Abstract
In this study, strain PSBB1 isolated from Vicia faba rhizosphere was identified as Burkholderia cepacia, by 16S rDNA sequence analysis and characterized. Strain PSBB1 tolerated glyphosate up to 3200 μg ml−1 and produced IAA (81.6 μg ml−1), ACC deaminase (69.3 mg−1 protein h−1), SA (39.3 μg ml−1) and 2,3-DHBA (26.6 μg ml−1), solubilized insoluble P (50.8 μg ml−1) and secreted 29.4 μg ml−1 exopolysaccharides, which decreased with increasing concentrations of glyphosate. Cell damage following glyphosate application was visible under SEM and CLSM. The phytotoxicity of glyphosate on chickpea was variable but significant. B. cepacia mitigated toxicity and enhanced the size, dry matter, symbiosis, seed attributes and nutritional contents of chickpea. Further, B. cepacia strain PSBB1 declined the levels of CAT, POD, APX and GPX and MDA contents at 4332 μg kg−1 soil glyphosate. Proline also increased under glyphosate stress but declined in B. cepacia inoculated plants. The ability to tolerate higher concentration of glyphosate, the capacity to secrete plant growth regulators even under herbicide stress and potential to reduce the level of proline and antioxidant enzymes makes B. cepacia as an interesting choice for enhancing chickpea production in soils contaminated even with herbicides.
Keywords: Chickpea, Herbicide toxicity, Burkholderia cepacia, Proline, Antioxidant enzymes, Bioremediation
Introduction
Herbicides are frequently and abruptly used in intensive cropping systems for optimizing crop production. Owing to widespread and inadvisable application, major portion of the herbicides used in excess amount, however, persist within soils (Curran 2016). Following accumulation within soils and later on uptake by plants, herbicides cause toxicity to many crops including legumes (Ugbe et al. 2016). Among legumes, chickpea is considered important due to its protein rich nutritional value. In addition, it is severely affected due to its inability to compete with weeds as it has limited growth rate and leaf area which grow slowly during initial growth stages (Goud et al. 2013). Moreover, it needs wider spacing during cultivation which facilitates crop weed competition which as a result pose a serious threat to crop quality and production unless it is controlled effectively. However, to eradicate weeds from cultivable fields, suitable weed control strategies involving mechanical practices, crop rotations, hand weeding and application of herbicides are available and practiced in agricultural practices. The herbicides besides exhibiting inhibitory effects also cause threat to the existence and physiological functions of rhizobacteria (Nandula and Tyler 2016) and, consequently, indirectly affects the soil fertility (Bitew and Alemayehu 2017). Glyphosate, a broad spectrum systemic herbicide which belongs to organophosphorus family is applied to destroy weeds, especially annual broadleaf weeds and grasses which in turn limit the growth of crops. While evaluating the impact of high concentration of glyphosate on nitrogenase activity of numerous rhizobial strains, Zablotowicz and Reddy (2007) observed that herbicides considerably declined nitrogenase activity of rhizobia. As a consequence, the symbiotic events leading to nodule formation and root morphogenesis of the test plants were drastically diminished (Adami et al. 2017). Similarly, the lethal impact of certain herbicides like pendimethalin, chlormuron, propaquizafop, oxyfluorfen and imazethapyr on nodulation, growth and yield parameters of chickpea plants has previously been reported by Raghavendra et al. (2017). In addition, the phyto-fatal effect of butachlor, alachlor and oxyfluorfen on oil contents and yield components of groundnut (Arachis hypogeae) is reported (Sahoo et al. 2017). Nevertheless, due to the involvement of more labour and high cost, environmental persistence, the emergence of herbicide resistance among weeds, herbicide drift and environmentally insecure nature, the search for inexpensive and ecologically sustainable option is an urgent need for the end users (practitioners) to minimise the risks caused by herbicides. To this end, numerous hard working and meticulous scientists have reported herbicide tolerant microbes with potential plant growth promoting activities. Chief among them belongs to genera Rhizobium (Sudharshana et al. 2013), Bacillus (Perez-Fernández and Alexander 2017), Azospirillum (Khalid and Khokhar 2013), Burkholderia (Tetard-Jones and Edwards 2016), etc. Sadly, such microbial inoculants when applied in fields are exposed to poisonous substances for instance herbicides inhibits leguminous crops and their associated nodule bacteria (Alori et al. 2017). Although studies highlighting the noxious effects of herbicides on cognate rhizobacteria and many agronomic crops (Parsa et al. 2013) including legumes are available, the reports on the result are, however, contradictory. In addition, the toxicity of herbicides especially glyphosate to plant growth promoting (PGP) features of Burkholderia cepacia and chickpea is unknown. Realizing these, the present study was designed to evaluate the—(i) toxic impact of glyphosate on bacteria isolated from rhizosphere soil (ii) influence of glyphosate on bioactive molecules of potent glyphosate tolerant B. cepacia strain PSBB1 (iii) influence of herbicide tolerant bacterial strain on the biochemical activities of chickpea plants raised in herbicide treated soils (iv) antioxidant response besides determination of stress alleviator proline in inoculated plants grown under herbicide stress and (v) impact of glyphosate on nutrient uptake and root morphology of chickpea plants.
Materials and methods
Physico-chemical properties and microbial composition of rhizosphere soil
The soil samples collected from rhizosphere of faba bean (Vicia faba) grown at the agricultural fields of Faculty of Agricultural Sciences, Aligarh Muslim University, Aligarh (27°53′N 78°05′E 27.88°N 78.08°E), Uttar Pradesh, India were processed and analysed for different physicochemical properties using standard and widely used methods. The soil samples obtained from rhizosphere were used further for assaying microbial compositions. The microbial composition including total bacterial communities, fungal abundance, actinomycetal populations, phosphate solubilizing microorganisms (PSM) involving both phosphate solubilising bacteria (PSB) and phosphate solubilising fungi (PSF) and asymbiotic nitrogen fixer (Azotobacter) were recovered employing standard methods. Moreover, PSB were maintained on Pikovskaya agar medium (Pikovskaya 1948). Isolated bacterial cultures were identified primarily by standard microbiological and biochemical methods (Holt et al. 1994).
Herbicide tolerance and strain identification
The bacterial strains were further exposed to varying concentration of glyphosate [(CAS No. 1071836, a.i = 41% of isopropylamine salt, molecular weight (g mol−1) 169.08, MP 200 °C)] to select herbicide tolerant bacteria. After sterility check, the minimal agar plates were amended with increasing rates (0–3200 μg ml−1) of glyphosate and overnight grown bacterial strains were spot inoculated. Plates were incubated at 28 ± 2 °C for 48 h and the colonies surviving at the highest concentration of glyphosate were picked and designated as herbicide tolerant strains. Of the total 20 bacterial strains expressing varying degree of P-solubilization, strain PSBB1 performing higher tolerance to glyphosate was chosen for further studies. Herbicide resistant bacterial isolate was identified by both biochemical and molecular method. The biochemical tests employed for presumptive identification of PSBB1 strain included, indole production, citrate utilization, Voges Proskauer, methyl red, nitrate reduction catalase and oxidase test, gelatine liquefaction, starch hydrolysis and carbohydrates (mannitol, dextrose and sucrose) utilization (Holt et al. 1994). Bacterial strain was later on identified to species level using 16S rRNA partial gene sequence analysis which was done commercially by a DNA sequencing service provided by Macrogen Inc., Seoul, South Korea, using universal primers 785F (5′-GGATTAGATACCCTGGTA-3′ and 907R (5′-CCGTCAATTCMTTTRAGTTT-3′). The nucleotide sequence data obtained from Macrogen was deposited in the GenBank sequence database. The BLASTn program available online was employed to find similar sequences with known taxonomic information accessible from the databank accessible at the NCBI website (http://www.ncbi.nlm.nih.gov/BLAST) to precisely recognize bacterial strain PSBB1. Sequence was aligned using bootstrapped neighbour-joining method and a phylogenetic tree was built using MEGA6.0 software.
Bioassay for plant growth regulators in glyphosate stress condition
Indole acetic acid, cyanogenic compounds and ammonia
The quantitative estimation of indole-3-acetic acid (IAA) produced by B. cepacia strain PSBB1 was performed as described by Gordon and Weber (1951) later modified by Brick et al. (1991). Here, B. cepacia PSBB1 was grown in Luria–Bertani (LB) broth (g l−1: tryptone 10; yeast extract 5; NaCl 10 and pH 7.5). Luria–Bertani broth (100 ml) containing 100 mg ml−1 tryptophan were treated with 0, normal (1×), double (2×) and three times (3×) more of normal concentrations of glyphosates. The normal concentration of glyphosate used throughout the experiment was 500 μg ml−1. The glyphosate containing LB was then inoculated with 100 μl culture (108 cells ml−1) of B. cepacia PSBB1 strain and incubated at 28 ± 2 °C for 7 days with shaking at 120 r min−1. Following complete incubation, culture (5 ml) was centrifuged (8000 r min−1) for 10 min. and two ml supernatant was added with 100 μl orthophosphoric acid and four ml Salkowsky reagent (2% 0.5 M FeCl3 prepared in 35% per-chloric acid) and incubated for 1 h at 28 ± 2 °C in dark. The absorbance of pink colour developed during the reaction was measured at 530 nm. The quantity of indole acetic acid was calibrated using pure IAA as a standard. HCN production by B. cepacia PSBB1 strain was evaluated by the method of Bakker and Schippers (1987). For HCN production, B. cepacia PSBB1 strain was inoculated on an HCN induction medium (g l−1: tryptic soy broth 30; glycine 4.4 and agar 15) supplemented with 0, 1×, 2× and 3× concentrations of glyphosate and incubated at 28 ± 2 °C for 4 days. A disk of Whatman filter paper No. 1 soaked in 0.5% picric acid and 2% Na2CO3 was placed under the lid of the Petri plates and sealed with parafilm. After 4 days incubation at 28 ± 2 °C, an orange brown colour of the paper confirmed the production of HCN. The NH3 production by B. cepacia PSBB1 strain was grown in peptone water with control (0), recommended dose and two and three times of recommended rates of glyphosate and incubated at 28 ± 2 °C for 4 days. Nessler reagent (1 ml) was added to each tube and the development of yellow colour showed ammonia production (Dye 1962).
