Abstract
The precise orchestration of hormonal regulation at all levels of the hypothalamic-pituitary-gonadal axis is essential for normal reproductive function and fertility. The pulsatile secretion of hypothalamic gonadotropin-releasing hormone (GnRH) stimulates the synthesis and release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) by pituitary gonadotropes. GnRH acts by binding to its high affinity seven-transmembrane receptor (GnRHR) on the cell surface of anterior pituitary gonadotropes. Different signaling cascades and transcriptional mechanisms are activated, depending on the variation in GnRH pulse frequency, to stimulate the synthesis and release of FSH and LH. While changes in GnRH pulse frequency may explain some of the differential regulation of FSH and LH, other factors, such as activin, inhibin and sex steroids, also contribute to gonadotropin production. In this review, we focus on the transcriptional regulation of the gonadotropin subunit genes and the signaling pathways activated by pulsatile GnRH.
Keywords: Gonadotropin-releasing hormone, follicle-stimulating hormone, luteinizing hormone, G proteins, GnRH receptor, signal transduction
1. Introduction
Regulation of the pituitary gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), is essential for normal mammalian sexual maturation and reproductive function (Marshall and Kelch, 1986). FSH and LH secretion from the gonadotrope is controlled primarily by the hypothalamic decapeptide, GnRH (Belchetz, Plant, Nakai et al., 1978). GnRH is synthesized in hypothalamic neurons and is secreted into the hypophyseal portal circulation to act primarily on the anterior pituitary. It binds to its G protein-coupled receptor, the gonadotropin-releasing hormone receptor (GnRHR), on the cell surface of a specific pituitary cell type, the gonadotrope cells, initiating downstream signaling that induces the production of these gonadotropins (Kaiser, Conn and Chin, 1997). LH and FSH, in turn, enter the peripheral circulation, acting at the ovaries and testes to regulate folliculogenesis, ovulation, spermatogenesis and steroidogenesis (Burger, Haisenleder, Dalkin et al., 2004). GnRH is released in a pulsatile manner and variations in GnRH pulse frequencies and amplitudes have differential effects on FSH and LH synthesis and release (Knobil, 1980, Savoy-Moore and Swartz, 1987, Wildt, Hausler, Marshall et al., 1981). FSH is preferentially stimulated at low GnRH pulse frequencies, whereas LH is preferentially stimulated at high GnRH pulse frequencies.
FSH and LH, are glycoprotein dimers that are comprised of two subunits, a common α-subunit (αGSU) and a distinct β-subunit (FSHβ or LHβ, respectively), which determines the biological specificity of the gonadotropins (Ciccone and Kaiser, 2009,Gharib, Wierman, Shupnik et al., 1990). The expression of the gonadotropin subunit genes is also dependent on GnRH pulse frequency. In rat models, Fshb gene expression is preferentially stimulated at low GnRH pulse frequencies (maximal at an interval of every 120 minutes) (Haisenleder, Dalkin, Ortolano et al., 1991, Kaiser, Jakubowiak, Steinberger et al., 1997, Dalkin, Haisenleder, Ortolano et al., 1989). Conversely, Lhb gene expression is preferentially stimulated at higher GnRH pulse frequencies (maximal at an interval of every 30 minutes) (Burger, Haisenleder, Aylor et al., 2008, Haisenleder, Burger, Walsh et al., 2008). Expression of Cga, the gene encoding αGSU, is stimulated by both pulsatile and continuous GnRH, with less frequency dependence (Weiss, Duca and Crowley, 1990, Ferris and Shupnik, 2006, Bedecarrats and Kaiser, 2003). The control of FSH and LH synthesis is closely correlated with the expression of the distinct β-subunits.
Many reproductive disorders are associated with disruption of GnRH, FSH, and/or LH signaling pathways (Seminara, Hayes and Crowley, 1998). For instance, persistently rapid GnRH pulses, which result in an increased LH:FSH ratio to contribute to excessive ovarian androgen production and ovulatory dysfunction, have been observed in polycystic ovarian syndrome (PCOS), a common disorder that affects 6%-10% of the female population of reproductive age (McCartney and Marshall, 2016, Dumesic, Oberfield, Stener-Victorin et al., 2015). This syndrome is also associated with cardiometabolic abnormalities (Baldani, Skrgatic and Ougouag, 2015), obesity, and impaired glucose tolerance (Wild, Carmina, Diamanti-Kandarakis et al., 2010, Blank, McCartney, Helm et al., 2007). Conversely, low GnRH pulse frequencies and abnormal serum gonadotropin levels are associated with hypothalamic amenorrhea (Reame, Sauder, Case et al., 1985, Marshall, Eagleson and McCartney, 2001). These examples highlight the importance of the proper functioning of HPG axis and of the differential control of FSH and LH secretion for normal reproductive function.
There are several excellent recent reviews of the signaling pathways activated by GnRH in the gonadotrope and of the regulation of gonadotropin subunit gene expression, but only a few focus on the differential regulation by GnRH pulse frequency (Thompson and Kaiser, 2014, Mugami, Kravchook, Rahamim-Ben Navi et al., 2017, Thackray, Mellon and Coss, 2010, Naor and Huhtaniemi, 2013, Coss, 2017, Stojilkovic, Bjelobaba and Zemkova, 2017). Several hypotheses regarding how gonadotropes decode patterns of pulsatile GnRH to differentially regulate FSH and LH production have been proposed. However, the exact mechanisms remain to be fully elucidated. This review focuses on the signaling pathways activated by different GnRH pulse frequencies to result in differential regulation of gonadotropin subunit gene expression. Studies using pulsatile GnRH in an effort to more closely emulate physiological responses of the pituitary form the basis of this review.
2. Experimental Models for Studying Gonadotrope Function
Several experimental models have been used to study the hormonal regulation of the gonadotropes, including primary pituitary cell culture, in vivo animal models and immortalized cell culture.
Primary cultures of mixed pituitary cells generated by dispersion of fresh pituitary tissue are frequently employed. However, several limitations of these models need to be mentioned. First, the anterior pituitary gland is a heterogeneous population of secretory cells including gonadotropes, thyrotropes, somatotropes, lactotropes and corticotropes, as well as folliculostellate cells, which each secrete distinct hormones. Gonadotropes represent only 10-15% of the adenohypophyseal cell populations (Ooi, Tawadros and Escalona, 2004). Second, the gonadotropes and lactotropes are densely represented near the intermediate lobe in several species, suggesting a possible paracrine relationship (Bliss, Navratil, Xie et al., 2010, Denef, 2008). Moreover, folliculostellate cells can affect experimental outcomes as they produce paracrine factors, such as follistatin and PACAP (Thackray et al., 2010, Kawakami, Fujii, Okada et al., 2002, Winters and Moore, 2007), which can modulate gonadotrope responses. These paracrine relationships may be disrupted in dispersed pituitary cultures. Third, it is important to consider the endocrine environment at the time of pituitary harvest, such as the estrous stage of female mice, as these conditions may affect experimental results (Fallest and Schwartz, 1991).
To overcome these limitations, investigators have developed novel strategies for identifying and purifying gonadotropes from transgenic mouse models. For example, FSH-producing gonadotropes have been tagged in vivo with a transgenic cell surface antigen (H-2Kk) so that they can be purified in vitro by immunologically-based cell enrichment using H-2Kk-specific antibodies (Wu, Su, Safwat et al., 2004). As another strategy, researchers used a gene targeted approach to express yellow fluorescent protein (YFP) in gonadotropes and thereby help in their visualization and identification. Mice were generated which co-express Cre-recombinase with GnRHR (GnRHR-IRES-Cre, or GRIC, mice), thereby directing Cre-mediated expression of a YFP reporter allele specifically in the gonadotropes of these-in mice (Wen, Schwarz, Niculescu et al., 2008). Nonetheless, some limitations persist, such as difficulty in acquiring sufficient yields of purified gonadotropes to perform detailed characterization studies.
Many in vivo animal models have been used to study the synthesis, secretion and action of gonadotropins, including gonadectomized rats (Haisenleder et al., 1991, Dalkin et al., 1989) and gain- and loss-of-function mouse models (Kumar, 2016). Mice with gonadotrope-specific deletion of genes encoding transcription factors, such as cFos and steroidogenic factor 1 (SF-1) (Xie, Jonak, Kauffman et al., 2015, Zhao, Bakke, Krimkevich et al., 2001, Zhao, Bakke and Parker, 2001, Tran, Zhou, Lafleur et al., 2013), or of other factors that are involved in the regulation of Fshb and Lhb transcription, such as ERK1/2 (Bliss, Miller, Navratil et al., 2009), have been developed. These animal models have provided the opportunity to study the effects of blocking specific signaling pathways in vivo.
The development of two murine gonadotrope-derived cell lines, αT3-1 and LβT2 cells, by the Mellon laboratory greatly benefited the studies of the hormonal signaling mechanisms that mediate expression of the gonadotropin genes. These immortalized cell lines were developed through targeted expression of SV40 T-antigen (Alarid, Windle, Whyte et al., 1996, Alarid, Holley, Hayakawa et al., 1998). αT3-1 cells represent immature gonadotropes and express only limited gonadotrope-associated proteins, including Cga, Gnrhr, and Nr5a1 (encoding SF-1), but lack expression of Fshb and Lhb (Windle, Weiner and Mellon, 1990). In contrast, LβT2 cells are characterized by a more mature gonadotropic phenotype, as they express Lhb and Fshb and secrete LH and FSH in response to hormonal stimulation (Graham, Nusser and Low, 1999, Pernasetti, Vasilyev, Rosenberg et al., 2001, Turgeon, Kimura, Waring et al., 1996). These characteristics validate the LβT2 cell line as a representative model for studying gonadotrope physiology. LβT2 cells also express activin, follistatin, and inhibin as well as their receptors, and receptors for steroid hormones (Thackray, McGillivray and Mellon, 2006, Takeda, Otsuka, Otani et al., 2007, Lewis, Gray, Blount et al., 2000). Studies conducted with LβT2 cells and other gonadotrope-derived cell lines may lack the effects of paracrine factors produced by other pituitary cells. In addition, although LβT2 cells represent more mature gonadotropes than the αT3-1 cell line, they differ slightly from primary gonadotropes in their profile of expressed genes (Yuen, Choi, Pincas et al., 2012). However, the great majority of the regulated genes were the same. The observed differences in the expressed genes may be due to species differences, or may be the result of differences in experimental design, microarray used, sensitivity, or due to differences in paracrine factors.
Despite these limitations, LβT2 cells currently represent the most widely used in vitro cellular model for studying gonadotrope signaling. Since LβT2 cells have become a popular model for studying gonadotropin synthesis, the majority of data presented in this review are based on studies that used these immortalized gonadotropic cells.
3. The GnRH Receptor (structural and functional aspects)
The GnRHR is a member of the G protein-coupled receptor family (GPCRs), characterized by seven transmembrane domains linked by extracellular and intracellular loops (Stojilkovic, Reinhart and Catt, 1994, Re, Pampillo, Savard et al., 2010). GPCRs represent the largest and most complex group of integral membrane protein receptors in the human genome (Huang and Tesmer, 2011). They are classically divided into 3 main classes: class A Rhodopsin related receptors, class B Secretin and Adhesion related receptors, class C Glutamate related receptors and the Taste2, Frizzled and Vomeronasal related receptors (Naor and Huhtaniemi, 2013, Sharman, Mpamhanga, Spedding et al., 2011). The GnRHR belongs to the Rhodopsin family of GPCRs (Tsutsumi, Zhou, Millar et al., 1992).
