Abstract
Since control of atopic dermatitis (AD) remains challenging but has not been adequately characterized, the objective of this study was to characterize disease control among patients with a history of moderate to severe AD. Data were from the 2014 Adelphi US AD Disease Specific Programme, a cross‐sectional survey of physicians (n = 202) and their patients with history of moderate to severe AD (n = 1064, 54% female, 75% white, mean age 40 years). Inadequately controlled AD as rated by the physician was defined as currently flaring; deteriorating/changeable AD; or physician dissatisfaction with current control. The overall inadequate control rate was 58.7% (n = 625), which increased with current AD severity and was observed in 53.4% and 83.4% of patients receiving immunosuppressants and systemic corticosteroids, respectively. Relative to controls, inadequately controlled patients had poorer disease‐specific quality of life, higher level of work impairment, greater itch and sleep interference with daily living (all P < 0.05). Multivariate analysis showed factors significantly associated with inadequate control (all P < 0.05), including Hispanic race, symptoms on the head/neck or lower limbs, itch and sleep interference with daily living. A limitation of the study was reliance on accuracy of reporting, potential selection bias and cross‐sectional study design. In summary, there was a high rate and substantial impact of physician‐rated inadequately controlled disease among patients with a history of moderate to severe AD, suggesting the need for more effective therapies.
Keywords: atopic dermatitis, burden, disease control, patient‐reported outcomes, quality of life
Introduction
Atopic dermatitis (AD) is clinically characterized by eczema and intense itching, with disease severity that is moderate and severe in 20–37% and 10–34% of patients, respectively.1, 2 Although adult onset can occur, AD generally initiates during early childhood, extending into adulthood in approximately half of the cases.3 AD is associated with a multidimensional patient burden including itch, sleep disturbances, anxiety/depression, reductions in function/productivity and lower quality of life (QoL) relative to patients without AD.4, 5, 6, 7, 8, 9, 10, 11, 12, 13
Control of AD remains challenging despite treatment guidelines, which recommend topical corticosteroids as first‐line therapy.14, 15, 16 However, these therapies have limited efficacy in moderate to severe AD, and their long‐term use is associated with side‐effects.14, 15
Current guidelines discourage general use of systemic immunomodulatory agents including oral corticosteroids, methotrexate, mycophenolate mofetil and azathioprine, with cyclosporin reserved for refractory cases.16 These agents are not approved in the USA for AD and have limited evidence of efficacy and safety.17 In an international survey of patients with moderate to severe AD, three‐quarters of patients and caregivers stated that effective control would be the single most important contribution to QoL.18
Chronic diseases are often treated to a specific target that defines disease control, such as glycated hemoglobin of less than 7% for diabetes or serum uric acid levels less than 6 mg/dL for gout. However, few studies have investigated AD control in the real‐world setting, especially among adults with moderate to severe disease. Furthermore, there are no data on factors that may be predictive of inadequately controlled AD. Therefore, the purposes of this study were to characterize uncontrolled AD in routine clinical practice among adults with a history of moderate to severe AD, and identify factors associated with failure to attain disease control.
Methods
Data source and populations
The Adelphi AD Disease Specific Programme (DSP) is a cross‐sectional, real‐world survey that captures data from the perspective of doctors and their consulting patients;19 collection of patient data is compliant with the Health Insurance Portability and Accountability Act of 1996 and the Health Information Technology for Economic and Clinical Health Act of 2009. Data in the current study were from the 2014 Adelphi US AD DSP. By checking a box, patients provided consent for the aggregated analysis and reporting of results.
Screening and recruitment of doctors reflect nationally representative samples subject to meeting DSP inclusion criteria, which for the current study were primary care physicians (PCP; including internal medicine specialists), dermatologists or allergists/immunologists who qualified between 1978 and 2011; were practicing in the USA with active involvement in the pharmaceutical management of AD patients; and saw a minimum number of moderate to severe (as assessed by the physician) AD patients per month (≥5 for PCP, ≥6 for dermatologists and ≥15 for allergists/immunologists). Physicians were requested to complete a Patient Record Form (PRF) for the next five prospective patients eligible for inclusion, and each specialist physician was requested to provide two additional prospective PRF for patients receiving a systemic immunosuppressant therapy.
For inclusion, patients were required to be adults (≥18 years) with, in the physician's subjective opinion, a confirmed diagnosis or history of moderate to severe AD and not currently enrolled in a clinical trial. Patients with mild AD at the time of consultation could be included, if at some point previously in their disease course they were considered moderate or severe. Physicians rated AD severity in response to the question, “What is your overall assessment of the severity of atopic dermatitis (AD) symptoms in this patient currently based on your own definitions of mild, moderate and severe?”.
