Abstract
Clopidogrel efficacy is influenced by genetic variation of cytochrome P450 (CYP)2C19, however, few studies have considered patients who have a stroke. We used electronic medical records (EMRs) linked to a bioresource to examine real‐world implications of clopidogrel pharmacogenetics in stroke. Patients hospitalized for any arterial thrombo‐occlusive (ATO) event who subsequently redeemed clopidogrel prescriptions in the community were entered into the study (n = 651). During 24‐month follow‐up, the primary endpoint of recurrent ATO or death occurred in 299 patients (46%). CYP2C19*2 loss‐of‐function allele carriers had an increased risk (hazard ratio (HR) = 1.29; 95% confidence interval (CI) = 1.04–1.59; P = 0.019). In the ischemic stroke subgroup (n = 94), the estimate of risk was greater (HR = 2.23; 95% CI = 1.17–4.24; P = 0.015), which was further supported by a meta‐analysis of available studies. In conclusion, we have demonstrated the clinical impact of CYP2C19*2 on clopidogrel efficacy using a purely EMR approach. This suggests that the risk in the ischemic stroke population may be particularly high.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ The loss‐of‐function variants of CYP2C19 are known to impair the bioactivation and therapeutic efficacy of clopidogrel. However, no studies have considered patients who have ischemic strokes treated with clopidogrel monotherapy in white populations.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ We set out to develop and test a model to investigate the association of CYP2C19*2 genotype and clinical outcomes in clopidogrel‐treated individuals using EMR linkage alone. Using this model, we investigated the risk associated with CYP2C19*2 specifically in a subgroup of patients who have an ischemic stroke.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
☑ We demonstrated that it is possible to detect an association between CYP2C19 genotype and clopidogrel efficacy in a real‐world setting using purely observational data. Furthermore, the risk associated with CYP2C19*2 seemed to be greater in the ischemic stroke population largely prescribed clopidogrel monotherapy.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE
☑ Similar methods could be used to investigate the pharmacogenetics of other antiplatelet or antithrombotic drugs in bioresources linked to EMRs. Pharmacogenetic‐guided antiplatelet therapy should be considered in patients who have an ischemic stroke.
Recurrent arterial thrombo‐occlusive (ATO) events are a major cause of disability and death in economically developed nations. Antiplatelet drugs can reduce the risk of recurrence.1 Clopidogrel is an antiplatelet prodrug with a broad clinical evidence‐base and is widely prescribed. Its active metabolite binds irreversibly to platelet adenosine diphosphate P2Y12 receptors inhibiting platelet aggregation. The activation of clopidogrel is a two‐step process catalyzed by several cytochrome P450 (CYP) enzymes with CYP2C19 playing the most important role in vivo.2, 3, 4 In European populations, much of the variability in clopidogrel active metabolite formation can be explained by a single genetic variant, designated CYP2C19*2 (c.681G>A, rs4244285).5 This is a loss‐of‐function allele that results in an inactive form of the CYP2C19 enzyme and impaired clopidogrel bioactivation.6 About 25% of the white population carry this variant and when these individuals are treated with clopidogrel they are at greater potential risk of ATO.7, 8, 9 Because of this, the US Food and Drug Administration issued a boxed warning in 2010 recommending “that healthcare professionals consider use of other antiplatelet medications or alternative dosing strategies for patients identified as poor metabolizers.” In spite of this recommendation, patients who are treated with clopidogrel largely do not get tested for their genetically determined metabolizer status and, thus, CYP2C19 poor metabolizers do not have their antiplatelet therapy adjusted.