In vertebrates, three cognate receptors or receptor-like sequences have been identified with distinct distributions and functions (Millar, 2005, Neill, Duck, Sellers et al., 2001). Only two types of GnRHR occur in mammals, though (Morgan and Millar, 2004). The mammalian type I GnRHR shares over 80% amino acid identity amongst rat, human, sheep and cow and pig (Millar, Lu, Pawson et al., 2004). The type II GnRHR is fully functional in monkeys, pigs and dogs, but absent in mice, sheep, and cows, as well as silenced in human and chimpanzee genomes (Millar, 2003, Hapgood, Sadie, van Biljon et al., 2005). The type I receptor is the type of receptor that is functional and predominant in the mammalian gonadotrope (in this review the term “GnRHR” refers to type I GnRHR). In some species, including humans, it is also expressed in reproductive tissues (e.g. breast, endometrium, ovary, prostate) and in tumors derived from these tissues (Cheung and Wong, 2008, Perrett and McArdle, 2013).
What is structurally unique in the mammalian GnRHR, compared to other GPCRs, is the lack of an intracellular cytoplasmic carboxyl-terminal tail (Finch, Green, Hislop et al., 2004, Davidson, Wakefield and Millar, 1994). The C-terminal tail normally plays a key role in rapid desensitization and receptor internalization (Ferguson, 2001). It is an important phosphorylation target of the GPCR kinases (GRKs). GRK-mediated phosphorylation generates a docking site for β-arrestin scaffolding proteins, which upon binding mediate rapid desensitization through uncoupling from G proteins and dynamin-dependent internalization of the receptor (Bliss et al., 2010, Perrett and McArdle, 2013, Bockaert, Marin, Dumuis et al., 2003). It has been demonstrated experimentally that the mammalian GnRHR is resistant to rapid desensitization upon GnRH stimulation and instead undergoes relatively slow internalization (Vrecl, Heding, Hanyaloglu et al., 2000, Finch, Caunt, Armstrong et al., 2009, Pawson, Faccenda, Maudsley et al., 2008). Furthermore, experiments fusing the C-terminal tail of various GPCRs to the mammalian GnRHR demonstrated rapid desensitization and internalization, like other members of the GPCR family (Heding, Vrecl, Bogerd et al., 1998, Hanyaloglu, Vrecl, Kroeger et al., 2001). Therefore, these results together establish that the absence of the C-terminal tail in the mammalian GnRHR accounts for its resistance to rapid desensitization and ligand-induced internalization, and the absence of recruitment of β-arrestin (McArdle, Davidson and Willars, 1999, Millar, Pawson, Morgan et al., 2008).
The atypical lack of GnRHR desensitization, compared to the other members of the GPCR family, suggests that different mechanisms occur that potentially modulate the cellular response to pulsatile GnRH, such as changes in receptor abundance or changes in the signaling pathways that are activated upon binding GnRH to its receptor. Several factors are believed to affect Gnrhr gene expression based on in vitro studies, such as gonadal steroids, activins and inhibins, but the most notable factor is GnRH itself (Nathwani, Kang, Cheng et al., 2000, Gregory and Kaiser, 2004). It has been demonstrated in rat pituitary cultures that Gnrhr gene expression is dependent on GnRH pulse frequency. Gnrhr mRNA levels are significantly increased at all pulse frequencies compared to untreated controls, with greater stimulation observed under conditions of high pulse frequency (e.g., every 30 minutes) (Kaiser et al., 1997). In addition, it has been shown that cell-surface GnRHR number in LβT2 cells follows similar pattern, with greater increases in number at higher frequencies of pulsatile GnRH (Bedecarrats and Kaiser, 2003). It has been shown that GnRH-regulated Gnrhr expression is PKC-dependent and involves the MAPK signaling pathway (Bjelobaba, Janjic, Tavcar et al., 2016). Furthermore, at high cell surface densities of GnRHRs, Lhb expression is optimally stimulated, whereas Fshb expression is preferentially stimulated at lower cellular densities of the receptor (Kaiser, Sabbagh, Katzenellenbogen et al., 1995). These studies support a model in which the number of cell surface GnRHRs plays a critical role in the differential responses to various GnRH pulse frequencies.
4. Signaling Pathways Activated by Pulsatile GnRH
In the pituitary, GnRH acts by binding to the G protein-coupled GnRHR on the cell surface of the gonadotrope, inducing interaction of the receptor with heterotrimeric G proteins and catalyzing GTP-GDP exchange on the G protein α subunit (Lambert, 2008, Oldham and Hamm, 2008). Four Gα subfamilies have been identified in the mammalian genome: Gαs, Gαq/11, Gα12/13 and Gαi/o (Simon, Strathmann and Gautam, 1991). It is well established that the GnRHR interacts with Gα proteins to activate a variety of distinct signaling pathways. However, questions remain about precisely which G proteins are involved in GnRHR signaling in the gonadotrope, how each G protein contributes to signaling, and how the GnRH pulses are decoded to activate signaling cascades that preferentially induce FSH or LH production.
Initially, even before gonadotrope-derived cell lines became available, it was suggested that the GnRHR couples to a pertussis toxin-insensitive G protein (Gp, later renamed as Gq), based on studies performed in cultured rat pituitary cells (Naor, Azrad, Limor et al., 1986). Subsequently, studies in αT3-1, CHO-K1 and COS-7 cells enhanced the hypothesis that the GnRHR initiates signaling pathway(s) by coupling exclusively to Gαq/11 (Hsieh and Martin, 1992, Grosse, Schmid, Schoneberg et al., 2000). On the other hand, studies in LβT2 cells indicated that both Gαq/11 and Gαs were involved in GnRHR signaling (Liu, Usui, Evans et al., 2002). In addition, studies from the same laboratory noticed a differential desensitization of Gαs and Gαq/11 in response to pulsatile GnRH stimulation (Tsutsumi, Mistry and Webster, 2010). In a more recent study, the expression and role of individual G proteins were investigated in LβT2 cells using siRNA and bacterial toxins. These studies indicated that GnRH signaling in gonadotrope cells involved primarily Gαq/11 and Gαs (and to a lesser extent Gα12/13 as well) and, more interestingly, that their depletion differentially affected GnRH-stimulated gonadotropin gene expression (Choi, Jia, Pfeffer et al., 2012). Gαq/11 knockdown reduced GnRH-stimulated Fshb mRNA levels, while Gαs knockdown reduced GnRH-stimulated Lhb mRNA levels. However, these results were not entirely consistent with the effects on the promoter activity of Fshb and Lhb. Although the Gαi/o subfamily is expressed in LβT2 cells, it was not found to be involved in regulation of gonadotropin gene expression (Choi et al., 2012, Krsmanovic, Mores, Navarro et al., 2003).
Therefore, it appears that the GnRHR can activate multiple Gα subfamilies, a factor to be taken into consideration when studying the signaling pathways activated by GnRH. Several signaling pathways are activated and may be preferentially regulated by different GnRH pulse frequencies. In this review, we will focus primarily on protein kinase C/MAPK, calcium-calmodulin dependent kinases, calcineurin/NFAT, and cAMP/PKA pathways.
4.1 Protein kinase C/MAPK pathways
After the activation of Gαq/11 proteins by the GnRHR, stimulation of phospholipase Cβ (PLCβ) occurs. PLCβ, in turn, cleaves phosphatidylinositol-4-5-bisphosphate (PIP2) into inositol trisphosphate (IP3) and diacylglycerol (DAG) (Naor, 2009). IP3 stimulates Ca2+ release from endoplasmic reticulum stores into the cytosol, whereas DAG activates protein kinase C (PKC) (Tsutsumi and Webster, 2009). Some PKC isoforms (PKCα, βI, βII, γ) are activated by Ca2+ as well (Kishimoto, Mikawa, Hashimoto et al., 1989). Many investigators have shown that GnRH induces increased intracellular calcium levels and PKC activation, which can activate several mitogen-activated protein kinase (MAPK) cascades in gonadotropes (Bliss et al., 2010, Naor, 2009, Caunt, Finch, Sedgley et al., 2006). The MAPKs can translocate to the nucleus and activate several transcription factors (Murphy and Blenis, 2006).
Activation of the family members MAPK1/3 (extracellular signal-regulated kinase, ERK1/2), MAPK8/9 (c-Jun N-terminal kinase, JNK1/2) and MAPK14 (p38-α) has been reported to mediate GnRH-induced gonadotropin subunit transcription (Burger et al., 2004, Haisenleder et al., 2008, Naor and Huhtaniemi, 2013, Kanasaki, Purwana, Oride et al., 2012, Ando, Hew and Urano, 2001, Bernard, Fortin, Wang et al., 2010). For the purposes of this review, these family members will be referred to as ERK, JNK and p38, respectively. The ERK pathway is comprised of MAPK kinase kinase (Raf1), MAPK kinases (MEK1 and MEK2) and MAPKs (ERK1 and ERK2) (Pearson, Robinson, Beers Gibson et al., 2001). However, a study of mice with pituitary-targeted deletion of Raf1 demonstrated that Raf1 may not be mandatory for ERK signaling in gonadotropes (Bliss, Navratil, Xie et al., 2012). Moreover, it has also been shown that ERK1/2 activation can be promoted via a pathway that includes c-Src and Ras as well (Kanasaki et al., 2012). On the other hand, GnRH stimulates JNK and P38 pathways via activation of c-Src and Rac1/Cdc42 and subsequent activation of MAP4K (Harris, Bonfil, D et al., 2002). These molecules in turn activate MAPK kinases, MKK4/7 and MKK3/6, which subsequently activate JNK and p38, respectively (Naor, 2009, Pearson et al., 2001). These MAPK cascades, especially the ERK pathway, have been implicated in regulating Fshb and Lhb promoter activity in response to pulsatile GnRH (Kanasaki, Bedecarrats, Kam et al., 2005).
Studies in both perifused LβT2 cells and primary gonadotropes demonstrated more rapid onset and sustained patterns of ERK1/2 phosphorylation following stimulation by low rather than high GnRH pulse frequencies (Kanasaki et al., 2005, Haisenleder, Cox, Parsons et al., 1998). In addition, higher levels of nuclear phosphorylated ERK have been reported following low GnRH pulse frequency stimulation. The different patterns of ERK activation/inactivation in response to various GnRH pulse frequencies imply that ERK phosphorylation may be important for GnRH pulse frequency-dependent differential stimulation of Fshb and Lhb expression (Kanasaki et al., 2005). Many previous studies demonstrated the importance of the ERK-dependent transcription factor, early growth response-1 protein (Egr1), for Lhb expression, supporting this hypothesis (Dorn, Ou, Svaren et al., 1999, Fortin, Lamba, Wang et al., 2009, Lawson, Tsutsumi, Zhang et al., 2007, Lee, Sadovsky, Swirnoff et al., 1996, Wolfe and Call, 1999, Halvorson, Kaiser and Chin, 1999). In contrast, the role of ERK signaling in mediating the GnRH-induced Fshb expression is more controversial, although ERK phosphorylation follows a pattern similar to that of Fshb expression. Studies using pharmacological inhibition of MEK1, the kinase that activates ERK1/2, demonstrated inhibition of GnRH-induced ovine, murine, rat and human Fshb/FSHB promoter activities in LβT2 cells (Burger et al., 2008, Bernard et al., 2010, Kanasaki et al., 2005, Bonfil, Chuderland, Kraus et al., 2004, Coss, Hand, Yaphockun et al., 2007, Coss, Jacobs, Bender et al., 2004). In addition, studies in perifused male rat primary pituitary cultures demonstrated that MEK1 inhibition attenuated GnRH-stimulated Fshb expression (Haisenleder et al., 1998). More recently, our group demonstrated that stimulation of both Fshb and Lhb gene expression by pulsatile GnRH was markedly attenuated by MEK1/2 inhibition at both high and low GnRH pulse frequencies (Kanasaki et al., 2005, Thompson, Ciccone, Zhou et al., 2016). Together, these data suggest a potential role of ERK1/2 in GnRH induction of both Lhb and Fshb gene expression. However, in vivo studies with gonadotrope-specific ERK1/2 double knock-out mice showed only partial impairment in Fshb expression and FSH secretion, with more marked effects on Lhb expression and secretion (Bliss et al., 2009). These data imply that ERK1/2 may be more important for LH than for FSH regulation in vivo, or that other GnRH-activated signaling pathways compensate for the loss of ERK1/2 function in these animals.
