Abstract
Objective
Chronic intestinal inflammation is a risk factor for colorectal cancer (CRC) initiation and development. Diets that are rich in Western style fats have been shown to promote CRC. This study was conducted to investigate the role of intestinal microbiome in American ginseng-mediated CRC chemoprevention in a mouse model. The population and diversity of enteric microbiome were evaluated after the ginseng treatment.
Methods
Using an azoxymethane (AOM)/dextran sulfate sodium (DSS)-induced gut inflammation and tumorigenesis mouse model, the effects of oral American ginseng on high fat diet-associated enteric pathology were determined. After establishment of a 16S rRNA illumina library from fecal samples, MiSeq sequencing was carried out to reveal the microbial population. The alpha and beta diversities of microbiome were analyzed.
Results
American ginseng significantly attenuated AOM/DSS-induced colon inflammation and tumorigenesis by reducing the colitis score and colon tumor multiplicity. The MiSeq results showed that the majority of sequences fell into three phyla, Firmicutes, Bacteroidetes and Verrucomicrobia. Further, two significant abundance shifts at the family level, Bacteroidaceae and Porphyromonadaceae, were identified to support ginseng’s anti-colitis and anti-tumor effects. In addition, alpha and beta diversity data demonstrated that ginseng led to a profound recovery from the AOM/DSS-induced dysbiosis in the microbial community.
Conclusion
Our results suggest that the CRC chemopreventive effects of American ginseng are mediated through enteric microbiome population-shift recovery and dysbiosis restoration. Ginseng’s regulation of the microbiome balance contributes to the maintenance of enteric homeostasis.
Keywords: Gut inflammation, Tumorigenesis, Colorectal cancer, AOM/DSS, American ginseng, Intestinal microbiome, 16S rRNA MiSeq sequencing, Dysbiosis
Introduction
Colorectal cancer (CRC) is one of the most common forms of malignancies worldwide with a substantial public health burden [1]. Previous studies have indicated that chronic gut inflammation is a risk factor for CRC initiation and development [2]. It has also been reported that compared to Asia countries, the incidence of CRC is higher in developed Western countries [1, 3]. This obvious difference in CRC epidemiology can be linked to different dietary patterns, such as the high fat in the Western diet, compared to the Oriental diet, which contains various vegetables and large amounts of carbohydrates. Diets that are rich in Western style fats have been shown to promote CRC [3, 4].
Natural products have been used as traditional medicines for thousands of years to prevent and treat various forms of diseases, including cancer [5, 6]. Ginseng is one of the world’s most widely used herbal medicines and one of the best selling natural products. We previously reported that, as an anti-inflammatory botanical, American ginseng possessed considerable actions against the CRC in animal models [7, 8].
The human body contains trillions of bacteria in the intestinal tract [9]. This dense enteric microbiota population has many physiological functions that maintain gut homeostasis and body health. In recent years, our understanding of CRC carcinogenesis has been greatly advanced. In addition to gut inflammation and diet, enteric microbiota have been demonstrated to influence CRC development and progression [10]. We previously reported that after the oral administration of American ginseng, the intestinal microbiome plays a key role in converting ginseng’s parent compounds into their active metabolites, and the latter showed significant CRC chemoprevention activities [11]. However, the effects of American ginseng on changes in the intestinal microbiome population with respect to enteric homeostasis and cancer prevention have not been investigated. Further, the role of ginseng-induced enteric microbiota species and profile shifts in animals fed with a high fat diet has not been evaluated [12].
In this study, an azoxymethane (AOM)/dextran sulfate sodium (DSS)-induced gut inflammation and tumorigenesis mouse model was used to observe the role of the intestinal microbiome in American ginseng-mediated CRC chemoprevention. The animal’s pathological conditions were exacerbated by feeding with high fat diet for better assessment for the CRC chemoprevention effects. After establishment of a 16S rRNA illumina library from fecal samples, MiSeq sequencing was carried out to reveal the microbial population. Specific attention has been paid to the effects of ginseng on changes in enteric microbiome structure at different taxa levels. In addition, enteric microbiome diversities in experimental animal groups were observed and compared with respect to the CRC chemoprevention.
Materials and methods
Chemicals
Ginsenosides Rb1, Rb2, Rb3, Rc, Rd, Rg1, Rg2, Re and Rh1 were obtained from Delta Information Center for Natural Organic Compounds (Xuancheng, AH, China). HPLC grade methanol, n-butanol, acetonitrile, and absolute ethanol were obtained from Fisher Scientific (Pittsburgh, PA). Mili Q water was supplied by a water purification system (US Filter, Palm Desert, CA).
Plant materials, extraction and high performance liquid chromatography (HPLC) analysis
The root of P. quinquefolius L. was collected from Roland Ginseng, LLC (Wausau, WI). The dried roots were ground and extracted with 70% ethanol. The solvent of the extract solution was evaporated under vacuum. The dried extract was dissolved in water and then extracted with water-saturated n-butanol. The n-butanol phase was evaporated under a vacuum and then lyophilized to produce ginseng extract.
HPLC analysis was conducted on a Waters 2960 instrument with a Waters 996 photodiode array detector (Milford, MA, USA). The separation was carried out on a Prodigy ODS (2) column (250 × 3.2 mm I.D.) with a ProdigyTM guard column (3.0 × 4.0 mm I.D.) (Phenomenex, Torrance, CA, USA). Acetonitrile (solvent A) and water (solvent B) were used. Gradient elution started with 17.5% solvent A and 82.5% solvent B, changed to 21% A for 20 min; to 26% A for 3 min and held for 19 min; to 36% A for 13 min; to 50% A for 9 min; to 95% A for 2 min and held for 3 min. The flow rate was 1.0 ml/min and the detection wavelength was set to 202 nm. All tested solutions were filtered through Millex 0.2-μm nylon membrane syringe filters before use. The contents of ginsenosides in each sample were calculated using standard curves of ginsenosides.
