Abstract
Introduction
Lysozyme is one of the salivary antimicrobial proteins which act as the “first line of defence” at the mucosal surface. The effects of prolonged exercise in the hot and cool environments among recreational athletes on salivary lysozyme responses are very limited in the literature, especially in the Asian countries.
Objective
To determine the effects of prolonged running in the hot and cool environments on selected physiological parameters and salivary lysozyme responses among recreational athletes.
Methods
Randomised and cross-over study design. Thirteen male recreational athletes (age: 20.9 ± 1.3 years old) from Universiti Sains Malaysia participated in this study. They performed two separate running trials; 90 min running at 60% of their respective maximum oxygen uptake One running trial was performed in the hot (31ºC) while the other was in the cool (18ºC) environment and this sequence was randomised. Each running trial was started with a 5 min warm-up at 50% of participant's respective Recovery period between these two trials was one week. In the both trials, saliva samples, blood samples, heart rate, ratings of perceived exertion, skin and tympanic temperatures, oxygen consumption, nude body weight, room temperature, and relative humidity were collected.
Results
Participants' skin temperature, tympanic temperature, body weight changes, heart rate, ratings of perceived exertion, and plasma volume changes were significantly higher (p < 0.05) in the hot trial compared to the cool trial. Saliva flow rate was not significantly (p = 0.949) different between the hot (0.32 ± 0.08 ml/min) and cool (0.27 ± 0.05 ml/min) trials. However, in each trial, it significantly decreased (p < 0.05) at post-exercise as compared to pre-exercise but it returned to baseline value at 1 h post-exercise. In addition, there were no significant differences between and within hot and cool trials in salivary lysozyme concentration (p = 0.925; 4.79 ± 1.37 and 4.44 ± 1.11 μg/ml respectively) and secretion rate (p = 0.843; 1.67 ± 1.1 and 1.17 ± 1.0 μg/min respectively).
Conclusion
This study found similar lysozyme responses between both hot and cool trials. Thus, room/ambient temperature did not affect lysozyme responses among recreational athletes. Nevertheless, the selected physiological parameters were significantly affected by room temperature.
Keywords: Athletes, Cold temperature, Exercise, Hot temperature, Immune function, Saliva
1. Introduction
In recent years, many researchers started to study the effects of exercise on the body immune functions. In general, regular exercise at a moderate intensity improves immune function whereas prolonged exercise at high intensity may suppress immune function.1 Depression of immune function is most pronounced when the exercise is continuous, prolonged, of moderate to high intensity, and performed without food intake.1 Suppressed immune function may lead to risk of infection, especially the upper respiratory tract infection (URTI). It is believed that athletes who are free from illness prior to and during the competition are likely to perform better than their peers with illness.2 This is because infection/illness may result in fever and dehydration, decrease energy and protein storage, decrease muscle strength, less motivation, and lead to stress. Therefore, athletes, coaches, and fitness enthusiasts are very concerned regarding this matter.
To our knowledge, investigations regarding the effects of exercise on innate mucosal immune secretions, specifically on antimicrobial proteins (AMPs) are scarce. It has been well-documented that reduced levels of salivary AMPs in athletes can contribute to increasing risk of URTI.4 Lysozyme is one of the salivary AMPs which serve as the “first line of defence” at the mucosal surface. Salivary lysozyme is produced by epithelial cells and salivary glands, and also localised in granules of neutrophils.6 It exerts immunological action via enzymatic effects on the peptidoglycan layer of gram-positive bacterial walls. It hydrolyses the bonds (beta 1-4 glucosidic linkages) in exposed bacterial cell walls and causes cell lysis and death.7 It has been reported that salivary lysozyme concentration may be reduced by an intense physical exercise where it is significantly reduced after exercise in elite swimmers.8
However, the effects of prolonged exercise in the hot and cool environments among recreational athletes on salivary lysozyme responses are very limited in the literature especially in the Asian countries. Exercising in extreme environmental temperature can stress the body's thermoregulatory capacities and compromise cardiovascular responses which in turn, can influence the immune function.9 It has been reported that performing the exercise in hot conditions with associated elevated circulating stress hormones and catecholamines, would cause greater immune disturbance compared with exercise in thermoneutral conditions.10 Similarly, it has been found that performing the exercise in cold temperature results in attenuated leukocytosis.11 Therefore, the purpose of this study is to investigate the effects of prolonged running in the hot and cool temperatures on salivary lysozyme responses among recreational athletes.
