Abstract
Oil spill responders require information on the absolute and relative toxicities of chemical dispersants to relevant receptor species to assess their use in spill response. However, little toxicity data are available for tropical marine species including reef-building corals. In this study, we experimentally assessed the sub-lethal toxicity of five dispersants to larvae of the coral Acropora millepora over three short exposure periods (2, 6 and 24 h) reflecting real-world spill response scenario durations. Inhibition of larval settlement increased rapidly between 2 and 6 h, and was highest at 24 h: EC50 Corexit EC9500A = 4.0 mg l−1; Ardrox 6120 = 4.0 mg l−1; Slickgone LTSW = 2.6 mg L−1; Slickgone NS = 11.1 mg L−1 and Finasol OSR52 = 3.4 mg L−1. Coral larvae were more sensitive to dispersants than most other coral life stages and marine taxa, but the toxic thresholds (EC10s) exceeded most realistic environmental dispersant concentrations. Estimating toxic threshold values for effects of dispersants on coral should benefit the decision-making of oil spill responders by contributing to the development of species sensitivity distributions (SSDs) for dispersant toxicity, and by informing net environmental benefit assessment (NEBA) for dispersant use.
Introduction
Chemical dispersants are used to reduce the impacts of petroleum oil and fuel spills by changing the chemical and physical properties of a surface slick1. Typically applied by air or surface vessels, dispersants enhance the mixing of oil into seawater by promoting oil solubility and dilution by forming smaller (less buoyant) oil droplets that become suspended or entrained in the water column. Ultimately the aim is to minimise the impacts of oil slicks on sea life at the water’s surface and the intertidal zone, and to enhance biodegradation1–3. The greatest application of dispersants to date was in response to the 2010 Deepwater Horizon wellhead blowout in the Gulf of Mexico, where almost 7 million litres of Corexit EC9500A and Corexit EC9527A were used to disperse slicks both at the surface and by injection at the wellhead, 1500 m below the surface4. The influence of this response on the ecological outcomes for shoreline and deep water ecosystems is debated3,5, but clearly more studies are needed to test the responses of sensitive biota to oil, dispersed oil and dispersants6,7.
Oil spill dispersants usually contain a combination of anionic or non-ionic surfactants with solvents such as glycol ethers and low-aromatic kerosene8. Surfactants are critical for enhancing slick dispersal, but they are also considered the more toxic component of dispersant formulations9. Surfactants impact organisms in a variety of ways but all are capable of denaturing and binding to proteins and altering the permeability of cell membranes10. Contemporary (third generation) dispersants are less toxic than earlier formulations which often contained higher proportions of surfactants or kerosene9 and are broadly considered less toxic than aromatic hydrocarbons, the primary contributor to the toxicity of oil and fuel spills1,11. The potential hazard posed by each dispersant will depend on the combined effects of the components in each formulation to marine species and this hazard is likely to be species-specific12,13.
Chemical dispersal changes the bioavailability of petroleum contaminants in the water column and on the sea floor, in some cases increasing risks to species occupying these habitats1,14. It is therefore generally recommended to use dispersants when: (i) the oil is physically and chemically “dispersible” (soon after a spill to maximise efficacy); (ii) the environmental conditions (i.e. depth, currents, and wave energy for mixing) are conducive and (iii) when the application is likely to reduce impacts to surface and shore species, taking into account any subsequent impacts to water column and benthic habitats1. Specific guidelines for dispersant use can vary widely; however, there are many situations where the net environmental benefits of chemical dispersion are not clear15,16. For example, a slick in close proximity to both a coral reef and a mangrove habitat may present the need to choose between the risks posed by a predominantly floating slick (which may persist in a mangrove system for many years) and dispersant use that increases the immediate, acute exposure of the sub-surface corals17,18. Resolving this issue requires information on the expected exposure type and duration as well as the hazards posed by oil, chemically dispersed oil and the dispersants themselves. If chemical dispersal of a spill is considered to provide a net environmental benefit, then the comparative efficacy, cost and potential toxicity of dispersants to sensitive “receptor” species should also be considered19.
Oil and gas extraction often occurs near coral reefs in the Middle East, throughout South East Asia, in the Caribbean and across north-western Australia and the Timor Sea, and shipping traffic continues to increase in close proximity to coral reefs, including the Great Barrier Reef20. While these activities continue, there is always a low likelihood but potentially catastrophic risk posed by uncontrolled spills to tropical coral reefs. It is generally not recommended to apply dispersants in shallow environments or near coral reefs1,17; however, dispersants were employed to reduce the surface slicks during the grounding of the Shen Neng 1 on a reef in the Great Barrier Reef Marine Park21,22. A range of six different dispersants (totalling 184 m3)23,24 were also applied in response to the estimated 4700 m3 medium crude spill from the Montara well head platform off tropical north Western Australia to minimise the risks of surface slicks reaching coral reefs23. The toxicity of oil and/or chemically dispersed oil to corals has been summarised and reviewed recently15,25,26. Although it is difficult to compare toxicities between studies due to inconsistencies in methodologies and toxicity metrics, in general, early life phases of corals are more sensitive to hydrocarbons and dispersants than adults. Table 1 summarises the toxicity of dispersants alone to various life phases of coral available from eight publications since 2000. While many of the dispersant formulations were not toxic to adult coral branches at concentrations of 10 mg l−1, concentrations below this often affected the survival and settlement of larvae for some species. The Australian National Plan benchmark for acceptable toxicity is based on a multiple day exposure to an EC50 or equivalent, depending on species and test27.
Table 1.
Summary of published dispersant toxicity studies to different life stages of corals since 2000. EC50 where 50% organisms are affected. NOEC = no observed effect concentration. LOEC = lowest observed effect concentration. NA = affected at the lowest concentration tested.
