Abstract
Aim
To investigate determinants of change in glycated haemoglobin (HbA1c) in patients with type 2 diabetes mellitus (T2DM) at 6 months after initiating uninterrupted second‐line glucose‐lowering therapies.
Materials and Methods
This cohort study utilized retrospective data from 10 256 patients with T2DM who initiated second‐line glucose‐lowering therapy (switch from or add‐on to metformin) between 2011 and 2014 in Germany and the UK. Effects of pre‐specified patient characteristics on 6‐month HbA1c changes were assessed using analysis of covariance.
Results
Patients had a mean (standard error [SE]) baseline HbA1c of 8.68% (0.02); 28.5% of patients discontinued metformin and switched to an alternative therapy and the remainder initiated add‐on therapy. Mean (SE) unadjusted 6‐month HbA1c change was −1.27% (0.02). When adjusted for baseline HbA1c, 6‐month changes depended markedly on the magnitude of the baseline HbA1c (HbA1c <9%, −0.45% per unit increase in HbA1c; HbA1c ≥9%, −0.87% per unit increase in HbA1c). Adjusted mean 6‐month HbA1c reductions showed slight treatment differences (range, 0.92–1.09%; P < .001). Greater reductions in HbA1c were associated with second‐line treatment initiation within 6 months of T2DM diagnosis (1.36% vs 1.03% [P < .001]) and advanced age (≥70 years, 1.13%; <70 years, 1.02% [P < .001]).
Conclusions
Many patients with T2DM have very high HbA1c levels when initiating second‐line therapy, indicating the need for earlier treatment intensification. Patient‐specific factors merit consideration when making treatment decisions.
Keywords: glycaemic control, observational study, primary care, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes mellitus (T2DM), a chronic and increasingly prevalent disease, poses a significant clinical and economic burden worldwide.1 The pathophysiology of T2DM is characterized by progressive decline in β‐cell function, with a subsequent reduction in glycaemic control. Given evidence for a causal link between dysglycaemia and the development of microvascular and macrovascular complications, controlling blood glucose levels is a major goal of therapy in patients with T2DM.2, 3, 4
Current clinical guidelines recommend the use of metformin (MET), in conjunction with lifestyle changes, as first‐line glucose‐lowering therapy in patients with no contraindications who can tolerate this therapy.5, 6, 7, 8 When MET monotherapy fails to control glycated haemoglobin (HbA1c), guidelines recommend the addition of a second glucose‐lowering agent such as a sulfonylurea (SU), a thiazolidinedione, a glucagon‐like peptide‐1 receptor antagonist (GLP‐1RA), a dipeptidyl peptidase‐4 inhibitor (DPP‐4i) or a sodium–glucose linked transporter 2 inhibitor.6, 9 A recent position statement proposed jointly by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommends treatment intensification if glycaemic control is not achieved after 3 months of initial therapy.6
In clinical practice, several second‐ and subsequent‐line therapies are used to treat patients with T2DM, because of a lack of clear consensus on the optimal treatment regimen for the management of hyperglycaemia, as well as inter‐patient differences in the efficacy and safety profiles of different therapies.6, 9 The global DISCOVER study programme (NCT02322762 and NCT02226822) is designed to address this knowledge gap.10 DISCOVER comprises 2 prospective observational studies of patients with T2DM who initiated second‐line glucose‐lowering therapy (defined as adding a glucose‐lowering drug or switching between therapies) after failure of first‐line oral treatment (mono‐, dual or triple therapy). The studies aim to describe disease management patterns and treatment outcomes in patients with T2DM worldwide, and over 16 000 patients in 38 countries across 6 continents have been enrolled. As part of the DISCOVER study programme, a retrospective analysis was conducted, using data from existing electronic medical record (EMR) databases in Canada, France, Germany and the UK.
This paper reports on an analysis using data from Germany and the UK. The aims of the present study are: (1) to describe the changes in HbA1c in patients with T2DM 6 months after initiating a second‐line therapy; and (2) to assess patient characteristics that influence changes in HbA1c after 6 months of second‐line treatment.
