Abstract
Leber congenital amaurosis (LCA) is a neurodegenerative disease of photoreceptor cells that causes blindness within the first year of life. It occasionally occurs in syndromic metabolic diseases and plurisystemic ciliopathies. Using exome sequencing in a multiplex family and three simplex case subjects with an atypical association of LCA with early-onset hearing loss, we identified two heterozygous mutations affecting Arg391 in β-tubulin 4B isotype-encoding (TUBB4B). Inspection of the atomic structure of the microtubule (MT) protofilament reveals that the β-tubulin Arg391 residue contributes to a binding pocket that interacts with α-tubulin contained in the longitudinally adjacent αβ-heterodimer, consistent with a role in maintaining MT stability. Functional analysis in cultured cells overexpressing FLAG-tagged wild-type or mutant TUBB4B as well as in primary skin-derived fibroblasts showed that the mutant TUBB4B is able to fold, form αβ-heterodimers, and co-assemble into the endogenous MT lattice. However, the dynamics of growing MTs were consistently altered, showing that the mutations have a significant dampening impact on normal MT growth. Our findings provide a link between sensorineural disease and anomalies in MT behavior and describe a syndromic LCA unrelated to ciliary dysfunction.
Keywords: Leber congenital amaurosis, early-onset sensorineural hearing loss, retino-cochlear tubulinopathy, TUBB4B, de novo mutations, dominant mutations, mosaicism, abnormal dynamics of microtubule growth
Main Text
Tubulins are the subunits of microtubules (MTs), dynamic polymers that participate in a striking variety of essential cellular functions. Eight α- and nine β-tubulin isotypes are encoded in humans, and there is compelling evidence that their tissue-specific expression patterns and post-translational modifications contribute to the generation of functionally specialized MTs.1 Mutations in tubulin isotypes have been implicated in a wide and overlapping range of brain malformations collectively known as the tubulinopathies (see GeneReviews in Web Resources). Hitherto, no mutations in TUBB4B (encoding the β-tubulin 4B isotype) (MIM: 602660) have been associated with any human disease. Here, we report that monoallelic TUBB4B mutations cause a severe neurodegenerative condition of the retina known as Leber congenital amaurosis (LCA), associated with sensorineural hearing loss (SHL).
LCA (MIM: PS204000) is a group of inherited photoreceptor-neuron degenerative diseases that results in severe early-onset visual dysfunction.2 It typically presents as an autosomal-recessive isolated ocular anomaly without systemic involvement. Diseases involving sensorineural hearing loss (SHL) are essentially monogenic non-syndromic conditions ascribable to inner-ear cell dysfunction (see Hereditary Hearing loss Homepage in Web Resources). The association of a degenerative disease of the retina with SHL is typically seen in multi-tissue syndromic disorders, in particular in metabolic disorders and ciliopathies.3 The specific association of the two anomalies is known as Usher syndrome (MIM: PS276900), but SHL has been reported in some individuals with retinal degeneration due to RPGR (MIM: 312610) mutations.4 In Usher syndrome, as in individuals with RPGR mutations, the retinal disease differs from LCA by a later onset and a less severe visual deficiency.
We identified five individuals from four families with a previously unreported association of LCA and early-onset SHL (families 1–4; Figure 1) and performed whole-exome sequencing (WES) to resolve these uncommon cases. WES informed consent was obtained from all participating individuals, and the study was approved by the Comité de Protection des Personnes “Ile-De-France II.” WES datasets were generated from individuals II1, II2, III2, and IV1 in family 1 and from the index case subject and his/her parents in families 2–4. Sequencing, image analysis, base calling, and genetic variation annotation were performed as described previously.5 WES coverage and read-depth were consistent with a high degree of base calling confidence (Table S1). Sequence variants were filtered according to the pedigree structure using the Imagine pipeline, POLYWEB.