| Dispersant | Species | Life stage | Endpoint | Reported toxicities (mg/L) | Reference |
|---|---|---|---|---|---|
| Corexit EC9500A | Porites astreoides | Larvae | Settlement 48 h | NA (NOEC), 25 (LOEC) | 58 |
| Porites astreoides | Mortality 72 h | 25 (NOEC), 50 (LOEC) | 58 | ||
| Montastraea faveolata | Settlement 48 h | NA (NOEC), 25 (LOEC) | 58 | ||
| Montastraea faveolata | Mortality 72 h | NA (NOEC), 25 (LOEC) | 58 | ||
| Xenia elongata a | Adult | Bleaching 24 & 72 h | NA (NOEC), 5 (LOEC) | 34 | |
| Swiftia exserta a | Healthb 48 h | 64 (LC10), 70 (LC50) | 33 | ||
| Paramuricea sp. | Healthb 96 h | NA (NOEC), 35 (LOEC) | 59 | ||
| Callogorgia delta | Healthb 96 h | NA (NOEC), 35 (LOEC) | 59 | ||
| Leiopathes glaberrima | Healthb 96 h | NA (NOEC), 35 (LOEC) | 59 | ||
| Corexit EC9527A | Acropora millepora | Gametes | Fertilisation, 4 h | 1 (NOEC), 10 (LOEC) | 49 |
| Acropora millepora | Settlement, 24 h | 1 (NOEC), 5 (LOEC) | 49 | ||
| Montastraea franksi | Adult | Gene expressionc, 8 h | 1 (NOEC), 5 (LOEC) | 60 | |
| Slickgone NS | Stylophora pistillata | Adult | Mortalityd 24 h | 125 (NOEC), 250 (LOEC)* | 61 |
| Pocillopora damicornis | Mortalityd 24 h | 25 (NOEC), 50 (LOEC)* | 61 | ||
| Dispolen 36S | Stylophora pistillata | Adult | Mortalityd 24 h | 50 (NOEC), 125 (LOEC)* | 61 |
| Pocillopora damicornis | Mortalityd 24 h | 25 (NOEC), 50 (LOEC)* | 61 | ||
| Inipol 90 | Stylophora pistillata | Larvae | Mortality 96 h | 5 (NOEC), 50 (LOEC)* | 62 |
| Stylophora pistillata | Mortality 96 h | 0.5 (NOEC), 5 (LOEC)* | 62 | ||
| Stylophora pistillata | Adult | Mortalityd 24 h | 50 (NOEC), 125 (LOEC)* | 61 | |
| Pocillopora damicornis | Mortalityd 24 h | 50 (NOEC), 125 (LOEC)* | 61 | ||
| Petrotech PTI-25 | Stylophora pistillata | Larvae | Mortality 96 h | 5 (NOEC), 50 (LOEC)* | 62 |
| Stylophora pistillata | Settlement 96 h | NA (NOEC), 0.5 (LOEC)* | 62 | ||
| Stylophora pistillata | Adult | Mortalityd 24 h | 50 (NOEC), 125 (LOEC)* | 61 | |
| Pocillopora damicornis | Mortalityd 24 h | 125 (NOEC), 250 (LOEC)* | 61 | ||
| Bioreico R-93 | Stylophora pistillata | Larvae | Mortality 96 h | 5 (NOEC), 50 (LOEC)* | 62 |
| Stylophora pistillata | Settlement 96 h | NA (NOEC), 0.5 (LOEC)* | 62 | ||
| Stylophora pistillata | Adult | Mortalityd 24 h | 50 (NOEC), 125 (LOEC)* | 61 | |
| Pocillopora damicornis | Mortalityd 24 h | 25 (NOEC), 50 (LOEC)* | 61 | ||
| Emulgal | Stylophora pistillata | Larvae | Mortality 96 h | 5 (NOEC), 50 (LOEC)* | 62 |
| Stylophora pistillata | Settlement 96 h | NA (NOEC), 0.5 (LOEC)* | 62 | ||
| Stylophora pistillata | Adult | Mortalityd 24 h | 50 (NOEC), 125 (LOEC)* | 61 | |
| Pocillopora damicornis | Mortalityd 24 h | 25 (NOEC), 50 (LOEC)* | 61 | ||
| Biosolve | Stylophora pistillata | Larvae | Survivorship 96 h | 5 (NOEC), 50 (LOEC)* | 62 |
| Stylophora pistillata | Settlement 96 h | NA (NOEC), 0.5 (LOEC)* | 62 |
*Assuming ~500 mg l−1 stock.
aOctocorals.
bBranch health 96 h incorporated partial mortality and other stress responses.
cHSP70 expression upregulation. HSP90 and P-GP were up-regulated at 10 mg l−1.
dSurvival one week after a 24 h exposure.
Surface oil slicks respond best to chemical dispersion prior to weathering changing the composition of the slick. Dispersion becomes less effective within hours to days, depending on the oil and conditions9. In most spill scenarios, exposures of marine organisms to peak concentrations of hydrocarbons and dispersants (when used) is expected to be short23,28 (minutes to hours), even when there is a prolonged discharge, because local marine and weather conditions dilute the potential toxicants through mixing, currents and tides. Therefore, it has been proposed that environmentally relevant toxicity tests should consider shorter exposures than the standard multiple day laboratory toxicity protocols14,15,19. In this study the toxicity of five dispersant formulations to coral larvae were tested over three short exposure periods (2, 6 and 24 h). The inhibition of larval settlement was selected as a potentially sensitive and ecologically relevant endpoint and we interpolated the toxicity thresholds (EC10 and EC50, where 10% and 50% of settlement is inhibited, respectively) from concentration-response curves29.
Results
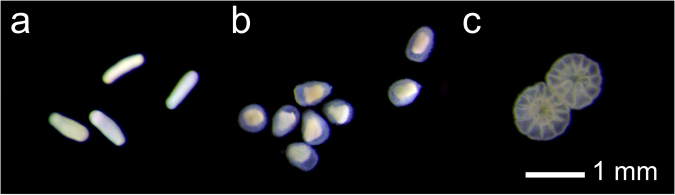
In uncontaminated water (control treatments) and in the presence of the lowest concentrations of dispersant, ~1 mm long larvae were typically cigar-shaped and highly motile (Fig. 1a). As the concentration and exposure time increased (for all dispersants) larvae became less motile, forming a squat, bullet shape with ill-defined and transparent outer membranes (Fig. 1b). Similar effects were observed for the reference toxicant SDS. After exposure to uncontaminated seawater (2, 6 and 24 h) and following the addition of chemical inducer, 85–95% of control coral larvae underwent successful attachment and metamorphosis into single polyp juveniles (Fig. 1c, Table 2).
Figure 1.
Photomicrographs of 7 d-old A. millepora larvae following 24 h exposure to (a) control seawater and (b) 10 mg l−1 Slickgone LTSW. Normal post-settlement metamorphosis of control larvae after an additional 18 h (c), with mesentery and tentacle formation evident in the single polyps.
Table 2.
Metamorphosis success (% ± SE, n = 6) of A. millepora larvae after 2, 6 and 24 h exposure to filtered seawater (control treatments only).
| Dispersant treatments | % metamorphosis controls | ||
|---|---|---|---|
| 2 h | 6 h | 24 h | |
| Ardrox 6120 | 95 ± 3 | 85 ± 4 | 89 ± 5 |
| Corexit EC9500A | 94 ± 2 | 86 ± 5 | 89 ± 4 |
| Slickgone LTSW | 90 ± 5 | 87 ± 5 | 87 ± 4 |
| Slickgone NS | 90 ± 4 | 86 ± 6 | 93 ± 2 |
| Finasol OSR 52 | 85 ± 5 | 88 ± 5 | 93 ± 3 |
| SDS | 85 ± 7 | 93 ± 3 | 90 ± 3 |
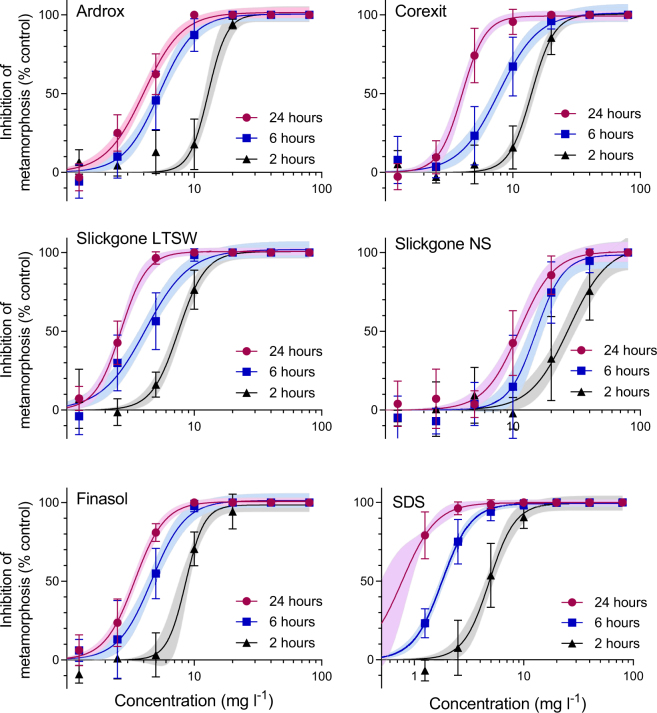
The effects of exposure to all dispersants and SDS followed typical concentration-response curves with increasing concentrations (Fig. 2). Fitting the % inhibition data to 4-parameter sigmoidal equations (R2 values 0.80–0.98, Table 3) enabled the interpolation of concentrations that inhibited metamorphosis in A. millepora larvae by 10% (EC10) and 50% (EC50) (Table 3). Of the five dispersants, Slickgone LTSW was the most toxic (24 h EC50 = 2.6 mg l−1), while Slickgone NS was the least toxic (24 h EC50 = 11.1 mg l−1). Exposure to the lowest concentration of SDS (1.25 mg l−1) at 24 h caused 79% inhibition so ECXX values for SDS were not reliable at this time point.
