Abstract
Background/Objective
Oxygen consumption during walking per unit distance (Cw; mL/kg/m) is known to be greater for older adults than younger adults, although its underlying process is controversial.
Methods
We measured the Cw values at six gait speeds from 30 m/min to 105 m/min on level ground and gradient slopes (±5%) in healthy younger and older male adults. A quadratic approximation was applied for a relationship between Cw and gait speeds (v; m/min). It gives a U-shaped Cw–v relationship, which includes a particular gait speed minimizing the Cw, the so-called economical speed (ES). The age-related difference of the Cw–v relationship was assessed by comparisons of ES and/or Cw.
Results
A significantly greater Cw at 30 m/min and slower ES were found for older adults at the downhill gradient, suggesting that a combination of leftward and upward shifts of the Cw–v relationship was found at that gradient. Only a slower ES was found for older adults at the uphill gradient, suggesting that a leftward shift was found for older adults at that gradient. Neither a significant leftward nor an upward shift was found at the level gradient. Leg length significantly correlated to the ES for younger adults at the level and downhill gradients, while such a significant relationship was observed only at the level gradient for older adults. The maximal quadriceps muscle strength significantly correlated to the ES for older adults at all gradients, but not for younger adults.
Conclusion
The age-related alteration of the Cw–v relationship depends on the gradient, and its related factors were different between age groups.
Keywords: Aging, Bipedal locomotion, Gait, Optimal speed, Oxygen consumption
Introduction
It is well known that there is a U-shaped relationship between oxygen consumption during walking per unit distance (Cw; mL/kg/m) and gait speeds (v; m/min).1 This indicates that there is a particular gait speed minimizing the Cw in each individual, and it has been called economical speed (ES)2, 3, 4, 5, 6 or optimal speed.7, 8, 9, 10
An age-related increase in the Cw values was found for healthy older adults,11 and it was explained by a difference in the muscle strength in association with gait instability. This can be described as an upward shift of the Cw–gait speeds (v) relationship (Figure 1A). Such an age-related increase in the Cw values has also been reported by other studies, whereas mechanisms underlying the phenomenon have been controversial. Some related studies reported that several potential factors, including, but not limited to, training status,12, 13 aerobic fitness levels,14 differences in individual-limb mechanical work,15, 16 and leg-muscle activity patterns,15, 17, 18 are comprehensively related to the phenomenon. Given the results of these previous studies, no single factor alone can fully explain the age-related increase in the Cw values for older adults.
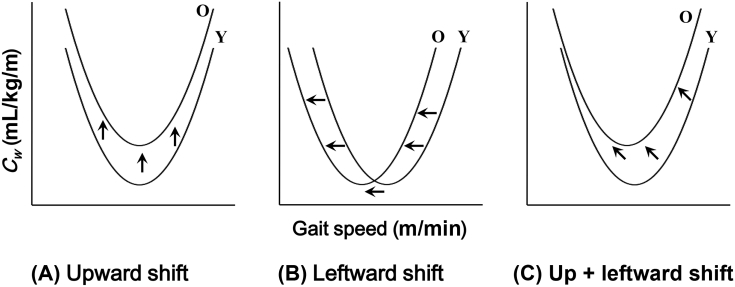
Figure 1.
Schematic illustration of (A) upward shift, (B) leftward shift, and (C) combination of upward and leftward shifts of the Cw–gait speed (v) relationship. Y and O represent younger and older adults, respectively. Arrows mean potential shifting directions. Cw–v = oxygen consumption during walking per unit distance and gait speeds.
