ABSTRACT
Salmonella is estimated to cause one million foodborne illnesses in the United States every year. Salmonella-contaminated poultry products are one of the major sources of salmonellosis. Given the critical role of the gut microbiota in Salmonella transmission, a manipulation of the chicken intestinal microenvironment could prevent animal colonization by the pathogen. In Salmonella, the global regulator gene fnr (fumarate nitrate reduction) regulates anaerobic metabolism and is essential for adapting to the gut environment. This study tested the hypothesis that an attenuated Fnr mutant of Salmonella enterica serovar Typhimurium (attST) or prebiotic galacto-oligosaccharides (GOS) could improve resistance to wild-type Salmonella via modifications to the structure of the chicken gut microbiome. Intestinal samples from a total of 273 animals were collected weekly for 9 weeks to evaluate the impact of attST or prebiotic supplementation on microbial species of the cecum, duodenum, jejunum, and ileum. We next analyzed changes to the gut microbiome induced by challenging the animals with a wild-type Salmonella serovar 4,[5],12:r:− (Nalr) strain and determined the clearance rate of the virulent strain in the treated and control groups. Both GOS and the attenuated Salmonella strain modified the gut microbiome but elicited alterations of different taxonomic groups. The attST produced significant increases of Alistipes and undefined Lactobacillus, while GOS increased Christensenellaceae and Lactobacillus reuteri. The microbiome structural changes induced by both treatments resulted in a faster clearance after a Salmonella challenge.
IMPORTANCE With an average annual incidence of 13.1 cases/100,000 individuals, salmonellosis has been deemed a nationally notifiable condition in the United States by the Centers for Disease Control and Prevention (CDC). Earlier studies demonstrated that Salmonella is transmitted by a subset of animals (supershedders). The supershedder phenotype can be induced by antibiotics, ascertaining an essential role for the gut microbiota in Salmonella transmission. Consequently, modulation of the gut microbiota and modification of the intestinal microenvironment could assist in preventing animal colonization by the pathogen. Our study demonstrated that a manipulation of the chicken gut microbiota by the administration of an attenuated Salmonella strain or prebiotic galacto-oligosaccharides (GOS) can promote resistance to Salmonella colonization via increases of beneficial microorganisms that translate into a less hospitable gut microenvironment.
KEYWORDS: chicken gut microbiome, gut microbiome modulation, prebiotics, Salmonella, Salmonella enterica serovar Typhimurium attenuated strain, galacto-oligosaccharides
INTRODUCTION
With an average annual incidence of 13.1 cases/100,000 individuals, salmonellosis has been deemed a nationally notifiable condition in the United States by the Centers for Disease Control and Prevention (CDC). Specifically, foodborne infections after the consumption of poultry meat or egg products contaminated with Salmonella enterica are a major public health concern (1–3). In 2009, the United States produced and sold 8.5 billion broilers (meat-type birds), 247 million turkeys, and 90.4 billion eggs from 337 million laying hens. It is estimated that 1 in 8 chickens are contaminated with Salmonella (4, 5). The two serotypes most commonly associated with foodborne illness in poultry foods are S. enterica serovar Typhimurium and S. enterica serovar Enteritidis, accounting for approximately 40 to 60% of all reported Salmonella infections (6, 7). Salmonella enterica is a Gram-negative intracellular pathogen that causes gastroenteritis in the human host. Although it is not life threatening in healthy adults, it can be fatal for children and immunocompromised individuals. The infection proceeds via two main stages: invasion and systemic infection. During the invasion stage, the pathogen adheres to and colonizes the intestine, gaining access to the epithelial cells. Subsequently, Salmonella crosses the epithelial cells and gets internalized by the macrophages, where it multiplies, spreads in the host, and causes systemic infection (8–10).
Considerable effort is made by farmers and food processors, both pre- and postharvest, to prevent Salmonella contamination of food products. One of the primary preharvest tools employed in poultry production has been the use of vaccines. The objective is to condition the chicken immune system to decrease the levels of Salmonella associated with the animals, consequently reducing the transmission of potentially pathogenic strains to human populations. Multiple Salmonella vaccines have been developed and used for this purpose, representing the three main categories of vaccines: inactivated, live attenuated, and subunit vaccines (11). While many of these vaccines are capable of eliciting strong serum antibody responses, vaccination does not seem adequate for producing immune responses that eliminate Salmonella from the intestinal tract, leaving a reservoir for reintroduction and contamination (12). Furthermore, most of the live attenuated strains used commercially are dependent on autotrophic mutations (e.g., harboring defects in amino acids, nucleic acid biosynthesis, and UDP-glucose 4-epimerase), which have the potential for reversion to the virulent phenotype and are influenced by the diet of the host (13). Indeed, the search continues for live attenuated Salmonella strains that can reduce colonization and invasion by the challenge strains (14). We have previously developed an attenuated Fnr mutant (NC983 [attST]) of Salmonella Typhimurium (15, 16) and showed that it is stable, elicits Salmonella-specific antibodies, and provides protection against S. Typhimurium in mice (our unpublished data). In Salmonella and other enteric bacteria, fumarate nitrate reduction (FNR) is a global regulator of anaerobic metabolism (17) and hence is essential for adapting to the gut environment.
Transmission of S. Typhimurium in mice occurs only via a subset of the infected mice, i.e., supershedders, that shed high levels of the virulent organism (>108 CFU/g) in their feces (18). The immune response is the main determinant of Salmonella levels in the colon, as immunosuppression of the infected mice does not induce suppershedding. However, altering the indigenous microbiota by antibiotics induces the supershedder phenotype (18). These findings clearly demonstrate the critical role of the intestinal microbiota and gut homeostasis in controlling Salmonella transmission, infection, and disease. In pigs, chickens, and mice, it has been shown that supershedders have increased innate inflammatory responses (19); however, there is limited data on the role of the chicken microbiota in controlling the spread of Salmonella spp. in poultry and poultry products (20, 21).
The gut microbiota plays important roles in the digestion of complex plant fibers and polysaccharides, the development of the host immune system, and protection against colonization by invasive pathogenic organisms (colonization resistance [CR]) (22–25). Abundant information is available on how the human gut microbiota can control pathogen colonization (26–28); however, limited information on the role of the chicken intestinal microbiota in resistance to disease (i.e., Salmonella) is available. According to recent reports, the cecum contains the most diverse bacterial populations, with Bacteroidetes and Firmicutes being the most prevalent phyla in the cecal microbiota of egg-laying hens (29). On the other hand, the microbiota in the ceca of meat-type birds are dominated by Firmicutes and to a lesser extent by Bacteroidetes (30, 31). The difference in the microbiota of broilers versus layers may be related to the diet (29). Indeed, diet modification through the inclusion of prebiotics has been shown to affect the intestinal microbiota, modifying transit time, luminal pH, and the production of microbial metabolites in humans and animal models (32–36), including poultry (37). Prebiotics have been identified as potential interventions for gut disorders due to their capacity to modulate the gut microbiota (38, 39) and act as soluble decoy receptors, preventing pathogen attachment to mucosal surfaces (40–44). The prebiotics inulin, fructo-oligosaccharides (FOS), and mannan-oligosaccharides (MOS) have been shown to confer a protective effect in chicks during the first few days postinfection, reducing the colonization by and shedding of Salmonella (37, 45, 46).
In the present study, we tested the hypothesis that the live attenuated Salmonella strain (attST) or prebiotic galacto-oligosaccharides (GOS) could improve resistance to wild-type Salmonella via modifications to the structure of the chicken gut microbiome.
RESULTS
Scope of the study.
