ABSTRACT
Water resources contaminated with wastewater are an important source for the dissemination of enteric viruses with an impact on the health of the population. The aim of the study was to assess the viral contamination of freshwater from a dam in Argentina by using infectious enterovirus detection, viral RNA amplification, and a genetic characterization of five enteric viruses associated with diarrhea and hepatitis. Enterovirus infectivity (iEV) was evaluated by cell culture and direct immunofluorescence. The detection of the viral genome of rotavirus (RV), human astrovirus (HAstV), norovirus (NoV), hepatitis A virus (HAV), and hepatitis E virus (HEV) was performed by reverse transcriptase PCR (RT-PCR). A total of 48 water samples from 4 monitoring points on the body of the dam from January to December 2012 and 66 water samples from 3 tourist beaches on the edge of the dam from October 2013 to October 2015 were collected monthly. During the first period, the overall viral frequency detection was 52.1% for group A RV, 50% for HAstV, 60.4% for NoV, 22.9% for HAV, 2.1% for HEV, and 64.6% for iEV. The overall frequency detection for the second sampling was 18.2% for RV and HAstV, 31.8% for NoV, 7.57% for HEV, and 66.7% for iEV. There was no detection of HAV during this period. The genotypes and genogroups detected through the study correlated with the most common genomic variants associated with human gastrointestinal and hepatitis illnesses. The results obtained could alert the health systems and environmental sanitation to make decisions for viral control and prevention in our environment.
IMPORTANCE The study shows the impact of anthropic contamination of one of the most important tourist water resources in Argentina. This course of recreational water would be a favorable scenario for infection, as well as a reservoir for the enteric viruses, creating a risk for the population exposed to these waters. The results obtained could alert the health systems and environmental sanitation to make decisions for the control and prevention of viral diseases in this environment.
KEYWORDS: genotype/genogroup variants, freshwater viral contamination, hepatitis viruses, RT-PCR, enteric viruses, gastrointestinal viruses, immunofluorescence, viable enterovirus
INTRODUCTION
Public attention towards the care and preservation of the environment, particularly of the watercourses, has grown in recent decades, becoming one of the priority issues of humanity. The impact of the population on the water resources has become more evident in recent years, highlighting the close relationship between the levels of microbiological contamination of water and community health.
Severe pathogen pollution, the rise of which is largely due to the expansion of sewer systems that discharge untreated wastewater into surface waters, is estimated to affect approximately a quarter of Latin American watercourses (1).
In this sense, viruses that infect humans prevail throughout the environment, causing public health concerns and leading to substantial economic losses. Many orally transmitted viruses produce subclinical infections, and symptoms are present only in a small proportion of the population. However, some viruses may give rise to life-threatening conditions, such as acute hepatitis in adults, as well as severe gastroenteritis in small children and the elderly.
The United Nations Environment Program (UNEP) estimates that approximately 3.4 million people die each year from diseases associated, among others, with enteric viruses in water, such as viral hepatitis and diarrheal diseases, and up to 25 million people are at risk of infection from these diseases in Latin America (1).
However, the development of the diseases is associated with the viral characteristics, such as the infective dose of the viral agent, the genetic variant of the strain, the endemicity and/or host factors, including the age, health, and immunological and nutritional statuses of the infected individuals, and the availability of health care.
Human-pathogenic viruses in urban wastewater include enterovirus (EV), adenovirus (AdV), norovirus (NoV), rotaviruses (RVs), astrovirus (HAstV), human polyomavirus (HPyV), hepatitis A virus (HAV), and hepatitis E virus (HEV), with variable prevalence in different geographical areas and/or periods of the year.
According to the last census in Argentina, only 48.9% of the population have sewage treatment systems and 62.2% have septic tanks and cesspools in their homes (2). As a result, untreated or poorly treated sewage and treated sewage are discharged together into the surface water, resulting in fecal contamination with a high viral load. This creates a risk to human health via waterborne enteric viruses. A high occurrence of infections, produced by NoV, group A RV (RVA), HAV, HEV, and human EV, has been reported recently in Argentina in a variety of water environment matrices (3–8).
The San Roque Dam, located in the Punilla Valley in Córdoba province (second touristic destination of Argentina), was constructed more than 70 years ago for flood control, water supply, recreation, and sport fishing. The recreational activities practiced in and around this watercourse promoted urbanization in the surroundings of the dam with the consequent discharge of poorly treated residual liquids. Despite its importance, this watercourse is currently one of the most problematic inland aquatic environments in Argentina because of the advanced state of eutrophication, with the anthropic activities as the main source of pollution, constituting a potential source for infections by pathogenic microorganisms in the exposed population.
The aim of the present study was to assess the viral contamination of freshwater from a dam in Argentina by using infectious enterovirus detection, viral RNA amplification, and a genetic characterization of five enteric viruses associated with diarrhea and hepatitis.
The findings will contribute to our understanding of the local epidemiology as well as the dynamic of the circulation of this extensive group of viruses in the community, promoting the creation of public policies oriented to the care of this resource.
RESULTS
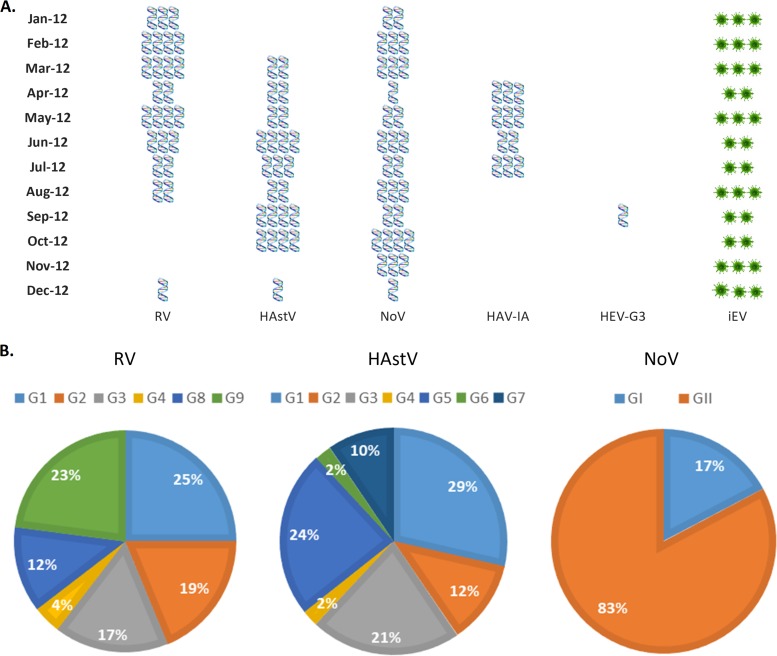
The presence of at least one of the different viruses analyzed was demonstrated in 100% of the samples belonging to the four studied points from the body of the dam (Fig. 1). During this period, the overall viral frequency of detection was 25/48 (52.1%) for RVA, 24/48 (50%) for HAstV, 29/48 (60.4%) for NoV, 11/48 (22.9%) for HAV, 1/48 (2.1%) for HEV, and 31/48 (64.6%) for enterovirus infectivity (iEV).