The distinct pattern of ERK phosphorylation in response to different GnRH pulse frequencies suggests a possible role of MAPK phosphatases (MKPs) as a mediator of this differential activity. MKPs are phosphatases that inactivate MAPKs through dephosphorylation of threonine and tyrosine residues (Kanasaki et al., 2012). Activation of ERK and JNK in pituitary gonadotropes promotes MKP1 and MKP2 (DUSP1 and DUSP4 respectively) activity, which then effectively inactivate ERK, JNK, and p38 (Zhang, Mulvaney and Roberson, 2001, Kondoh and Nishida, 2007, Franklin and Kraft, 1997). Studies in αT3-1 cells and in vivo in mice demonstrated an increase in MKP1 and MKP2 expression in response to GnRH (Zhang and Roberson, 2006). Furthermore, studies performed in perifused LβT2 cells showed increased MKP1 expression after high rather than low GnRH pulse frequency (Nguyen, Intriago, Upadhyay et al., 2010, Purwana, Kanasaki, Mijiddorj et al., 2011). This regulation pattern is distinct compared to ERK1/2 activation, which is more rapidly induced and more sustained at low frequency GnRH pulses (Kanasaki et al., 2005). Interestingly, knockdown of MKP1 in LβT2 cells promoted activation of ERK and Lhb expression by GnRH, while overexpression of MKP1 attenuated ERK and Lhb promoter activation (Nguyen et al., 2010).
Collectively these studies suggest a potential role of MKPs in a negative feedback loop to control MAPK activity in response to different GnRH pulse frequencies. Nevertheless, a study by Armstrong et al. using live cell imaging to track ERK2-GFP translocation in heterologous HeLa cells argues against this potential role. Using pharmacological and molecular genetic approaches to suppress MKP activity, they demonstrated little or no effect on the rapid and transient translocation of ERK2-GFP to the nucleus after pulsatile stimulation with GnRH (Armstrong, Caunt, Fowkes et al., 2010).
4.2 Calcium/Calmodulin-dependent kinase II (CaMK II) pathway
Binding of GnRH to the GnRHR initiates several intracellular signaling cascades, including calcium influx via L-type voltage-gated calcium channels and IP3-induced mobilization of calcium from intracellular stores (Duran-Pasten and Fiordelisio, 2013). The GnRH-induced rapid increase in intracellular calcium is essential for rapid gonadotropin secretion and for gonadotropin subunit gene expression (Naor, 2009). Previous studies have shown that blockade of Ca+2 channels prevents the increase in Cga, Lhb and Fshb expression in rat pituitary cells (Burger et al., 2004) and in Lhb promoter activity in LβT2 cells (Weck, Fallest, Pitt et al., 1998). Interestingly, perifusion studies in rat primary cells with a calcium channel agonist revealed a pulse frequency-dependent differential regulation of subunit gene expression similar to that induced by pulsatile GnRH. High frequency pulses preferentially stimulated Lhb while low frequency pulses favored Fshb (Haisenleder, Yasin and Marshall, 1997, Haisenleder, Workman, Burger et al., 2001).
The downstream mediators of calcium action within the gonadotrope have not been fully elucidated. Elevation of intracellular calcium levels is required for MAPK activation and for activation of certain PKC isoforms (Mugami et al., 2017, Roberson, Bliss, Xie et al., 2005). However, calcium also signals via the calcium/calmodulin-dependent kinase II (CaMK II) pathway (Roberson et al., 2005, Melamed, Savulescu, Lim et al., 2012). CaMK II, which is a common mediator of calcium signaling and pulse frequency decoder in many cell types, requires two molecules of calcium-bound calmodulin (Ca-CALM1) to bind to the enzyme in order to be activated (Ferris and Shupnik, 2006). The higher the frequency of Ca2+ influxes, the higher the percentage of CaMK II activated, likely because there is less time for Ca-CALM1 to dissociate from CaMK II while awaiting a second Ca-CALM1 molecule to bind (De Koninck and Schulman, 1998). In addition, it has been demonstrated that a single GnRH pulse can induce a rapid and transient increase in CaMK II activation in both primary pituitary and LβT2 cells. Another interesting finding is that inhibition of CaMK II inhibits Cga, Lhb and Fshb expression (Haisenleder, Burger, Aylor et al., 2003, Haisenleder, Ferris and Shupnik, 2003), suggesting a potential role of CaMK II in decoding GnRH pulse frequency within the gonadotrope.
On the other hand, it was reported that activation of CaMK II is upregulated by GnRH but not in a frequency-dependent manner (Burger et al., 2008). This observation does not exclude the potential role of CaMK II, but indicates that it is not the only mechanism involved. The rapid inactivation kinetics of CaMK II implies that extended CaMK II activity may occur preferentially at high GnRH pulse frequency and hence result in greater Lhb than Fshb expression (Thompson and Kaiser, 2014).
4.3 Calcineurin/NFAT pathway
In addition to CaMK II, another calcium/calmodulin target is the serine/threonine protein phosphatase, Calcineurin (Natarajan, Ness, Wooge et al., 1991, Lim, Luo, Koh et al., 2007). It has been demonstrated that Calcineurin is involved in the GnRH-induced derepression of FSHβ gene expression in αT3-1 gonadotrope cells, possibly by activation of Nur77 (Lim et al., 2007). More recently, the nuclear factor of activated T cells (NFAT), which is activated by Calcineurin, has been reported to be regulated by GnRH in αT3-1, LβT2 and HeLa cells (Gardner, Maudsley, Millar et al., 2007, Armstrong, Caunt, Fowkes et al., 2009, Pnueli, Luo, Wang et al., 2011). This is of interest, as NFAT is a known transcriptional activator of Nur77 (Pnueli et al., 2011), indicating a possible role of Calcineurin/NFAT pathway in GnRH-induced Fshb expression. A study using a Calcineurin-specific inhibitor and siRNA for NFAT demonstrated that this signaling cascade is also necessary for the transcriptional regulation of the immediate early genes, Jun, Fos and Atf3, which are important for GnRH-regulated expression of Cga and Fshb (Binder, Grammer, Herndon et al., 2012). In contrast, induction of Lhb transcription by GnRH, which is dependent on Egr1 (Wolfe and Call, 1999), remained unaffected by changes in Calcineurin activation (Binder et al., 2012).
In addition, another study using the nuclear translocation of an emerald fluorescent protein-tagged NFAT (NFAT-EFP) as a downstream readout for the activation of the Calcineurin/NFAT pathway demonstrated translocation into the nucleus upon pulsatile GnRH stimulation in a dose- and frequency-dependent manner (Armstrong et al., 2009). The effect was reversible, but much slower in onset and in reversibility compared to ERK2-GFP (Armstrong et al., 2010, Armstrong et al., 2009). Pulsatile GnRH also caused a pulse frequency dependent activation of Cga-, Lhb-, Fshb-luciferase reporters, which was attenuated by the Calcineurin inhibitor, cyclosporine A. New mathematical models of GnRH signaling, which accurately reflect these experimental observations, demonstrate that the NFAT and ERK pathways can mediate genuine frequency decoding when they act in a cooperative manner at the transcriptome (Tsaneva-Atanasova, Mina, Caunt et al., 2012, Pratap, Garner, Voliotis et al., 2016).
4.4 cAMP/PKA/CREB pathway
The GnRHR can also activate the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA)/cAMP response element binding protein (CREB) pathway (Thompson and Kaiser, 2014). GnRH-stimulated cAMP production has been reported in rat pituitary cells, in LβT2 cells, and in many heterologous cell lines, including HeLa, GH3 and COS-7 cells (Perrett and McArdle, 2013, Cohen-Tannoudji, Avet, Garrel et al., 2012). The coupling mechanism between the GnRHR and the cAMP pathway has been studied extensively. It has been shown in LβT2 cells that GnRH stimulation can activate cAMP production via Gαs recruitment (Liu et al., 2002). In support of this mechanism, a recent study using cAMP and calcium biosensors in αT3-1 cells has demonstrated that GnRH increases cAMP production and that the GnRHR interacts with the proto-oncogene SET, which inhibits receptor coupling to the calcium signaling pathway and increases coupling to the cAMP pathway. Its action is probably mediated by preventing Gαq/11 binding to the GnRHR (Avet, Garrel, Denoyelle et al., 2013). On the other hand, the same group showed that the PKC δ and ε isoforms can mediate cAMP production via activation of adenylate cyclase 5 and 7 in LβT2 cells, indicating that this pathway can also be activated via Gαq/11 (Lariviere, Garrel, Simon et al., 2007). Several studies have demonstrated GnRH stimulation of PKA in rat pituitary cells and in LβT2 cells as well (Garrel, Simon, Thieulant et al., 2010, Grafer, Thomas, Lambrakos et al., 2009).
The majority of these studies, however, have used tonic GnRH treatment to activate signaling. Studies using pulsatile GnRH, which are closer to the physiological response of the pituitary, are more limited. The Webster laboratory, using cAMP and PKA-dependent FRET reporters, monitored the dynamics of responses in LβT2 cells after pulsatile GnRH treatment. They demonstrated that pulses of GnRH, at both high and low frequencies, can cause pulses of cAMP elevation and PKA activation, which are rapid and transient with no obvious desensitization to subsequent pulses (Tsutsumi et al., 2010). Our group, using PKA inhibitors and measuring Fshb mRNA levels, Fshb-luciferase activity, and CREB phosphorylation, has shown that PKA activity and CREB phosphorylation are significantly increased to the greatest extent by low GnRH pulse frequencies. Moreover, after using a PKA inhibitor, both CREB phosphorylation and Fshb expression, but not Lhb expression, were attenuated at both high and low GnRH pulse frequencies, implicating PKA as an important mediator of GnRH stimulation of FSHβ production (Thompson, Ciccone, Xu et al., 2013). Despite minor differences, both of these studies support a role for the cAMP/PKA pathway in decoding pulsatile GnRH inputs.
5. Regulation of Gonadotropin Gene Expression by Pulsatile GnRH
The signaling pathways described previously affect Cga, Fshb and Lhb gene expression. These genes are differentially regulated by varying GnRH pulse frequencies, although the α-subunit is produced in excess, regardless of GnRH pulse frequency (Haisenleder et al., 1991, Dalkin et al., 1989). Therefore, the control of FSH and LH synthesis is closely correlated with the production of the distinct β-subunits. This section of this review will focus on transcription factors that are activated by pulsatile GnRH and mediate Fshb and Lhb expression.