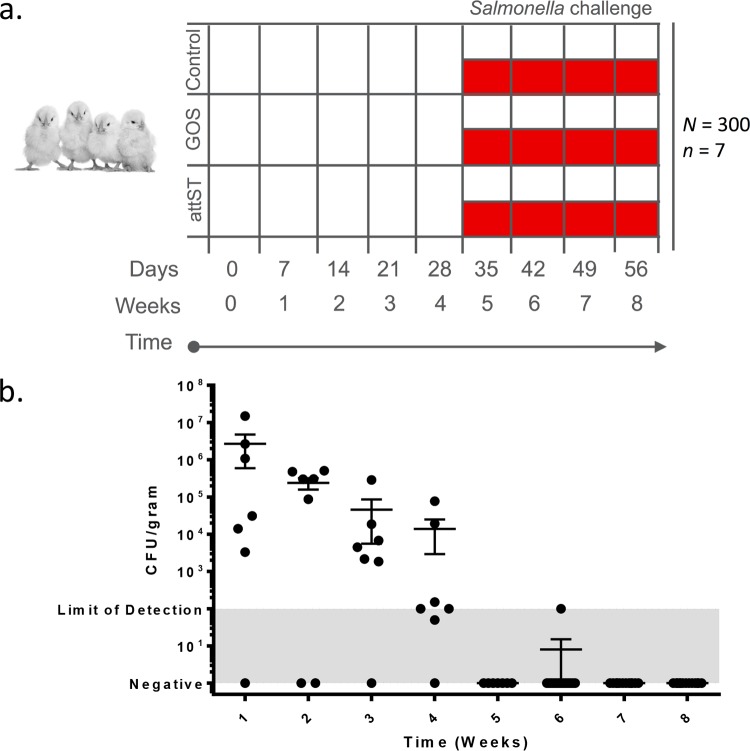
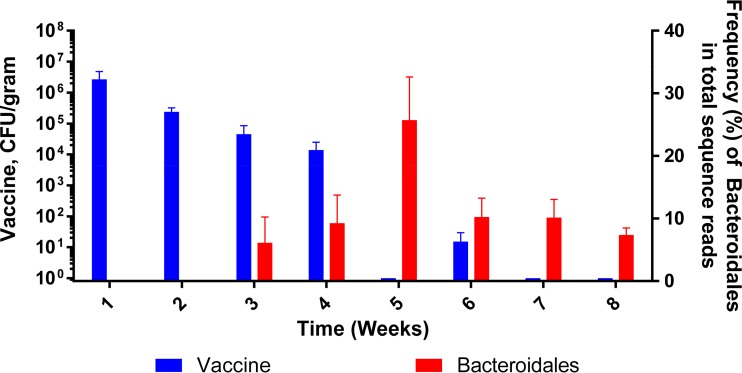
A total of 273 1-day-old female commercial white leghorn chicks were randomly assigned to three groups, namely, control, prebiotics (GOS), and attST (Rifr, NC983), and samples were collected weekly (Fig. 1a). Each week, 7 animals from each group were euthanized and sampled for microbiome and bacteriological analyses as outlined in Materials and Methods. After collecting week 4 samples, half of the remaining birds in all groups were challenged with Salmonella serovar 4,[5],12:r:− (Nalr), and samples were collected weekly until week 8. An analysis of the attST strain clearance rate, by culturing on XLT4-MOPS (xylose-lysine-tergitol 4 medium with morpholinepropanesulfonic acid) containing rifampin (100 μg/ml), showed that the attenuated strain was undetectable or at the lower limit of detection in the ceca of treated birds by week 5 (Fig. 1b).
FIG 1.
(a) Experimental design. A total of 273 1-day-old chicks were assigned to the following groups: control, prebiotics (galacto-oligosaccharides [GOS]), and attST. A total of 7 animals were sampled at 0, 7, 14, 21, and 28 days. After sampling on day 28, half of the remaining birds in each treatment group were challenged with the virulent Salmonella strain. There are 3 groups from 0 to 28 days (i.e., control, GOS, and attST) and 6 groups after challenging with the virulent Salmonella (i.e., control challenged, GOS challenged, and attST challenged). After the challenge, birds were sampled weekly (n = 7 birds) for up to 8 weeks (56 days). (b) Kinetics of clearance of the attST strain in the ceca of chickens in the treated group. Determination of the number of CFU was done by culturing.
Effect of attST and GOS treatments on Salmonella challenge.
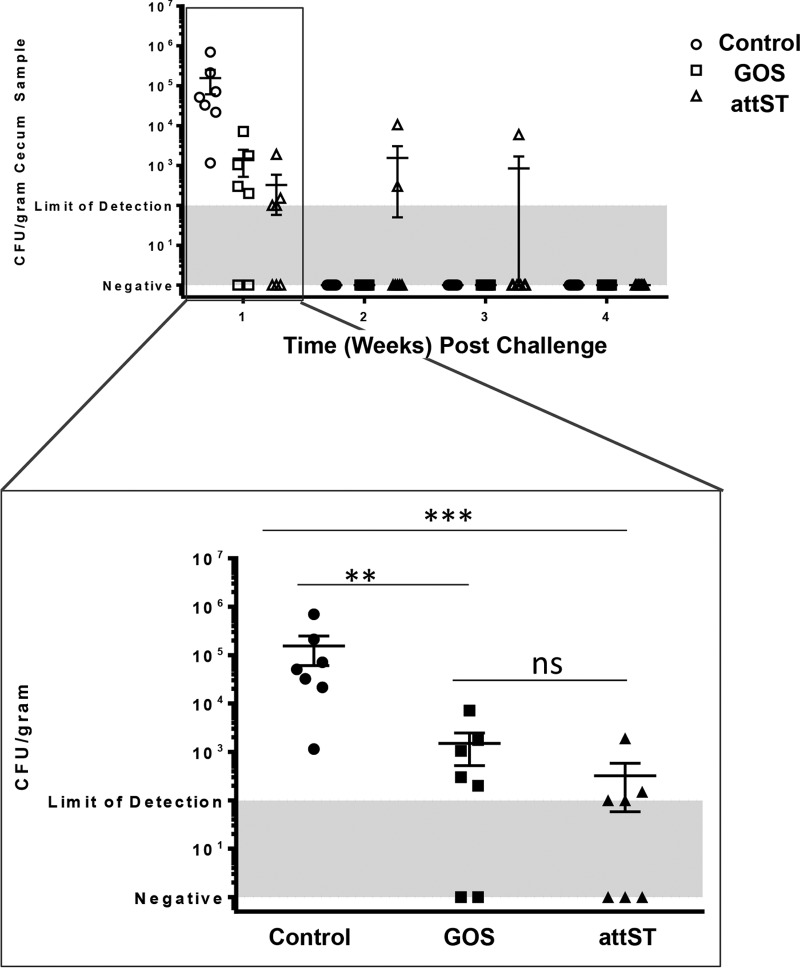
We hypothesized that the attST strain or prebiotic GOS would improve resistance to wild-type Salmonella via modifications to the structure of the chicken gut microbiome. To test our hypothesis, after 4 weeks of treatment, one-half of each group (attST, GOS, and control) was administered 1.7 × 109 CFU of Nalr Salmonella serovar 4,[5],12:r:− by oral gavage. Figure 2 shows that at 1 week postchallenge, the attST and GOS groups had reduced levels of the Salmonella serovar 4,[5],12:r:− (Nalr) compared to that in the control. By weeks 2 to 4 postchallenge, the challenge strain was undetectable in animals in all treatment groups.
FIG 2.
Clearance of the challenge Salmonella strain from the ceca of control, GOS-treated, and attST-treated animals during the 4-week period postchallenge. **, P < 0.01; ***, P < 0.001; ns, nonsignificant (P > 0.05).
Chicken gut microbiome.
We used 16S rRNA amplicon sequencing to determine the effects of the attST strain and prebiotic supplementation on the diversity of the microbial species in the intestinal tract of chickens, as well as the correlation between microbiome structural changes and resistance to Salmonella challenges. We first determined the compositions of the microbiomes of 273 cecum samples by 16S rRNA amplicon pyrosequencing targeting the V1–V2 region of the 16S rRNA gene. Pyrosequencing of amplicons resulted in a total of 2,355,189 reads after quality filtering, representing 5,810 operational taxonomic units (OTUs). Samples yielded 8,627 ± 3,295 reads on average.
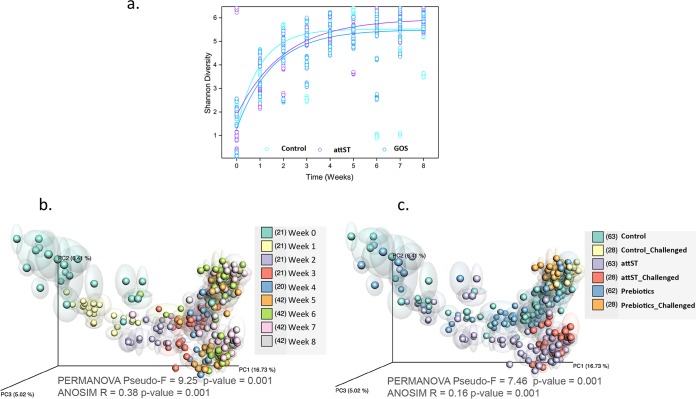
Confirming previous reports (21, 47), the early cecum microbiome was characterized by a low bacterial diversity (Fig. 3), which increased in all treatment groups until it reached a plateau at week 4. Accordingly, an unweighted UniFrac principal-coordinate analysis (PCoA) of samples showed statistically significant clustering of samples first by age (in weeks) (Fig. 3b) and then by treatment (Fig. 3c), with significant differences between the attST/attST-challenged group and the other groups: control/control challenged and GOS/GOS challenged. In our study, interindividual differences, i.e., differences between animals, were not statistically significant (analysis of similarities [ANOSIM], R = −0.015, P > 0.05).
FIG 3.
