Abstract
Background
Diagnosing Airway hyper-responsiveness (AHR) requires bronchial provocation tests that are performed at rest and after exercise or hyperventilation in either a lab or field setting. Presently, it is unclear whether the proposed AHR field test for swimming induces sufficient provocation due to lack of intensity. Thus we aimed to examine how the 8 minute field swim test compared to all out racing and a lower intensity practice exposure affected AHR. We hypothesized that the race would affect AHR the most thereby highlighting the importance of maximal effort in swim AHR.
Methods
10 female and 15 male swimmers completed three conditions (sanctioned race of different distances, 8 min field swim challenge and swim practice). Forced vital capacity (FVC), forced expired volume in 1 second (FEV1) and forced expiratory flow (FEF25-75) were measured at rest and after each exercise condition (at 6 and 10 min) in accordance with standard protocols. AHR was defined as a decrease in FEV1 of ≥10% post exercise.
Results
A significant increase in FEV1 and FEF25-75 was observed for both post swim field test and post-race. The practice condition reduced FEV1 in 44% of swimmers although the magnitude of change was small. There was a wide variability in the individual responses to the 3 conditions and AHR was diagnosed in one swimmer (race condition).
Conclusion
All conditions have poor sensitivity to diagnose EIB and total accumulated ventilation (distance swum) did not influence AHR. These results also indicate that elite swimmers, despite many risk factors, are not limited by respiratory function in race conditions. It is proposed that the swim field test not be used for AHR assessment in swimmers due to too high relative humidity.
Keywords: Exercise-induced bronchospasm, Airway hyper-responsiveness, Cough, Exercise induced asthma, Athletic performance, Respiratory health
1. Introduction
Airway dysfunction is the most prevalent chronic medical condition facing athletes (8% of all Olympic athletes)1 and the incidence increases (up to 76%) in what is described as high-risk sports such as swimming.2 Airway hyper-responsiveness (AHR) is a specific type of airway dysfunction in which the airways respond “too much and too easily to stimuli”.3
Elite swimmers undergo high volume and high intensity training in a chlorinated pool environment on most days of the week for several hours, sometimes accompanied by high levels of fatigue from inadequate sleep, illness symptoms, and other life stressors.4 Additionally, their pool training is rather unfavourable to overall lung health5 and can result in AHR. AHR is most often associated with acute airway narrowing post intense exercise6 and has been defined as exercise induced bronchoconstriction (EIB).7 For swimmers the high prevalence of EIB is likely due to a combination of ventilatory demand and airborne chlorine derivatives8 which have been shown to damage or cause remodeling in the airway epithelium.5 Swimmers have a higher prevalence of EIB and maximal decrease in FEV1 compared to other “high ventilation” athletes despite having larger forced vital capacities (FVC).9, 10
EIB can be assessed with bronchial provocation tests, which are both lab based11 and field tests12 that have been identified as both direct13 and indirect14 challenges to the airway. Ultimately, both direct and indirect challenges lead to constriction of the airways from either direct contraction of smooth muscle or indirect via inflammation leading to smooth muscle constriction.15 In swimmers the majority of research has focused on indirect laboratory challenges to determine EIB; however an eight minute field swim test as an indirect bronchial provocation test for swimmers10 has been proposed.
In general, sport specific field tests are sensitive and specific similar to lab based (exercise and non-exercise) bronchial provocation tests in identifying EIB.16 More importantly sport specific field tests replicate the degree of EIB that occurs in high ventilation training and racing situations (prolonged periods of heavy ventilation) providing direct insight on how EIB influences performance.12 Furthermore, given the unique environment that swimmers train and compete in heightens the importance of validating a swim specific field test. Yet only one swimming specific field test has been reported in the literature where it was found to be a poor surrogate for EIB compared to a lab based test.10 In this study, the degree of hyperpnoea was discussed as potentially inadequate limiting the magnitude of airway provocation because the prescribed intensity was not all out race pace. This speculation may be true because post-race EIB in youth swimmers provided similar EIB prevalence and magnitude of EIB to lab based challenges.17 Thus the role of intensity and the degree of hyperpnoea associated with a specific intensity requires further examination in the manifestation of EIB in swimmers.
Thus, our main aim was to examine the influence of swim specific intensity on EIB in elite swimmers. To answer this question we replicated the 8 min field swim challenge test which is high intensity and purported to induce sufficient hyperpnoea to provoke airways. These results were compared to all out intensity from a race and from a practice where the intensity was lower but of longer duration. Finally, to understand whether cumulative training volume influenced responsiveness to these specific intensities we grouped swimmers into sprint, middle and long distance. It was hypothesized that greater prevalence and magnitude of EIB would occur in the race condition compared to the field swim challenge test and that the field swim challenge test would induce more EIB than a normal practice. We hypothesized that the distance swimmers would have greater prevalence of EIB and the magnitude of the response would be greater compared to middle distance or sprint swimmers.