Patients were classified whether they had inadequate AD control based on physician assessment. Inadequate control was defined as either: currently flaring AD; deteriorating or changeable AD; or physician dissatisfaction with current control evaluated on a 1–7 scale (1 = extremely dissatisfied, 7 = extremely satisfied), with scores 1–3 considered “dissatisfied”.
The analyses carried out in this study were conducted on an existing database. All analyses were conducted on the existing database; no new information was gathered for analysis purposes. Prior to receipt for analysis, all data were fully de‐identified. The original data collection for the database was conducted per the EphMRA code of conduct; no personal identifiable information was collected. The code stipulates that “market research relating to market or consumer behavior of the sort that pharmaceutical companies routinely commission, whether involving healthcare professionals, patients, carers or members of the public does not require Clinical Research Ethics Committee or Independent Review Board approval (Institutional Review Board in the USA)”. As such, no approval was required by an institutional review board.
Physician‐reported outcomes
The physician‐completed PRF objectively assessed AD including all information required to derive the Eczema Area and Severity Index (EASI)20 for each patient. Physicians reported the presence of anxiety, depression and stress based on the statement, “Provide your assessment of this patient's current status as a result of their AD against the below criteria, on a scale of 0 to 6 where 0 = none and 6 = severe” (cutoff >0 indicating presence). Itch interference with daily living and sleep disturbance were evaluated by the physician according to the question, “Based on your discussions with the patient or perceptions during the last week, how much interference has each of the following aspects of the patient's condition caused to their activities of daily living (excluding work)?” with responses on a scale of 0 (“none at all”) to 6 (“complete interference”); a value of 4 or more was considered “significant interference”.
Patient‐reported outcomes
At the time of consultation, patients were invited to complete a Patient Self‐Completion (PSC) form, independently of the physician, and return it in a sealed envelope. The PSC included questions and validated questionnaires on demographics and details relating to the patient's AD, QoL and productivity. Patients self‐rated their current AD severity based on the question, “Generally how bad was your atopic dermatitis (AD) in the past 24 h?” with responses of mild, moderate and severe. The validated Patient‐Oriented Eczema Measure (POEM) evaluated the presence of AD signs and symptoms in the past week and their impact on sleep (score ranging 0–28; higher scores indicating greater severity).21 QoL was evaluated using the Dermatology Life Quality Index (DLQI; score ranging 0–30; higher scores indicating greater impact on QoL).22 The Work Productivity and Activity Impairment Questionnaire for Specific Health Problems (WPAI:SHP)23 evaluated AD effects on productivity during the past 7 days.24
Statistical analyses
Bivariate analysis compared inadequately controlled versus controlled patients. The Mann–Whitney U‐test was used for numeric and ordinal data, and Fisher's exact test and Pearson's χ2‐test for categorical data. Multivariate analysis (logistic regression) was used to identify factors predictive of physician‐rated inadequate control, expressed as odds ratios (OR) with their 95% confidence interval (CI). Variables for inclusion in the model were guided by results from the bivariate analysis in conjunction with disease knowledge. Standard errors were adjusted in the model to allow intragroup correlation (or clustering) of patients within physician, using the Huber and White sandwich estimator of variances.25 Level of significance was set at P < 0.05. All analyses were performed using Stata Statistical Software: Release 14 (StataCorp, College Station, TX, USA).
Results
Population
After excluding patients whose condition was only ever mild and who were either diagnosed “today” or whose flare status was unknown, data were available for 1064 patients with a history of moderate to severe AD, representing 49 PCP, 52 internal medicine specialists, 73 dermatologists and 25 allergists/immunologists. The patient population was 54% female and 75% white, with a mean age of 40 years (Table 1). Overall, 655 (62%) patients agreed to complete the PSC.
Table 1.
Demographic characteristics of the patient population (n = 1064)
| Variable | Value |
|---|---|
| Age, years | 40.3 ± 16.0 |
| Race, n (%) | |
| White/Caucasian | 800 (75.2) |
| Non‐white/Caucasian | 264 (24.8) |
| African American | 130 (12.2) |
| Hispanic/Latino | 65 (6.1) |
| Other | 69 (6.5) |
| Sex, n (%) | |
| Male | 495 (46.5) |
| Female | 569 (53.5) |
| Employment status, n (%) | |
| Unemployed | 204 (19.6) |
| Employed | 836 (80.4) |
| Missing | 24 |
| Body mass index, n (%) | |
| Normal (18.5–24.9 kg/m2) | 432 (40.8) |
| Overweight (25.0–29.9 kg/m2) | 439 (41.4) |
| Obese (≥30 kg/m2) | 189 (17.8) |
| Missing | 4 |
| Time since diagnosis, years† | 12.4 ± 12.4 |
| No. of other atopic conditions‡ | 1.1 ± 1.1 |
| Charlson Comorbidity Index | 0.1 ± 0.6 |
| Flares in last 12 months§ | 1.9 ± 2.0 |
†n = 789, ‡ n = 980, § n = 883. Data are presented as mean ± standard deviation unless otherwise stated.