Phosphate solubilization and siderophore production
The quantitative estimation of phosphate solubilization activity (PSA) of PSBB1 strain was done using Pikovskaya broth medium amended with three concentrations of glyphosate. The amount of solubilized P was evaluated by chlorostannous reduced molybdophosphoric acid blue method (King 1932; Jackson 1976). Solubilization index (SI) and solubilization efficiency (SE) was calculated by the formula of Premono et al. (1996) and Nguyen et al. (1992), respectively.
Secretion of siderophores by the B. cepacia strain PSBB1 was determined qualitatively by FeCl3 test (Atkin et al. 1970) and by the Chrome Azurol S (CAS) method (Alexander and Zuberer 1991) using the three doses of glyphosate, added to CAS agar plates. Siderophore secreted by B. cepacia PSBB1 was quantitatively assayed by growing bacterial culture in Modi medium added with three concentrations of glyphosate for 5 days and Catechol-type phenolates was determined (Reeves et al. 1983). For detection, equal volume of the Hathway’s reagent and sample were mixed, and absorbance was measured at 560 nm for salicylates (SA) and at 700 nm for dihydroxy phenols using sodium salicylate and 2,3-dihydroxy benzoic acid (DHBA) as a standard, respectively.
Extraction of exo-polysaccharide and ACC deaminase
The exopolysaccharide (EPS) released by B. cepacia PSBB1 was extracted by the method of Mody et al. (1989). For this, PSBB1 was grown in basal medium supplemented with 5% sucrose and treated with recommended, two times and three times doses of glyphosate and incubated for 5 days at 28 ± 2 °C at 120 rpm. Culture broth was centrifuged (8000 rpm min−1) for 20 min and EPS was extracted by mixing chilled acetone (CH3COCH3) and supernatant in a ratio of 3:1. The precipitated EPS so obtained was washed three times alternately with distilled water and acetone, transferred to filter paper and weighed after overnight drying at room temperature. The bacterial enzyme ACC deaminase (EC 4.1.99.4) secreted by B. cepacia strain PSBB1 was qualitatively detected by spot inoculation method using DF salts minimal medium (Dworkin and Foster 1958) containing 3 mM ACC as the only source of N. Plates containing DF medium without ACC and with (NH4)2SO4 (0.2% w/v) served as negative and positive control, respectively. Plates maintained at 28 ± 2 °C for 72 h were examined each day for bacterial growth. Mesorhizobium LMS-1 containing pRKACC plasmid (Shah et al. 1998) was used as a positive control. The quantity of ACC deaminase was also extracted following the methods of Honma and Shimomura (1978) and Penrose and Glick (2003). The quantity of α-ketobutyrate generated due to ACC deaminase activity was measured spectrophotometrically against a standard curve of α-ketobutyrate. The activity of ACC deaminase was presented as the quantity of α-ketobutyrate released/mg of protein/h. All experiments were conducted three times.
Cellular damage induced by glyphosate observed under SEM and CLSM
Cellular damage to the bacterial strain B. cepacia PSBB1 was observed under SEM (Saleem et al. 2017) after growing B. cepacia PSBB1 in NB (g l−1: peptone 10; beef extract 10; NaCl 5; pH 7) treated with 1000 μg ml−1 glyphosate. In addition, the toxicity of glyphosate to strain PSBB1 was observed under Confocal Laser Scanning Microscopy (CLSM).
Crop-based experiments
Seed inoculation, herbicide treatment and plant culture
The surface of healthy seeds of chickpea (cv. avarodhi) was sterilized with 70% ethanol for 3 min.; 3% sodium hypochlorite (NaOCl) for 3 min.; washed six times with sterile water and dried. Sterilized seeds of chickpea were bacterized with B. cepacia PSBB1 by dipping seeds in liquid culture medium for 2 h using 10% gum arabic as sticker to achieve 108 cells seed−1. The un-inoculated sterilized seeds submerged in sterile water only were taken as control. Non-bacterized and bio primed ten seeds were sown in each earthen pot containing 3 kg of conventional soils. The experimental soil (non-sterilized) was: sandy clay loam and had organic C 6.2 g kg−1, Kjeldahl N 0.75 g kg−1, Olsen P 16 mg kg−1, pH 7.2 and WHC 0.44 ml g−1, cation exchange capacity 11.7 and 5.1 cmol kg−1 anion exchange capacity. Glyphosate (μg kg−1) at 1444 (1×), 2888 (2×) and 4332 (3×) were added to each experimental pot. Pots without herbicide served as control. Soils after adding herbicide were mixed homogenously. There were eight treatments and individual treatment was repeated three times. Experimental pots were set up in a complete randomized design and three plants were retained in every pot 7 days after emergence. Pots were watered regularly and were kept in an open field conditions (9 h photoperiod/15 h dark cycle). The crop experiments were carried out regularly for 2 years to achieve consistency in results.
Assessment of chickpea growth, symbiosis, and grain features
All plants in three pots for each treatment were uprooted at 80 days after sowing and remaining plants in three pots were harvested at 120 DAS. The detached plants were used for the measurement of growth and symbiotic attributes. Plants collected at 80 and 120 days after sowing were oven-dried and dry matter accumulated within plant tissues was measured. Chlorophyll a, b, total chlorophyll and carotenoid contents in chickpea leaves (Arnon 1949) and accumulation of leghaemoglobin (LHb) in nodule tissues of inoculated/non-inoculated plants were determined 80 days after growth (Sadasivum and Manickam 1992). Grain features such as the formation of seeds and seed protein (Lowry et al. 1951) was estimated at 120 DAS (at harvest).
Bioassay of proline, malondialdehyde (MDA) and antioxidant enzymes
Proline content in fresh plant tissues collected from different organs (root, shoot and leaves) were determined at 80 DAS while in grains it was estimated after harvest (120 DAS) as suggested by Bates et al. (1973). The extent of lipid peroxidation was evaluated by measuring MDA by the method of Heath and Packer (1968) and the result was expressed as μ mol MDA g−1 fresh weight. Antioxidant enzymes such as CAT, POD, APX and GPX in fresh leaves were detected at 80 DAS following the previously described methods (Ahmed et al. 2017; Leonard et al. 2004; Hammerschmidt et al. 1982; Zhang and Kirkham 1996). All enzyme assays were performed three times with three replicates.
Root morphology, bacterial colonization and nutrient uptake
The toxic and destructive impact of glyphosate on root morphology of chickpea plants grown on soft agar plates treated with and without 1000 μg ml−1 of glyphosate was monitored under SEM (JSM 6510 LV, JEOL, Japan). Uninoculated and PSBB1 inoculated chickpea roots were used further for assessing B. cepacia colonization ability. For this, root samples were thoroughly washed with water and phosphate buffer. Root samples were then fixed for 12 h in 2% (v/v) glutaraldehyde prepared in 0.1 M phosphate buffer, pH 7.0, washed three times with the same buffer and dehydrated in a graded series of ethanol at 4 °C. Samples were dried in critical point dryer (CPD). Dried samples were fixed in gold stubs and examined under SEM. The nutrient uptake (nitrogen and phosphorous content) by roots and shoots of chickpea plants detached at 120 DAS were determined following the method of Jackson (1976) and Iswaran and Marwah (1980).
Statistical analysis
The experiments were performed with plants exposed to identical treatments for two successive years under similar experimental conditions. Each experiment was done in three groups with three replicates per treatment and data was presented as mean ± SD. Data were pooled, and TWO-WAY analysis of variance was applied using statistical program Minitab 17. The significant differences and similarities among the treatments were compared using Duncan’s Multiple Range Test (DMRT) by two-way analysis of variance at 5% significance.
