Abstract
Background/Objective
The beneficial effects of physical activity (PA) are well known, but it remains challenging to increase PA among physically inactive and overweight young individuals. The present study aimed to examine how selected psychological and physical characteristics assessed at baseline predict the increase in total PA over a 6-month follow-up among 51 physically inactive and overweight adults (20 women, 31 men; age 26–40 years) who participated in a lifestyle counselling study without supervised PA sessions.
Methods
Baseline measurements included a questionnaire assessment of sense of coherence and psychological flexibility, heart rate monitoring-based stress/recovery from stress (stress%/recovery% during 24 hours), and body composition. PA volume was elicited through interview. Participants who increased their PA by ≥ 500 metabolic equivalent of task-minutes/week during the follow-up compared with their prebaseline PA level were regarded as able to increase PA. Logistic regression was used to analyze associations of baseline characteristics with PA increase.
Results
During the 6-month follow-up, 41% of the participants increased their total PA by ≥ 500 metabolic equivalent of task-minutes/week. The best predictors of the increase in PA were high meaningfulness subscores of the sense of coherence questionnaire (multivariate adjusted odds ratio 1.57, 95% confidence interval 1.04–2.35) and high recovery% during a day off (odds ratio 1.15, 95% confidence interval 1.02–1.30).
Conclusion
A strong sense of meaningfulness and better recovery from stress predict an increase in PA among physically inactive and overweight young adults. Therefore, participants with a low sense of meaningfulness and low recovery from stress may require support from other interventions to be able to increase their PA.
Keywords: Obesity, Physical activity, Physical inactivity, Recovery, Sense of coherence
Introduction
Physical activity (PA) has many health benefits including reducing the risks of obesity and type 2 diabetes1 and favorably impacting psychological well-being2 and stress.3 Accordingly; regular PA plays important roles in preventing chronic diseases and promoting physical and psychological well-being. However, one third of adults worldwide do not reach the current PA recommendation4 (i.e., ≥ 150 minutes of moderate intensity PA weekly).5
Physical inactivity together with increasing prevalence of being overweight6 predisposes people to increased risk of cardiometabolic diseases. Thus, healthcare workers and other professionals strive to increase people's participation in regular PA. However, this is a challenging goal. Despite exercise counselling or other strategies to increase PA (such as internet- or mobile phone/smartphone-based applications), not all physically inactive and overweight individuals manage to increase their PA. Therefore, it is important to study the factors that influence a person's likelihood of starting/increasing their PA after exercise counselling or other interventions. Such knowledge could help in selecting additional interventions (such as stress management) necessary to successfully increase PA.
Both physical and psychosocial factors are associated with successfully increasing PA within populations of physically inactive and overweight adults. Of the physical factors, older age,7 male sex,7, 8 higher9 or lower7 body mass index (BMI), and higher physical fitness8 at baseline have been associated with increased PA observed during follow-up periods within physically inactive and overweight (or predominantly overweight) study populations (with a mean age of around 40–50 years). Psychosocial factors that predict an increase of PA in these study populations include a higher stage of readiness to change PA habits,7, 9 greater motivation to increase PA, and higher self-efficacy for PA.9 Higher self-efficacy is also associated with an increase of PA in other target groups, e.g., among patients with type 2 diabetes10 and arthritis.11 However, less is known about the factors that predict an increase in PA among younger overweight and physically inactive adults.
The psychological factor of a strong sense of coherence (SOC) is also associated with a greater prevalence of PA among healthy adults12, 13 and with higher PA volume in adult type 1 diabetics.14 A 13-year follow-up study by Myers et al15 showed that a low SOC was associated with decreased engagement in PA among patients with myocardial infarction. SOC could also be associated with PA in other target groups, such as among overweight and physically inactive adults. SOC reflects psychological characteristics formed from three components: comprehensibility (one's ability to understand what happens around him/her), manageability (the extent to which one can manage a situation alone or through significant others in one's social network), and meaningfulness (ability to find meaning in the situation).16, 17, 18 SOC is also associated with other health-related factors, such as morbidity19, 20 and mortality.21, 22, 23
Another potential predictor of PA is psychological flexibility, which reflects an individual's willingness to endure negative private events (e.g., discomfort during physical activity), acceptance of these events, and ability to live according to his/her values. A person with greater psychological flexibility usually also shows higher acceptance skills and less experiential avoidance.24 It is suggested that psychological flexibility is associated with health-related benefits, while psychological inflexibility is considered to be associated with psychopathology.25 For example, high psychological flexibility has been reported to be associated with better chronic pain management26 and fewer symptoms of disordered eating.27, 28 Additionally, physically active adults show better mindfulness skills (a component of psychological flexibility) than less physically active adults.29 This suggests that high psychological flexibility might also be associated with an increase of PA.
Stress may also be an important determinant of PA. Prospective studies show that psychological stress predicts less PA/exercise or more sedentary behaviour.30 High job strain is also a risk factor for leisure time physical inactivity.31 Both positive and negative stress may cause physiological alterations in the body. Stress can influence heart rate variability (HRV), with higher effort at work associated with lower daytime HRV, and lower levels of perceived stress at work associated with higher HRV during work time.32 The association between perceived stress and reduced PA raises the question of whether objectively measured stress is also associated with PA.