Inadequate AD control
Physicians rated 625 (58.7%) patients as inadequately AD controlled. Flares were the most common criterion for defining inadequate control (76.5%), followed by disease state (45.8%), and physician dissatisfaction (23.7%) (Fig. 1a). Some patients satisfied multiple criteria, with the greatest overlap between flares and changeable/deteriorating AD; all three criteria were satisfied in 8.5% of these patients (53/625). Higher proportions of patients met each criterion as physician‐ and patient‐reported severity increased (Fig. 1b).
Figure 1.
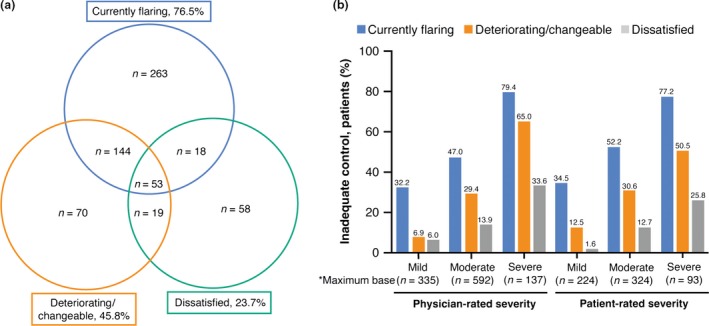
Inadequately controlled disease by criteria satisfied. (a) Frequency of inadequately controlled disease by criteria satisfied (n = 625). (b) Criteria satisfied by disease severity. *Base sizes across some variables are lower than reported due to missing data. Greatest overall level of missing data is 3.1%.
The proportion of patients for whom physicians reported dissatisfaction with disease control, 23.7%, was similar to the rate of treatment dissatisfaction reported by patients; 24.6% overall and 27.9% of those identified by physicians as having inadequate control. Among patients who reported being dissatisfied with treatment, a significantly higher proportion were rated as inadequately controlled by the physicians (66.9% vs 33.1%, P = 0.0276).
Rate of inadequate control significantly increased with current AD severity, regardless of whether severity was assessed objectively using derived EASI cut‐off scores (Fig. 2a)26 or subjectively based on physician or patient ratings (all P < 0.0001) (Fig. 2b). Physician ratings of control were not dependent on the physician specialty (Table 2). The majority of patients were rated by the physicians as being inadequately controlled despite current treatment across all evaluated treatment regimens including systemic therapies (Fig. 3). Although the frequency of inadequate control was significantly higher among patients not receiving systemic immunosuppressants relative to those receiving such drugs (60.5% vs 53.4%, P = 0.0441), the use of any systemic agent or phototherapy was associated with a higher rate of inadequate control relative to those not using these therapies (65.0% vs 53.3%, P = 0.0001) (Fig. 3).
Figure 2.
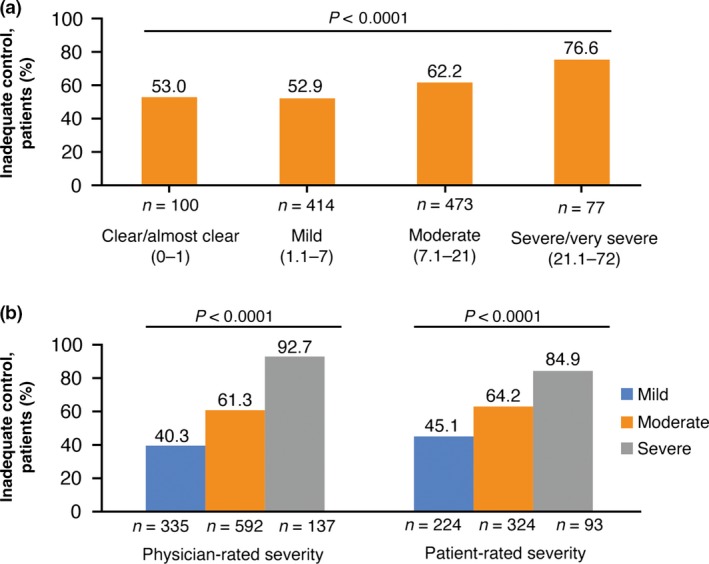
Inadequate control by measures of disease severity. (a) Severity assessed objectively based on derived Eczema Area and Severity Index score. (b) Subjective assessment of severity by physicians and patients.