2. Methods
2.1. Overview
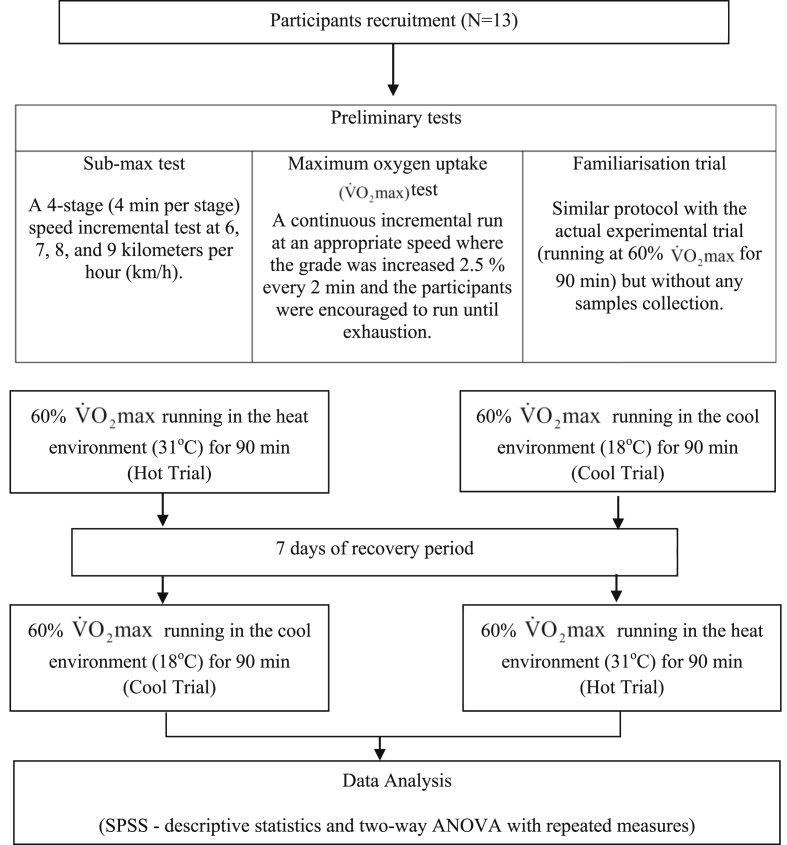
A randomised, cross-over design was employed for this study. Participants performed two running trials; one trial in the hot environment (Hot trial) followed by another in the cool environment (Cool trial) or vice versa. Recovery period between these two trials was one week. Flowchart of the study protocol was summarised in Fig. 1. All the tests were conducted in the Sports Science Laboratory, Universiti Sains Malaysia (USM). Participation in this study was on a voluntary basis. This study was been approved by the Human Research Ethics Committee, USM (USM/JEPeM/14100164). The Committee adopts research ethics guidelines outlined by the Helsinki Declaration agreed by the World Medical Association and Council for International Organizations of Medical Sciences (CIOMS).
Fig. 1.
Flowchart of the study protocol.
2.2. Participants
Opportunistic or convenience sampling was used, whereby 13 active recreational athletes were recruited among USM students. Participants were healthy males, aged 18-30 years old, non-smokers, and exercising regularly (at least three times/week with at least 30 min/session). Those who were having cold or respiratory tract infection at least two weeks prior to the study and on medication were excluded from this study. Throughout the study period, participants were required to abstain from taking any supplements that are known to affect immune function, e.g. probiotics, vitamin C, vitamin D and quercetin.
2.3. Exercise protocols
During the first three visits to the laboratory, participants performed three preliminary tests which included sub-maximal test, maximal oxygen uptake test, and familiarisation trial. The preliminary tests were carried out on a treadmill (TrackMaster TMX425CP, USA) to establish a relationship between speed and oxygen uptake, determine participant's , calculate each participant's speed at 60% , and familiarise them with the running trial protocol.
For the actual running trial, participants were randomised to run for 90 min at 60% ; either in the hot (31°C) or cool (18°C) environments. The order of the running trials in the two different environments was randomized. Hot environment was maintained at 31°C using halogen lamps (Philips-500W, France) whereby cool environment was set at 18°C by adjusting the temperature on the air conditioner (York, USA). The relative humidity in both running trials was maintained at 70% using a heated water-bath (Memment W350t, Germany).
During each running trial, participants came to the laboratory in the morning after an overnight fast. Upon arrival, participants were asked to measure their nude body using a body composition analyser (TBF- 410 Tanita, Japan) in a closed room. Participants were given a standardised breakfast; two pieces of white bread (Gardenia®, Malaysia) and 250 ml of cool plain water.
The running trial was started by a 5 min warm-up at 50% of participant's respective followed by 90 min running at 60% . During the 90 min of exercise, participants were asked to drink 3 ml/kg body weight of cool water at every 20 min to avoid any adverse effects of dehydration. Heart rate (Heart rate sensor: Sport Tester PE3000, Finland), oxygen uptake (gas analyser system: VMax-SensorMedics, USA), Borg's rating of perceived exertion (RPE), skin temperature (thermistors: Yellow springs Instrument, USA), tympanic temperature (ear thermometer: Microlife 1R1DB1, Switzerland), room temperature and relative humidity (psychrometer: Extech Instruments RH305, USA) were measured before warm-up, after warm-up, at every 20 min during exercise and post-exercise. After exercise, participants were asked to rest in a comfortable room for 1 h before dismissed.
Participant's saliva sample (∼2 ml) was collected by 5 min of un-stimulated dribbling into sterile bijou tube (Sterilin, UK). They were asked to sit on a chair, lean the head forward and let the saliva passively dribble into the tube; without using their tongue or any mouth movement. The bijou tube with the saliva collected was weighed and recorded. Saliva samples were collected before breakfast (baseline), immediately post-exercise, and 1 h post-exercise. Saliva samples were centrifuged (centrifuge: Hettich Zentrifugen, Germany) at 12,000 rpm for 5 min. Then, the upper layer (liquid part) was transferred into eppendorf tube and kept in the freezer (Acson® ACF 300, Malaysia) at -20°C until further analysis were carried out. Saliva samples were analysed in duplicates for lysozyme concentrations via an enzyme-linked immunosorbent assay (ELISA) method using a commercially available reagent kit (AssayMax Human Lysozyme ELISA kit, USA). Before analysis was carried out, saliva samples were diluted 1000 times with diluent solution. The microplate used was read using a microplate reader (Molecular Devices; Versamax tunable microplate reader, USA) at a wavelength of 450 nm. The intra- and inter-assay coefficients of variability were 4.2% and 8.2% respectively.