Most of the data about the clinical impact of clopidogrel pharmacogenetics is derived from patients with coronary artery disease in whom dual antiplatelet therapy, usually comprising aspirin and clopidogrel, has been the norm. However, clopidogrel monotherapy is recommended as first‐line therapy for secondary prevention for patients with noncardioembolic ischemic stroke in the United Kingdom.10, 11 Patients with ischemic stroke treated with clopidogrel monotherapy may, therefore, be more susceptible to the impact of genetically determined clopidogrel poor metabolizer status. The American Heart Association guidelines for stroke from 2014 indicate the option for the use of clopidogrel and aspirin only during the first 21 days after minor stroke or transient ischemic attack (TIA).12 Very recently, a meta‐analysis of large studies from China, including clopidogrel‐treated patients with stroke or TIA, indicated increased risk of recurrent stroke and vascular endpoints for the carriers of CYP2C19 loss‐of‐function allele.13 However, few European studies have directly considered the impact of genetic variation on outcomes specifically in a stroke population treated with clopidogrel monotherapy, and thus genotype‐based dosing guidelines for coronary artery disease cannot be directly applied to patients who have a stroke.5, 14, 15, 16
Bioresources linked to electronic medical records (EMRs) are potentially powerful and flexible platforms for exploring the real‐world potential of genomics for patient stratification to improve the efficacy of drugs. However, models need to be developed to make the best use of data collected for routine clinical practice. Our objective was to use a purely record linkage approach in the Genetics of Diabetes Audit and Research Tayside Study (GoDARTS) to optimally detect the risk of recurrent ATO in clopidogrel‐treated individuals who had been Mendelian‐randomized to possession of the CYP2C19*2 allele compared to those not possessing this variant. We subsequently determined the risk in an ischemic stroke subgroup of patients.
RESULTS
Clopidogrel prescribed for secondary prevention of ATO
2,496 subjects participating in GoDARTS had genotype information for CYP2C19*2 available and a record of having redeemed at least one prescription for clopidogrel in a community pharmacy. There were 1,657 individuals who redeemed at least one prescription for clopidogrel following hospitalization for ATO (myocardial infarction, ischemic stroke, or peripheral artery disease). Restricting inclusion of patients to those who redeemed the prescription within the first 21 days from the date of hospitalization resulted in a final study population of 651 individuals. Of these, the qualifying ATO to be included in the analysis was myocardial infarction for 555 individuals (85%) and ischemic stroke for 91 individuals (14%). For 2 individuals, the qualifying event was peripheral artery disease, and 3 individuals had experienced both myocardial infarction and stroke. The allele frequency of CYP2C19*2 was 14.3% and the observed genotype frequencies were consistent with Hardy‐Weinberg equilibrium. In the group that possessed no loss‐of‐function alleles (CYP2C19*1/*1; n = 477), 209 (44%) had a recurrent ATO or death during the subsequent 24‐month follow‐up period from the date of the first clopidogrel prescription. In the group that possessed at least one loss‐of‐function allele (CYP2C19*1/*2 or *2/*2 genotype; n = 174), there were 90 (52%) recurrent events (Table 1). This gave an unadjusted incidence rate ratio of 1.36 (P = 0.017) with the population attributable risk for being a CYP2C19 loss‐of‐function allele carrier of about 8%. A Cox regression additive model for CYP2C19 genotype adjusted for age at study entry, sex, exposure at baseline to aspirin, and/or proton pump inhibitors, found an increased risk of CYP2C19*2 allele carrier status with a hazard ratio (HR) of 1.29 (95% confidence interval (CI) = 1.04–1.59; P = 0.019; Table 2). A Kaplan‐Meier plot of time to first event is shown in Figure 1 a. The results remained just significant when limiting the study population to those whose qualifying event was myocardial infarction only (HR = 1.25; 95% CI = 1.00–1.57; P = 0.049).
Table 1.
Characteristics of the full study population and the ischemic stroke subpopulation by genotype and recurrent events (all thrombo‐occlusive events and death)
| Mean age, years | Male sex (%) | Total years of follow‐up (no. of individuals) | No. of events | Yearly incidence rate | |
|---|---|---|---|---|---|
| Full population | |||||
| CYP2C19*1/*1 | 70 | 301 (63) | 560 (477) | 209 | 0.37 |
| CYP2C19*1/*2 or *2/*2 | 71 | 114 (66) | 178 (174) | 90 | 0.51 |
| Total | 70 | 415 (64) | 737 (651) | 299 | 0.41 |
| Ischemic stroke subpopulation | |||||
| CYP2C19*1/*1 | 74 | 42 (63) | 93.1 (67) | 17 | 0.18 |
| CYP2C19*1/*2 or *2/*2 | 76 | 16 (59) | 29.4 (27) | 11 | 0.37 |
| Total | 74 | 58 (62) | 122 (94) | 28 | 0.23 |
Table 2.