Figure 2.
Concentration response curves for inhibition of metamorphosis of A. millepora larvae by dispersants measured following 2, 6 and 24 h exposures. Bars represent SD ± n = 6. Shaded areas represent the 95% confidence boundaries of each 4 parameter sigmoidal model. All effect concentrations (EC10 and EC50) for each dispersant at each time point are provided in Table 3.
Table 3.
Concentrations of dispersants that inhibited the metamorphosis success of A. millepora larvae by 50% and 10% (EC50 and EC10), over three exposure periods and calculated from concentration-response curves presented in Fig. 2. 95% confidence intervals in parentheses. F-test results demonstrating the significant effect of exposure duration on EC50 values. PC95 = 95% species protection values (=HC5) derived from species sensitivity distributions for Ardrox, Slickgone LTSW, Slickgone NS, Finasol36 and Corexit EC9500A37.
| Dispersant | EC50 (mg l−1) | EC10 (mg l−1) | P | PC95 (mg l−1) | ||||
|---|---|---|---|---|---|---|---|---|
| 2 h | 6 h | 24 h | 2 h | 6 h | 24 h | F(df) | (No. species) | |
| Ardrox 6120 | 12.8 | 5.3 | 4.0 | 8.9 | 2.6 | 1.9 | <0.0001 | 0.013 |
| (11.8–14.4) | (4.8–5.9) | (3.6–4.3) | (8.0–9.9) | (2.1–3.2) | (1.6–2.3) | 157 (2, 138) | (3) | |
| Corexit C9500A | 14.0 | 7.7 | 4.0 | 8.9 | 3.5 | 2.5 | <0.0001 | 6.6 |
| (13.0–15.1) | (6.8–8.6) | (3.7–4.3) | (8.0–10.1) | (2.8–4.5) | (2.2–3.0) | 196 (2, 138) | (18) | |
| Slickgone LTSW | 7.5 | 4.1 | 2.6 | 4.4 | 1.7 | 1.6 | <0.0001 | 0.017 |
| (6.8–8.2) | (3.3–5.1) | (2.5–2.8) | (3.8–5.2) | (1.1–2.5) | (1.4–1.8) | 80.1 (2, 138) | (4) | |
| Slickgone NS | 26.4 | 15.4 | 11.1 | 12.9 | 8.8 | 5.6 | <0.0001 | 0.1 |
| (22.7–30.9) | (13.1–17.7) | (10.0–12.5) | (9.9–17.1) | (6.9–11.5) | (4.5–7.1) | 37.2 (2, 138) | (7) | |
| Finasol OSR 52 | 8.6 | 4.6 | 3.4 | 5.9 | 2.4 | 1.9 | <0.0001 | 0.13 |
| (7.5–9.3) | (4.1–5.1) | (3.2–3.6) | (4.7–7.4) | (1.9–3.0) | (1.7–2.1) | 122 (2, 138) | (7) | |
| SDS | 4.9 | 1.8 | 0.77 | 2.7 | 0.91 | 0.35 | <0.0001 | — |
| (4.4–5.4) | (1.7–1.9) | (0.48–0.95) | (2.1–3.4) | (0.80–1.0) | (0.20–0.65) | 195 (2, 135) | ||
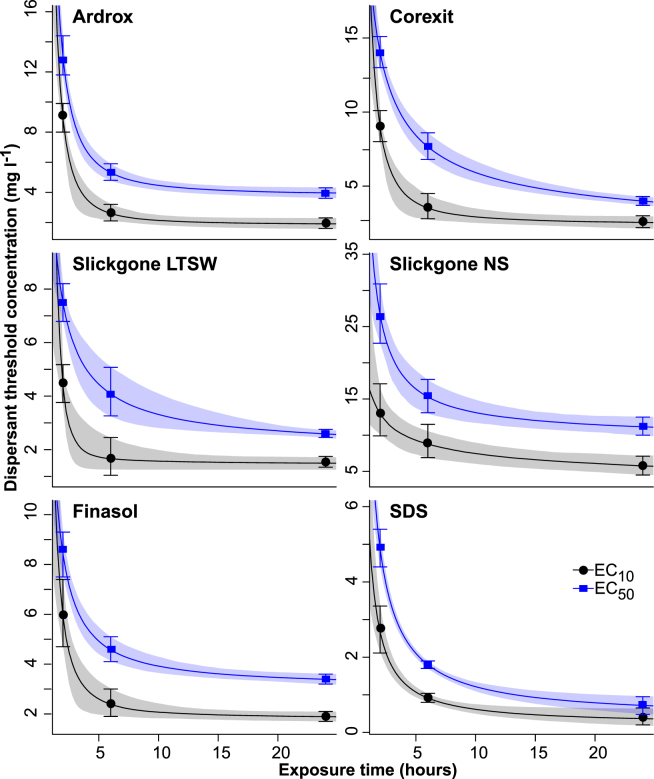
The exposure duration had a strong effect on the toxicity of all five dispersants and SDS to coral larvae, with EC50 values decreasing by an average 2.9-fold as the exposure duration increased from 2 to 24 h (Table 3). The EC50 for SDS also decreased dramatically as the duration of exposure increased from 2 h to 6 h (Table 3). F-test comparisons from the fitted curves demonstrated a significant effect of exposure duration on EC50 values for each dispersant and SDS (Table 3). The time-dependent relationship between the ECx values and concentration was captured using a three parameter exponential decay function (Fig. 3). The ECx values can be predicted for other exposure times using Equation 1 with the estimated parameters in Table 4.
Figure 3.
Time dependence of estimated EC10 and EC50 concentrations for each dispersant, with values and 95% confidence bars taken from Table 4. Solids lines are fitted three parameter exponential decay relationships, with shaded areas representing 95% confidence bands based on 1000 iterations of a monte-carlo simulation sampling from gamma distributions fitted to the estimated threshold concentration values and their 95% confidence bands.
Table 4.