Table 2.
Frequency of inadequate atopic dermatitis control based on physician specialty
| Specialty | No. (%) of patients | |
|---|---|---|
| Inadequate control (n = 625) | Controlled (n = 439) | |
| Primary care physiciana | 263 (42.1) | 188 (42.8) |
| Dermatologist | 278 (44.5) | 178 (40.5) |
| Allergist | 84 (13.4) | 73 (16.6) |
Includes internal medicine specialists.
Figure 3.
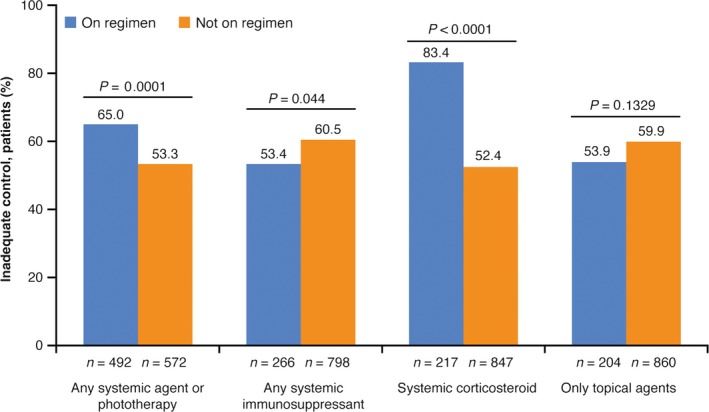
Inadequate control by treatment regimen.
Burden of inadequate control
The AD burden was greater among patients rated by the physicians as inadequately controlled compared with those rated as controlled for physician‐ and patient‐reported outcomes including mental health, itch and sleep interference with daily living, QoL and work productivity (Table 3). The differences were significant for all outcomes except physician‐reported anxiety. Additionally, inadequately controlled patients reported significantly greater disease activity on POEM (Table 3).
Table 3.
Burden of inadequately controlled atopic dermatitis
| Outcome | Inadequate control | Controlled | P |
|---|---|---|---|
| Physician‐reported, n/total (%)† | n = 625 | n = 439 | |
| Depression | 383/598 (64.0) | 224/426 (52.6) | 0.0003 |
| Anxiety | 445/605 (73.6) | 293/429 (68.3) | 0.0696 |
| Stress | 469/609 (77.0) | 292/431 (67.7) | 0.0011 |
| Itch interference with daily living | 293/609 (48.1) | 121/433 (27.9) | <0.0001 |
| Sleep disturbance interfering with daily living | 141/593 (23.8) | 36/425 (8.5) | <0.0001 |
| Patient‐reported | n = 398 | n = 257 | |
| POEM score | 11.7 ± 6.8 (n = 382) | 8.4 ± 5.8 (n = 252) | <0.0001 |
| DLQI score | 8.1 ± 6.6 (n = 366) | 5.6 ± 5.0 (n = 242) | <0.0001 |
| WPAI:SHP Overall Work Impairment, %‡ | 22.8 ± 22.2 (n = 243) | 18.4 ± 19.9 (n = 164) | 0.0398 |
†Reflects actual number of patients because data were missing for some outcomes. ‡Among employed patients who completed the Work Productivity and Impairment Index for Specific Health Problem (WPAI:SHP). Data are presented as mean ± standard deviation unless otherwise stated. DLQI, Dermatology Life Quality Index; POEM, Patient‐Oriented Eczema Measure.
Among employed patients who completed the WPAI:SHP (n = 407), those with inadequate control reported significantly greater overall work impairment relative to controlled patients (22.8% vs 18.4%, P = 0.0398) (Table 3).
Factors associated with inadequate control
In the bivariate analysis (Table 4), only for race among demographic characteristics was there a significant difference between physician ratings of inadequately controlled and controlled patients. While several clinical variables were significantly associated with inadequate control, including sites affected and the derived EASI score (both P < 0.0001), the percentage of body surface area affected was not significantly different between inadequately controlled and controlled patients (Table 4).
Table 4.