The full Cox regression model for the endpoint of any thrombo‐occlusive event or death in the full study population and the ischemic stroke subpopulation
| HR | 95% CI | P value | |
|---|---|---|---|
| Full population | |||
| CYP2C19, additive | 1.29 | 1.04–1.59 | 0.019 |
| Age, years | 1.01 | 1.00–1.02 | 0.206 |
| Male sex | 0.93 | 0.74–1.18 | 0.561 |
| Aspirin use | 0.92 | 0.73–1.16 | 0.483 |
| PPI use | 1.02 | 0.77–1.36 | 0.887 |
| Ischemic stroke subpopulation | |||
| CYP2C19, additive | 2.23 | 1.17–4.24 | 0.015 |
| Age, years | 1.01 | 0.97–1.05 | 0.653 |
| Male sex | 0.66 | 0.30–1.43 | 0.287 |
| Aspirin use | 0.25 | 0.03–1.95 | 0.186 |
| PPI use | 2.12 | 0.83–5.38 | 0.114 |
CI, confidence interval; HR, hazard ratio; PPI, proton pump inhibitor.
Figure 1.
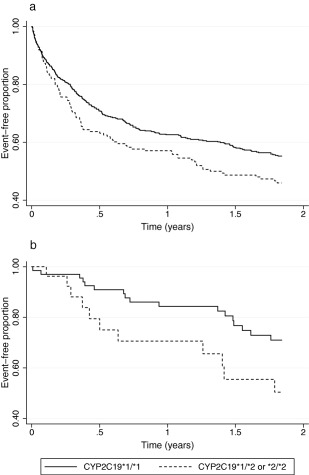
Kaplan‐Meier curves of all arterial thrombo‐occlusive events and death for (a) the whole clopidogrel‐treated population and (b) the ischemic stroke subpopulation.
Clopidogrel‐treated ischemic stroke subpopulation
435 members of the study cohort had experienced an ischemic stroke prior to the first prescription of clopidogrel. However, again restricting inclusion of patients to those redeeming a first prescription for clopidogrel in the community within the first 21 days after the date of hospitalization resulted in a study population of 94 individuals (including the three individuals who had a record of both ischemic stroke and myocardial infarction within 21 days). The allele frequency of CYP2C19*2 in this smaller group was 16.0% and the genotype frequencies were consistent with Hardy‐Weinberg equilibrium. In this population, we found a higher estimation of risk by CYP2C19 genotype (Table 1, Figure 1 b) compared to the full study population. In a Cox regression model, which included the same covariates as the full population, the HR was 2.23 (95% CI = 1.17–4.24; P = 0.015; Table 2). Only 10 of these individuals were prescribed aspirin concomitantly.
DISCUSSION
We have performed a real‐world study using purely EMR linkage of routine administrative clinical data in GoDARTS in the Tayside region of Scotland. We have demonstrated the feasibility of investigating the clinical impact of Mendelian‐randomization to possession of the CYP2C19*2 loss‐of‐function allele in patients redeeming prescriptions for clopidogrel following hospitalization for an ATO. Although we have only considered a single loss‐of‐function allele, this study further underscores the uncomfortable implication that at least one in every four patients who are prescribed clopidogrel will receive significantly reduced benefit due to their genotype and remain at higher risk of future ATOs. It also suggests that patients with ischemic stroke who subsequently receive clopidogrel monotherapy, which due to current guidelines is largely the case in the United Kingdom, where this study was undertaken, may be even more exposed to further acute events compared with patients with myocardial infarction who usually receive dual antiplatelet therapy. Given that the recent trial for use of ticagrelor in acute ischemic stroke17 has not been encouraging, it is likely that these guidelines will remain unchanged in the foreseeable future. Interestingly, however, in the PLATO genetic substudy, ticagrelor was not, in fact, significantly better than clopidogrel in the group with no CYP2C19 loss‐of‐function alleles.9
By demonstrating the clinical impact of a well‐established pharmacogenetic paradigm in a bioresource linked to extensive EMRs using a purely record linkage approach, this study indicates the general utility of such resources to investigate real‐world clinical implications of genetic variability influencing drug efficacy and safety across a wide array of clinical situations.18 Indeed, we have demonstrated this previously in GoDARTS in the context of efficacy and side effects of statins.19, 20 To the best of our knowledge, the only two other studies that have used a bioresource linked to EMRs to investigate CYP2C19 genotype in the context of clopidogrel therapy also utilized manual review of electronically defined recurrent cardiovascular events.21, 22 Our HR is lower than that estimated in the study by Delaney et al.,21 although the use of a case‐control methodology in that study meant that a population‐based HR was not possible to be determined and, therefore, cannot be directly compared with our data. As we had access to hospital admission records and a prescription dataset, which includes all community‐dispensed prescriptions, we could reliably identify patients who were hospitalized due to an acute ATO and were subsequently initiated on clopidogrel using a straightforward model. As such, our approach could be applied to test other hypotheses, such as pharmacogenetics of other antiplatelet drugs.