2. METHODS
2.1. Study design and data source
In this longitudinal cohort study, EMR patient‐level data from 2 countries were extracted from 2 databases: the IMS Disease Analyzer in Germany compiles drug prescriptions, diagnoses and basic medical and demographic data from the computer systems of a representative sample of primary care physicians and internal medicine practices in Germany11; The Health Improvement Network (THIN) contains healthcare information from patients registered with participating primary healthcare practices in the UK.12 Studies have shown THIN to be representative of the UK population in terms of patient demographics and the prevalence of major conditions.13, 14 No ethical approval is required for studies based on anonymized databases in Germany. Studies using THIN have been approved by a nationally accredited ethics committee12 and specific approval was obtained for this study from the relevant Scientific Review Committee before study initiation (reference number: 14THIN052).
2.2. Study population
Inclusion and exclusion criteria were based on key criteria used in the prospective DISCOVER studies. Patients (aged ≥18 years) with T2DM (identified by International Statistical Classification of Diseases and Related Health Problems, 10th Revision codes or Quality and Outcomes Framework [QOF] Read codes) (Text S1 of S1) who were receiving MET monotherapy as first‐line treatment and initiating a second‐line therapy (add‐on or switching) were eligible for inclusion in the study. Patients were required to have a minimum of 12 months of EMR data available before MET initiation, as well as HbA1c measurements both at second‐line treatment initiation and after 6 months of uninterrupted treatment (defined as no reported change or cessation of treatment over the 6 months following initiation of second‐line therapy). Major exclusion criteria included a diagnosis of type 1 diabetes mellitus, use of insulin as first‐line treatment, pregnancy, initiation of a third‐line therapy before the 6‐month HbA1c measurement, and treatment with chemotherapy or oral/intravenous steroids (based on either prescription or relevant diagnostic codes within the past 6 months).
Eligible patients were identified from the databases during the periods May 2011 to April 2014 (Germany) and May 2011 to November 2013 (UK). The index date was defined as the date of initiation of second‐line therapy. Information on demographics and clinical characteristics was recorded using data from the baseline period before the index date. Variables collected included the type of second‐line treatment initiated, time since diagnosis of T2DM, time since first‐line treatment initiation, age, body mass index (BMI), sex and HbA1c. Patients were followed until their 6‐month HbA1c measurement, as defined below.
The main outcome of interest was change in HbA1c at 6 months after initiation of second‐line therapy. Data on HbA1c were not always available for the exact time points of interest (ie, initiation of second‐line therapy and/or after 6 months of follow‐up). In such cases, the HbA1c values used for the analysis were those closest to the time points of interest, within the following time periods: from 90 days before to 14 days after initiation of second‐line therapy for baseline HbA1c; and from 90 to 270 days after initiation for 6‐month HbA1c. Any HbA1c result <3.5% was considered unrealistically low and was removed from the data set before patient selection.
2.3. Statistical analysis
2.3.1. Baseline characteristics
Baseline variables and clinical characteristics were reported as mean (standard deviation [SD]) or median (interquartile range [IQR]). The numbers of patients in different categories for each variable were reported as number (percentage). Patients were classified according to the second‐line therapy initiated as follows: (1) add‐on oral therapy (either combinations of MET and an SU [MET + SU] or combinations of MET and a DPP‐4i [MET + DPP‐4i]); (2) switch to alternative oral therapy (either SU or DPP‐4i monotherapy); (3) switch to or addition of insulin (insulin monotherapies/combinations of insulin with other therapies, with or without MET discontinuation); (4) switch to other monotherapies or combination therapies (including other monotherapies, eg, GLP‐1RAs, and other combination therapies).
2.3.2. Assessment of baseline, 6‐month change and baseline‐adjusted 6‐month change in HbA1c according to second‐line treatment and other variables
Mean HbA1c (standard error) at baseline and 6 months was used to calculate crude 6‐month changes in HbA1c. For each second‐line therapy, the 6‐month change in HbA1c was determined using analysis of covariance models adjusted for baseline HbA1c using variance‐weighted least‐squares estimation. The relationship between baseline HbA1c and subsequent 6‐month change in HbA1c was explored to determine the best way to adjust for HbA1c in the analysis. This included an assessment of linearity, with subsequent exploration of other types of relationship, including quadratic and spline models (linear and restricted cubic with differing numbers and locations of knots), as well as a model using baseline HbA1c as a categorical variable (full details are provided in Text 2 of S1).