Bivariate analysis of demographic and clinical characteristics between inadequately controlled and controlled patients
| Variable | Inadequate control (n = 625) | Controlled (n = 439) | P |
|---|---|---|---|
| Age, years | 40.0 ±16.4 | 40.7 ±15.5 | 0.2691 |
| Race, n (%) | |||
| White/Caucasian | 441 (70.6) | 359 (82.3) | <0.0001* |
| Non‐white/Caucasian | 184 (29.4) | 80 (18.3) | |
| African American | 88 (14.1) | 42 (9.6) | |
| Hispanic/Latino | 49 (7.8) | 16 (3.6) | |
| Other | 47 (7.5) | 22 (5.0) | |
| Sex, n (%) | 0.1512 | ||
| Male | 279 (44.6) | 216 (49.2) | |
| Female | 346 (55.4) | 223 (50.8) | |
| Employment status, n (%)† | 0.2677 | ||
| Unemployed | 127 (20.8) | 77 (17.9) | |
| Employed | 484 (79.2) | 352 (82.1) | |
| Body mass index, n (%) | 0.0843 | ||
| Normal (18.5–24.9 kg/m2) | 273 (43.9) | 159 (36.3) | |
| Overweight (25.0–29.9 kg/m2) | 236 (37.9) | 203 (46.3) | |
| Obese (≥30 kg/m2) | 113 (18.2) | 76 (17.4) | |
| Head and neck currently affected, n (%) | 283 (45.3) | 141 (32.1) | <0.0001 |
| Upper limbs currently affected, n (%) | 498 (79.7) | 346 (78.8) | 0.7586 |
| Trunk currently affected, n (%) | 379 (60.6) | 225 (51.3) | 0.0026 |
| Lower limbs currently affected, n (%) | 424 (67.8) | 269 (61.3) | 0.0310 |
| EASI score | 10.4 ± 9.9 | 7.8 ± 6.7 | <0.0001 |
| BSA affected, % | 16.5 ± 15.8 | 14.0 ± 11.0 | 0.2794 |
| No. of flares in last 12 months | 2.5 ± 2.0‡ | 1.3 ± 1.7§ | <0.0001 |
| No. of baseline symptoms (day‐to‐day) | 4.9 ± 4.7¶ | 5.9 ± 3.5 | <0.0001 |
| No. of other atopic conditions | 1.2 ± 1.2†† | 1.0 ± 1.0‡‡ | 0.0175 |
| Charlson Comorbidity Index | 0.2 ± 0.6 | 0.1 ± 0.6 | 0.4882 |
*P‐value for non‐Hispanic white versus all others; P = 0.0003 when comparing all racial groups. †Non‐missing data n = 611 for inadequate control group and n = 429 for controlled group. ‡ n = 476, § n = 407, ¶ n = 592, †† n = 572, ‡‡ n = 408. Data are presented as mean ± standard deviation unless otherwise stated. BSA, body surface area; EASI, Eczema Area and Severity Index.
Variables that were identified in the regression model as significantly associated with a greater likelihood of inadequate control (Fig. 4) included Hispanic race, with an OR of 2.6 (95% CI = 1.2–5.7), indicating that Hispanics were 2.6‐times more likely to have inadequately controlled AD relative to non‐Hispanic whites, and symptom locations on the head/neck (OR = 1.6, 95% CI = 1.2–2.3) and on lower limbs (OR = 1.5, 95% CI = 1.1–2.1). Itch interference with daily living and sleep disturbance also showed an approximate two‐times higher likelihood of inadequately controlled AD. Only the number of baseline symptoms appeared to be significantly associated with controlled AD (OR = 0.9, 95% CI = 0.8–0.9).
Figure 4.
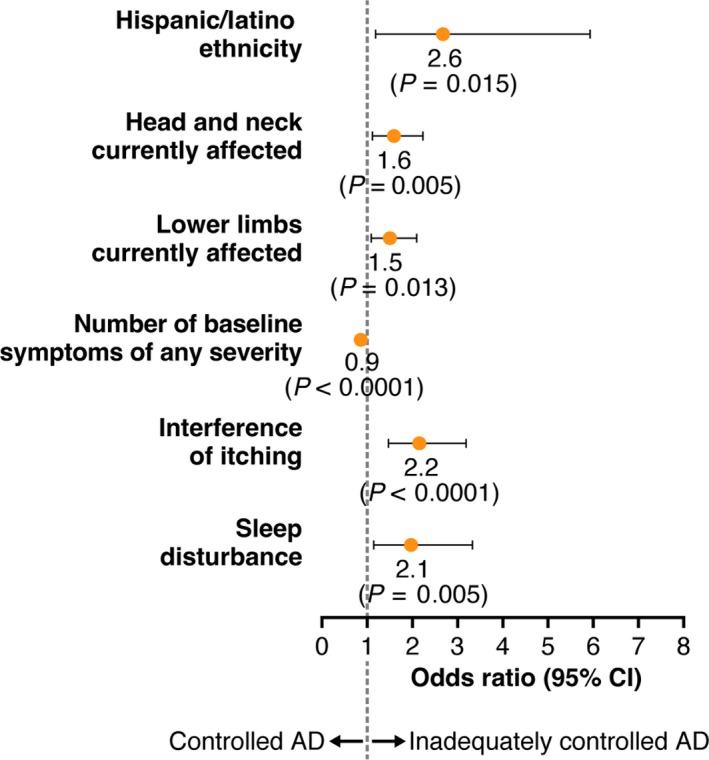
Variables significantly associated with disease control in multivariate analysis. AD, atopic dermatitis; CI, confidence interval.
