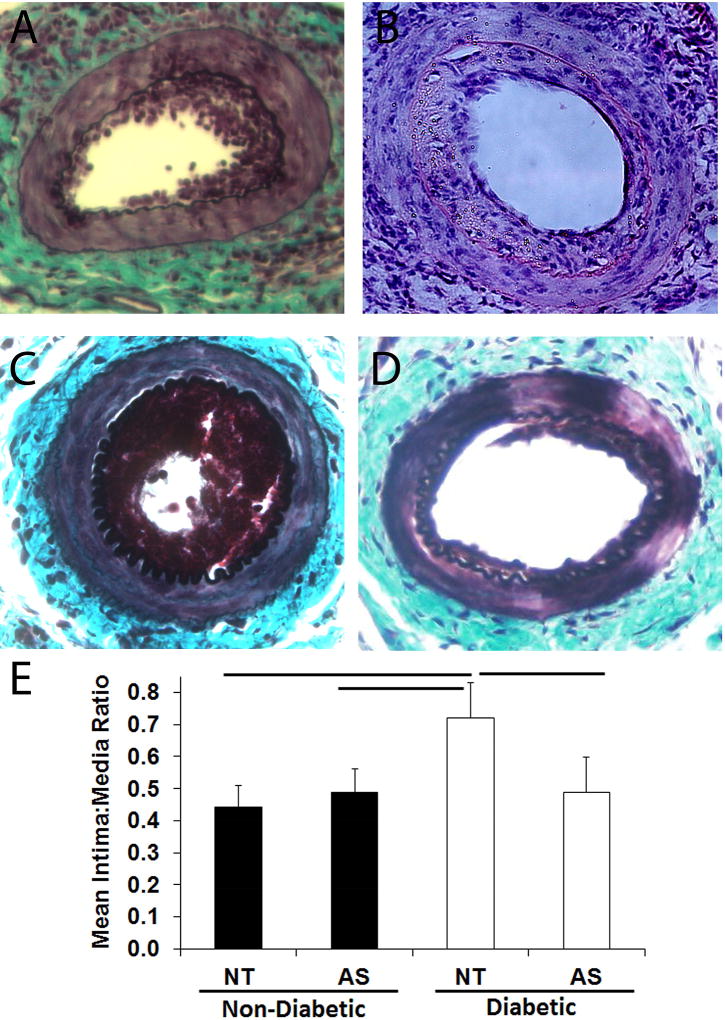
Our goal in this study is to investigate the impact of diabetes induced increased miR-221 and -222, we observed in patients on intimal thickening in the vasculature. To do this, we chose a murine model of type 2 diabetes, the NONcNZO10. This mouse develops an obesity associated hyperglycemia and insulin resistance at 12–14 weeks of age, when fed a moderately elevated fat diet (11–12%). In our hands, DM mice developed a significantly higher body weight (37.7 ± 2.8 g vs. 30.0 ± 5.9 g, p < 0.05), mean fed blood glucose (406 ± 76 mg/dL vs. 241 ± 31 mg/dL, p < 0.05), and fasting blood glucose (156.2 ± 7.8 mg/dL vs. 90.7 ± 12.0 mg/dL, p < 0.05). First, we confirmed that arteries of these mice exhibit elevated miR-221 and -222 and that we can restore normal miR-221 and -222 levels using perivascular administration of an antisense inhibitor of both miR-221 and -222 (2’-OMe-miR-222). We measured miR-221 and -222 and their targets in the femoral arteries of ND and DM mice treated perivascularly with either 2’-OMe-miR-222 or control oligo. DM mice exhibited increased miR-221 and -222 as well as reduced levels of p27Kip1 mRNA in uninjured femoral arteries, and treatment with 2’OMe-miR-222 restored normal levels of miR-221 and -222 and their targets (Fig. 1A and B). Similarly, p27Kip1 protein levels are restored by 2’OMe-miR-222 treatment (Fig. 1C). Finally, treatment with 2’OMe-miR-222 inhibited the elevated level of neointimal hyperplasia following wire injury (Fig. 2). These data demonstrate that increased miR-221 and -222 in the arteries promote an increase in neointimal hyperplasia.