An age-related increase in the Cw values for older adults will relate to a slower ES. However, such an increased Cw does not always result in a slower ES if the Cw–v relationship shifted only upward, as shown in Figure 1A. It is worth noting that most of the previous studies, except for Hortobágyi et al,18 tested only at the level gradient. Indeed, the Cw values increased steeply at faster walking speeds for older adults at the level gradient.11, 16 These previous results suggest that the physical characteristics, particularly in muscle strength, would relate to an alteration of the Cw–v relationship by aging, and it will result not only in a leftward shift (Figure 1B), but also in a combination of upward and leftward shifts of the Cw–v relationship (Figure 1C) at the gradient environments. However, as far as we know, no information has been available with regard to the ES in healthy older adults at the gradient environments. We further questioned whether the Cw–v relationship actually showed to be greater for healthy, older active manual workers than younger adults. We hypothesized that the age-related increase in the Cw values for older adults would result in an upward shift, or a combination of leftward and upward shifts of the Cw–v relationship at each gradient. The purpose of the present study was to investigate whether the Cw–v relationship could be affected by either aging or other factors, such as physical characteristics and environmental factors.
Methods
Participants
To eliminate the effects of different leg lengths on the Cw–v relationship, eight younger and eight older healthy male adults with equivalent body heights were recruited (Table 1). All older adults were nonretired full-time manual workers. After an explanation of all procedures, possible risks, and benefits of participation, written informed consent was obtained from each participant. This study conformed to the Declaration of Helsinki, and an ethical committee established in Kyushu Sangyo University approved the purpose and all procedures of this study (H240324).
Table 1.
Physical characteristics and muscle strength in two groups.
| Younger (n = 8) | Older (n = 8) | |
|---|---|---|
| Age (y) | 22.5 ± 0.6 | 64.8 ± 1.2* |
| Height (cm) | 172.4 ± 1.9 | 170.2 ± 2.8 |
| BM (kg) | 63.4 ± 2.2 | 62.9 ± 2.4 |
| Leg length (cm) | 91.1 ± 1.3 | 87.7 ± 1.1 |
| Ratio of length to height (%) | 52.8 ± 0.4 | 51.6 ± 0.6 |
| Muscle strength per BM | 0.99 ± 0.05 | 0.72 ± 0.03* |
Values are presented as mean ± standard error of the mean (S.E.M.).
*A significant difference (p < 0.05).
BM = body mass.
Exercise protocols and measurements
All experiments were carried out on a motor-driven treadmill of 4.0 m in length and 2.0 m in width (Biomill 1300; S&ME, Tokyo, Japan). Under all experimental conditions, each participant walked on the same treadmill with a freely chosen step frequency at each gait speed and gradient. The participants wore underwear, shirts, socks, shorts, and lightweight training shoes.19 To become accustomed to normal treadmill walking while wearing a gas-collection mask, each participant walked at least three preliminary practices (15-minute duration) on the same treadmill at several gait speeds and gradients. As a result, all participants were fully familiarized with walking on the treadmill.
The gait speeds were set at 30 m/min, 45 m/min, 60 m/min, 75 m/min, 90 m/min, and 105 m/min, and the treadmill gradient was set at 0% (level), −5% (downhill), and +5% (uphill) based on a recent work.6 The total number of trials performed by each participant was 18 (3 gradients × 6 speeds), and all participants were tested, at most, six gait speeds/d, each of which lasted for 4 minutes. The pulmonary oxygen uptake (; mL/kg/min) and heart rate (HR) were measured throughout the study. All participants performed a series of walking trials, which were separated by a resting period of sitting in a chair for 4–5 minutes until the HR recovered to the baseline level. The order of the gradient trials was randomized.
The ventilation and gas-exchange variables were measured using an online computerized breath-by-breath method (Vmax; SensorMedics, CareFusion Corp., Yorba Linda, CA, USA). The inspired- and expired-gas volumes were measured using a hot-wire respiratory-flow system. Flow signals were electrically integrated for the duration of each breath to calculate minute ventilation. The expired fractions of O2 and CO2 were analyzed using a zirconium solid-electrolyte oxygen analyzer and an infrared carbon-dioxide analyzer, respectively. The standard known gases (O2 16.0%, CO2 4.0%, and N2 80.0%) and room air were used for the calibration of the gas analyzer. The HR was recorded using an electrocardiogram monitor, which was attached to the gas-analyzer system.