2. Methods
2.1. Study population
Twenty five varsity swimmers with 10 years or more of competitive swimming experience were recruited (10 female and 15 males). All swimmers were preparing for the National University Swimming Championships and were recruited to the program from high performance clubs. All were currently training and free from any diagnosed illness, respiratory infection or injury which prohibited them from their normal training program. Their training program for the 3 months prior to testing included 7-9 swim sessions, 2 dryland sessions, and one day off per week (Sunday). All participants had normal baseline FEV1 and FVC values for their age, height and gender (ATS/ERS task force: Standardization of lung function testing).18 Swimmers with a history of asthma or respiratory symptoms associated with exercise were not excluded. Based on expected prevalence from previous research on field or lab EIB testing we could conservatively estimate 10 percent prevalence with a precision of 5% and 95% level of confidence the sample size estimate would be 6.9 participants.
2.2. Experimental design
Participants were assessed at three different time points over a 4 week period. The order of testing was practice, swim field challenge, race. First swimmers were measured before and after swim practice in the pool environment. The swimmers were exposed to the pool environment for approximately 10 minutes before pre-practice spirometry was taken. Practice was approximately 90 minutes long and included some low intensity/kick sets as well as some “hard intensity intervals (duration between 30 seconds and 1 minute)”. Three days after practice intensity spirometry was completed swimmers completed the 8 min swim field test using the recommended protocol10 in an indoor 25 m pool. On the day of the 8 min swim field test participants had not completed any training or strenuous exercise a minimum 24 hours prior to their arrival at the pool. Swimmers were allowed to warm up for 400 m before starting their swim field challenge test. The participants were asked to “swim as far as possible in 8 minutes” and they all employed an even pace strategy based on their own assessment of fitness. To explain the swimmers decided upon a target 100 m pace they wanted to hold to achieve their farthest distance possible in the 8 minutes. Once 8 minutes had been surpassed swimmers were notified by placing a kick board in the water as they were about to complete their next flip turn. At that time the swimmer exited the pool and completed their post field test spirometry. Participants were asked on a scale of 0 -10 how hard they swam in the field test. This question was asked post spirometry to reduce the chance of immediate acute fatigue from biasing their perspective on how hard the entire 8 min swim was. Participants were asked to refrain from medications that might influence lung function (24 hours for short acting β2 agonists and 72 hours for inhaled corticosteroids) and abstain from caffeine 6 hours prior to their test.
The race condition spirometry was measured at a National level meet that was the key qualifier for the University Championships. Swimmers were measured after their “best/strongest race” which was decided a priori by the swimmers, the head coach and the research staff. The distance ranged from 50 m to 1500 m and the races were spread over a 2 day period. Given the range of distances raced swimmers were grouped into sprint (50, 100 m), middle (200, 400 m) and long distance (800, 1500 m) groups to determine influence of distance raced on EIB. The pool environment conditions were similar for both the practice and 8 min swim field challenge testing (28°C and 75 % relative humidity). The race condition ambient conditions were 30°C and 90 % relative humidity. The study received Institutional Research Ethics Board approval and all participants provided informed consent for all tests and procedures prior to starting the study.
2.3. Spirometry measures
Spirometry measures were performed on a portable spirometer (Spirolab III, Medical International Research, Rome, Italy) using recommended manufacturer guidelines. Participants completed baseline spirometry assessments of FEV1 and FVC to determine lung function according to “ATS/ERS Task Force: Standardisation of Lung Function Testing guidelines”.19 FEF25-75 was also recorded to provide an estimation of small airway function in athletes.20 Post condition spirometry was completed as follows. FEV1 was measured in duplicate at 6 and 10 minutes post condition in accordance with standard protocols.21 Minimum post-exercise FEV1 was the lowest recorded value post exercise regardless of time point using previously published protocols for determination of post exertion spirometry measures.22 The time point of 6 minutes was chosen post exercise to address the logistics of especially the race condition where swimmers are held until all competitors in their heat complete the race. This allowed for all participants to be ready to perform the post-race spirometry at 6 min. In addition the degree of severe hyperpnoea was evident (both in short and long races) after some swim field tests and some race distances due to expression of additional CO2 from the lungs due to anaerobic energy system buffering and Exercise Post Oxygen Consumption mechanisms. Thus, by having the first time point at 6 minutes respiration rate had returned to normal in all participants.