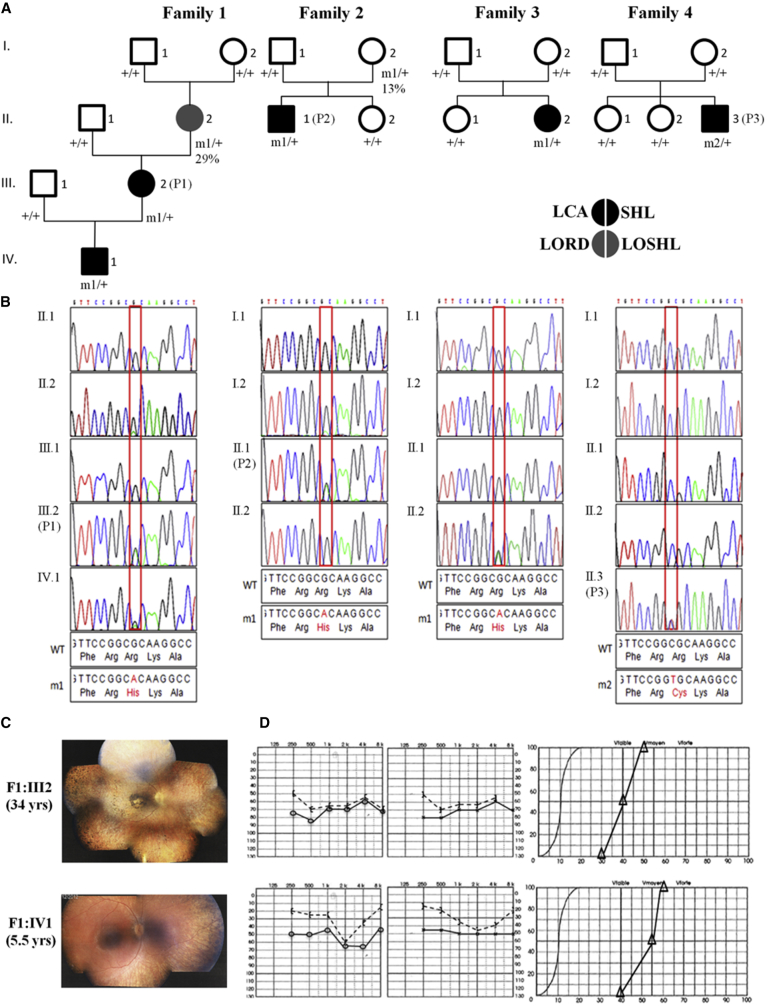
Figure 1.
Heterozygous TUBB4B Substitutions Affecting Arg391 Cause LCA with Hearing Deficiency
(A) Pedigrees of families and segregation analysis of the mutations. m1, c.1172G>A (p.Arg391His); m2, c.1171C>T (p.Arg391Cys); +, wild-type allele. P1, P2, and P3 are affected individuals whose fibroblasts were analyzed. The percentage below the genotype of individuals I2 in families 1 and 2 shows the relative abundance of mutant reads in genomic DNA from lymphocytes.
(B) Sanger sequence traces around the mutation are shown below the corresponding pedigree.
(C and D) Representative eye fundus views and audiograms in two individuals with TUBB4B substitutions affecting Arg391. The eldest individual’s funduscopy image displays features of advanced retinitis pigmentosa with a marked reduction in the caliber of retinal vessels, generalized choroid atrophy, marked macular rearrangements, and numerous pigmentary deposits in the periphery. The fundus of the youngest individual displays similar abnormalities, although to a much lesser extent (C). Left and right ear pure-tone audiograms of the two individuals displaying moderate symmetric hearing loss (left and central panels, respectively). Vocal audiograms (right panels) are consistent with pure-tone traces, suggesting that the two individuals have endocochlear deafness (D).
In family 1, the index case subject (IV1) and his mother (III2) were both affected, supporting a dominant transmission of sensory symptoms. The maternal grandmother (II2) was known to have SHL since her 30s but she did not complain of visual problems. Variants with a minor allele frequency (MAF) exceeding 0.01% were filtered out. Analysis of WES datasets driven by the hypothesis of the occurrence of a de novo mutation in a grandparental gamete, transmitted in a dominant manner from III2 to IV1, identified no variant (Table S2). In contrast, considering the transmission of the disease through the deaf maternal grandmother based on the hypothesis of a single disease with variable symptomatic expression, we identified 40 unreferenced or extremely rare heterozygous changes (Table S2). None of them affected a gene involved in eye or ear diseases, except for the unreferenced and likely damaging c.1771T>C (p.Cys591Arg [GenBank: NM_201253.2]) missense substitution identified in CRB1 (Table S2). Approximately 200 CRB1 mutations are referenced in the Human Gene Mutation Database. All but one of them cause recessive LCA or retinitis pigmentosa 12 (RP12 [MIM: 600105]). The p.Val162Met substitution within the fourth EGF-like domain has been reported in a unique family with dominant pigmented paravenous chorioretinal atrophy (PRCA [MIM: 172872]), an unusual retinal degeneration characterized by accumulation of pigmentation along retinal veins.6 By analogy with other dominant retinal diseases caused by mutations in EGF-like domains of other proteins, the uncommon heredity and presentation associated with the p.Val162Met substitution were ascribed to its localization. The localization of the p.Cys591Arg substitution in the laminin G domain involved in several LCA/RP12 