The present study aimed to investigate how selected psychological (SOC and psychological flexibility) and physical (body composition, objectively measured stress, and recovery) factors measured at baseline predict an increase in PA among physically inactive and overweight healthy young adults during an intervention study without supervised PA sessions.
Methods
Study design
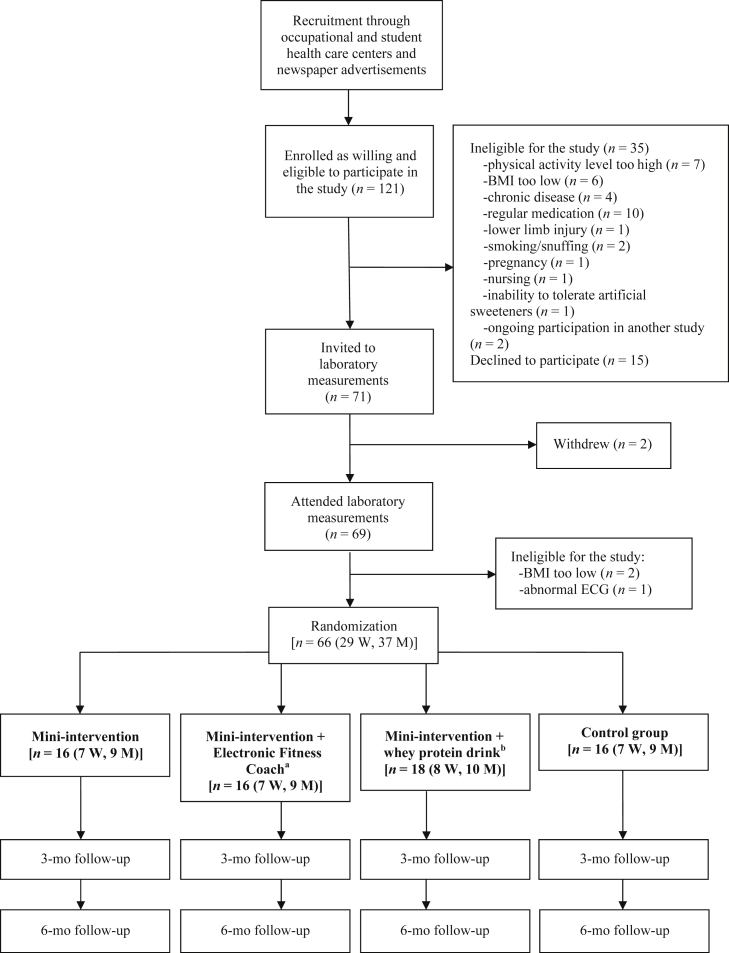
The present investigation is part of the Body and Future Health study, which aims to examine the effects of independently executed lifestyle changes (nutrition and PA) on body composition and metabolic and psychological stress (see www.controlled-trials.com/ISRCTN92130721/kujala). The complete project is a randomized controlled trial with four experimental groups (Appendix Figure 1): mini-intervention, mini-intervention + electronic fitness coach, mini-intervention + whey protein drink, and control. Briefly, the mini-intervention included simple instructions for improving nutrition (e.g., “eat regularly”, “avoid sugar”) and increasing PA (“perform relaxing and enjoyable exercise daily”). PA advice was based on PA recommendations for healthy adults: ≥ 150 minutes moderate intensity or 75 minutes vigorous intensity exercise weekly.5 Control participants were informed about the study aims but not instructed to make any specific lifestyle changes (although they were not advised to avoid lifestyle changes). The electronic fitness coach and whey protein drink are explained briefly in Appendix Figure 1.
The intervention period lasted 6 months. Laboratory and real-life measurements were performed at baseline (before starting the intervention), mid-intervention (at the 3-month follow-up), and following completion of the intervention (at the 6-month follow-up). PA during the past 3 months was ascertained via a detailed interview at baseline (prebaseline 3-month PA level) and at the 3- and 6-month follow-up visits.
Preliminary analyses revealed no between-group differences in increases in PA. Therefore, in the present substudy, we examined individuals from all four study groups together using study group information as a covariate. Increase in PA (compared to prebaseline PA level) was assessed for the whole 6-month follow-up and predictors of increases in PA were obtained from the baseline measurements.
Participants
Inclusion criteria for the study were as follows: age 25–40 years, BMI 25.0–35.0 kg/m2, waist circumference of ≥ 80 cm (women) or ≥ 94 cm (men), able to tolerate lactose-free milk products, access to the internet, and not exercising vigorously (> 20 minutes per session) more than twice a week. Exclusion criteria were: chronic disease requiring regular medication, other regular medication (except for contraceptive drugs), abnormal electrocardiogram, pregnancy or planning pregnancy during the next 12 months, < 12 months postpartum, milk allergy, eating disorder, bodybuilding, smoking/snuffing, heavy alcohol use (> 3 drinks/day), drug use, and weight change of > 5 kg during the previous 6 months.
A total of 121 individuals were initially recruited. A telephone interview found 35 to be ineligible and 15 declined to participate. Thus, 71 individuals were invited to undergo laboratory measurements. Withdrawals and additional exclusions based on laboratory measurements left 66 participants (29 women and 37 men) eligible for the study (Appendix Figure 1). Four individuals were excluded from statistical analyses due to medical problems that emerged during the 6-month follow-up (not related to intervention), and 11 individuals (16.7%) were lost to follow-up. The final number of participants analyzed was 51 (20 women and 31 men).