Parameter estimates for the time-dependence of EC10 and EC50 concentrations for each dispersant for the three parameter exponential decay function (y = m + a * exp(−b * log(x)) where y is the EC10 or EC50 threshold concentration, x is exposure time in hours, exp(−b * log(x)) is the exponential function, m is the concentration at which an infinite exposure time would theoretically yield no effect, a is the theoretical threshold concentration at zero exposure (y-intercept) and b is the exponential rate of decay. 95% confidence intervals in parentheses.
| Dispersant | EC50 curve parameters | EC10 curve parameters | ||||
|---|---|---|---|---|---|---|
| a | b | m | a | b | m | |
| Ardrox 6120 | 29.9 | 1.67 | 3.79 | 40.2 | 2.22 | 1.85 |
| (20.3–43.2) | (1.21–2.23) | (3.3–4.25) | (17.5–182) | (1.38–4.82) | (1.42–2.29) | |
| Corexit EC9500A | 20.6 | 0.644 | 0.698 | 27.3 | 1.76) | 2.35 |
| (18–24.4) | (0.275–1.02) | (−5.67–3.19) | (12.3–97.3) | (0.832–3.84 | (1.47–2.9) | |
| Slickgone LTSW | 12 | 0.899 | 0.835 | 82.7 | 3.61 | 1.48 |
| (8.6–26.3) | (0.0985–1.8) | (−14.7–2.63) | (5.63–320) | (0.793–6.71) | (1.12–1.75) | |
| Slickgone NS | 38.5 | 1.06 | 8.29 | 39.5 | 0.561 | −18.7 |
| (24.1–70) | (0.281–2.03) | (−2.79–12.2) | (10.5–137) | (0.0208–2.08) | (−113–6.74) | |
| Finasol OSR 52 | 12.4 | 1.18 | 3.02 | 23.3 | 2.06 | 1.81 |
| (8.62–18.2) | (0.653–1.77) | (2.13–3.48) | (7.22–128) | (0.911–4.98) | (1.4–2.08) | |
| SDS | 9.43 | 1.06 | 0.384 | 5.83 | 1.2 | 0.219 |
| (7.72–11.6) | (0.823–1.36) | (−0.0524–0.808) | (3.74–9.47) | (0.711–1.92) | (−0.196–0.6) | |
Discussion
Coral larval settlement was sensitive to acute exposures to all five individual oil spill dispersants formulations, with the threshold concentrations for toxicity (EC10s) all relatively low in comparison with other coral life stages and marine taxa. The toxicity of each dispersant increased with exposure duration until 6 h, indicating rapid uptake of the toxic components of the formulations and this could be related to the small size and high surface area to volume of the larvae. Although the toxic thresholds were low, they were higher than dispersant concentrations that would be expected in the real-world following applications in tropical waters. Differences in the toxicity observed between dispersants was likely due to the combined toxic effects of different formulation components and their relative concentrations. The toxic threshold values determined here for each dispersant should contribute to the development of species sensitivity distributions (SSDs) for dispersant toxicity and support net environmental benefit assessments (NEBAs) for dispersant applications, to improve decision making by oil spill responders.
Comparative toxicity between dispersants
All of the dispersant formulations were observed to affect larvae in a similar way. Settlement and metamorphosis was reduced at low concentrations, while impaired mobility, changes to larval shape and increased membrane transparency were typically observed at higher concentrations. Although each dispersant formulation is different, similar toxic modes of action between formulations might be expected as all contain the anionic surfactant DOSS, which is likely to be a major contributor to toxicity9,15,30. The reference toxicant SDS caused a similar response pattern and (like DOSS) was likely to have damaged membrane integrity, leading to a failure of cellular homeostasis30. Additional impacts on membrane proteins, lipopolysaccharides and other cellular functions cannot be discounted31. Surfactants have a wide range of toxicities, with no effect concentrations of environmentally relevant endpoints reported between 0.01 and 100 mg l−1, and sub-lethal responses often occurring at concentrations an order of magnitude lower32. The 24 h thresholds for toxicity (EC10) of between 1.6–5.6 mg l−1 for the five dispersants were similar to the toxicities of other dispersants to coral larvae summarised in Table 1, but direct comparisons with these other studies are difficult as EC10s have not previously been calculated for corals from concentration response curves. The only other study to publish EC10 or EC50 values on the effects of dispersants to coral, indicated that Corexit EC9500A was more than 10-fold less toxic to adult fragments of a temperate octocoral33 than to A. millepora larvae. The current study allowed direct comparisons of toxicity between the five dispersants to the same species under identical conditions. This matched dataset indicated that although there were differences in toxicity between the dispersants to coral larvae, the range of EC50s was relatively narrow (2.6 and 11 mg l−1) after 24 h. The acute toxicity of each formulation should be linked to the mixture toxicity (combined potency and concentration of surfactants and other components) of the dissolved fraction30, but without detailed chemical analysis (not available here), assigning specific reasons for these differences would be speculative.
Time-dependent toxicity
To examine the time-dependency of toxicity, coral larvae were exposed to dispersants over three short periods representing short peaks in concentration expected during an oil spill response19,28. There was a rapid exponential change in toxicity between 2 and 24 h with the majority of the ~3-fold increase occurring between 2 and 6 hours (Fig. 3). The minor changes in toxicity between 6 and 24 h exposures indicate that maximum acute toxicity to the small coral larvae occurred rapidly for all of the dispersants tested. In adult soft corals, Corexit EC9500A exposure caused bleaching (the loss of symbionts) but no time-dependent effects were observed between 24 and 72 h34. The rapid onset of toxicity for larvae could be related to their small size and high surface area to mass ratio, leading to fast uptake kinetics35. The acute toxicity results reported here are relevant to short-term field exposures expected in the majority of response scenarios where applications are short or tides and currents reduce exposure from longer applications23,28. The consistent exposures applied over three short periods were followed by settlement in uncontaminated water. Additional short and long term exposures tests should be conducted to assess a variety of other exposure scenarios. Acute exposures of larvae to spikes of dispersant followed by gradual dilution during settlement is a more likely natural scenario and should be tested, but deriving effect thresholds for comparison with other studies is more difficult. Extended low concentration exposure tests should also be conducted to assess the potential chronic effects posed by longer term dispersant responses4.
Toxicity relative to regulatory values
Ideally, responders to an oil spill would know the relative toxicity of each available dispersant in combination with the specific oil type for species relevant to the spill site19. Since these data are rarely available, the comparative toxicity of dispersant formulations to the relevant taxa (as measured here for corals) may be valuable for combination with modelled concentrations to assess risk and to help choose the least harmful dispersant for the response. Each national or state jurisdiction has its own regulations or guidelines on the appropriate use of dispersants. For example, some European nations require toxicity tests to demonstrate that dispersant formulations are less toxic than a reference toxicant or do not enhance the toxicity of oil in comparison to mechanically dispersed oil17. Guidelines set by the Australian Maritime Safety Authority (AMSA) under the National Plan limit the acceptability for use of dispersant products that show a toxic effect (EC50, LC50 or similar) to a diversity of marine species, including microalgae, copepods, amphipods, sea urchins, scallops and fish, at concentrations of 10 mg l−1 or less27. Inhibition of larval settlement and metamorphosis is considered an ecologically relevant endpoint for water quality derivation29. If the 24 h coral larval exposure results (Table 3) were to be included within this AMSA multi-taxa approach, then only Slickgone NS would have met this requirement.