In 2010, UK guidelines for the secondary prevention of stroke were updated to recommend clopidogrel monotherapy and, prior to, that most ischemic strokes would have been treated with a combination of aspirin and dipyridamole meaning that our substudy was relatively small but comprised patients treated predominantly with clopidogrel monotherapy.10 When comparing our results with previous studies, it should be noted that there are differences in treatment practices between countries and guidelines.12, 23 Initial dual antiplatelet therapy with aspirin and clopidogrel is recommended in some cases of, for example, intracranial stenosis. Also, dual antiplatelet therapy is suggested for initiation within 24 hours of a minor ischemic stroke or TIA and for continuation for 21 days in the American Heart Association guidelines.12
A recent meta‐analysis of studies, including 4,762 clopidogrel‐treated patients with stroke or TIA, indicated an increased risk for recurrent stroke in CYP2C19 loss‐of‐function allele carriers.13 The overall estimate of the risk ratio for composite vascular events was 1.51 (95% CI = 1.10–2.06), which is very similar to results obtained in the two meta‐analyses of studies in patients with coronary artery disease with HRs of 1.57 (95% CI = 1.13–2.16) and 1.50 (95% CI = 1.21–1.87).7, 8 It is noteworthy that the stroke meta‐analysis included patients with various states of cerebrovascular disease, and more than half of the patients were treated with dual antiplatelet therapy. Most of the patients included in the analysis were Chinese and only 386 patients (8% of the total 4,762) were of white origin and of these over 90% were from two studies using dual antiplatelet therapy.22, 24 Interestingly, the risk of vascular endpoints in relation to CYP2C19 genotype seems to be lower in studies in which the study protocol clearly included dual antiplatelet therapy for 90 days or longer than in studies using clopidogrel monotherapy.13 Indeed, excluding the small number of individuals who were co‐prescribed aspirin in our study further increased the HR (data not shown). A meta‐analysis of our study with the one study clearly including patients solely on clopidogrel monotherapy, which had a very similar point estimate of risk compared to our study, provides an overall estimate of the HR for CYP2C19 loss‐of‐function allele carriers of 2.28 (95% CI = 1.53–3.40; P = 0.00005; Figure 2). Notably, this point estimate is higher than the upper 95% CI limits in the meta‐analyses of patients with coronary artery disease.7, 8 These findings suggest that patients who have ischemic strokes treated with clopidogrel monotherapy may be at greater risk based on their CYP2C19 genotype than patients treated with dual antiplatelet therapy regardless of indication. It is biologically plausible that individuals on clopidogrel monotherapy in whom its bioactivation is impaired are especially vulnerable to recurrent events, whereas the use of aspirin concomitantly could diminish the CYP2C19 genotype effect. These results underline the fact that the implications of CYP2C19 genotype should not be directly extrapolated to different disease states or treatment strategies (e.g. clopidogrel monotherapy vs. dual antiplatelet therapy).
Figure 2.
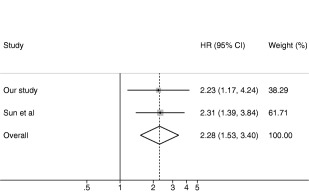
Meta‐analysis of our study with the study by Sun et al.16 HR, hazard ratio; CI, confidence interval.