The study was conducted according to the ethical rules stated in the Declaration of Helsinki. All participants were informed about the study and signed a written informed consent prior to any measurements. The study plan was approved by the ethics committee of the Central Finland Health Care District.
Assessment of outcome
The outcome variable was an increase in total PA across the 6-month follow-up as a dichotomous variable (yes/no). Baseline and both follow-up visits included interviews in which a modified version of the Kuopio Ischemic Heart Disease Risk Factor Study Questionnaire33 was used to retrospectively elicit the participant's PA level over the previous 3 months including leisure time, daily (nonexercise activities, e.g., gardening), and commuting PA. Participants were asked to state the monthly frequency and average duration of each form of activity, as well as the average intensity of each activity using the following descriptions: the activity resembles “outdoors activities”, “light PA”, “moderate PA”, or “strenuous/competitive PA”. The reported intensities were then converted to multiples of the resting metabolic rate [metabolic equivalent of task (METs)].
The 3-month MET-index for each form of PA was calculated by multiplying the activity intensity (MET) by the session duration (minutes) and monthly frequency. The resulting indices were summed to obtain a 3-month total PA MET-index. Activity volume was expressed as METminutes/week. The 6-month total PA MET-index was calculated as the average of the 3-month MET-indices from the 3- and 6-month follow-up visits. This index describes the volume of total PA over the whole 6-month follow-up.
The increase in total PA during the 6-month follow-up was calculated as the difference between the 6-month and prebaseline 3-month MET-indices. Participants were considered able to increase PA if they showed an increase of ≥ 500 METminutes/week (corresponding to 150 minutes moderate intensity or 75 minutes vigorous intensity PA weekly, which is the current PA recommendation for healthy adults5 and is regarded as the minimum volume of PA required to induce health-related benefits) compared with the prebaseline level, while those who increased PA by < 500 METminutes/week were considered unable to increase PA. As mentioned earlier, participants were required to not exercise vigorously for > 20 minutes per session more than twice a week at study inclusion. At baseline, the participants showed a mean (± standard deviation) of 103 ± 204 METminutes/week (10 ± 20% of the total PA) vigorous PA, 272 ± 421 METminutes/week (29 ± 30% of the total PA) moderate PA, and 375 ± 430 METminutes/week (38 ± 31% of the total PA) moderate-to-vigorous PA. Thus, most of the PA at baseline comprised of light intensity PA. The participants who were able to increase their total PA during follow-up (≥500 METminutes/week) were those who increased their moderate and/or vigorous PA in accordance with the recommendation for health-enhancing PA.
Assessment of predictors
SOC was assessed using Antonovsky's 13-item Sense of Coherence Scale (SOC-13).16, 17, 18 Each item is answered on a seven-point Likert scale ranging from “never” to “very often/always”. The resulting total sum score ranges from 13 to 91, with a higher total score associated with a stronger SOC. In this scale, the total SOC score is formed from three components: comprehensibility (one's ability to understand what happens around him/her), manageability (the extent to which one can manage a situation alone or through significant others in one's social network), and meaningfulness (ability to find meaning in the situation). Subscores can be calculated separately for the three components of the SOC (comprehensibility subscore, range 5–35; manageability subscore, range 4–28; and meaningfulness subscore, range 4–28) and are interpreted in the same manner as the total score.
Psychological flexibility was measured using the Acceptance and Action Questionnaire (AAQ-II),24 which measures experiential avoidance. The AAQ-II is an index of the participant's willingness to endure negative private events, acceptance of these events, and whether he/she can live according to his/her values. The AAQ-II comprises 10 items with seven response options ranging from “never true” to “always true”. The total summed score ranges from 10 to 70, with a higher score indicating greater psychological flexibility, e.g., higher acceptance skills and less experiential avoidance.
Stress and recovery from stress were estimated based on heart rate (HR) and HRV.34, 35, 36, 37, 38, 39 HR and HRV were recorded in the course of normal everyday life during 2 workdays and 1 day off from work (altogether 72 hours) using a Bodyguard-device (Firstbeat Technologies Ltd., Jyväskylä, Finland).
Physiological variables describing stress and recovery were analyzed using the program Firstbeat Analysis Server, version 5.3.0.4, which calculates HRV indices second-by-second using the short-time Fourier transform-method, as well as HR- and HRV-derived variables of respiration rate and oxygen consumption using neural network data modeling. The program also calculates second-by-second indices of stress and recovery, reflecting activities of the sympathetic (absolute stress vector; ASV) and parasympathetic (absolute relaxation vector; ARV) nervous systems. ASV is calculated from the HR, high frequency power, low frequency power, and respiratory variables. ASV is high when HR is elevated, HRV is reduced, and there are inconsistencies in the HRV frequency distribution due to changes in respiratory period. ARV is calculated from HR and high frequency power, and is high when HR is near the basic resting level and when HRV is great and regular. These variables can be used to detect different physiological states of the body, including stress state, recovery state, and physical activity.34, 35, 36, 37, 38, 39
For nonexercise data segments, continuous indices of stress (ASV) were used to identify when the body was in a stress state defined as increased activation of the body with dominant activity of the sympathetic nervous system and decreased activity of the parasympathetic system. Stress can be induced by both external and internal stressors, and the stress state definition does not account for the nature of the stress response, i.e., positive or negative. However, continuous indices of recovery (ARV) identify when the body is in a recovery state defined as decreased activation of the body during recovery, rest, and/or peaceful working. This state is related to a lack of external and internal stressors, and parasympathetic activation is dominant.34, 35, 36, 37, 38, 39
Stress- and recovery-related variables (stress% and recovery%) were determined for each 24-hour period, separately for the workdays and day off from work. For workdays, the mean of both days was calculated. Stress% during workdays describes the percentage of stress time during working, leisure, and sleeping time. Stress% during the day off describes the percentage of stress time during both waking and sleeping time. Recovery% was determined similarly for workdays and the days off. During the measurement period, participants were asked to complete a diary, recording information about working hours, sleeping time, PA, and psychologically stressful situations.