Animals and treatment protocols
The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Chicago. Approximately 6-week old male A/J mice, weighing between 18 and 22 g, were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were maintained under controlled room temperature, humidity and light (12/12 hr light/dark cycle) and allowed ad libitum access to standard mouse chow and tap water. The mice were allowed to acclimate to these conditions for at least 7 days before inclusion in the experiments.
As shown in Figure 1, 24 animals were separated into 3 groups (n = 8 per group): control, model and ginseng (30 mg/kg/day) groups. Except control group, animals in model and ginseng groups were initially received a single intraperitoneal injection of azoxymethane (AOM, 7.5 mg/kg). One week after the AOM injection (set as Day 1 or Week 0), the animals began to receive 2.5% dextran sulfate sodium (DSS) in drinking water for 7 consecutive days. The ginseng group animals also received American ginseng extract at 300 ppm in high fat diet chow for up to 77 consecutive days. We calculated that the daily dose of the ginseng was approximately 30 mg/kg/day. Bodyweight was recorded from Day 0 to Day 77. The stool samples were collected at Days 0, 28, 42, 56 and 70. The animals were sacrificed at Day 77 and gut tissue samples were obtained.
Fig. 1.
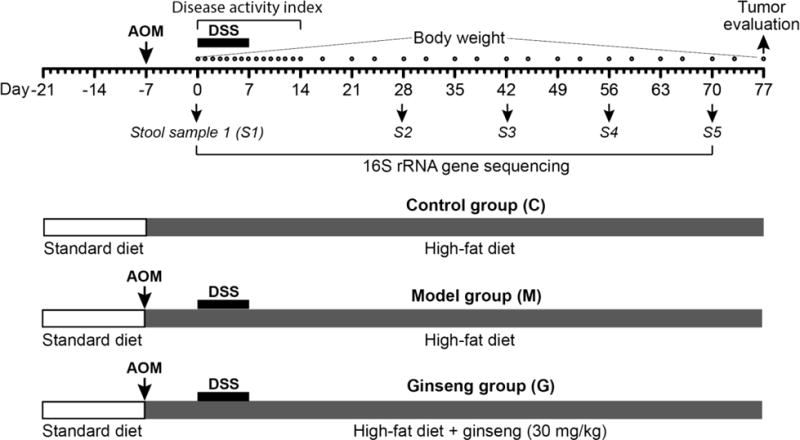
Experimental protocol. Experimental A/J mice were divided into three groups, i.e., control (or negative control) group, model group, and American ginseng group. Animals in the model and ginseng groups initially received a single intraperitoneal injection of azoxymethane (AOM; 7.5 mg/kg). One week after the AOM administration (set as Day 0), mice in the model and ginseng groups received 2.5% dextran sodium sulfate (DSS) in drinking water for 7 consecutive days. Before AOM injection, mice received a standard diet. After AOM injection, mice received a high fat diet, and mice in the ginseng group also received oral American ginseng extract 30 mg/kg/day from Day -7 to Day 77. Fecal samples were collected on Day 0, 28, 42, 56 and 70.
Paraffin-embedded gut tissue samples were serially sectioned and stained with hematoxylin and eosin (H&E). The stained sections were subsequently examined for histopathological changes by a gastrointestinal pathologist.
Disease activity index and histological assessment
AOM/DSS-induced acute colitis was scored as the disease activity index (DAI), as described previously [13]. In brief, the DAI was the combined score of weight loss (0, none; 1, 0–5%; 2, 5–10%; 3, 10–20%; 4, >20%), stool consistency change (0, none; 2, loose stool; 4, diarrhea), and bleeding (0, none; 1, trace; 2, mild hemoccult; 3, obvious hemoccult; 4, gross bleeding), and then divided by 3. The animals were scored for the DAI at the same time each day, by personnel blind to the treatment. The minimum score was 0 and the maximum score was 4. DAI scores were recorded daily from Day 0 to Day 14.
Fecal DNA extraction and 16S rRNA sequencing-based phylogenetic analysis
Before extraction, fecal samples collected from each group at each time point were mixed and homogenized. Fecal samples were mixed with 1 ml of extraction buffer [50 mM Tris (pH 7.4), 100 mM EDTA (pH 8.0), 400 mM NaCl, 0.5% SDS] containing 20 ul proteinase K (20 mg/ml). 500 μl of a slurry of 0.1-mm-diameter zirconia/silica beads (BioSpec Products, Bartlesville, OK) were added into the extraction tubes and a Mini-Beadbeater-8k Cell Disrupter (BioSpec Products) set on 5 min was used to lyse the microbial cells. After overnight incubation at 55°C, standard DNA extraction with phenol:chloroform:isoamyl alcohol and precipitation with ethanol were performed. Isolated DNA was dissolved in TE buffer and stored at −80°C [14].
16S rRNA-based illumina library preparation and MiSeq sequencing was used to investigate the microbial structure. The PCR primers used were specific for the 515–806 bp region of the 16S rRNA encoding gene (338F: 5′-GTGCCAGCMGCCGCGGTAA-3′ and 806R: 5′-GGACTACHVGGGTWTCTAAT-3′) and contain Illumina 3′ adapter sequences as well as a 12-bp barcode. This barcode-based primer approach allows sequencing of multiple samples in a single 454 sequencing run without the need for physical partitioning. Sequencing was performed by an Illumia MiSeq DNA sequencer at the Argonne National Laboratory. Sequences were then trimmed and classified with the QIIME toolkit. Using the QIIME wrappers, OTUs were picked at 97% sequence identity using cdhit and a representative sequence was then chosen for each OTU by selecting the most abundant sequence in that OTU. These representative sequences were aligned using PyNAST and taxonomy was assigned to them using the RDP Classifier. The PyNAST-aligned sequences were also used to build a phylogenetic tree with FastTree and unweighted UniFrac distances were then computed between all samples for additional ecological analyses, including principal coordinates analysis (PCoA) [14].