The leg length, defined as the length from the trochanter major to the ground, was measured in both legs for each participant using a steel tape measure. The individual leg length was defined as the average length of both legs. The leg-muscle strength (quadriceps muscles) was measured using a concentric-only knee-extension machine (Cybex VR2-1411 leg extension; Cybex International, Inc., Medway, MA, USA). The participants sat on an adjustable chair attached to the machine, and they were stabilized with straps across the shoulders, waist, and thighs throughout the test to prevent additional body movements. All participants crossed their arms across their chest to eliminate additional body twists using a handrail of the knee-extension machine. The participants were instructed to lift the load through the range of motion. After a warm-up, a maximal muscle-strength test using both legs was started. The maximal quadriceps muscle strength (MQMS; kg) was determined using increments of 10 kg until 60–80% of the perceived maximum. Then, the load was gradually increased by 1–5 kg weights until lift fail, in which the participant was not able to maintain proper form, or to completely lift the weight. Each trial was conducted with an enough-rest interval period in order to eliminate individual muscle fatigue and/or pain. The final acceptable lift with the highest possible load was determined as the individual MQMS.20 The individual MQMS was expressed as the ratio of the individual body mass (BM)—MQMS/BM.
Determination of ES
A single sample with an average final 1-minute value (mL/kg/min) at each gait speed was calculated to obtain the oxygen consumption during walking per unit distance (Cw; mL/kg/m).6 Each particular Cw was determined by the ratio of the steady-state to the gait speed (v; m/min):
| (1) |
The relationship between gait speed and Cw can be well approximated using a quadratic equation.5, 6, 9, 19, 21 The Cw–v relationship can be mathematically described by the following equation:
| (2) |
where the constants a, b, and c are determined by the least-square regressions with the actually observed Cw values at each gait speed. A differential function of the original quadratic equation (2) of each individual can be described as follows:
| (3) |
Then, the individual ES was determined at the gait speed when equals zero, that is, the individual ES can be observed using the following equation:
| (4) |
A subtraction of standing or sitting from the gross (absolute) for calculating the ES has been argued.21, 22 Wall-Scheffler and Myers21 emphasized that numerous studies have found that people preferred walking speeds at or near the speed associated with their ES calculated by the gross .23, 24, 25 The ES calculated from the gross was equivalent to a gait speed, at which the maximal transfer occurred between kinetic gravitational potential energy in adults and children.8 Indeed, other related studies did not subtract sitting or standing when calculating the individual ES3, 4, 8, 9, 10, 21, 24 or Cw values.26 Thus, we used gross for calculating the ES or each Cw value for the purpose of this study. A comparison of the ES between age groups was used to examine whether the Cw–v relationship shifted leftward.
Comparison of Cw between age groups
The Cw values obtained from six gait speeds at each gradient were further averaged to determine a percent difference of the Cw–v relationship between age groups. We also compared the Cw values at each gradient between age groups to examine whether the Cw–v relationship shifted upward for older adults.
Statistics
All data are presented as mean ± standard error of the mean (S.E.M.), because the S.E.M. has been known to present a within-participant deviation,27 which is substantially related to the “true” mean value. The Cw values obtained from six gait speeds tested were averaged in each age group to show a percent difference of the Cw values at each gradient between age groups, meaning that a statistical comparison could not be performed in those comparisons. A regression analysis using a quadratic equation was performed on the Cw–v relationship obtained from each gradient.3, 5, 6, 9, 19, 21 An unpaired t test was conducted for a comparison of the physical characteristics and muscle strength between age groups. A three-way repeated-measures analysis of variance (ANOVA) within participants was used to compare the Cw (age × speed × gradient) using online software ANOVA 4. A two-way repeated-measures ANOVA within participants was also conducted for a comparison of the ES (age × gradient). If a significant F value was obtained, Ryan's28 multiple comparison as a post hoc test was applied to the appropriate data set to establish the significant mean differences. Ryan's28 post hoc test is practical for human experiments, because it can be used regardless of sample size. Its statistical power for detecting statistical significance is equivalent to one of the most popular post hoc tests, Tukey's multiple comparison,28 which is known as one of the most powerful post hoc tests.29 Pearson's correlation coefficient was used to evaluate the relationship between ES and influencing factors, such as leg length and muscle strength. The statistical significance was set at p = 0.05 probability level.