Other studies have reported the validity and accuracy of the R-R interval-derived parameters utilized here.39 Uusitalo et al32 reported that higher work effort was associated with lower daytime HRV and recovery%. Additionally, lower perceived stress at work was associated with higher HRV during work time, and higher perceived irritation at work was associated with lower night- time HRV. Teisala et al34 reported that objectively measured stress at work was associated with subjective occupational burnout symptoms.
The presently used method to assess stress and recovery has some strengths compared with other objective measurement tools. One such strength is its ability to separate PA from other stress factors. It also enables individual analysis of both stress and recovery.39 Compared with subjective stress assessment methods (e.g., questionnaires or interviews), objective measures may reveal physiological stress reactions in situations even when the person does not perceive stress.
Anthropometric measurements included height, weight, and waist circumference. Waist circumference was determined as the mean of three measurements taken from mid-way between the lowest ribs and the top of the iliac bones after a light exhalation. BMI was calculated by dividing weight in kg by height squared in m. Body composition (including whole-body fat%) was assessed with dual-energy X-ray absorptiometry (Prodigy, GE Lunar Corp., Madison, WI, USA). Picture handling and analysis was performed using enCORE 2009, version 13.20. Anthropometric measurements and body composition assessment were performed after fasting for 10–12 hours.
Statistical analysis
Descriptive data for continuous variables were calculated as mean ± standard deviation. Mean differences were analyzed using an independent samples t-test and analysis of variance for parametric variables, and the Mann-Whitney U-test and Kruskal-Wallis test for nonparametric variables. A Chi-square test was used to compare proportions. Associations between baseline characteristics and increase in PA were investigated using binary logistic regression analysis to obtain an odds ratio (OR) and 95% confidence interval (CI). First, an unadjusted analysis was performed for each baseline characteristic. Thereafter, the analyses were adjusted for age, sex, study group, prebaseline 3-month total PA MET-index, and BMI using the enter method. All p values were two-sided and p < 0.05 was considered to denote statistical significance. Data were analyzed using IBM SPSS Statistics 20 (SPSS Inc., Chicago, IL, USA).
Results
Table 1 presents baseline characteristics for all participants and according to increases in total PA (yes vs. no) throughout the 6-month follow-up. Baseline characteristics did not significantly differ between the study groups – except the manageability subscore of the SOC-13 questionnaire, which was significantly higher (p = 0.02) in the mini-intervention + whey protein group (21.4 ± 2.8 points) compared with the control group (18.2 ± 3.0 points).
Table 1.
Baseline characteristics for all participants and according to increases in total physical activity of ≥ 500 METminutes/week throughout the 6-month follow-up.
| Characteristic | All participants (n = 51) | Increase in total physical activity of ≥ 500 METmin/wk |
p | |
|---|---|---|---|---|
| Yes (n = 21) | No (n = 30) | |||
| Sex | 0.47 | |||
| Men, n (%) | 31 (60.8) | 14 (66.7) | 17 (56.7) | |
| Women, n (%) | 20 (39.2) | 7 (33.3) | 13 (43.3) | |
| Age (y) | 34.1 ± 4.2 | 33.0 ± 4.7 | 34.8 ± 3.8 | 0.18 |
| Height (cm) | 174.3 ± 10.8 | 174.1 ± 11.9 | 174.4 ± 10.2 | 0.94 |
| Weight (kg) | 87.4 ± 12.0 | 89.2 ± 13.1 | 86.4 ± 11.3 | 0.45 |
| Body mass index (kg/m2) | 28.6 ± 2.5 | 29.1 ± 2.5 | 28.4 ± 2.5 | 0.38 |
| Waist circumference (cm) | 99.6 ± 8.8 | 99.4 ± 9.9 | 99.7 ± 8.1 | 0.28 |
| Whole-body fat% | 33.8 ± 8.2 | 32.5 ± 9.5 | 34.6 ± 7.2 | 0.34 |
| 3-mo total physical activity (METmin/wk)a | 1003 ± 703 | 782 ± 490 | 1158 ± 791 | 0.06 |
| SOC-13b total score (range 13–91) | 65.0 ± 7.2 | 66.1 ± 6.6 | 64.3 ± 7.7 | 0.38 |
| SOC-13b comprehensibility subscore (range 5–35) | 24.0 ± 3.5 | 24.3 ± 3.2 | 23.8 ± 3.8 | 0.74 |
| SOC-13b manageability subscore (range 4–28) | 20.2 ± 3.0 | 20.2 ± 2.7 | 20.2 ± 3.2 | 0.99 |
| SOC-13b meaningfulness subscore (range 4–28) | 20.8 ± 2.6 | 21.6 ± 2.3 | 20.2 ± 2.7 | 0.10 |
| AAQ-IIc (range 10–70) | 58.9 ± 6.4 | 59.7 ± 5.1 | 58.3 ± 7.2 | 0.65 |
| Stress% work d | 53.2 ± 10.6 | 52.7 ± 9.2 | 53.7 ± 11.6 | 0.76 |
| Stress% d off | 52.5 ± 12.8 | 50.1 ± 10.0 | 54.2 ± 14.5 | 0.28 |
| Recovery% work d | 25.2 ± 6.8 | 27.7 ± 6.1 | 23.3 ± 6.9 | 0.03 |
| Recovery% d off | 24.9 ± 9.7 | 29.4 ± 8.3 | 21.6 ± 9.4 | 0.004 |
Values are mean ± standard deviation unless otherwise stated.