Statistical analysis
All data were expressed as mean ± S.D. A student’s t-test and a one-way ANOVA with Tukey’s post hoc test were used to test the significance of the differences between ginseng treatment and model groups. The statistical significance was set at P < 0.05.
Results
Chemical analysis of ginseng root using HPLC
The chemical structures of nine ginsenosides are shown in Figure 2A. Based on the HPLC procedures described previously, reverse-phase HPLC appeared efficient for the qualitative and quantitative determination of ginsenosides. The HPLC chromatogram of the ginseng extract is shown in Figure 2B. The contents of ginsenosides Rb1, Rb2, Rb3, Rc, Rd, Re, Rg1, Rg2 and Rh1 in the root were 28.9%, 0.6%, 0.6%, 5.2%, 8.8%, 13.4%, 1.7%, 0.1% and 0.2%, respectively. The total ginsenoside content in the root was 59.4%, Ginsenoside Rb1, Rc, Rd and Re were the major constituents in the root.
Fig. 2.
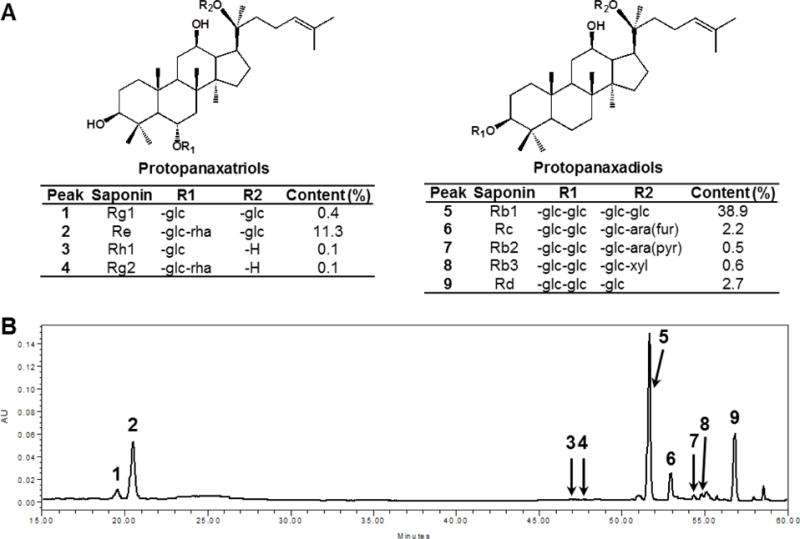
Chemical structures and HPLC analysis of ginsenosides in American ginseng extract. The structures of determined ginsenosides are shown in (A). HPLC chromatogram of American ginseng extract recorded at 202 nm is shown in (B). HPLC conditions are described in the Materials and Methods. Nine ginsenosides were determined. Ginsenosides (peak numbers) in the chromatogram: Rg1 (1), Re (2), Rh1 (3), Rg2 (4), Rb1 (5), Rc (6), Rb2 (7), Rb3 (8) and Rd (9).
Ginseng ameliorates AOM/DSS-induced colitis and body weight reduction
After DSS treatments, animals showed diarrhea and rectal bleeding starting from Day 3 in the model group. As the treatment continued, the presence and development of inflammation were clearly demonstrated. The disease severity, scored by disease activity index (DAI) reached highest level on Day 8. Figure 3A shows that ginseng feeding reduced the DAI score. One-way ANOVA was used to compare the differences between groups. Statistical significance was found between the model and ginseng groups (P = 0.0268). This suppression of the colitis was not only evident during DSS treatment, but also obvious several days after the cessation of its administration, suggesting that ginseng significantly promoted the recovery from the colitis.
Fig. 3.
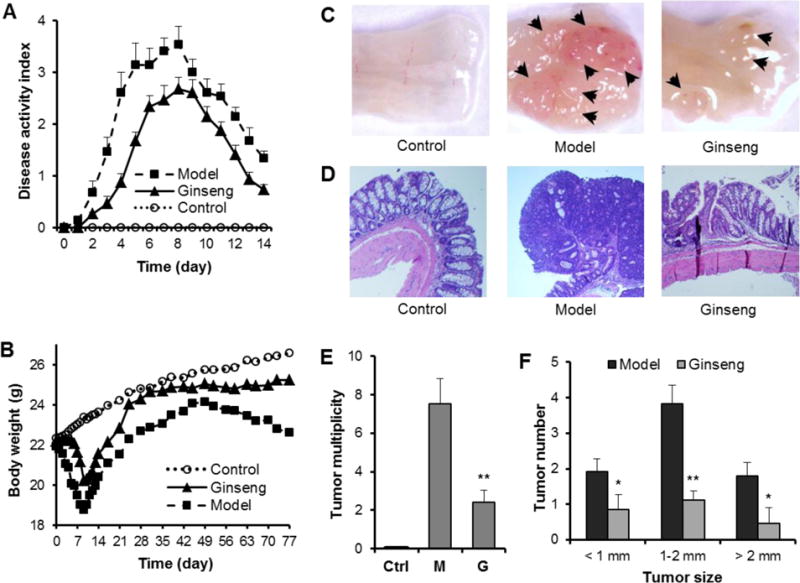
Effects of American ginseng on AOM/DSS-induced colitis and colon tumorigenesis in A/J mice. (A) American ginseng attenuated the experimental colitis, expressed as disease activity index (DAI). Data from the control group are all zeros from Day 0 to Day 14. P = 0.0268, model vs ginseng; P = 1.75 × 10−7, model vs control; P = 0.0002, ginseng vs control. (B) American ginseng reduced DSS-induced body weight changes. While the model group showed significant weight reduction from Day 3, American ginseng treatment significantly reduced the DSS-induced weight reduction. P = 0.0021, model vs ginseng; P = 0.0010, model vs control; P = 0.0123, ginseng vs control; one-way ANOVA with Tukey’s post hoc test. (C) Representative macroscopic morphology of colon tumors in the control (or negative control), model, and ginseng groups. Arrows indicate the azoxymethane/dextran sodium sulfate-induced tumors. (D) Representative hematoxylin and eosin stained histological sections of the control, model, and ginseng groups. (E) Tumor multiplicity was reduced very significantly in the ginseng group compared to the model group. (F) Tumor size distribution. * P < 0.05; **, P < 0.01 vs, model group; student’s t-test.