5.1 Lhb
The structure of the Lhb gene promoter has been characterized to a great extent, providing much information about Lhb gene expression following GnRH stimulation. The proximal Lhb promoter region contains two binding sites for the immediate early gene protein Egr1, two binding sites for steroidogenic factor 1 (SF-1) and a homeodomain element, which is a binding site for Pitx1/Otx1 (Halvorson, Kaiser and Chin, 1996, Quirk, Lozada, Keri et al., 2001). The interactions among Egr1, SF-1 and Pitx1/Otx1 are required for GnRH induction of the promoter (Dorn et al., 1999, Tremblay and Drouin, 1999). The distal Lhb promoter region, which is necessary for full GnRH induction of the promoter, contains binding sites for Sp1 and NFY and an overlapping CArG box (Kaiser, Sabbagh, Chen et al., 1998, Weck, Anderson, Jenkins et al., 2000). The transcriptional co-activators SNURF and p300 connect the distal and proximal GnRH response regions of the Lhb promoter and may enhance Egr1 action (Mouillet, Sonnenberg-Hirche, Yan et al., 2004, Curtin, Ferris, Hakli et al., 2004).
Egr1 is a key factor regulating Lhb expression after pulsatile GnRH stimulation (Dorn et al., 1999, Fortin et al., 2009, Lawson et al., 2007). In vivo studies with Egr1 deficient mice demonstrated reduced expression of the Lhb gene and infertility (Lee et al., 1996, Topilko, Schneider-Maunoury, Levi et al., 1998). Pituitary-specific SF-1 knock-out mice are infertile as well and demonstrated markedly decreased LH levels, but treatment with high doses of GnRH induced Lhb expression (Zhao et al., 2001). Studies in perifused LβT2 cells demonstrated that Egr1 expression is stimulated to a greater extent at high GnRH pulse frequency, consistent with increased Lhb transcription at high GnRH pulse frequency (Kanasaki et al., 2005). In contrast, Nab1/2 (Ngfi-A binding proteins), corepressors of the Egr family, are preferentially stimulated at low GnRH pulse frequency (Lawson et al., 2007). The differential regulation of responses of Egr1 and Nab1/2 to varying frequencies of pulsatile GnRH suggest that regulation of Egr1 synthesis and bioavailability may play a role in the differential regulation of the Lhb gene following pulsatile GnRH stimulation (Tsutsumi and Webster, 2009) (Figure 1).
Figure 1. Proposed model of regulation of Lhb transcription by pulsatile GnRH.
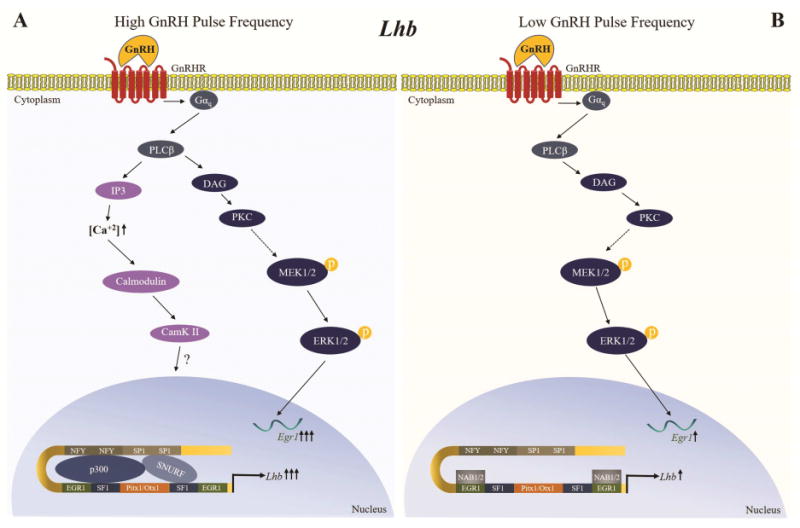
High and low GnRH pulse frequencies stimulate different signaling pathways to mediate the synthesis of transcription factors and repressors controlling Lhb transcription. A) At a high GnRH pulse frequency, the PKC/MAPK and Calcium/Calmodulin-dependent kinase II (CaMK II) pathways are activated, causing an increase in Egr1 expression. Egr1 in turn increases activity of the Lhb promoter. B) At a low GnRH pulse frequency, the PKC/MAPK pathway is activated but the transient increase in Egr1 expression is insufficient for maximal activation of the Lhb promoter, an effect further antagonized by increased expression of Nab1/2, corepressors of the Egr family, which are preferentially stimulated at low GnRH pulse frequency.
5.2 Fshb
In contrast to the Lhb gene promoter, the Fshb gene promoter has been less well characterized, due in part to the lack of ideal cellular models for study. The development of LβT2 cells, which produce both LHβ and FSHβ, provided a tool for the study of the Fshb gene promoter and hence a greater understanding of the gonadotropin regulation. Our group (Ciccone, Lacza, Hou et al., 2008) and others (Coss et al., 2004, Wang, Fortin, Lamba et al., 2008) have identified a GnRH-responsive element in the proximal Fshb promoter region, which is highly conserved in humans (Wang et al., 2008) and contains a partial cAMP response element (CRE)/AP1 site. We have demonstrated that the binding of USF1 and USF2 transcription factors mediate basal rat Fshb transcription, whereas GnRH stimulates CREB phosphorylation, which in turn binds to the homologous CRE/AP-1 half site and recruits the histone acetyltransferase CREB-binding protein (CBP) to the promoter (Ciccone et al., 2008). Several studies have demonstrated that GnRH-induced activation of MAPKs, including ERK1/2, JNK and p38, results in activation of members of the AP1 family, such as cFos, cJun and ATFs (Coss et al., 2004, Liu, Austin, Mellon et al., 2002, Xie, Bliss, Nett et al., 2005). The role of AP1 transcription factors in the stimulation of Fshb expression by GnRH is controversial. Miller's laboratory has extensively studied the role of AP1 sites in GnRH-induced FSHB transcription in vitro and in vivo. In vitro experiments demonstrated that GnRH signals through the AP1 sites to increase ovine FSHB transcription in gonadotropes (Strahl, Huang, Pedersen et al., 1997, Strahl, Huang, Sebastian et al., 1998). On the other hand, in vivo studies using mice called into question the importance of these AP1 sites in the GnRH regulation of the ovine FSHB promoter (Huang, Sebastian, Strahl et al., 2001).
Our group further explored the role of CREB in perifusion studies in vitro. It was demonstrated in LβT2 cells that the preferential Fshb expression at low GnRH pulse frequencies is dependent on CREB binding to the CRE/AP1 site (Ciccone, Xu, Lacza et al., 2010). In addition, we showed that CREB phosphorylation and PKA activity are increased to a greater extent at low GnRH pulse frequencies (Figure 2). Interestingly, PKA inhibition attenuated both CREB phosphorylation and Fshb expression, indicating that CREB phosphorylation is mediated by PKA (Thompson et al., 2013).
Figure 2. Proposed model of regulation of Fshb transcription by pulsatile GnRH.

High and low GnRH pulse frequencies stimulate different signaling pathways that mediate the synthesis of transcription factors and repressors controlling Fshb transcription. A) At a high GnRH pulse frequency, the GnRHR preferentially couples to Gαq/11 proteins, activating the PKC/MAPK pathway. This pathway stimulates ICER expression and synthesis. ICER attenuates GnRH stimulation of Fshb expression by antagonizing the binding of CREB to the Fshb CRE-AP1 site. SKIL and TGIF1 are also induced at high GnRH pulse frequencies and also bind to the Fshb gene promoter to prevent any potential action of cFos and cJun. B) At a low GnRH pulse frequency, the GnRHR preferentially couples to Gαs protein, activating the cAMP/PKA/CREB pathway (indicated by bold arrows). The phosphorylated CREB protein binds to the homologous CRE-AP1 site of the Fshb promoter and recruits the histone acetyltransferase CREB-binding protein (CBP), stimulating Fshb transcription. PKC/MAPK and Calcineurin/NFAT pathways are also activated, but to a lesser extent. They contribute to induction of Fshb transcription by stimulating cFos and cJun expression.
Mutation of this GnRH responsive element within the Fshb promoter resulted in loss of preferential Fshb expression at low GnRH pulse frequencies (Ciccone et al., 2010), leading to the hypothesis that activation of repressors of CREB and AP1 transcription factors at high GnRH pulse frequencies attenuates Fshb expression. The inducible cAMP early repressor (ICER) was identified as a potential repressor of Fshb expression. ICER expression and synthesis are preferentially stimulated at high GnRH pulse frequencies, and ICER attenuates GnRH stimulation of Fshb expression by antagonizing the binding of CREB to the Fshb CRE site (Ciccone et al., 2010) (Figure 2). Our group demonstrated that induction of ICER by pulsatile GnRH is regulated by ERK1/2 (Thompson et al., 2016). Inhibition of ICER by inhibition of ERK1/2 activation did not augment the induction of Fshb expression at high GnRH pulse frequencies, however, likely because MEK1/2 inhibition attenuated cFos and cJun induction as well (Thompson et al., 2016). This observation suggests that stimulation of Fshb expression by pulsatile GnRH is in part driven by activation of AP1 transcription factors, which bind to the corresponding CRE/AP1 element (Coss et al., 2004). Interestingly, a study of cFos deficient mice showed markedly reduced Fshb, Lhb, Cga and Gnrhr mRNA levels, which did not recover after GnRH treatment (Xie et al., 2015). Thus, it is not clear if the reduction of Fshb expression is due to a direct effect of cFos on the Fshb promoter or due to a decrease in GnRHR levels. More recently, cJun dimerization protein 2 (Jdp2) has been identified as a novel repressor of GnRH-mediated Fshb induction. Studies in LβT2 cells and in Jdp2 null mice demonstrated that Jdp2 serves as negative regulator, directly by binding to the Fshb promoter to form a complex with cJun, thereby preventing cJun-cFos dimerization, and indirectly by reducing cJun expression (Jonak, Lainez, Roybal et al., 2017).
As mentioned, AP1 proteins are induced by GnRH. Mistry et al. demonstrated that cFos and cJun protein expression is increased at both high and low GnRH pulse frequencies, but to a greater extent at high GnRH pulse frequencies (Mistry, Tsutsumi, Fernandez et al., 2011). This is not consistent with the preferential Fshb expression at low GnRH pulse frequencies. However, other negative effectors of Fshb expression, SKIL and TGIF1, are also induced at high GnRH pulse frequencies. These repressors have been shown to bind to the Fshb gene promoter to inhibit any potential action of cFos and cJun (Mistry et al., 2011).
The signaling pathways activated to induce CREB, ICER, and AP1 proteins to decode GnRH pulse frequency and regulate FSH synthesis are not yet fully elucidated. In addition, it needs to be taken into consideration that many other factors contribute to the regulation of Fshb expression, such as activin, follistatin, steroids, VGF-derived peptide NERP1 and epigenetic modifications (Choi, Wang, Jia et al., 2016).