(a) Shannon diversity index of samples by time (weeks) and treatment, including all time points. (b and c) Unweighted UniFrac principal-coordinate analysis (PCoA) plot of chicken cecal samples colored by time (b) and treatment (c). PERMANOVA and ANOSIM statistics for each category are indicated. The numbers between brackets in the legends indicate the numbers of samples included in the analyses.
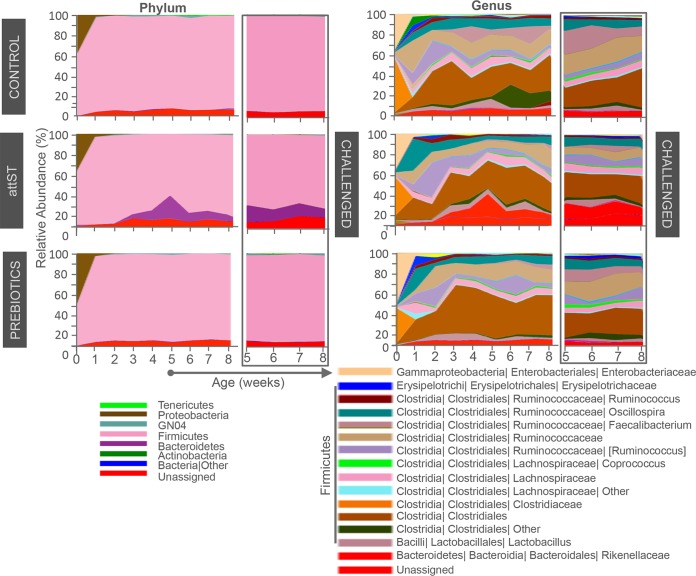
At the phylum level, nonchallenged groups were characterized by a relatively high abundance of Proteobacteria, from 38.4% in the control to 57.7% in the prebiotics group (Fig. 4), mostly of uncharacterized species of the family Enterobacteriaceae. The other main phylum characterizing the early cecum microbiome was Firmicutes of the family Clostridiaceae (uncharacterized). Both Enterobacteriaceae and Clostridiaceae declined at week one, with corresponding increases in the phylum Firmicutes and a significant proportion of unknown taxa. The main lineages within Firmicutes increasing from week 1 included Lactobacillus, Clostridiales_other, uncharacterized Clostridiales, uncharacterized Lachnospiraceae, Ruminococcus, uncharacterized Ruminococcaceae, Faecalibacterium, and Oscillospira. The main difference between groups was the noticeable spike in the abundance of uncharacterized Rikenellaceae (Bacteroidetes) in the attST-treated group at 5 weeks. A further BLAST analysis showed that the main contributor to this difference was an OTU assigned to the genus Alistipes, although no clear identification of species was made. The increased abundance of Rikenellaceae was inversely correlated with the clearance of the attST strain (Fig. 5). Additionally, the Clostridiales_other group was reduced in the GOS and the attST groups compared to that in the control at 6, 7, and 8 weeks.
FIG 4.
Relative abundance of phyla and genera over time in nonchallenged and challenged groups. For clarity, only taxa represented at >1% were included in the legends.
FIG 5.
Detection of the order Bacteroidales in the ceca of attST-treated birds in relation to the clearing of the attenuated strain.
Location and treatment, but not Salmonella challenge, had major impacts on the structure of the gut microbiome.
We next analyzed the structural changes to the gut microbiome induced by challenging the animals at 4 weeks with a wild-type strain of Salmonella serovar 4,[5],12:r:− (Nalr). The structure of the microbiome was determined by sequencing the V1–V2 region of the 16S rRNA genes of 432 samples from the cecum and three locations of the small intestine (duodenum, jejunum, and ileum) at weeks 5 through 8 using the Ion Torrent PGM sequencing platform, as outlined in Materials and Methods. Sequencing of the amplicons yielded 18,989,684 reads after quality filtering. The samples yielded 43,957 ± 27,354 reads per sample.
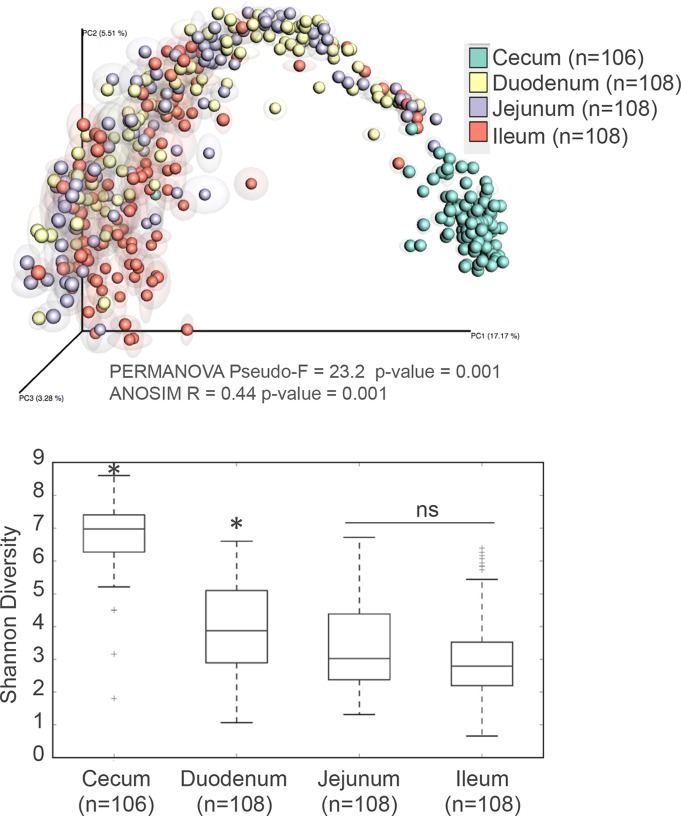
Our analysis revealed marked differences between the cecum and small intestine sections (ileum, duodenum, and jejunum) (Fig. 6, top), with the highest diversity and number of species found in the cecum (Fig. 6, bottom). Moreover, significant decreases in diversity were observed when progressing from the duodenum to the ileum. Supervised learning methods using even rarefied biological observation matrix (BIOM) tables were applied to identify highly discriminant OTUs (see Table S2 in the supplemental material). Regardless of treatment and time point, 3 Lactobacillus crispatus OTUs were discriminant between the duodenum and the cecum, being clearly more abundant in the duodenum. Conversely, 13 Clostridiales OTUs were significantly associated with the cecum. A further BLAST analysis putatively assigned those OTUs to the following taxa: Eisenbergiella tayi, Ihubacter massiliensis, Sporacetigenium, Romboutsia, Clostridium sphenoides, Clostridium indolis, Desulfotomaculum guttoideum, Ruminococcus, and Pseudoflavonifractor capillosus. Four OTUs within the phylum Proteobacteria were discriminant between the cecum and duodenum, being clearly overrepresented in the duodenum. These OTUs were designated Ralstonia pickettii (or Ralstonia insidiosa), a waterborne organism, and Janthinobacterium lividum, a soil-dwelling bacterium. Likewise, 10 OTUs of the order Streptophyta, most probably originating from the feed, were identified as more prevalent in the duodenum (Table S2).
FIG 6.
Effect of intestinal location on composition and diversity of the microbiome. (Top) PCoA analysis of samples with repeated resampling according to location. Only time points from week 5 and later (i.e., weeks 5 to 8) were included in this analysis. PERMANOVA and ANOSIM statistics are indicated in the figure. (Bottom) Shannon diversity indices of samples by location starting at week 5.
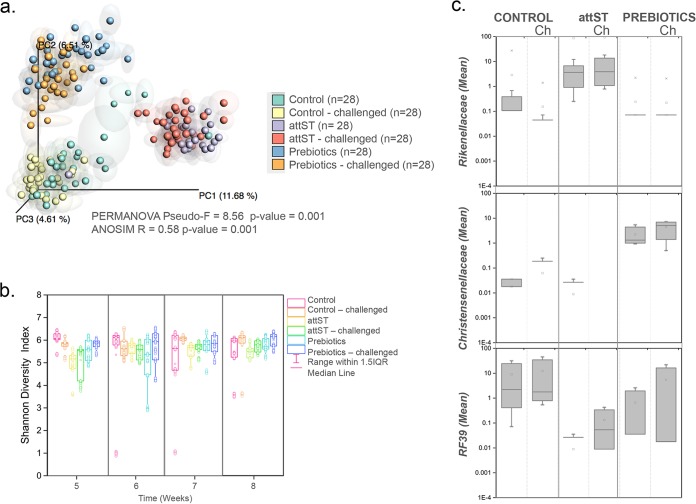
The PCoA plot depicted in Fig. 7 (top left) shows clear clustering by treatment in the cecum but not a noticeable impact of the Salmonella challenge on the different groups. Moreover, no significant differences (analysis of variance [ANOVA], P > 0.05) were observed in sample diversity between the groups (Fig. 7, bottom left). Data analysis showed that 18 defined taxonomic groups (not including unassigned reads) contained OTUs that were differentially represented in the sample groups formed by treatment and Salmonella challenge based on Kruskal-Wallis tests (see Table S3). A further exploration of the data by supervised learning methods using even rarefied BIOM tables confirmed the analysis by identifying lineages significantly discriminant between the groups (Table 1). The taxa with the highest discriminant power included Rikenellaceae (Bacteroidetes phylum), which was overrepresented in the attST-treated group, Christensenellaceae (Firmicutes phylum), overrepresented in the prebiotics (GOS) group, and RF39 (Tenericutes phylum), overrepresented in the control (Fig. 7, right). Also, the genus Faecalibacterium was discriminant between unchallenged and challenged subgroups (not shown).