Figure 3B showed body weight changes in different experiment groups. Compared to the control group, which slowly gained weight, the model group had significant weight reduction starting from Day 3. This reduced weight continued up to Day 9, two day after cessation of the DSS. However, the ginseng group significantly reduced the body weight reduction (P = 0.0021 compared to the model group).
Ginseng treatment decreased colon tumorigenesis
AOM/DSS-induced tumor multiplicity was evaluated at Day 77. The representative macroscopic morphology for the control, model and ginseng treatment group are shown in Figure 3C. Obvious tumorigenesis was observed in the model group. However, in the ginseng group, there were fewer tumors and the tumors were smaller in size. The representative histological H&E staining sections of the four groups are shown in Figure 3D. In the colon tissue, multifocal adenomatous lesions were observed, mild inflammation with cryptitis, goblet cell loss and fibrosis changes were observed in the model group. In the ginseng group, the mucosa showed tightly packed glands with a normal amount of goblet cells, and crypt architecture remained normal. In addition, the histological sections of the ginseng treatment group are more similar to the normal control group. Tumor multiplicity data are shown in Figure 3E. Compared to the model group, ginseng treatment significantly reduced the tumor number (P <0.01). As shown in Figure 3F, the majority of tumors in the model group are 1–2 mm in size. Ginseng treatment significantly reduced tumor numbers in different size ranges (P<0.05).
Effects of ginseng on intestinal microbiome structures in phylum and class levels
To determine whether tumor multiplicity between the model and ginseng treatment groups could be correlated with differences in bacterial colonization, we analyzed the bacterial communities in stool samples. 16S rRNA gene clone libraries were established and sequenced. After DSS treatment, at Day 28, the majority of sequences were classified into three phyla Firmicutes, Bacteroidetes and Verrucomicrobia (Figure 4A). Compared to the control (40.1% and 0%), the abundance of Firmicutes was obviously lower (8.0%), and Verrucomicrobia was much higher in model group (10.4%). For the ginseng group, although the trend of abundance of the two phyla was similar to model group, the proportion was more similar to the control group (17.5% and 3.0%, respectively).
Fig. 4.
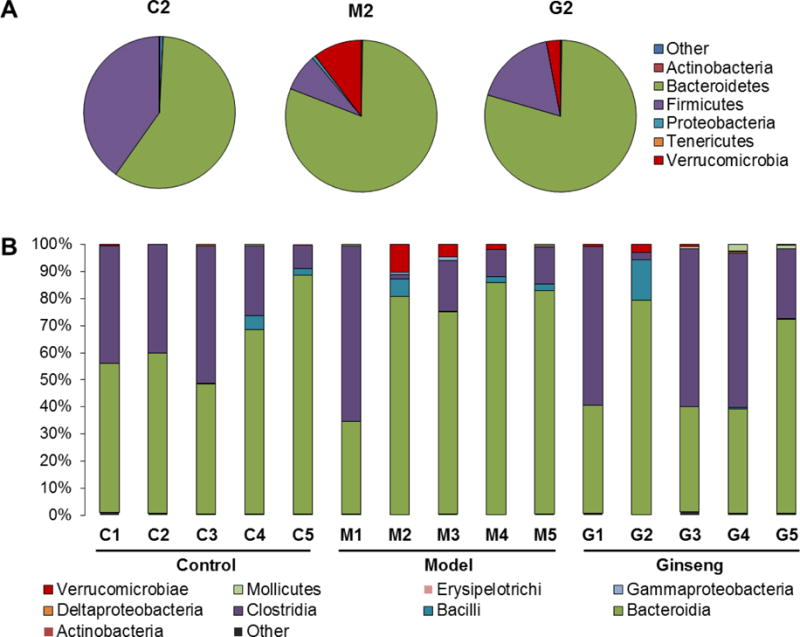
Distribution of phyla and class by time. (A) Distribution of phyla at Day 28 (Sample #2). Six phyla (Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, Tenericutes, Verrucomicrobia) represented at least 99% of the reads. (B) Distribution of class at different time points. After DSS treatment, the model group harbored higher proportions of Bacteroidaceae and Porphyromonadaceae. American ginseng treatment restored microbiome populations that closed to the control group. Numbers 1, 2, 3, 4, 5 represent sampling time points at Day 0, 28, 42, 56 and 70, respectively.