Results
Physical characteristics and muscle strength
No significant differences were found for body height, BM, leg length, or the ratio of leg length to body height between age groups (Table 1). The MQMS/BM ratio was significantly lower for older adults (Table 1).
Comparison of Cw between age groups
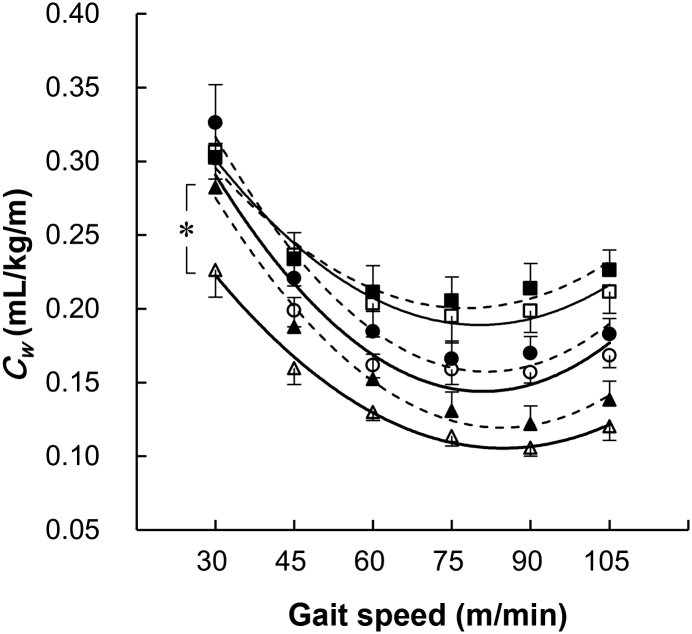
The averaged Cw values obtained from six gait speeds at each gradient were 9.0% greater at the level, +18.3% at the downhill, and +3.1% at the uphill gradient for older adults than for younger adults, although the two-way ANOVA failed to reveal the significant differences at each gradient between groups (Table 2). Figure 2 shows the quadratic relationships between Cw and gait speeds for the examined conditions. The Cw–v relationship showed a clear U-shape over the range of gait speeds tested, and the regression analysis showed that there was a strong correlation between gait speeds and Cw values for younger adults (r = 0.944–0.990) and older adults (r = 0.964–0.996). A significantly greater Cw value was obtained at 30 m/min at the downhill gradient for older adults (Figure 2). However, there were no significant differences in the Cw values at the level and uphill gradients at any gait speeds (Figure 2).
Table 2.
Comparisons of averaged oxygen consumption (Cw) and ES obtained at each gradient between younger and older adults.
| Averaged Cw (mL/kg/m) |
Difference (%) | ES (m/min) |
Difference (%) | |||
|---|---|---|---|---|---|---|
| Younger | Older | Younger | Older | |||
| Level | 0.190 ± 0.009a | 0.201 ± 0.015 | +9.0 | 81.8 ± 1.3a | 77.6 ± 1.3a | −5.1 |
| Downhill | 0.139 ± 0.009a,b | 0.170 ± 0.017a,b | +18.3 | 88.2 ± 2.0a,b,c | 79.7 ± 1.4a,b | −9.6 |
| Uphill | 0.225 ± 0.020 | 0.232 ± 0.017 | +3.1 | 78.2 ± 1.6c | 71.8 ± 0.9 | −8.2 |
Values are presented as mean ± S.E.M.
ES = economical speed.
Significant differences versus uphill gradient within the same age group. The averaged Cw is “uphill > level > downhill” and the ES is “downhill > level > uphill” in both age groups. Signs (+/–) indicate the difference between younger and older groups: “+” is “older > younger,” and “–” is “younger > older,” respectively.