One limitation of our study is that we investigated the effect of only a single CYP2C19 variant. Other CYP2C19 loss‐of‐function alleles have been described, but their minor allele frequencies are low in white populations, making their relevance limited for the current study. On the other hand, CYP2C19*17 allele has been associated with enhanced clopidogrel response and increased risk of bleeding in some studies.25, 26, 27, 28 Variants in other genes, including ABCB1, CES1, and PON1, have been suggested to play a role in the interindividual variability of clopidogrel response, but these results have not been widely replicated.27, 29, 30 A further weakness is that we were unable to include data in relation to medical or other treatments during the time period in the hospital with information on treatment coming entirely from redemption of prescriptions from community pharmacies. This was the principal reason for requiring undertaking the sensitivity analysis for optimizing the time between hospital admission and the first community‐redeemed clopidogrel prescription. Finally, due to the limited sample size and limitations of the EMR data, we could not include all environmental or genetic factors that could influence clopidogrel response.31, 32, 33 However, it is unlikely that these factors would be differently distributed between the CYP2C19 genotype groups, as there is no evidence linking CYP2C19 with any diseases or conditions independent of drug metabolism and response, and, thus, they are not likely to introduce bias to our analysis.5
In conclusion, we have shown that using purely observational routinely collected healthcare data and record linkage methodology, it is possible to demonstrate the real‐world impact of CYP2C19 genotype on clopidogrel efficacy. Approximately one quarter of patients with ischemic stroke or TIA who are treated with clopidogrel remain at significantly greater risk of future events because of their CYP2C19 genotype. There are potentially several alternative treatments available for such individuals, including increasing the dose of clopidogrel, however, one of the major impediments to clinically identifying them is the lack of evidence for clinical effectiveness of specifically genotyping all patients to identify this subgroup. With the growing number of individuals now taking part in large biorepositories linked to de‐identified patient EMR data, there is an ideal opportunity and arguably an ethical imperative to perform such studies by linking pre‐existing genotypes in these bioresources directly to hospital systems so that genotype data can be used to alter therapy appropriately.
METHODS
Source population
The GoDARTS is a large TENOVUS and Wellcome Trust funded study derived from the Tayside region of Scotland for investigating genetic determinants of type 2 diabetes, response to therapy, and its complications. GoDARTS comprises approximately 10,500 patients with type 2 diabetes and 7,500 individuals with a similar demographic but with no diagnosis of type 2 diabetes at recruitment. Recruitment into GoDARTS commenced in 1997 and was complete in 2007 and, therefore, individuals can have up to 19 years of follow‐up. All individuals provided a blood sample for DNA extraction and genotyping as well as consent for linkage of this information to detailed longitudinal EMRs that are available through an established regional clinical health informatics research infrastructure. Genotyping for CYP2C19*2 had taken place previously as component of genetic studies in the GoDARTS bioresource. There were 15,317 subjects who had genotype information available for the CYP2C19*2 polymorphism. Of these, 11,346 (74.1%) were noncarriers of the CYP2C19*2 allele and were designated as CYP2C19*1/*1, 3,656 (23.9%) had the CYP2C19*1/*2 genotype, and 315 (2.1%) had the CYP2C19*2/*2 genotype (CYP2C19*2 allele frequency = 0.14). GoDARTS was approved by the Tayside Medical Ethics Committee. Informed consent was obtained from all participants.
Clinical datasets used
The main clinical datasets used for this study were the Tayside prescribing dataset, originally developed through the Medicines Monitoring Unit (MEMO) at the University of Dundee, which contains a longitudinal record of all prescriptions dispensed by community pharmacies; the Scottish Morbidity Records (SMR01), which contains all hospital discharge diagnoses coded as International Classification of Disease (ICD)‐9 and ICD‐10, and General Register Office (GRO) information containing date and cause of death (also ICD coded). These data are provided in anonymized form through the Health Informatics Centre at the University of Dundee and linked to genetic data assimilated by the Centre for Pharmacogenomics.
Study population
The study population comprised individuals in GoDARTS who had been genotyped for CYP2C19*2 polymorphism and who had also redeemed at least one prescription for clopidogrel. As our study relied upon hospital admission data for ATO but community redemption of medicines following hospital discharge, this meant that there was a time period between the date of qualifying event (date of hospital admission) and the date of the first knowledge of exposure to clopidogrel. It is established that the majority of recurrent ATO occur rapidly within the first few weeks of the index event. Therefore, we undertook a sensitivity analysis to determine the optimum time difference between the date of hospital admission for the index event and the first community encashment of clopidogrel for inclusion in the analysis. This balanced larger numbers of individuals for inclusion who had been in the hospital longer with potentially more severe disease and greater treatment intensity against smaller numbers of patients who were more ambulatory with lower treatment intensities and earlier risk. To ensure the population had active disease, the sensitivity analysis resulted in an optimum time between hospital admission and prescription encashment to be no greater than 21 days (3 weeks) following a hospital admission for any ATO (myocardial infarction, ischemic stroke, or peripheral artery disease) derived from the ICD codes on hospital admission. A substudy of this population was restricted to those individuals whose qualifying ischemic event had been an ischemic stroke.