To further investigate the microbiome populations, at the class level, among the three groups, relatively stable composition was observed in the normal control group during the experiment. However, there was a clear difference in the representation of those class in mice in the other two groups, especially at Day 28. After receiving DSS, the proportion of Bacteroidia was significantly increased in the model group, while the level of Clostridia obviously decreased, and then slowly increased. In contrast, in the ginseng treatment group, although the proportion of Clostridia was obviously decreased at Day 28, its level was quickly increased at Day 42, and the level of Bacteroidia was decreased. Interesting, the level of Verrucomicrobiae was obviously increased after DSS treatment in the model group (10.4% at Day 28); it slowly decreased at Day 42 (4.6%) and Day 56 (1.8%). For the ginseng treatment group (3.0% at Day 28), the level of Verrucomicrobiae was quickly decreased at Day 42 (0.7%) (Figure 4B). This result suggests that DSS significantly changed the intestinal microbiota compositions, while ginseng treatment quickly restored the gut microbiota population, so that it resembled that of the normal control group.
Intestinal microbiome abundance changes in family level
In order to gain a deeper insight into the specific bacterial phylotype changes of the mouse feces from different groups, the abundance of the intestinal microbiome at a species level was analyzed. As indicated above, a class-level shift was evident in AOM/DSS-treated model mice (Figure 4B), with obviously increased Bacteroidia and Verrucomicrobiae, and decreased Clostridia. These trends were also detectable at lower taxonomic levels, including increased Bacteroidaceae, Porphyromonadaceae, Enterobacteriaceae and Verrucomicrobiaceae (Figure 5A) and decreased Clostridiaceae, Catabacteriaceae, Lachnospiraceae and Ruminococcaceae (Figure 5B). Ginseng treatment restored the changes induced by AOM/DSS treatment, to depress/elevate the abundance of these microbial species so that they resembled that of the normal control group.
Fig. 5.
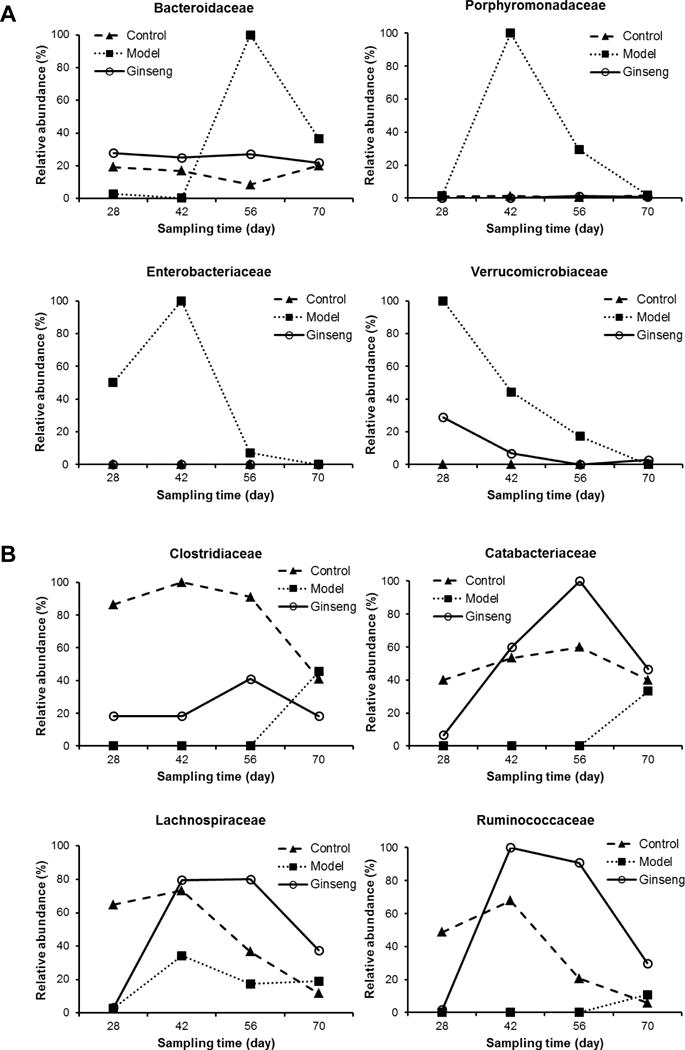
Relative microbial abundance of fecal microbiota changes in family level throughout treatment process. (A) Higher relative abundance in the model group after DSS treatment. (B) Lower relative abundance in the model group after DSS treatment. Ginseng treatment restored microbial abundance that resembled that of the control group.
Alpha diversity of intestinal microbiota in different groups
Alpha diversity was assessed by observed species, which reflects the richness by measuring the number of operational taxonomic units (OTUs), and Shannon diversity index based on 16S rRNA gene deep sequencing data. As shown in Figure 6A, in all three groups, at beginning of the experiment (Day 0), the observed species were approx. 200. For the control group, there was little change during the experiment. However, for the model group, after the administration of DSS, the observed species was obviously decreased. Then, the observed species slowly increased, and at Day 70, it was similar to that of the control group. For the ginseng treatment group, although the observed species was reduced (Day 28), it quickly returned to the normal value at Day 42. Ginseng treatment attenuated the species reduction by the DSS treatment.
Fig. 6.