Blood samples (5 ml) were collected into a K2EDTA collection tube (Sekusui Insepack, Japan) before breakfast (baseline), after warm-up, at every 30 min during the running trial, immediately post exercise, and 1 h post-exercise. All blood samples were taken in standing posture. Participants were cannulated for blood withdrawal purposes. Patency of the cannula was maintained by heparinised saline whereby 0.2 ml of heparinised saline was injected into the extension tube after each blood withdrawal to avoid blood clotting. Blood samples were analysed for haemoglobin (Hb) concentration whereby the values were used to calculate plasma volume. The analysis was performed within a few hours after samples collection using an automated haematology analyser (Sysmex XS-800i, Germany).
2.4. Calculations
The following formula was used to calculate the skin temperature: 0.3 (Tchest + T biceps) + 0.2 (TThigh + Tcalf), where T = mean temperature.36
Plasma volume was calculated by using the following equation: 100 X Hbi (100 - Hbp)/Hbp (100 – Hbi) – 100, where Hbi = initial Hb level and Hbp = post Hb level.37
The saliva volume, flow rate, and lysozyme secretion rate were calculated using the formulae below:
-
•
Saliva volume (ml) = Difference in weight (g) of bijou tube before and after saliva's collection (saliva density = 1.0 g/ml)
-
•
Saliva flow rate (ml/min) = Saliva volume (ml)/Collection time (min)
-
•
Lysozyme secretion rate (μg/min) = Saliva flow rate (ml/min) x Lysozyme concentration (μg/ml)
2.5. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 22.0. Descriptive statistics were performed on physiological characteristics. Room temperature, relative humidity, body weight changes were analysed by paired t-test. Two-way analysis of variance (ANOVA) with repeated measures was performed to measure significant differences between and within trials for saliva flow rate, lysozyme responses, oxygen uptake, and heart rate. Whenever the differences between groups were found significant, a Bonferroni (post-hoc) test was carried out to confirm where the differences occurred. The accepted level of significance was set at p < 0.05. Results were reported as means ± standard deviation (SD).
3. Results
3.1. Physical and physiological characteristics of participants
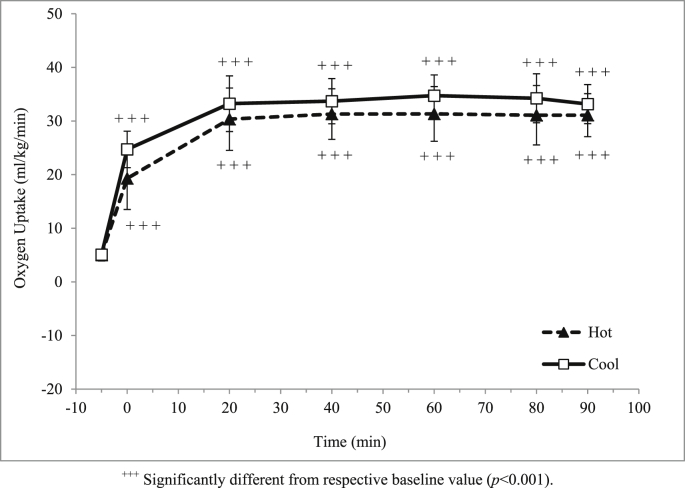
Mean age, height, body weight, body mass index (BMI), and maximum oxygen uptake of the participants were tabulated in Table 1. Oxygen uptake during exercise in both hot and cool trials increased (p < 0.001) from a baseline value to warm-up (Fig. 2), but it was relatively stable throughout exercise at approximately 60% of participants' (31.1 ± 4.0 and 33.2 ± 3.6 ml/kg/min respectively). In addition, body weight changes were significantly greater (p < 0.001) in the hot trial compared to cool trial (Table 2).
Table 1.
Physical and physiological characteristics of the participants.
| Variables | Mean ± SD |
|---|---|
| Age (years old) | 20.9 ± 1.3 |
| Body mass index (kg/m2) | 22.4 ± 2.1 |
| (ml/kg/min) | 47.0 ± 4.1 |
Fig. 2.
Mean oxygen uptake (ml/kg/min) in hot and cool trials.
Table 2.
Body weight changes of the participants.
| Variables | Hot trial | Cool trial |
|---|---|---|
| Pre-exercise body weight (kg) | 63.2 ± 7.8 | 63.3 ± 7.9 |
| Post-exercise body weight (kg) | 62.3 ± 7.9 | 62.8 ± 8.0 |
| Body weight changes (%) | 1.5 ± 0.6 | 0.7 ± 0.5a |
Significantly different compared to the Hot Trial (p < 0.001).