Results and discussion
Soil composition and microbial characterization and identification
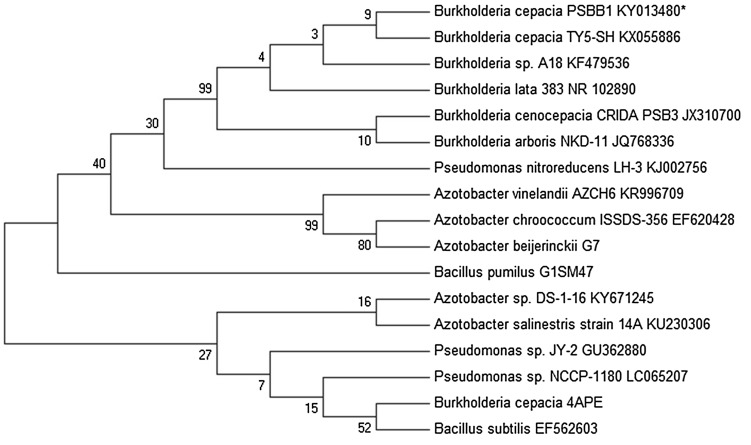
The physico-chemical properties of soils determined in this study showed: pH 8.9, electrical conductivity (EC) 0.995 mv cm−2, organic carbon (OC) 0.4%, total available N, P and K 0.077, 18, and 319.5 kg ha−1, respectively. Sulphur (S) and Boron (B) was 11.5 and 5.2 mg kg−1, respectively, while micronutrients like Zn, Fe, Mn and Cu were 1.14, 9.26, 4.32 and 0.54 mg kg−1, respectively. Bacterial strain PSBB1 isolated from V. faba rhizosphere was identified as Gram-negative bacterium which showed positive reaction to citrate, oxidase, catalase, nitrate reduction, hydrolysed starch and gelatin. Strain PSBB1, however, showed a variable preference for carbohydrates. Based on the morphological and biochemical characteristics, strain PSBB1 belonged to genus Burkholderia. However, this genus was further identified to species level by 16S rRNA gene sequence analysis. The nucleotide sequence of 16S rRNA of PSBB1 (approximately 823 bp in size) was submitted to GenBank (Accession Number KY013480). A similarity search was performed using BLASTn programme which indicated that strain PSBB1 was closely related to B. cepacia NR114491.1 (16S: 99% similarity). Due to highest similarity, the strain PSBB1 was confirmed as B. cepacia. Later on, a phylogenetic tree was constructed by MEGA 6.0 software, based on 16S rRNA partial gene sequence (Fig. 1).
Fig. 1.
Phylogenetic tree constructed from the 16S rRNA gene sequence (823 bp) of Burkholderia cepacia strain PSBB1 (GenBank Accession No. KY013480) and related organisms using Clustal W and MEGA 6.0 software
Glyphosate tolerance
The application of herbicides in agronomic practices is a common practice to protect crops from undesirable weed competition and hence, to optimize food production. However, excessive use of such chemicals leads both to reduction in crop production and the emergence of resistance to such chemicals among beneficial soil microflora. To solve these problems, we tried to find herbicide tolerant bacteria which could be used as microbial inoculant for enhancing crop production in herbicide enriched soil. In this study, the rhizobacterial strain B. cepacia PSBB1 when exposed to varying concentrations of glyphosate was found to tolerate exceptionally higher level (3200 μg ml−1) of glyphosate and grew well on carbon- and nitrogen-source-free minimal salt agar plates treated with herbicide. Principally, the sensitivity or resistance toward herbicides is controlled by physiological activity and genetic composition of microbiota (Herman et al. 2005). Hence, microorganisms capable of tolerating higher level of pesticides have been report as frequent degrader of such chemicals (Karishma and Prasad 2016). Since the medium used in our study to identify glyphosate tolerant strain of B. cepacia had no C and N other than herbicide, it is convincingly presumed that B. cepacia strain PSBB1 probably used up herbicides as a sole energy source by biodegrading glyphosate and hence, showed maximum tolerance to this herbicide. This feature of higher glyphosate tolerance is considered important for different reasons—(i) glyphosate tolerant B. cepacia can thrive well under herbicide stressed environment and (ii) if applied as inoculant under herbicide stress, can facilitate crop production (Table 1).
Table 1.
Morphological and biochemical properties of Burkholderia cepacia strain PSBB1
| Characteristics | Burkholderia cepacia strain PSBB1 |
|---|---|
| Morphology | Medium, circular, rounded whitish colony, entire margin |
| Gram reaction | −ve |
| Shape | Short rods |
| Biochemical reactions | |
| Citrate utilization | − |
| Indole | − |
| Methyl red | + |
| Nitrate reduction | + |
| Oxidase | + |
| Catalase | + |
| Voges Proskauer | − |
| Carbohydrate utilization | |
| Glucose | + |
| Lactose | + |
| Fructose | + |
| Sucrose | + |
| Hydrolysis | |
| Starch | + |
| Gelatin | + |
| Tolerance to glyphosate (μg ml−1) | |
| At minimal agar plate | 3200 |
| GenBank Accession No. | KY013480 |
Plant growth regulators under glyphosate stress
IAA, HCN and NH3 production
Glyphosate tolerant bacterial strain PSBB1 revealed a variable production of plant growth regulators when grown both with and without herbicide (Table 2). Generally, the amount of active biomolecules secreted by B. cepacia PSBB1 decreased with increasing rates of glyphosate. For instance, PSBB1 produced IAA 81.6 μg ml−1 in the absence of glyphosate which, however, declined regularly with consistent increase in glyphosate concentration and a maximum reduction in IAA synthesis was recorded at 3× (59.3 μg IAA ml−1). Similarly, Nithyakalyani et al. (2016) observed comparable secretion of IAA by Bradyrhizobium. A constant enhancement in IAA secretion in medium containing tryptophan is not shocking since tryptophan is used for indole acetic acid production. However, secretion of IAA even at higher glyphosate level is interesting because such herbicide tolerant microbes when applied under herbicide polluted soil are likely to continue secreting IAA. And, therefore, the phytohormone requirement of crops can be fulfilled even under herbicide polluted soils. This finding thus suggests that the glyphosate tolerant and IAA positive strain PSBB1 may be applied to facilitate many important biochemical activities of plants, for example, growth and ramification of cells, root morphogenesis, symbiosis, apical dominance, phototropism and geotropisms even in herbicide contaminated soils (Deinum et al. 2016). Among other microbial metabolites, cyanogenic compounds like HCN are also secreted by several microorganisms, which can be produced directly from glycine and cyanogenic glycosides (Rijavec and Lapanje 2016). Ammonia is yet another metabolite produced by bacteria via degradation of amino acids and by nitrite ammonification, urease-mediated hydrolytic degradation of urea and decarboxylation of amino acids. Both HCN and NH3 have been found in root exudates. The HCN and ammonia production is found to be a common trait of various PGPR strains including Burkholderia, Aeromonas, Pseudomonas and Bacillus (Rahman et al. 2017). The secretion of cyanogenic compounds in varied rhizosphere gives HCN producing bacteria a competitive and selective advantage over other HCN sensitive bacterial populations. Production of cyanogenic compound and ammonia by bacterial strain was, however, not detected at the 3× concentration of glyphosate. Similarly, hydrogen cyanide and NH3 limitation by rhizosphere microorganisms in polluted soil is reported (Rani and Kumar 2017). Reduction in HCN and ammonia production by rhizobacterial strains under stressed environmental conditions could possibly be due to the impairment of various metabolic activities (Azarmi et al. 2016).
Table 2.
Plant growth promoting (PGP) activities of strain PSBB1 in the absence and presence of different concentration of glyphosate
| Treatment | Dose rate (µg ml−1) | IAA (µg ml−1) | Phosphate solubilization | EPS (µg ml−1) | ACC deaminase activity (μM α-ketobutyrate mg−1 protein h−1) | Siderophore | HCN | NH3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liquid broth (µg ml−1) | Solid media | Phenolate (µg ml−1) | CAS agar (mm) | FeCl3 test | |||||||||
| SE | SI | SA | 2,3-DHBA | ||||||||||
| Control | 0 | 81.6a | 50.8a | 2.4a | 129.3a | 29.4a | 69.3a | 39.3a | 26.6a | 16.3a | +++ | ++ | +++ |
| 1× | 500 | 72.6b | 23.5b | 2.3a | 126a | 28.9ab | 60.5b | 39a | 25.1b | 16.0ab | ++ | ++ | ++ |
| 2× | 1000 | 62.2c | 19.2c | 2.1b | 111b | 27b | 55.9c | 34.3b | 20.7c | 15.3b | ++ | + | + |
| 3× | 1500 | 59.3c | 14.1d | 2.0c | 108b | 22.3c | 49.1d | 26.1c | 18.3d | 14.0c | ++ | ND | ND |
| LSD | 3.01 | 1.72 | 0.098 | 5.08 | 2.37 | 2.53 | 3.33 | 1.27 | 0.88 | − | − | − | |
Value indicates the means of three independent replicates. Means followed by similar alphabets are not significantly different from each other according to DMRT
Phosphate solubilization and production of siderophores
P-solubilizing efficiency of rhizobacterial strain PSBB1, decreased as the dose rate of glyphosate increased. The maximum decrease in P-solubilization was recorded at 3× of glyphosate (14.1 μg ml−1), which diminished the solubilized phosphorous by 72% over control (50.8 μg ml−1). The SE of strain PSBB1 varied between 2.3 (1×) and 2.0 (3×) while the SI differed between 126 (1×) and 108 (3×). In many studies, the PSA of rhizobacteria has been due to the release of low molecular weight organic acids that results in drop in pH (Ditta and Khalid 2016). Like other plant growth regulators, the production of siderophores involving salicylic acid and DHBA also decreased considerably under varying rates of glyphosate. For example, the SA and DHBA secreted by B. cepacia PSBB1 in herbicide free medium was 29.3 and 26.6 μg ml−1, respectively, which, however, declined by 45 and 26%, respectively, at 3× of glyphosate. Siderophores, an iron chelating compound produced by bacterial communities under iron starved conditions (Dorjey et al. 2017) provide Fe to plants when grown in limited Fe deficient environment (Kurth et al. 2016). Under aerobic environments, iron occurs principally as insoluble hydroxide and oxyhydroxide, which becomes inaccessible/unavailable to microbial communities. Therefore, the synthesis of siderophores under iron starved condition could be advantageous because such siderophores producing strains could be used in the management of phytopathogens.