2.4. Statistical analysis
Descriptive statistics (means and standard deviations) were calculated for FEV1, FVC, FEV1/FVC and FEF25-75 and predicted spirometry measures. Paired t-tests were used to compare pre-post difference in FEV1, FVC, FEF25-75 in each condition. A one way repeated measures ANOVA (Condition) determined delta change between conditions for FEV1, FVC, FEF25-75. Pairwise comparisons were made with 95 % confidence intervals. A two-way repeated measures ANOVA (Condition x Group) determined influence of distance background on delta change for each condition. Pearsons Product correlational determined any relationships between race distance, baseline characteristics and delta change for spirometry measures. Statistical analysis was performed using SPSS version 21 (IBM Corp., Armonk, NY, USA). P-values below 0.05 were considered significant.
3. Results
3.1. Baseline anthropometric and spirometry measures
Male swimmers were 20.5 ± 2.4 years of age, 180.5 ± 6.7cm in height, and weighed 78.8 ± 9.4 kg. Female swimmers were 20.5 ± 2.4 years of age, 168.3 ± 4.8 cm in height, and weighed 68.1 ± 7.0 kg. One male swimmer had been diagnosed with asthma and used a short acting β2 agonist when required. Individual results are reported in Table 1 for baseline spirometry. There was a wide range in the FEV1/FVC ratio with 5 swimmers less than 0.70 (70%) the most common FEV1/FVC cutoff for indication of airway obstruction. There were 3 swimmers whose baseline FEV1 was less than predicted (87.1 – 93.0 % of predicted) and all swimmers FVC was greater than predicted (see Table 1). There were no differences in percent of predicted baseline spirometry values between sprint, middle and long distance groups except FVC between long and middle distance groups (151.4 % long and 125.8 % for middle distance).
Table 1.
Participant baseline spirometry and individual change for FEV1, FVC and FEF 25-75 in response to practice, swim test and race conditions. Baseline spirometry expressed in percent of predicted for age, height and gender. Practice, swim and race delta change expressed as percent change from pre-test values. Range, Minimum, Maximum, Mean and Standard Deviation are provided for the entire sample (n = 25). Statistical significance (p) is provided as the pre-post difference for each measure at each condition.
| Group | Event Time | Gender | FEV% | FVC% | FEV1/FVC% | FEV1 Δ Practice (%) | FVC Δ Practice (%) | FEF25-75 Δ Practice (%) | FEV1 Δ Swim Test (%) | FVC Δ Swim Test (%) | FEF25-75 Δ Swim Test (%) | FEV1 Δ Race (%) | FVC Δ Race (%) | FEF25-75Δ Race (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sprint | 23.7 | M | 134.8 | 128.4 | 108.4 | -2.1 | -1.5 | -5.4 | 7.7 | -1.8 | 27.7 | 3.9 | 1.7 | -2.8 |
| Sprint | 26.0 | M | 93.0 | 128.4 | 74.7 | 6.1 | 6.9 | 9.2 | 6.8 | 1.1 | 16.3 | 6.4 | 2.2 | 13.5 |
| Sprint | 27.2 | M | 141.6 | 162.5 | 89.6 | 3.1 | -0.3 | 4.9 | -0.2 | -1.0 | -1.8 | 4.8 | -2.1 | 13.0 |
| Sprint | 27.6 | M | 132.4 | 167.0 | 81.7 | 0.0 | 1.2 | -0.4 | 1.7 | -1.0 | 5.8 | 3.6 | -1.2 | 11.9 |
| Sprint | 30.3 | F | 178.0 | 198.2 | 91.9 | -5.8 | -2.4 | -16.0 | 4.3 | -1.5 | 15.1 | 2.6 | 2.7 | 1.9 |
| Sprint | 32.6 | F | 124.5 | 131.3 | 97.5 | -1.8 | 2.4 | -4.0 | 1.7 | -2.9 | 5.3 | -0.9 | -0.7 | -3.7 |
| Sprint | 33.0 | M | 146.0 | 152.8 | 98.7 | -0.7 | 1.3 | -1.6 | -2.9 | -2.1 | -5.5 | 9.6 | -4.0 | 44.3 |