missense mutations (HGMD: CM124913, HM0648, HM060031, CM130790, CM043269, CM040711) and the retinal presentation in the mother and her son both differ strikingly from PRCA. These observations argue strongly against the involvement of this gene in the disease in family 1. Of the 38 of the remaining variants, 37 were dismissed because (1) they had high probabilities to be benign and/or they were detected in individuals with no overlapping symptoms in our in-house WES database (DejaVu, > 10,000 exomes) (32/38) or (2) they were detected by Sanger sequencing of the DNA of the asymptomatic great-grandparents (I1 or I2, Figure 1) (6/38) (Table S2). The only variant which circumvented filtering was the unreferenced TUBB4B c.1172G>A (p.Arg391His [GenBank: NM_006088.5]) substitution predicted to be damaging by PolyPhen-2, SIFT, and MutationTaster (Table S2). This change drew our attention because of a reduced number of variant reads produced from the deaf maternal grandmother’s DNA (29% versus 50% of reads produced from the index case subject and his mother’s DNAs). This observation suggested a mosaicism that was confirmed by PCR-based deep next-generation sequencing of TUBB4B exon 4 (570/1,956 reads generated from the maternal grandmother’s DNA versus 1,063/2,215 from the mother’s DNA) (Table S3). Consistent with these data, the variant was undetected in the maternal great-grandparents’ (I1, I2) DNA, as determined by Sanger sequencing (primer sequences available in Table S4).
Interestingly, inspection of our DejaVu database identified the TUBB4B c.1172G>A variant in 3 out of more than 10,000 individuals: the index case subjects of families 2 and 3 and the mother in family 2. These individuals carried 50% and 13% of mutant reads, respectively. Deep sequencing analysis were consistent with WES data (Table S3), supporting mosaicism and de novo mutational events, respectively. WES datasets were further analyzed in a search for ultra-rare likely damaging de novo dominant and rare recessive (MAF < 0.001 and 0.01, respectively) variants, absent in heterozygosity, homozygosity, or hemizygosity (depending on the genetic model) in individuals with no overlapping symptoms (DejaVu database). In family 2, this filtering strategy pointed to four genes—GUCY1B3 (MIM: 139397), MLST8 (MIM: 612190), MUC16 (MIM: 606154), and CDK16 (MIM: 311550)—none of which had a known role in the eye or ear (the TUBB4B variant present in 13% of the reads produced from the mother’s DNA was not detected) (Table S2). In families 3 and 4, unique variants circumvented filtering: the TUBB4B c.1172G>A change and another TUBB4B unreferenced and likely damaging (PolyPhen-2, SIFT, and Mutation Taster) variant affecting the adjacent nucleotide (c.1171C>T [p.Arg391Cys]), respectively (Figure 1 and Table S2). The c.1172G>A and c.1171C>T substitutions were absent in parental DNA as determined by WES and deep sequencing, suggesting a de novo event (families 3 and 4, respectively; Figure 1 and Table S3). Familial segregation analysis of the TUBB4B c.1172G>A and c.1171C>T mutations with the disease was further confirmed by Sanger sequencing (Figure 1).