Exopolysaccharides and ACC deaminase
Realizing the significance of EPS in biological nitrogen fixation (BNF) (Ghosh and Maiti 2016) soil aggregation (Vogel et al. 2017) and capability of PGPR to adapt to environmental stressor molecules, the strain PSBB1 was also examined for EPS producing ability under herbicide stress. Fascinatingly, PSBB1 released a substantial quantity of EPS which may affect the growth of many plants directly and/or indirectly even under stressed situation. In the absence of glyphosate, 29.4 μg ml−1 EPS was produced by PSBB1 strain, which declined gradually with increasing concentrations of glyphosate (Table 2). The release of EPS by bacterial strains both in the absence or presence of stressor molecules could be advantageous both for producing bacteria and crops. Exopolysaccharides on one hand protects bacteria from harsh environment such as desiccation, phagocytosis and phage attack (Zeidan et al. 2017) by forming a polymeric network around growing culture while on the other hand it protects plants from pathogen attack (Rodríguez-Navarro et al. 2014). Also, invasion process, formation of infection thread, bacteroid, and nodules during Rhizobium–legume interactions is greatly influenced by EPS (Yuan et al. 2017). Due to these, the interest in identifying EPS producing organism has been increased in recent times (Kaushal and Wani 2016).
ACC deaminase secreted by many bacteria is an important biochemical trait which decreases unusually the higher concentration of ethylene and, therefore, facilitates performance of plants growing under unfavourable environments (Han et al. 2015; Sharma et al. 2016). Here, B. cepacia PSBB1 strain exhibited a positive ACC deaminase reaction grown even with the different concentration of herbicide. A gradual decline in the amount of α-ketobutyrate was, however, observed as the concentration of herbicide was increased (Table 2). It was found that, 3× dose of glyphosate had the largest inhibitory effect and decreased ACC deaminase synthesis by 29% over control. Secretion of ACC Deaminase by B. cepacia PSBB1, however, even in herbicide stress could agronomically be a beneficial feature for raising the productivity of crops under herbicide stress (Glick et al. 2007). Conclusively, the ability of B. cepacia PSBB1 to survive under glyphosate stress and its potential to secrete plant growth promoting substances even under herbicide stress makes this organism an interesting and most promising choice for crop production even under the environment polluted with herbicides.
Assessment of glyphosate induced toxicity to B. cepacia PSBB1 under SEM and CLSM
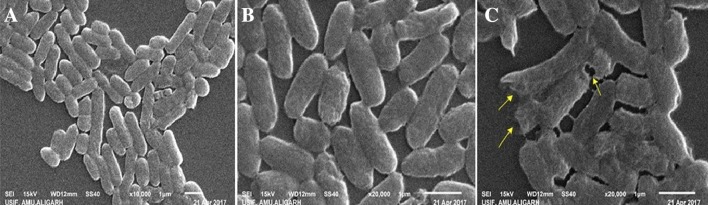
After estimating the lethal effect of glyphosate on B. cepacia PSBB1 growth under in vitro conditions, the glyphosate impact on strain PSBB1 was evaluated further by SEM. The SEM showed a distinct and unruptured bacterial cell when grown without herbicide (Fig. 2a, b). However, strain PSBB1 when grown in the presence of 1000 μg ml−1 of glyphosate had damaged cell surface (Fig. 2c). The CLSM images further confirmed the toxicity of herbicides resulting in increasing number of dead cells (Fig. 3) which increased with increasing concentration of glyphosate. Thus, both SEM and CLSM used for analyzing glyphosate toxicity confirmed the inhibitory effect of glyphosate observed under in vitro conditions. Similar destructive impact of herbicides and consequential cellular damage to other rhizobacterial cells such as, Bradyrhizobium and Pseudomonas and observed under SEM has recently been reported (Shahid and Khan 2017).
Fig. 2.
SEM micrographs of B. cepacia grown in the absence of glyphosate (a, b) and in the presence of 1000 μg ml−1 of glyphosate (c). Yellow arrows indicate the damage/rupture caused by glyphosate
Fig. 3.
CLSM images of B. cepacia PSBB1 in the absence of glyphosate a control cells and b 500 μg ml−1, c 1000 μg ml−1 and d 1500 μg ml−1 of glyphosate. The image shows an increasing number of dead cells (stained red with propidium iodide) which enhanced with increasing glyphosate concentrations
Herbicide and inoculation impact on chemical and biological characteristics of chickpea plants
Considering the threat that herbicide adversely affects the production of legumes on one hand and herbicide tolerant soil microflora may enhance the legume production even under herbicide stressed soil, on the other hand, the current research was designed. Although, B. cepacia is a known pathogen, yet, it also has some agronomic importance and have previously been used as a PGPR due largely to its nitrogen fixing, phosphate solubilizing and ACC deaminase activity (Sandanakirouchenane et al. 2017; Rahman et al. 2017; Arthee and Marimuthu 2017) for enhancing the production of crops. For instance, B. cepacia as biofertilizer have been applied to protect crops from damaging impact of fungal pathogens leading eventually to the increase in crop yields (Holmes et al. 1998). Apart from these, B. cepacia strain CH9 is also known to reduce the toxicity of pesticides by degrading them. For example, the degradation of imidacloprid and metribuzin (Gopal et al. 2011) and parathion (Fernández-López et al. 2017) following B. cepacia application is reported. Considering these, the glyphosate tolerant B. cepacia PSBB1 was included in this study as inoculant for augmenting chickpea production in glyphosate treated soils.
Plant growth under glyphosate stress
The bacterized and uninoculated chickpea plants cultivated in soils treated with varying level of glyphosate had variable plant growth (Table 3). In general, the measured biological properties declined with rising concentration of glyphosate. Bacterized chickpea plants in contrast had superior growth relative to uninoculated plants but the biological characteristics of even bio primed plants declined gradually when raised along the herbicide compared to those grown in glyphosate free soil (Table 3). For example, B. cepacia PSBB1 strain when used as a bioinoculant with 2× of glyphosate, increased the dry biomass of roots and shoots by 12 and 41% at 80 days after sowing and 54 and 4% after 120 days of plant growth, respectively, relative to non-bacterized plants grown under herbicide stressed soils. While assessing the impact of bacterial culture (PSBB1) used with 3× concentration of glyphosate and comparing with those of only herbicide amended soil, a highest enhancement of 24 and 31% in shoot biomass at 80 DAS, and at 120 DAS, respectively, was observed in plants grown without glyphosate and non-inoculated treatment. The ANOVA (two-way) analysis indicated a significant (P ≤ 0.05) interaction between bacterial culture application and herbicide (glyphosate). The effect of biopriming and glyphosate on biological and chemical characteristics of test plant was synergistically significant at 80 DAS and 120 DAS (Table 3). Like many traditional PGPR, glyphosate tolerant B. cepacia PSBB1 used as bacterial inoculant in our study also resulted in a significant improvement in the functioning of chickpea which could possibly be due to the synthesis of plant growth regulators (Premachandra et al. 2016). Of these, growth regulators, IAA for example, stimulates elongation or division of cells and promotes root growth (Pandey et al. 2017). As a result of expanded roots, the plant absorbs more water and minerals (Rijavec and Lapanje 2016) which consequently leads to enhanced growth. Other factors that might have contributed in the overall development of chickpea growth could be the availability of other essential nutrient such as P, siderophores, HCN, etc. to chickpea plants directly or indirectly in the chickpea rhizosphere.
Table 3.