Figure 1. p27Kip1 levels are reduced in the arteries of DM mice in response to elevated miR-221 and -222.
ND and DM mice were treated with a perivascular Pluronic gel containing non-targeting oligonucleotides (NT) or 2’OMe-miR-222 (AS, n=3 for all groups). Relative levels of (A) miR-221 and -222 levels and (B) p27Kip1 mRNA in the femoral arteries of ND and DM mice. Data is normalized to the ND NT group. † p < 0.05. (C) p27Kip1 protein levels in the aortae of ND and DM mice. † p < 0.05.
Figure 2. Intimal thickening following wire injury is increased in DM mice in a miR-221 and -222 dependent manner.
ND and DM mice underwent femoral artery wire injury coupled with perivascular implantation of a Pluronic gel with either non-targeting oligonucleotide (NT) or 2’OMe-miR-222 (AS). (A–D) Representative micrographs of injured femoral arteries from ND treated with NT (A, n = 8), AS (B, n = 5), and DM mice treated with NT (C, n = 11) and AS (D, n = 6). (E) Intima to media ratio for ND and DM mice. Bar p < 0.05.
3.2 VSMCs isolated from diabetic mice exhibit increased rates of proliferation and migration and a relative resistance to rapamycin
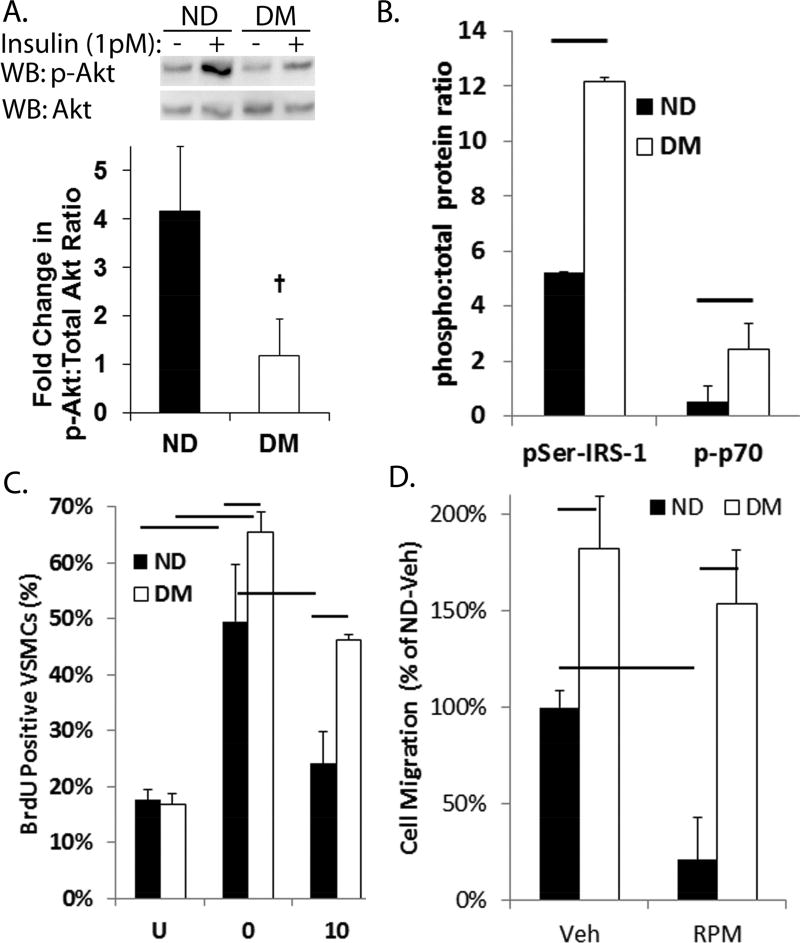
VSMCs were isolated from DM and ND mice to directly measure the effect of diabetes on VSMC proliferation and migration. Insulin resistance of DM-VSMCs was confirmed by a significant decrease in Akt phosphorylation in response to insulin stimulation compared to ND-VSMCs (Fig. 3A). Phosphorylation of insulin receptor substrate-1 (IRS-1) at serine 307 residue, and serine 421 and serine 424 residues of p70S6kinase, both of which are associated with obesity related insulin resistance, is increased in basal DM-VSMCs (Fig. 3B).27–29 IRS-1 contains multiple phosphorylation sites and phosphorylation of these sites is complex under insulin resistant conditions.30 However, taken together, these data suggest the diabetic phenotype is maintained in vitro. The DM-VSMCs exhibited increased cell proliferation in response to serum stimulation and migration toward platelet derived growth factor compared to ND-VSMCs (Fig. 3C and D). As expected, inhibition of the mTOR pathway with the macrolide antibiotic, rapamycin and its analogs, is highly effective at blocking VSMC proliferation and migration under normal conditions. The DM-VSMCs, however, exhibited a relative resistance to the effects of rapamycin treatment on VSMC proliferation and migration, suggesting a change in the mechanisms controlling VSMC proliferation and migration.