Discussion
This study provides an initial effort at defining and characterizing patients with inadequately controlled moderate to severe AD, which was observed in more than half of the patients. Although the physician ratings of inadequate control, which relied on three criteria, has not been validated neither was it based on clinical measures, these criteria likely reflect real‐world clinical practice, especially because physicians report low awareness and use of clinical or patient‐reported measures for the assessment of AD and its severity.27 However, robustness of these criteria was supported by the observation that the proportion of patients with inadequate control for each criterion increased with AD severity regardless of whether severity was rated by the physicians or the patients themselves; inadequate control was also observed in a substantial proportion of patients currently rated as mild.
Current flares were the most common reason for inadequate control, which may not be surprising, because flares could potentially have been the driver of the physician consultation. Of greater clinical relevance, inadequate control occurred regardless of treatment, with more than half of the patients treated with each medication class, including systemic agents, rated by the physicians as being inadequately controlled. It is possible that the high rate of inadequate control among patients on systemic corticosteroids might have resulted from use of these drugs in response to current flares. While the lack of control may be interpreted as current therapies being less than optimal, there appeared to be low dissatisfaction with treatment by both physicians (23.7%) and patients (24.6%). These results suggest there may be disparity between disease control and perceptions of disease management, and also reflect a need for greater understanding of what may be expected during treatment.
Inadequate control was associated with higher burdens relative to adequately controlled patients, including significantly greater work impairment and lower disease‐specific QoL. Inadequately controlled patients also had a higher prevalence of depression and stress, with a trend toward higher anxiety. These mental health outcomes are important because they not only contribute to QoL,28, 29 but have additional implications, because suicidal ideation has been reported in AD patients.29, 30, 31
Physician‐reported itch interference and sleep disturbance were independent factors associated with inadequate control, and the OR of more than 2 indicated that inadequate control was twice as likely among patients with these outcomes compared with those without. These results support the interrelationship of these outcomes.8, 12, 32 In particular, sleep disturbance is frequently reported by patients with AD and its importance to patients is increasingly recognized,9, 33 as it correlates with QoL.8, 32
In the bivariate analysis, patients rated as inadequately controlled by the physicians were more likely to be currently affected by AD on their head and neck, trunk and lower limbs, had an overall higher severity rating as measured by EASI score and physician‐perceived severity, and had experienced a greater number of flares in the previous 12 months. In the multivariate analysis, the only variables related to symptom location that retained predictive significance for inadequate control were head and neck, and lower limbs. The multivariate analysis also identified Hispanic race as a significant predictor of inadequate control, which may be explained, at least in part, by observations that race may impact AD presentation,34 with suggestions of increased AD prevalence or severity in some non‐white racial groups. While black skin may present more challenges in diagnosis and symptom evaluation because of lack of recognition of symptoms/signs (redness/erythema),35 it is not known if similar issues arise in Hispanics or other racial groups who are also characterized by non‐white skin. Additional studies are needed to elucidate the relationship of skin color with AD symptoms (presentation, recognition and evaluation), diagnosis and disease control. Notably, body surface area, which is generally considered an indicator of disease severity, was not a significant predictor of inadequate disease control, suggesting that disease control encompasses more than just management of the skin condition.
Study limitations include selection bias; there were minimum requirements for physician participation, and physicians and patients who agreed to participate might have had characteristics different from those who did not agree. To minimize selection bias, physician participants in this study included PCP, allergists and dermatologists, and they recruited the next five consecutive consulting AD patients. However, findings from this study may still not be generalizable to the general AD population. For example, physicians treating small numbers of patients are likely to have been excluded due to the entry criterion stating they must have a minimum workload of AD patients. In addition, some physicians (e.g. PCP) may not provide all treatment choices such as phototherapy; and the clinical visit by the patients might have been triggered by a flare or some other immediate need for a consultation, and thus potential underrepresentation of stable mild patients who may consult less frequently or not at all. Furthermore, those treated with systemic immunosuppressants were oversampled, potentially underestimating the rate of control due to better efficacy relative to other medications. While the analysis may be potentially criticized for the use of non‐validated criteria to define inadequate control, the purpose of this analysis was to explore the concept of disease control in AD, because no definitions have been developed that can be used for characterizing such patients. However, the consistency of results across outcomes and disease severity supports the appropriateness of the physician rating criteria. In this regard, the current analysis only defined control from the physician's perspective. Because a previous study suggested discordance between the perspectives of physicians and their patients, at least with regard to disease severity,36 additional studies may be warranted to further explore similarities and differences in perceptions of AD and its control. A similar limitation is that patients who were recruited in the current study were those with a history of moderate to severe AD as evaluated by the clinician rather than through use of validated clinical scales. However, this subjective evaluation likely reflects real‐world clinical practice, because clinical scales are not necessarily used in every patient at each visit, and there has been no standardization of severity assessment;37 interpretation of severity scales may be dependent on their content.38 Lastly, because the DSP is cross‐sectional, relationships should be considered associative rather than causal.