Phytotoxic effect of different concentration of glyphosate on biological and symbiotic characteristics of chickpea grown in absence and presence of bioinoculant Burkholderia cepacia strain PSBB1
| Treatments | Dose rate (μg kg−1 soil) | Plant length (cm) | Fresh weight (g plant−1) | Dry biomass (g plant−1) | Symbiotic attributes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | Shoot | Root | No. of nodules/plant | Nodule biomass | ||||||||||
| 80 DAS | 120 DAS | 80 DAS | 120 DAS | 80 DAS | 120 DAS | 80 DAS | 120 DAS | 80 DAS | 120 DAS | 80 DAS | 120 DAS | 80 DAS | 120 DAS | 80 DAS | 120 DAS | ||
| Uninoculated | 0 | 35.3bc | 63.6b | 19bc | 37.6b | 1.5bc | 9.7bc | 1.2b | 3.6a | 0.38c | 3.3b | 0.1c | 0.42d | 8.6bc | 7.0b | 0.17b | 0.02c |
| 1444 | 32.3c | 41.0 g | 17.6c | 27.6c | 1.7b | 9.2c | 0.96d | 3.5a | 0.31e | 2.7c | 0.36a | 0.21 g | 11.3b | 6.0b | 0.11c | 0.03a | |
| 2888 | 21.0ef | 47.3e | 17.3c | 21.0e | 1.5bc | 6.4e | 0.85d | 2.8bc | 0.21 g | 2.4d | 0.09 cd | 0.25f | 5.6d | 5.0bc | 0.10c | 0.01d | |
| 4332 | 18.0f | 44.0f | 11.6d | 13.6f | 1.1c | 3.7f | 0.79d | 2.5c | 0.19 h | 1.2f | 0.07de | 0.34e | 1.6e | 4.0c | 0.11c | 0.01d | |
| Inoculated | 0 | 43.0a | 74.0a | 37.3a | 41.0a | 2.5a | 11.7a | 2.7a | 2.1d | 0.49a | 4.0a | 0.06e | 0.51b | 21.6a | 10.0a | 0.23a | 0.01e |
| 1444 | 33.6c | 56.0c | 18.3c | 26.3 cd | 2.4a | 10.6b | 1.2bc | 3.1b | 0.47b | 2.9c | 0.14b | 0.54a | 8.0 cd | 6.0b | 0.13c | 0.02b | |
| 2888 | 28.35 | 57.0c | 21b | 25.3 cd | 2.3a | 8.0d | 1.01c | 2.6c | 0.36d | 2.3d | 0.11c | 0.46c | 9.3bc | 6.0b | 0.11c | 0.017c | |
| 4332 | 27d | 52.6d | 13.6d | 23.3de | 1.4bc | 4.4f | 0.84d | 2.0d | 0.25f | 1.5e | 0.08de | 0.44d | 11.0bc | 4.0c | 0.12c | 0.01d | |
| LSD | 3.981 | 2.763 | 2.45 | 31.6 | 0.34 | 1.04 | 0.25 | 1.04 | 0.008 | 0.37 | 0.02 | 0.026 | 2.98 | 1.35 | 0.002 | 0.011 | |
|
F value Un inoculated (df = 1) |
142.6 | 79.54 | 137 | 228.95 | 75.79 | 34.35 | 77.49 | 75.4 | 3610 | 9.87 | 173.0 | 854.9 | 101.2 | 15.7 | 40.3 | 25.9 | |
| Inoculated (df = 3) | 58.09 | 127.0 | 132 | 73.5 | 20.17 | 67.7 | 84.71 | 31.7 | 1769 | 115 | 299.7 | 54.79 | 33.85 | 29.5 | 215.9 | 5.59 | |
| Un inoculated × inoculated (df = 3) | 3.92 | 175.2 | 40.2 | 15.59 | 4.42 | 88.3 | 33.96 | 14.3 | 553 | 3.25 | 133.6 | 81.9 | 12.43 | 5.11 | 63.6 | 1.34 | |
Each value is a mean of three replicates where each replicate constituted three plants/pot. Mean values are significant at P ≤ 0.05. Means followed by similar alphabets are not significantly different from each other according to DMRT test
Photosynthetic pigments and symbiotic attributes
The chlorophyll content and symbiotic attributes such as nodule numbers, nodule biomass and leghaemoglobin of fresh nodules detached from non-bacterized chickpea plants uprooted at 80 DAS declined constantly with subsequent increase in concentration of glyphosate (Table 4). Glyphosate at 4332 μg kg−1 decreased the chl a, chl b, total chlorophyll and carotenoids contents maximally by 13, 15, 11, 24%, respectively, in contrast to uninoculated chickpea plants. On comparing the effect of 1444 μg kg−1 glyphosate on inoculated and non-inoculated plants, a greatest increase of 8, 14, 9 and 13% in chl a, chl b, total chlorophyll and carotenoids content, respectively, was observed over glyphosate treated and non-inoculated control plants. In contrast, the bioinoculant increased the chl a, chl b, total chlorophyll and carotenoids content by 4, 16, 6 and 17%, respectively, at 2888 μg kg−1 soil relative to the herbicide treated but un-inoculated plants. A similar impact of glyphosate and PSBB1 strain on symbiotic attributes of chickpea plant was observed. Nodule number, nodule biomass and leghaemoglobin content were decreased by 17, 8 and 35%, respectively, at 3× concentration of glyphosate at 80 DAS. However, in the presence of 2× glyphosate concentration, bioinoculant (PSBB1) enhanced the nodule number, nodule dry biomass and leghaemoglobin content by 49, 16 and 14%, respectively, at 80 DAS. Owing to the toxic effect of glyphosate on the nutritional contents in legumes the efficiency of PSBB1 strain to enhance the nutritional uptake of chickpea was assessed. It was found that there was a marginal increase in N and P contents in plant organs as compared to glyphosate treated and PSBB1 inoculated plants. Two factor ANOVA showed that the single effect of inoculation and their interaction (inoculation × glyphosate) were significant (P ≤ 0.05) for the calculated factors. Also, the leghaemoglobin concentration in nodule tissues detached from PSBB1 inoculated chickpea plants was better. The enhanced nodule numbers and leghaemoglobin in bacterized legumes raised in glyphosate stressed soil is suggestive of the microbial colonization and existence inside herbicide contaminated soil. Similar, enhancement of different growth attributes of bio inoculated legumes grown under herbicides stressed soils has been reported by Sarkar et al. (2005).
Table 4.
Phytotoxic effect of different concentration of glyphosate on photosynthetic pigments, leghaemoglobin content, proline accumulation, seed attributes and nutritional content of chickpea grown in absence and presence of bioinoculant Burkholderia cepacia strain PSBB1
| Treatments | Dose rate (μg kg−1 soil) | Chlorophyll content (μg mg−1) | Leghaemoglobin content (mM (gf m)−1) | Proline content (mg (g fw)−1) | Seed attributes | N content (μg mg−1) | P content (μg mg−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chl a | Chl b | Total chl | Carotenoid | Shoot | Root | Leaves | Grains | No. of seeds/plant | Seed yield (g plant−1) | Seed protein (mg g−1) | Shoot | Root | Shoot | Root | |||
| Uninoculated | 0 | 0.31b | 0.19b | 0.42c | 1.25ab | 0.37b | 9.1f | 20.4e | 11.7f | 54.4a | 10.3d | 0. 9b | 341.3bc | 22.4b | 14.4b | 0.49b | 0.29b |
| 1444 | 0.29c | 0.18c | 0.41c | 1.15bc | 0.23c | 25.8d | 23.4 cd | 20.2d | 21.1e | 13.0bc | 0.88b | 350abc | 21.9b | 13.5b | 0.47b | 0.25c | |
| 2888 | 0.27c | 0.16d | 0.37d | 0.95d | 0.24e | 41.2b | 29.2b | 27.6b | 21.1e | 14.6ab | 0.71c | 290.6d | 20.2bc | 12.5c | 0.45d | 0.24d | |
| 4332 | 0.23d | 0.14e | 0.26e | 0.84d | 0.22e | 47.4a | 33.7a | 32.4a | 26.6c | 12.0c | 0.52e | 244.6e | 18.3bc | 12.3c | 0.42e | 0.24d | |
| Inoculated | 0 | 0.37a | 0.20a | 0.55a | 1.37a | 0.46a | 24.5d | 19.5e | 9.6 g | 44.5b | 7.0e | 0.99a | 395.0a | 27.2a | 18.0a | 0.54a | 0.33a |
| 1444 | 0.34b | 0.21a | 0.45b | 1.33ab | 0.28d | 16.1e | 22.8d | 14.1e | 19.2f | 16.0a | 0.89b | 354.3ab | 22.7b | 14.0b | 0.49bc | 0.26c | |
| 2888 | 0.28c | 0.19b | 0.39c | 1.15bc | 0.28d | 25.2d | 25.4c | 23.0c | 18.8f | 13.6bc | 0.73c | 305.0 cd | 21.6b | 14.0b | 0.45 cd | 0.23d | |
| 4332 | 0.22d | 0.17c | 0.28e | 1.0 cd | 0.27d | 32.0c | 20.0e | 26.0b | 23.1d | 9.6d | 0.59d | 268.6de | 19.0c | 12.0 cc | 0.43e | 0.22d | |
| LSD | 0.025 | 0.008 | 0.016 | 0.138 | 0.027 | 1.52 | 2.11 | 1.25 | 1.45 | 0.065 | 43.12 | 4.25 | 1.05 | 0.022 | 3.20 | ||
|
F value Un inoculated (df = 1) |
47.09 | 53.19 | 625 | 26.06 | 28.63 | 201 | 24.51 | 580.8 | 1560 | 4.92 | 172.9 | 5.74 | 1.68 | 29.5 | 4.11 | 1.02 | |
| Inoculated (df = 3) | 83.42 | 104.26 | 430.8 | 18.71 | 141.77 | 367.19 | 24.5 | 488.1 | 784.1 | 45.73 | 60.06 | 25.8 | 9.65 | 47.3 | 54.1 | 0.97 | |
| Un-inoculated × inoculated (df = 3) | 24.93 | 5.26 | 42.62 | 11.57 | 23.04 | 1008 | 84.97 | 257.3 | 517.3 | 11.33 | 16.9 | 1.12 | 1.87 | 11.7 | 5.11 | 0.99 | |
Each value is a mean of three replicate where each replicate constituted three plants/pot. Mean values are significant of P ≤ 0.05. Means followed by similar alphabets not significantly different from each other according to DMRT test
Seed attributes
Seed yield (SY) and grain protein (GP) of chickpea plants assayed at harvest (120 DAS) exhibited gradual decrease with successive increasing in concentrations of glyphosate (Table 4). In contrast, the SY and GP of inoculated chickpeas enhanced by 9 and 13%, in comparison to non-inoculated chickpea plants. In contrast, the glyphosate tolerant strain B. cepacia PSBB1 increased the SY and GP marginally by 3 and 5%, respectively, at 2888 μg kg−1 soil, comparatively uninoculated chickpea raised in soil amended with identical doses of glyphosate. On the contrary, herbicide tolerant strain B. cepacia (PSBB1) enhanced the SY and GP by 12 and 9%, respectively, at 3× concentration of glyphosate, relative to the un-inoculated chickpea plants grown with the identical dose rate of herbicide. In a similar study, Madariaga-Navarrete et al. (2017) found an enhancement in different growth attributes of Phaseolus vulgaris when grown in the presence of consortium comprising of Trichoderma and Rhizobium sp. under the influence of herbicide atrazine. The statistical analysis significantly showed the interactive consequence of inoculation and glyphosate (inoculation × glyphosate) on the measured parameters.