Significant differences versus level gradient within the same age group.
Significant differences between younger and older.
Figure 2.
Oxygen consumption of walking (Cw) in younger and older adults at each gradient. ○: level in younger adults; ●: level in older adults; △: downhill in younger adults; ▲: downhill in older adults; □: uphill in younger adults; and ■: uphill in older adults. * p < 0.05 between younger and older adults at a gait speed of 30 m/min at the downhill gradient. Values are mean ± standard error of the mean.
Comparison of ES
A comparison of the ES observed at each gradient is shown in Table 2. The ES seemed to be slower, but not significantly, for older adults than for younger adults at the level gradient (p = 0.058). By contrast, the ES observed at the downhill gradient was significantly slower for older adults (p < 0.001). The ES at the uphill gradient was also significantly slower for older adults (p = 0.006).
ES and physical characteristics
Table 3 summarizes the correlation coefficients between leg length and ES at each gradient. There was a significant positive relationship between leg length and ES at the level and downhill gradients for younger adults, while such a significant relationship was found only at the level gradient for older adults. Table 2 also shows that there was a significant relationship between MQMS/BM and ES only for older adults at all gradients; however, such a significant relationship was not observed for younger adults at any gradients.
Table 3.
Correlation coefficient between measured ES and leg length or muscle strength.
| Measured ES |
||||||
|---|---|---|---|---|---|---|
| Younger |
Older |
|||||
| Level | Downhill | Uphill | Level | Downhill | Uphill | |
| Leg length | r = 0.714 | r = 0.729 | r = 0.593 | r = 0.777 | r = 0.693 | r = 0.506 |
| p < 0.05 | p < 0.05 | n.s. | p < 0.05 | n.s. | n.s. | |
| Muscle strength per BM | r = 0.595 | r = 0.305 | r = 0.470 | r = 0.732 | r = 0.719 | r = 0.753 |
| n.s. | n.s. | n.s. | p < 0.05 | p < 0.05 | p < 0.05 | |
Bold and italic r values show a significant relationship (p < 0.05).
BM = body mass; ES = economical speed; n.s. = nonsignificant relationship.
Discussion
Upward and leftward shifts of the Cw–v relationship
A significantly greater averaged Cw value obtained from six gait speeds for older adults was supported by a previous study.18 A greater Cw value was also found for older adults at 30 m/min at the downhill gradient (Figure 2). These results indicate that our hypothesis for the upward shift of the Cw–v relationship for older adults was partly supported. A significantly slower ES for older adults was found at the downhill and uphill gradients, but not at the level gradient (Table 2), indicating that the Cw–v relationship showed a leftward shift at the downhill and uphill gradients for older adults. In this matter of slower ES for older adults, our hypothesis for the leftward shift of the Cw–v relationship was supported at these sloped gradients.
Some considerations are further necessary with regard to the leftward shift alone of the Cw–v relationship (Figure 1B), because such a leftward shift alone results in a decrease in the Cw values particularly at slower gait speeds. This is a contradiction of our proposed shifting model of the Cw–v relationship due to aging. As shown in Figure 2, our data show a trend for a steeper increase in the Cw–v relationship for older adults at the uphill gradient, particularly at faster gait speeds, which is supported by other previous studies.3, 6 A striking finding of the present study was that a significantly slower ES was not found for older adults only at the level gradient (p = 0.058, Table 2). Another remarkable finding was that a significantly greater Cw value was found for older adults only at 30 m/min at the downhill gradient, meaning that any particular Cw values were not significantly greater for older adults at the level and uphill gradients (Figure 2). A sample size of this study was equivalent to that of some previous studies.11, 14, 16 The statistical power of the post hoc test used in this study has been reported to be strong enough to detect statistical differences.28, 29 The differences between gross and net Cw could partly explain such nonsignificant differences in the Cw values at the level and uphill gradients. If the resting is subtracted from the gross (absolute) for calculating the net Cw, then such net Cw values are likely to lead to significant differences, particularly at slower gait speeds. This is because the fraction of the resting amounted to approximately 60% of the gross at slower gait speeds.22 These facts indicate that a nonsignificant difference of the average ES at the level gradient and Cw values at the level and uphill gradients should not be due to effects of sample size and/or statistical power.
