Paroxysmal nocturnal haemoglobinuria (PNH) and idiopathic aplastic anaemia (AA) share common pathogenetic aspects which eventually diverge and thus their clinical course may overlap and cross (Maciejewski et al 2001a). PNH mutant cells seem to be present in a majority of patients with AA already at presentation and a substantial proportion of these patients will evolve to a frank, haemolytic PNH (Maciejewski et al 2001b). PNH is a marker of immune disease and immune responsiveness in AA (Maciejewski et al 2001b, Young et & Maciejewski 2000).
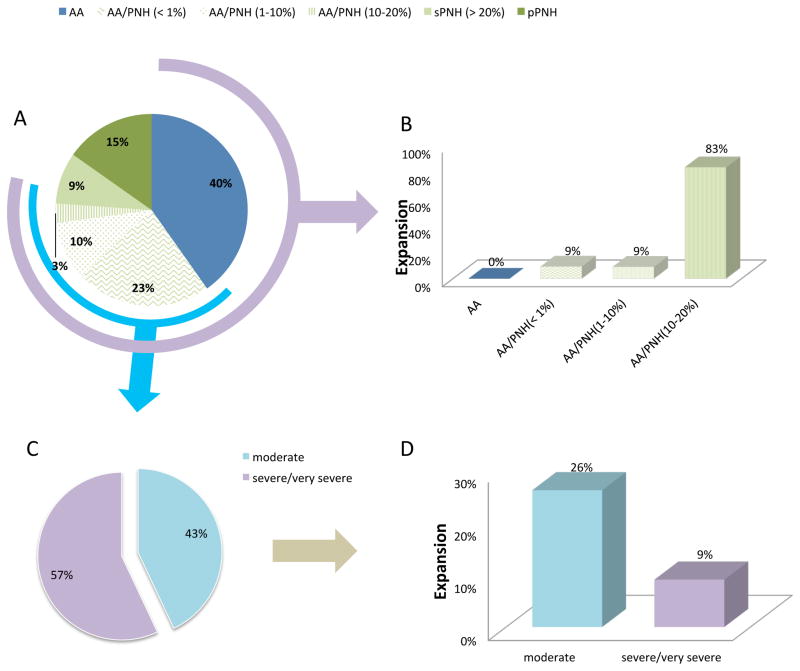
This study describes a total of 319 bone marrow failure patients evaluated at the Cleveland Clinic between July 2000 and February 2017 according to the governing protocols of the Cleveland Clinic Foundation Institutional Review Board. One hundred and seventeen patients were diagnosed with AA without a PNH clone, 132 had AA with a PNH clone (AA/PNH), 26 were post-AA/PNH (secondary PNH; sPNH) and 44 were diagnosed with primary PNH (primary PNH; pPNH) based on diagnostic criteria (Table I, Figure 1A, Table SI). AA and PNH patients divided based on their initial clone size (Figure 1A). AA/PNH patients were defined as “expanders” and “stable” according to the change in their granulocyte PNH clone size (Table SII). In total, 319 patients (median age 46 years) had one of these diagnoses and a minimum of 61 months (0–510) follow-up. We first compared 249 patients with of AA (n=117) and AA/PNH (n=132, Table SIII) with regard to their clinical characteristics. Thirty patients evolved to myelodysplastic syndrome (MDS), with the rate being significantly higher in AA vs. PNH patients (P=0.035). Glycosylphosphatidylinositol-anchored protein (GPI-AP) deficiency in granulocytes had a favourable impact on survival (Figure S1A, 1B and 1C). In AA/PNH (n=132) and PNH (sPNH+pPNH) patients (n=70; Table SI) per definition, clone size differed in AA/PNH vs. PNH (2.5% vs. 60%). PNH patients had higher absolute neutrophil counts (ANC) and platelet counts at diagnosis (P<0.0001). To understand differences in clinical presentation and complications in patients who manifest haemolytic PNH, we sub-grouped patients as sPNH (n=48) and pPNH (n=44; Table SIV). ANC and platelet counts were higher in pPNH vs. sPNH (P=0.02 and P=0.0001, respectively). Thrombotic complications were more common (20/44 vs. 9/20 P=0.05) in pPNH. No difference in eculizumab use was found (P=0.6). Clonal complications have important prognostic implications. We thus determined which biological features were predictive of clonal expansion. For this, patients were grouped as stable (n=122) or as expanders (n=22), based on arbitrary cut-off of >10% of GPI-AP-deficient granulocytes increases in ~1 year. Patients were ranked based on their initial GPI-AP-deficient granulocytes as <1 and >0.1, 1–10%, 10–20% (Figure 1A). Overall expansion of each subgroup is illustrated in Figure 1B and Figure S2. Notably, a PNH clone evolved in only 5/117 AA patients (0% of PNH granulocytes at diagnosis), with a median of 0.05% (0.003%–0.1%), but these new clones did not expand over the 43 months of follow-up (Figure 1B). Patients with <1% GPI-AP-deficient granulocytes at presentation (7/75 or 9%) showed a lower risk of expansion (Odds Ratio [OR]=0.22) than those with a higher percentage of PNH granulocytes at presentation, whereas those with 10–20% GPI-AP-deficient granulocytes at presentation (8/9 or 90%) showed a higher risk of further expansion (OR = 0.37) (Figure 1B and Figure S3A). To illustrate the dynamics of clonal evolution and determine the average increase over the follow-up period -(67 months), we compared serial measurements and demonstrated that it was 0.8% (Δ1.8–2.6%) in the stable group and 51.7% (Δ 7.2–58.5%) among expanders (P<0.001) (Figure S3B). When clonal dynamics for the type II vs type III (0.7 vs. 38.2%) clones were examined in expanders, the bulk of the expansion was accounted for by type III over type II clones (1.9% vs. 85.5%) during follow-up (Figure S3C). The presence of severe AA vs. moderate AA (OR= 0.28) or therapy with antithymocyte globulin (ATG) vs