FIG 1.
Frequencies of infectious human enterovirus (iEV), viral RNA amplification (RV, HAstV, NoV, HAV, and HEV), and genetic characterization from four monitoring sites at the San Roque Dam, Cordoba, Argentina. (A) Monthly frequencies of detection of RV, HAstV, NoV, HAV, and HEV during 2012. HAV-IA, hepatitis A virus/genotype IA; HEV-GE, hepatitis E virus/genotype 3. The quantity of blue symbols (RNA virus detection) or green asterisks (infectious human enterovirus) represents the numbers of sampling points for the indicated virus. (B) Proportional distributions of rotavirus, norovirus, and astrovirus genogroups/genotypes.
The detections of RVA, HAstV, and HAV showed temporal behaviors; no RV was detected in the spring (September, October, and November 2012). HAstV had a drop in detection during the summer months, specifically in January and February, and HAV genotype IA was detected only during cold seasons (Fig. 1A).
HEV genotype 3 was only detected in September, showing a low frequency of circulation. Meanwhile, NoV was detected during all the sampling periods.
iEV was detected during all the studied months from at least in 2 sampling points (Fig. 1A and 2A). The molecular characterization demonstrated a cocirculation of different genotypes/genogroups for RV, HAstV, and NoV. Among the RVs, the most prevalent genotypes were G1 and G9, followed by G2, G3, and G8, and to a lesser extent G4. A high genotype variability was observed for HAstV: genotypes G1, G5, and G3 were the most prevalent, followed by G2 and G7, and to a lesser extent by G4 and G6. For NoV, a higher proportion was genogroup GII than GI (Fig. 1B).
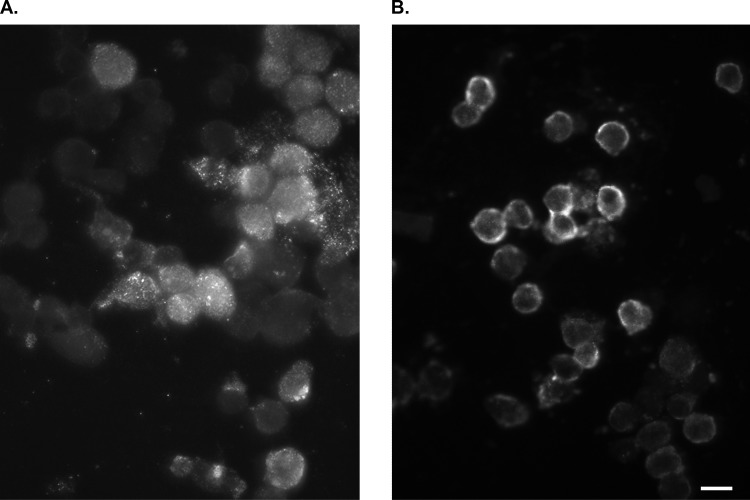
FIG 2.
Detection of enterovirus VP1 protein by immunofluorescence staining on HEp-2 cells. (A) Positive (infected) cells show punctate fluorescence, mainly in the cytoplasmic region. Samples are from the body of the dam. (B) Positive (infected) cells show fluorescence at the cellular periphery (membranous ring). Samples are from the beaches of the dam. Scale bar, 20 μm.
Results obtained during the second sampling, carried out on the beaches (October 2013 to October 2015), are depicted in Fig. 3. In accordance with that observed for the body of the lake, the presence of at least one of the different viruses analyzed was demonstrated in 100% of the samples. However, the overall frequency of detection for the three monitoring points was lower than that detected before, except for iEV. For RV and HAstV, the frequency of detection was 12/66 (18.2%), whereas it was 21/66 (31.8%) for NoV, 5/66 (7.57%) for HEV, and 44/66 (66.7%) for iEV. There was no detection of HAV during this period (Fig. 3A). Nevertheless, it is important to highlight that during October 2013 to October 2014, there was a significant decrease in RV detection (5.55%) compared with the RV frequency detected in the period from January to October 2015 (33.5%) and an absence of HEV detection (Fig. 3A). In contrast, the detection frequencies for HAstV and iEV were similar during the periods October 2013 to October 2014 (19.4% and 69.4%, respectively) and January to October 2015 (16.6% and 63.3%, respectively; P > 0.05). Although NoV detection in the period from October 2013 to October 2014 (25.0%) was less than its detection in the period from January to October 2015 (40.0%), this difference was not statistically significant (P > 0.05).
FIG 3.
Frequencies of infectious human enterovirus (iEV), viral RNA amplification (RV, HAstV, NoV, HAV, and HEV), and genetic characterization from three monitoring sites at the beaches located on the banks of the San Roque Dam, Cordoba, Argentina. (A) Monthly frequencies of detection of RV, HAstV, NoV, HAV, and HEV during October 2013 to October 2015. HAV-IA, hepatitis A virus/genotype IA; HEV-GE, hepatitis E virus/genotype 3. The quantity of blue symbols (RNA virus detection) or green asterisks (infectious human enterovirus) represents the numbers of sampling points for the indicated virus. (B) Proportional distributions of rotavirus, norovirus, and astrovirus genogroups/genotypes.
HAstV was more frequently detected in the warm months than in the cold months (Fig. 3A). Viable (infective) enterovirus was detected throughout all the studied periods from at least one of the three sample sites (Fig. 2B and 3A).
Figure 3B shows the molecular characterization of RV, HAstV, and NoV. A decrease in the circulation of RV genotypes was observed, with G1 being the most prevalent, followed by G2 and to a lesser extent, G3 and G4.
For HAstV, three genotypes were detected: genotype G3, the most prevalent, and genotypes G5 and G7. The circulating genogroups belonging to NoV had proportions similar to those detected in the body of the lake: 80% for genogroup II and 20% for genogroup I (Fig. 3B).