The individual ES depends on the leg length.6 The leg length was not significantly different between age groups (Table 1), although the average ESs obtained at the downhill and uphill gradients were significantly slower for older adults (Table 2). Our present study focused on nonretired full-time manual workers, who were approximately 10 years younger than those in previous studies.13, 15, 16, 18 No single factor alone may fully explain the age-related increase in the Cw; however, as shown in our study, healthy older manual workers did not always show greater Cw values compared with those of younger adults. A combined effect of a 5.4% slower ES (as a leftward shift) and a 9.0% greater Cw value obtained from six gait speeds (as an upward shift) in association with a 27.3% lesser muscle strength comprehensively influenced the Cw–v relationship for older adults at the level gradient.
What determines individual ES?
Leg-muscle strength declines with aging.11, 30 The preferred and/or self-selected gait speed slows with aging.11, 31 Changes in physiological functions with aging, such as decreasing aerobic capacity and increasing difficulty for common daily activities, are associated with an elevated energy cost of walking.32 These previous findings could relate to the individual ES for older adults. In line with the results of a previous study,11 the older adults exhibited 27.3% lesser MQMS/BM compared with younger adults (Table 1), even though they were habitually working as manual workers. It was interesting to note that a long-term training-based increase in the muscle strength of the quadriceps muscles for older adults did not conduct any alterations in the individual Cw values at any gait speeds.13 These previous and our present findings suggest that the muscular work strategy during walking at different gradient environments would be different between age groups. Indeed, older adults performed more positive work of the trailing leg using muscles crossing the hip during double-support phase at the level and steeper uphill gradients (+15.8%).33 During the single-support phase, they did more positive work of the supporting leg than younger adults using muscles crossing the knee at the level and steeper uphill gradients (+10.5% and +15.8%).34 These findings indicate that older adults were more dependent on the positive work of the quadriceps muscles during walking.
Table 2 summarizes the correlation coefficients for the relationships between the ES and leg length or MQMS/BM. The ES was significantly related to leg length for younger adults at the level and downhill gradients, but not at the uphill gradient. For older adults, the ES was significantly related to leg length only at the level gradient. It was worth noting that a significant relationship was found between the ES and MQMS/BM for older adults at all gradients, whereas this was not found at any gradients for younger adults (Table 3). These results indicate that leg length explained the individual ES for younger adults, while muscle strength in association with leg length explained the individual ES for older adults. Recent studies showed that the gait pattern for older people was characterized by a greater and/or longer coactivation of the agonists and their antagonists in the lower leg extremities during one gait cycle.15, 17, 18 This could result in a greater Cw–v relationship and slower ES for older adults in a negative manner, although antagonist coactivation presumably increases joint stiffness, and it may thereby improve joint stability in a positive manner. These considerations were supported by the fact that the MQMS/BM was significantly correlated to the individual ES only for older adults (Table 3).
Conclusion
The results of the present study suggest that the Cw–v relationship exhibited a combination of upward and leftward shifts for older adults at the downhill gradient. Only a leftward shift of the Cw–v relationship was found at the uphill gradient, being explained by a steeper increase in the Cw–v relationship particularly at faster gait speeds. Neither a significant leftward nor an upward shift was found at the level gradient. The leg length significantly correlated to the ES for younger adults at the level and downhill gradients, while such a significant relationship was observed only at the level gradient for older adults (Table 2). The quadriceps muscle strength significantly correlated to the ES for older adults at all gradients, but not for younger adults (Table 3). These comprehensive results indicate that the age-related alteration of the Cw–v relationship depends on the gradient, and its related factors are different between age groups.
Conflicts of interest
The authors declare no conflicts of interest.