In conclusion, this study suggests that in the clinical setting, physicians rate a high proportion of patients with AD as having inadequately controlled disease across all disease severity levels and despite current use of a variety of therapies. Inadequate control was observed even among patients being treated by dermatologists and allergists. The burden of AD appeared to be higher in patients with inadequate disease control than those whose AD was controlled. These results demonstrate a need for more effective therapies, and emphasize the importance of incorporating measures of function and QoL for characterizing and assessing AD control.
Conflict of Interest
S. S. and E. G. are employees and stockholders of Sanofi; W. W. was an employee of Sanofi at the time of study and is now an employee of Regeneron Pharmaceuticals Inc., and stockholders of Sanofi and Regeneron Pharmaceuticals Inc.; A. G. and J. G. are employees and stockholders of Regeneron Pharmaceuticals Inc.; and P. A., S. B., R. M. and J. P. are employees of Adelphi Real World, a company that received research funding for the current study.
Acknowledgments
This study was funded by Regeneron Pharmaceuticals Inc., and Sanofi. Editorial support in the preparation of this publication was provided by E. Jay Bienen and sponsored by Sanofi and Regeneron Pharmaceuticals Inc. The authors were responsible for all content and editorial decisions and received no honoraria related to the development/presentation of this publication.
References
- 1. Kim JP, Chao LX, Simpson EL, Silverberg JI. Persistence of atopic dermatitis (AD): a systematic review and meta‐analysis. J Am Acad Dermatol 2016; 75: 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The World Allergy Organization (WAO) . The World Allergy Organization (WAO) White Book on Allergy 2013 Update. Available from: http://www.worldallergy.org/UserFiles/file/WhiteBook2-2013-v8.pdf [Accessed July 4, 2017].
- 3. Napolitano M, Megna M, Patruno C, Gisondi P, Ayala F, Balato N. Adult atopic dermatitis: a review. G Ital Dermatol Venereol 2016; 151: 403–411. [PubMed] [Google Scholar]
- 4. Fivenson D, Arnold RJ, Kaniecki DJ, Cohen JL, Frech F, Finlay AY. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm 2002; 8: 333–342. [DOI] [PubMed] [Google Scholar]
- 5. Barbeau M, Bpharm HL. Burden of atopic dermatitis in Canada. Int J Dermatol 2006; 45: 31–36. [DOI] [PubMed] [Google Scholar]
- 6. Chamlin SL. The psychosocial burden of childhood atopic dermatitis. Dermatol Ther 2006; 19: 104–107. [DOI] [PubMed] [Google Scholar]
- 7. Dalgard FJ, Gieler U, Tomas‐Aragones L et al The psychological burden of skin diseases: a cross‐sectional multicenter study among dermatological outpatients in 13 European countries. J Invest Dermatol 2015; 135: 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bender BG, Leung SB, Leung DY. Actigraphy assessment of sleep disturbance in patients with atopic dermatitis: an objective life quality measure. J Allergy Clin Immunol 2003; 111: 598–602. [DOI] [PubMed] [Google Scholar]
- 9. Wittkowski A, Richards HL, Griffiths CE, Main CJ. Illness perception in individuals with atopic dermatitis. Psychol Health Med 2007; 12: 433–444. [DOI] [PubMed] [Google Scholar]
- 10. Yano C, Saeki H, Ishiji T et al Impact of disease severity on sleep quality in Japanese patients with atopic dermatitis. J Dermatol Sci 2013; 72: 195–197. [DOI] [PubMed] [Google Scholar]
- 11. Yano C, Saeki H, Ishiji T et al Impact of disease severity on work productivity and activity impairment in Japanese patients with atopic dermatitis. J Dermatol 2013; 40: 736–739. [DOI] [PubMed] [Google Scholar]
- 12. Simpson EL, Bieber T, Eckert L et al Patient burden of moderate‐to‐severe atopic dermatitis: insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol 2016; 74: 491–498. [DOI] [PubMed] [Google Scholar]
- 13. Kiebert G, Sorensen SV, Revicki D et al Atopic dermatitis is associated with a decrement in health‐related quality of life. Int J Dermatol 2002; 41: 151–158. [DOI] [PubMed] [Google Scholar]
- 14. Ring J, Alomar A, Bieber T et al Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol 2012; 26: 1045–1060. [DOI] [PubMed] [Google Scholar]