3.2. Room conditions and participants' body temperature
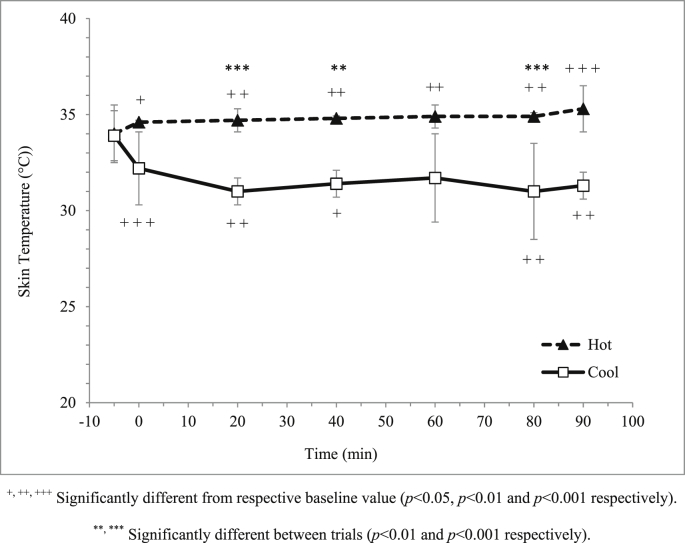
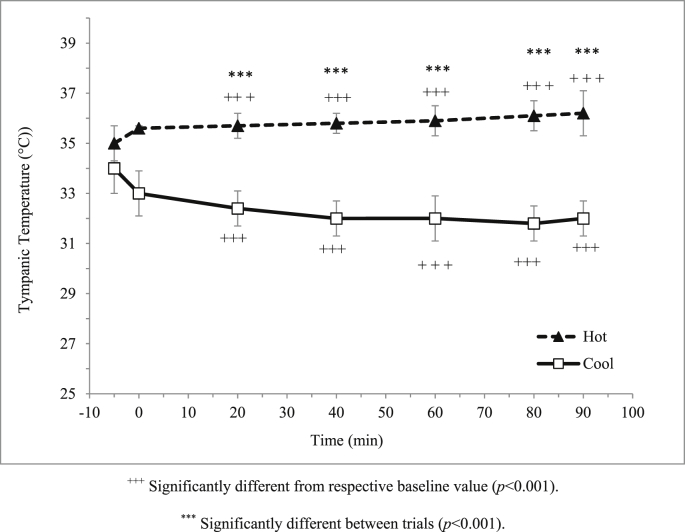
Room temperature for hot and cool trials was maintained at 31.0 ± 0.2ºC and 18.2 ± 0.3ºC respectively. Relative humidity was similar (p = 0.227) between the hot and cool trials whereby it was closely maintained at 70%; 70.3 ± 1.0% and 70.8 ± 1.0% respectively. Participants' skin temperature was significantly higher (p < 0.001) in the hot trial compared to the cool trial (Fig. 3). Similarly, participants' tympanic temperature was significantly higher (p < 0.001) in the hot trial compared to the cool trial (Fig. 4).
Fig. 3.
Mean skin temperature (°C) in hot and cool trials.
Fig. 4.
Mean tympanic temperature (°C) in hot and cool trials.
3.3. Heart rate, haemoglobin, plasma volume changes and ratings of perceived exertion
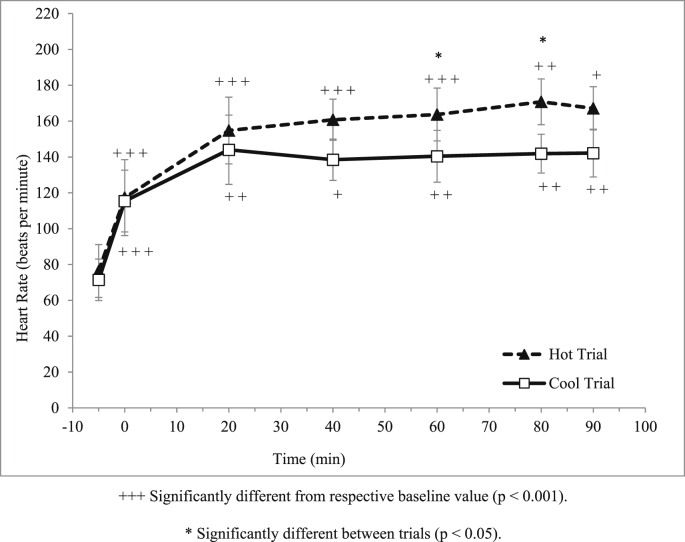
There was a significant main effect of time (p < 0.001) on heart rate during trials where it was increased over time (Fig. 5). There was also significant interaction between time and trial on heart rate (p < 0.001). Moreover, there was also a significant difference on heart rate between trials (p < 0.001) where, heart rate at min 80 and 90 during the 90 min running trial were significantly higher in the hot trial compared to cool trial.
Fig. 5.
Mean heart rate (beats/min) in hot and cool trials.
There was a significant main effect of time on haemoglobin concentration during the trials (p < 0.001). It was increased from baseline (Hot trial: 15.0 g/dl; Cool trial: 14.9 g/dl) to the end (Hot trial: 15.8 g/dl; Cool trial: 15.3 g/dl) of the trials. However, there was no interaction between time and trial on haemoglobin concentration (p = 0.224).