6. Conclusions
Normal reproductive function and fertility require the precise regulation of LH and FSH. Identifying the molecular mechanisms that regulate gonadotropin synthesis may help us to better understand the ovulatory and menstrual cycles, puberty, and even the pathophysiology of reproductive disorders such as polycystic ovarian syndrome. Several signaling pathways have been implicated in both LH and FSH synthesis. It appears that the GnRHR differentially activates multiple distinct signaling pathways in response to varying GnRH pulse frequencies. Considerable progress has been made during the past decade, but much more remains to be elucidated to fully understand the enigma of the GnRH pulse frequency decoder. More in vivo studies are necessary to confirm and refine results obtained in immortalized gonadotropic cell lines. In vivo studies using mice with gonadotrope-specific deletion of Gαs or Gαq/11 will help to further elucidate the role of each G protein in gonadotropin regulation. Furthermore, additional studies of the GnRHR are needed to understand the differential G protein coupling of the GnRHR upon pulsatile GnRH stimulation. In addition, other factors regulating gonadotropins, including inhibin, activin, follistatin, sex steroids, and other neuropeptides, as well as epigenetic contributions to regulation, need to be integrated into this model in order to fully understand the regulation of gonadotropins in health and disease.
Highlights.
Experimental Models for Studying Gonadotrope Function
The GnRH Receptor (Structural and Functional Aspects)
Signaling Pathways Activated by Pulsatile GnRH
Regulation of Gonadotropin Gene Expression by Pulsatile GnRH
Acknowledgments
We thank Dr. Rona Carroll for her contribution to the preparation of the manuscript and Dr. Iosif Pediaditakis for his contribution to the preparation of the figures. This work was supported by NIH R01 HD019938 to U.B.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Marshall JC, Kelch RP. Gonadotropin-releasing hormone: role of pulsatile secretion in the regulation of reproduction. N Engl J Med. 1986;315:1459–68. doi: 10.1056/NEJM198612043152306. [DOI] [PubMed] [Google Scholar]
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202:631–3. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Conn PM, Chin WW. Studies of gonadotropin-releasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocr Rev. 1997;18:46–70. doi: 10.1210/edrv.18.1.0289. [DOI] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol. 2004;33:559–84. doi: 10.1677/jme.1.01600. [DOI] [PubMed] [Google Scholar]
- Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. doi: 10.1016/b978-0-12-571136-4.50008-5. [DOI] [PubMed] [Google Scholar]
- Savoy-Moore RT, Swartz KH. Several GnRH stimulation frequencies differentially release FSH and LH from isolated, perfused rat anterior pituitary cells. Adv Exp Med Biol. 1987;219:641–5. doi: 10.1007/978-1-4684-5395-9_35. [DOI] [PubMed] [Google Scholar]
- Wildt L, Hausler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109:376–85. doi: 10.1210/endo-109-2-376. [DOI] [PubMed] [Google Scholar]
- Ciccone NA, Kaiser UB. The biology of gonadotroph regulation. Curr Opin Endocrinol Diabetes Obes. 2009;16:321–7. doi: 10.1097/MED.0b013e32832d88fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharib SD, Wierman ME, Shupnik MA, Chin WW. Molecular biology of the pituitary gonadotropins. Endocr Rev. 1990;11:177–99. doi: 10.1210/edrv-11-1-177. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Dalkin AC, Ortolano GA, Marshall JC, Shupnik MA. A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology. 1991;128:509–17. doi: 10.1210/endo-128-1-509. [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology. 1997;138:1224–31. doi: 10.1210/endo.138.3.4968. [DOI] [PubMed] [Google Scholar]
- Dalkin AC, Haisenleder DJ, Ortolano GA, Ellis TR, Marshall JC. The frequency of gonadotropin-releasing-hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinology. 1989;125:917–24. doi: 10.1210/endo-125-2-917. [DOI] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Aylor KW, Marshall JC. Regulation of intracellular signaling cascades by GNRH pulse frequency in the rat pituitary: roles for CaMK II, ERK, and JNK activation. Biol Reprod. 2008;79:947–53. doi: 10.1095/biolreprod.108.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haisenleder DJ, Burger LL, Walsh HE, Stevens J, Aylor KW, Shupnik MA, Marshall JC. Pulsatile gonadotropin-releasing hormone stimulation of gonadotropin subunit transcription in rat pituitaries: evidence for the involvement of Jun N-terminal kinase but not p38. Endocrinology. 2008;149:139–45. doi: 10.1210/en.2007-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J, Duca KA, Crowley WF., Jr Gonadotropin-releasing hormone-induced stimulation and desensitization of free alpha-subunit secretion mirrors luteinizing hormone and follicle-stimulating hormone in perifused rat pituitary cells. Endocrinology. 1990;127:2364–71. doi: 10.1210/endo-127-5-2364. [DOI] [PubMed] [Google Scholar]
- Ferris HA, Shupnik MA. Mechanisms for pulsatile regulation of the gonadotropin subunit genes by GNRH1. Biol Reprod. 2006;74:993–8. doi: 10.1095/biolreprod.105.049049. [DOI] [PubMed] [Google Scholar]
- Bedecarrats GY, Kaiser UB. Differential regulation of gonadotropin subunit gene promoter activity by pulsatile gonadotropin-releasing hormone (GnRH) in perifused L beta T2 cells: role of GnRH receptor concentration. Endocrinology. 2003;144:1802–11. doi: 10.1210/en.2002-221140. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Hayes FJ, Crowley WF., Jr Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann's syndrome): pathophysiological and genetic considerations. Endocr Rev. 1998;19:521–39. doi: 10.1210/edrv.19.5.0344. [DOI] [PubMed] [Google Scholar]
- McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med. 2016;375:54–64. doi: 10.1056/NEJMcp1514916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr Rev. 2015;36:487–525. doi: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldani DP, Skrgatic L, Ougouag R. Polycystic Ovary Syndrome: Important Underrecognised Cardiometabolic Risk Factor in Reproductive-Age Women. Int J Endocrinol. 2015;2015:786362. doi: 10.1155/2015/786362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95:2038–49. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- Blank SK, McCartney CR, Helm KD, Marshall JC. Neuroendocrine effects of androgens in adult polycystic ovary syndrome and female puberty. Semin Reprod Med. 2007;25:352–9. doi: 10.1055/s-2007-984741. [DOI] [PubMed] [Google Scholar]
- Reame NE, Sauder SE, Case GD, Kelch RP, Marshall JC. Pulsatile gonadotropin secretion in women with hypothalamic amenorrhea: evidence that reduced frequency of gonadotropin-releasing hormone secretion is the mechanism of persistent anovulation. J Clin Endocrinol Metab. 1985;61:851–8. doi: 10.1210/jcem-61-5-851. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Eagleson CA, McCartney CR. Hypothalamic dysfunction. Mol Cell Endocrinol. 2001;183:29–32. doi: 10.1016/s0303-7207(01)00611-6. [DOI] [PubMed] [Google Scholar]
- Thompson IR, Kaiser UB. GnRH pulse frequency-dependent differential regulation of LH and FSH gene expression. Mol Cell Endocrinol. 2014;385:28–35. doi: 10.1016/j.mce.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugami S, Kravchook S, Rahamim-Ben Navi L, Seger R, Naor Z. Differential roles of PKC isoforms (PKCs) and Ca2+ in GnRH and phorbol 12-myristate 13-acetate (PMA) stimulation of p38MAPK phosphorylation in immortalized gonadotrope cells. Mol Cell Endocrinol. 2017;439:141–154. doi: 10.1016/j.mce.2016.10.031. [DOI] [PubMed] [Google Scholar]
- Thackray VG, Mellon PL, Coss D. Hormones in synergy: regulation of the pituitary gonadotropin genes. Mol Cell Endocrinol. 2010;314:192–203. doi: 10.1016/j.mce.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor Z, Huhtaniemi I. Interactions of the GnRH receptor with heterotrimeric G proteins. Front Neuroendocrinol. 2013;34:88–94. doi: 10.1016/j.yfrne.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Coss D. Regulation of reproduction via tight control of gonadotropin hormone levels. Mol Cell Endocrinol. 2017 doi: 10.1016/j.mce.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojilkovic SS, Bjelobaba I, Zemkova H. Ion Channels of Pituitary Gonadotrophs and Their Roles in Signaling and Secretion. Front Endocrinol (Lausanne) 2017;8:126. doi: 10.3389/fendo.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi GT, Tawadros N, Escalona RM. Pituitary cell lines and their endocrine applications. Mol Cell Endocrinol. 2004;228:1–21. doi: 10.1016/j.mce.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Bliss SP, Navratil AM, Xie J, Roberson MS. GnRH signaling, the gonadotrope and endocrine control of fertility. Front Neuroendocrinol. 2010;31:322–40. doi: 10.1016/j.yfrne.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denef C. Paracrinicity: the story of 30 years of cellular pituitary crosstalk. J Neuroendocrinol. 2008;20:1–70. doi: 10.1111/j.1365-2826.2007.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami S, Fujii Y, Okada Y, Winters SJ. Paracrine regulation of FSH by follistatin in folliculostellate cell-enriched primate pituitary cell cultures. Endocrinology. 2002;143:2250–8. doi: 10.1210/endo.143.6.8857. [DOI] [PubMed] [Google Scholar]
- Winters SJ, Moore JP. Paracrine control of gonadotrophs. Semin Reprod Med. 2007;25:379–87. doi: 10.1055/s-2007-984744. [DOI] [PubMed] [Google Scholar]
- Fallest PC, Schwartz NB. Acute inhibitory effects of 17 beta-estradiol are observed on gonadotropin secretion from perifused pituitary fragments of metestrous, but not proestrous, rats. Endocrinology. 1991;128:273–9. doi: 10.1210/endo-128-1-273. [DOI] [PubMed] [Google Scholar]
- Wu JC, Su P, Safwat NW, Sebastian J, Miller WL. Rapid, efficient isolation of murine gonadotropes and their use in revealing control of follicle-stimulating hormone by paracrine pituitary factors. Endocrinology. 2004;145:5832–9. doi: 10.1210/en.2004-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S, Schwarz JR, Niculescu D, Dinu C, Bauer CK, Hirdes W, Boehm U. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149:2701–11. doi: 10.1210/en.2007-1502. [DOI] [PubMed] [Google Scholar]
- Kumar TR. Mouse Models for the Study of Synthesis, Secretion, and Action of Pituitary Gonadotropins. Prog Mol Biol Transl Sci. 2016;143:49–84. doi: 10.1016/bs.pmbts.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Xie C, Jonak CR, Kauffman AS, Coss D. Gonadotropin and kisspeptin gene expression, but not GnRH, are impaired in cFOS deficient mice. Mol Cell Endocrinol. 2015;411:223–31. doi: 10.1016/j.mce.2015.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, Parker KL. Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development. 2001;128:147–54. doi: 10.1242/dev.128.2.147. [DOI] [PubMed] [Google Scholar]