Statistical analysis
Cox's proportional hazards model was used to compare the rate of endpoints between individuals carrying one or two CYP2C19*2 alleles and noncarriers of CYP2C19*2 during exposure to clopidogrel. Because of the limitations of using purely observational data available through medical record linkage, we considered the study population to have become at risk due to the presence of the CYP2C19*2 allele after community encashment of their first clopidogrel prescription. Subjects were followed until the primary endpoint, which was either a hospitalization for a further ATO or their death from any cause. Censoring was at 24 months (96 weeks), or when no further data was available in the record (i.e., the subject had left the Tayside area or the end of study date occurred, which was 14 February 2016). Exposure to clopidogrel was modeled based on the first community‐redeemed prescription and we did not censor for apparent further changes in clopidogrel prescribing, as it would have been impossible to know whether the events occurred on or off treatment. We also included in the model exposure to aspirin and proton pump inhibitors because both of these may influence outcome. Due to the relatively small numbers available, we simply modeled exposure to any proton pump inhibitor. All analyses were adjusted for age at becoming at risk (first dispensed clopidogrel prescription) and gender. STATA version 11.2 (StataCorp LP, College Station, TX) was used for all analyses.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
A.T., C.N.A.P., T.M.D., and A.S.F.D. wrote the manuscript. R.F., C.N.A.P., T.M.D., and A.S.F.D. designed the research. A.T., R.F., S.M., E.V., and A.S.F.D. performed the research. A.T. and A.S.F.D. analyzed the data.
ACKNOWLEDGMENTS
We acknowledge the support of the Health Informatics Centre, University of Dundee for managing and supplying the anonymized data. A.T. was supported by a Fellowship grant from Sigrid Jusélius Foundation (Helsinki, Finland).
References
- 1. Antithrombotic Trialists' Collaboration . Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324, 71–86 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dansette, P.M. , Rosi, J. , Bertho, G. & Mansuy, D. Cytochromes P450 catalyze both steps of the major pathway of clopidogrel bioactivation, whereas paraoxonase catalyzes the formation of a minor thiol metabolite isomer. Chem. Res. Toxicol. 25, 348–356 (2012). [DOI] [PubMed] [Google Scholar]
- 3. Hulot, J.S. et al Cytochrome P450 2C19 loss‐of‐function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood 108, 2244–2247 (2006). [DOI] [PubMed] [Google Scholar]
- 4. Kazui, M. et al Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab. Dispos. 38, 92–99 (2010). [DOI] [PubMed] [Google Scholar]
- 5. Scott, S.A. et al Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin. Pharmacol. Ther. 94, 317–323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brandt, J.T. et al Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J. Thromb. Haemost. 5, 2429–2436 (2007). [DOI] [PubMed] [Google Scholar]
- 7. Mao, L. et al Cytochrome CYP2C19 polymorphism and risk of adverse clinical events in clopidogrel‐treated patients: a meta‐analysis based on 23,035 subjects. Arch. Cardiovasc. Dis. 106, 517–527 (2013). [DOI] [PubMed] [Google Scholar]
- 8. Mega, J.L. et al Reduced‐function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta‐analysis. JAMA 304, 1821–1830 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wallentin, L. et al Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet 376, 1320–1328 (2010). [DOI] [PubMed] [Google Scholar]
- 10. National Institute for Health and Care Excellence . Clopidogrel and modified‐release dipyridamole for the prevention of occlusive vascular events. NICE technology appraisal guidance. <https://www.nice.org.uk/guidance/ta210>. Accessed 23 January 2017. [DOI] [PubMed]
- 11. Diener, H.C. et al Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial. Lancet 364, 331–337 (2004). [DOI] [PubMed] [Google Scholar]
- 12. Kernan, W.N. et al Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45, 2160–2236 (2014). [DOI] [PubMed] [Google Scholar]