non-ATG (OR = 0.32) were associated with a lower risk of a subsequent PNH expansion (Figure 1D and Figure S3A). Most importantly, the degree of increase in the PNH granulocyte clone over time was higher in patients who initially presented with >10% vs. <1% PNH clone, in whom PNH clones were more likely to remain small. The presence of PNH clone at presentation or its expansion was not associated with subsequent progression to MDS. Preferential expansion of type III over type II clones suggests a conditional growth advantage by evading an immune attack directed against normal progenitor cells. By ranking patients according to initial PNH counts, we determined that those with >10% GPI-AP-deficient granulocytes at presentation were more likely to expand than those who presented with smaller clones, which remained relatively stable. Patients who received intense immunosuppressive therapy (with ATG) were also less likely to experience expansion while those not treated or who received ciclosporin alone were more likely to evolve. In summary, our experience indicates that the presence of a PNH clone at the initial diagnosis of AA, together with blood counts, could suggest that application of intense immunosuppressive therapy in the form of ATG treatment is warranted, not only to improve blood counts but also to reduce the risk of clonal expansion.
Table I.
Characteristics of study cohort
| Characteristic | N (%) |
|---|---|
| Total number of patients | 319 |
|
| |
| Sex, female | 165 (51%) |
|
| |
| Age at diagnosis, years | 45.7±20 |
|
| |
| Initial presentation* (n) | |
| AA | 249 |
| AA | 117/249 (47%) |
| AA with PNH clone | 132/249 (53%) |
| PNH | 70 |
| sPNH | 26/70 (37%) |
| pPNH | 44/70 (63%) |
|
| |
| Size of PNH clone | |
| AA | 0% |
| AA with PNH clone | 2.5% (0.02–18.3) |
| sPNH | 54.1% (21.1–99.6) |
| pPNH | 62.9% (2.1–99.3) |
|
| |
| Treatment | |
| Drugs | |
| ATG/ciclosporin | 145 |
| Ciclosporin alone | 28 |
| Danazol/prednisone | 14 |
| Eculizumab | 53 |
| BMT | 59 |
|
| |
| Thrombosis | 39 (12%) |
|
| |
| Transfusion dependency at presentation | 119 (37%) |
AA, aplastic anaemia; ATG, anti-thymocyte globulin; BMT, bone marrow transplantation; PNH, paroxysmal nocturnal haemoglobinuria; pPNH, primary paroxysmal nocturnal haemoglobinuria; sPNH, secondary paroxysmal nocturnal haemoglobinuria.
Figure 1. Classification of all patients based on initial diagnosis and presence of PNH clone.
A. Distribution of patients according to diagnosis and clone range, B. Expansion rate in each group including AA, AA/PNH, C. Distribution of AA/PNH patients according to severity (for definition please see text), D. Expansion rate according to severity
AA, aplastic anaemia; AA/PNH, aplastic anaemia with paroxysmal nocturnal haemoglobinuria clone; PNH, paroxysmal nocturnal haemoglobinuria; pPNH, primary paroxysmal nocturnal haemoglobinuria; sPNH, secondary paroxysmal nocturnal haemoglobinuria.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (R01HL118281, R01HL123904, and R01HL128425, to J.P. Maciejewski). TB partly supported by the Evans MDS foundation.
Footnotes
CONTRIBUTION
TB, JM designed and wrote the article. ONA, SB, AM, TR helped for design and writing, MC performed the PNH essays.
References
- Maciejewski JP, Rivera C, Kook H, Dunn D, Young NS. Relationship between bone marrow failure syndromes. and the presence of glycophosphatidyl inositol-anchored protein-deficient clones. British journal of haematology. 2001a;115:1015–1022. doi: 10.1046/j.1365-2141.2001.03191.x. [DOI] [PubMed] [Google Scholar]
- Maciejewski JP, Follmann D, Nakamura R, Saunthararajah Y, Rivera CE, Simonis T, Brown KE, Barrett JA, Young NS. Increased frequency of HLA-DR2 in patients with paroxysmal nocturnal hemoglobinuria and the PNH/aplastic anemia syndrome. Blood. 2001b;98:3513–3519. doi: 10.1182/blood.v98.13.3513. [DOI] [PubMed] [Google Scholar]
- Young NS, Maciejewski JP. Genetic and environmental effects in paroxysmal nocturnal hemoglobinuria: this little PIG-A goes “Why? Why? Why?”. The Journal of clinical investigation. 2000;106:637–641. doi: 10.1172/JCI11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.