- Zhao L, Bakke M, Parker KL. Pituitary-specific knockout of steroidogenic factor 1. Mol Cell Endocrinol. 2001;185:27–32. doi: 10.1016/s0303-7207(01)00621-9. [DOI] [PubMed] [Google Scholar]
- Tran S, Zhou X, Lafleur C, Calderon MJ, Ellsworth BS, Kimmins S, Boehm U, Treier M, Boerboom D, Bernard DJ. Impaired fertility and FSH synthesis in gonadotrope-specific Foxl2 knockout mice. Mol Endocrinol. 2013;27:407–21. doi: 10.1210/me.2012-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss SP, Miller A, Navratil AM, Xie J, McDonough SP, Fisher PJ, Landreth GE, Roberson MS. ERK signaling in the pituitary is required for female but not male fertility. Mol Endocrinol. 2009;23:1092–101. doi: 10.1210/me.2009-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122:3319–29. doi: 10.1242/dev.122.10.3319. [DOI] [PubMed] [Google Scholar]
- Alarid ET, Holley S, Hayakawa M, Mellon PL. Discrete stages of anterior pituitary differentiation recapitulated in immortalized cell lines. Mol Cell Endocrinol. 1998;140:25–30. doi: 10.1016/s0303-7207(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Windle JJ, Weiner RI, Mellon PL. Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol. 1990;4:597–603. doi: 10.1210/mend-4-4-597. [DOI] [PubMed] [Google Scholar]
- Graham KE, Nusser KD, Low MJ. LbetaT2 gonadotroph cells secrete follicle stimulating hormone (FSH) in response to active A. J Endocrinol. 1999;162:R1–5. doi: 10.1677/joe.0.162r001. [DOI] [PubMed] [Google Scholar]
- Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang HJ, Miller WL, Mellon PL. Cell-specific transcriptional regulation of follicle-stimulating hormone-beta by activin and gonadotropin-releasing hormone in the LbetaT2 pituitary gonadotrope cell model. Endocrinology. 2001;142:2284–95. doi: 10.1210/endo.142.6.8185. [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Kimura Y, Waring DW, Mellon PL. Steroid and pulsatile gonadotropin-releasing hormone (GnRH) regulation of luteinizing hormone and GnRH receptor in a novel gonadotrope cell line. Mol Endocrinol. 1996;10:439–50. doi: 10.1210/mend.10.4.8721988. [DOI] [PubMed] [Google Scholar]
- Thackray VG, McGillivray SM, Mellon PL. Androgens, progestins, and glucocorticoids induce follicle-stimulating hormone beta-subunit gene expression at the level of the gonadotrope. Mol Endocrinol. 2006;20:2062–79. doi: 10.1210/me.2005-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Otsuka F, Otani H, Inagaki K, Miyoshi T, Suzuki J, Mimura Y, Ogura T, Makino H. Effects of peroxisome proliferator-activated receptor activation on gonadotropin transcription and cell mitosis induced by bone morphogenetic proteins in mouse gonadotrope LbetaT2 cells. J Endocrinol. 2007;194:87–99. doi: 10.1677/JOE-07-0138. [DOI] [PubMed] [Google Scholar]
- Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, Vale W. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404:411–4. doi: 10.1038/35006129. [DOI] [PubMed] [Google Scholar]
- Yuen T, Choi SG, Pincas H, Waring DW, Sealfon SC, Turgeon JL. Optimized amplification and single-cell analysis identify GnRH-mediated activation of Rap1b in primary rat gonadotropes. Mol Cell Endocrinol. 2012;350:10–9. doi: 10.1016/j.mce.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojilkovic SS, Reinhart J, Catt KJ. Gonadotropin-releasing hormone receptors: structure and signal transduction pathways. Endocr Rev. 1994;15:462–99. doi: 10.1210/edrv-15-4-462. [DOI] [PubMed] [Google Scholar]
- Re M, Pampillo M, Savard M, Dubuc C, McArdle CA, Millar RP, Conn PM, Gobeil F, Jr, Bhattacharya M, Babwah AV. The human gonadotropin releasing hormone type I receptor is a functional intracellular GPCR expressed on the nuclear membrane. PLoS One. 2010;5:e11489. doi: 10.1371/journal.pone.0011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Tesmer JJ. Recognition in the face of diversity: interactions of heterotrimeric G proteins and G protein-coupled receptor (GPCR) kinases with activated GPCRs. J Biol Chem. 2011;286:7715–21. doi: 10.1074/jbc.R109.051847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman JL, Mpamhanga CP, Spedding M, Germain P, Staels B, Dacquet C, Laudet V, Harmar AJ, Nc I. IUPHAR-DB: new receptors and tools for easy searching and visualization of pharmacological data. Nucleic Acids Res. 2011;39:D534–8. doi: 10.1093/nar/gkq1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M, Zhou W, Millar RP, Mellon PL, Roberts JL, Flanagan CA, Dong K, Gillo B, Sealfon SC. Cloning and functional expression of a mouse gonadotropin-releasing hormone receptor. Mol Endocrinol. 1992;6:1163–9. doi: 10.1210/mend.6.7.1324422. [DOI] [PubMed] [Google Scholar]
- Millar RP. GnRHs and GnRH receptors. Anim Reprod Sci. 2005;88:5–28. doi: 10.1016/j.anireprosci.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Neill JD, Duck LW, Sellers JC, Musgrove LC. A gonadotropin-releasing hormone (GnRH) receptor specific for GnRH II in primates. Biochem Biophys Res Commun. 2001;282:1012–8. doi: 10.1006/bbrc.2001.4678. [DOI] [PubMed] [Google Scholar]
- Morgan K, Millar RP. Evolution of GnRH ligand precursors and GnRH receptors in protochordate and vertebrate species. Gen Comp Endocrinol. 2004;139:191–7. doi: 10.1016/j.ygcen.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25:235–75. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- Millar RP. GnRH II and type II GnRH receptors. Trends Endocrinol Metab. 2003;14:35–43. doi: 10.1016/s1043-2760(02)00016-4. [DOI] [PubMed] [Google Scholar]
- Hapgood JP, Sadie H, van Biljon W, Ronacher K. Regulation of expression of mammalian gonadotrophin-releasing hormone receptor genes. J Neuroendocrinol. 2005;17:619–38. doi: 10.1111/j.1365-2826.2005.01353.x. [DOI] [PubMed] [Google Scholar]
- Cheung LW, Wong AS. Gonadotropin-releasing hormone: GnRH receptor signaling in extrapituitary tissues. FEBS J. 2008;275:5479–95. doi: 10.1111/j.1742-4658.2008.06677.x. [DOI] [PubMed] [Google Scholar]
- Perrett RM, McArdle CA. Molecular mechanisms of gonadotropin-releasing hormone signaling: integrating cyclic nucleotides into the network. Front Endocrinol (Lausanne) 2013;4:180. doi: 10.3389/fendo.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch AR, Green L, Hislop JN, Kelly E, McArdle CA. Signaling and antiproliferative effects of type I and II gonadotropin-releasing hormone receptors in breast cancer cells. J Clin Endocrinol Metab. 2004;89:1823–32. doi: 10.1210/jc.2003-030787. [DOI] [PubMed] [Google Scholar]
- Davidson JS, Wakefield IK, Millar RP. Absence of rapid desensitization of the mouse gonadotropin-releasing hormone receptor. Biochem J. 1994;300(Pt 2):299–302. doi: 10.1042/bj3000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Bockaert J, Marin P, Dumuis A, Fagni L. The ‘magic tail’ of G protein-coupled receptors: an anchorage for functional protein networks. FEBS Lett. 2003;546:65–72. doi: 10.1016/s0014-5793(03)00453-8. [DOI] [PubMed] [Google Scholar]
- Vrecl M, Heding A, Hanyaloglu A, Taylor PL, Eidne KA. Internalization kinetics of the gonadotropin-releasing hormone (GnRH) receptor. Pflugers Arch. 2000;439:R19–20. [PubMed] [Google Scholar]
- Finch AR, Caunt CJ, Armstrong SP, McArdle CA. Agonist-induced internalization and downregulation of gonadotropin-releasing hormone receptors. Am J Physiol Cell Physiol. 2009;297:C591–600. doi: 10.1152/ajpcell.00166.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Faccenda E, Maudsley S, Lu ZL, Naor Z, Millar RP. Mammalian type I gonadotropin-releasing hormone receptors undergo slow, constitutive, agonist-independent internalization. Endocrinology. 2008;149:1415–22. doi: 10.1210/en.2007-1159. [DOI] [PubMed] [Google Scholar]
- Heding A, Vrecl M, Bogerd J, McGregor A, Sellar R, Taylor PL, Eidne KA. Gonadotropin-releasing hormone receptors with intracellular carboxyl-terminal tails undergo acute desensitization of total inositol phosphate production and exhibit accelerated internalization kinetics. J Biol Chem. 1998;273:11472–7. doi: 10.1074/jbc.273.19.11472. [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, Vrecl M, Kroeger KM, Miles LE, Qian H, Thomas WG, Eidne KA. Casein kinase II sites in the intracellular C-terminal domain of the thyrotropin-releasing hormone receptor and chimeric gonadotropin-releasing hormone receptors contribute to beta-arrestin-dependent internalization. J Biol Chem. 2001;276:18066–74. doi: 10.1074/jbc.M009275200. [DOI] [PubMed] [Google Scholar]
- McArdle CA, Davidson JS, Willars GB. The tail of the gonadotrophin-releasing hormone receptor: desensitization at, and distal to, G protein-coupled receptors. Mol Cell Endocrinol. 1999;151:129–36. doi: 10.1016/s0303-7207(99)00024-6. [DOI] [PubMed] [Google Scholar]
- Millar RP, Pawson AJ, Morgan K, Rissman EF, Lu ZL. Diversity of actions of GnRHs mediated by ligand-induced selective signaling. Front Neuroendocrinol. 2008;29:17–35. doi: 10.1016/j.yfrne.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani PS, Kang SK, Cheng KW, Choi KC, Leung PC. Regulation of gonadotropin-releasing hormone and its receptor gene expression by 17beta-estradiol in cultured human granulosa-luteal cells. Endocrinology. 2000;141:1754–63. doi: 10.1210/endo.141.5.7447. [DOI] [PubMed] [Google Scholar]
- Gregory SJ, Kaiser UB. Regulation of gonadotropins by inhibin and activin. Semin Reprod Med. 2004;22:253–67. doi: 10.1055/s-2004-831901. [DOI] [PubMed] [Google Scholar]
- Bjelobaba I, Janjic MM, Tavcar JS, Kucka M, Tomic M, Stojilkovic SS. The relationship between basal and regulated Gnrhr expression in rodent pituitary gonadotrophs. Mol Cell Endocrinol. 2016;437:302–311. doi: 10.1016/j.mce.2016.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser UB, Sabbagh E, Katzenellenbogen RA, Conn PM, Chin WW. A mechanism for the differential regulation of gonadotropin subunit gene expression by gonadotropin-releasing hormone. Proc Natl Acad Sci U S A. 1995;92:12280–4. doi: 10.1073/pnas.92.26.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NA. Dissociation of heterotrimeric g proteins in cells. Sci Signal. 2008;1:re5. doi: 10.1126/scisignal.125re5. [DOI] [PubMed] [Google Scholar]
- Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–8. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Naor Z, Azrad A, Limor R, Zakut H, Lotan M. Gonadotropin-releasing hormone activates a rapid Ca2+-independent phosphodiester hydrolysis of polyphosphoinositides in pituitary gonadotrophs. J Biol Chem. 1986;261:12506–12. [PubMed] [Google Scholar]
- Hsieh KP, Martin TF. Thyrotropin-releasing hormone and gonadotropin-releasing hormone receptors activate phospholipase C by coupling to the guanosine triphosphate-binding proteins Gq and G11. Mol Endocrinol. 1992;6:1673–81. doi: 10.1210/mend.6.10.1333052. [DOI] [PubMed] [Google Scholar]