DISCUSSION
In recent years, investigations on environmental virology have placed an emphasis on monitoring the viral quality of wastewater and surface water to try to reduce the risk of viral diseases. Viruses are released into the environment through sewage and come into contact with recreational and consumption waters, which are the sources for human infections. To enrich our understanding of the local epidemiology and the dynamic of circulation of waterborne viruses, as well as to increase the poor existing data and underline the need of environmental surveillance programs in Argentina, two different monitoring campaigns were conducted in recreational waters during 3 years (2012 to 2015) to examine the presence of viruses that are associated with diarrhea and enteric hepatitis.
At least one type of virus was detected in each of the analyzed samples, although there were differences in viral detection frequencies according to the sampling site and the analyzed period, with fewer detections from coastal areas of the dam, especially in the first months of the year 2014 for RV.
Although molecular techniques are the primary choice for environmental viral surveillance due to their high sensitivity, specificity, and practicality, in this study, the presence of iEV was additionally investigated and was found to be a good indicator for determining viable and infectious viruses in environmental samples, with a high sensibility. The evidence of EV viability could indicate adequate matrix conditions for maintaining the infectivity of the other viruses analyzed by genomic detection. A limitation of this investigation is that an internal control was not used during the viral genomic molecular analysis, and so the viral detection frequency could be underestimated. However, the high frequency and diversity of viral detections would indicate the absence (or at least an insufficient concentration) of inhibitors in the samples analyzed.
In the present study, group A RV, HAstV, NoV, HAV, and HEV were detected and genetically characterized. The high iEV frequency of detection found during this study is in accordance with previous investigations performed in Córdoba Province, Argentina, which reported iEV frequencies between 99% and 86% (9). In the work mentioned above, poliovirus (PV) was found in 56% of the samples, the other ones (44%) were positive for only non-PV enterovirus. In addition, among the poliovirus-positive samples, 19% of them were also positive for non-PV enteroviruses. Also, in a study conducted in river recreational waters from Córdoba, 78.6% from the Suquia river and 87.5% from the Xanaes river were iEV-positive samples (8). No seasonal pattern was found for iEV circulation.
It is important to highlight that in Argentina, since the end of 2015, a sequential vaccination schedule of inactivated PV vaccine ([IPV] at 2 and 4 months of age), followed by two doses of oral PV vaccine ([OPV] at 9 and 18 months of age) replaced the OPV scheme in the routine childhood immunization program. As a consequence, many studies have reported a reduction of poliovirus shedding in children who received at least 2 doses of IPV before exposure to OPV (10–12). So, it is expected that this may affect the detection of infectious enteroviruses (by picking less-vaccine-derived polioviruses). However, our results, together with the previously mentioned environmental studies of Argentina, indicate a high circulation of non-PV enterovirus in watercourses and the population.
Group A RV is widely known as one of the most important childhood diarrheal pathogens worldwide. It is estimated that RV causes more than approximately 600,000 deaths annually in children all over the world. In Argentina, it is responsible for 40% of hospital admissions of acute diarrhea, and it is estimated that this virus may cause between 30 and 50 deaths annually in the country (13).
Seasonal fluctuations in rotavirus infection are documented, with an increase of the viral activity in cold and dry months (6, 13–17). However, in accordance with other studies that describe RV as circulating throughout the year (6, 18, 19), in our study, the presence of RV was demonstrated during the four seasons of the 3 years sampled, with a decrease in the frequency of detection in the spring. Despite this, the low RV detection performed during the first monitoring year (October 2013 to October 2014) of the second sampling, becoming higher again in the summer and autumn of 2015, was surprising. This could be explained, in part, by the increased rainfall. Global climate change is expected to affect the frequency, intensity, and duration of extreme water-related weather events, such as excessive precipitation, floods, and drought (20). In this sense, it is important to note that during the first 14 months of sampling on the beaches in Argentina, there was a global weather phenomenon called El Niño. It was characterized by intense rains that exceeded the annual historical average of rainfall levels in Córdoba province in the year 2014, according to rainfall records of the Córdoba Grain Exchange (21). In fact, in the year 2014, the average precipitation during the months corresponding to autumn and winter (dry season) was comparable to that found in the months corresponding to spring and summer (wet season). In the dry season of the year 2014, the average precipitation was 102.5 mm, and the historical peak of precipitation during January through April in Cordoba Province is around 100 to 200 mm of rainfall.
Thus, our findings agree with those obtained by many authors who report a drop in the detection of RV during the wet months compared with that during the dry ones (6, 14, 16, 22, 23). However, these results contrast with other studies carried out in Brazil, in which an increase in virus concentration was detected in samples collected during the rainfall events (24, 25).
Our environmental study reflects a local complex dynamic of RV “G” genotype circulation characterized by the cocirculation of different genotypes. In this sense, the waters of San Roque Dam provide a possible source of infection, with the population exposed to a wide variety of RV genotypes.
During the first period of the study, the most prevalent RV G genotypes (G1 and G9) correlated with the most common genomic variants associated with human gastrointestinal illness in Argentina (13) and other regions worldwide (6, 26–29). On the beaches, the pattern of circulating genotypes was different, although G1 was the most frequent.
Two live-attenuated vaccines (one pentavalent, RotaTeq by Merck, and one monovalent, Rotarix by GlaxoSmithKline) have been successfully introduced in a growing number of countries since 2006 (30, 31). In January 2015, the monovalent RV vaccine (G1 [P8]; GSK) was introduced in the Argentine National Immunization Program. The second sampling of this work was conducted between October 2013 and October 2015, and so it involved 10 months during which the RV immunization program was working and infants were vaccinated (vaccination schedule at 2 to 4 months of age). However, this virus was detected during all of the 10 months studied during this period in the same way that is was detected during the period from January to December 2012. Previous investigations have demonstrated the transmission of rotavirus vaccine strains from vaccinated children to nonvaccinated siblings. RV vaccine strain RNA was persistently detected in stool samples collected from vaccine recipients. The quantitative real-time reverse transcriptase PCR (RT-PCR) data revealed a peak viral RNA load 1 week after vaccination, followed by a gradual decrease. Therefore, rotavirus vaccination does not appear to restrict virus circulation and, in turn, viral detection in surface waters contaminated with fecal matter. Moreover, during the two sampling periods, RV-G1 type 1 was the most prevalent genotype. It would be necessary to sequence the VP7 viral genome region to establish whether the circulating virus belongs to the vaccine or to wild-type RV strains. It would also be important to carry out clinical and environmental investigations in the near future to evaluate the impact of the vaccine introduction on the dynamics of circulation and the diversity of RV strains in the community.