These linear regression models were also used to assess the impact of patient characteristics (time since T2DM diagnosis, age, BMI, sex, country) on 6‐month HbA1c changes. Finally, multivariable analyses adjusted for the aforementioned patient characteristics, baseline HbA1c and second‐line therapies were used to calculate adjusted estimates of 6‐month changes in HbA1c. All statistical analyses were conducted using STATA 14 software (StataCorp, College Station, Texas).
3. RESULTS
3.1. Patient characteristics
This analysis comprised 10 256 patients with T2DM initiating second‐line glucose‐lowering therapy after first‐line treatment with MET in the period 2011 to 2014. Baseline and treatment characteristics are presented in Table 1. The patients were from Germany (30.4%) and the UK (69.6%). The mean age (SD) of the patients was 62.3 (12.2) years; 85.0% were aged >50 years and 42.3% were women. Mean (SD) BMI was 32.36 (6.4) kg/m2; 61.0% of patients were obese (BMI ≥30 kg/m2) and 29.6% were overweight (BMI 25 to <30 kg/m2). Mean (SD) baseline HbA1c at initiation of second‐line therapy was 8.68% (1.8). Notably, 11.0% of patients had baseline HbA1c <7.0%. The median (IQR) time between T2DM diagnosis and second‐line treatment initiation was 3.30 (1.29–5.93) years overall, and 13.8% of patients started second‐line treatment during the 6 months following T2DM diagnosis (Table 1). A total of 34.8% of patients had remained on MET monotherapy for >3 years and 16.4% had remained on MET monotherapy for ≥5 years.
Table 1.
Characteristics of 10 256 patients with T2DM initiating second‐line therapy after first‐line treatment with MET
| Number of patients (%), by baseline HbA1c category | ||||
|---|---|---|---|---|
| Characteristic | Overall number of patients (%) | <7%, n = 1129 | ≥7% and <9%, n = 5794 | ≥9%, n = 3333 |
| Second‐line treatment | ||||
| MET + SU | 4191 (40.9) | 130 (11.5) | 2253 (38.9) | 1808 (54.2) |
| MET + DPP‐4i | 3147 (30.7) | 344 (30.5) | 1961 (33.8) | 842 (25.3) |
| SU monotherapy | 1278 (12.5) | 255 (22.6) | 718 (12.4) | 305 (9.2) |
| DPP‐4i monotherapy | 1031 (10.1) | 295 (26.1) | 586 (10.1) | 150 (4.5) |
| Insulin (monotherapy or in combination) | 178 (1.7) | 26 (2.3) | 60 (1.0) | 92 (2.8) |
| Other monotherapies or combination therapies | 431 (4.2) | 79 (7.0) | 216 (3.7) | 136 (4.1) |
| Time between T2DM diagnosis and second‐line treatment initiation (index date) | ||||
| Median (IQR), years | 3.30 (1.29–5.93) | 3.04 (1.23–5.57) | 3.85 (1.08–6.40) | 2.40 (0.56–4.98) |
| Germany | 3.37 (1.42–5.72) | 3.23 (1.44–5.55) | 3.61 (1.65–6.03) | 2.85 (0.46–4.93) |
| UK | 3.29 (1.25–5.99) | 2.78 (0.82–5.75) | 3.95 (1.85–6.48) | 2.33 (0.57–5.01) |
| Proportion of overall cohort in each time category | ||||
| <6 months | 1313 (13.8) | 121 (13.3) | 426 (7.9) | 766 (24.0) |
| 6 months to <1 year | 684 (7.2) | 82 (9.0) | 362 (6.7) | 240 (7.5) |
| 1 to <3 years | 2404 (25.3) | 244 (26.7) | 1363 (25.3) | 797 (24.9) |