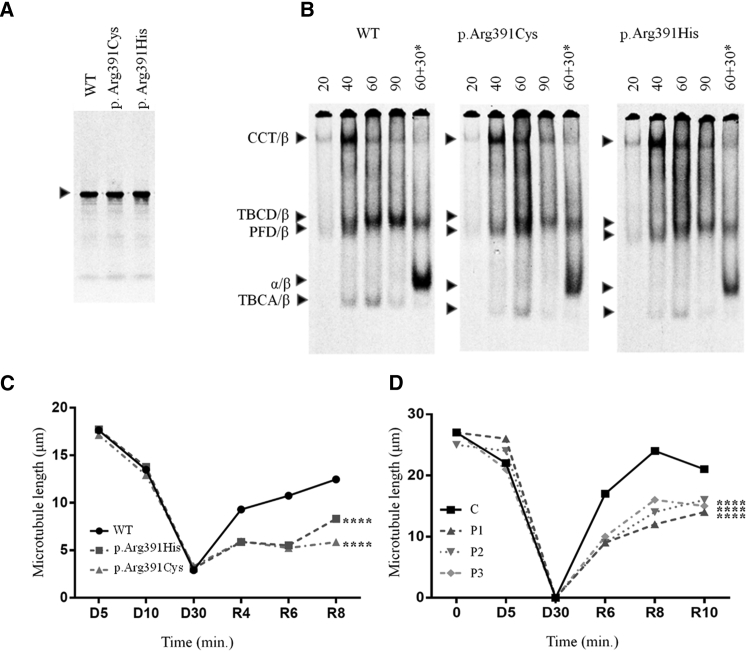
The folding and assembly of the αβ-tubulin heterodimer depends on the concerted action of several molecular chaperones. Nascent tubulin polypeptides are first recognized and stabilized by the heterohexameric chaperone prefoldin, which delivers its bound target protein to the cytosolic chaperonin CCT.7 The latter provides a sequestered chamber in which the target protein can partition on a productive folding pathway via multiple ATP-dependent iterations of binding and release from the chaperonin’s internal surfaces. Quasi-native tubulins discharged from the chaperonin interact with a series of downstream tubulin-specific chaperones termed TBCA-TBCE that act in concert as an αβ-tubulin heterodimer assembly nanomachine controlled by hydrolysis of β-tubulin-bound GTP.8, 9 To determine the influence of the mutations we discovered on the chaperone-dependent αβ-heterodimer assembly pathway, we analyzed the products of coupled in vitro transcription/translation reactions driven by full-length cDNA constructs encoding wild-type and mutant forms of TUBB4B.9 As expected for single amino acid substitutions, we found that the wild-type and mutant proteins were transcribed and translated with the same efficiency (Figure 2A). However, kinetic analysis of these reactions done under native conditions revealed some differences with respect to wild-type, most conspicuously in the diminished yield of the TBCD/β-tubulin folding intermediate from t = 60 mins onward and of de novo folded αβ-tubulin heterodimers in the case of TUBB4B p.Arg391Cys and p.Arg391His (Figure 2B). A reduction in the yield of TBCD/β-tubulin implies a reduced stability or a reduced affinity of CCT-generated mutant-containing folding intermediates for TBCD, in either case leading to a reduced relative yield of mutant-containing αβ-heterodimers (quantitated from 6 independent experiments, Figure S1). There is also a possibility that the production of mutant αβ-heterodimers might have a dominant-negative effect on overall de novo heterodimer production.
Figure 2.
Functional Analysis of TUBB4B Mutations In Vitro, in Cultured Cells, and in Skin Fibroblasts
(A and B) Folding in vitro. Plasmids encoding full-length TUBB4B (wild-type or containing the mutations shown) were expressed in rabbit reticulocyte lysate containing 35S-methionine.
(A) Labeled reaction products analyzed by SDS-PAGE. Arrow: migration position of β-tubulin.
(B) Kinetic analysis by native 4.5% PAGE of labeled reaction products done for the times shown (in min).
In each panel, the right-hand track (marked with an asterisk) shows reactions chased with unlabeled native αβ-tubulin heterodimers, included so as to drive the heterodimer assembly reaction. Arrows (top to bottom): CCT/β-tubulin binary complex, TBCD/β-tubulin folding intermediate, prefoldin (PFD)/β-tubulin binary complex, αβ-tubulin heterodimer, and TBCA/β-tubulin folding intermediate.
(C and D) Depressed MT growth rates in transfected COS7 cells (C) and in patient-derived skin fibroblasts (D) following recovery from cold-induced depolymerization.
See Figures S2 and S3 for methods and statistical analyses.
Typically, different tubulin isotypes can freely intermingle into mosaic MTs upon expression in cultured cells.10 To determine the ability of the p.Arg391His and p.Arg391Cys substitutions to incorporate into the MT lattice in vivo, we co-transfected COS7 cells with wild-type and mutant pcDNA3.1-TUBB4B-C-(K)DYK constructs and a pCAGG-GFP plasmid. FLAG-tagged mutant and wild-type TUBB4B protein levels were similar as determined by computed-densitometry analysis of western blots using anti-FLAG and anti-GFP antibodies (Figure S2A). Immune staining using an end-binding 1 (EB1) protein antibody to label the +ends of polymerizing protofilaments11 showed comparable MT lattices in cells overexpressing mutant and wild-type TUBB4B (Figure S2B). Consistent with our folding data (Figures 2B and S1), these experiments show that the mutant TUBB4B proteins were able to fold, form αβ dimers, and co-assemble into the endogenous MT lattice. While the mutations did not alter the cold-driven depolymerization of these MT lattices, we found that they had a significant impact on MT repolymerization dynamics (Figures 2C, 2D, and S2B). We conclude that the p.Arg391His and p.Arg391Cys substitutions have a dampening effect on normal MT growth.