Another approach that should be considered to compare and regulate dispersant safety involves the application of species sensitivity distributions (SSD); probability models of the sensitivity of multiple taxa to dispersant exposure. Adams36 recently developed preliminary SSDs for a range of dispersants registered for use in Australia based on the very limited set of toxicity data available for marine species. The SSDs were used to determine the predicted no-effect concentration which was assigned as 95% species protection (PC95, this is equivalent to the HC5 where 5% of species are affected). The PC95s (Table 3), were generally derived from chronic EC10 values and were of low to moderate quality due to the lack of toxicity data (only 3 to 7 species available per dispersant). More toxicity data is available for Corexit EC9500A and a PC95 of 6.6 mg l−1 was derived by Barron et al.37. A comparison of the acute EC10s for coral larval settlement with the sensitivity data reported by Adams36 and reviewed by Hook and Lee15, indicates that coral larvae are relatively sensitive to Ardrox, Slickgone LTSW, Slickgone NS and Finasol compared with many other species tested, especially if the acute toxicity thresholds derived here were converted to chronic values (by dividing by a factor of 2–5)29. However, the PC95 values derived from limited toxicity data would be broadly protective of coral larvae to these dispersants. Coral larvae were also relatively sensitive to Corexit EC9500A in comparison to many other fish, mollusc and crustacean species37,38 but would not be protected by the PC95 derived by Barron et al.37.
When a NEBA is undertaken to assess whether to apply dispersants to help degrade oil slicks and protect intertidal and shoreline habitats, it requires input of a very broad range of information that includes the type and size of a spill, weather conditions, hydrographic and oceanographic conditions, the relative presence and distribution of vulnerable receptors to both dispersed and undispersed oil, accessibility and availability of application, and monitoring resources and predictive modelling9,15. Dispersant application may increase the exposure of sub-surface species to greater doses of petroleum hydrocarbons, and this additional exposure and potential harm from dispersants themselves is also a critical consideration for the NEBA process19. While specific studies should continue to be conducted to compare the toxicity of physically versus chemically dispersed oil, the current study demonstrated that these five dispersants were approximately an order of magnitude less toxic to coral larvae than dissolved aromatic hydrocarbons from a water accommodated fraction of light crude oil in very similar tests25. This is consistent with the general notion that dispersants alone are less toxic than oil, but chemically dispersed oil is more toxic due to greater concentrations of oil in water5,12,39.
Comparing toxic thresholds for dispersants derived from laboratory studies with concentrations measured in the field are particularly difficult as dispersant formulations are comprised of multiple potentially toxic components. These components are likely to retain their original proportions in short acute experiments, but these proportions will change rapidly when formulations are applied in the field. Field and laboratory comparisons are further complicated as concentrations can be expressed as dilutions of the formulation (which can only be modelled in the field) or as concentrations of marker/proxy components, such as DOSS (which doesn’t account for different behaviour and toxicity of other components). The highest concentration of Corexit EC9500A at the water surface soon after application by air (dispersant:oil ratio 1:20) has been estimated as ~5 mg l−140 and up to 13 mg l−1 of dispersant measured in sea trials41. The EC10 thresholds for 24 h exposure of coral larvae to dispersants were 1.6–5.6 mg l−1, indicating that coral larvae in the immediate vicinity of dispersant application could be at risk. However, rapid dilution at sea is expected to reduce exposure concentrations1,28,41. For example, measured concentrations of DOSS as a presumed marker for Corexit EC9500A and EC9527A (since other sources of DOSS were identified in the Gulf42,43) reported in weeks following the Deepwater Horizon spill were far less than the EC10 values for dispersant formulations reported here for coral larval settlement44,45. Nevertheless, more tropical species toxicity data, especially for sensitive reproductive stages of reef-building corals and for relevant exposure durations, are crucial to improve the SSD approach to assess environmental safety and to contribute to a more robust information base for the NEBA process and decision support for oil responders15,16,36,37. Other operational considerations reinforce this conclusion. For example, Australian dispersant operations protocols recommend that dispersants not be applied without sufficient depth of water, or water exchange, or near coral reefs1,9,46. The most sensitive reproductive processes for many reef-building corals occur during and soon after discrete mass spawning events that usually occur annually at a given reef, and where spawn and larvae are present in the water column between reefs for days to weeks47. This limits the likelihood of exposing mobile coral larvae to oil spills for most of the year, but dispersant applications during the annual coral spawning seasons (even long distances from coral reefs) should be carefully considered in the NEBA process.
Materials and Methods
Coral larvae were exposed to eight concentrations of five different dispersants for durations of 2, 6 or 24 h. After these exposure periods the larvae were rinsed and the larvae allowed to settle and undergo metamorphosis over an additional 18 h in uncontaminated seawater. The concentrations of dispersant that inhibited metamorphosis by 10% and 50% (EC10 and EC50) were calculated from concentration-response curves for each exposure duration.
Coral collection and larval culture
Colonies of the common Indo-Pacific broadcast spawning coral Acropora millepora (Ehrenberg, 1834) >20 cm were collected from ~3 m depth in October 2015 from Esk Island, on the central Great Barrier Reef (GBR, 18°46.420′S 146°31.372′E). This species has been used in similar larval assays for over a decade and has predictable spawning and settlement behaviour48–52. Gravid colonies were transported to the National Sea Simulator (SeaSim) at the Australian Institute of Marine Science (Townsville, Australia) and placed in flow-through tanks at ~27 °C until spawning. Gametes were collected from seven parental colonies on a single night, fertilized and the symbiont-free larvae were cultured at less than 500 larvae l−1 in flow through tanks as previously described49. A. millepora larvae reach maximum competency for settlement after six days (reviewed in Jones et al.47) and seven day old larvae were used in these exposure experiments.
Dispersant preparation
Dispersants (Table 5) were provided by AMSA. The dispersants chosen were those listed at the time on the Australian National Plan Register of Oil Spill Control Agents and so available for deployment in an oil spill. Subsequently, Dasic Slickgone LTSW and Ardrox 6120 have been removed from operational stockpiles as obsolete stock. Concentrated stock solutions (4 g l−1) of each dispersant were prepared in 1 µm filtered seawater (FSW) and the homogeneity of stocks was maintained by rapidly stirring with a Teflon-coated magnetic bar. Solutions of eight nominal concentrations (0, 1.25, 2.5, 5, 10, 20, 40 and 80 mg l−1) were prepared by dilution of the stirring stock with FSW and each of these exposure solutions were used within 2 h of preparation. Nominal concentrations of each formulation were reported in the experiment as the more toxic components, including DOSS, are water soluble and little degradation and loss of volatiles would occur over the short assay periods. The alternative (using measured concentrations) would require estimation of potential dispersant losses from concentrations of a proxy component, and this could be misleading in the case of the complex dispersant formulations where minor components may behave differently and/or contribute more or less toxicity than the measured component. Furthermore, direct comparisons between the toxicity of distinct dispersant formulations are also assisted by expressing the treatments in mg (formulation) l−1. Sodium dodecyl sulphate (SDS), an anionic surfactant, was prepared in the same way and used as a reference toxicant.
Table 5.