| 3 to <5 years | 1977 (20.8) | 188 (20.6) | 1189 (22.1) | 600 (18.8) |
| ≥5 years | 3110 (32.8) | 278 (30.4) | 2038 (37.9) | 794 (24.8) |
| Missing | 768 | 216 | 416 | 136 |
| Time between first‐ and second‐line treatment initiation (index date) | ||||
| Median (IQR), years | 1.96 (0.64–3.93) | 1.78 (0.56–3.69) | 2.35 (0.96–4.26) | 1.39 (0.28–3.20) |
| Germany | 1.90 (0.57–3.88) | 1.85 (0.64–3.63) | 2.10 (0.75–4.07) | 1.39 (0.17–3.34) |
| UK | 2.00 (0.67–3.95) | 1.41 (0.41–3.80) | 2.43 (1.03–4.36) | 1.39 (0.31–3.17) |
| Proportion of overall cohort in each time category | ||||
| <6 months | 2229 (21.7) | 251 (22.2) | 912 (15.7) | 1066 (32.0) |
| 6 months to <1 year | 1088 (10.6) | 156 (13.8) | 591 (10.2) | 341 (10.2) |
| 1 to <3 years | 3369 (32.8) | 370 (32.8) | 1979 (34.2) | 1020 (30.6) |
| 3 to <5 years | 1884 (18.4) | 175 (15.5) | 1197 (20.7) | 512 (15.4) |
| ≥5 years | 1686 (16.4) | 177 (15.7) | 1115 (19.2) | 394 (11.8) |
| Age, years | ||||
| Mean (SD) | 62.3 (12.2) | 65.93 (11.54) | 63.51 (11.58) | 59.10 (12.65) |
| <50 | 1540 (15.0) | 98 (8.7) | 698 (12.0) | 744 (22.3) |
| 50 to <60 | 2657 (25.9) | 217 (19.2) | 1417 (24.5) | 1023 (30.7) |
| 60 to <70 | 2982 (29.1) | 340 (30.1) | 1785 (30.8) | 857 (25.7) |
| ≥70 | 3077 (30.0) | 474 (42.0) | 1894 (32.7) | 709 (21.3) |
| Body mass index (kg/m2) | ||||
| Mean (SD) | 32.36 (6.4) | 31.07 (6.01) | 32.31 (6.21) | 32.73 (6.70) |
| <25 | 663 (9.4) | 80 (14.6) | 337 (8.3) | 246 (10.0) |
| 25 to <30 | 2089 (29.6) | 174 (31.8) | 1246 (30.8) | 669 (27.1) |
| 30 to <35 | 2240 (31.7) | 167 (30.5) | 1331 (32.9) | 762 (30.8) |
| ≥35 | 2068 (29.3) | 127 (23.2) | 1147 (28.4) | 794 (32.1) |
| Missing | 3196 | 581 | 1753 | 862 |
| Sex | ||||
| Female | 4335 (42.3) | 541 (47.9) | 3298 (56.9) | 2082 (62.5) |
| Male | 5921 (57.7) | 588 (52.1) | 2496 (43.1) | 1251 (37.5) |
| Country | ||||
| Germany | 3120 (30.4) | 831 (73.6) | 1711 (29.5) | 578 (17.3) |
| UK | 7136 (69.6) | 298 (26.4) | 4083 (70.5) | 2755 (82.7) |
| Baseline HbA1c (%) | ||||
| Mean (SD) | 8.68 (1.8) | – | – | – |
| <7.0 | 1129 (11.0) | – | – | – |
| 7.0 to <8.0 | 3055 (29.8) | – | – | – |
| 8.0 to <9.0 | 2739 (26.7) | – | – | – |
| ≥9.0 | 3333 (32.5) | – | – | – |
| Absolute reduction in HbA1c at 6 months (%) | ||||
| Mean (SD) | 1.27 (1.8) | 0.03 (0.71) | 0.69 (0.94) | 2.69 (2.19) |
| No reduction | 1857 (18.1) | 482 (42.7) | 1093 (18.9) | 282 (8.5) |
| <0.5 | 1818 (17.7) | 456 (40.4) | 1200 (20.7) | 162 (4.9) |
| 0.5 to <1.0 | 1926 (18.8) | 161 (14.3) | 1512 (26.1) | 253 (7.6) |
| 1.0 to <2.0 | 2377 (23.2) | 30 (2.7) | 1663 (28.7) | 684 (20.5) |
| 2.0 to <3.0 | 963 (9.4) | 0 | 306 (5.3) | 657 (19.7) |
| 3.0 to <5.0 | 854 (8.3) | 0 | 20 (0.3) | 834 (25.0) |
| ≥5.0 | 461 (4.5) | 0 | 0 | 461 (13.8) |
Abbreviations: DPP‐4i, dipeptidyl peptidase‐4 inhibitor; HbA1c, glycated haemoglobin; IQR, interquartile range; MET, metformin; SD, standard deviation; SU, sulfonylurea; T2DM, type 2 diabetes mellitus.