- 15. Eichenfield LF, Tom WL, Berger TG et al Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014; 71: 116–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sidbury R, Davis DM, Cohen DE et al Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 2014; 71: 327–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roekevisch E, Spuls PI, Kuester D, Limpens J, Schmitt J. Efficacy and safety of systemic treatments for moderate‐to‐severe atopic dermatitis: a systematic review. J Allergy Clin Immunol 2014; 133: 429–438. [DOI] [PubMed] [Google Scholar]
- 18. Zuberbier T, Orlow SJ, Paller AS et al Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol 2006; 118: 226–232. [DOI] [PubMed] [Google Scholar]
- 19. Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real‐world physician and patient behaviour across countries: disease‐specific programmes–a means to understand. Curr Med Res Opin 2008; 24: 3063–3072. [DOI] [PubMed] [Google Scholar]
- 20. Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001; 10: 11–18. [DOI] [PubMed] [Google Scholar]
- 21. Charman CR, Venn AJ, Williams HC. The patient‐oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004; 140: 1513–1519. [DOI] [PubMed] [Google Scholar]
- 22. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 23. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993; 4: 353–365. [DOI] [PubMed] [Google Scholar]
- 24. Reilly MC. Work productivity and activity impairment questionnaire: Specific health problem V2.0 (WPAI:SHP). Available from: http://www.reillyassociates.net/WPAI_SHP.html [Accessed July 4, 2017]. [PubMed]
- 25. Huber PJ, editor The behavior of maximum likelihood estimates under nonstandard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability; 1967.
- 26. Leshem YA, Hajar T, Hanifin JM, Simpson EL. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol 2015; 172: 1353–1357. [DOI] [PubMed] [Google Scholar]
- 27. Wei W, Anderson P, Gadkari A et al Extent and consequences of inadequate disease control among adults with a history of moderate‐to‐severe atopic dermatitis [abstract]. J Invest Dermatol 2016; 136(suppl): S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wittkowski A, Richards HL, Griffiths CE, Main CJ. The impact of psychological and clinical factors on quality of life in individuals with atopic dermatitis. J Psychosom Res 2004; 57: 195–200. [DOI] [PubMed] [Google Scholar]
- 29. Zachariae R, Zachariae C, Ibsen HH, Mortensen JT, Wulf HC. Psychological symptoms and quality of life of dermatology outpatients and hospitalized dermatology patients. Acta Derm Venereol 2004; 84: 205–212. [DOI] [PubMed] [Google Scholar]
- 30. Dieris‐Hirche J, Gieler U, Kupfer JP, Milch WE. Suicidal ideation, anxiety and depression in adult patients with atopic dermatitis [Article in German]. Hautarzt 2009; 60: 641–646. [DOI] [PubMed] [Google Scholar]
- 31. Kimata H. Prevalence of suicidal ideation in patients with atopic dermatitis. Suicide Life Threat Behav 2006; 36: 120–124. [DOI] [PubMed] [Google Scholar]
- 32. Beikert FC, Langenbruch AK, Radtke MA, Kornek T, Purwins S, Augustin M. Willingness to pay and quality of life in patients with atopic dermatitis. Arch Dermatol Res 2014; 306: 279–286. [DOI] [PubMed] [Google Scholar]
- 33. Yu SH, Attarian H, Zee P, Silverberg JI. Burden of sleep and fatigue in US adults with atopic dermatitis. Dermatitis 2016; 27: 50–58. [DOI] [PubMed] [Google Scholar]
- 34. Torrelo A. Atopic dermatitis in different skin types. What is to know? J Eur Acad Dermatol Venereol 2014; 28(Suppl 3): 2–4. [DOI] [PubMed] [Google Scholar]
- 35. Ben‐Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol 2002; 147: 920–925. [DOI] [PubMed] [Google Scholar]
- 36. Wei W, Anderson P, Gadkari A et al Discordance between physician‐ and patient‐reported disease severity in adults with atopic dermatitis: a US cross‐sectional survey. Am J Clin Dermatol 2017; 18: 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hill MK, Kheirandish Pishkenari A, Braunberger TL, Armstrong AW, Dunnick CA. Recent trends in disease severity and quality of life instruments for patients with atopic dermatitis: a systematic review. J Am Acad Dermatol 2016; 75: 906–917. [DOI] [PubMed] [Google Scholar]
- 38. Chopra R, Vakharia PP, Sacotte R et al Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br J Dermatol 2017; 177: 1316–1321. [DOI] [PubMed] [Google Scholar]