FIG 7.
Effect of treatment on composition and diversity of the cecum microbiome. (Top left) PCoA analysis of samples with repeated resampling, colored according to treatment. Only time points from week 5 and later were included in this analysis. PERMANOVA and ANOSIM statistics are indicated in the figure. (Bottom left) Shannon diversity indices of samples by treatment starting at week 5. (Right) Highly discriminant bacterial taxa determined by random forest analysis differentially represented in the different treatments (Kruskal-Wallis test, Bonferroni's correction, P < 0.05).
TABLE 1.
Bacterial taxa differentially represented under different treatment conditionsa
| Lineageb | No. of OTUs | Mean count (SD) |
RFc importance score | |||||
|---|---|---|---|---|---|---|---|---|
| Control |
FNR |
Prebiotics |
||||||
| Unchallenged | Challenged | Unchallenged | Challenged | Unchallenged | Challenged | |||
| Bacteroidetes, Bacteroidia, Bacteroidales, Rikenellaceae | 10 | 2.88 (8.1) | 0.15 (0.4) | 86.01 (247.4) | 91.91 (263.2) | 0.24 (0.7) | 0.22 (0.6) | 0.06 |
| Firmicutes, Clostridia, Clostridiales, Christensenellaceae | 4 | 0.02 (0.02) | 0.06 (0.1) | 0.01 (0.02) | 0.00 (0.0) | 2.25 (2.1) | 4.48 (2.9) | 0.06 |
| Tenericutes, Mollicutes, RF39 | 4 | 8.99 (15.0) | 12.29 (21.9) | 0.01 (0.02) | 0.13 (0.2) | 0.66 (1.3) | 5.46 (10.9) | 0.03 |
| Firmicutes, Clostridia, Clostridiales, Ruminococcaceae, Faecalibacterium | 6 | 94.54 (117.0) | 209.77 (282.2) | 15.79 (16.4) | 29.71 (27.9) | 43.89 (46.3) | 147.97 (133.5) | 0.02 |
| Firmicutes, Clostridia, Clostridiales, Ruminococcaceae | 52 | 13.02 (37.7) | 27.34 (86.5) | 4.75 (14.8) | 4.51 (12.6) | 10.96 (31.8) | 19.25 (69.4) | 0.01 |
| Firmicutes, Clostridia, Clostridiales, Ruminococcaceae, Anaerotruncus | 1 | 11.46 | 15.50 | 7.32 | 5.89 | 4.68 | 2.79 | 0.01 |
| Firmicutes, Clostridia, Clostridiales | 61 | 18.16 (38.3) | 20.63 (42.9) | 29.77 (76.0) | 22.04 (64.0) | 35.32 (90.5) | 23.41 (45.3) | 0.01 |
| Firmicutes, Erysipelotrichi, Erysipelotrichales, Erysipelotrichaceae | 3 | 5.69 (3.8) | 7.87 (2.9) | 6.08 (4.6) | 7.35 (9.1) | 5.99 (6.2) | 26.70 (39.9) | 0.01 |
| Firmicutes, Clostridia, Clostridiales, Lachnospiraceae, Dorea | 3 | 1.45 (1.7) | 1.74 (0.9) | 0.10 (0.2) | 0.14 (0.2) | 0.51 (0.9) | 4.48 (4.8) | 0.01 |
| Firmicutes, Clostridia, Clostridiales, Lachnospiraceae, Blautia | 1 | 8.61 | 1.82 | 0.21 | 0.39 | 0.04 | 0.43 | 0.01 |
| Firmicutes, Clostridia, Clostridiales, Ruminococcaceae, Oscillospira | 10 | 21.76 (34.4) | 24.28 (35.2) | 7.80 (13.3) | 9.02 (11.4) | 12.18 (18.7) | 19.48 (26.7) | 0.01 |
| Firmicutes, Clostridia, Clostridiales, Lachnospiraceae, Coprococcus | 5 | 10.04 (17.9) | 18.12 (35.6) | 11.08 (16.9) | 11.26 (13.9) | 11.99 (14.3) | 36.88 (59.6) | 0.01 |
Differential representation determined by Kruskal-Wallis tests with Bonferroni's correction (P < 0.05). Only lineages considered to be highly discriminative by random forest analysis (importance score ≥0.01) are included in the table.
Listed by phylum, class, order, family (if available), and genus (if available).
RF, random forest.
DISCUSSION
Salmonella contaminations in poultry, and hence the potential for foodborne outbreaks, are highly prevalent due to the traditional processing practices that can dramatically impact the animal gut microbiome structure and function (48, 49). Our study aimed to determine the efficacy of a new attenuated Salmonella strain on Salmonella clearance after a challenge with a virulent strain of Salmonella and to evaluate its impact on the gut microbiota of poultry. We also determined how galacto-oligosaccharides (GOS) modified the structure of the gut microbiome and the impact of this modification on Salmonella infection clearance. We applied 16S rRNA amplicon sequencing to determine the effects of the attST strain and prebiotic supplementation on the diversity of the microbial species in the chicken intestinal tract and the potential association between microbiome diversity and resistance to Salmonella serovar 4,[5],12:r:− (Nalr) challenges.
As the new hatchling exchanges its nutrient source from the yolk for carbohydrates and proteins during the early posthatching period, the intestinal tract provides an ideal niche for microbial colonization. In our study, the first-week microbiome was characterized by a high abundance of uncharacterized species of the families Enterobacteriaceae (Proteobacteria) and Clostridiaceae (Firmicutes). Similar to our study, recent analyses (21, 50) reported that at day 1, animals had a dominance of Proteobacteria of the Enterobacteriaceae family and Firmicutes of the Enterococcaceae family. Also confirming previous reports (51), the cecum had the highest diversity, while the ileum had the lowest. Our analysis revealed that Lactobacillus crispatus was significantly overrepresented in the small intestine compared to the cecum regardless of treatment. L. crispatus could be considered a biomarker of a healthy gut. It has been isolated from the chicken gut and shown to competitively exclude Salmonella enterica serovar Enteritidis in vitro when tested in a coculture with Clostridium lactatifermentans (52), as well as Salmonella Typhimurium and Escherichia coli O157:H7 (53), in vitro. Other taxa significantly associated with the small intestine included the Proteobacteria Ralstonia pickettii (or Ralstonia insidiosa), a waterborne organism, and Janthinobacterium lividum, a soil-dwelling bacterium. Conversely, 13 Clostridiales taxa were significantly associated with the cecum, including Eisenbergiella tayi, Ihubacter massiliensis, Sporacetigenium, Romboutsia, Clostridium sphenoides, Clostridium indolis, Desulfotomaculum guttoideum, Ruminococcus, and Pseudoflavonifractor capillosus. The order Clostridiales comprises a heterogeneous group of genera characterized by their capacity to form endospores, their strictly anaerobic metabolism, and their inability to reduce sulfate to sulfite (54). Of the Clostridiales taxa listed above, Clostridium indolis has been described as having a high prevalence in the ceca of commercial Ross-hybrid broilers fed a vegetarian corn-soy broiler diet devoid of feed additives (31).