Figure 3. DM-VSMCs exhibit increased proliferation and migration coupled with a relative resistance to rapamycin.
(A) Phosphorylation of Akt at threonine 308 residue (p-Akt) is reduced in DM-VSMCs compared to ND-VSMCs following stimulation with 1 pmol/L of insulin. P-Akt is normalized to total Akt. The upper panel shows a representative Western blot and the lower panel shows the densitometric analysis. + p < 0.05. (B) Basal phosphorylation of IRS-1 at serine 307 residue and p70S6kinase at tyrosine 421 and serine 424 residues in serum starved DM-VSMCs and ND-VSMCs. (C) Percent of BrdU positive VSMCs following serum starvation overnight (U) and stimulation with 20% serum/DMEM with rapamycin (10 nmol/L) or vehicle (0). Bar p < 0.05. (D) Migration of VSMCs toward 10 ng/mL PDGF in a modified Boyden chamber. VSMCs were incubated in normal media with vehicle (Veh) or 10 nM rapamycin (RPM) overnight prior to seeding in the chamber. All studies were performed in triplicate. Data is expressed as percentage of ND-VSMCs treated with vehicle. Bar p < 0.05.
3.3. p27Kip1 protein and mRNA are reduced in the diabetic mice and VSMCs
As resistance to the anti-proliferative and anti-migratory effects of rapamycin is associated with loss of p27Kip1 expression9, 18, we measured the ability of rapamycin treatment to restore p27Kip1 levels. Rapamycin treatment was effective at inhibiting mTOR activity, as evidenced by a reduction in the phosphorylation of 4E-BP1, a downstream target of the mTOR complex 1 pathway, in the injured arteries of both DM and ND mice (Fig. 4A). In contrast, the injured arteries of DM mice did not exhibit an increase in p27Kip1 protein in response to rapamycin treatment (Fig 4A and B). Similarly, p27Kip1 protein levels are elevated in ND-VSMCs in the presence of rapamycin, but are maintained low in DM-VSMCs (Fig 4C and D). Previous reports have linked resistance to rapamycin to a dysregulation of mTOR and p27Kip1 9, 31. To determine if p27Kip1 expression was reduced in DM mice in the absence of injury, we compared p27Kip1 protein in the uninjured abdominal aortae of DM and ND mice. p27Kip1 protein and mRNA levels are reduced in the aortae of DM mice compared to ND mice (Fig 4E and F). This latter point suggests that p27Kip1 is repressed at the mRNA level under diabetic conditions in an mTOR-independent manner.
Figure 4. Regulation of p27Kip1 by the mTOR pathway is lost under diabetic conditions.