Funding/support
This study was financially supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (26440268 to Masahiro Horiuchi, and 22770248 and 26440266 to Daijiro Abe).
References
- 1.Saibene F., Minetti A.E. Biomechanical and physiological aspects of legged locomotion in humans. Eur J Appl Physiol. 2003;88:297–316. doi: 10.1007/s00421-002-0654-9. [DOI] [PubMed] [Google Scholar]
- 2.Saibene F. The mechanisms for minimizing energy expenditure in human locomotion. Eur J Clin Nutr. 1990;44(suppl 1):65–71. [PubMed] [Google Scholar]
- 3.Abe D., Muraki S., Yasukouchi A. Ergonomic effects of load carriage on energy cost of gradient walking. Appl Ergon. 2008;39:144–149. doi: 10.1016/j.apergo.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Abe D., Muraki S., Yasukouchi A. Ergonomic effects of load carriage on the upper and lower back on metabolic energy cost of walking. Appl Ergon. 2008;39:392–398. doi: 10.1016/j.apergo.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Wezenberg D., van der Woude L.H., Faber W.X. Relation between aerobic capacity and walking ability in older adults with a lower-limb amputation. Arch Phys Med Rehabil. 2013;94:1714–1720. doi: 10.1016/j.apmr.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Horiuchi M., Muraki S., Horiuchi Y. Energy cost of pushing a wheelchair on various gradients in young men. Int J Ind Ergon. 2014;44:442–447. [Google Scholar]
- 7.Cavagna G.A., Willems P.A., Heglund N.C. The role of gravity in human walking: pendular energy exchange, external work and optimal speed. J Physiol. 2000;528:657–668. doi: 10.1111/j.1469-7793.2000.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeJaeger D., Willems P.A., Heglund N.C. The energy cost of walking in children. Pflügers Arch. 2001;441:538–543. doi: 10.1007/s004240000443. [DOI] [PubMed] [Google Scholar]
- 9.Bastien G.J., Willems P.A., Schepens B. Effect of load and speed on the energetic cost of human walking. Eur J Appl Physiol. 2005;94:76–83. doi: 10.1007/s00421-004-1286-z. [DOI] [PubMed] [Google Scholar]
- 10.Leurs F., Ivanenko Y.P., Bengoetxea A. Optimal walking speed following changes in limb geometry. J Exp Biol. 2011;214:2276–2282. doi: 10.1242/jeb.054452. [DOI] [PubMed] [Google Scholar]
- 11.Malatesta D., Simar D., Dauvilliers Y. Energy cost of walking and gait instability in healthy 65- and 80-yr-olds. J Appl Physiol. 2003;95:2248–2256. doi: 10.1152/japplphysiol.01106.2002. [DOI] [PubMed] [Google Scholar]
- 12.Martin P.E., Rothstein D.E., Larish D.D. Effects of age and physical activity status on the speed–aerobic demand relationship of walking. J Appl Physiol. 1992;73:200–206. doi: 10.1152/jappl.1992.73.1.200. [DOI] [PubMed] [Google Scholar]
- 13.Mian O.S., Thom J.M., Ardigo L.P. Effect of a 12-month physical conditioning programme on the metabolic cost of walking in healthy older adults. Eur J Appl Physiol. 2007;100:499–505. doi: 10.1007/s00421-006-0141-9. [DOI] [PubMed] [Google Scholar]
- 14.Malatesta D., Simar D., Dauvilliers Y. Aerobic determinants of the decline in preferred walking speed in healthy, active 65- and 80-year-olds. Pflügers Arch. 2004;447:915–921. doi: 10.1007/s00424-003-1212-y. [DOI] [PubMed] [Google Scholar]
- 15.Mian O.S., Thom J.M., Ardigò L.P. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol (Oxf) 2006;186:127–139. doi: 10.1111/j.1748-1716.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- 16.Ortega J.D., Farley C.T. Individual limb work does not explain the greater metabolic cost of walking in older adults. J Appl Physiol. 2007;102:2266–2273. doi: 10.1152/japplphysiol.00583.2006. [DOI] [PubMed] [Google Scholar]