| Sprint | 56.3 | M | 106.9 | 141.3 | 77.8 | 2.4 | -0.5 | 5.3 | 1.3 | -1.3 | 3.3 | -2.8 | -3.7 | -1.6 |
| Sprint | 57.7 | M | 119.5 | 145.0 | 85.1 | 2.2 | 3.5 | 1.7 | -2.4 | 0.9 | -3.9 | 11.2 | 2.3 | 26.8 |
| Sprint | 59.0 | F | 142.0 | 149.1 | 97.8 | -3.3 | -4.0 | -5.0 | 2.4 | -1.1 | 7.5 | 2.4 | -11.2 | 36.8 |
| Middle | 127.7 | F | 108.0 | 112.9 | 98.3 | 0.5 | 1.1 | -0.2 | 2.1 | -1.8 | 8.8 | -2.3 | -1.1 | 1.2 |
| Middle | 133.0 | F | 117.2 | 122.3 | 99.1 | 0.5 | 2.5 | 3.6 | 0.8 | 1.4 | -5.3 | -2.6 | 0.0 | -1.7 |
| Middle | 136.6 | F | 87.1 | 106.5 | 84.0 | -1.7 | -4.8 | 7.5 | 7.6 | 11.4 | 5.3 | 10.2 | -0.5 | 25.0 |
| Middle | 140.3 | F | 92.0 | 101.1 | 94.4 | 2.7 | 1.9 | 9.5 | -1.7 | -0.5 | -1.9 | 1.7 | 0.2 | 7.0 |
| Middle | 142.9 | F | 120.9 | 128.5 | 96.9 | 2.0 | 0.7 | 7.0 | 2.4 | -1.1 | 1.3 | 7.7 | -1.8 | 24.6 |
| Middle | 146.3 | M | 115.6 | 140.6 | 84.7 | 2.8 | 1.4 | 8.2 | 6.4 | -0.2 | 16.8 | 13.1 | -3.9 | 56.9 |
| Middle | 273.6 | M | 131.9 | 176.1 | 77.6 | 2.9 | 1.9 | 7.9 | -1.3 | -0.1 | -4.5 | 7.1 | -1.0 | 15.3 |
| Long | 541.8 | F | 125.9 | 133.9 | 96.9 | -1.5 | -1.8 | -0.4 | 1.7 | 0.2 | 0.5 | 0.9 | 0.0 | 10.3 |
| Long | 552.4 | F | 126.9 | 137.0 | 94.8 | -1.2 | -2.2 | -1.4 | 12.2 | 11.0 | 12.0 | 4.5 | -1.2 | 14.2 |
| Long | 949.0 | M | 150.9 | 161.1 | 96.8 | -1.2 | 1.6 | -3.1 | 2.2 | 0.4 | 6.4 | -14.0 | 0.9 | 15.4 |
| Long | 950.0 | M | 121.3 | 162.9 | 76.6 | 1.8 | -2.6 | 9.6 | 7.1 | -2.2 | 16.9 | 1.3 | -1.9 | 2.8 |
| Long | 989.1 | M | 138.3 | 156.7 | 91.0 | 2.5 | 1.8 | 4.4 | 4.7 | -1.6 | 16.0 | 6.7 | -0.7 | 20.4 |
| Long | 989.9 | M | 127.9 | 155.3 | 85.2 | 3.2 | -0.5 | 6.9 | -5.6 | -0.7 | -2.4 | 3.3 | -0.3 | 10.3 |
| Long | 996.9 | M | 143.1 | 150.8 | 97.8 | -0.4 | -0.2 | -0.2 | 1.5 | 1.1 | 3.1 | 5.0 | -2.3 | 19.3 |
| Long |
1007.7 |
M |
137.9 |
153.2 |
92.2 |
-2.7 |
0.2 |
-8.1 |
1.7 |
0.7 |
8.0 |
4.1 |
-1.0 |
10.4 |
| Range | 90.9 | 97.1 | 33.7 | 11.8 | 11.7 | 25.5 | 17.8 | 14.4 | 33.2 | 27.0 | 13.9 | 60.7 | ||
| Min | 87.1 | 101.1 | 74.7 | -5.8 | -4.8 | -16.0 | -5.6 | -2.9 | -5.5 | -14.0 | -11.2 | -3.7 | ||
| Max | 178.0 | 198.2 | 108.4 | 6.1 | 6.9 | 9.6 | 12.2 | 11.4 | 27.7 | 13.1 | 2.7 | 56.9 | ||
| Mean | 126.5 | 144.1 | 90.8 | 0.4 | 0.3 | 1.6 | 2.5 | 0.3 | 6.0 | 3.5 | -1.1 | 14.9 | ||
| SD | 20.1 | 22.1 | 8.7 | 2.6 | 2.5 | 6.4 | 3.9 | 3.5 | 8.6 | 5.5 | 2.8 | 14.9 | ||
| p | 0.56 | 0.43 | 0.70 | 0.02 | 0.83 | 0.01 | 0.03 | 0.55 | 0.00 |
3.2. Pre-post changes in spirometry for all conditions
The post-practice FEV1 was decreased in 11 (44 %) swimmers (see Table 1) as well as FVC (11 (44 %) swimmers with decreases in FVC post practice; see Table 1) and 12 swimmers (48 %) decreased FEF 25-75 post practice (one swimmer -16 %). The field swim post-test FEV1 was decreased in 6 (24 %) swimmers (range -5.6 to –0.2 %); FVC was decreased in 16 (64 %) swimmers (all decreases -3 to 0 %) and 7 swimmers decreased FEF 25-75 post-test (range between -5.5 to -1.8 %). In the race condition one swimmer demonstrated a decrease in FEV1 of ≥10% indicating EIB (-14.0 % after his 1500 m race). Four other swimmers saw a decrease in FEV1 post-race, 4 (16 %) swimmers decreased FEF 25-75 (<5 % decrease) and 17 (68 %) swimmers decreased FVC post-race (changes in most swimmers; see Table 1) with one of these 17 swimmers decreasing FVC 11.2 % (100 m Freestyle race).