Proline, malondialdehyde (MDA) and antioxidant enzymes
Formation and accumulation of proline within plant tissues is a physiological reaction to stressor molecules present in the environment. Proline, an extremely water-soluble amino acid biomolecule defends the membranes from the destructive effects of high concentrations of inorganic ions. In addition, it can function both as a protein-compatible hydro trope (Sharma and Verslues 2010) and as a hydroxyl radical scavenger (Kaul et al. 2008). The increase in free cellular proteins under different abiotic and abiotic stresses provide a multifarious defensive role in most plant species (Hossain et al. 2014). Considering these attributes of proline, we also determined proline in various plant organs of chickpea. Here, we found a substantial build-up of proline in roots, shoots, leaves and seeds of chickpea grown under glyphosate stress. Proline concentration in roots, shoots and foliage recorded at 80 DAS and grains at 120 days after planting enhanced with increasing rate of glyphosate (Table 4). A maximum uptake of 33.7, 47.7 and 32.4 mg g−1 fresh weight was found in roots, shoots and leaves after 80 days of chickpea growth under glyphosate tress. Similar to this, an increase in the proline content in other legumes for example, faba bean developed with herbicide fusillade has been observed (Osman et al. 2016). However, in the presence of strain PSBB1, the level of proline was substantially reduced, and it was found as 20.4, 32 and 26.9 mg g−1 fresh weight in roots, shoots and leaves, respectively, at 4332 μg kg−1 glyphosate. The bioinoculant PSBB1 strain considerably decreased the proline content in roots and shoots of chickpea plants by 38 and 14%, respectively, at 80 days after sowing relative to plants grown in soils treated with only 2× of glyphosate (Fig. 4). Similar reduction in proline concentration in inoculated faba bean cultivated under herbicide stress is reported by Osman et al. (2016). The reduction in the concentration of proline in different organs of inoculated chickpea plants grown in glyphosate amended soil could probably be due to the detoxifying/bioremediation ability of PSBB1 strain (Fig. 5).
Fig. 4.
Proline accumulation in shoots (a), root (b) and leaves (c) at 80 DAS and grains at 120 DAS (d) of B. cepacia PSBB1 inoculated and uninoculated chickpea grown in soils treated with different dose of glyphosate. The values indicate the mean SD of three replicates
Fig. 5.
Effect of glyphosate and bioinoculant B. cepacia strain PSBB1 on photosynthetic pigments chl a, chl b, total chlorophyll and carotenoids
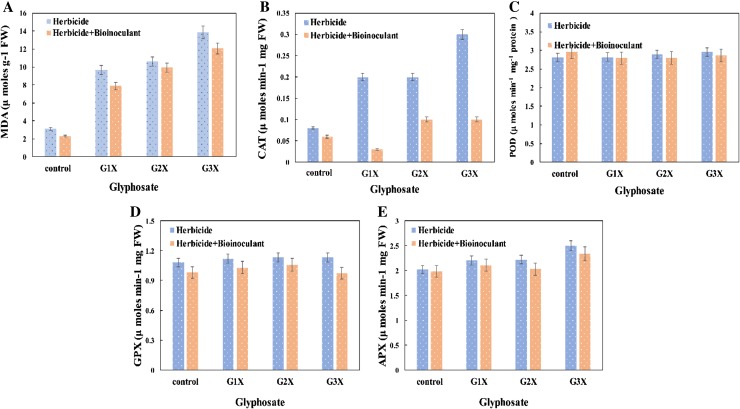
Malondialdehyde (MDA) is frequently used as an index of lipid peroxidation under harsh environmental conditions. In this study, the efficiency of glyphosate in prompting lipid peroxidation and causing variations in antioxidants enzymes was assayed. Here, the MDA contents increased progressively with increasing concentration of herbicide. Glyphosate at 3×, produced the maximum amount (13.8 μ mol g−1 fw) of MDA in leaves. However, PSBB1 primed chickpea plants exhibited a marginal decrease in MDA content following herbicide application (Fig. 6). In a similar investigation, Curá et al. (2017) have showed significant enhancement in MDA content of seedlings under stress condition compared to control. Like MDA, CAT, POD, APX and GPX of leaves varied between bacterized and uninoculated plants raised along with tested herbicide. Generally, the concentration of antioxidant enzymes increased with increasing rates of herbicides. In contrast, B. cepacia PSBB1 strain reduced the CAT activity maximally by 66% at 3× concentration of glyphosate. However, at normal rate of glyphosate, CAT activity was found to be reduced by 85% by strain PSBB1 (Fig. 6). The POD activity increased maximally by 18% when plants were grown exclusively at 3× of glyphosate amended soil compared with uninoculated control. Moreover, APX declined maximally by 9% at two times more concentration of glyphosate. The GPX activity of leaves increased by 4% at 2× concentration of glyphosate over non-inoculated control. On the contrary, B. cepacia PSBB1, caused a 14% reduction in the GPX activity even in the presence of 3× concentration of glyphosate. In agreement to our findings, the CAT activity in leaf and root tissue of other legume seedling have also been found to increase significantly in a concentration dependent manner (Chehelpar et al. 2016). Moreover, the decline in oxidative stress (CAT and POD) in PGPR Bacillus aryabhattai strain SRB02 inoculated soybean is also reported by Park et al. (2017).
Fig. 6.
Inoculation effect of strain PSBB1 on oxidative stress in leaves of chickpea under glyphosate stress: a membrane lipid peroxidation (MDA content), b catalase, c peroxidase, d guaiacol peroxidase, and e ascorbate peroxidase
Morphological distortion and bacterial colonization
Distortion/damage to root tips was observed under SEM when chickpea plants were developed in the presence of 1000 μg ml−1 of glyphosate (Fig. 7). The glyphosate inhibition to roots was more pronounced in the radical regions of growing chickpea plants. Similarly, it has been reported by Mondal et al. (2013) where they observed a significant damage in the morphological structure of chickpea/legumes roots grown in the presence of pesticide. The PSBB1 strain used as inoculant were able to colonize the chickpea roots while non-inoculated root surface showed no colonization under SEM after 10 days of inoculation on the surface of primary and lateral roots. Aggregation of cells was centred/localized on root tips and at the elongation zone (Fig. 8). Similarly, the colonizing ability of bacteria the root surface and hence, to enhance the plant growth is reported (del Gallo et al. 2017).
Fig. 7.
SEM micrograph of chickpea roots. a, b Root tip of chickpea grown without glyphosate while c, d indicates glyphosate toxicity to chickpea roots
Fig. 8.
Colonization of B. cepacia PSBB1 on chickpea root surface observed under SEM a represents the uninoculated root surface, b, c represents the colonization of bacteria on root surface
Conclusion
The phytotoxic effect of glyphosate on survival, cellular morphology and biomolecules, secreted by of B. cepacia PSBB1 differed considerable. Interestingly, the ability of strain PSBB1 to excrete such growth enhancers was not abolished completely even at higher glyphosate concentration; though the amounts of plant growth regulators declined regularly. Moreover, the inhibitory impact of glyphosate was greater on uninoculated chickpea plants compared to bacterized plants. Herbicide tolerant B. cepacia PSBB1 when used as bioinoculant, safeguarded the plants from the inhibitory effect of glyphosate and concomitantly augmented the whole dry matter production, nutrient accumulation and seed attributes of chickpeas. The overall enhancement in chickpea production due to inoculation of strain PSBB1 in combination with glyphosate could possibly be due to multiple variables such as—(i) firm colonization and establishment of inoculated bacterial culture (ii) secretion of phytohormone, siderophores, EPS and ACC deaminase by B. cepacia PSBB1 (iii) reduction in proline level in herbicide stressed environment and (iv) suppression of antioxidant enzymes and MDA level. Considering the herbicide tolerance ability, capacity to express plant growth regulators even under herbicide stress and potential to mitigate the toxicity of herbicide (bioremediation), this bacterial strain PSBB1 of B. cepacia could safely and inexpensively be developed as a bioinoculant for enhancing chickpea production in soils contaminated even with herbicides.
Acknowledgements
The authors would like to thank Macrogen Seoul, Korea, for providing 16SrRNA gene Sequencing analysis and University Sophisticated Instrument facility (USIF) for providing SEM and CLSM facilities.
Authors contributions
MSK conceived and designed the experiments. MS performed the experiments and analyzed the data statistically. MS and MSK prepared the manuscript and approved the final draft.