MET = metabolic equivalent.
Includes leisure time, daily, and commuting physical activity.
Sense of Coherence-13 scale.
Acceptance and Action Questionnaire-II (psychological flexibility).
During follow-up, 21 participants (41%) increased their total PA by ≥ 500 METminutes/week (1186 ± 506 METminutes/week), while 30 participants (59%) did not (−41 ± 497 METminutes/week) (p < 0.001). The proportion of participants showing a total PA increase did not differ with study groups or sex.
The best predictors of an increase in total PA across follow-up were HR monitoring-based recovery% during the day off and the meaningfulness subscore of the SOC-13 questionnaire (Table 2). Multivariate-adjusted analysis (adjusted for age, sex, study group, prebaseline 3-month total PA, and BMI) showed that greater likelihood of increasing total PA was associated with higher recovery% during the day off (OR 1.15; 95% CI 1.02–1.30) and with higher meaningfulness subscore (OR 1.57; 95% CI 1.04–2.35). Results for the recovery% of the workdays were very similar to those for the day off (multivariate adjusted OR 1.14; 95% CI 0.98–1.32).
Table 2.
Association of baseline characteristics with increases in total physical activity during the 6-month follow-up.
| Characteristic | Unadjusted model OR (95% CI) |
Model 1c OR (95% CI) |
Model 2d OR (95% CI) |
|---|---|---|---|
| SOC-13a total score | 1.04 (0.96–1.12) | 1.03 (0.95–1.12) | 1.07 (0.97–1.18) |
| SOC-13a comprehensibility subscore | 1.04 (0.88–1.22) | 1.01 (0.85–1.20) | 1.11 (0.89–1.37) |
| SOC-13a manageability subscore | 1.00 (0.83–1.21) | 0.97 (0.79–1.19) | 1.02 (0.79–1.31) |
| SOC-13a meaningfulness subscore | 1.26 (0.98–1.62) | 1.39 (1.03–1.88) | 1.57 (1.04–2.35) |
| AAQ-IIb | 1.04 (0.95–1.14) | 1.03 (0.93–1.13) | 1.03 (0.92–1.14) |
| Stress% work d | 0.99 (0.94–1.05) | 0.99 (0.94–1.05) | 1.00 (0.94–1.06) |
| Stress% d off | 0.98 (0.93–1.02) | 0.98 (0.93–1.03) | 0.96 (0.90–1.03) |
| Recovery% work d | 1.11 (1.01–1.23) | 1.10 (0.99–1.22) | 1.14 (0.98–1.32) |
| Recovery% d off | 1.11 (1.03–1.21) | 1.11 (1.02–1.20) | 1.15 (1.02–1.30) |
| Weight | 1.02 (0.97–1.07) | 1.02 (0.96–1.08) | 1.01 (0.92–1.12) |
| Body mass index | 1.12 (0.88–1.42) | 1.20 (0.92–1.56) | 1.25 (0.92–1.69) |
| Waist circumference | 1.00 (0.93–1.06) | 1.00 (0.93–1.08) | 0.90 (0.77–1.06) |
| Whole-body fat% | 0.97 (0.90–1.04) | 0.98 (0.87–1.11) | 0.88 (0.74–1.05) |
CI = confidence interval; MET = metabolic equivalent; OR = odds ratio.
Sense of Coherence-13 scale.
Acceptance and Action Questionnaire-II (psychological flexibility).
Adjusted for age and sex.
Adjusted for age, sex, study group, prebaseline 3-month total physical activity MET-index, and body mass index.
Discussion
Our present results showed that a strong sense of meaningfulness, and a high recovery from work and other stressors significantly predicted an increase in total PA among physically inactive and overweight healthy young adults.