Tested dispersants and the hazardous constituents listed in their safety data sheets; percentages are included, where available. DOSS (dioctyl sodium succinate) is an anionic surfactant and CXX denotes the carbon number of hydrocarbons (HCs).
| Dispersant | Hazardous Constituents Listed in Safety Data Sheet | CAS No. |
|---|---|---|
| Corexit EC9500A | 10–30% DOSS | 577-11-7 |
| 10–30% hydrotreated petroleum distillate | 64742-47-8 | |
| 1–5% 1,2-propanediol | 57-55-6 | |
| Finasol OSR 52 | 20–25% DOSS | 577-11-7 |
| 15–20% C11-C14 HCs (<2% aromatics) | 926-141-6 | |
| 15–20% (2-methoxymethylethoxy)propanol | 34590-94-8 | |
| Slickgone NS | DOSS (unspecified %) | 577-11-7 |
| hydrotreated petroleum distillate (unspecified %) | 64742-47-8 | |
| Slickgone LTSW | 10–20% DOSS | 577-11-7 |
| 30–40% 2-butoxyethanol | 111-76-2 | |
| Ardrox 6120 | 10–30% Anionic surfactants (including soaps) | mixture |
| 10–30% 2-butoxyethanol | 111-76-2 |
Settlement assays
Coral larvae were statically exposed in acetone-rinsed 20 ml glass vials containing 10–12 coral larvae and 15 ml dispersant solutions with six replicate vials used for each dispersant concentration. Vials were sealed with aluminium-lined caps and a ~7 ml headspace allowed oxygen exchange. Vials were then transferred to an orbital incubator/shaker (50 rpm) to maintain gentle water movement and to discourage settlement25. Temperature was set at 27 °C and light at 40 µmol quanta m−2 s−1. Under these conditions dissolved oxygen was maintained at >7 mg l−1, and pH (8.0–8.2) and salinity 33–35 psu (Table S-1, Supplementary information). Three exposure durations were tested with vials removed from the incubator after 2, 6 or 24 h exposures. To enable the testing of multiple treatments, the start times for each group of exposure durations (24 h, 6 h and 2 h in sequence) were offset by 2 h. Control (uncontaminated seawater) exposures were conducted (n = 6) for each dispersant type at each time point. Following the exposures, larvae were transferred into small (15 mm wide) 100 µm nylon mesh filters which were partially submerged and the larvae gently washed with an excess of uncontaminated FSW. The dispersant-free larvae were then transferred into individual 6-well cell culture plates containing 10 ml FSW (12 ml, Nunc, NY, USA).
Settlement and metamorphosis of coral larvae in the plastic well plates was initiated by the addition of a slightly sub-optimal (to maximise the sensitivity of the assay) concentration (5 µl) of crustose coralline algae (CCA) extract51 prepared using 4 g of the crustose coralline algae Porolithon onkodes48. Following exposure the settlement inducer, larvae cease swimming within minutes and elongate before undergoing early metamorphosis within 12 h48,53. Metamorphosis was assessed after 18 h and larvae scored as normal and functional if they had changed from either the free swimming or casually attached pear-shaped forms to squat, firmly attached, disc-shaped structures with pronounced flattening of the oral–aboral axis and with septal mesenteries radiating from the central mouth region48. This assay therefore assessed whether larvae were functional following the dispersant exposure. Larval settlement above 70% in the controls was considered acceptable as an endpoint based upon several previous studies using CCA or extracts of CCA to initiate settlement of Acropora spp.25,54–56.
Data analysis
Inhibition of metamorphosis (% inhibition relative to 0% WAF control) was calculated from treatment data as Inhibition (%) = 100 × [(% metamorphosiscontrol − % metamorphosistreatment)/% metamorphosiscontrol]. The concentration of dispersant that inhibited 10% and 50% of metamorphosis (EC10 and EC50) was calculated from concentration-response curves (four-parameter sigmoidal models) fitted to the % inhibition and total aromatics data of each treatment using the program GraphPad Prism (v7, San Diego, USA). The probability that EC50 values generated by the logistic curves were statistically different was tested by applying the F test in Graph Pad Prism v6. EC50 values were considered different when p < 0.05.
A Monte Carlo approach was carried out using the R software57 to propagate uncertainty in the EC10 and EC50 thresholds (calculated independently for each experimental exposure time) and to provide parametric model fits (and associated uncertainty) for estimating thresholds as a continuous function of exposure time that might be more readily incorporated into oil spill modelling. This was achieved in two steps: (i) a gamma distribution was fitted to the mean, lower and upper confidence bands for the EC10 and EC50 estimated threshold concentrations for each experimental time; (ii) secondly 1000 random new concentration values were generated using these fitted distributions for each of the three exposure times, and fitted these using a three parameter exponential decay function:
| 1 |
where y is the EC10 or EC50 threshold concentration, x is exposure time in hours, a is the initial value, exp(−b * log(x)) is the exponential function and m is the concentration at which an infinite exposure time would theoretically yield no effect. Both the gamma and non-linear relationships were fitted using simple least squares procedures via the function optim in R.
Electronic supplementary material
Acknowledgements
The authors would like to thank the efforts and expertise of staff in the National Sea Simulator at AIMS
Author Contributions
A.P.N., H.M.L., D.L.B. and P.I. designed the study, A.P.N. and H.M.L. performed the study, A.P.N. and R.F. analyzed the data. All authors wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20709-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.NRC. Oil Spill Dispersants: Efficacy and Effects, Committee on Understanding Oil SpillDispersants: Efficacy and Effects, National Research Council, The National Academies Press. (2005)Available at: http://www.nap.edu/catalog/11283.html, Accessed: 21st October 2017 (2005).
- 2.NRC. Responding to Oil Spills in Arctic Marine Environments. Report of the Committee on Responding to Oil Spills in Arctic Marine Environments. Transportation Research Board, Ocean Studies Board, Marine Board and Polar Research Board; Division on Earth and Life Studies; National Research Council. The National Academies Press, Washington, D.C., 250 pp. https://www.nap.edu/catalog/18625/responding-to-oil-spills-in-the-us-arctic-marine-environment, Accessed: 21st October 2017 (2014).
- 3.Prince RC, Coolbaugh TS, Parkerton TF. Oil dispersants do facilitate biodegradation of spilled oil. Proc. Natl. Acad. Sci. 2016;113:E1421. doi: 10.1073/pnas.1525333113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NRC. National Research Council (NRC). 2013. An ecosystem services approach to assessing the impacts of the Deepwater Horizon oil spill in the Gulf of Mexico. The National Academies Press, Washington, D.C., USA. https://www.nap.edu/catalog/18387/an-ecosystem-services-approach-to-assessing-the-impacts-of-the-deepwater-horizon-oil-spill-in-the-gulf-of-mexico, Accessed: 21st October 2017 (2013). [PubMed]
- 5.Prince RC. Oil spill dispersants: boon or bane? Environ. Sci. Tech. 2015;49:6376–6384. doi: 10.1021/acs.est.5b00961. [DOI] [PubMed] [Google Scholar]
- 6.John V, Arnosti C, Field J, Kujawinski E, McCormick A. The role of dispersants in oil spill remediation: fundamental concepts, rationale for use, fate, and transport issues. Oceanography. 2016;29:108–117. doi: 10.5670/oceanog.2016.75. [DOI] [Google Scholar]
- 7.Bagby SC, Reddy CM, Aeppli C, Fisher GB, Valentine DL. Persistence and biodegradation of oil at the ocean floor following DeepwaterHorizon. Proc. Natl. Acad. Sci. 2017;114:E9–E18. doi: 10.1073/pnas.1610110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiocco RJ, Lewis A. Oil spill dispersants. Pure and Applied Chemistry. 1999;71:27–42. doi: 10.1351/pac199971010027. [DOI] [Google Scholar]
- 9.EMSA. Manual on the applicability of oil spill dispersants. European Maritime Safety Agency. Manual prepared as part of EMSA contract EMSA 05-679-RES/04/2005 and updated under EMSA contract EMSA/146/2008. http://www.emsa.europa.eu/opr-documents/opr-manual-a-guidelines/item/719-manual-on-the-applicability-of-oil-spill-dispersants.html, Accessed 21st October, 2017 (2010).