We obtained and analyzed skin-derived fibroblasts from three affected individuals (F1:III2, F2:II1, F4:II3) and three control subjects (C1–C3). RT-qPCR analysis using TUBB4B-specific primers showed that cells from control subjects and affected individuals expressed similar mRNA amounts (Figure S3A). We were unable to analyze TUBB4B protein levels due to the very high degree of homology among tubulin isotypes and the absence of any non-cross-reacting isotype-specific antibodies. Fibroblasts from the affected individuals had unremarkable steady-state MT lattices and displayed similar MT depolymerization dynamics upon either cold exposure (which induces MT depolymerization [Figures 2D and S3B]) or by incubation at 37°C with the MT-depolymerizing drug nocodazole (Figure S4). However, as in the case of transfected COS7 cells, fibroblasts from affected individuals showed dramatically diminished MT growth rates (Figures 2D, S2B, and S3C).
Inspection of the atomic structure of β-tubulin12, 13 within a MT protofilament shows that Arg391 and the adjacent Arg390 and Lys392 residues form a binding pocket that interacts with α-tubulin in the longitudinally adjacent tubulin αβ-heterodimer.14 The diminished MT growth rates found in cells expressing the TUBB4B mutations suggests that substitution of p.Arg391 in the H11 helix of β-tubulin by either a histidine or a cysteine residue destabilizes hydrophobic interactions between the β-tubulin H11’ helix and the T7 loop and H8 helix of α-tubulin contained in the adjacent αβ-heterodimer15 (Figure S5). This predicted destabilization is consistent with the observed effect of the p.Arg391 variant on the dynamics of MT growth.
The anomalous MT polymerization rates in fibroblasts from affected individuals had no impact on cell proliferation as determined by an automated live cell proliferation assay (Figure S6), nor was ciliogenesis affected as determined by measuring the abundance and size of acetylated α-tubulin-stained cilia axonemes in serum-starved cells (Figures S7A and S7B). Ciliary trafficking, assessed by immune staining of the centrosomal CEP290 and the intraflagellar IFT 20, 25, 81, and 140 proteins, was also unaffected (Figure S8).
All individuals carrying the c.1171C>T or c.1172G>A mutations except F1:II2 and F2:I2 had early-onset and severe visual and auditory symptoms (Table 1). The youngest (F1:IV1, F3:II2, F4:II3, ages < 10 years) presented with retinal disease consistent with the rod-cone dystrophy subtype of LCA or LCA-type 2 (Table 1).16 No early ophthalmologic data were available for F1:III2 and F2:II1 (aged 34 and 31 years, respectively). However, current clinical data (in particular the retinitis pigmentosa appearance of the fundus) were consistent with advanced LCA-type 2 (Table 1 and Figures 1C and S9). All individuals had high hypermetropia, which is atypical in LCA-type 2.16 Hearing defects were diagnosed from birth to age 8 years regardless of the mutation (Table 1 and Figures 1D and S10). All individuals had normal neuro-psychomotor development, and a cerebral MRI available for F4:II3 at age 2 months showed no anomalies (Figure S11).
Table 1.
Genotype and Phenotype of Families with TUBB4B Mutations
| Family | Individual (Age in Years) | Origin | Nucleotide Alteration | Deduced Protein Change | Retinal Disease | Auditory Defects |
|---|---|---|---|---|---|---|
| 1 | II2 (61) | France | c.1172G>A (mosaic 29%) | p.Arg391His | normal ERG VA 20/20 (RE), 20/29 (LE) hypermetropia +6 (RE), +9 (LE) pigmentary deposits and atrophy in the peripheral retina, peripapillar atrophy, and macular alterations (Figure S9A) | diagnosis at 30 years A: 75/70 |
| 1 | III2 (P1) (34) | France | c.1172G>A (het) | p.Arg391His | diagnosis at birth nystagmus flat ERG complete blindness photophobia hypermetropia +6 (LRE) thin retinal vessels, salt and pepper appearance of the posterior pole, yellowish peripheral retina with round pigmented spots at the fundus, macular reorganization (30 year; Figure S9A) | diagnosis at 7 years A: 70/70 ABR: 100/65 |
| 1 | IV1 (6) | France | c.1172G>A (het) | p.Arg391His | diagnosis at birth flat ERG VA 20/63 (LRE) constricted VF hypermetropia +10 (LRE) thin retinal vessels, salt and pepper appearance of the posterior pole, yellowish peripheral retina with round pigmented spots at the fundus (Figure S9A) | diagnosis at birth A: 45/45 |