There was a significant main effect of time on plasma volume changes (p < 0.001) during the trials where it was increased over time. However, there was no interaction between time and trial on plasma volume changes (p = 0.271). Mean plasma volume changes at the end of the hot and cool trials were -4.9 ± 3.6% and -2.2 ± 4.0% respectively.
There was a significant main effect of time on RPE during the trials (p < 0.01) where it was increased over time. However, there was no interaction between time and trial on RPE (p = 0.087). The data revealed that the highest RPE scale of the participants was 13 ± 2.6 Borg's unit during the hot trial and 12.0 ± 2.0 Borg's unit during the cool trial.
3.4. Saliva flow rate and lysozyme concentration and secretion rate
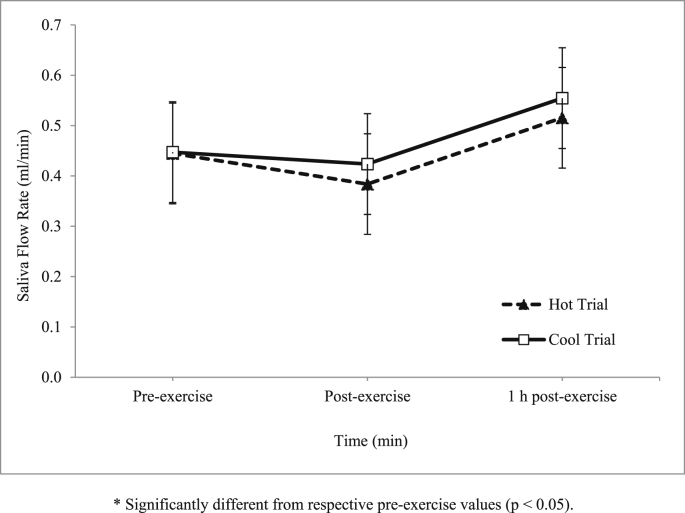
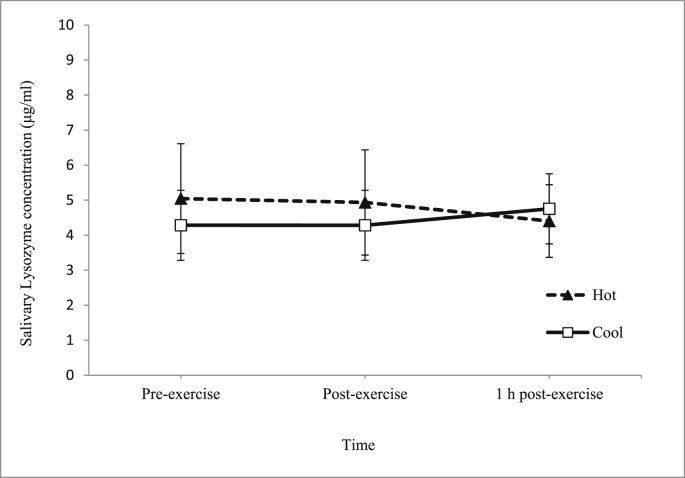
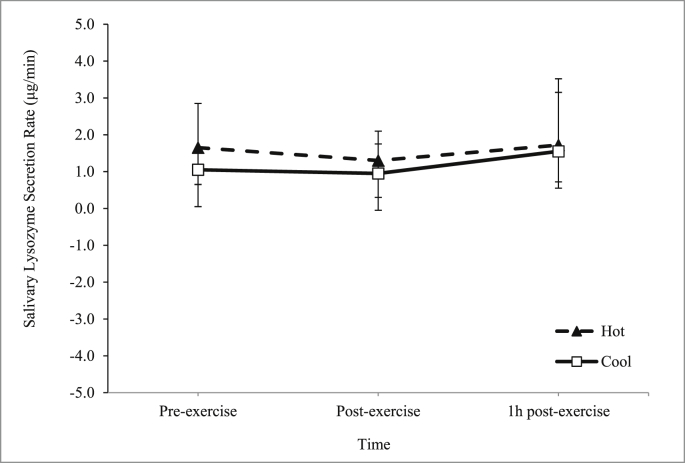
There was an increase in the main effect of time on saliva flow rate during the trials (p = 0.025) (Fig. 6). However, there was no interaction between time and trial on saliva flow rate (p = 0.949). As for salivary lysozyme responses, there was no significant main effect of time on salivary lysozyme concentration (p > 0.05) (Fig. 7) and secretion rate (p > 0.05) (Fig. 8) during the trials.
Fig. 6.
Mean saliva flow rate (ml/min) in hot and cool trials.
Fig. 7.
Mean salivary lysozyme concentration (μg/ml) in hot and cool trials.
Fig. 8.
Mean salivary lysozyme secretion rate (μg/min) in hot and cool trials.
4. Discussion
The main finding in this study was that salivary lysozyme responses were not significantly different between hot and cool trials (Fig. 7, Fig. 8). Although limited, there were a few of previous studies reported the effects of exercise in the hot environments and also in the and cool environments on lysozyme responses. However, to our knowledge, no studies have been carried out to compare the effects of exercise in the hot and cool environments on lysozyme responses. Thus, this might be the first study conducted to compare two similar running trials conducted in hot and cool environments on salivary lysozyme responses. Throughout the year, the temperature of Malaysia ranges from 20°C to 30°C on an average, meaning that participants recruited were acclimatised to both trials conditions (Hot trial: 31°C; Cool trial:18°C). Thus, this factor might explain the insignificant effects between trials found on most of the measured parameters, especially the salivary lysozyme responses.