During the last century, the incorporation of growth promoters, including antibiotics, probiotics, and prebiotics, into the feed of productive animals has resulted in improvements to health conditions and a decrease of food production costs. However, several research studies have revealed concerning effects of subtherapeutic antibiotics in animal feed, leading to the search for nonantibiotic additives that improve animal health without contributing to the spread of bacterial antibiotic resistance genes. In our study, the early microbiome (until 4 weeks of age) was not impacted by treatment, the only difference being an increase in the Alistipes genus in the attST group. This genus was previously identified in the chicken gut microbiota (55, 56), although isolates have not been characterized and its role in the gut has not been clearly defined. From week 5, we observed a clear separation between samples from different treatments. Our study demonstrated that both the attST strain and GOS treatment modified the structure of the gut microbiome; however, the treatments elicited increases in different taxonomic groups. Importantly, the changes from both treatments resulted in a faster clearance after Salmonella infection (Fig. 2). Treatment with the attST strain resulted in a significant increase of Rikenellaceae, specifically of the genus Alistipes, and three OTUs of the Lactobacillus genus, among others, while GOS feeding was associated with increases of Christensenellaceae and Lactobacillus reuteri. The family Christensenellaceae has been associated with a healthy body mass index (BMI) (57) and shown to be enhanced by GOS in humans (35). Likewise, L. reuteri is a species containing numerous strains of recognized probiotic properties. Research has shown that L. reuteri administration in ovo singly or in combination with gentamicin followed by L. reuteri administration via drinking water or feed appeared to have the potential to control enteric pathogens in poultry (58). We also observed a trending increase of bifidobacteria, although changes in abundances did not reach statistical significance (data not shown). A previous study reported a 21-fold increase in Bifidobacterium in response to a diet containing a high concentration of GOS (3 kg per 25 kg; 12% GOS) and Bifidobacterium lactis in comparison to that of the control-fed birds (59). Of note is that while our study was under way, the sequencing primers used for 16S rRNA amplicon sequencing were reported to underrepresent the phylum Actinobacteria and, specifically, species of Bifidobacterium (60).
This study identified microorganisms impacted by attST (Alistipes, Lactobacillus) and by GOS (Christensenellaceae, L. reuteri) that could be regarded as beneficial in the acceleration of Salmonella clearance rates in poultry. GOS administration resulted in the expected enrichment of the species that can metabolize the β1-4 linkage available in the polysaccharide. Since Salmonella and the host lack the enzymes required for GOS utilization, one could appreciate the presence of Christensenellaceae and L. reuteri, which do possess such enzymes (61, 62), in GOS-treated animals. However, an elucidation of the mechanisms involved in the enrichment of Alistipes and Lactobacillus upon administration of attST will require further studies. A recent report demonstrated the use of a metabolically competent but attenuated strain of Salmonella as a probiotic to prevent Salmonella infection in mice (63). The authors suggested that the attenuated strain of Salmonella could compete with the virulent strain for the Salmonella-specific nutrients available in the gut. The attenuated strain used in this study elicited Salmonella-specific antibodies and provided protection against S. Typhimurium challenges in mice (our unpublished results); however, no antibody response was observed in chickens (unpublished results). Indeed, future mechanistic studies will be essential to determine if competitive exclusion mechanisms are responsible for the observed effect of the attST on accelerating the rate of Salmonella clearance in poultry.
MATERIALS AND METHODS
Animals and IACUC approval.
Day-old female commercial white leghorn chicks (W-36; Hy-Line North America, Mansfield, GA) were used in this study. The birds were housed in climate-controlled HEPA-filtered isolation units, 50 birds per isolator (934-1 WP; Federal Designs, Inc., Comer, GA). Water and feed were provided ad libitum. Each week, 7 animals from each group (control, prebiotic, and attST) were euthanized according to a protocol (15-065-A) approved by the Institutional Animal Care and Use Committee at NC State University (OLAW D16-00214) and sampled for gut microbiome and Salmonella analyses.
Bacterial strains and growth conditions.
A live attenuated strain of Salmonella enterica serovar Typhimurium (attST NC983; Rifr) derived from strain ATCC 14028s (15) was used in this study. The complete genome sequence of NC983 (16) and its efficacy and protection in mice against virulent S. Typhimurium have been established (unpublished data).
A nalidixic acid-resistant Salmonella enterica serovar 4,[5],12:r:− (Nalr) strain was used for challenging the birds. This strain was obtained from the laboratory of B. W. Sheldon (Poultry Science Department, NC State University); it was originally isolated from North Carolina commercial turkey farms. The antigenic formula for this strain, according to the Kauffmann-White classification scheme, was determined by the National Veterinary Service Laboratories, Ames, IA. The Salmonella strains {attST NC983 (Rifr) and the challenge strain, 4,[5],12:r:− (Nalr)} were grown statically for approximately 17 h (overnight) at 37°C in Luria-Bertani (LB) medium without antibiotics. The concentration of nalidixic acid used in this study was based on previous publications (64, 65).
Prebiotics.
Oligomate 55 from Yakult Pharmaceutical Industry (Japan) was used in this study. This product contains 55 to 56% GOS, and the remainder (44 to 45%) is monosaccharides and lactose. GOS was added to a standard poultry feed diet (see Table S1 in the supplemental material) at 1% (equivalent to 0.55% pure GOS).
Experimental design and sample collection.
A scheme of the experimental design is depicted in Fig. 1. A total of 300 1-day-old female commercial white leghorn chicks were used in this study. Upon arrival, 100 birds were assigned to each of the following treatment groups: control, prebiotics (GOS), and attenuated Salmonella (attST NC983). Each group was placed in two isolators (50 birds/isolator) and housed in separate animal biosafety level 2 (ABSL-2) rooms (i.e., separate rooms per treatment). All groups were sampled (7 birds per group) on day zero. Also, on day zero, birds in the attST group received 0.1 ml per chick of a phosphate-buffered saline (PBS)-washed cell suspension containing 8.6 × 109 CFU/ml by oral gavage (i.e., 8.6 × 108 CFU per bird). All groups were provided ad libitum water and feed; the prebiotic group received a standard diet supplemented with 0.55% GOS (i.e., 1% Oligomate 55), while the control and attST groups received a standard diet supplemented with 0.45% d-glucose to account for the monosaccharides present in Oligomate 55. Subsequently, all groups were sampled weekly (7 birds from each group) for 4 weeks. After week 4 samples were obtained, half of the remaining birds from each group were challenged with 1.7 × 109 CFU of Salmonella serovar 4,[5],12:r:− (Nalr) per bird via oral gavage in 0.1 ml. The other half of the birds in each treatment group received 0.1 ml of PBS.
After the challenge, samples (7 birds) were collected weekly (i.e., at weeks 5, 6, 7, and 8) from the different treatment groups and their corresponding challenged groups. In summary, samples were collected at the following time points: 0, 1, 2, 3, 4, 5, 6, 7, and 8 weeks. There were 3 groups from 0 to 4 weeks (i.e., control, GOS, and attST) and 6 groups from 5 to 8 weeks (i.e., control, control challenged, GOS, GOS challenged, attST, and attST challenged). Intestinal (cecum and small intestine) contents were collected in duplicate tubes for microbiome analyses and a single tube for bacteriological analysis.
Bacteriological analysis.
Samples collected for the enumeration of the attST (Rifr) strain and the 4,[5],12:r:− challenge strain (Nalr) were individually weighed and suspended at 100 mg/ml in PBS containing 25% glycerol and 2 mM MgSO4. The suspended samples were serially diluted and plated on XLT4 solid medium containing 100 mM MOPS (pH 7.4) and 100 μg/ml rifampin or 200 μg/ml nalidixic acid for the enumeration of attST or the challenge strain, respectively. The plates were incubated at 37°C for 24 h before colonies were counted. The data are reported as CFU/g of cecum content.
DNA isolation.
The isolation of total genomic DNA was carried out on a Qiagen BioRobot universal system (Qiagen, Valencia, CA) using an E.Z.N.A. stool DNA kit (Omega Bio-Tek, Norcross, GA) according to the manufacturer's instructions with bead-beating modifications. Briefly, 200 mg of intestinal content was added to a tube containing 540 μl of SLB buffer (Qiagen) and 200 mg of 100-μm glass beads (Sigma, St. Louis, MO). Samples were homogenized using a TissueLyser II (Qiagen, Germantown, MD) for 5 min at 30 Hz, and then 20 μl of proteinase K (Qiagen) was added according to the manufacturer's instructions. The mixture was then incubated at 70°C for 10 min, followed by another incubation at 95°C for 5 min. Subsequent purification steps were as described by the manufacturer, with the following modifications: 300 μl of the supernatant was then transferred to a new tube containing 300 μl of BL buffer (Qiagen) and 300 μl 100% ethanol. The elution step was performed twice in 25 μl of elution buffer at 65°C. The quality of isolated DNA was validated by agarose gel electrophoresis, and the purity verified using A260/A280 and A260/A230 ratios measured by a NanoDrop 1000 instrument (Thermo Fisher Scientific, Pittsburgh, PA). The DNA concentration was quantified using Quant-iT PicoGreen dsDNA reagent (Molecular Probes, Life Technologies division, Grand Island, NY).
Amplicon sequencing of the V1–V2 region of the 16S rRNA gene.