HAstVs have been associated with endemic diarrheal episodes and outbreaks of gastroenteritis in industrialized and nonindustrialized countries and have been detected in sewage as well as in surface water worldwide (32–35). In this study, HAstV was detected at a high frequency during the dry months of the first period of monitoring (year 2012), in agreement with previous studies in Latin America which detected HAstV at a high rate during the dry season of the year (34). During the second monitoring period, the particular meteorological phenomenon during the year 2014 made it difficult to describe the true circulation pattern of HAstV on the beaches of the lake. Despite this, it is important to highlight that in central Argentina, a longitudinal study carried out in a river of the same region demonstrated the presence of HAstV in surface waters, with differences in the detection rates between the dry and wet seasons (36). Although there are few data on the frequency of genotypes in superficial waters (33), our results show a profile similar to those of the most prevalent genotypes in the world (32, 37), with the exception of genotype 8, suggesting a circulation of a wide diversity of HAstV genotypes in recreational waters of Argentina.
NoV was found in high proportions in the two studied monitoring periods. These viruses have a high impact on human health all over the world, but in Argentina, NoV outbreak investigations and routine diagnosis of acute gastroenteritis cases are uncommon. The only data available are on an outbreak of acute gastroenteritis by NoV caused by the ingestion of water contaminated by sewage (38). The CDC estimates that each year, NoV causes 19 to 21 million illnesses, 56,000 to 71,000 hospitalizations, and 570 to 800 deaths. NoVs are associated with outbreaks due to contact with infected persons or the ingestion of contaminated food or water (39). Therefore, the study of this viral group and its potential sources of infection in a region with scarce data acquires great relevance.
Our detection of the genogroups I and II (GI and GII) of NoV agree with previous reports from different water matrices in the same region and worldwide (40–42).
In this study, the presence of HEV-3 on an environmental watercourse was demonstrated again in this region, adding evidence to the previously reported detection in riverine samples (4). Waterborne transmission of HEV to humans in direct contact with watercourses is possible and has important implications in public health in Córdoba province. HEV has been associated with serious acute hepatitis disease in pregnant women and in patients with preexisting chronic liver disease. Chronic HEV infections with clinical relevance have been described in immunocompromised patients infected with HEV-3, especially in organ transplant recipients (43). Previous studies in the central region of Argentina have reported the detection of HEV-3 RNA in 6% of sewage and a seroprevalence of anti-HEV IgG of 4.4% among the general population, 5.8% among solid-organ transplant patients, 16% in HIV-infected individuals with severe immunosuppression, and 10.2% among dialysis patients, which demonstrate that HEV circulates in this area (4, 44, 45). Although the sources of transmission are yet little understood, this study indicates that the courses of recreational water would be a favorable scenario for the infection, as well as a reservoir for the virus.
HAV has shown intermediate endemicity in Argentina, but the notification of clinical cases has been severely limited since the introduction of the vaccine in 2005. Vizzotti et al. (46) reported that a single-dose universal hepatitis A immunization in infants resulted in low HAV circulation and persistent immunologic protection up to 4 years in Argentina. However, differences in seroprotection among vaccinated children were related to differences in hygiene habits in settings with residual viral circulation. In this study, in agreement with what was previously documented by Blanco Fernández et al. (3) and Yanez et al. (5), we demonstrated the circulation of HAV in the year 2012 in a recreational water source not previously investigated, indicating viral maintenance and a potential risk for susceptible individuals during this period. The absence of detection of HAV on the beaches could be linked to the spatiotemporal characteristics of the sampling (described above for RV with regard to climate change in the different monitoring years and different places) but also to a decrease in the viral excretion of the population, as a consequence of the continuity and effectiveness of the vaccination program, which provides cumulative immunity.
Conclusion.
Data obtained in our study show the impact of anthropic contamination of one of the most important tourist water resources in Argentina, which is in close contact with the local population, constituting a possible viral infectious source for the population, especially in the summertime when these water sources can be used recreationally. The results obtained could alert the health systems and environmental sanitation to make decisions for viral control and prevention in this environment.
In this sense, the National Ministry of Health of Argentina is committed to making decisions based on local evidence and to share its experiences to promote cost-effective strategies of control and prevention. The environmental viral monitoring and genetic variability evidence of local circulating strains should be part of this policy. Thereby, data reported in the present study, added to previous local reports on environmental virology, could (a) contribute to active surveillance and monitoring of the effectiveness of the immunization programs implemented in the region, (b) anticipate outbreaks in unvaccinated populations or those with low vaccination coverage, (c) contribute to the surveillance of emerging viruses, (d) identify sources of viral contamination, and (e) describe the circulation of poorly known and underdiagnosed viruses potentially hazardous to health.
MATERIALS AND METHODS
Study area and monitoring sites.
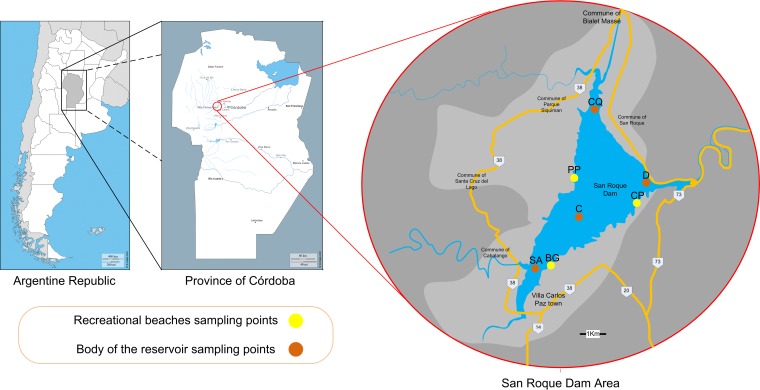
The study was conducted at the San Roque Dam (31°22′S, 64°28′W; 608 m above sea level [asl]), located in a mountain range in the Punilla Valley, next to Carlos Paz city, 40 km west from Córdoba city, in the province of Córdoba, Argentina (Fig. 4). The Dam has two main tributaries: the San Antonio River (with an estimated annual mean flow of 4.0 m3/s) and the Cosquin River (with an estimated annual mean flow of 16.5 m3/s). Both rivers are subjected to a seasonal fluctuation in water flow. The surface area of this reservoir covers around 16 km2. Four monitoring points located at the San Roque Dam were selected: one central monitoring site named center C, one at the east side of the dam near the spillway and the dock walls named Dam D, and two monitoring stations at the mouths of the tributaries: one monitoring site at the San Antonio River mouth (named SA) and the other one at the Cosquin River mouth (named CQ) (Fig. 4). Besides, 3 beaches on the banks of the dam were monitored: Perelli Beach (PP) that is a great bay located in a residential area, used as a wharf by those who practice sailing; Bahía del Gitano Beach (BG), located in an urban area, used by boats, jet skis, and fishermen; and Club de Pescadores Beach (CP), used for sport fishing and swimming (Fig. 4).