- Grosse R, Schmid A, Schoneberg T, Herrlich A, Muhn P, Schultz G, Gudermann T. Gonadotropin-releasing hormone receptor initiates multiple signaling pathways by exclusively coupling to G(q/11) proteins. J Biol Chem. 2000;275:9193–200. doi: 10.1074/jbc.275.13.9193. [DOI] [PubMed] [Google Scholar]
- Liu F, Usui I, Evans LG, Austin DA, Mellon PL, Olefsky JM, Webster NJ. Involvement of both G(q/11) and G(s) proteins in gonadotropin-releasing hormone receptor-mediated signaling in L beta T2 cells. J Biol Chem. 2002;277:32099–108. doi: 10.1074/jbc.M203639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi R, Mistry D, Webster NJ. Signaling responses to pulsatile gonadotropin-releasing hormone in LbetaT2 gonadotrope cells. J Biol Chem. 2010;285:20262–72. doi: 10.1074/jbc.M110.132662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SG, Jia J, Pfeffer RL, Sealfon SC. G proteins and autocrine signaling differentially regulate gonadotropin subunit expression in pituitary gonadotrope. J Biol Chem. 2012;287:21550–60. doi: 10.1074/jbc.M112.348607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krsmanovic LZ, Mores N, Navarro CE, Arora KK, Catt KJ. An agonist-induced switch in G protein coupling of the gonadotropin-releasing hormone receptor regulates pulsatile neuropeptide secretion. Proc Natl Acad Sci U S A. 2003;100:2969–74. doi: 10.1073/pnas.0535708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol. 2009;30:10–29. doi: 10.1016/j.yfrne.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J. 2009;56:729–37. doi: 10.1507/endocrj.k09e-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto A, Mikawa K, Hashimoto K, Yasuda I, Tanaka S, Tominaga M, Kuroda T, Nishizuka Y. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain) J Biol Chem. 1989;264:4088–92. [PubMed] [Google Scholar]
- Caunt CJ, Finch AR, Sedgley KR, McArdle CA. Seven-transmembrane receptor signalling and ERK compartmentalization. Trends Endocrinol Metab. 2006;17:276–83. doi: 10.1016/j.tem.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci. 2006;31:268–75. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Kanasaki H, Purwana I, Oride A, Mijiddorj T, Miyazaki K. Extracellular Signal-Regulated Kinase (ERK) Activation and Mitogen-Activated Protein Kinase Phosphatase 1 Induction by Pulsatile Gonadotropin-Releasing Hormone in Pituitary Gonadotrophs. J Signal Transduct. 2012;2012:198527. doi: 10.1155/2012/198527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando H, Hew CL, Urano A. Signal transduction pathways and transcription factors involved in the gonadotropin-releasing hormone-stimulated gonadotropin subunit gene expression. Comp Biochem Physiol B Biochem Mol Biol. 2001;129:525–32. doi: 10.1016/s1096-4959(01)00356-6. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Fortin J, Wang Y, Lamba P. Mechanisms of FSH synthesis: what we know, what we don't, and why you should care. Fertil Steril. 2010;93:2465–85. doi: 10.1016/j.fertnstert.2010.03.034. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Bliss SP, Navratil AM, Xie J, Miller A, Baccarini M, Roberson MS. ERK signaling, but not c-Raf, is required for gonadotropin-releasing hormone (GnRH)-induced regulation of Nur77 in pituitary gonadotropes. Endocrinology. 2012;153:700–11. doi: 10.1210/en.2011-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D, Bonfil D, D CH, Kraus S, Seger R, Naor Z. Activation of MAPK Cascades by GnRH: ERK and Jun N-Terminal Kinase Are Involved in Basal and GnRH-Stimulated Activity of the Glycoprotein Hormone LHbeta-Subunit Promoter. Endocrinology. 2002;143:1018–1025. doi: 10.1210/endo.143.3.8675. [DOI] [PubMed] [Google Scholar]
- Kanasaki H, Bedecarrats GY, Kam KY, Xu S, Kaiser UB. Gonadotropin-releasing hormone pulse frequency-dependent activation of extracellular signal-regulated kinase pathways in perifused LbetaT2 cells. Endocrinology. 2005;146:5503–13. doi: 10.1210/en.2004-1317. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Cox ME, Parsons SJ, Marshall JC. Gonadotropin-releasing hormone pulses are required to maintain activation of mitogen-activated protein kinase: role in stimulation of gonadotrope gene expression. Endocrinology. 1998;139:3104–11. doi: 10.1210/endo.139.7.6091. [DOI] [PubMed] [Google Scholar]
- Dorn C, Ou Q, Svaren J, Crawford PA, Sadovsky Y. Activation of luteinizing hormone beta gene by gonadotropin-releasing hormone requires the synergy of early growth response-1 and steroidogenic factor-1. J Biol Chem. 1999;274:13870–6. doi: 10.1074/jbc.274.20.13870. [DOI] [PubMed] [Google Scholar]
- Fortin J, Lamba P, Wang Y, Bernard DJ. Conservation of mechanisms mediating gonadotrophin-releasing hormone 1 stimulation of human luteinizing hormone beta subunit transcription. Mol Hum Reprod. 2009;15:77–87. doi: 10.1093/molehr/gan079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson MA, Tsutsumi R, Zhang H, Talukdar I, Butler BK, Santos SJ, Mellon PL, Webster NJ. Pulse sensitivity of the luteinizing hormone beta promoter is determined by a negative feedback loop Involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol. 2007;21:1175–91. doi: 10.1210/me.2006-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G, Milbrandt J. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1) Science. 1996;273:1219–21. doi: 10.1126/science.273.5279.1219. [DOI] [PubMed] [Google Scholar]
- Wolfe MW, Call GB. Early growth response protein 1 binds to the luteinizing hormone-beta promoter and mediates gonadotropin-releasing hormone-stimulated gene expression. Mol Endocrinol. 1999;13:752–63. doi: 10.1210/mend.13.5.0276. [DOI] [PubMed] [Google Scholar]
- Halvorson LM, Kaiser UB, Chin WW. The protein kinase C system acts through the early growth response protein 1 to increase LHbeta gene expression in synergy with steroidogenic factor-1. Mol Endocrinol. 1999;13:106–16. doi: 10.1210/mend.13.1.0216. [DOI] [PubMed] [Google Scholar]
- Bonfil D, Chuderland D, Kraus S, Shahbazian D, Friedberg I, Seger R, Naor Z. Extracellular signal-regulated kinase, Jun N-terminal kinase, p38, and c-Src are involved in gonadotropin-releasing hormone-stimulated activity of the glycoprotein hormone follicle-stimulating hormone beta-subunit promoter. Endocrinology. 2004;145:2228–44. doi: 10.1210/en.2003-1418. [DOI] [PubMed] [Google Scholar]
- Coss D, Hand CM, Yaphockun KK, Ely HA, Mellon PL. p38 mitogen-activated protein kinase is critical for synergistic induction of the FSH(beta) gene by gonadotropin-releasing hormone and activin through augmentation of c-Fos induction and Smad phosphorylation. Mol Endocrinol. 2007;21:3071–86. doi: 10.1210/me.2007-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss D, Jacobs SB, Bender CE, Mellon PL. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone beta gene by gonadotropin-releasing hormone. J Biol Chem. 2004;279:152–62. doi: 10.1074/jbc.M304697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson IR, Ciccone NA, Zhou Q, Xu S, Khogeer A, Carroll RS, Kaiser UB. GnRH Pulse Frequency Control of Fshb Gene Expression Is Mediated via ERK1/2 Regulation of ICER. Mol Endocrinol. 2016;30:348–60. doi: 10.1210/me.2015-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Mulvaney JM, Roberson MS. Activation of mitogen-activated protein kinase phosphatase 2 by gonadotropin-releasing hormone. Mol Cell Endocrinol. 2001;172:79–89. doi: 10.1016/s0303-7207(00)00378-6. [DOI] [PubMed] [Google Scholar]
- Kondoh K, Nishida E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim Biophys Acta. 2007;1773:1227–37. doi: 10.1016/j.bbamcr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Franklin CC, Kraft AS. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem. 1997;272:16917–23. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- Zhang T, Roberson MS. Role of MAP kinase phosphatases in GnRH-dependent activation of MAP kinases. J Mol Endocrinol. 2006;36:41–50. doi: 10.1677/jme.1.01881. [DOI] [PubMed] [Google Scholar]
- Nguyen KA, Intriago RE, Upadhyay HC, Santos SJ, Webster NJ, Lawson MA. Modulation of gonadotropin-releasing hormone-induced extracellular signal-regulated kinase activation by dual-specificity protein phosphatase 1 in LbetaT2 gonadotropes. Endocrinology. 2010;151:4882–93. doi: 10.1210/en.2009-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwana IN, Kanasaki H, Mijiddorj T, Oride A, Miyazaki K. Induction of dual-specificity phosphatase 1 (DUSP1) by pulsatile gonadotropin-releasing hormone stimulation: role for gonadotropin subunit expression in mouse pituitary LbetaT2 cells. Biol Reprod. 2011;84:996–1004. doi: 10.1095/biolreprod.110.088526. [DOI] [PubMed] [Google Scholar]
- Armstrong SP, Caunt CJ, Fowkes RC, Tsaneva-Atanasova K, McArdle CA. Pulsatile and sustained gonadotropin-releasing hormone (GnRH) receptor signaling: does the ERK signaling pathway decode GnRH pulse frequency? J Biol Chem. 2010;285:24360–71. doi: 10.1074/jbc.M110.115964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Pasten ML, Fiordelisio T. GnRH-Induced Ca(2+) Signaling Patterns and Gonadotropin Secretion in Pituitary Gonadotrophs. Functional Adaptations to Both Ordinary and Extraordinary Physiological Demands. Front Endocrinol (Lausanne) 2013;4:127. doi: 10.3389/fendo.2013.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck J, Fallest PC, Pitt LK, Shupnik MA. Differential gonadotropin-releasing hormone stimulation of rat luteinizing hormone subunit gene transcription by calcium influx and mitogen-activated protein kinase-signaling pathways. Mol Endocrinol. 1998;12:451–7. doi: 10.1210/mend.12.3.0070. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Yasin M, Marshall JC. Gonadotropin subunit and gonadotropin-releasing hormone receptor gene expression are regulated by alterations in the frequency of calcium pulsatile signals. Endocrinology. 1997;138:5227–30. doi: 10.1210/endo.138.12.5611. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Workman LJ, Burger LL, Aylor KW, Dalkin AC, Marshall JC. Gonadotropin subunit transcriptional responses to calcium signals in the rat: evidence for regulation by pulse frequency. Biol Reprod. 2001;65:1789–93. doi: 10.1095/biolreprod65.6.1789. [DOI] [PubMed] [Google Scholar]
- Roberson MS, Bliss SP, Xie J, Navratil AM, Farmerie TA, Wolfe MW, Clay CM. Gonadotropin-releasing hormone induction of extracellular-signal regulated kinase is blocked by inhibition of calmodulin. Mol Endocrinol. 2005;19:2412–23. doi: 10.1210/me.2005-0094. [DOI] [PubMed] [Google Scholar]
- Melamed P, Savulescu D, Lim S, Wijeweera A, Luo Z, Luo M, Pnueli L. Gonadotrophin-releasing hormone signalling downstream of calmodulin. J Neuroendocrinol. 2012;24:1463–75. doi: 10.1111/j.1365-2826.2012.02359.x. [DOI] [PubMed] [Google Scholar]