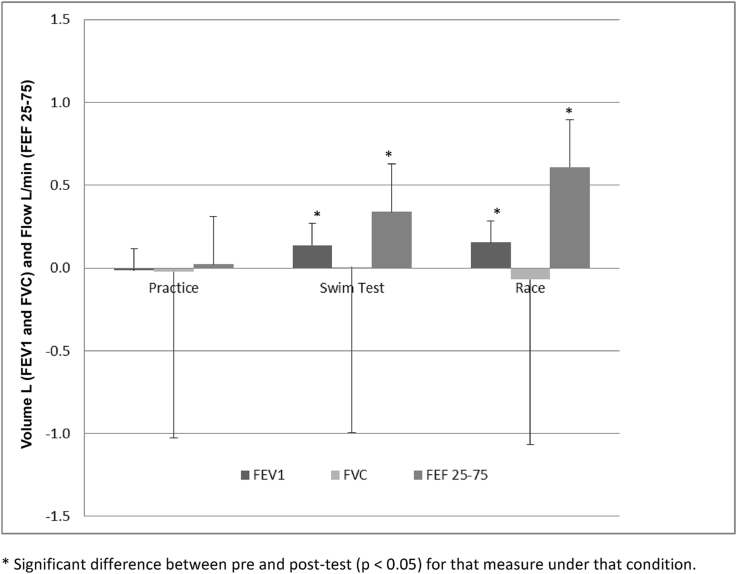
Overall there was a significant increase in FEV1 and FEF 25-75 post-race (0.15 ± 0.32 L, p = 0.03 and 0.61 ± 0.61 L/min, p = 0.00 respectively). FEV1 and FEF 25-75 significantly increased post-test as well (0.14 ± 0.20 L, p = 0.00 and 0.34 ± 0.45 L/min, p = 0.00 respectively). There were no other significant changes in pre-post spirometry overall for any condition. The mean delta change for spirometry measures for each condition overall is shown in Fig. 1.
Fig. 1.
Mean absolute difference (±SD) in FEV1, FVC, and FEF 25-75 for each condition (Practice, Swim Field Test and Race; n = 25 for each condition).
There was a main effect for distance on FEV1 (F(2, 48) = 10.4, p < 0.05). Pairwise comparison found a difference between middle and long distance group's delta change in the test condition (p < 0.05). There was a main effect for distance on FVC (F(2,48) = 92.6, p < 0.05) and pairwise comparison revealed the middle distance groups delta change in FVC was significantly different than sprint and distance groups for the test condition (p < 0.05). There was a main effect for distance on FEF 25-75 (F(2,48) = 13.2, p < 0.05) and pairwise comparison found that in the practice condition the middle distance group FEF 25-75 change was significantly different from the sprint and distance group (p < 0.05). Overall (collapsed across condition) no significant differences between groups in delta change for FEV1, FVC or FEF 25-75 were found.
Post hoc correlational analysis found no significant relationships between event (race) time and baseline characteristics (FEV1, FVC, FEV1/FVC percent of predicted) or delta change for FEV1, FVC, FEF 25-75 for any of the conditions (practice, test, race).
4. Discussion
The aim of this study was to understand how the swim field challenge test compared to race pace intensity and a standard practice in the determination of the magnitude and prevalence of EIB. To understand whether training volume influenced the EIB response participants were grouped in sprint, middle distance and long distance groups. The results indicate that the sensitivity of the 8 min swim field challenge test to determine EIB was low (no positive EIB tests) similar to others who found that a swim field test as an indirect bronchial provocation test to determine EIB is limited in its present protocol of >85 % maximum heart rate for 8 minutes.10 In this study the swimmers all completed the swim field test adequately and as shown in Table 1 some swimmers decreased post-test spirometry (FEV1, FVC and FEF 25-75). However the implication from these results is that the swim field is not severe enough to induce enough stress on the airway to activate mechanisms responsible for EIB.