Previous studies were in agreement with the present study main findings. For instance, one study was carried out to determine the salivary antimicrobial responses and hydration status of 23 ultra-endurance runners during a 230 km multistage ultramarathon in hot ambient conditions (32-40°C).12 It was found that there was no change in salivary lysozyme secretion rate. Similarly, in a separate study, it was found that 2 h running exercise at 60% in hot ambient conditions (34.0°C and 32% room humidity) among 8 endurance trained male volunteers did not significantly affect salivary lysozyme responses.13 With regards to exercise in a cool temperature, one study has been carried out whereby saliva samples were collected from 54 highly active men and women engaged in endurance-based physical activities during 4 months of spring (10-16°C) training.4 This study reported that lysozyme concentrations were unchanged over the course of the study.
In addition to insignificant effects of ambient/room temperature on the salivary lysozyme responses observed in this study, it was also noted that prolonged exercise, running at 60% for 90 min, have no significant effects on the salivary lysozyme responses. Similarly, a previous study has reported no change in salivary lysozyme after exercise,14 while other investigators have reported significant increases16, 17 or decreases.18 This discrepancy may be attributed to the different methods of expressing lysozyme, nutritional status of the participants, and the exercise protocol employed. Nevertheless, it has been proposed that acute increase in salivary lysozyme after a high-intensity exercise is due to increase sympathetic nervous system activity.19 Furthermore, it was reported that lysozyme secretion rate depends on the exercise intensity where, exercise intensity is directly proportional to the lysozyme secretion rate.20
It has been suggested that an increase in the concentration of salivary AMPs, which include salivary lysozyme, after exercise might confer improved immunity to infection.19 Hydration during exercise may affect the ability to maintain adequate salivary AMPs secretion rates despite lower concentrations.21 Therefore, maintaining hydration may be an important factor in maintaining mucosal immunity. In this study, body weight changes (Table 2) and plasma volume changes in the hot trial was significantly higher compared to the cool trial, demonstrating that hydration strategy used in this study (3 ml/kg body weight at every 20 min during the 90 min running) was not effective during the hot trial. Nevertheless, dehydration state induced in the hot trial was not severe enough to affect the lysozyme concentration.
In the present study, saliva flow rate during exercise in the hot and cool trials was not significantly different (Fig. 2). This observation was consistent with a previous study which reported that saliva flow rate among cyclists in hot conditions was not statistically different compared to cooler conditions.23 Similarly, running at 55% in the in hot temperature (40°C) was also found to have no effect on saliva flow rate.24 Other study also reported the same findings where, saliva flow rate was not affected by hot environment.25
In this study, prolonged exercise seems to affect saliva flow rate whereby it significantly decreased at post-exercise in both trials. Previous study also reported the same findings, for example, decreased saliva flow rate was observed at post-competition in runners with no changes being observed in controls.18 Similarly, another study reported that salivary flow rate decreased after an ultra-endurance exercise test.26 However, a few other studies reported differently whereby one study found that saliva flow rate was not significantly reduced post-50 km ultra-marathon.27 Another study also reported that saliva flow rate was not significantly changed in any trials conducted; cycling trials at 50% and cycling trials at 75% , and an incremental test to exhaustion.20 Theoretically, it was suggested that sympathetic nervous system activity influenced decrease of saliva flow rate.5 It is well known that exercise stimulates sympathetic nervous system22; hence this may explain the occurrence of reduced saliva flow rate found in the present study.
The present study found that skin and tympanic temperatures were significantly higher in the hot trial (31°C) compared to cool trial (18°C). During exercise, thermoregulatory system allows excess heat generated during exercise is dissipating through the skin31 where, if impaired, will results in an increase in body temperature. The present study also found that body weight changes were only 0.8% in the hot trial compared to the cool trial therefore shows that these athletes were accustomed to exercise in the hot. Body weight reductions post-exercise indicated that body water was loss during exercise, and this loss was mainly through sweating.32 The impairment of the exercise performance can occur even when an individual is dehydrated by as little as 2% of body weight. Therefore, shows that the participants were accustomed to exercise in the hot.
In addition, we found that heart rate was significantly increased during exercise in both trials and was significantly higher in the hot trial compared to a cool trial. A similar finding has also been observed in previous studies.33 Heart rate increases in direct proportion to exercise intensity in order to increase the cardiac output, therefore, the blood flows to the skin increases during exercise, especially in a hot environment. The higher cardiac output is needed during exercise to cope with the skeletal muscles' oxygen demand.
Ratings of perceived exertion (RPE) was one of the methods that can be used for describing and monitoring exercise intensity.34 Based on the present study, the intensity of the exercise can be assumed to be maintained at moderate intensity (60% )throughout both trials. Previous studies reported that RPE was higher when exercising in the hot compared to cool environment.35 However, the present study did not find significant difference in RPE between exercise in the hot and cool trial. Plasma volume decreased during exercise in both trials. This is because, dehydration induced by prolonged exercise causes water to move from the plasma compartment into the interstitial and intracellular fluid compartments of the contracting muscle.15 However, plasma volume returns to baseline value 1 h after exercise.