(A) Representative Western blots for p27Kip1, β-actin, and 4E-BP1 in ND and DM mice undergoing femoral artery wire injury, coupled with intraperitoneal injection of either vehicle or rapamycin. The contralateral artery underwent a sham procedure. (B) Densitometry of Western blots for p27Kip1 for ND (n=4 for vehicle, n=6 for rapamycin) and DM (n=3 for both groups) mice. Data is presented as mean of the percentage of p27Kip1 in the injured artery compared to the sham. This results in a reduction of the number of comparisons to 6. (C) Representative Western blots for p27Kip1 and β-actin in ND-VSMCs and DM-VSMCs. VSMCs were serum starved overnight (U), stimulated with DMEM/20% FBS supplemented with the indicated dose of rapamycin (RPM). (D) Densitometry of Western blots for p27Kip1 for ND-VSMCs and DM-VSMCs treated as indicated in C. All studies were performed in triplicate. Bar p < 0.05. (E) p27Kip1 protein levels in the aortae of ND and DM mice (n=4 for both groups) as measured by ELISA assay. † p < 0.05. (F) p27Kip1 mRNA in the aortae of ND and DM mice (n=3 for both groups).
3.4. p27Kip1 mRNA is decreased through increased extracellular signal response kinase 1/2 activation in DM mice
Our laboratory and others have demonstrated an increase in activation of the ERK-1/2 pathway in the arteries of diabetic animal models and patients17, 20. Similarly, ERK-1/2 phosphorylation is significantly increased in DM mice compared to ND mice (Fig. 5A). Our previous work has shown that under insulin resistant conditions, the half-life of p27Kip1 mRNA is reduced in an ERK-1/2 dependent manner17. The half-life of p27Kip1 mRNA in DM-VSMCs following insulin stimulation was approximately half of that of ND-VSMCs (Fig. 5B and C). Treatment with an ERK pathway inhibitor (PD98059) or siRNA targeting ERK2 increased the half-life of p27Kip1 mRNA in DM-VSMCs to levels similar to those of controls. Treatment of DM-VSMCs, but not ND-VMSCs, with the ERK pathway inhibitor (U0126) abolished the increase in miR-221 and -222 (Fig. 5F) and both U0126 and PD98059 reduced the elevation of miR-221 and -222 in arteries of DM mice ex vivo (Supplementary Fig. 1). Finally, we confirmed that p27Kip1 was repressed in an ERK-1/2 dependent manner in vivo, by measuring p27Kip1 mRNA and protein in the aortae of ND and DM mice treated with the ERK-1/2 pathway inhibitor, U0126. p27Kip1 mRNA and protein were reduced in the DM aortae and U0126 treatment restored normal levels (Fig. 5D and E).
4. Discussion
Diabetics are at greater risk of cardiovascular disease and complications of cardiovascular interventions32–36. Our previous work has suggested that the response of VSMCs to mitogenic stimuli may be augmented in the setting of diabetes17, 37. Additionally, we have reported that miR-221 and -222 are elevated in both healthy and atherosclerotic arteries of diabetic patients.13, 14 This study was conducted to elucidate the role of miR-221 and -222 in the regulation of VSMC cell proliferation and migration under diabetic conditions that serve to promote intimal hyperplasia. We demonstrate that intimal hyperplasia in response to wire injury is increased in response to elevated arterial miR-221 and -222 levels reducing p27Kip1 expression. This increase in miR-221 and -222 occurs in response to elevated ERK-1/2 activity and produces a dysregulation of p27Kip1 and mTOR.
Previous studies linked dysregulation of mTOR and p27Kip1 to a relative resistance to rapamycin9, 11, 31. BC3H1 cells, an immortalized myogenic cell line, develop resistance to rapamycin characterized by normal inhibition of p70S6Kinase and 4E-BP1 phosphorylation, but lack of increase in p27Kip1 in response to rapamycin treatment after repeated passaging in the presence of high doses of rapamycin (>100 nM)9. VSMCs and endothelial cells lacking p27Kip1 exhibit a relative resistance to rapamycin’s anti-migratory effects7, 10. VSMCs obtained from diabetic subjects and VSMCs lacking the insulin receptor both exhibit the increased proliferation and migration coupled with a dysregulation of mTOR and p27Kip1 17, 37. The present study demonstrates that increased arterial expression of miR-221 and -222 levels induces a similar dysregulation of mTOR and p27Kip1.