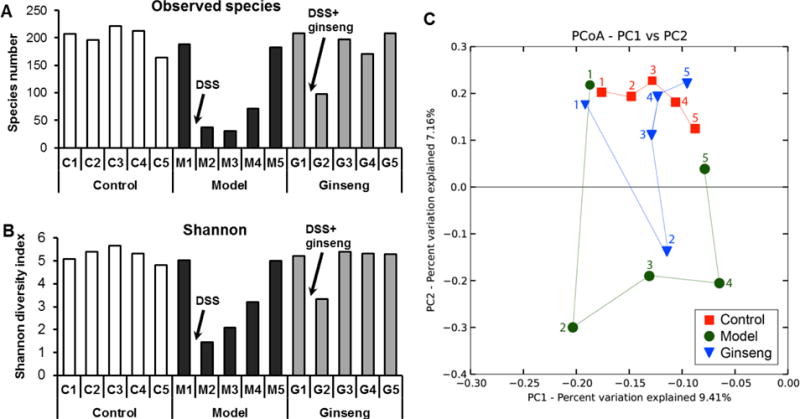
Microbial structural comparison of fecal microbiota. (A) Species richness of fecal microbiota in different groups. In model group, after DSS treatment, the number of species was reduced obviously. American ginseng treatment attenuated the influence of DSS treatment. (B) Shannon diversity index of fecal microbiota in different groups. Similar to the species richness results, American ginseng treatment restored the negative influence by DSS. (C) Principal coordinate analysis (PCoA) scores plot. Each point represents the principal component score of all rats in a group at a certain time point. The abbreviations of group names are as follows: C: control; M: model; G: ginseng treatment.
The Shannon index, which indicates the diversity by taking account of both the number of OTUs and the evenness of distribution of reads among the OTU categories, was also estimated (Figure 6B). Diversity was obviously decreased in DSS model group at Day 28, and then slowly increased. Similar to the observed species, ginseng treatment increased the diversity, suggesting that ginseng treatment restored the diversity of microbial community. Therefore, alpha diversity was obviously associated with colitis. A greater severity of colitis was negatively associated with the number of observed bacterial species and Shannon index.
Beta diversity of intestinal microbiota among three groups
Beta diversity analysis considers biodiversity between groups of samples, focusing on the elements that are either unique to or shared among groups of samples. In this study, beta diversity analysis was based on 454 pyrosequencing. The Hellinger distance was used to generate a matrix of pairwise sample dissimilarities between communities; a scatter plot (Figure 6C) was then generated from the matrix of distances using principal coordinates analysis (PCoA) [15]. As shown in Figure 6C, at Day 0, the plots of three groups were located very close together, suggesting the similarity of intestinal microbiome communities of three groups. After Day 28, there was an obvious separation of control and model groups. A similar difference was observed in the ginseng treatment groups at Day 28. However, from Day 42 to Day 70, the plots of the ginseng group were very close to those of the control group. This result suggests that ginseng treatment resolved AOM/DSS-induced abnormalities in the microbiome community.
Discussion
CRC is the second leading cause of cancer-related deaths in the United States, and it is responsible for over 10% of all cancer treatment expenditures in the U.S. [1]. Inflammatory bowel disease (IBD) is a group of inflammatory conditions in the intestine in humans, and chronic inflammation is recognized as a risk factor for CRC initiation and development [2]. The clinical management of CRC invariably involves diverse conventional modalities, including surgery, radiation, and chemotherapy. However, the complex characteristics of CRC require some alternative management to improve the therapeutic efficacy of conventional treatment and the quality life of cancer patients. Natural products, including herbal medicines have recently gained attention for cancer management.
Asian ginseng (Panax ginseng), a commonly used herbal medicine in Asia, has many reported health benefits [16, 17]. A case-control study on over one thousand pairs in Korea showed that Asian ginseng intakers had a decreased risk for many different cancers compared with non-intakers [18]. American ginseng (Panax quinquefolius) is a major ginseng species produced in North America. Saponins in American ginseng have been identified and fall into two major groups: the 20(S)-protopanaxadiol ginsenosides (PPDs; e.g., Rb1, Rb2, Rb3, Rc, Rd) and the 20(S)-protopanaxatriol ginsenosides (PPTs; e.g., Re, Rg1, Rg2, Rh1) [19]. We previously observed that after oral administration, parent ginseng compounds were biotransformed by the enteric microbiome to metabolites such as Rg3, Rh1, CK and PPD [20]. Interestingly, we recently observed significant ginsenoside Rb1 and compound K plasma level differences in human subjects consuming Asian and Western diets, suggesting that the differences in enteric microbiota structures could be determined by cultural norms (e.g. diet), or host factors (e.g. genetics) influencing ginseng compound bioavailability [21]. We have also shown that those ginseng metabolites have potent anticancer effects on CRC [11]. Recently, we observed that American ginseng inhibited chemically-induced colitis and tumorigenesis [12]; However, in these studies, the ginseng dose was difficult to control because the extract was added to the drinking water. In addition, the diet contained low levels of fat, which is different from the Western diet.
In this study, the effects of American ginseng on gut colitis and colorectal tumorigenesis were investigated using an AOM/DSS mouse model. AOM/DSS is a chemical compound induced model, characterized by early inflammation followed by tumor development [22]. Azoxymethane (AOM, methyl-methylimino-oxidoazanium) is a pro-carcinogen that is metabolized in the liver and further metabolized in the colon to produce an active carcinogenetic alkylating agent. AOM targets proto-oncogenes to activate mutations in β-catenin and K-Ras [23]. DSS is a polysulfated polymer that arrests colonic crypt cell re-generation, leading to acute mucosal ulceration and clinical colitis that enhances tumorigenesis. The AOM/DSS model is a commonly-used experimental gut-specific animal model for pharmacological studies [22].