Data are reported as n (%) unless otherwise stated.
3.2. Type 2 diabetes mellitus treatments
For most patients, second‐line treatments initiated at baseline comprised add‐on therapies in combination with MET (MET + SU, 40.9%; MET + DPP‐4i, 30.7%). Another 22.6% of patients switched from MET to an alternative single agent, the most common agents being SU (12.5%) and DPP‐4i (10.1%). A small proportion of patients switched to or added insulin therapy to MET (1.7%) or switched to other monotherapies or combination therapies (4.2%) (Table 1).
Second‐line treatment choices differed between countries. Insulin was more commonly chosen in Germany (4.8%) than in the UK (0.3%) and second‐line monotherapies were more common in Germany (46.3%) than in the UK (12.1%). While MET + SU was the most common second‐line treatment choice in the UK (56.8%), it was rarely chosen in Germany (4.4%), where DPP‐4i treatment predominated (data not shown).
3.3. Changes in HbA1c at 6 months
The mean (SD) change in HbA1c at 6 months after initiation of second‐line therapy was −1.27% (1.8) (Table 1). The change was directly, but non‐linearly, related to baseline HbA1c (Figure 1). We explored various statistical modelling options and compared the fit of each model using measures such as R2; we also conducted adjusted analyses with each modelling type to compare the results (Text 2 of S1). On the basis of our findings, we used linear spline regression to model the relationship between baseline and 6‐month change in HbA1c as two straight lines connected at a baseline HbA1c of 9.0%, as this was the simplest reliable model. We conducted sensitivity analyses by reproducing baseline adjusted mean changes in HbA1c using the various modelling approaches and found agreement between the estimates from our modelling approach and more complex models (Text 2 of S1).
Figure 1.
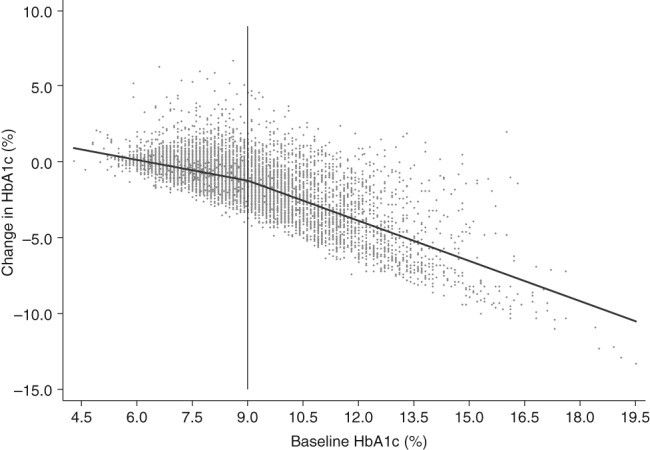
Association between 6‐month change in HbA1c and baseline HbA1c. HbA1c, glycated haemoglobin
3.4. Variation in 6‐month change in HbA1c according to choice of second‐line treatment
Mean baseline HbA1c varied substantially across treatment groups (Table 2); values were highest in patients whose second‐line treatment was insulin (9.48%) or MET + SU (9.22%), and lowest in those who switched to DPP‐4i monotherapy (7.72%). After 6 months of second‐line treatment, HbA1c decreased in all treatment groups and there were small differences in the magnitude of this decrease between groups (Table 2 and Figure S1). The combination of MET + SU was associated with the largest decrease in HbA1c (1.09%); SU reduced HbA1c to a greater extent than did DPP‐4i, both when used alone (difference of 0.08%, P = .023) and in combination with MET (difference of 0.07%, P < .001). Combining MET with SU or DPP‐4i reduced HbA1c more than either monotherapy alone (SU alone: difference of 0.06%, P = .049; DPP‐4i alone: difference of 0.12%, P < .001; overall difference from monotherapy: 0.09%, P < .001). Data analysed separately for Germany and the UK are presented in Tables S1a and S1b (S); data showing the proportions of patients achieving HbA1c <7.0% after 6 months, according to second‐line treatment and baseline HbA1c category, are presented in Tables S2a and S2b (S1). Additionally, a sensitivity analysis was conducted in a cohort in which patients with very high baseline HbA1c (≥12.0%) were excluded. Estimates of 6‐month mean HbA1c change for each second‐line treatment group agreed closely with the overall findings in all patients (Text 2 of S1).