- De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–30. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Burger LL, Aylor KW, Dalkin AC, Marshall JC. Gonadotropin-releasing hormone stimulation of gonadotropin subunit transcription: evidence for the involvement of calcium/calmodulin-dependent kinase II (Ca/CAMK II) activation in rat pituitaries. Endocrinology. 2003;144:2768–74. doi: 10.1210/en.2002-0168. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Ferris HA, Shupnik MA. The calcium component of gonadotropin-releasing hormone-stimulated luteinizing hormone subunit gene transcription is mediated by calcium/calmodulin-dependent protein kinase type II. Endocrinology. 2003;144:2409–16. doi: 10.1210/en.2002-0013. [DOI] [PubMed] [Google Scholar]
- Natarajan K, Ness J, Wooge CH, Janovick JA, Conn PM. Specific identification and subcellular localization of three calmodulin-binding proteins in the rat gonadotrope: spectrin, caldesmon, and calcineurin. Biol Reprod. 1991;44:43–52. doi: 10.1095/biolreprod44.1.43. [DOI] [PubMed] [Google Scholar]
- Lim S, Luo M, Koh M, Yang M, bin Abdul Kadir MN, Tan JH, Ye Z, Wang W, Melamed P. Distinct mechanisms involving diverse histone deacetylases repress expression of the two gonadotropin beta-subunit genes in immature gonadotropes, and their actions are overcome by gonadotropin-releasing hormone. Mol Cell Biol. 2007;27:4105–20. doi: 10.1128/MCB.00248-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S, Maudsley S, Millar RP, Pawson AJ. Nuclear stabilization of beta-catenin and inactivation of glycogen synthase kinase-3beta by gonadotropin-releasing hormone: targeting Wnt signaling in the pituitary gonadotrope. Mol Endocrinol. 2007;21:3028–38. doi: 10.1210/me.2007-0268. [DOI] [PubMed] [Google Scholar]
- Armstrong SP, Caunt CJ, Fowkes RC, Tsaneva-Atanasova K, McArdle CA. Pulsatile and sustained gonadotropin-releasing hormone (GnRH) receptor signaling: does the Ca2+/NFAT signaling pathway decode GnRH pulse frequency? J Biol Chem. 2009;284:35746–57. doi: 10.1074/jbc.M109.063917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnueli L, Luo M, Wang S, Naor Z, Melamed P. Calcineurin mediates the gonadotropin-releasing hormone effect on expression of both subunits of the follicle-stimulating hormone through distinct mechanisms. Mol Cell Biol. 2011;31:5023–36. doi: 10.1128/MCB.06083-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder AK, Grammer JC, Herndon MK, Stanton JD, Nilson JH. GnRH regulation of Jun and Atf3 requires calcium, calcineurin, and NFAT. Mol Endocrinol. 2012;26:873–86. doi: 10.1210/me.2012-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaneva-Atanasova K, Mina P, Caunt CJ, Armstrong SP, McArdle CA. Decoding GnRH neurohormone pulse frequency by convergent signalling modules. J R Soc Interface. 2012;9:170–82. doi: 10.1098/rsif.2011.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratap A, Garner KL, Voliotis M, Tsaneva-Atanasova K, McArdle CA. Mathematical modeling of gonadotropin-releasing hormone signaling. Mol Cell Endocrinol. 2016 doi: 10.1016/j.mce.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Tannoudji J, Avet C, Garrel G, Counis R, Simon V. Decoding high Gonadotropin-releasing hormone pulsatility: a role for GnRH receptor coupling to the cAMP pathway? Front Endocrinol (Lausanne) 2012;3:107. doi: 10.3389/fendo.2012.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avet C, Garrel G, Denoyelle C, Laverriere JN, Counis R, Cohen-Tannoudji J, Simon V. SET protein interacts with intracellular domains of the gonadotropin-releasing hormone receptor and differentially regulates receptor signaling to cAMP and calcium in gonadotrope cells. J Biol Chem. 2013;288:2641–54. doi: 10.1074/jbc.M112.388876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariviere S, Garrel G, Simon V, Soh JW, Laverriere JN, Counis R, Cohen-Tannoudji J. Gonadotropin-releasing hormone couples to 3′,5′-cyclic adenosine-5′-monophosphate pathway through novel protein kinase Cdelta and -epsilon in LbetaT2 gonadotrope cells. Endocrinology. 2007;148:1099–107. doi: 10.1210/en.2006-1473. [DOI] [PubMed] [Google Scholar]
- Garrel G, Simon V, Thieulant ML, Cayla X, Garcia A, Counis R, Cohen-Tannoudji J. Sustained gonadotropin-releasing hormone stimulation mobilizes the cAMP/PKA pathway to induce nitric oxide synthase type 1 expression in rat pituitary cells in vitro and in vivo at proestrus. Biol Reprod. 2010;82:1170–9. doi: 10.1095/biolreprod.109.082925. [DOI] [PubMed] [Google Scholar]
- Grafer CM, Thomas R, Lambrakos L, Montoya I, White S, Halvorson LM. GnRH stimulates expression of PACAP in the pituitary gonadotropes via both the PKA and PKC signaling systems. Mol Endocrinol. 2009;23:1022–32. doi: 10.1210/me.2008-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson IR, Ciccone NA, Xu S, Zaytseva S, Carroll RS, Kaiser UB. GnRH pulse frequency-dependent stimulation of FSHbeta transcription is mediated via activation of PKA and CREB. Mol Endocrinol. 2013;27:606–18. doi: 10.1210/me.2012-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorson LM, Kaiser UB, Chin WW. Stimulation of luteinizing hormone beta gene promoter activity by the orphan nuclear receptor, steroidogenic factor-1. J Biol Chem. 1996;271:6645–50. doi: 10.1074/jbc.271.12.6645. [DOI] [PubMed] [Google Scholar]
- Quirk CC, Lozada KL, Keri RA, Nilson JH. A single Pitx1 binding site is essential for activity of the LHbeta promoter in transgenic mice. Mol Endocrinol. 2001;15:734–46. doi: 10.1210/mend.15.5.0628. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Drouin J. Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone beta gene transcription. Mol Cell Biol. 1999;19:2567–76. doi: 10.1128/mcb.19.4.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser UB, Sabbagh E, Chen MT, Chin WW, Saunders BD. Sp1 binds to the rat luteinizing hormone beta (LHbeta) gene promoter and mediates gonadotropin-releasing hormone-stimulated expression of the LHbeta subunit gene. J Biol Chem. 1998;273:12943–51. doi: 10.1074/jbc.273.21.12943. [DOI] [PubMed] [Google Scholar]
- Weck J, Anderson AC, Jenkins S, Fallest PC, Shupnik MA. Divergent and composite gonadotropin-releasing hormone-responsive elements in the rat luteinizing hormone subunit genes. Mol Endocrinol. 2000;14:472–85. doi: 10.1210/mend.14.4.0453. [DOI] [PubMed] [Google Scholar]
- Mouillet JF, Sonnenberg-Hirche C, Yan X, Sadovsky Y. p300 regulates the synergy of steroidogenic factor-1 and early growth response-1 in activating luteinizing hormone-beta subunit gene. J Biol Chem. 2004;279:7832–9. doi: 10.1074/jbc.M312574200. [DOI] [PubMed] [Google Scholar]
- Curtin D, Ferris HA, Hakli M, Gibson M, Janne OA, Palvimo JJ, Shupnik MA. Small nuclear RING finger protein stimulates the rat luteinizing hormone-beta promoter by interacting with Sp1 and steroidogenic factor-1 and protects from androgen suppression. Mol Endocrinol. 2004;18:1263–76. doi: 10.1210/me.2003-0221. [DOI] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Trembleau A, Gourdji D, Driancourt MA, Rao CV, Charnay P. Multiple pituitary and ovarian defects in Krox-24 (NGFI-A, Egr-1)-targeted mice. Mol Endocrinol. 1998;12:107–22. doi: 10.1210/mend.12.1.0049. [DOI] [PubMed] [Google Scholar]
- Ciccone NA, Lacza CT, Hou MY, Gregory SJ, Kam KY, Xu S, Kaiser UB. A composite element that binds basic helix loop helix and basic leucine zipper transcription factors is important for gonadotropin-releasing hormone regulation of the follicle-stimulating hormone beta gene. Mol Endocrinol. 2008;22:1908–23. doi: 10.1210/me.2007-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fortin J, Lamba P, Bonomi M, Persani L, Roberson MS, Bernard DJ. Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone beta-promoter activity. Endocrinology. 2008;149:5577–91. doi: 10.1210/en.2008-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Austin DA, Mellon PL, Olefsky JM, Webster NJ. GnRH activates ERK1/2 leading to the induction of c-fos and LHbeta protein expression in LbetaT2 cells. Mol Endocrinol. 2002;16:419–34. doi: 10.1210/mend.16.3.0791. [DOI] [PubMed] [Google Scholar]
- Xie J, Bliss SP, Nett TM, Ebersole BJ, Sealfon SC, Roberson MS. Transcript profiling of immediate early genes reveals a unique role for activating transcription factor 3 in mediating activation of the glycoprotein hormone alpha-subunit promoter by gonadotropin-releasing hormone. Mol Endocrinol. 2005;19:2624–38. doi: 10.1210/me.2005-0056. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Huang HJ, Pedersen NR, Wu JC, Ghosh BR, Miller WL. Two proximal activating protein-1-binding sites are sufficient to stimulate transcription of the ovine follicle-stimulating hormone-beta gene. Endocrinology. 1997;138:2621–31. doi: 10.1210/endo.138.6.5205. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Huang HJ, Sebastian J, Ghosh BR, Miller WL. Transcriptional activation of the ovine follicle-stimulating hormone beta-subunit gene by gonadotropin-releasing hormone: involvement of two activating protein-1-binding sites and protein kinase C. Endocrinology. 1998;139:4455–65. doi: 10.1210/endo.139.11.6281. [DOI] [PubMed] [Google Scholar]
- Huang HJ, Sebastian J, Strahl BD, Wu JC, Miller WL. Transcriptional regulation of the ovine follicle-stimulating hormone-beta gene by activin and gonadotropin-releasing hormone (GnRH): involvement of two proximal activator protein-1 sites for GnRH stimulation. Endocrinology. 2001;142:2267–74. doi: 10.1210/endo.142.6.8203. [DOI] [PubMed] [Google Scholar]
- Ciccone NA, Xu S, Lacza CT, Carroll RS, Kaiser UB. Frequency-dependent regulation of follicle-stimulating hormone beta by pulsatile gonadotropin-releasing hormone is mediated by functional antagonism of bZIP transcription factors. Mol Cell Biol. 2010;30:1028–40. doi: 10.1128/MCB.00848-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak CR, Lainez NM, Roybal LL, Williamson AD, Coss D. c-JUN Dimerization Protein 2 (JDP2) Is a Transcriptional Repressor of Follicle-stimulating Hormone beta (FSHbeta) and Is Required for Preventing Premature Reproductive Senescence in Female Mice. J Biol Chem. 2017;292:2646–2659. doi: 10.1074/jbc.M116.771808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry DS, Tsutsumi R, Fernandez M, Sharma S, Cardenas SA, Lawson MA, Webster NJ. Gonadotropin-releasing hormone pulse sensitivity of follicle-stimulating hormone-beta gene is mediated by differential expression of positive regulatory activator protein 1 factors and corepressors SKIL and TGIF1. Mol Endocrinol. 2011;25:1387–403. doi: 10.1210/me.2011-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SG, Wang Q, Jia J, Chikina M, Pincas H, Dolios G, Sasaki K, Wang R, Minamino N, Salton SR, Sealfon SC. Characterization of Gonadotrope Secretoproteome Identifies Neurosecretory Protein VGF-derived Peptide Suppression of Follicle-stimulating Hormone Gene Expression. J Biol Chem. 2016;291:21322–21334. doi: 10.1074/jbc.M116.740365. [DOI] [PMC free article] [PubMed] [Google Scholar]