Lifestyle, especially dietary factors, are believed to contribute substantially to the risk of CRC development [3, 4]. In this regard Western diets that are rich in Western-style fats have been shown to promote CRC [24]. Recent studies has demonstrated that high fat Western diet induces gut barrier dysfunction and systemic chronic inflammation, which is associated with the dysbiosis of intestinal microbiota, while these changes could be restored to normal levels by standard diet with fiber supplements [25, 26]. To mimic the diet in Western societies, in this study, a high fat diet (contains 20% of fats) was used. In the acute phase, ginseng significantly suppressed DSS-induced colitis. Ginseng treatment also reduced AOM/DSS-induced bodyweight loss. Chronic ginseng feeding significantly decreased tumor multiplicity. These results confirmed our previous observations in AOM/DSS mice fed with a standard low fat diet. In addition, DAI data and histologic evidence suggested that ginseng’s actions on AOM/DSS mice were exerted via inhibition of gut inflammation.
Microbes are an important component of the human body. Approximately 70% of human microbial cosmos is localized in the gut, while the colon is the site where the gut microbiota reaches its highest concentration [9]. Gut microbes produce a large number of bioactive compounds that can influence health. In some disease states such as IBD, a reduction in overall microbiome diversity is common in dysbiosis, in which the composition and activities of the microbiota are altered to a state that may be deleterious to the host. Nutrient or drug intervention may influence the structure of microbiota. Elucidating the mechanisms behind the host-microbe interaction involved in health and CRC is a prerequisite for understanding processes and the subsequent development of drugs and therapies.
To investigate the role of microbiota in ginseng-mediated anticancer activity, 16S rRNA gene clone libraries were established and MiSeq sequencing was carried out. Alpha diversity of gut microbiota revealed a profound difference between the control and AOM/DSS model groups. In the model group, a much higher tumor burden was characterized by lower alpha diversity, evidenced by lower numbers of observed species and Shannon index. Beta analysis showed that compared to the control, in the model group after AOM/DSS, the gut microbiome profile was severely changed. This adds support to the view that AOM-induced DNA damage and DSS-induced inflammatory perturbations may contribute to the development of a dysbiosis in the microbial community that contributes to the formation of tumors in mice. After ginseng treatment, numbers of species observed and Shannon index were obviously increased. Ginseng treatment restored the microbiota profiles, and preserved the gut homeostasis.
Taxa analyses showed that the composition of the microbiome community in each group was very different. AOM/DSS-induced colitis and tumorigenesis may have contributed to dysbiosis in the model group. The majority of sequences fell into three phyla, Firmicutes, Bacteroidetes and Verrucomicrobia, while the proportions of these three phyla were different between the healthy and pathologic groups. At the species level, Bacteroidaceae was obviously increased in the model group, especially after Day 56. In an in vivo study, colon tumor-bearing mice showed enrichment in OTUs affiliated with members of the Bacteroidaceae. Conventionalization of germ free mice with microbiota from tumor-bearing mice significantly increased tumorigenesis in the colon compared to that of animals colonized with a healthy gut microbiome [27]. In another study, Porphyromonadaceae were shown to be positively related to the tumorigenesis rate in mice transplanted with fecal microbiota from CRC patients [28]. In previous studies, lower levels of Clostridiaceae and Lachnospiraceae were observed in CRC patients [29]. A recent clinical study data suggested that stools from CRC patients were depleted in a network of Clostridia operational taxonomic units from several families including Clostridiaceae [30]. Further studies are needed to investigate the role of these microbiome families in CRC initiation and development. Nevertheless, our results suggested that gut inflammation and colorectal tumorigenesis are associated with microbial dysbiosis, which can be restored by ginseng treatment.
In conclusion, we observed that American ginseng significantly attenuated high fat diet associated chemically-induced colitis and colon tumorigenesis, which is evidenced by the colitis score and tumor multiplicity data. The 16S rRNA gene-based analysis showed that for the fecal microbial communities, a greater severity of colitis was negatively associated with alpha diversity. With beta diversity, there was an obvious separation of the control and model groups induced by AOM/DSS, however, the plots of the ginseng treatment group were very close to those of the healthy control group. Chemically induced colitis and colon tumorigenesis was correlated with augment of Bacteroidaceae and Porphyromonadaceae, while the proportion of these species was diminished by the treatment of ginseng. Our results suggest that the CRC chemopreventive effects of American ginseng are mediated through enteric microbiome population-shift recovery and dysbiosis restoration. Ginseng’s regulation of the microbiome balance contributes to the maintenance of enteric homeostasis.
Acknowledgments
Funding
This work was supported in part by the grants of NIH AT004418, AT005362, GM120046 and 5P30DK042086.
Footnotes
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Animals were cared for in the animal facility at the University of Chicago. This facility follows NIH guidelines for the humane care of animals. Use of these animals were approved under the guidelines of the Animal Care and Use Committee and those of the University of Chicago, both of which comply with the guidelines outlined by the National Institutes of Health.