FIG 4.
Geographical representation of the area under study: the San Roque Dam, located in the province of Córdoba in Argentina. The sampling points are indicated. The brown circles mark the samplings carried out on the body of the reservoir: central (C), dam (D), and the San Antonio River (SA) and Cosquin River (CQ) (mouths of the tributaries). The yellow circles show the sampling points on the recreational beaches: Perelli Beach (PP), Club de Pescadores Beach (CP), and Bahía del Gitano Beach (BG). Map templates can be found at http://d-maps.com/carte.php?num_car=4732&lang=en, http://d-maps.com/carte.php?num_car=140164&lang=en, and https://www.google.com.ar/maps/place/Dique+San+Roque.
Sample collection and concentration.
A total of 48 water samples from the 4 monitoring points of the dam from January to December 2012 and 66 water samples from the 3 beaches from October 2013 to October 2015 were collected monthly.
All water samples (1.5 liter each) were collected during the daytime and were typically gathered in the morning between the 12th and 17th days of the month. Samples were taken at a depth of 0.20 m using sterile bottles and were transported within 12 h at 4°C to 8°C to the laboratory for further processing and analysis.
Specimens were concentrated by using the polyethylene glycol (PEG) precipitation method, previously described by Lewis and Metcalf (47) and Greening et al. (48) as modified by Huang et al. (49). The 1.5-liter water samples were concentrated 100-fold to 15 ml by high-speed centrifugation (two centrifugation steps at 10,700 × g for 20 to 25 min), elution (two steps at room temperature for 1 h), and PEG precipitation (10% PEG 6000, overnight at 4°C). The concentrated samples were further treated with chloroform to obtain a clear sample for cell culture virus isolation.
The recovery efficiencies of the nucleic acid extraction method and PEG concentration procedure were previously evaluated using PP7 as a comparator virus (50).
Infectious human enterovirus.
Infectious enteroviruses were detected according to the WHO polio laboratory manual (51). In summary, after the concentration technique, the sample clarification was performed with the double chloroform organic treatment followed by the addition of penicillin (100 units/ml), streptomycin (100 μg/ml), and amphotericin B ([Fungizone] 250 μg/ml). After this, 150 μl from each sample was inoculated in a 24-well plate containing HEp-2 cells. For this, the maintenance medium was removed and the sample was deposited on the cell monolayer. Then, plates were incubated at 37°C for 60 min. Subsequently, 150 μl of minimal essential medium (MEM) supplemented with 2% fetal bovine serum ([FBS] inoculation medium) was added. Samples were incubated at 37°C for 7 days or until the cytopathic effect (CPE) occurred. In general, the CPE produced by enteroviruses is manifested by the widespread destruction of the monolayer.
Infectious EV was confirmed by a direct immunofluorescence assay. The monoclonal antibody solution used for iEV detection consisted of monoclonal antibodies against coxsackievirus type A9, coxsackievirus type B (B1, B2, B3, B4, B5, and B6), echovirus (serotypes 4, 6, 9, 11, 30, and 34), poliovirus (serotypes 1, 2, and 3), and enterovirus (serotypes 60, 71, and Cox A16). The monoclonal antibody reagents were commercially prepared and were purchased from Chemicon International (Temecula, CA).
Detection of viral genomic RNA.
In all the cases, viral RNA was extracted from 140 μl of the concentrated water sample using a commercial QIAamp viral RNA kit (Qiagen Inc., Hilden, Germany). The manufacturer's protocol was followed, and the purified viral RNA was eluted in 60 μl of elution buffer. Extracted RNA was reverse transcribed into cDNA using random hexamer primers and an ImProm-II reverse transcription system (Promega Corp.). The primer characteristics and references for the amplification conditions of the PCR protocol used for nucleic acid detection of all viral groups are shown in Table 1.
TABLE 1.
Oligonucleotide primers used for the detection of genotypes/genogroups of enteric viruses from recreational water of the San Roque Dam
| Virusa | Primer name (directionb) | Sequence (5′→3′) | Genome region | Nucleotides | Annealing temp (°C) | Fragment size (bp) | Reference |
|---|---|---|---|---|---|---|---|
| RVc | Beg9 (F) | GGCTTTAAAAGAGAGAATTTCCGTCTGG | VP7 | 1–28 | 42d | 1.062 | 52 |
| End9 (R) | GGTCACATCATACAATTCTAATCTAAG | 1,062–1,036 | |||||
| aBT1 (F) | CAAGTACTCAATCAATGATGG | VP7 | 314–335 | 749 | |||
| aCT2 (F) | CAATGATATTAACACATTTTCTGTG | 411–435 | 652 | ||||
| aET3 (F) | CGTTTGAAGAAGTTGCAACAG | 689–709 | 374 | ||||
| aDT4 (F) | CGTTTCTGGTGAGGAGTTG | 480–498 | 583 | ||||
| aAT8 (F) | GTCACACCATTTGTAAATTCG | 178–198 | 885 | ||||
| aFT9 (F) | CTAGATGTAACTACAACTAC | 757–776 | 306 | ||||
| HAV | HAV1 (F) | CTCTCCCCTTGCGCTAGGCTCT | 5′ UTRg | 208–229 | 65 | 230 | 3 |
| HAV1 (R) | CAGTCCTCCGGCGTTGAATGGT | 463–484 | |||||
| HAV2 (F) | CTTGCCCTAGGCTCTGGCCGT | 215–236 | 45 | ||||
| HAV2 (R) | CAATATCCGCCGCTGTTACCCTAT | 420–443 | |||||
| HEV | 3156N (F) | AATTATGCYCAGTAYCGRGTTG | ORF-2 | 5,687–5,708 | 45d | 348 | 49 |
| 3157NR (R) | CCCTTRTCYTGCTGMGCATTCTC | 6,395–6,417 | |||||
| 3158N (F) | GTWATGCTYTGCATWCATGGCT | 5,972–5,993 | |||||
| 3159N (R) | AGCCGACGAAATCAATTCTGTC | 6,298–6,319 | |||||
| NoVe | NV1 (F) | ATACCACCTATGATGCWGAYTA | Region A polymerase | 4,279–4,299 | 45 | 237 | 53 |
| NV2 (R) | ATYTCATCACCATARAAIGA | 4,585–4,605 | |||||
| NV3 G1 (F) | TCNGAAATGGATGTTGG | 4,691–4,707 | 37 | 188 | |||
| NV4 (R) | AGCCAGTTTTCGATGGARTTC | 4,495–4,515 | |||||
| HAstVf | PreCap1 (F) | GGACTGCAAAGCAGCTTCCTG | ORF-2 | 62–82 | 48d | 2,473 | 54 |
| 12GR (R) | TTTTTTTTTTTTTTTTTTTTTGC | 2,454–2,473 | |||||
| HAsTV-1 (F) | AACCAAGGAATGACAATGAC | 2,166–2,185 | 212 | ||||
| HAsTV-2 (F) | ACCTGCGCTGAGAAACTG | 2,247–2,185 | 158 | ||||
| HAsTV-3 (F) | CTGCTTGCATCTGGTCTTTCA | 2,283–2,303 | 119 | ||||
| HAsTV-4 (F) | TGATGATGAAGACTCTAATAC | 2,071–2,091 | 258 | ||||
| HAsTV-5 (F) | TAGTAACTTATGATAGCC | 2,014–2,031 | 388 | ||||
| HAsTV-6 (F) | TGGCCACCCTTGTTCCTCAGA | 1,951–1,971 | 427 | ||||
| HAsTV-7 (F) | CTAGACAACAACAACCCG | 1,842–1,859 | 548 | ||||
| HAstV-8 (F) | GGTAAGTGGTACCTGCTAACTAG | 1,753–1,775 | 599 | ||||
| End (R) | TCCTACTCGGCGTGGCCGC | 2,377–2,359 | 212 |
RV, rotavirus; HAV, hepatitis A virus; HEV, hepatitis E virus; NoV, norovirus; HAstV, human astrovirus.