Conversely it was hypothesized that race pace intensity would increase stress on the airway resulting on greater airway dysfunction post-race and more EIB prevalence in these elite swimmers. However, race pace intensity was only sufficient to induce EIB in one participant (-14 % FEV1 decrease after 1500 m race). It is possible that this cohort of elite swimmers has low AHR but it is more likely that a race (race pace intensity) is also not as sufficient a provocation to induce EIB in elite swimmers given the fact that prevalence of AHR has been shown to be as high as 76 %8 in the sport of swimming. Further in a similar cohort of elite swimmers others have found that EIB diagnosed with the gold standard EVH lab test was 55 % compared to 3 % for a swim test.10 It is notable that the swimmer that was EIB positive swam a 1500 m race implicating time in combination with maximal intensity are important triggers for this individual especially because his swim field test did not induce EIB. Yet the correlation between race time and change in spirometry measures overall was poor (FEV1: r = -0.29, p > 0.05; n = 25) which strengthens the idea that triggers for EIB/AHR are individual and can vary amongst a similar cohort of elite swimmers.
To our knowledge only one other study has examined post-race changes in airway function17 in swimmers under similar conditions race conditions. In their study 5 swimmers were EIB positive (>10 % decrease in FEV1) and age, training background and training status were all similar to our study except that all participants in the Pedersen study were female. Although gender has been implicated in greater mechanical stress for fit females compared to similarly fit men23 our study showed that no female participants had a significant decrease in FEV1 post-race. In addition, gender as a between subject factor showed no significant difference across conditions for any of the spirometry measures. Thus gender in our case likely did not increase the chance of EIB despite this potential gender factor and the fact that Pedersen did find EIB due to race in female swimmers.
We did measure in addition to FEV1; FVC and FEF 25-75 to better understand global airway function in response to these provocation tests. Similar to FEV1, there was a wide range of changes to both FVC and FEF 25-75 in the swim field test and race conditions (see Table 1). On average as shown in Fig. 1 FEV1 and FEF 25-75 increased after both the swim field test and the race conditions indicating normal airway function in response to both provocation conditions. On an individual level the importance of those who saw changes (both positive and negative) in their airway function is not useful for EIB diagnosis. However the results do highlight that in a cohort of elite swimmers there is a diverse response in airway function to intense swimming exercise. Furthermore with the exception of one swimmer the decreases were well within what would be classified as normal airway function post exercise2 despite the large exposure to triggers of airway dysfunction.8 It has been implied previously that although repetitive exposure to pool environments is one factor for development of AHR, intense exercise in a pool environment (specifically chlorine) may not induce AHR acutely.10 Although discordant with the view that swimmers are one of the sports with the poorest lung health,3 these results indicate that intense swimming exercise does not influence airway function significantly. More importantly from a performance perspective these results show that EIB is likely not a major factor in limiting performance in a race. Thus, our results support the concept that elite swimmers are not unduly influenced by large exposure to known triggers of airway dysfunction including the pool environment and being high ventilation athletes.24
Overall these swimmers baseline characteristics were above predicted norms (Table 1) and no distinct baseline characteristics were found between sprint, middle and distance groups. Given the fact that exposure to chlorine in combination with high ventilation has been shown to induce AHR over time we wanted to determine whether total volume of air inhaled might influence baseline characteristics and the subsequent response to either provocation test. Although we cannot provide a direct estimate of ventilation for the training year we can accurately say that the middle distance group did 1.2 times the distance of the sprint group and the distance group did 1.5 times the distance of the sprint group. On average the swimmers weekly exposure to a pool environment was 7-9 workouts totaling 13-15 hours plus 2 dryland workouts totaling 3 hours. Thus over the 19 weeks of the training season prior to the start of data collection the middle and distance groups had respectively significantly more ventilation and thus total exposure to known triggers of EIB. We did find that the middle and distance groups varied in their response to both practice and provocation conditions; however the changes were increases in FEV1, FVC and FEF 25-75. Thus despite greater total ventilation exposure the middle and distance groups had improved airway function to provocation tests than the sprint swimmers. This is potentially reasonable where a standardized 8 min test may induce greater stress in a sprinter compared to middle or distance swimmer. Yet like others10, 17 we did not measure ventilation nor have a good means of estimating ventilation during the swim field test. Post hoc analysis of effort (RPE) for the swim field test revealed no differences in RPE between groups and intensity was either 8 or 9 (out of 10) for all swimmers indicating that the required intensity for a valid swim field test was achieved. Thus despite greater exposure the middle and distance group are not significantly AHR positive compared to the sprint group.