Table 2.
Changes in HbA1c by second‐line treatment
| Second‐line treatment | Baseline HbA1c, % | 6‐month change in HbA1c, % | Baseline‐adjusted 6‐month change in HbA1c, %a | Mean (SE) baseline‐adjusted 6‐month change in HbA1c (%), for the subgroup with baseline HbA1c <9.0%b (n = 6923) | Mean (SE) baseline‐adjusted 6‐month change in HbA1c (%), for the subgroup with baseline HbA1c ≥9.0%c (n = 3333) |
|---|---|---|---|---|---|
| MET + SU | 9.22 (0.03) | −1.68 (0.03) | −1.09 (0.02) | −0.65 (0.02) | −2.74 (0.04) |
| MET + DPP‐4i | 8.42 (0.03) | −1.04 (0.03) | −1.02 (0.02) | −0.58 (0.02) | −2.56 (0.05) |
| SU monotherapy | 8.27 (0.05) | −1.00 (0.05) | −1.00 (0.03) | −0.55 (0.03) | −2.70 (0.09) |
| DPP‐4i monotherapy | 7.72 (0.05) | −0.59 (0.05) | −0.92 (0.03) | −0.47 (0.03) | −2.78 (0.12) |
| Insulin (monotherapy or in combination) | 9.48 (0.13) | −2.11 (0.13) | −1.00 (0.08) | −0.45 (0.09) | −3.05 (0.17) |
| Other monotherapies or combination therapies | 8.48 (0.08) | −1.11 (0.08) | −1.05 (0.05) | −0.61 (0.05) | −2.65 (0.13) |
| Overall | 8.68 (0.02) | −1.27 (0.02) | −1.05 (0.02) | −0.58 (0.01) | −2.70 (0.03) |
Abbreviations: DPP‐4i, dipeptidyl peptidase‐4 inhibitor; HbA1c, glycated haemoglobin; MET, metformin; SE, standard error; SU, sulfonylurea.
Data are reported as mean (SE).
For patients with mean baseline HbA1c (8.68%), estimated from a linear regression model with slope −0.45% (0.01) per unit increase in HbA1c for baseline HbA1c <9.0% and −0.86% (0.02) per unit increase in HbA1c for baseline HbA1c ≥9.0%.
Estimates for patients with mean baseline HbA1c 7.69% for the subgroup <9.0% HbA1c at baseline. Slope is −0.45% (0.01) per unit increase in HbA1c.
Estimates for patients with mean baseline HbA1c 10.75% for the subgroup ≥9.0% HbA1c at baseline. Slope is −0.87% (0.02) per unit increase in HbA1c.
We next examined whether the relative treatment effects were dependent on baseline HbA1c by splitting the overall cohort into patients with baseline HbA1c <9.0% (n = 6923 [67.5%]) and ≥9.0% (n = 3333 [32.5%]) (Table 2). The results of this subgroup analysis were generally consistent with findings from the overall cohort. However, there were several differences; while mean baseline‐adjusted HbA1c reduction was smallest with DPP‐4i monotherapy in the main analysis, this was not the case in the subgroup of patients with HbA1c ≥9.0%. It is important to note that the number of patients with HbA1c ≥9.0% who received DPP‐4i monotherapy was small (n = 150 vs 881 in the <9.0% group).
In patients with baseline HbA1c ≥9.0%, the mean baseline‐adjusted reduction in HbA1c was slightly greater in patients who initiated insulin as second‐line treatment (3.05%), compared with the other treatment groups (P = .036 vs all other treatment groups combined). However, the number of patients in this insulin‐treated subgroup was small (n = 86).
3.5. Determinants of change in HbA1c following initiation of second‐line glucose‐lowering therapies
3.5.1. Country
There was weak evidence that the median (IQR) time between initiation of first‐ and second‐line treatments differed between countries, with a shorter time to treatment intensification in Germany than in the UK (1.90 [0.57–3.88] vs 2.00 [0.67–3.95] years; P = .075). When adjusted for baseline HbA1c, the 6‐month changes in HbA1c were slightly greater in Germany than in the UK (−1.11% vs −1.04%; P = .001; Table 3).