F, forward; R, reverse.
Genotypes G1 to G4, G8, and G9.
The cycling conditions for the nested PCR were the same as those described for the first amplification round.
NoV genogroups I and II.
Genotypes G1 to G8.
UTR, untranslated region.
The heminested protocols used for RVA, HAstV, and NoV detections were all described previously (52–54).
Statistical analysis.
Statistical analyses of the data were performed to estimate the frequencies of detection of the studied viral groups.
Fisher's tests were used to compare the rates of virus detection in the samples studied. A P value of <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This work was supported by the National Agency for Scientific and Technical Promotion (PICT 2012-0998 and PICT 2014-3221).
G.M., M.B.P., and V.R. are members of the researcher career program of CONICET, Argentina. V.E.P. is a recipient of a CONICET fellowship.
REFERENCES
- 1.UNEP. 2016. UNEP annual report. UNEP, Nairobi, Kenya: http://web.unep.org/annualreport/2016/index.php?page=0&lang=en. [Google Scholar]
- 2.Instituto Nacional de Estadísticas y Censos. 2010. Publication of the National Census of Population and Housing 2010. Bicentennial census. Instituto Nacional de Estadísticas y Censos, Buenos Aires, Argentina. [Google Scholar]
- 3.Blanco Fernández MD, Torres C, Riviello-López G, Poma HR, Rajal VB, Nates S, Cisterna DM, Campos RH, Mbayed VA. 2012. Analysis of the circulation of hepatitis A virus in Argentina since vaccine introduction. Clin Microbiol Infect 18:E548–E551. doi: 10.1111/1469-0691.12034. [DOI] [PubMed] [Google Scholar]
- 4.Martínez Wassaf MG, Pisano MB, Barril PA, Elbarcha OC, Pinto MA, Mendes de Oliveira J, DiGiusto P, Nates SV, Ré VE. 2014. First detection of hepatitis E virus in central Argentina: environmental and serological survey. J Clin Virol 61:334–339. doi: 10.1016/j.jcv.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Yanez LA, Lucero NS, Barril PA, Díaz MDP, Tenaglia MM, Spinsanti LI, Nates SV, Isa MB, Ré VE. 2014. Evidence of hepatitis A virus circulation in central Argentina: seroprevalence and environmental surveillance. J Clin Virol 59:38–43. doi: 10.1016/j.jcv.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Barril PA, Fumian TM, Prez VE, Gil PI, Martínez LC, Giordano MO, Masachessi G, Isa MB, Ferreyra LJ, Ré VE, Miagostovich M, Pavan JV, Nates SV. 2015. Rotavirus seasonality in urban sewage from Argentina: effect of meteorological variables on the viral load and the genetic diversity. Environ Res 138:409–415. doi: 10.1016/j.envres.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Ferreyra LJ, Giordano MO, Martínez LC, Barril PA, Masachessi G, Isa MB, Poma R, Rajal V, Biganzoli P, Nates SV, Pavan JV. 2015. Tracking novel adenovirus in environmental and human clinical samples: no evidence of endemic human adenovirus type 58 circulation in Córdoba city, Argentina. Epidemiol Infect 143:1427–1431. doi: 10.1017/S0950268814002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prez VE, Gil PI, Temprana CF, Cuadrado PR, Martínez LC, Giordano MO, Masachessi G, Isa MB, Ré VE, Paván JV, Nates SV, Barril PA. 2015. Quantification of human infection risk caused by rotavirus in surface waters from Córdoba, Argentina. Sci Total Environ 538:220–229. doi: 10.1016/j.scitotenv.2015.08.041. [DOI] [PubMed] [Google Scholar]
- 9.Muller JE, Bessaud M, Huang QS, Martinez LC, Barril PA, Morel V, Balanant J, Bocacao J, Hewitt J, Gessner BD, Delpeyroux F, Nates SV. 2009. Environmental poliovirus surveillance during oral poliovirus vaccine and inactivated poliovirus vaccine use in Córdoba Province, Argentina. Appl Environ Microbiol 75:1395–1401. doi: 10.1128/AEM.02201-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plotkin SA, Vidor E. 2004. Poliovirus vaccine-inactivated, p 625–49. In Plotkin SA, Orenstein WA (ed), Vaccines, 4th ed Elsevier, Philadelphia, PA. [Google Scholar]
- 11.Laassri M, Lottenbach K, Belshe R, Wolff M, Rennels M, Plotkin S, Chumakov K. 2005. Effect of different vaccination schedules on excretion of oral poliovirus vaccine strains. J Infect Dis 192:2092–2098. doi: 10.1086/498172. [DOI] [PubMed] [Google Scholar]
- 12.Asturias EJ, Dueger EL, Omer SB, Melville A, Nates SV, Laassri M, Chumakov K, Halsey NA. 2007. Randomized trial of inactivated and live polio vaccine schedules in Guatemalan infants. J Infect Dis 196:692–698. doi: 10.1086/520546. [DOI] [PubMed] [Google Scholar]
- 13.Degiuseppe JI, Reale EA, Stupka JA, Argentine Rotavirus Surveillance Network. 2017. Rotavirus epidemiology and surveillance before vaccine introduction in Argentina, 2012–2014. J Med Virol 89:423–428. doi: 10.1002/jmv.24650. [DOI] [PubMed] [Google Scholar]
- 14.Cook SM, Glass RI, LeBaron CW, Ho MS. 1990. Global seasonality of rotavirus infections. Bull World Health Organ 68:171–177. [PMC free article] [PubMed] [Google Scholar]
- 15.D'Souza RM, Hall G, Becker NG. 2008. Climatic factors associated with hospitalizations for rotavirus diarrhoea in children under 5 years of age. Epidemiol Infect 136:56–64. doi: 10.1017/S0950268807008229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy K, Hubbard AE, Eisenberg JN. 2009. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol 38:1487–1496. doi: 10.1093/ije/dyn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagai JS, Sarkar R, Castronovo D, Kattula D, McEntee J, Ward H, Kang G, Naumova EN. 2012. Seasonality of rotavirus in South Asia: a meta-analysis approach assessing associations with temperature, precipitation, and vegetation index. PLoS One 7:e38168. doi: 10.1371/journal.pone.0038168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teixeira Masukawa MDL, Moriwaki AM, Uchimura NS, Souza EM, Uchimura TT. 2014. Intervention analysis of introduction of rotavirus vaccine on hospital admissions rates due to acute diarrhea. Cad Saude Publica 30:2101–2111. doi: 10.1590/0102-311X00124713. [DOI] [PubMed] [Google Scholar]