Reasons for the lack of change or rather severity of the provocation to induce EIB is likely tied to ambient humidity where airway drying is the most provocative mechanism for airway injury and subsequent bronchoconstriction.25 This has been shown in previous studies where intense lab based provocation protocols with a relative humidity of 60%10, 26 did not provoke the same EIB magnitude or prevalence compared to EVH (which requires inhalation of 0% humidity air)10 or outdoor field tests in dry environments.26 Given the fact that low humidity is likely the most important factor for EIB the utility of a swim test in a humid pool environment is low. Conversely, race pace swimming intensity will likely induce less EIB than a low humidity lab test such as EVH despite the fact that ventilation is maximal (a requirement for a valid field based bronchoprovocation test). Further, the practice condition where total exposure to the pool environment at moderate ventilations (with short bouts of heavy) induces the greatest prevalence of EIB indicates that other factors besides heavy acute ventilation influence airway function.
In summary this study provides greater evidence that a swim field test to diagnoses EIB is limited despite the fact the test is sport specific. This study also provides new evidence that race pace swimming intensity does not induce greater airway dysfunction in elite swimmers despite the hypothesis that maximal heavy ventilation in a chlorinated pool environment should increase EIB.8 Further we have shown that the length of the heavy ventilation in the race condition does not duly increase EIB and that those swimmers with greater exposure to inhaled chlorinated air likely are not more hyper responsive (except for an athlete with underlying asthma). In fact the practice condition results highlight that typical swim training likely induces airway reduction in some swimmers and should be considered as an important cumulative exposure over training blocks. Having a standard EIB lab test would have helped elucidate the prevalence of EIB in this cohort of swimmers. But our primary purpose was not to evaluate EIB with a gold standard test but rather evaluate the proposed field test for swimming to race pace intensity and a typical practice. In all conditions in can be stated that there is wide variability in the response to swimming. Furthermore these results indicate that if AHR is to be diagnosed in a swimmer who may be experiencing respiratory symptoms or adverse airway dysfunction that a 0% relative humidity exercise challenge or EVH test be completed.
Conflicts of interest
The author(s) have no conflicts of interest relevant to this article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
None to declare.
Contributor Information
Michael D. Kennedy, Email: kennedy@ualberta.ca.
Jessie M.S. Gill, Email: gill4@ualberta.ca.
Alastair N.H. Hodges, Email: Alastair.Hodges@ufv.ca.
References
- 1.Fitch K.D. An overview of asthma and airway hyper-responsiveness in olympic athletes. Br J Sports Med. 2012;46(6):413–416. doi: 10.1136/bjsports-2011-090814. [DOI] [PubMed] [Google Scholar]
- 2.Price O.J., Hull J.H., Ansley L. Advances in the diagnosis of exercise-induced bronchoconstriction. Expert Rev Respir Med. 2014;8(2):209–220. doi: 10.1586/17476348.2014.890517. [DOI] [PubMed] [Google Scholar]
- 3.Mountjoy M., Fitch K., Boulet L. Prevalence and characteristics of asthma in the aquatic disciplines. J Allergy Clin Immunol. 2015;136(3):588–594. doi: 10.1016/j.jaci.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy M.D., Tamminen K.A., Holt N.L. Factors that influence fatigue status in canadian university swimmers. J Sports Sci. 2013;31(5):554–564. doi: 10.1080/02640414.2012.738927. [DOI] [PubMed] [Google Scholar]
- 5.Bougault V., Turmel J., St-Laurent J. Asthma, airway inflammation and epithelial damage in swimmers and cold-air athletes. Eur Respir J. 2009;33(4):740–746. doi: 10.1183/09031936.00117708. [DOI] [PubMed] [Google Scholar]
- 6.Parsons J.P., Hallstrand T.S., Mastronarde J.G. American thoracic society documents. Am J Respir Crit Care Med. 2013;187(9):1016–1027. doi: 10.1164/rccm.201303-0437ST. [DOI] [PubMed] [Google Scholar]