| 2 | I2 (64) | Algeria | c.1172G>A (mosaic 13%) | p.Arg391His | asymptomatic | asymptomatic |
| 2 | II1 (P2) (31) | Algeria | c.1172G>A (het) | p.Arg391His | diagnosis at 2.5 years nystagmus VA reduced to LP (22yrs) photophobia hypermetropia +8 (LRE) narrowed retinal vessels and a beaten-bronze appearance of retinal periphery with some pigment migration and macular atrophic changes (Figure S9A) | diagnosis at 8 years A: 60/55 ABR: 70/60 |
| 3 | II2 (6) | France | c.1172G>A (het) | p.Arg391His | diagnosis at 3 years severely altered photopic and scotopic ERG VA 20/40 (LRE) hemeralopia constricted VF hypermetropia +7 thin retinal vessels, dull retina at the fundus (textual description) | diagnosis at 3 years A: 20 drained serous otitis |
| 4 | II3 (P3) (9) | Denmark | c.1171C>T (het) | p.Arg391Cys | diagnosis at birth flat ERG VA 20/50 (RE), 20/63 (LE) hemeralopia constricted VF hypermetropia + 8.25 (RE) +8.75 (RE) slightly pale disc, narrowed retinal vessel, pale retinal periphery with bronze-beaten appearance, absence of pigment migration, and perimacular retinal atrophy at the fundus (Figure S9A) | diagnosis at birth A: 50/50 ABR: 60/45 OAE: absent |
Segregation analysis confirmed the de novo status of the mutation in families 3 and 4 and dominant transmission in families 1 and 2. Abbreviations: ERG, electroretinogram; LE, left eye; RE, right eye; LRE, left and right eyes; LP, light perception; VF, visual field; VA, visual acuity; A, audiogram (HdB level) right ear/left ear; OAE, oto-acoustic emissions; ABR, auditory brainstem evoked response (HdB level).
F1:II2, aged 61 and carrying the c.1172G>A mutation on 29% of TUBB4B alleles, had SHL in her 30s and a history of highly asymmetric hypermetropia with left eye amblyopia, although she claimed she had no visual problems. Upon examination she presented with hypermetropia of +6 and + 9 diopters and her best corrected visual acuities were 20/20 and 20/29 (right and left eye, respectively; Table 1). Interestingly, fundus examination and optic coherence tomography (OCT) showed objective signs of retinal degeneration (Figure S9), but her photopic and scotopic electroretinograms (ERGs) were normal (Figure S12). F2:I2, aged 57 and carrying the same mutation on 13% of TUBB4B alleles, had no auditory symptoms upon examination. She did not complain of visual problems and had no sign of retinal involvement upon detailed ophthalmological examination, including funduscopy, OCT, and ERG recordings.
Evidence of mosaicism in blood cells of the two women, and presumably in the ear and retina of F1:II2 presenting with mild retinal disease and SHL, is consistent with a somatic mosaicism. On the other hand, transmission of the disease through these women to the descendant demonstrates that the mutation affects the germline. Such a situation is typically seen when the mutation occurs very early in development. Interestingly, the two TUBB4 mutations change a cytosine involved in CpG dinucleotides (+ and – strands, respectively) into a thymine. It is likely that the mutation occurred by the spontaneous deamination of the methylated versions of these cytosines, a mechanism known to contribute to nucleotide hypermutability.17 The eye and ear involvement in F1:II2 suggest mosaicism in the retinal and cochlear cells of the individual with the highest proportion of mutant-bearing blood cells, questioning the correlation between mosaic ratio and disease expression. The TUBB4B-related sensorineural phenotype raises the question of the mechanisms involved in cell-type-specific effects. While most tubulin mutations causing tissue- and cell-type-specific pathologies occur in broadly expressed isotypes,18 mutations in TUBB1 (MIM: 612901),19 TUBB3 (MIM: 602661),20 and TUBB8 (MIM: 616768)21 cause symptoms that correlate with tissue-specific expression. We analyzed the abundance of tubb4b mRNA by RT-qPCR in a spectrum of mouse tissues to assess tissue-specific expression. tubb4b mRNA was detected in all analyzed embryonic and adult tissues, including the retina and cochlea. We note that compared to other embryonic and adult tissues, the retina expressed a significantly higher level of tubb4b mRNA (Figure S13). This could reflect a specific requirement for TUBB4B in retinal development; however, the low relative expression of tubb4b in the cochlea questions the existence of a universal correlation between tissue-specific expression and phenotypic disease.