- 17.Peterson D.S., Martin P.E. Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait Posture. 2010;31:355–359. doi: 10.1016/j.gaitpost.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Hortobágyi T., Finch A., Solnik S. Association between muscle activation and metabolic cost of walking in young and old adults. J Gerontol A Biol Sci Med Sci. 2011;66:541–547. doi: 10.1093/gerona/glr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe D., Yanagawa K., Niihata S. Effects of load carriage, load position, and walking speed on energy cost of walking. Appl Ergon. 2004;35:329–335. doi: 10.1016/j.apergo.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Suga T., Okita K., Morita N. Dose effect on intramuscular metabolic stress during low-intensity resistance exercise with blood flow restriction. J Appl Physiol. 2010;108:1563–1567. doi: 10.1152/japplphysiol.00504.2009. [DOI] [PubMed] [Google Scholar]
- 21.Wall-Scheffler C.M., Myers M.J. Reproductive costs for everyone: how female loads impact human mobility strategies. J Hum Evol. 2013;64:448–456. doi: 10.1016/j.jhevol.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weyand P.G., Smith B.R., Sandell R.F. Assessing the metabolic cost of walking: the influence of baseline subtractions. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:6878–6881. doi: 10.1109/IEMBS.2009.5333126. [DOI] [PubMed] [Google Scholar]
- 23.Browning R.C., Kram R. Energetic cost and preferred speed of walking in obese versus normal weight women. Obes Res. 2005;13:891–899. doi: 10.1038/oby.2005.103. [DOI] [PubMed] [Google Scholar]
- 24.Browning R.C., Baker E.A., Herron J.A. Effects of obesity and sex on the energetic cost and preferred speed of walking. J Appl Physiol. 2006;100:390–398. doi: 10.1152/japplphysiol.00767.2005. [DOI] [PubMed] [Google Scholar]
- 25.Peyrot N., Thivel D., Isacco L. Why does walking economy improve after weight loss in obese adolescents? Med Sci Sports Exerc. 2012;44:659–665. doi: 10.1249/MSS.0b013e318236edd8. [DOI] [PubMed] [Google Scholar]
- 26.Castillo E.R., Lieberman G.M., McCarty L.S. Effects of pole compliance and step frequency on the biomechanics and economy of pole carrying during human walking. J Appl Physiol. 2014;117:507–517. doi: 10.1152/japplphysiol.00119.2014. [DOI] [PubMed] [Google Scholar]
- 27.Hopkins W.G. Measures of reliability in sports medicine and science. Sports Med. 2000;30:1–15. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Ryan T.A. Significance tests for multiple comparison of proportions, variances, and other statistics. Psychol Bull. 1960;57:318–328. doi: 10.1037/h0044320. [DOI] [PubMed] [Google Scholar]
- 29.Ludbrook J. On making multiple comparisons in clinical and experimental pharmacology and physiology. Clin Exp Pharmacol Physiol. 1991;18:379–392. doi: 10.1111/j.1440-1681.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- 30.Marcell T.J., Hawkins S.A., Wiswell R.A. Leg strength declines with advancing age despite habitual endurance exercise in active older adults. J Strength Cond Res. 2014;28:504–513. doi: 10.1519/JSC.0b013e3182a952cc. [DOI] [PubMed] [Google Scholar]
- 31.Studenski S., Perera S., Patel K. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wert D.M., Brach J.S., Perera S. The association between energy cost of walking and physical function in older adults. Arch Gerontol Geriatr. 2013;57:198–203. doi: 10.1016/j.archger.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franz J.R., Kram R. Advanced age and the mechanics of uphill walking: a joint-level, inverse dynamic analysis. Gait Posture. 2014;39:135–140. doi: 10.1016/j.gaitpost.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franz J.R., Kram R. Advanced age affects the individual leg mechanics of level, uphill, and downhill walking. J Biomech. 2013;46:535–540. doi: 10.1016/j.jbiomech.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]