The initial amplification of the hypervariable V1–V2 region of the bacterial 16S rRNA gene was performed on total DNA from collected samples as previously described (66, 67). The reaction master mixes contained the Qiagen HotStar HiFidelity polymerase kit reagents (Qiagen, Valencia CA), with a forward primer composed of the Roche Titanium fusion primer A (5′-CCATCTCATCCCTGCGTGTCTCCGACTCAG-3′), a 10-bp multiplex identifier (MID) sequence (Roche, Indianapolis, IN) unique to each of the samples, and the universal bacterial primer 8F (5′-AGAGTTTGATCCTGGCTCAG-3′). The reverse primer was composed of the Roche Titanium primer B (5′-CCTATCCCCTGTGTGCCTTGGCAGTCTCAG-3′), the 10-bp MID sequence identical to that in the forward primer, and the reverse bacterial primer 338R (5′-GCTGCCTCCCGTAGGAGT-3′). The thermal profile for amplification had an initial denaturing step at 94°C for 5 min, followed by a cycling of denaturation at 94°C for 45 s, annealing at 50°C for 30 s, and a 1-min 30-s extension at 72°C (35 cycles), and a 10-min final extension at 72°C. Negative controls, not containing template, were amplified for all barcode-primer sets. Each sample was gel purified individually using the E-Gel electrophoresis system (Thermo Fisher Scientific, Life Technologies division, Grand Island, NY) and standardized prior to pooling. The 16S rRNA gene amplicons from the pooled sample were sequenced on a 454 genome sequencer FLX Titanium instrument (Roche, Indianapolis, IN) at the Microbiome Core Facility (University of North Carolina, Chapel Hill, NC) using the GS FLX Titanium XLR70 sequencing reagents and the corresponding protocol.
For the analysis of the structure of the gut microbiome from weeks 5 to 8 in different locations (cecum, duodenum, jejunum, and ileum), we transitioned to the Ion Torrent PGM sequencing platform from Life Sciences. Before performing the analysis, we compared the Ion Torrent PGM and Roche 454 GS FLX Titanium platforms with standard and modified protocols for library preparation and showed that while there were differences in the depth of coverage and phylogenetic diversity, all workflows demonstrated comparable treatment effects on microbial diversity. Moreover, the platforms compared were able to discriminate samples by treatment, despite differences in diversity and abundance, leading to similar biological conclusions (68). PGM sequencing libraries were prepared from total genomic DNA by adding the adapter sequences during PCR using a fusion primer method for amplification of the V1–V2 hypervariable region of the 16S rRNA gene (66, 67). The forward primer was composed of the Ion Torrent adapter, a 10-bp Ion Xpress barcode unique to each sample (Thermo Fisher Scientific, Life Technologies division, Grand Island, NY), and the universal bacterial primer 8F. The reverse primer consisted of the Ion Torrent trP1 adapter followed by the reverse bacterial primer 338R.
The complete primer sequences were 8F, 5′-CCATCTCATCCCTGCGTGTCTCCGACTCAGNNNNNNNNNNAGAGTTTGATCCTGGCTCAG-3′; and 338R, 5′-CCTCTCTATGGGCAGTCGGTGATGCTGCCTCCCGTAGGAGT; where NNNNNNNNNN is the Ion Xpress barcode sequence. The PCR mixtures contained 50 ng of DNA template, 2.5 units of HotStar HiFidelity DNA polymerase (Qiagen, Valencia, CA), 1× HotStar HiFidelity PCR buffer containing deoxynucleoside triphosphates (dNTPs), and 0.6 μM each primer. The reaction conditions consisted of an initial denaturation for 5 min at 94°C followed by 35 cycles of denaturing at 94°C for 60 s, annealing at 57°C for 60 s, and extension at 72°C for 60 s, with a final extension of 72°C for 10 min. All libraries were purified, mixed at equimolar concentrations, and clonally amplified onto the proprietary Ion Sphere particles. Clonal amplification was accomplished by emulsion PCR using an Ion PGM template OT2 400 kit (Life Technologies) according to the manufacturer's instructions. The prepared template was sequenced on the Ion Torrent PGM instrument (Life Technologies) using the Ion PGM 400 sequencing reagents. The initial data analysis, base pair calling, and trimming of each sequence were performed on the Ion Torrent browser to yield high-quality reads.
Sequencing data analysis.
Roche 454 sequencing results were initially processed using a GS data analysis software package (69). Demultiplexing and quality filtering were performed on the joined results. MID and linker primer sequences were trimmed, and the reads were subsequently filtered for quality. The quality control of both raw and processed sequencing reads was verified by FastQC (70). For Roche 454 and PGM sequencing data, the sequences were clustered into OTUs based on the de novo OTU picking algorithm using the QIIME 1.8.0 (71) implementation of UCLUST (72) at a similarity threshold of 97%. OTUs identified as chimeric by ChimeraSlayer (73) and those composed of a single read (singletons) were eliminated. The remaining OTUs were assigned taxonomic identifiers with respect to the Greengenes database (74), their sequences were aligned using template alignment through PyNAST (75), and a phylogenetic tree was built with FastTree 2.1.3 (76).
The phylogenetic diversity whole tree, Shannon index, Chao1, and observed species number metrics were estimated using QIIME at a rarefaction depth of 2,265 sequences per sample for Roche 454 data and 4,487 sequences per sample for PGM data. Beta diversity estimates were calculated within QIIME using weighted and unweighted UniFrac distances (77). The results were summarized and visualized with a principal-coordinate analysis as implemented in QIIME. Supervised classification with the random forest classifier was done using the QIIME script supervised_learning.py. We ran 10-fold cross-validation on a directory of OTU tables rarefied at even depths to obtain more robust estimates of the generalization error and feature importance (including standard deviations). We then produced a single file of results that contained the average estimated generalization error of the classifications and the pooled standard deviation. The baseline error for random guessing was 80%.
Statistical analysis.
A one-way ANOVA using Holm-Sidak's multiple comparisons (GraphPad Prism 7.03) was used to evaluate the clearance of Salmonella serovar 4,[5],12:r:− from the chicken cecum at 6 days postchallenge (dpc). To evaluate similarities or dissimilarities between the groups, we computed the distance matrix between OTUs using an analysis of similarities (ANOSIM) and permutational multivariate analysis of variance (PERMANOVA) within QIIME. ANOVAs with pairwise comparisons were used to identify significant differences in alpha diversity between the different time points, while Kruskal-Wallis tests with Bonferroni's correction for multiple comparisons were used to identify significant differences in bacterial taxa between groups. P values of less than 0.05 were considered significant unless otherwise stated.
Supplementary Material
ACKNOWLEDGMENTS
Funding was provided by USDA-NIFA 2012-68003-19621. The Microbiome Core Facility is supported in part by NIH/National Institute of Diabetes and Digestive and Kidney Diseases grant P30 DK34987.
The funding agencies had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02526-17.