- 19.Pitzer VE, Viboud C, Lopman BA, Patel MM, Parashar UD, Grenfell BT. 2011. Influence of birth rates and transmission rates on the global seasonality of rotavirus incidence. J R Soc Interface 8:1584–1593. doi: 10.1098/rsif.2011.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cann KF, Thomas DR, Salmon RL, Wyn-Jones AP, Kay D. 2013. Extreme water-related weather events and waterborne disease. Epidemiol Infect 141:671–686. doi: 10.1017/S0950268812001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolsa de Cereales de Córdoba. 2014. Historical facts. Bolsa de Cereales de Córdoba, Córdoba, Argentina: http://www.bccba.com.ar/datos-historicos-6393.html. [Google Scholar]
- 22.Bresee JS, Marcus R, Venezia RA, Keene WE, Morse D, Thanassi M, Brunett P, Bulens S, Beard RS, Dauphin LA, Slutsker L, Bopp C, Eberhard M, Hall A, Vinje J, Monroe SS, Glass RI, U.S. Acute Gastroenteritis Etiology Study Team . 2012. The etiology of severe acute gastroenteritis among adults visiting emergency departments in the United States. J Infect Dis 205:1374–1381. doi: 10.1093/infdis/jis206. [DOI] [PubMed] [Google Scholar]
- 23.Kane EM, Turcios RM, Arvay ML, Garcia S, Bresee JS, Glass RI. 2004. The epidemiology of rotavirus diarrhea in Latin America. Anticipating rotavirus vaccines. Rev Panam Salud Publica 16:371–377. doi: 10.1590/S1020-49892004001200002. [DOI] [PubMed] [Google Scholar]
- 24.Victoria M, Fumian TM, Rocha MS, Dalmao F, Leite JP, Girones R, Miagostovich MP. 2014. Gastroenteric virus dissemination and influence of rainfall events in urban beaches in Brazil. J Appl Microbiol 117:1210–1218. doi: 10.1111/jam.12592. [DOI] [PubMed] [Google Scholar]
- 25.Haramoto E, Katayama H, Oguma K, Yamashita H, Tajima A, Nakajima H, Ohgaki S. 2006. Seasonal profiles of human noroviruses and indicator bacteria in a wastewater treatment plant in Tokyo, Japan. Water Sci Technol 54:301–308. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Vlasova A, Velasquez DE, Saif LJ, Kandasamy S, Kochba E, Levin Y, Jiang B. 2016. Skin vaccination against rotavirus using microneedles: proof of concept in gnotobiotic piglets. PLoS One 11:e0166038. doi: 10.1371/journal.pone.0166038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruggeri FM, Bonomo P, Ianiro G, Battistone A, Delogu R, Germinario C, Chironna M, Triassi M, Campagnuolo R, Cicala A, Giammanco GM, Castiglia P, Serra C, Gaggioli A, Fiore L. 2015. Rotavirus genotypes in sewage treatment plants and in children hospitalized with acute diarrhea in Italy in 2010 and 2011. Appl Environ Microbiol 81:241–249. doi: 10.1128/AEM.02695-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kargar M, Javdani N, Najafi A, Tahamtan Y. 2013. First molecular detection of group A rotavirus in urban and hospital sewage systems by nested-RT PCR in Shiraz, Iran. J Environ Health Sci Eng 11:4. doi: 10.1186/2052-336X-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barril PA, Giordano MO, Isa MB, Masachessi G, Ferreyra LJ, Castello AA, Glikmann G, Nates SV. 2010. Correlation between rotavirus A genotypes detected in hospitalized children and sewage samples in 2006, Córdoba, Argentina. J Med Virol 82:1277–1281. doi: 10.1002/jmv.21800. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, Cervantes Y, Linhares AC, López P, Macías-Parra M, Ortega-Barría E, Richardson V, Rivera-Medina DM, Rivera L, Salinas B, Pavía-Ruz N, Salmerón J, Rüttimann R, Tinoco JC, Rubio P, Nuñez E, Guerrero ML, Yarzábal JP, Damaso S, Tornieporth N, Sáez-Llorens X, Vergara RF, Vesikari T, Bouckenooghe A, Clemens R, De Vos B, O'Ryan M, Human Rotavirus Vaccine Study Group. 2006. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 31.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, Shinefield HR, Christie CD, Ylitalo S, Itzler RF, Coia ML, Onorato MT, Adeyi BA, Marshall GS, Gothefors L, Campens D, Karvonen A, Watt JP, O'Brien KL, DiNubile MJ, Clark HF, Boslego JW, Offit PA, Heaton PM, Rotavirus Efficacy and Safety Trial (REST) Study Team. 2006. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 32.Nadan S, Walter JE, Grabow WOK, Mitchell DK, Taylor MB. 2003. Molecular characterisation of astroviruses by reverse transcriptase PCR and sequence analysis: comparison of clinical and environmental isolates from South Africa. Appl Environ Microbiol 69:747–753. doi: 10.1128/AEM.69.2.747-753.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miagostovich MP, Ferreira FF, Guimarães FR, Fumian TM, Diniz-Mendes L, Luz SL, Silva LA, Leite JP. 2008. Molecular detection and characterization of gastroenteritis viruses occurring naturally in the stream waters of Manaus, central Amazonia, Brazil. Appl Environ Microbiol 74:375–382. doi: 10.1128/AEM.00944-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez RA, Pepper IL, Gerba CP. 2009. Application of PCR-based methods to assess the infectivity of enteric viruses in environmental samples. Appl Environ Microbiol 75:297–307. doi: 10.1128/AEM.01150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones TH, Brassard J, Topp E, Wilkes G, Lapen DR. 2017. Waterborne viruses and F-specific coliphages in mixed-use watersheds: microbial associations, host specificities, and affinities with environmental/land use factors. Appl Environ Microbiol 83:e02763-16. doi: 10.1128/AEM.02763-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giordano MO, Ferreyra LJ, Nates SV. 2017. Astrovirus humanos en aguas recreacionales de Córdoba, Argentina. Editorial Académica Española, Saarbrücken, Germany. [Google Scholar]