It is known that tympanic and skin temperature are not the best measures of core temperature, thus this become one of the limitations in the present study. Future study might consider measuring core temperature instead of tympanic and skin temperature. In addition, pre-trial hydration status was not properly controlled and tested before each running trial. Thus, this becomes another limitation in this study. It is important to controlled participants' hydration status because hydration level may affect salivary lysozyme responses. Another limitation was that nutritional dietary intake and physical activities of the participants throughout the study period was not controlled. Hence, it is recommended to limit these confounding factors in the future research.
5. Conclusions
The present study found that prolonged running at moderate intensity in the hot (31°C) and cool (18°C) environments among acclimatised recreational athletes did not suppress salivary lysozyme. However, physiological parameters were much more affected following exercise in the hot compared to exercise in the cool temperatures.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Funding
This work was supported by the Universiti Sains Malaysia (USM) [short-term grant: 304/PPSP/61313029].
Acknowledgements
We would like to thank all participants involved and staff of Sports Science Laboratory USM for helping us in this study.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jesf.2017.08.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Gleeson M. Immune function and exercise. Eur J Sport Sci. 2005;4(3):15. [Google Scholar]
- 2.Pyne D.B., McDonald W.A., Gleeson M., Flanagan A., Clancy R.L., Fricker P.A. Mucosal immunity, respiratory illness, and competitive performance in elite swimmers. Med Sci Sports Exerc. 2001;33(3):348–353. doi: 10.1097/00005768-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Gleeson M., Bishop N., Oliveira M., McCauley T., Tauler P., Muhamad A.S. Respiratory infection risk in athletes: association with antigen-stimulated IL-10 production and salivary IgA secretion. Scand J Med Sci Sports. 2012;22(3):410–417. doi: 10.1111/j.1600-0838.2010.01272.x. [DOI] [PubMed] [Google Scholar]
- 5.Ford J., Trevatt C., Dix A., Fallowfield J.L. The effect of fluid replacement and heat on salivary flow rate and optical density at 280nm in response to exercise. J Sports Sci. 1997;15:49–50. [Google Scholar]
- 6.Dubin R., Robinson S., Widdicombe J. Secretion of lactoferrin and lysozyme by cultures of human airway epithelium. Am J Physiol. 2004;286:L750–L755. doi: 10.1152/ajplung.00326.2003. [DOI] [PubMed] [Google Scholar]
- 7.McKenzie H.A., White F.H., Jr. Lysozyme and alpha-lactalbumin: structure, function, and interrelationships. Adv Protein Chem. 1991;41:173–315. doi: 10.1016/s0065-3233(08)60198-9. [DOI] [PubMed] [Google Scholar]
- 8.Koutedakis Y., Sabin E., Perera S. Modulation of salivary lysozyme by training in elite male swimmers. J Sports Sci. 1996;14:90. [Google Scholar]
- 9.Walsh N.P., Oliver S.J. Exercise, immune function and respiratory infection: an update on the influence of training and environmental stress. Immunol Cell Biol. 2016;94:132–139. doi: 10.1038/icb.2015.99. [DOI] [PubMed] [Google Scholar]
- 10.Walsh N.P. Immune response to exercise in extreme environments. In: Neil Spurway D.M., editor. Immune Function in Sport and Exercise. Churchill Livingstone; China: 2006. pp. 139–160. [Google Scholar]
- 11.Rhind S.G., Gannon G.A., Shek P.N., Brenner I.K., Severs Y., Zamecnik J., Buguet A., Natale V.M., Shephard R.J., Radomski M.W. Contribution of exertional hyperthermia to sympathoadrenal-mediated lymphocyte subset redistribution. J Appl Physiol. 1999;87(3):1178–1185. doi: 10.1152/jappl.1999.87.3.1178. [DOI] [PubMed] [Google Scholar]
- 12.Gill S.K., Teixeira A.M., Rama L., Rosado F., Hankey J., Scheer V., Robson-Ansley P., Costa R.J. Salivary antimicrobial protein responses during multistage ultramarathon competition conducted in hot environmental conditions. Appl Physiol Nutr Metab. 2013;38(9):977–987. doi: 10.1139/apnm-2013-0005. [DOI] [PubMed] [Google Scholar]
- 13.Gill S.K., Teixeira A.M., Rosado F., Cox M., Costa R.J. High-dose probiotic supplementation containing lactobacillus casei for 7 Days does not enhance salivary antimicrobial protein responses to exertional heat stress compared with placebo. Int J Sport Nutr Exerc Metab. 2016;26(2):150–160. doi: 10.1123/ijsnem.2015-0171. [DOI] [PubMed] [Google Scholar]
- 14.Muhamad A., Gleeson M. Effects of a 14-strain probiotics supplement on salivary antimicrobial proteins at rest and in response to an acute bout of prolonged exercise. Int J Sports Sci. 2014;4(2):7. [Google Scholar]