Our studies in two different in vitro models of diabetes, VSMCs lacking the insulin receptor and VSMCs obtained from diabetic subjects, link an increase in ERK-1/2 activation to a reduction in p27Kip1 mRNA17, 37. Jonas et al. have previously demonstrated that ERK-1/2 activity is increased in the arteries of diabetic animal models20. Despite clear evidence that ERK-1/2 is activated following arterial injury38, the efficacy of ERK-1/2 inhibitors for prevention of intimal hyperplasia is unclear. An initial report using the synthetic MEK1 inhibitor, PD98059, found no effect of ERK-1/2 pathway inhibition on intimal hyperplasia39. In contrast, administration of an adenovirus encoding a dominant negative mutant of p44 ERK did reduce intimal hyperplasia40. Here, we link the increased ERK-1/2 in the arteries of diabetic animal models to increased miR-221 and -222 expression and intimal hyperplasia.
As described above, increased miR-221 and -222 expression promotes intimal thickening in VSMCs through a reduction in p27Kip1 mRNA levels. Additionally, elevated miR-221 and -222 inhibit endothelial cell proliferation and migration through down-regulation of a second target, c-Kit.15, 16 miR-222 has also been reported to down-regulate signal transducer and activator of transcription 5A signaling in endothelial cells, reducing inflammation driven neovascularization.41 Thus, the impact of elevated miR-221 and -222 on an artery may extend beyond the effects on VSMCs described here.
For these studies, we chose the NONcNZO10/LtJ (RCS10) mouse, a recombinant cogenic mouse model of type 2 diabetes23. Compared with monogenic strains that develop diabetes based on the manipulation of a single gene, this strain develops an obesity associated form of diabetes due to multiple genetic mutations, making it more representative of the human disease. This strain develops a visceral obesity, insulin resistance in the skeletal muscle, liver, and heart, and hyperglycemia when fed a moderate fat (MF) diet of 10–11% fat as opposed to a low fat (LF, 4%) diet22, 23. It has also been used as tool in studying diabetic nephropathy and impaired wound healing42, 43. Importantly for our studies, the diabetic NONcNZO10 mouse exhibits increased arterial miR-221 and -222 expression as seen in type 2 diabetic patients.14 This increase was also seen in the New Zealand Obese mouse following feeding of a high fat diet to induce a type 2 diabetic state (unpublished data). Here, we demonstrate that diabetic NONcNZO10 mice recapitulate the increased intimal thickening seen in the diabetic population. As such, this study extends the use of this model into the field of the cardiovascular complications of diabetes.
Changes in the regulation of intimal thickening have the potential to greatly impact the progression of cardiovascular complications of diabetes. Diffuse intimal thickening provides a supportive environment for lipid deposition in the artery wall, initiating plaque formation44, 45. Pathologic intimal thickening occurs as part of an artery’s response to the inflammation of plaque formation46. Type 2 diabetes is accompanied by an increase in carotid intimal-medial thickness47, 48. The data presented here suggest that increased ERK-1/2 activity in the arteries of diabetic subjects promotes intimal thickening via increased miR-221 and -222 expression under diabetic conditions. Thus, inhibition of this pathway may be efficacious in reducing the cardiovascular complications of diabetes. It should be noted, however, that a previous report from our laboratory demonstrates a decrease in miR-221 and -222 in carotid plaques acutely following stroke, suggesting a potential role in maintaining plaque stability.13 Thus, such a therapy should aim to restore normal expression of these miRNAs rather than completely block their activity. For example, while the internal mammary artery of type 2 diabetic patients exhibits elevated miR-221 and -222 expression, we found that type 2 diabetic patients on metformin had similar expression levels as that of non-diabetic patients.14 Such normalization of miR-221 and -222 expression may prevent the increase in intimal thickening in response to diabetes while avoiding effects on plaque vulnerability.
In conclusion, we observed an increase in intimal hyperplasia in the NONcNZO10 type 2 diabetic mouse that was dependent on elevated expression of miR-221 and -222. The increased miR-221 and -222 expression is linked to an increase in ERK-1/2 activity and results in destabilization of p27Kip1 mRNA. Loss of p27Kip1 protein expression results in a relative resistance to the effects of inhibition of mTOR on VSMC proliferation and migration. Additional studies are required to examine the potential effects of elevated miR-221 and -222 in other vascular cells (e.g. endothelial cells) as well as potential effects on other signaling pathways in VSMCs. Nonetheless, these data describe a role for ERK-1/2 and miR-221 and -222 in the cardiovascular complications of diabetes.