In summary, we have identified TUBB4B mutations as the cause of a previously unreported autosomal-dominant syndrome manifesting as early-onset and severe photoreceptor and cochlear cell loss. Our data provide a link between a sensorineural disease and anomalies in MT behavior and describe an early-onset and severe retinal disease with extraocular associated features unrelated to cilia or metabolic dysfunctions. Currently, no means exist to prevent photoreceptor loss in individuals with TUBB4B mutations. However, early identification of hearing loss and early intervention have proven highly important for language development.22, 23 Based on our findings, systematic auditory explorations merit consideration in infants with LCA to provide optimal care to those in need.
Acknowledgments
We are grateful to the families for their participation in the study. This work was supported by “S’entendre,” by grants from Retina France to I.P., UNADEV-AVIESAN ITMO MNP (R16073KS) to J.-M.R., FRM (DEQ20160334869) to J.D., and by a grant from the NIH (GM097376) to N.J.C.
Published: November 30, 2017
Footnotes
Supplemental Data include 13 figures and 4 tables and can be found with this article online at https://doi.org/10.1016/j.ajhg.2017.10.010.
Contributor Information
Jean-Michel Rozet, Email: jean-michel.rozet@inserm.fr.
Sandrine Marlin, Email: sandrine.marlin@aphp.fr.
Web Resources
1000 Genomes, http://www.internationalgenome.org/
Alamut Interpretation Software 2.0 (gateway for Align DGVD, PolyPhen-2, SIFT, SpliceSiteFinder-like, MaxEntScan, NNSPLICE, and Human Splicing Finder), http://www.interactive-biosoftware.com
ExAC Browser, http://exac.broadinstitute.org/
GeneReviews, Bahi-Buisson, N., and Cavallin, M. (1993). Tubulinopathies Overview. https://www.ncbi.nlm.nih.gov/books/NBK350554/
Hereditary Hearing loss Homepage, http://hereditaryhearingloss.org/
Human Gene Mutation Database, http://www.hgmd.cf.ac.uk/ac/index.php
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
OMIM, http://www.omim.org/
UCSF Chimera, http://www.cgl.ucsf.edu/chimera/
Supplemental Data
Exhaustive list of rare SNVs/indels de novo and recessive variants identified in the four families. The deleterious effect of variants was assessed using the Alamut Mutation Interpretation software which uses Polyphen 2, SIFT, Mutation Taster, GVGD, SpliceSiteFinder-like, MaxEntScan, NNSPLICE, and/or Human Splicing Finder. MAF: minor allele frequency.
References
- 1.Gadadhar S., Bodakuntla S., Natarajan K., Janke C. The tubulin code at a glance. J. Cell Sci. 2017;130:1347–1353. doi: 10.1242/jcs.199471. [DOI] [PubMed] [Google Scholar]
- 2.Leber T. Ueber Retinitis pigmentosa und angeborene Amaurose. Archiv fur Opthalmologie. 1869;15:1–25. [Google Scholar]
- 3.Mysore N., Koenekoop J., Li S., Ren H., Keser V., Lopez-Solache I., Koenekoop R.K. A review of secondary photoreceptor degenerations in systemic disease. Cold Spring Harb. Perspect. Med. 2014;5 doi: 10.1101/cshperspect.a025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zito I., Downes S.M., Patel R.J., Cheetham M.E., Ebenezer N.D., Jenkins S.A., Bhattacharya S.S., Webster A.R., Holder G.E., Bird A.C. RPGR mutation associated with retinitis pigmentosa, impaired hearing, and sinorespiratory infections. J. Med. Genet. 2003;40:609–615. doi: 10.1136/jmg.40.8.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerber S., Alzayady K.J., Burglen L., Brémond-Gignac D., Marchesin V., Roche O., Rio M., Funalot B., Calmon R., Durr A. Recessive and dominant de novo ITPR1 mutations cause Gillespie syndrome. Am. J. Hum. Genet. 2016;98:971–980. doi: 10.1016/j.ajhg.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKay G.J., Clarke S., Davis J.A., Simpson D.A.C., Silvestri G. Pigmented paravenous chorioretinal atrophy is associated with a mutation within the crumbs homolog 1 (CRB1) gene. Invest. Ophthalmol. Vis. Sci. 2005;46:322–328. doi: 10.1167/iovs.04-0734. [DOI] [PubMed] [Google Scholar]
- 7.Vainberg I.E., Lewis S.A., Rommelaere H., Ampe C., Vandekerckhove J., Klein H.L., Cowan N.J. Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin. Cell. 1998;93:863–873. doi: 10.1016/s0092-8674(00)81446-4. [DOI] [PubMed] [Google Scholar]