- 15.Kowalchuk J.M., Heigenhauser G.J., Lindinger M.I., Obminski G., Sutton J.R., Jones N.L. Role of lungs and inactive muscle in acid-base control after maximal exercise. J Appl Physiol. 1988;65(5):2090–2096. doi: 10.1152/jappl.1988.65.5.2090. [DOI] [PubMed] [Google Scholar]
- 16.Gillum T.L., Kuennen M.R., Castillo M.N., Williams N.L., Jordan-Patterson A.T. Exercise, but not acute sleep loss, increases salivary antimicrobial protein secretion. J Strength Cond Res. 2015;29(5):1359–1366. doi: 10.1519/JSC.0000000000000828. [DOI] [PubMed] [Google Scholar]
- 17.Ligtenberg A.J., Brand H.S., van den Keijbus P.A., Veerman E.C. The effect of physical exercise on salivary secretion of MUC5B, amylase and lysozyme. Arch Oral Biol. 2015;60(11):1639–1644. doi: 10.1016/j.archoralbio.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Gill S.K., Teixeira A.M., Rosado F., Hankey J., Wright A., Marczak S., Murray A., Costa R.J. The impact of a 24-h ultra-marathon on salivary antimicrobial protein responses. Int J Sports Med. 2014;35(11):966–971. doi: 10.1055/s-0033-1358479. [DOI] [PubMed] [Google Scholar]
- 19.West N.P., Pyne D.B., Kyd J.M., Renshaw G.M., Fricker P.A., Cripps A.W. The effect of exercise on innate mucosal immunity. Br J Sports Med. 2010;44(4):227–231. doi: 10.1136/bjsm.2008.046532. [DOI] [PubMed] [Google Scholar]
- 20.Allgrove J.E., Gomes E., Hough J., Gleeson M. Effects of exercise intensity on salivary antimicrobial proteins and markers of stress in active men. J Sports Sci. 2008;26(6):653–661. doi: 10.1080/02640410701716790. [DOI] [PubMed] [Google Scholar]
- 21.Fortes M.B., Diment B.C., Di Felice U., Walsh N.P. Dehydration decreases saliva antimicrobial proteins important for mucosal immunity. Appl Physiol Nutr Metab. 2012;37(5):850–859. doi: 10.1139/h2012-054. [DOI] [PubMed] [Google Scholar]
- 22.Murray D.R., Irwin M., Rearden C.A., Ziegler M., Motulsky H., Maisel A.S. Sympathetic and immune interactions during dynamic exercise. Mediation via a beta 2-adrenergic-dependent mechanism. Circulation. 1992;86(1):203–213. doi: 10.1161/01.cir.86.1.203. [DOI] [PubMed] [Google Scholar]
- 23.Tharp G.D., Barnes M.W. Reduction of saliva immunoglobulin levels by swim training. Eur J Appl Physiol. 1990;60:61–64. doi: 10.1007/BF00572187. [DOI] [PubMed] [Google Scholar]
- 24.McDowell S.L., Hughes R.A., Hughes R.J., Housh T.J., Johnson G.O. The effect of exercise training on salivary immunoglobulin-a and cortisol responses to maximal exercise. Int J Sports Med. 1992;13:577–580. doi: 10.1055/s-2007-1024568. [DOI] [PubMed] [Google Scholar]
- 25.Nieman D.C., Henson D.A., Fagoaga O.R., Utter A.C., Vinci D.M., Davis J.M., Nehlsen-Cannarella S.L. Change in salivary IgA following a competitive marathon race. Int J Sports Med. 2002;23(1):69–75. doi: 10.1055/s-2002-19375. [DOI] [PubMed] [Google Scholar]
- 26.Tauler P., Martinez S., Moreno C., Martínez P., Aguilo A. Changes in salivary hormones, immunoglobulin A, and C-reactive protein in response to ultra-endurance exercises. Appl Physiol Nutr Metab. 2014;39(5):560–565. doi: 10.1139/apnm-2013-0466. [DOI] [PubMed] [Google Scholar]
- 27.Gillum T.L., Kuennen M., Gourley C., Schneider S., Dokladny K., Moseley P. Salivary antimicrobial protein response to prolonged running. Biol Sport. 2013;30:3–8. doi: 10.5604/20831862.1029814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim C.L., Byrne C., Lee J.K. Human thermoregulation and measurement of body temperature in exercise and clinical settings. Ann Acad Med Singapore. 2008;37(4):347–353. [PubMed] [Google Scholar]
- 32.Sawka M.N., Noakes T.D. Does dehydration impair exercise performance? Med Sci Sports Exerc. 2007;39(8):1209–1217. doi: 10.1249/mss.0b013e318124a664. [DOI] [PubMed] [Google Scholar]
- 33.Ramezani A., Barati A.H., Azarbaijani M.A., Tohidi M., Abbaszadegan M. The effect of one bout of incremental exercise on salivary immunoglobulin A (IgA) of high school students. Int J Sports Med. 2012;6:5. [Google Scholar]
- 34.Borg G.A. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 35.Taylor L., Fitch N., Castle P., Watkins S., Aldous J., Sculthorpe N., Midgely A., Brewer J., Mauger A. Exposure to hot and cold environmental conditions does not affect the decision making ability of soccer referees following an intermittent sprint protocol. Front Physiol. 2014;5:185. doi: 10.3389/fphys.2014.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramanathan N.L. A new weighting system for mean surface temperature of the human body. J Appl Physiol. 1964;19:531–533. doi: 10.1152/jappl.1964.19.3.531. [DOI] [PubMed] [Google Scholar]
- 37.Dill D.B., Costill D.L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;98:1799–1804. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.