Stronger sense of meaningfulness (one of the three components of SOC) was associated with a greater likelihood of increasing total PA. This is in line with results of previous cross-sectional studies showing that strong SOC is associated with greater PA prevalence among healthy adults,12, 13 and higher PA volume among adult type 1 diabetics.14 A 13-year follow-up study by Myers et al15 revealed an association between low SOC and decreased engagement in PA among patients with myocardial infarction. Our present results suggest that the meaningfulness component could be the most important SOC component with regard to the ability to increase PA among previously inactive and overweight individuals. Of the questions assessing the meaningfulness component, “How often do you feel that you don't care what is happening around you?” was the best predictor of increases in PA, with those who often cared what was happening around them being more likely to increase their PA. A strong sense of meaningfulness may explain greater PA participation in many ways. For example, the likelihood of regular PA participation may increase, if the individual has a feeling that he/she has a meaning for it (e.g., improvement in physical fitness, prevention of chronic diseases) or he/she has a meaning for his/her life in general. Meaningfulness has been regarded as “a driving force for a life” and thus the most important factor among the SOC components strengthening the other two components (comprehensibility and manageability).40
Higher HR monitoring-based recovery% during a day off also predicted an increase in total PA, i.e., the participants who better recovered from work and other stressors were more likely to increase their PA. We also performed these analyses with combined recovery% from workdays and days off (results not shown) and the results were similar to those for only a day off with combined recovery% significantly predicting increase in total PA. Previous studies among working-aged adults report that perceived work stress41, 42 and high job strain31 increased the likelihood for being physically inactive. In addition to work stress, other kinds of stress (such as major life events) reportedly predict less PA.30 In our study, high stress% was not associated with reduced likelihood to increase PA, but the observation that a high recovery% (which is associated with a smaller stress%) significantly predicted increases in PA can be considered as a reverse interpretation of a same phenomenon. Thus, our results showing that objectively measured recovery/absence of stress was associated with an increase in PA resemble previous results showing association between perceived stress and reduced PA.30, 31, 41, 42
It is understandable that recovery would be a determinant of increase in PA. While modern working life can predispose an individual to acute/chronic stress, leisure time may also include activities and duties (e.g., household work, taking care of small children) that increase stress risk, especially among young adults. If leisure time also includes many stress-predisposing factors, inadequate time may be available for complete recovery. In turn, incomplete recovery may lead to a lack of the physical and/or psychological resources needed to participate in physically demanding activities since extra effort may be needed to perform adequately at work.43 Incomplete recovery is also associated with other health-related factors, such as increased risk of cardiovascular death.44
Higher psychological flexibility has previously been reported to be associated with more active lifestyle,29 but no such association was observed with PA in our study. The AAQ-II questionnaire used in our study is a broad measure that especially addresses emotions such as anxiety and depression. If a specifically adapted AAQ to assess difficulties in participation in regular PA had been available, it could have been more suitable for measuring physically inactive and overweight individuals' psychological flexibility than the general AAQ. Previous studies of different health problems have shown that the impact of psychological flexibility is better assessed by modifying the general AAQ to target the specific area being studied, such as type 2 diabetes management45 or smoking.46
One strength of the present study is its prospective design. Most previous studies addressing SOC and PA have been cross-sectional, making it difficult to interpret the causal nature of this association. Our study strengthens previous observations that strong SOC (especially its meaningfulness component) may influence participation in PA. We also measured stress and recovery objectively using HR/HRV-based monitoring, providing new insights into the roles of stress and recovery in participation in PA.
Our study also has some limitations. One is the small sample size, which may have led to a lack of statistical power. Additionally, the study groups were not given exactly the same advice on how to increase PA. However, the proportion of participants able to increase their total PA did not differ significantly between the study groups. Moreover, we used the study group information as a covariate in the statistical analyses; therefore, the differences in advice provided should not have affected the results.
In conclusion, here we show that a strong sense of meaningfulness and high recovery from work and other stressors are significant predictors of increase in total PA among physically inactive and overweight healthy young adults. Participants with a low sense of meaningfulness and incomplete recovery from stress may thus require support from other interventions to be able to increase PA to the recommended level. Therefore, the presence of these characteristics should be determined before initiating exercise counselling or other strategies to increase PA. Studies in larger sample sizes are needed to confirm these results. It is also important to study the effects of (intervention-induced) improvements in SOC and recovery on increases in PA. Such interventions could help achieve more persistent results in attempts to increase PA in previously inactive individuals.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Funding/support
Firstbeat Technologies Ltd. provided measurement devices and an analysis environment for the stress and recovery measurements. This work was funded by the SalWe Research Program for Mind and Body under Tekes – the Finnish Funding Agency for Technology and Innovation, grant number 1104/10.
Appendix Figure 1. Study design.
a Electronic Fitness Coach is a computer program (Firstbeat Technologies Ltd., Jyväskylä, Finland), which gives physical training advice (duration, intensity) based on the individual's personal background information (e.g., previous and actual physical activity habits) and aims for physical activity (maintaining or improvement in fitness level).
b Participants were advised to consume 5 dL/day of the whey protein drink (Valio Ltd., Helsinki, Finland) that provides 40 g protein daily.