- 10.Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: not just a soap opera. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2004;1666:105–117. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 11.French-McCay, D., Beegle-Krause, C., Rowe, J., Rodriguez, W. & Schmidt Etkin, D. Oil spill risk assessment: relative impact indices by oil type and location. Proceedings of the 32. AMOP technical seminar on environmental contamination and response. Volume 2, Canada, Ottawa, 601–653 (2009).
- 12.Bejarano AC, Clark JR, Coelho GM. Issues and challenges with oil toxicity data and implications for their use in decision making: A quantitative review. Environ. Toxicol. Chem. 2014;33:732–742. doi: 10.1002/etc.2501. [DOI] [PubMed] [Google Scholar]
- 13.Wise J, Wise JP. A review of the toxicity of chemical dispersants. Rev. Environ. Health. 2011;26:281–300. doi: 10.1515/REVEH.2011.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aurand, D. & Coelho, G. Cooperative aquatic toxicity testing of dispersed oil and the “Chemical Response to Oil Spills: Ecological Effects Research Forum (CROSERF)”. Technical Report 07-03 (2005) 105, p Available at: https://www.bsee.gov/sites/bsee.gov/files/osrr-oil-spill-response-research/296ac.pdf, Accessed 21st October, 2017 (2005).
- 15.Hook, S. E. & Lee, K. A review of the ecotoxicological implications of oil dispersant use in Australian waters. CSIRO, Oceans and Atmosphere, Lucas Heights, NSW Australia. https://www.amsa.gov.au/environment/marine-pollution-response/scientific-info/dispersants/Documents/csiro-dispersant-report.pdf, Accessed: 21st October 2017 (2015).
- 16.Irving, P. & Lee, K. Improving Australia’s dispersant response strategy. Proceedings of the 38th AMOP Technical Seminar, Environment Canada, Ottawa, Ontario, 973–987 (2015).
- 17.REMPEC. Guidelines for the use of dispersants for combating oil pollution at sea in the Mediterranean region Part II: Basic information on dispersants and their application. Regional Marine Pollution Emergencey Response Centre for the Mediterranean Sea (REMPEC). http://www.rempec.org/admin/store/wyswigImg/file/Information%20resources/Guidelines/RIS%20D2%20(Dispersants)/EN/DispersantsGuidelinesPartII.pdf, (Accessed October 21st 2017) (2011).
- 18.Merlin, F. X. Draft Guidelines for the use of dispersants for combating oil pollution at sea, Part 1, Basic information on dispersants and their application, as presented to the OPRC-HNS Technical Group of IMO, Session 16, Agenda Item 3 (2014).
- 19.Coelho G, Clark J, Aurand D. Toxicity testing of dispersed oil requires adherence to standardized protocols to assess potential real world effects. Environ. Pollut. 2013;177:185–188. doi: 10.1016/j.envpol.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 20.PGM. Great Barrier Reef shipping: review of environmental implications, PGM Environment, Safety Bay, Western Australia. https://www.environment.gov.au/system/files/pages/884f8778-caa4-4bd9-b370-318518827db6/files/23qrc-doc3.pdf, Accessed: October 21st 2017 (2012).
- 21.ATSB. Grounding of the bulk carrier Shen Neng 1 at Douglas Shoal, Queensland. Australian Transport Safety Bureau Report Marine Occurrence Investigation No. 274, Australian Government. http://www.atsb.gov.au/media/2478832/mo2010003.pdf, Accessed: October 21st, 2017 (2011).
- 22.Irving, P. Australian revised national plan policy and process for the recognition, application and monitoring of oil spill control agents, including dispersants, in Proceedings of the 36th AMOP Technical Seminar on Environmental Contamination and Response, Environment Canada, Ottawa, ON. pp:797–808 (2013).
- 23.Storrie, J. Montara Wellhead Platform Oil Spill – A Remote Area Response. International Oil Spill Conference Proceedings. March 2011. pp 159. Available at: http://www.ioscproceedings.org/toc/iosc/2011/1, Accessed October 21st 2017. 10.7901/2169-3358-2011-1-159 (2011).
- 24.Borthwick, D. Report of the Montara Commission ofInquiry, Montara Commission of Inquiry, Commonwealth of Australia, page 301. https://industry.gov.au/resource/UpstreamPetroleum/MontaraInquiryResponse/Pages/default.aspx. Accessed 21st October, 2017 (2010).
- 25.Negri AP, et al. Acute ecotoxicology of natural oil and gas condensate to coral reef larvae. Sci. Rep. 2016;6:21153. doi: 10.1038/srep21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner NR, Renegar DA. Petroleum hydrocarbon toxicity to corals: A review. Mar. Pollut. Bull. 2017;119:1–16. doi: 10.1016/j.marpolbul.2017.04.050. [DOI] [PubMed] [Google Scholar]
- 27.AMSA. National Plan for Maritime Environmental Emergencies; Register of Oil Spill Control Agents for maritime response use – Reference: NP-POL-04. Australian Maritime Safety Authority, 1 May 2015 (2015), https://www.amsa.gov.au/forms-and-publications/Publications/national_plan.pdf, Accessed October 21st, 2017 (2015).
- 28.Lee K, Nedwed T, Prince RC, Palandro D. Lab tests on the biodegradation of chemically dispersed oil should consider the rapid dilution that occurs at sea. Mar. Pollut. Bull. 2013;73:314–318. doi: 10.1016/j.marpolbul.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Warne, M. S. et al. Revised method for deriving Australian and New Zealand water quality guideline values for toxicants. Prepared for the Council of Australian Government’s Standing Council on Environment and Water (SCEW). Department of Science Information Technology and Innovation, Brisbane, Queensland. https://publications.csiro.au/rpr/pub?pid=csiro:EP159161, Accessed October 21st, 2017 (2015).
- 30.Singer MM, George S, Tjeerdema RS. Relationship of some physical properties of oil dispersants and their toxicity to marine organisms. Arch. Environ. Contam. Toxicol. 1995;29:33–38. doi: 10.1007/BF00213084. [DOI] [Google Scholar]
- 31.Sikkema J, De Bont J, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis MA. Chronic and sublethal toxicities of surfactants to aquatic animals: A review and risk assessment. Water Res. 1991;25:101–113. doi: 10.1016/0043-1354(91)90105-Y. [DOI] [Google Scholar]
- 33.Frometa J, DeLorenzo ME, Pisarski EC, Etnoyer PJ. Toxicity of oil and dispersant on the deep water gorgonian octocoral Swiftia exserta, with implications for the effects of the Deepwater Horizon oil spill. Mar. Pollut. Bull. 2017;122:91–99. doi: 10.1016/j.marpolbul.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Studivan MS, Hatch WI, Mitchelmore CL. Responses of the soft coral Xenia elongata following acute exposure to a chemical dispersant. SpringerPlus. 2015;4:80. doi: 10.1186/s40064-015-0844-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rand, G. M. Fundamentals of aquatic toxicology: effects, environmental fate and risk assessment. CRC Press 1148 p (1995).