Ethical statement
The manuscript does not contain clinical studies or patient data
References
- 1.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–93. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Lewis B, Lin J, Wu X, Xie H, Shen B, Lai K, et al. Crohn’s disease-like reaction predicts favorable prognosis in colitis-associated colorectal cancer. Inflamm Bowel Dis. 2013;19(10):2190–8. doi: 10.1097/MIB.0b013e31829e13e1. [DOI] [PubMed] [Google Scholar]
- 3.Whittemore AS, Wu-Williams AH, Lee M, Zheng S, Gallagher RP, Jiao DA, et al. Diet, physical activity, and colorectal cancer among Chinese in North America and China. J Natl Cancer Inst. 1990;82(11):915–26. doi: 10.1093/jnci/82.11.915. [DOI] [PubMed] [Google Scholar]
- 4.Willett WC. Diet and cancer. Oncologist. 2000;5(5):393–404. doi: 10.1634/theoncologist.5-5-393. [DOI] [PubMed] [Google Scholar]
- 5.Wang CZ, Anderson S, Yuan CS. Phytochemistry and anticancer potential of notoginseng. Am J Chin Med. 2016;44(1):23–34. doi: 10.1142/S0192415X16500026. [DOI] [PubMed] [Google Scholar]
- 6.Jiao R, Liu Y, Gao H, Xiao J, So KF. The anti-oxidant and antitumor properties of plant polysaccharides. Am J Chin Med. 2016;44(3):463–88. doi: 10.1142/S0192415X16500269. [DOI] [PubMed] [Google Scholar]
- 7.Yu C, Wen XD, Zhang Z, Zhang CF, Wu X, He X, et al. American ginseng significantly reduced the progression of high-fat-diet-enhanced colon carcinogenesis in Apc (Min/+) mice. J Ginseng Res. 2015;39(3):230–7. doi: 10.1016/j.jgr.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang CZ, Aung HH, Ni M, Wu JA, Tong R, Wicks S, et al. Red American ginseng: ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007;73(7):669–74. doi: 10.1055/s-2007-981524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 10.Sinha R, Ahn J, Sampson JN, Shi J, Yu G, Xiong X, et al. Fecal Microbiota, Fecal Metabolome, and Colorectal Cancer Interrelations. PLoS One. 2016;11(3):e0152126. doi: 10.1371/journal.pone.0152126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang CZ, Du GJ, Zhang Z, Wen XD, Calway T, Zhen Z, et al. Ginsenoside compound K, not Rb1, possesses potential chemopreventive activities in human colorectal cancer. Int J Oncol. 2012;40(6):1970–6. doi: 10.3892/ijo.2012.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu C, Wen XD, Zhang Z, Zhang CF, Wu XH, Martin A, et al. American ginseng attenuates azoxymethane/dextran sodium sulfate-induced colon carcinogenesis in mice. J Ginseng Res. 2015;39(1):14–21. doi: 10.1016/j.jgr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131(4):1122–30. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3(8):944–54. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riehle K, Coarfa C, Jackson A, Ma J, Tandon A, Paithankar S, et al. The Genboree Microbiome Toolset and the analysis of 16S rRNA microbial sequences. BMC Bioinformatics. 2012;13(Suppl 13):S11. doi: 10.1186/1471-2105-13-S13-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58(11):1685–93. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 17.Bing SJ, Ha D, Hwang I, Park E, Ahn G, Song JY, et al. Protective effects on central nervous system by acidic polysaccharide of Panax ginseng in relapse-remitting experimental autoimmune encephalomyelitis-induced SJL/J mice. Am J Chin Med. 2016;44(6):1099–110. doi: 10.1142/S0192415X16500610. [DOI] [PubMed] [Google Scholar]
- 18.Yun TK. Panax ginseng–a non-organ-specific cancer preventive? Lancet Oncol. 2001;2(1):49–55. doi: 10.1016/S1470-2045(00)00196-0. [DOI] [PubMed] [Google Scholar]
- 19.Qi LW, Wang CZ, Yuan CS. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry. 2011;72(8):689–99. doi: 10.1016/j.phytochem.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan JY, Liu P, Wang HY, Qi LW, Wang CZ, Li P, et al. Biotransformation and metabolic profile of American ginseng saponins with human intestinal microflora by liquid chromatography quadrupole time-of-flight mass spectrometry. J Chromatogr A. 2013;1286:83–92. doi: 10.1016/j.chroma.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 21.Wan JY, Wang CZ, Zhang QH, Liu Z, Musch MW, Bissonnette M, et al. Significant difference in active metabolite levels of ginseng in humans consuming Asian or Western diet: The link with enteric microbiota. Biomed Chromatogr. 2017;31(4) doi: 10.1002/bmc.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, et al. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guda K, Upender MB, Belinsky G, Flynn C, Nakanishi M, Marino JN, et al. Carcinogen-induced colon tumors in mice are chromosomally stable and are characterized by low-level microsatellite instability. Oncogene. 2004;23(21):3813–21. doi: 10.1038/sj.onc.1207489. [DOI] [PubMed] [Google Scholar]
- 24.Dougherty U, Mustafi R, Wang Y, Musch MW, Wang CZ, Konda VJ, et al. American ginseng suppresses Western diet-promoted tumorigenesis in model of inflammation-associated colon cancer: role of EGFR. BMC Complement Altern Med. 2011;11:111. doi: 10.1186/1472-6882-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang T, Gao X, Wu C, Tian F, Lei Q, Bi J, et al. Apple-derived pectin modulates gut microbiota, improves gut barrier function, and attenuates metabolic endotoxemia in rats with diet-induced obesity. Nutrients. 2016;8(3):126. doi: 10.3390/nu8030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson JS, Opiyo MN, Thomson M, Gharbi K, Seckl JR, Heger A, et al. 11beta-hydroxysteroid dehydrogenase-1 deficiency alters the gut microbiome response to Western diet. J Endocrinol. 2017;232(2):273–83. doi: 10.1530/JOE-16-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zackular JP, Baxter NT, Iverson KD, Sadler WD, Petrosino JF, Chen GY, et al. The gut microbiome modulates colon tumorigenesis. MBio. 2013;4(6):e00692–13. doi: 10.1128/mBio.00692-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baxter NT, Ruffin MTt, Rogers MA, Schloss PD. Microbiota-based model improves the sensitivity of fecal immunochemical test for detecting colonic lesions. Genome Med. 2016;8(1):37. doi: 10.1186/s13073-016-0290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 2013;105(24):1907–11. doi: 10.1093/jnci/djt300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters BA, Dominianni C, Shapiro JA, Church TR, Wu J, Miller G, et al. The gut microbiota in conventional and serrated precursors of colorectal cancer. Microbiome. 2016;4(1):69. doi: 10.1186/s40168-016-0218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