- 8.Cowan N.J., Lewis S.A. Type II chaperonins, prefoldin, and the tubulin-specific chaperones. Adv. Protein Chem. 2001;59:73–104. doi: 10.1016/s0065-3233(01)59003-8. [DOI] [PubMed] [Google Scholar]
- 9.Tian G., Cowan N.J. Tubulin-specific chaperones: components of a molecular machine that assembles the α/β heterodimer. Methods Cell Biol. 2013;115:155–171. doi: 10.1016/B978-0-12-407757-7.00011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis S.A., Gu W., Cowan N.J. Free intermingling of mammalian beta-tubulin isotypes among functionally distinct microtubules. Cell. 1987;49:539–548. doi: 10.1016/0092-8674(87)90456-9. [DOI] [PubMed] [Google Scholar]
- 11.Dixit R., Barnett B., Lazarus J.E., Tokito M., Goldman Y.E., Holzbaur E.L. Microtubule plus-end tracking by CLIP-170 requires EB1. Proc. Natl. Acad. Sci. USA. 2009;106:492–497. doi: 10.1073/pnas.0807614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nogales E., Wolf S.G., Downing K.H. Structure of the alpha beta tubulin dimer by electron crystallography. Nature. 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 13.Nogales E., Whittaker M., Milligan R.A., Downing K.H. High-resolution model of the microtubule. Cell. 1999;96:79–88. doi: 10.1016/s0092-8674(00)80961-7. [DOI] [PubMed] [Google Scholar]
- 14.Breuss M.W., Nguyen T., Srivatsan A., Leca I., Tian G., Fritz T., Hansen A.H., Musaev D., McEvoy-Venneri J., James K.N. Uner Tan syndrome caused by a homozygous TUBB2B mutation affecting microtubule stability. Hum. Mol. Genet. 2017;26:258–269. doi: 10.1093/hmg/ddw383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang R., Alushin G.M., Brown A., Nogales E. Mechanistic origin of microtubule dynamic instability and its modulation by EB proteins. Cell. 2015;162:849–859. doi: 10.1016/j.cell.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perrault I., Rozet J.M., Gerber S., Ghazi I., Leowski C., Ducroq D., Souied E., Dufier J.L., Munnich A., Kaplan J. Leber congenital amaurosis. Mol. Genet. Metab. 1999;68:200–208. doi: 10.1006/mgme.1999.2906. [DOI] [PubMed] [Google Scholar]
- 17.Zemojtel T., Kielbasa S.M., Arndt P.F., Chung H.-R., Vingron M. Methylation and deamination of CpGs generate p53-binding sites on a genomic scale. Trends Genet. 2009;25:63–66. doi: 10.1016/j.tig.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Tischfield M.A., Cederquist G.Y., Gupta M.L., Jr., Engle E.C. Phenotypic spectrum of the tubulin-related disorders and functional implications of disease-causing mutations. Curr. Opin. Genet. Dev. 2011;21:286–294. doi: 10.1016/j.gde.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiore M., Goulas C., Pillois X. A new mutation in TUBB1 associated with thrombocytopenia confirms that C-terminal part of β1-tubulin plays a role in microtubule assembly. Clin. Genet. 2017;91:924–926. doi: 10.1111/cge.12879. [DOI] [PubMed] [Google Scholar]
- 20.Tischfield M.A., Baris H.N., Wu C., Rudolph G., Van Maldergem L., He W., Chan W.M., Andrews C., Demer J.L., Robertson R.L. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng R., Sang Q., Kuang Y., Sun X., Yan Z., Zhang S., Shi J., Tian G., Luchniak A., Fukuda Y. Mutations in TUBB8 and human oocyte meiotic arrest. N. Engl. J. Med. 2016;374:223–232. doi: 10.1056/NEJMoa1510791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moeller M.P. Early intervention and language development in children who are deaf and hard of hearing. Pediatrics. 2000;106:E43. doi: 10.1542/peds.106.3.e43. [DOI] [PubMed] [Google Scholar]
- 23.Yoshinaga-Itano C. Benefits of early intervention for children with hearing loss. Otolaryngol. Clin. North Am. 1999;32:1089–1102. doi: 10.1016/s0030-6665(05)70196-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Exhaustive list of rare SNVs/indels de novo and recessive variants identified in the four families. The deleterious effect of variants was assessed using the Alamut Mutation Interpretation software which uses Polyphen 2, SIFT, Mutation Taster, GVGD, SpliceSiteFinder-like, MaxEntScan, NNSPLICE, and/or Human Splicing Finder. MAF: minor allele frequency.