Funding
The author (Mohammad Shahid) would like to acknowledge the financial support received in the form of UGC Non-NET fellowship granted by University Grants Commission, New Delhi.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Adami MFF, Modolo AJE, Adami PF. White clover tolerance to herbicides applied at different rates and phenological stages. Afr J Agric Res. 2017;12(28):2336–2341. doi: 10.5897/AJAR2016.11905. [DOI] [Google Scholar]
- Ahmed B, Dwivedi S, Abdin MZ, Azam A, Al-Shaeri M, Khan MS, Musarrat J. Mitochondrial and chromosomal damage induced by oxidative stress in Zn2+ ions, ZnO-bulk and ZnO-NPs treated Allium cepa roots. Sci Rep. 2017;7:40685. doi: 10.1038/srep40685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DB, Zuberer DA. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils. 1991;12(1):39–45. doi: 10.1007/BF00369386. [DOI] [Google Scholar]
- Alori ET, Dare MO, Babalola OO (2017) Microbial inoculants for soil quality and plant health. In: Sustainable agriculture reviews. Springer International Publishing, Berlin, pp 281–307
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Appl Environ Microbiol. 1949;55:1665–1669. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthee R, Marimuthu P. Studies on endophytic Burkholderia sp. From sugarcane and its screening for plant growth promoting potential. J Exp Biol. 2017;5:2. [Google Scholar]
- Atkin CL, Neilands JB, Phaff HJ. Rhodotorulic acid from species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a new alanine-containing ferrichrome from Cryptococcus melibiosum. J Bacteriol. 1970;103(3):722–733. doi: 10.1128/jb.103.3.722-733.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarmi F, Mozafari V, Dahaji PA, Hamidpour M. Biochemical, physiological and antioxidant enzymatic activity responses of pistachio seedlings treated with plant growth promoting rhizobacteria and Zn to salinity stress. Acta Physiol Plant. 2016;38:121. doi: 10.1007/s11738-015-2032-3. [DOI] [Google Scholar]
- Bakker AW, Schippers B. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp-mediated plant growth-stimulation. Soil Biol Biochem. 1987;19(4):451–457. doi: 10.1016/0038-0717(87)90037-X. [DOI] [Google Scholar]
- Bates LS, Woldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–208. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bitew Y, Alemayehu M (2017) Review article impact of crop production inputs on soil health: a review. 10.3923/ajps.2017.109.131
- Brick JM, Bostock RM, Silverstone SE. Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol. 1991;57(2):535–538. doi: 10.1128/aem.57.2.535-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehelpar N, Tohidi-Moghadam HR, Ghoushchi F. Hexaconazole foliar application alleviates water deficit effects in common bean. Pes Agro Tropic. 2016;46(3):301–310. doi: 10.1590/1983-40632016v4641432. [DOI] [Google Scholar]
- Curá JA, Franz DR, Filosofía JE, Balestrasse KB. Inoculation with Azospirillum sp. and Herbaspirillum sp. bacteria increases the tolerance of maize to drought stress. Microorganisms. 2017;5(3):41. doi: 10.3390/microorganisms5030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran WS. Persistence of herbicides in soil. Crop Soils. 2016;49(5):16–21. doi: 10.2134/cs2016-49-0504. [DOI] [Google Scholar]
- Deinum EE, Kohlen W, Geurts R. Quantitative modelling of legume root nodule primordium induction by a diffusive signal of epidermal origin that inhibits auxin efflux. BMC Plant Boil. 2016;16(1):254. doi: 10.1186/s12870-016-0935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Gallo M, Ercole C, Matteucci F (2017) Plant–bacteria interaction at the microscope. In: Méndez-Vilas A (ed) Microscopy and imaging science: practical approaches to applied research and education, pp 312–317
- Ditta A, Khalid A (2016) Bio-organo-phos: a sustainable approach for managing phosphorus deficiency in agricultural soils. In: Organic fertilizers-from basic concepts to applied outcomes. InTech, Rijeka, Croatia. 10.5772/62473
- Dorjey S, Dolkar D, Sharma R. Plant growth promoting rhizobacteria Pseudomonas: a review. Int J Curr Microbiol App Sci. 2017;6(7):1335–1344. doi: 10.20546/ijcmas.2017.607.160. [DOI] [Google Scholar]
- Dworkin M, Foster J. Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol. 1958;75:592–601. doi: 10.1128/jb.75.5.592-603.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye DW. The inadequacy of the usual determinative tests for the identification of Xanthomonas spp. New Zeal J Sci. 1962;5(4):393–416. [Google Scholar]
- Fernández-López MG, Popoca-Ursino C, Sánchez-Salinas E, Tinoco-Valencia R, Folch-Mallol JL, Dantán-González E, Laura Ortiz-Hernández M (2017) Enhancing methyl parathion degradation by the immobilization of Burkholderia sp. isolated from agricultural soils. Microbiol Open 6(5). 10.1002/mbo3.507 [DOI] [PMC free article] [PubMed]
- Ghosh PK, Maiti TK. Structure of extracellular polysaccharides (EPS) produced by rhizobia and their functions in legume–bacteria symbiosis: a review. Achiev Life Sci. 2016;10(2):136–143. doi: 10.1016/j.als.2016.11.003. [DOI] [Google Scholar]
- Glick BR, Todorovic B, Czarny J, Cheng Z. Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci. 2007;26(5–6):227–242. doi: 10.1080/07352680701572966. [DOI] [Google Scholar]
- Gopal M, Dutta D, Jha SK, Kalra S, Bandyopadhyay S, Das SK. Biodegradation of imidacloprid and metribuzin by Burkholderia cepacia strain CH9. Pestic Res J. 2011;23(1):36–40. [Google Scholar]
- Gordon S, Weber RP. The colorimetric estimation of IAA. Plant Physiol. 1951;26:192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goud VV, Murade NB, Khakre MS. Efficacy of imazethapyr and quizalofop-ethyl herbicides on growth and yield of chickpea. Bioscan. 2013;8(3):1015–1018. [Google Scholar]
- Hammerschmidt R, Nuckles EM, Kuć J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Plant Pathol. 1982;20(1):977–1082. [Google Scholar]
- Han Y, Wang R, Yang Z, Zhan Y. 1-aminocyclopropane 1- carboxylate deaminase from Pseudomonas stutzeri A 1501 facilitates the growth of rice in the presence of salts or heavy metals. J Microbiol Biotechnol. 2015;2:1119–1128. doi: 10.4014/jmb.1412.12053. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Herman PL, Behrens M, Chakraborty S. A three-component dicamba O-demethylase from Pseudomonas maltophilia, strain DI-6 gene isolation, characterization, and heterologous expression. J Biol Chem. 2005;280(26):24759–24767. doi: 10.1074/jbc.M500597200. [DOI] [PubMed] [Google Scholar]
- Holmes A, Govan J, Goldstein R. Agricultural use of Burkholderia (Pseudomonas) cepacia: a threat to human health. Emerg Infect Dis. 1998;4(2):221. doi: 10.3201/eid0402.980209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt GJ, Krieg NR, Sneath PHA (1994) Gram negative aerobic/microaerophilic rods and cocci. In: Bergey’s manual of determinative bacteriology, 9th edn. Williams and Wilkins, Lippincott, Philadelphia
- Honma M, Shimomura T. Metabolism of 1-aminocyclopropane 1-carboxylate deaminase. Agric Biol Chem. 1978;42:1825–1831. [Google Scholar]
- Hossain MA, Hoque MA, Burritt DJ (2014) Proline protects plants against abiotic oxidative stress: biochemical and molecular mechanisms. In: Oxidative damage to plants. Elsevier, San Diego, pp 477–522
- Iswaran V, Marwah TS. A modified rapid Kjeldahl method for determination of total nitrogen in agricultural and biological materials. Geobios. 1980;7(6):281–282. [Google Scholar]
- Jackson ML. Soil chemical analysis. New Delhi: Prentice Hall; 1976. [Google Scholar]
- Karishma B, Prasad SH. Isolation, characterization and growth studies of malathion insecticide degrading bacteria. Int J Environ Sci. 2016;6(5):697–706. [Google Scholar]
- Kaul S, Sharma SS, Mehta IK. Free radical scavenging potential of l-proline: evidence from in vitro assays. Amino Acids. 2008;34(2):315–320. doi: 10.1007/s00726-006-0407-x. [DOI] [PubMed] [Google Scholar]
- Kaushal M, Wani SP. Plant-growth-promoting rhizobacteria: drought stress alleviators to ameliorate crop production in drylands. Ann Microbiol. 2016;66(1):35–42. doi: 10.1007/s13213-015-1112-3. [DOI] [Google Scholar]
- Khalid S, Khokhar SN. Interaction of herbicides and bio-inoculants with agricultural crops and weeds. Pak J Agric Res. 2013;26(4):299–308. [Google Scholar]
- King JE. The colorimetric determination of phosphorus. Biochem J. 1932;26:292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth C, Kage H, Nett M. Siderophores as molecular tools in medical and environmental applications. Org Biomol Chem. 2016;14(35):8212–8227. doi: 10.1039/C6OB01400C. [DOI] [PubMed] [Google Scholar]