Supplementary Material
Highlights.
Diabetic mice exhibit increased intimal thickening in response to loss of p27Kip1.
Loss of p27Kip1 is due to an increased expression of miRNA-221 and -222.
A change in the molecular regulation of p27Kip1 in response to diabetes is proposed.
Acknowledgments
Financial support
Research reported in this publication was supported by National Institutes of Health (R01HL127092, P30GM103337 and U54GM104940) and the American Diabetes Association (1-13-BS-210).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Author contributions
D.J.L. and S.CM. performed animal care, sample collection, assays and data analysis T.C.W. designed the study, interpreted the results, performed animal care and sample collection, and edited the manuscript. All authors contributed to the final version of the manuscript. T.C.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Faries PL, Rohan DI, Takahara H, et al. Human vascular smooth muscle cells of diabetic origin exhibit increased proliferation, adhesion, and migration. J Vasc Surg. 2001;33:601–607. doi: 10.1067/mva.2001.111806. [DOI] [PubMed] [Google Scholar]
- 2.Kornowski R, Mintz GS, Kent KM, et al. Increased restenosis in diabetes mellitus after coronary interventions is due to exaggerated intimal hyperplasia. A serial intravascular ultrasound study. Circulation. 1997;95:1366–1369. doi: 10.1161/01.cir.95.6.1366. [DOI] [PubMed] [Google Scholar]
- 3.Niskanen L, Rauramaa R, Miettinen H, et al. Carotid artery intima-media thickness in elderly patients with NIDDM and in nondiabetic subjects. Stroke. 1996;27:1986–1992. doi: 10.1161/01.str.27.11.1986. [DOI] [PubMed] [Google Scholar]
- 4.Yamasaki Y, Kawamori R, Matsushima H, et al. Asymptomatic hyperglycaemia is associated with increased intimal plus medial thickness of the carotid artery. Diabetologia. 1995;38:585–591. doi: 10.1007/BF00400728. [DOI] [PubMed] [Google Scholar]
- 5.Chung J, Kuo CJ, Crabtree GR, et al. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- 6.Marx SO, Jayaraman T, Go LO, et al. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995;76:412–417. doi: 10.1161/01.res.76.3.412. [DOI] [PubMed] [Google Scholar]
- 7.Poon M, Marx SO, Gallo R, et al. Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest. 1996;98:2277–2283. doi: 10.1172/JCI119038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallo R, Padurean A, Jayaraman T, et al. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation. 1999;99:2164–2170. doi: 10.1161/01.cir.99.16.2164. [DOI] [PubMed] [Google Scholar]
- 9.Luo Y, Marx SO, Kiyokawa H, et al. Rapamycin resistance tied to defective regulation of p27Kip1. Mol Cell Biol. 1996;16:6744–6751. doi: 10.1128/mcb.16.12.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moss SC, Lightell DJ, Jr, Marx SO, et al. Rapamycin regulates endothelial cell migration through regulation of the cyclin-dependent kinase inhibitor p27Kip1. J Biol Chem. 2010;285:11991–11997. doi: 10.1074/jbc.M109.066621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun J, Marx SO, Chen HJ, et al. Role for p27(Kip1) in Vascular Smooth Muscle Cell Migration. Circulation. 2001;103:2967–2972. doi: 10.1161/01.cir.103.24.2967. [DOI] [PubMed] [Google Scholar]
- 12.Stone GW, Kedhi E, Kereiakes DJ, et al. Differential clinical responses to everolimus-eluting and Paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation. 2011;124:893–900. doi: 10.1161/CIRCULATIONAHA.111.031070. [DOI] [PubMed] [Google Scholar]