REFERENCES
- 1.Basler C, Nguyen TA, Anderson TC, Hancock T, Behravesh CB. 2016. Outbreaks of human Salmonella infections associated with live poultry, United States, 1990–2014. Emerg Infect Dis 22:1705–1711. doi: 10.3201/eid2210.150765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Glass K, Liu B, Hope K, Kirk M. 2016. Salmonella infection in middle-aged and older adults: incidence and risk factors from the 45 and up study. Foodborne Pathog Dis 13:689–694. doi: 10.1089/fpd.2016.2170. [DOI] [PubMed] [Google Scholar]
- 3.Gieraltowski L, Higa J, Peralta V, Green A, Schwensohn C, Rosen H, Libby T, Kissler B, Marsden-Haug N, Booth H, Kimura A, Grass J, Bicknese A, Tolar B, Defibaugh-Chavez S, Williams I, Wise M, Salmonella Heidelberg Investigation Team. 2016. National outbreak of multidrug resistant Salmonella Heidelberg infections linked to a single poultry company. PLoS One 11:e0162369. doi: 10.1371/journal.pone.0162369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley SL, Lynne AM. 2008. Food animal-associated Salmonella challenges: pathogenicity and antimicrobial resistance. J Anim Sci 86:E173–E187. doi: 10.2527/jas.2007-0447. [DOI] [PubMed] [Google Scholar]
- 5.Foley SL, Lynne AM, Nayak R. 2008. Salmonella challenges: prevalence in swine and poultry and potential pathogenicity of such isolates. J Anim Sci 86:E149–E162. doi: 10.2527/jas.2007-0464. [DOI] [PubMed] [Google Scholar]
- 6.Altekruse SF, Bauer N, Chanlongbutra A, DeSagun R, Naugle A, Schlosser W, Umholtz R, White P. 2006. Salmonella Enteritidis in broiler chickens, United States, 2000–2005. Emerg Infect Dis 12:1848–1852. doi: 10.3201/eid1212.060653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boore AL, Hoekstra RM, Iwamoto M, Fields PI, Bishop RD, Swerdlow DL. 2015. Salmonella enterica infections in the United States and assessment of coefficients of variation: a novel approach to identify epidemiologic characteristics of individual serotypes, 1996–2011. PLoS One 10:e0145416. doi: 10.1371/journal.pone.0145416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallis TS, Galyov EE. 2000. Molecular basis of Salmonella-induced enteritis. Mol Microbiol 36:997–1005. doi: 10.1046/j.1365-2958.2000.01892.x. [DOI] [PubMed] [Google Scholar]
- 9.Morgan E, Campbell JD, Rowe SC, Bispham J, Stevens MP, Bowen AJ, Barrow PA, Maskell DJ, Wallis TS. 2004. Identification of host-specific colonization factors of Salmonella enterica serovar Typhimurium. Mol Microbiol 54:994–1010. doi: 10.1111/j.1365-2958.2004.04323.x. [DOI] [PubMed] [Google Scholar]
- 10.Galán JE. 2001. Salmonella interactions with host cells: type III secretion at work. Annu Rev Cell Dev Biol 17:53–86. doi: 10.1146/annurev.cellbio.17.1.53. [DOI] [PubMed] [Google Scholar]
- 11.Desin TS, Koster W, Potter AA. 2013. Salmonella vaccines in poultry: past, present and future. Expert Rev Vaccines 12:87–96. doi: 10.1586/erv.12.138. [DOI] [PubMed] [Google Scholar]
- 12.Kogut MH, Arsenault RJ. 2017. Immunometabolic phenotype alterations associated with the induction of disease tolerance and persistent asymptomatic infection of Salmonella in the chicken intestine. Front Immunol 8:372. doi: 10.3389/fimmu.2017.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Methner U, Haase A, Berndt A, Martin G, Nagy B, Barrow PA. 2011. Exploitation of intestinal colonization-inhibition between Salmonella organisms for live vaccines in poultry: potential and limitations. Zoonoses Public Health 58:540–548. doi: 10.1111/j.1863-2378.2011.01400.x. [DOI] [PubMed] [Google Scholar]
- 14.Braukmann M, Barrow PA, Berndt A, Methner U. 2016. Combination of competitive exclusion and immunisation with a live Salmonella vaccine in newly hatched chickens: immunological and microbiological effects. Res Vet Sci 107:34–41. doi: 10.1016/j.rvsc.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Fink RC, Evans MR, Porwollik S, Vazquez-Torres A, Jones-Carson J, Troxell B, Libby SJ, McClelland M, Hassan HM. 2007. FNR is a global regulator of virulence and anaerobic metabolism in Salmonella enterica serovar Typhimurium (ATCC 14028s). J Bacteriol 189:2262–2273. doi: 10.1128/JB.00726-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Troxell B, Fink RC, Dickey AN, Scholl EH, Hassan HM. 2016. Complete genome sequence of NC983, a live attenuated strain of Salmonella enterica serovar Typhimurium. Genome Announc 4:e01074-. doi: 10.1128/genomeA.01074-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang Y, Weber KD, Qiu Y, Kiley PJ, Blattner FR. 2005. Genome-wide expression analysis indicates that FNR of Escherichia coli K-12 regulates a large number of genes of unknown function. J Bacteriol 187:1135–1160. doi: 10.1128/JB.187.3.1135-1160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawley TD, Bouley DM, Hoy YE, Gerke C, Relman DA, Monack DM. 2008. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect Immun 76:403–416. doi: 10.1128/IAI.01189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gopinath S, Carden S, Monack D. 2012. Shedding light on Salmonella carriers. Trends Microbiol 20:320–327. doi: 10.1016/j.tim.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Pedroso AA, Hurley-Bacon AL, Zedek AS, Kwan TW, Jordan AP, Avellaneda G, Hofacre CL, Oakley BB, Collett SR, Maurer JJ, Lee MD. 2013. Can probiotics improve the environmental microbiome and resistome of commercial poultry production? Int J Environ Res Public Health 10:4534–4559. doi: 10.3390/ijerph10104534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballou AL, Ali RA, Mendoza MA, Ellis JC, Hassan HM, Croom WJ, Koci MD. 2016. Development of the chick microbiome: how early exposure influences future microbial diversity. Front Vet Sci 3:2. doi: 10.3389/fvets.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macpherson AJ, Harris NL. 2004. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol 4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 23.Kosiewicz MM, Zirnheld AL, Alard P. 2011. Gut microbiota, immunity, and disease: a complex relationship. Front Microbiol 2:180. doi: 10.3389/fmicb.2011.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng T, Elson CO. 2011. Adaptive immunity in the host-microbiota dialog. Mucosal Immunol 4:15–21. doi: 10.1038/mi.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atarashi K. 2011. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stecher B, Hardt WD. 2011. Mechanisms controlling pathogen colonization of the gut. Curr Opin Microbiol 14:82–91. doi: 10.1016/j.mib.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Stelter C, Kappeli R, Konig C, Krah A, Hardt WD, Stecher B, Bumann D. 2011. Salmonella-induced mucosal lectin RegIIIbeta kills competing gut microbiota. PLoS One 6:e20749. doi: 10.1371/journal.pone.0020749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gill N, Finlay BB. 2011. The gut microbiota: challenging immunology. Nat Rev Immunol 11:636–637. doi: 10.1038/nri3061. [DOI] [PubMed] [Google Scholar]
- 29.Nordentoft S, Molbak L, Bjerrum L, De Vylder J, Van Immerseel F, Pedersen K. 2011. The influence of the cage system and colonisation of Salmonella Enteritidis on the microbial gut flora of laying hens studied by T-RFLP and 454 pyrosequencing. BMC Microbiol 11:187. doi: 10.1186/1471-2180-11-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjerrum L, Engberg RM, Leser TD, Jensen BB, Finster K, Pedersen K. 2006. Microbial community composition of the ileum and cecum of broiler chickens as revealed by molecular and culture-based techniques. Poult Sci 85:1151–1164. doi: 10.1093/ps/85.7.1151. [DOI] [PubMed] [Google Scholar]
- 31.Lu J, Idris U, Harmon B, Hofacre C, Maurer JJ, Lee MD. 2003. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl Environ Microbiol 69:6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cummings JH. 1981. Short-chain fatty acids in the human colon. Gut 22:763–779. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rastall RA, Gibson GR, Gill HS, Guarner F, Klaenhammer TR, Pot B, Reid G, Rowland IR, Sanders ME. 2005. Modulation of the microbial ecology of the human colon by probiotics, prebiotics and synbiotics to enhance human health: an overview of enabling science and potential applications. FEMS Microbiol Ecol 52:145–152. doi: 10.1016/j.femsec.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, Brown D, Stares MD, Scott P, Bergerat A, Louis P, McIntosh F, Johnstone AM, Lobley GE, Parkhill J, Flint HJ. 2011. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J 5:220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azcarate-Peril MA, Ritter AJ, Savaiano D, Monteagudo-Mera A, Anderson C, Magness ST, Klaenhammer TR. 2017. Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proc Natl Acad Sci U S A 114:E367–E375. doi: 10.1073/pnas.1606722113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monteagudo-Mera A, Arthur JC, Jobin C, Keku TO, Bruno Barcena JM, Azcarate-Peril MA. 2016. High purity galacto-oligosaccharides enhance specific Bifidobacterium species and their metabolic activity in the mouse gut microbiome. Benef Microbes 7:247–264. doi: 10.3920/BM2015.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patterson JA, Burkholder KM. 2003. Application of prebiotics and probiotics in poultry production. Poult Sci 82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- 38.Takemura N, Ozawa K, Kimura N, Watanabe J, Sonoyama K. 2010. Inulin-type fructans stimulated the growth of exogenously administered Lactobacillus plantarum no. 14 in the mouse gastrointestinal tract. Biosci Biotechnol Biochem 74:375–381. doi: 10.1271/bbb.90794. [DOI] [PubMed] [Google Scholar]