- 13. Pan, Y. et al Genetic polymorphisms and clopidogrel efficacy for acute ischemic stroke or transient ischemic attack: a systematic review and meta‐analysis. Circulation 135, 21–33 (2017). [DOI] [PubMed] [Google Scholar]
- 14. Scott, S.A. et al Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450‐2C19 (CYP2C19) genotype and clopidogrel therapy. Clin. Pharmacol. Ther. 90, 328–332 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jia, D.M. et al CYP2C19 polymorphisms and antiplatelet effects of clopidogrel in acute ischemic stroke in China. Stroke 44, 1717–1719 (2013). [DOI] [PubMed] [Google Scholar]
- 16. Sun, W. et al Variant recurrent risk among stroke patients with different CYP2C19 phenotypes and treated with clopidogrel. Platelets 26, 558–562 (2015). [DOI] [PubMed] [Google Scholar]
- 17. Johnston, S.C. et al Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N. Engl. J. Med. 375, 35–43 (2016). [DOI] [PubMed] [Google Scholar]
- 18. Sherman, R.E. et al Real‐world evidence ‐ what is it and what can it tell us? N. Engl. J. Med. 375, 2293–2297 (2016). [DOI] [PubMed] [Google Scholar]
- 19. Donnelly, L.A. et al Common nonsynonymous substitutions in SLCO1B1 predispose to statin intolerance in routinely treated individuals with type 2 diabetes: a go‐DARTS study. Clin. Pharmacol. Ther. 89, 210–216 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Donnelly, L.A. et al Apolipoprotein E genotypes are associated with lipid‐lowering responses to statin treatment in diabetes: a Go‐DARTS study. Pharmacogenet. Genomics 18, 279–287 (2008). [DOI] [PubMed] [Google Scholar]
- 21. Delaney, J.T. et al Predicting clopidogrel response using DNA samples linked to an electronic health record. Clin. Pharmacol. Ther. 91, 257–263 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoh, B.L. et al CYP2C19 and CES1 polymorphisms and efficacy of clopidogrel and aspirin dual antiplatelet therapy in patients with symptomatic intracranial atherosclerotic disease. J. Neurosurg. 124, 1746–1751 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piepoli, M.F. et al 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 37, 2315–2381 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDonough, C.W. et al CYP2C19 metabolizer status and clopidogrel efficacy in the Secondary Prevention of Small Subcortical Strokes (SPS3) study. J. Am. Heart Assoc. 4, e001652 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sibbing, D. et al Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel‐treated patients with coronary stent placement. Circulation 121, 512–518 (2010). [DOI] [PubMed] [Google Scholar]
- 26. Lewis, J.P. et al The CYP2C19*17 variant is not independently associated with clopidogrel response. J. Thromb. Haemost. 11, 1640–1646 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simon, T. et al Genetic determinants of response to clopidogrel and cardiovascular events. N. Engl. J. Med. 360, 363–375 (2009). [DOI] [PubMed] [Google Scholar]
- 28. Li, Y. , Tang, H.L. , Hu, Y.F. & Xie, H.G. The gain‐of‐function variant allele CYP2C19*17: a double‐edged sword between thrombosis and bleeding in clopidogrel‐treated patients. J. Thromb. Haemost. 10, 199–206 (2012). [DOI] [PubMed] [Google Scholar]
- 29. Trenk, D. & Hochholzer, W. Genetics of platelet inhibitor treatment. Br. J. Clin. Pharmacol. 77, 642–653 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tarkiainen, E.K. et al Carboxylesterase 1 c.428G>A single nucleotide variation increases the antiplatelet effects of clopidogrel by reducing its hydrolysis in humans. Clin. Pharmacol. Ther. 97, 650–658 (2015). [DOI] [PubMed] [Google Scholar]
- 31. Holmberg, M.T. , Tornio, A. , Neuvonen, M. , Neuvonen, P.J. , Backman, J.T. & Niemi, M. Grapefruit juice inhibits the metabolic activation of clopidogrel. Clin. Pharmacol. Ther. 95, 307–313 (2014). [DOI] [PubMed] [Google Scholar]
- 32. Scott, S.A. et al Exome sequencing of extreme clopidogrel response phenotypes identifies B4GALT2 as a determinant of on‐treatment platelet reactivity. Clin. Pharmacol. Ther. 100, 287–294 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Samant, S. et al Identifying clinically relevant sources of variability: the clopidogrel challenge. Clin. Pharmacol. Ther. 101, 264–273 (2017). [DOI] [PubMed] [Google Scholar]