References
- 1.Reiner M., Niermann C., Jekauc D. Long-term health benefits of physical activity – a systematic review of longitudinal studies. BMC Public Health. 2013;13:813. doi: 10.1186/1471-2458-13-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conn V.S. Depressive symptoms outcomes of physical activity interventions: meta-analysis findings. Ann Behav Med. 2010;39:128–138. doi: 10.1007/s12160-010-9172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conn V.S., Hafdahl A.R., Cooper P.S. Meta-analysis of workplace physical activity interventions. Am J Prev Med. 2009;37:330–339. doi: 10.1016/j.amepre.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallal P.C., Andersen L.B., Bull F.C., for the Lancet Physical Activity Series Working Group Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380:247–257. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . World Health Organization; Geneva, Switzerland: 2010. Global Recommendations on Physical Activity for Health. [PubMed] [Google Scholar]
- 6.Stevens G.A., Singh G.M., Lu Y. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10:22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez A., Grandes G., Sánchez-Pinilla R.O. Predictors of long-term change of a physical activity promotion programme in primary care. BMC Public Health. 2014;14:108. doi: 10.1186/1471-2458-14-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons R.K., van Sluijs E.M.F., Hardeman W. Who will increase their physical activity? Predictors of change in objectively measured physical activity over 12 months in the ProActive cohort. BMC Public Health. 2010;10:226. doi: 10.1186/1471-2458-10-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steptoe A., Rink E., Kerry S. Psychosocial predictors of changes in physical activity in overweight sedentary adults following counseling in primary care. Prev Med. 2000;31:183–194. doi: 10.1006/pmed.2000.0688. [DOI] [PubMed] [Google Scholar]
- 10.Plotnikoff R.C., Trinh L., Courneya K.S. Predictors of physical activity in adults with type 2 diabetes. Am J Health Behav. 2011;35:359–370. doi: 10.5993/ajhb.35.3.9. [DOI] [PubMed] [Google Scholar]
- 11.Peeters G., Brown W.J., Burton N.W. Psychosocial factors associated with increased physical activity in insufficiently active adults with arthritis. J Sci Med Sport. 2014 Aug 15 doi: 10.1016/j.jsams.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Poppius E., Tenkanen L., Kalimo R. The sense of coherence, occupation and the risk of coronary heart disease in the Helsinki Heart Study. Soc Sci Med. 1999;49:109–120. doi: 10.1016/s0277-9536(99)00105-7. [DOI] [PubMed] [Google Scholar]
- 13.Wainwright N.W.J., Surtees P.G., Welch A.A. Healthy lifestyle choices: could sense of coherence aid health promotion? J Epidemiol Community Health. 2007;61:871–876. doi: 10.1136/jech.2006.056275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahola A.J., Mikkilä V., Saraheimo M. Sense of coherence, food selection and leisure time physical activity in type 1 diabetes. Scand J Public Health. 2012;40:621–628. doi: 10.1177/1403494812460346. [DOI] [PubMed] [Google Scholar]
- 15.Myers V., Drory Y., Gerber Y. Sense of coherence predicts post-myocardial infarction trajectory of leisure time physical activity: a prospective cohort study. BMC Public Health. 2011;11:708. doi: 10.1186/1471-2458-11-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antonovsky A. Jossey-Bass; San Francisco: 1979. Health, Stress and Coping. [Google Scholar]
- 17.Antonovsky A. Jossey-Bass; San Francisco: 1987. Unraveling the Mystery of Health. How People Manage Stress and Stay Well. [Google Scholar]
- 18.Eriksson M., Lindström B. Validity of Antonovsky's sense of coherence scale: a systematic review. J Epidemiol Community Health. 2005;59:460–466. doi: 10.1136/jech.2003.018085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kouvonen A.M., Väänänen A., Woods S.A. Sense of coherence and diabetes: a prospective occupational cohort Study. BMC Public Health. 2008;8:46. doi: 10.1186/1471-2458-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouvonen A.M., Väänänen A., Vahtera J. Sense of coherence and psychiatric morbidity: a 19-year register-based prospective study. J Epidemiol Community Health. 2010;64:255–261. doi: 10.1136/jech.2008.083352. [DOI] [PubMed] [Google Scholar]
- 21.Surtees P., Wainwright N., Luben R. Sense of coherence and mortality in men and women in the EPIC-Norfolk United Kingdom prospective cohort study. Am J Epidemiol. 2003;158:1202–1209. doi: 10.1093/aje/kwg272. [DOI] [PubMed] [Google Scholar]
- 22.Super S., Verschuren W.M.M., Zantinge E.M. A weak sense of coherence is associated with a higher mortality risk. J Epidemiol Community Health. 2014;68:411–417. doi: 10.1136/jech-2013-203085. [DOI] [PubMed] [Google Scholar]
- 23.Mattisson C., Horstmann V., Bogren M. Relationship of SOC with sociodemographic variables, mental disorders and mortality. Scand J Public Health. 2014;42:434–445. doi: 10.1177/1403494814527188. [DOI] [PubMed] [Google Scholar]