- 39.Davis LM, Martinez I, Walter J, Goin C, Hutkins RW. 2011. Barcoded pyrosequencing reveals that consumption of galactooligosaccharides results in a highly specific bifidogenic response in humans. PLoS One 6:e25200. doi: 10.1371/journal.pone.0025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shoaf K, Mulvey GL, Armstrong GD, Hutkins RW. 2006. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect Immun 74:6920–6928. doi: 10.1128/IAI.01030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tzortzis G, Goulas AK, Gee JM, Gibson GR. 2005. A novel galactooligosaccharide mixture increases the bifidobacterial population numbers in a continuous in vitro fermentation system and in the proximal colonic contents of pigs in vivo. J Nutr 135:1726–1731. [DOI] [PubMed] [Google Scholar]
- 42.Searle LE, Best A, Nunez A, Salguero FJ, Johnson L, Weyer U, Dugdale AH, Cooley WA, Carter B, Jones G, Tzortzis G, Woodward MJ, La Ragione RM. 2009. A mixture containing galactooligosaccharide, produced by the enzymic activity of Bifidobacterium bifidum, reduces Salmonella enterica serovar Typhimurium infection in mice. J Med Microbiol 58:37–48. doi: 10.1099/jmm.0.004390-0. [DOI] [PubMed] [Google Scholar]
- 43.Quintero M, Maldonado M, Perez-Munoz M, Jimenez R, Fangman T, Rupnow J, Wittke A, Russell M, Hutkins R. 2011. Adherence inhibition of Cronobacter sakazakii to intestinal epithelial cells by prebiotic oligosaccharides. Curr Microbiol 62:1448–1454. doi: 10.1007/s00284-011-9882-8. [DOI] [PubMed] [Google Scholar]
- 44.Sarabia-Sainz HM, Armenta-Ruiz C, Sarabia-Sainz JA, Guzman-Partida AM, Ledesma-Osuna AI, Vazquez-Moreno L, Ramos-Clamont Montfort G. 2013. Adhesion of enterotoxigenic Escherichia coli strains to neoglycans synthesised with prebiotic galactooligosaccharides. Food Chem 141:2727–2734. doi: 10.1016/j.foodchem.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 45.Fukata T, Sasai K, Miyamoto T, Baba E. 1999. Inhibitory effects of competitive exclusion and fructooligosaccharide, singly and in combination, on Salmonella colonization of chicks. J Food Prot 62:229–233. doi: 10.4315/0362-028X-62.3.229. [DOI] [PubMed] [Google Scholar]
- 46.Baurhoo B, Letellier A, Zhao X, Ruiz-Feria CA. 2007. Cecal populations of lactobacilli and bifidobacteria and Escherichia coli populations after in vivo Escherichia coli challenge in birds fed diets with purified lignin or mannanoligosaccharides. Poult Sci 86:2509–2516. doi: 10.3382/ps.2007-00136. [DOI] [PubMed] [Google Scholar]
- 47.Simon K, Verwoolde MB, Zhang J, Smidt H, de Vries Reilingh G, Kemp B, Lammers A. 2016. Long-term effects of early life microbiota disturbance on adaptive immunity in laying hens. Poult Sci 95:1543–1554. doi: 10.3382/ps/pew088. [DOI] [PubMed] [Google Scholar]
- 48.Logue CM, Sherwood JS, Olah PA, Elijah LM, Dockter MR. 2003. The incidence of antimicrobial-resistant Salmonella spp. on freshly processed poultry from U.S. midwestern processing plants. J Appl Microbiol 94:16–24. doi: 10.1046/j.1365-2672.2003.01815.x. [DOI] [PubMed] [Google Scholar]
- 49.Rajan K, Shi Z, Ricke SC. 2017. Current aspects of Salmonella contamination in the U.S. poultry production chain and the potential application of risk strategies in understanding emerging hazards. Crit Rev Microbiol 43:370–392. doi: 10.1080/1040841X.2016.1223600. [DOI] [PubMed] [Google Scholar]
- 50.Schokker D, Jansman AJ, Veninga G, de Bruin N, Vastenhouw SA, de Bree FM, Bossers A, Rebel JM, Smits MA. 2017. Perturbation of microbiota in one-day-old broiler chickens with antibiotic for 24 hours negatively affects intestinal immune development. BMC Genomics 18:241. doi: 10.1186/s12864-017-3625-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sergeant MJ, Constantinidou C, Cogan TA, Bedford MR, Penn CW, Pallen MJ. 2014. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS One 9:e91941. doi: 10.1371/journal.pone.0091941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Wielen PW, Lipman LJ, van Knapen F, Biesterveld S. 2002. Competitive exclusion of Salmonella enterica serovar Enteritidis by Lactobacillus crispatus and Clostridium lactatifermentans in a sequencing fed-batch culture. Appl Environ Microbiol 68:555–559. doi: 10.1128/AEM.68.2.555-559.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Xu J, Shuai J, Chen J, Zhang Z, Fang W. 2007. The S-layer proteins of Lactobacillus crispatus strain ZJ001 is responsible for competitive exclusion against Escherichia coli O157:H7 and Salmonella Typhimurium. Int J Food Microbiol 115:307–312. doi: 10.1016/j.ijfoodmicro.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Gottschalk G, Andreesen JR, Hippe H. 1981. The genus Clostridium (nonmedical aspects), p 1767–1803. In Starr MP, Stolp H, Truper HG, Balows A (ed), The prokaryotes, vol 2 Springer, Berlin, Germany. [Google Scholar]
- 55.Polansky O, Sekelova Z, Faldynova M, Sebkova A, Sisak F, Rychlik I. 2015. Important metabolic pathways and biological processes expressed by chicken cecal microbiota. Appl Environ Microbiol 82:1569–1576. doi: 10.1128/AEM.03473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torok VA, Hughes RJ, Mikkelsen LL, Perez-Maldonado R, Balding K, MacAlpine R, Percy NJ, Ophel-Keller K. 2011. Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials. Appl Environ Microbiol 77:5868–5878. doi: 10.1128/AEM.00165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, Ley RE. 2014. Human genetics shape the gut microbiome. Cell 159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edens FW, Parkhurst CR, Casas IA, Dobrogosz WJ. 1997. Principles of ex ovo competitive exclusion and in ovo administration of Lactobacillus reuteri. Poult Sci 76:179–196. doi: 10.1093/ps/76.1.179. [DOI] [PubMed] [Google Scholar]
- 59.Jung SJ, Houde R, Baurhoo B, Zhao X, Lee BH. 2008. Effects of galacto-oligosaccharides and a Bifidobacteria lactis-based probiotic strain on the growth performance and fecal microflora of broiler chickens. Poult Sci 87:1694–1699. doi: 10.3382/ps.2007-00489. [DOI] [PubMed] [Google Scholar]
- 60.Sim K, Cox MJ, Wopereis H, Martin R, Knol J, Li MS, Cookson WO, Moffatt MF, Kroll JS. 2012. Improved detection of bifidobacteria with optimised 16S rRNA gene-based pyrosequencing. PLoS One 7:e32543. doi: 10.1371/journal.pone.0032543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morotomi M, Nagai F, Watanabe Y. 2012. Description of Christensenella minuta gen. nov., sp. nov., isolated from human faeces, which forms a distinct branch in the order Clostridiales, and proposal of Christensenellaceae fam. nov. Int J Syst Evol Microbiol 62:144–149. doi: 10.1099/ijs.0.026989-0. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen TH, Splechtna B, Steinbock M, Kneifel W, Lettner HP, Kulbe KD, Haltrich D. 2006. Purification and characterization of two novel beta-galactosidases from Lactobacillus reuteri. J Agric Food Chem 54:4989–4998. doi: 10.1021/jf053126u. [DOI] [PubMed] [Google Scholar]
- 63.Sabag-Daigle A, Blunk HM, Gonzalez JF, Steidley BL, Boyaka PN, Ahmer BM. 2016. Use of attenuated but metabolically competent Salmonella as a probiotic to prevent or treat Salmonella infection. Infect Immun 84:2131–2140. doi: 10.1128/IAI.00250-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Natrajan N, Sheldon BW. 2000. Efficacy of nisin-coated polymer films to inactivate Salmonella Typhimurium on fresh broiler skin. J Food Prot 63:1189–1196. doi: 10.4315/0362-028X-63.9.1189. [DOI] [PubMed] [Google Scholar]
- 65.Yang X, Brisbin J, Yu H, Wang Q, Yin F, Zhang Y, Sabour P, Sharif S, Gong J. 2014. Selected lactic acid-producing bacterial isolates with the capacity to reduce Salmonella translocation and virulence gene expression in chickens. PLoS One 9:e93022. doi: 10.1371/journal.pone.0093022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Edwards U, Rogall T, Blocker H, Emde M, Bottger EC. 1989. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res 17:7843–7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fierer N, Hamady M, Lauber CL, Knight R. 2008. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc Natl Acad Sci U S A 105:17994–17999. doi: 10.1073/pnas.0807920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allali I, Arnold JW, Roach J, Cadenas MB, Butz N, Hassan HM, Koci M, Ballou A, Mendoza M, Ali R, Azcarate-Peril MA. 2017. A comparison of sequencing platforms and bioinformatics pipelines for compositional analysis of the gut microbiome. BMC Microbiol 17:194. doi: 10.1186/s12866-017-1101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anonymous. GS data analysis software. Roche Applied Science, Indianapolis, IN. [Google Scholar]
- 70.Anonymous. 2014. FastQC 0.11.2. Babraham Institute, Cambridge, UK. [Google Scholar]
- 71.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 73.Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, Ciulla D, Tabbaa D, Highlander SK, Sodergren E, Methe B, DeSantis TZ, Human Microbiome Consortium, Petrosino JF, Knight R, Birren BW. 2011. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res 21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. 2010. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Price MN, Dehal PS, Arkin AP. 2010. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lozupone C, Knight R. 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.