- 13.Bazan HA, Hatfield SA, O'Malley CB, et al. Acute Loss of miR-221 and miR -222 in the Atherosclerotic Plaque Shoulder Accompanies Plaque Rupture. Stroke. 2015;46:3285–3287. doi: 10.1161/STROKEAHA.115.010567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman CB, Lightell DJ, Jr, Moss SC, et al. Elevation of miR-221 and -222 in the internal mammary arteries of diabetic subjects and normalization with metformin. Molecular and cellular endocrinology. 2013;374:125–129. doi: 10.1016/j.mce.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Cheng Y, Yang J, et al. Cell-specific effects of miR-221/222 in vessels: Molecular mechanism and therapeutic application. J Mol Cell Cardiol. 2012;52:645–655. doi: 10.1016/j.yjmcc.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Cheng Y, Zhang S, et al. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lightell DJ, Jr, Moss SC, Woods TC. Loss of Canonical Insulin Signaling Accelerates Vascular Smooth Muscle Cell Proliferation and Migration Through Changes in p27Kip1 Regulation. Endocrinology. 2011;152:651–658. doi: 10.1210/en.2010-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Totary-Jain H, Sanoudou D, Dautriche CN, et al. Rapamycin resistance is linked to defective regulation of Skp2. Cancer Res. 2012;72:1836–1843. doi: 10.1158/0008-5472.CAN-11-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods TC, Moss SC. Defective Insulin Signaling Induces a Relative Resistance to Rapamycin via a Loss of mTOR Regulation of p27Kip1 Protein Levels. Am J Cardiol. 2007;100:43L. [Google Scholar]
- 20.Jonas M, Edelman ER, Groothuis A, et al. Vascular neointimal formation and signaling pathway activation in response to stent injury in insulin-resistant and diabetic animals. Circ Res. 2005;97:725–733. doi: 10.1161/01.RES.0000183730.52908.C6. [DOI] [PubMed] [Google Scholar]
- 21.Terasawa K, Ichimura A, Sato F, et al. Sustained activation of ERK1/2 by NGF induces microRNA-221 and 222 in PC12 cells. Febs J. 2009;276:3269–3276. doi: 10.1111/j.1742-4658.2009.07041.x. [DOI] [PubMed] [Google Scholar]
- 22.Leiter EH, Reifsnyder PC, Xiao Q, et al. Adipokine and insulin profiles distinguish diabetogenic and non-diabetogenic obesities in mice. Obesity (Silver Spring) 2007;15:1961–1968. doi: 10.1038/oby.2007.234. [DOI] [PubMed] [Google Scholar]
- 23.Cho YR, Kim HJ, Park SY, et al. Hyperglycemia, maturity-onset obesity, and insulin resistance in NONcNZO10/LtJ males, a new mouse model of type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;293:E327–336. doi: 10.1152/ajpendo.00376.2006. [DOI] [PubMed] [Google Scholar]
- 24.Reifsnyder PC, Leiter EH. Deconstructing and reconstructing obesity-induced diabetes (diabesity) in mice. Diabetes. 2002;51:825–832. doi: 10.2337/diabetes.51.3.825. [DOI] [PubMed] [Google Scholar]
- 25.Roque M, Fallon JT, Badimon JJ, et al. Mouse model of femoral artery denudation injury associated with the rapid accumulation of adhesion molecules on the luminal surface and recruitment of neutrophils. Arterioscler Thromb Vasc Biol. 2000;20:335–342. doi: 10.1161/01.atv.20.2.335. [DOI] [PubMed] [Google Scholar]
- 26.Jain M, Singh A, Singh V, et al. Involvement of interleukin-1 receptor-associated kinase-1 in vascular smooth muscle cell proliferation and neointimal formation after rat carotid injury. Arterioscler Thromb Vasc Biol. 2015;35:1445–1455. doi: 10.1161/ATVBAHA.114.305028. [DOI] [PubMed] [Google Scholar]
- 27.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 28.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 29.Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 30.Langlais P, Yi Z, Finlayson J, et al. Global IRS-1 phosphorylation analysis in insulin resistance. Diabetologia. 2011;54:2878–2889. doi: 10.1007/s00125-011-2271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marx SO, Marks AR. Cell cycle progression and proliferation despite 4BP-1 dephosphorylation. Mol Cell Biol. 1999;19:6041–6047. doi: 10.1128/mcb.19.9.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franco OH, Steyerberg EW, Hu FB, et al. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007;167:1145–1151. doi: 10.1001/archinte.167.11.1145. [DOI] [PubMed] [Google Scholar]