- 24.Bond F.W., Hayes S.C., Baer R.A. Preliminary psychometric properties of the Acceptance and Action Questionnaire – II: a revised measure of psychological flexibility and experiential avoidance. Behav Ther. 2011;42:676–688. doi: 10.1016/j.beth.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Kashdan T.B., Rottenberg J. Psychological flexibility as a fundamental aspect of health. Clin Psychol Rev. 2010;30:865–878. doi: 10.1016/j.cpr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes S.C., Luoma J.B., Bond F.W. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44:1–25. doi: 10.1016/j.brat.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Lillis J., Hayes S.C., Levin M.E. Binge eating and weight control: the role of experiential avoidance. Behav Modif. 2011;35:252–264. doi: 10.1177/0145445510397178. [DOI] [PubMed] [Google Scholar]
- 28.Masuda A., Boone M.S., Timko C.A. The role of psychological flexibility in the relationship between self-concealment and disordered eating symptoms. Eating Behaviors. 2011;12:131–135. doi: 10.1016/j.eatbeh.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Kangasniemi A., Lappalainen R., Kankaanpää A. Mindfulness skills, psychological flexibility, and psychological symptoms among physically less active and active adults. Ment Health Phys Act. 2014;7:121–127. [Google Scholar]
- 30.Stults-Kolehmainen M.A., Sinha R. The effects of stress on physical activity and exercise. Sports Med. 2014;44:81–121. doi: 10.1007/s40279-013-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fransson E.I., Heikkilä K., Nyberg S.T. Job strain as a risk factor for leisure-time physical inactivity: an individual-participant meta-analysis of up to 170,000 men and women. Am J Epidemiol. 2012;176:1078–1089. doi: 10.1093/aje/kws336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uusitalo A., Mets T., Martinmäki K. Heart rate variability related to effort at work. Appl Ergon. 2011;42:830–838. doi: 10.1016/j.apergo.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Lakka T.A., Salonen J.T. The physical activity questionnaires of the Kuopio Ischemic Heart Disease Study (KIHD). A collection of physical activity questionnaires for health related research. Med Sci Sports Exerc. 1997;29:S46–S58. [PubMed] [Google Scholar]
- 34.Teisala T., Mutikainen S., Tolvanen A. Associations of physical activity, fitness, and body composition with heart rate variability-based indicators of stress and recovery on workdays: a cross-sectional study. J Occup Med Toxicol. 2014;9:16. doi: 10.1186/1745-6673-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Firstbeat Technologies Ltd. VO2estimation method based on heart rate measurement. [White paper, published Feb 2005, last update March 2012] Available from: http://www.firstbeat.fi/userData/firstbeat/download/white_paper_vo2_estimation.pdf.
- 36.Kettunen J, Saalasti S, inventors. Procedure for deriving reliable information on respiratory activity from heart period measurement. US Patent 7,460,901 B2. 2 Dec 2008. Available from: http://worldwide.espacenet.com/publicationDetails/originalDocument?CC=US&NR=7460901B2&KC=B2&FT=D&ND=4&date=20081202&DB=EPODOC&locale=fi_FI.
- 37.Kettunen J, Saalasti S, inventors. Procedure for detection of stress by segmentation and analyzing heart beat signal. US Patent 7,330,752 B2. 12 Feb 2008. Available from: http://worldwide.espacenet.com/publicationDetails/originalDocument?FT=D&date=20080212&DB=EPODOC&locale=fi_FI&CC=US&NR=7330752B2&KC=B2&ND=4.
- 38.Saalasti S. University of Jyvaskyla; Jyvaskyla, Finland: 2003. Neural Networks for Heart Rate Time Series Analysis.https://jyx.jyu.fi/dspace/bitstream/handle/123456789/13267/951391707X.pdf?sequence=1 [PhD thesis] Available from: [Google Scholar]
- 39.Firstbeat Technologies Ltd. Stress and recovery analysis method based on 24-hour heart rate variability. [White paper, published Sep 2014, last update Nov 2014] Available from: http://www.firstbeat.fi/userData/firstbeat/research-publications/Stress-and-recovery_white-paper_2014.pdf.
- 40.Lindström B., Eriksson M. Folkhälsan Research Center: Health Promotion Research; 2010. The Hitchhiker's Guide to Salutogenesis. Salutogenic Pathways to Health Promotion. Research Report No. 2. [Google Scholar]
- 41.Chandola T., Britton A., Brunner E. Work stress and coronary heart disease: what are the mechanisms? Eur Heart J. 2008;29:640–648. doi: 10.1093/eurheartj/ehm584. [DOI] [PubMed] [Google Scholar]
- 42.Kouvonen A., Vahtera J., Oksanen T. Chronic workplace stress and insufficient physical activity: a cohort study. Occup Environ Med. 2013;70:3–8. doi: 10.1136/oemed-2012-100808. [DOI] [PubMed] [Google Scholar]
- 43.Geurts S.A.E., Sonnentag S. Recovery as an explanatory mechanism in the relation between acute stress reactions and chronic health impairment. Scand J Work Environ Health. 2006;32:482–492. doi: 10.5271/sjweh.1053. [DOI] [PubMed] [Google Scholar]
- 44.Kivimäki M., Leino-Arjas P., Kaila-Kangas L. Is incomplete recovery from work a risk marker of cardiovascular death? Prospective evidence from industrial employees. Psychosom Med. 2006;68:402–407. doi: 10.1097/01.psy.0000221285.50314.d3. [DOI] [PubMed] [Google Scholar]
- 45.Gregg J.A., Callaghan G.M., Hayes S.C. Improving diabetes self-management through acceptance, mindfulness, and values: a randomized controlled trial. J Consult Clin Psychol. 2007;75:336–343. doi: 10.1037/0022-006X.75.2.336. [DOI] [PubMed] [Google Scholar]
- 46.Gifford E.V., Kohlenberg B.S., Hayes S.C. Acceptance-based treatment for smoking cessation. Behav Ther. 2004;35:689–705. [Google Scholar]