- 37.Meleg E, Bányai K, Martella V, Jiang B, Kocsis B, Kisfali P, Melegh B, Szucs G. 2008. Detection and quantification of group C rotaviruses in communal sewage. Appl Environ Microbiol 74:3394–3399. doi: 10.1128/AEM.02895-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poma HR, Gutiérrez Cacciabue D, Garcé B, Gonzo EE, Rajal VB. 2012. Towards a rational strategy for monitoring of microbiological quality of ambient waters. Sci Total Environ 433:98–109. doi: 10.1016/j.scitotenv.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glass RI, Parashar UD, Estes MK. 2009. Norovirus gastroenteritis. N Engl J Med 361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyn-Jones AP, Carducci A, Cook N, D'Agostino M, Divizia M, Fleischer J, Gantzer C, Gawler A, Girones R, Höller C, de Roda Husman AM, Kay D, Kozyra I, López-Pila J, Muscillo M, Nascimento MS, Papageorgiou G, Rutjes S, Sellwood J, Szewzyk R, Wyer M. 2011. Surveillance of adenoviruses and noroviruses in European recreational waters. Water Res 45:1025–1038. doi: 10.1016/j.watres.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanco Fernández MD, Torres C, Martínez LC, Giordano MO, Masachessi G, Barril PA, Isa MB, Campos RH, Nates SV, Mbayed VA. 2011. Genetic and evolutionary characterization of norovirus from sewage and surface waters in Córdoba City, Argentina. Infect Genet Evol 11:1631–1637. doi: 10.1016/j.meegid.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Lee S-G, Cho H-G, Paik S-Y. 2015. Molecular epidemiology of norovirus in South Korea. BMB Rep 48:61–67. doi: 10.5483/BMBRep.2015.48.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murali AR, Kotwal V, Chawla S. 2015. Chronic hepatitis E: a brief review. World J Hepatol 7:2194–2201. doi: 10.4254/wjh.v7.i19.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Debes JD, Pisano MB, Lotto M, Re V. 2016. Hepatitis E virus infection in the HIV-positive patient. J Clin Virol 80:102–106. doi: 10.1016/j.jcv.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Pisano MB, Balderramo D, Wassaf MM, Lotto M, Carlino Y, Ré VE, Debes JD. 2017. Hepatitis E virus infection in patients on dialysis and in solid organ transplant recipients in Argentina: exploring associated risk factors. Arch Virol 162:787–792. doi: 10.1007/s00705-016-3171-6. [DOI] [PubMed] [Google Scholar]
- 46.Vizzotti C, González J, Gentile A, Rearte A, Ramonet M, Cañero-Velasco MC, Pérez Carrega ME, Urueña A, Diosque M. 2014. Impact of the single-dose immunization strategy against hepatitis A in Argentina. Pediatr Infect Dis J 33:84–88. doi: 10.1097/INF.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 47.Lewis GD, Metcalf TG. 1988. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl Environ Microbiol 54:1983–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greening GE, Hewitt J, Lewis GD. 2002. Evaluation of integrated cell 3 culture-PCR (C-PCR) for virological analysis of environmental samples. J Appl Microbiol 93:745–750. doi: 10.1046/j.1365-2672.2002.01741.x. [DOI] [PubMed] [Google Scholar]
- 49.Huang QS, Greening G, Baker MG, Grimwood K, Hewitt J, Hulston D, van Duin L, Fitzsimons A, Garrett N, Graham D, Lennon D, Shimizu H, Miyamura T, Pallansch M. 2005. Persistence of oral polio vaccine virus after its removal from the immunisation schedule in New Zealand. Lancet 366:394–396. doi: 10.1016/S0140-6736(05)66386-6. [DOI] [PubMed] [Google Scholar]
- 50.Poma HR, Rajal VB, Blanco Fernández MD, Barril PA, Giordano MO, Masachessi G, Martínez LC, Isa MB, Freire MC, López Riviello G, Cisterna D, Nates SV, Mbayed VA. 2013. Evaluation of concentration efficiency of the Pseudomonas aeruginosa phage PP7 in various water matrixes by different methods. Environ Monit Assess 185:2565–2576. doi: 10.1007/s10661-012-2731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization. 2004. Polio laboratory manual, 4th ed World Health Organization, Geneva, Switzerland. [Google Scholar]
- 52.Gouvea V, Glass R, Woods P, Taniguchi K, Clark H, Forrester B, Fang Z. 1990. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol 28:276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vennema H, de Bruin E, Koopmans M. 2002. Rational optimization of generic primers used for Norwalk-like virus detection by reverse transcriptase polymerase chain reaction. J Clin Virol 25:233–235. doi: 10.1016/S1386-6532(02)00126-9. [DOI] [PubMed] [Google Scholar]
- 54.Sakamoto T, Negishi H, Wang QH, Akihara S, Kim B, Nishimura S, Kaneshi K, Nakaya S, Ueda Y, Sugita K, Motohiro T, Nishimura T, Ushijima H. 2000. Molecular epidemiology of astroviruses in Japan from 1995 to 1998 by reverse transcription-polymerase chain reaction with serotype-specific primers (1 to 8). J Med Virol 61:326–331. [PubMed] [Google Scholar]