- 33.Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 34.Cutlip DE, Chhabra AG, Baim DS, et al. Beyond restenosis: five-year clinical outcomes from second-generation coronary stent trials. Circulation. 2004;110:1226–1230. doi: 10.1161/01.CIR.0000140721.27004.4B. [DOI] [PubMed] [Google Scholar]
- 35.Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 36.Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369:667–678. doi: 10.1016/S0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- 37.Lightell DJ, Jr, Woods TC. Relative Resistance to mTOR Inhibition in Vascular Smooth Muscle Cells of Diabetic Donors. Ochsner J. 2013;13:56–60. [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Y, Cheng L, Hochleitner BW, et al. Activation of mitogen-activated protein kinases (ERK/JNK) and AP-1 transcription factor in rat carotid arteries after balloon injury. Arterioscler Thromb Vasc Biol. 1997;17:2808–2816. doi: 10.1161/01.atv.17.11.2808. [DOI] [PubMed] [Google Scholar]
- 39.Koyama H, Olson NE, Dastvan FF, et al. Cell replication in the arterial wall: activation of signaling pathway following in vivo injury. Circ Res. 1998;82:713–721. doi: 10.1161/01.res.82.6.713. [DOI] [PubMed] [Google Scholar]
- 40.Izumi Y, Kim S, Namba M, et al. Gene transfer of dominant-negative mutants of extracellular signal-regulated kinase and c-Jun NH2-terminal kinase prevents neointimal formation in balloon-injured rat artery. Circ Res. 2001;88:1120–1126. doi: 10.1161/hh1101.091267. [DOI] [PubMed] [Google Scholar]
- 41.Dentelli P, Rosso A, Orso F, et al. microRNA-222 controls neovascularization by regulating signal transducer and activator of transcription 5A expression. Arterioscler Thromb Vasc Biol. 2010;30:1562–1568. doi: 10.1161/ATVBAHA.110.206201. [DOI] [PubMed] [Google Scholar]
- 42.Reifsnyder PC, Doty R, Harrison DE. Rapamycin ameliorates nephropathy despite elevating hyperglycemia in a polygenic mouse model of type 2 diabetes, NONcNZO10/LtJ. PLoS One. 2014;9:e114324. doi: 10.1371/journal.pone.0114324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang RC, Kryger ZB, Buck DW, 2nd, et al. Limitations of the db/db mouse in translational wound healing research: Is the NONcNZO10 polygenic mouse model superior? Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2010;18:605–613. doi: 10.1111/j.1524-475X.2010.00634.x. [DOI] [PubMed] [Google Scholar]
- 44.Nakashima Y, Fujii H, Sumiyoshi S, et al. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler Thromb Vasc Biol. 2007;27:1159–1165. doi: 10.1161/ATVBAHA.106.134080. [DOI] [PubMed] [Google Scholar]
- 45.Nakashima Y, Wight TN, Sueishi K. Early atherosclerosis in humans: role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc Res. 2008;79:14–23. doi: 10.1093/cvr/cvn099. [DOI] [PubMed] [Google Scholar]
- 46.Otsuka F, Kramer MC, Woudstra P, et al. Natural progression of atherosclerosis from pathologic intimal thickening to late fibroatheroma in human coronary arteries: A pathology study. Atherosclerosis. 2015;241:772–782. doi: 10.1016/j.atherosclerosis.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Temelkova-Kurktschiev TS, Koehler C, Leonhardt W, et al. Increased intimal-medial thickness in newly detected type 2 diabetes: risk factors. Diabetes Care. 1999;22:333–338. doi: 10.2337/diacare.22.2.333. [DOI] [PubMed] [Google Scholar]
- 48.Folsom AR, Eckfeldt JH, Weitzman S, et al. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:66–73. doi: 10.1161/01.str.25.1.66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.