- 7.Anderson S.D., Kippelen P. Assessment of EIB: what you need to know to optimize test results. Immunol Allergy Clin North America. 2013;33(3):363–380. doi: 10.1016/j.iac.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Bougault V., Boulet L.P. Airway dysfunction in swimmers. Br J Sports Med. 2012;46(6):402–406. doi: 10.1136/bjsports-2011-090821. [DOI] [PubMed] [Google Scholar]
- 9.Seys S., Marijsse G., Dilissen E. 2013. Exercise-induced bronchoconstriction in young athletes. [Google Scholar]
- 10.Castricum A., Holzer K., Brukner P. The role of the bronchial provocation challenge tests in the diagnosis of exercise-induced bronchoconstriction in elite swimmers. Br J Sports Med. 2010;44(10):736–740. doi: 10.1136/bjsm.2008.051169. [DOI] [PubMed] [Google Scholar]
- 11.Argyros G.J., Roach J.M., Hurwitz K.M. Eucapnic voluntary hyperventilation as a bronchoprovocation technique: development of a standardized dosing schedule in asthmatics. CHEST J. 1996;109(6):1520–1524. doi: 10.1378/chest.109.6.1520. [DOI] [PubMed] [Google Scholar]
- 12.Rundell K.W., Anderson S.D., Spiering B.A. Field exercise vs laboratory eucapnic voluntary hyperventilation to identify airway hyperresponsiveness in elite cold weather athletes. CHEST J. 2004;125(3):909–915. doi: 10.1378/chest.125.3.909. [DOI] [PubMed] [Google Scholar]
- 13.Cockcroft D., Davis B. Direct and indirect challenges in the clinical assessment of asthma. Ann Allergy Asthma Immunol. 2009;103(5):363–370. doi: 10.1016/S1081-1206(10)60353-5. [DOI] [PubMed] [Google Scholar]
- 14.Anderson S.D., Brannan J.D. Methods for “indirect” challenge tests including exercise, eucapnic voluntary hyperpnea, and hypertonic aerosols. Clin Rev Allergy Immunol. 2003;24(1):27–54. doi: 10.1385/CRIAI:24:1:27. [DOI] [PubMed] [Google Scholar]
- 15.Kippelen P., Fitch K.D., Anderson S.D. Respiratory health of elite athletes - preventing airway injury: a critical review. Br J Sports Med. 2012;46(7):471–476. doi: 10.1136/bjsports-2012-091056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randolph C. Diagnostic exercise challenge testing. Curr Allergy Asthma Rep. 2011;11(6):482–490. doi: 10.1007/s11882-011-0225-4. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen L., Winther S., Backer V. Airway responses to eucapnic hyperpnea, exercise, and methacholine in elite swimmers. Med Sci Sports Exerc. 2008;40(9):1567–1572. doi: 10.1249/MSS.0b013e31875719a. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrino R., Viegi G., Brusasco V. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. doi: 26/5/948 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 20.Dickinson J.W., Whyte G.P., McConnell A.K. Mid-expiratory flow versus FEV1 measurements in the diagnosis of exercise induced asthma in elite athletes. Thorax. 2006;61(2):111–114. doi: 10.1136/thx.2005.046615. doi: thx.2005.046615 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitch K.D., Sue-Chu M., Anderson S.D. Asthma and the elite athlete: summary of the international olympic committee's consensus conference, lausanne, Switzerland, january 22-24, 2008. J Allergy Clin Immunol. 2008;122(2) doi: 10.1016/j.jaci.2008.07.003. 254–260. e7. [DOI] [PubMed] [Google Scholar]
- 22.Stensrud T., Berntsen S., Carlsen K. Exercise capacity and exercise-induced bronchoconstriction (EIB) in a cold environment. Respir Med. 2007;101(7):1529–1536. doi: 10.1016/j.rmed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 23.McClaran S.R., Harms C.A., Pegelow D.F. Smaller lungs in women affect exercise hyperpnea. J Appl Physiol (1985) 1998;84(6):1872–1881. doi: 10.1152/jappl.1998.84.6.1872. [DOI] [PubMed] [Google Scholar]
- 24.Rundell K.W., Slee J.B. Exercise and other indirect challenges to demonstrate asthma or exercise-induced bronchoconstriction in athletes. J Allergy Clin Immunol. 2008;122(2):238–246. doi: 10.1016/j.jaci.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Anderson S.D., Kippelen P. Airway injury as a mechanism for exercise-induced bronchoconstriction in elite athletes. J Allergy Clin Immunol. 2008;122(2):225–235. doi: 10.1016/j.jaci.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Rundell K.W., Wilber R.L., Szmedra L. Exercise-induced asthma screening of elite athletes: field versus laboratory exercise challenge. Med Sci Sports Exerc. 2000;32(2):309–316. doi: 10.1097/00005768-200002000-00010. [DOI] [PubMed] [Google Scholar]