- 36.Adams, M. S. Species sensitivity distributions as a risk evaluation tool for oil dispersants for use in Australian marine waters. CSIRO report prepared for the Australian Maritime Safety Authority (AMSA). Available from www.amsa.gov.au (2014).
- 37.Barron MG, Hemmer MJ, Jackson CR. Development of aquatic toxicity benchmarks for oil products using species sensitivity distributions. Integrated Environ. Assess. Manag. 2013;9:610–615. doi: 10.1002/ieam.1420. [DOI] [PubMed] [Google Scholar]
- 38.George-Ares A, Clark J. Aquatic toxicity of two Corexit® dispersants. Chemosphere. 2000;40:897–906. doi: 10.1016/S0045-6535(99)00498-1. [DOI] [PubMed] [Google Scholar]
- 39.Berninger JP, Williams ES, Brooks BW. An initial probabilistic hazard assessment of oil dispersants approved by the United States National Contingency Plan. Environ. Toxicol. Chem. 2011;30:1704–1708. doi: 10.1002/etc.532. [DOI] [PubMed] [Google Scholar]
- 40.Lewis, A. & Aurand, D. Putting Dispersants toWork: Overcoming Obstacles. Technical Report #IOSC-004.23 API 4652A, American Petroleum Institute, Washington, DC (1997).
- 41.Bocard, C., Castaing, G. & Gatellier, C. In Oil Spill Chemical Dispersants: Research, Experience, and Recommendations. https://www.astm.org/DIGITAL_LIBRARY/STP/PAGES/STP30233S.htm, (Accessed: 21st October, 2017) (ASTM International, 1984).
- 42.Wilson, M. et al. Oil sill science: persistence, fate, and effectiveness of dispersants used during the Deepwater Horizon Oil spill. Sea Grant Programs of the Gulf of Mexico. http://edis.ifas.ufl.edu/sg150, Accessed November 2nd, 2017 (2016).
- 43.Hayworth JS, Clement TP. Provenance of Corexit-related chemical constituents found in nearshore and inland Gulf Coast waters. Mar. Pollut. Bull. 2012;64:2005–2014. doi: 10.1016/j.marpolbul.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 44.Kujawinski EB, et al. Fate of dispersants associated with the Deepwater Horizon oil spill. Environ. Sci. Tech. 2011;45:1298–1306. doi: 10.1021/es103838p. [DOI] [PubMed] [Google Scholar]
- 45.Gray JL, et al. Presence of the Corexit component dioctyl sodium sulfosuccinate in Gulf of Mexico waters after the 2010 Deepwater Horizon oil spill. Chemosphere. 2014;95:124–130. doi: 10.1016/j.chemosphere.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 46.AMSA. Dispersant response. Australian Maritime Safety Authority. https://www.amsa.gov.au/environment/marine-pollution-response/scientific-info/dispersants/, Accessed 21st October, 2017 (2017).
- 47.Jones R, Ricardo G, Negri AP. Effects of sediments on the reproductive cycle of corals. Mar. Pollut. Bull. 2015;100:13–33. doi: 10.1016/j.marpolbul.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 48.Heyward AJ, Negri AP. Natural inducers for coral larval metamorphosis. Coral Reefs. 1999;18:273–279. doi: 10.1007/s003380050193. [DOI] [Google Scholar]
- 49.Negri AP, Heyward AJ. Inhibition of fertilization and larval metamorphosis of the coral Acropora millepora (Ehrenberg, 1834) by petroleum products. Mar. Pollut. Bull. 2000;41:420–427. doi: 10.1016/S0025-326X(00)00139-9. [DOI] [Google Scholar]
- 50.Negri AP, Heyward AJ. Inhibition of coral fertilisation and larval metamorphosis by tributyltin and copper. Mar. Environ. Res. 2001;51:17–27. doi: 10.1016/S0141-1136(00)00029-5. [DOI] [PubMed] [Google Scholar]
- 51.Negri AP, et al. Effects of the herbicide diuron on the early life history stages of coral. Mar. Pollut. Bull. 2005;51:370–383. doi: 10.1016/j.marpolbul.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 52.Shaw M, Negri A, Fabricius K, Mueller JF. Predicting water toxicity: Pairing passive sampling with bioassays on the Great Barrier Reef. Aquat. Toxicol. 2009;95:108–116. doi: 10.1016/j.aquatox.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Tebben J, et al. Chemical mediation of coral larval settlement by crustose coralline algae. Sci. Rep. 2015;5:10803. doi: 10.1038/srep10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Negri AP, Hoogenboom MO. Water contamination reduces the tolerance of coral larvae to thermal stress. PLoS ONE. 2011;6:e19703. doi: 10.1371/journal.pone.0019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Negri AP, Harford A, Parry D, van Dam RA. Effects of an alumina refinery discharge and its key metal constituents at the upper thermal tolerance of: 2. The early life stages of the coral Acropora tenuis Mar. Pollut. Bull. 2011;62:474–482. doi: 10.1016/j.marpolbul.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 56.Heyward AJ, Negri AP. Plasticity of larval pre-competency in response to temperature: observations on multiple broadcast spawning coral species. Coral Reefs. 2010;29:631–636. doi: 10.1007/s00338-009-0578-5. [DOI] [Google Scholar]
- 57.R Development Core Team. R:A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org (2015).
- 58.Goodbody-Gringley G, et al. Toxicity of Deepwater Horizon source oil and the chemical dispersant, Corexit® 9500, to coral larvae. PLoS ONE. 2013;8:e45574. doi: 10.1371/journal.pone.0045574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeLeo DM, Ruiz-Ramos DV, Baums IB, Cordes EE. Response of deep-water corals to oil and chemical dispersant exposure. Deep Sea Res. Part II: Tropical Studies in Oceanog. 2016;129:137–147. doi: 10.1016/j.dsr2.2015.02.028. [DOI] [Google Scholar]
- 60.Venn AA, Quinn J, Jones R, Bodnar A. P-glycoprotein (multi-xenobiotic resistance) and heat shock protein gene expression in the reef coral Montastraea franksi in response to environmental toxicants. Aquat. Toxicol. 2009;93:188–195. doi: 10.1016/j.aquatox.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Shafir S, Van Rijn J, Rinkevich B. Short and long term toxicity of crude oil and oil dispersants to two representative coral species. Environ. Sci. Tech. 2007;41:5571–5574. doi: 10.1021/es0704582. [DOI] [PubMed] [Google Scholar]
- 62.Epstein N, Bak RPM, Rinkevich B. Toxicity of third generation dispersants and dispersed egyptian crude oil on red sea coral larvae. Mar. Pollut. Bull. 2000;40:497–503. doi: 10.1016/S0025-326X(99)00232-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.