- Leonard SS, Harris GK, Shi X. Metal-induced oxidative stress and signal transduction. Free Radic Biol Med. 2004;37:1921–1942. doi: 10.1016/j.freeradbiomed.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL. Protein measurement with the Folin phenol reagent. J Boil Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- Madariaga-Navarrete A, Rodríguez-Pastrana BR, Villagómez-Ibarra JR. Bioremediation model for atrazine contaminated agricultural soils using phytoremediation (using Phaseolus vulgaris L.) and a locally adapted microbial consortium. J Environ Sci Health Part B. 2017;52(6):367–375. doi: 10.1080/03601234.2017.1292092. [DOI] [PubMed] [Google Scholar]
- Mody B, Bindra M, Modi V. Extracellular polysaccharides of cowpea rhizobia: compositional and functional studies. Arch Microbiol. 1989;153(1):38–42. doi: 10.1007/BF00277538. [DOI] [Google Scholar]
- Mondal NK, Das C, Roy S, Datta JK. Effect of varying cadmium stress on chickpea (Cicer arietinum L.) seedlings: an ultrastructural study. Ann Environ Sci. 2013;7:59–70. [Google Scholar]
- Nandula VK, Tyler HL. Effect of new auxin herbicide formulations on control of herbicide resistant weeds and on microbial activities in the rhizosphere. Am J Plant Sci. 2016;7(17):429. doi: 10.4236/ajps.2016.717212. [DOI] [Google Scholar]
- Nguyen C, Yan W, Le Tacon F, Lapeyrie F. Genetic variability of phosphate solubilizing activity by monocaryotic and dicaryotic mycelia of the ectomycorrhizal fungus Laccaria bicolor (Maire) PD Orton. Plant Soil. 1992;143(2):193–199. doi: 10.1007/BF00007873. [DOI] [Google Scholar]
- Nithyakalyani V, Kannan M, Anandan R. Insecticide and salt tolerance of plant growth promoting root nodule bacteria. Int J Curr Microbiol App Sci. 2016;5(4):942–956. doi: 10.20546/ijcmas.2016.504.107. [DOI] [Google Scholar]
- Osman MEH, Abo-Shady AM, El-Nagar MM. Cyanobacterial Arthrospira (Spirulina platensis) as safener against harmful effects of fusilade herbicide on faba bean plant. Re Lincei. 2016;27(3):455–462. doi: 10.1007/s12210-015-0498-y. [DOI] [Google Scholar]
- Pandey SP, Srivastava S, Goel R, Lakhwani D. Simulated herbivory in chickpea causes rapid changes in defense pathways and hormonal transcription networks of JA/ethylene/GA/auxin within minutes of wounding. Sci Rep. 2017;7:44729. doi: 10.1038/srep44729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YG, Mun BG, Kang SM, Hussain A. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE. 2017;12(3):e173203. 93–168. doi: 10.1371/journal.pone.0173203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa M, Aliverdi A, Hammami H. Effect of the recommended and optimized doses of haloxyfop-P-methyl or imazethapyr on soybean-Bradyrhizobium japonicum symbiosis. Ind Crops Prod. 2013;50:197–202. doi: 10.1016/j.indcrop.2013.07.019. [DOI] [Google Scholar]
- Penrose DM, Glick BR. Method for isolating and characterizing ACC deaminase containing plant growth promoting rhizobacteria. Physiol Plant. 2003;118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- Perez-Fernández M, Alexander V. Enhanced plant performance in Cicer arietinum L. Due to the addition of a combination of plant growth-promoting bacteria. Agriculture. 2017;7(5):40. doi: 10.3390/agriculture7050040. [DOI] [Google Scholar]
- Pikovskaya RI. Mobilization of phosphorous in soil in connection with vital activity of some microbial species. Mikrobiologiya. 1948;17:362–370. [Google Scholar]
- Premachandra D, Hudek L, Brau L. Bacterial modes of action for enhancing of plant growth. J Biotechnol Biomater. 2016;6(3):1–8. [Google Scholar]
- Premono ME, Moawad AM, Vlek PLG (1996) Effect of phosphate-solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere
- Raghavendra KS, Gundappagol RC, Santhosh GP (2017) Impact of herbicide application on beneficial soil microbial community, nodulation and yield parameters of chickpea (Cicer arietinum L.)
- Rahman CH, Ahcene B, Miloud B, Rachid D. Screening and characterization of plant growth promoting traits of phosphate solubilizing bacteria isolated from wheat rhizosphere of Algerian saline soil. Malays J Biol. 2017;13(2):124–131. [Google Scholar]
- Rani R, Kumar V. Endosulfan degradation by selected strains of plant growth promoting rhizobacteria. Bull Environ Contam Toxicol. 2017;99(1):138–145. doi: 10.1007/s00128-017-2102-x. [DOI] [PubMed] [Google Scholar]
- Reeves MW, Pine L, Neilands JB, Balows A. Absence of siderophore activity in Legionella species grown in iron-deficient media. J Bacteriol. 1983;154(1):324–329. doi: 10.1128/jb.154.1.324-329.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijavec T, Lapanje A. Hydrogen cyanide in the rhizosphere: not suppressing plant pathogens, but rather regulating availability of phosphate. Front Microbiol. 2016;7:1785. doi: 10.3389/fmicb.2016.01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Navarro DN, Rodríguez-Carvajal MA, Acosta-Jurado S. Structure and biological roles of Sinorhizobium fredii HH103 exopolysaccharide. PLoS ONE. 2014;9(12):115391. doi: 10.1371/journal.pone.0115391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivum S, Manickam A. Biochemical methods. New Delhi: New Age International Publishers (P) Ltd.; 1992. [Google Scholar]
- Sahoo SK, Pradhan J, Kuruwanshi VB, Guhey A, Rout GR, Dash R. Phytotoxic effect of pre-emergence herbicides on oil content and yield components of groundnut (Arachis hypogeae) Int J Curr Microbiol App Sci. 2017;6(9):1738–1748. doi: 10.20546/ijcmas.2017.609.215. [DOI] [Google Scholar]
- Saleem S, Ahmed B, Khan MS, Al-Shaeri M, Musarrat J. Inhibition of growth and biofilm formation of clinical bacterial isolates by NiO nanoparticles synthesized from Eucalyptus globulus plants. Microb Pathog. 2017 doi: 10.1016/j.micpath.2017.09.019. [DOI] [PubMed] [Google Scholar]
- Sandanakirouchenane A, Haque E, Geetha T. Recent studies on N2 fixing Burkholderia isolates as a biofertilizer for the sustainable agriculture. Int J Curr Microbiol App Sci. 2017;6(11):2780–2796. doi: 10.20546/ijcmas.2017.611.329. [DOI] [Google Scholar]
- Sarkar A, Mukherjee PK, Bhattacharya PM. Bio-efficacy of pendimethalin and fluchloralin in mustard. Ind J Weed Sci. 2005;37:275–276. [Google Scholar]
- Shah S, Li J, Moffatt BA, Glick BR. Isolation and characterization of ACC deaminase genes from two different plant growths promoting rhizobacteria. Can J Microbiol. 1998;44:833–843. doi: 10.1139/w98-074. [DOI] [PubMed] [Google Scholar]
- Shahid M, Khan MS. Assessment of glyphosate and quizalofop mediated toxicity to greengram [Vigna radiata (L.) Wilczek], stress abatement and growth promotion by herbicide tolerant Bradyrhizobium and Pseudomonas species. Int J Curr Microbiol App Sci. 2017;6(12):3001–3016. doi: 10.20546/ijcmas.2017.612.351. [DOI] [Google Scholar]
- Sharma S, Verslues PE. Mechanisms independent of abscisic acid (ABA) or proline feedback have a predominant role in transcriptional regulation of proline metabolism during low water potential and stress recovery. Plant Cell Environ. 2010;33(11):1838–1851. doi: 10.1111/j.1365-3040.2010.02188.x. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kulkarni J, Jha B. Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Front Microbiol. 2016;7:1600. doi: 10.3389/fmicb.2016.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudharshana C, Prakash TR, Jayasree G, Reddy APK. Effect of pendimethalin and imazethapyr on nodulation, nitrogen fixation and nitrogenase activity and yield in groundnut (Arachis Hypogaea L.) Int J Bioresour Stress Manag. 2013;4:309–313. [Google Scholar]
- Tetard-Jones C, Edwards R. Potential roles for microbial endophytes in herbicide tolerance in plants. Pest Manag Sci. 2016;72:203–209. doi: 10.1002/ps.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugbe LA, Nadaeyo NU, Envyong JF. Efficacy of selected herbicides on weed control, cowpea (Vigna unguiculata L. Walp) performance and economic returns in Akamkpa, Southeastern Nigeria. Int J Res Agri Forest. 2016;19:19–27. [Google Scholar]
- Vogel C, Rehschuh S, Kemi Olagoke F (2017) Interactions between extracellular polymeric substances and clay minerals affect soil aggregation. In: 19th EGU General Assembly, EGU2017, proceedings from the conference held 23–28 April, 2017 in Vienna, Austria, p 18653
- Yuan SL, Li R, Chen HF, Zhang CJ. RNA-Seq analysis of nodule development at five different developmental stages of soybean (Glycine max) inoculated with Bradyrhizobium japonicum strain 113-2. Sci Rep. 2017;7:42248. doi: 10.1038/srep42248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablotowicz RM, Reddy KN. Nitrogenase activity, nitrogen content, and yield responses to glyphosate in glyphosate-resistant soybean. Crop Prot. 2007;26(3):370–376. doi: 10.1016/j.cropro.2005.05.013. [DOI] [Google Scholar]
- Zeidan AA, Poulsen VK, Janzen T. Polysaccharide production by lactic acid bacteria: from genes to industrial applications. FEMS Microbiol Rev. 2017;41(Supp_1):S168–S200. doi: 10.1093/femsre/fux017. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kirkham MB. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996;132(3):361–373. doi: 10.1111/j.1469-8137.1996.tb01856.x. [DOI] [PubMed] [Google Scholar]