Table 3.
Other potential influences on 6‐month change in HbA1c
| Number of patients | Baseline HbA1c, % | Baseline‐adjusted change in HbA1c, %a | |
|---|---|---|---|
| Country | |||
| Germany | 3120 | 7.91 (0.03) | −1.11 (0.02) |
| UK | 7136 | 9.02 (0.02) | −1.04 (0.02) |
| Time between T2DM diagnosis and second‐line treatment initiation | |||
| <6 months | 1313 | 9.88 (0.05) | −1.36 (0.03) |
| 6 months to <1 year | 684 | 8.70 (0.07) | −0.97 (0.04) |
| ≥1 year | 7491 | 8.55 (0.02) | −1.02 (0.02) |
| Missing | 768 | 7.84 (0.06) | −1.08 (0.03) |
| Age (years) | |||
| <50 | 1540 | 9.24 (0.05) | −0.82 (0.03) |
| 50 to <60 | 2657 | 8.89 (0.03) | −1.04 (0.02) |
| 60 to <70 | 2982 | 8.59 (0.03) | −1.11 (0.02) |
| ≥70 | 3077 | 8.31 (0.03) | −1.13 (0.02) |
| Body mass index (kg/m2) | |||
| <25 | 663 | 8.99 (0.07) | −1.19 (0.04) |
| 25 to <30 | 2089 | 8.77 (0.04) | −1.11 (0.02) |
| 30 to <35 | 2240 | 8.79 (0.04) | −1.04 (0.02) |
| ≥35 | 2068 | 8.88 (0.04) | −0.94 (0.02) |
| Missing | 3196 | 8.35 (0.03) | −1.09 (0.02) |
| Sex | |||
| Female | 4335 | 8.52 (0.03) | −0.98 (0.02) |
| Male | 5921 | 8.80 (0.02) | −1.10 (0.02) |
Abbreviations: HbA1c, glycated haemoglobin; SE, standard error; T2DM, type 2 diabetes mellitus.
Data are reported as mean (SE).
For patients with mean baseline HbA1c (8.68%).
3.5.2. Time between diagnosis of type 2 diabetes mellitus and initiation of second‐line therapy
Mean HbA1c at baseline was higher in patients starting second‐line treatment during the 6 months following diagnosis (9.88%) than in those starting treatment beyond this time point (8.70% and 8.55% at 6 months to <1 year and at ≥1 year, respectively; Table 3). The mean baseline‐adjusted 6‐month change in HbA1c was significantly greater in patients who initiated treatment during the 6 months following T2DM diagnosis, than in those who initiated treatment ≥6 months after T2DM diagnosis (−1.36% vs −1.03%; P < .001).
3.5.3. Age
Patients who switched to another monotherapy as second‐line treatment tended to be older than those receiving second‐line combination therapies (mean age of patients initiating non‐MET monotherapy [SU or DPP‐4i] vs MET combination therapy [MET + SU or MET + DPP‐4i], 66.79 years vs 61.06 years). The mean age of patients receiving an SU was 62.99 years, compared with 61.69 years in those taking a DPP‐4i, a difference of 1.3 years (P < .001). The mean baseline‐adjusted change in HbA1c was lower in patients aged <70 years (−1.02%) than in patients aged ≥70 years (−1.13%; P < .001) (Table 3).
3.5.4. Body mass index
Mean BMI was highest in patients initiating MET + DPP‐4i and lowest in patients initiating insulin (33.8 kg/m2 vs 29.7 kg/m2; P < .001). While there was little association between baseline HbA1c and BMI (Table 3), there was an inverse association between mean baseline‐adjusted 6‐month change in HbA1c and BMI (<35 kg/m2, −1.09%; ≥35 kg/m2, −0.94%; P < .001).
3.5.5. Sex
In patients receiving an SU or DPP‐4i as monotherapy or in combination with MET, women were more likely than men to switch to another monotherapy (SU or DPP‐4i; 52.5% vs 47.5%; P < .001), rather than adding either drug to MET. Mean baseline HbA1c and mean baseline‐adjusted 6‐month change in HbA1c were slightly higher in men than in women (mean baseline HbA1c, 8.80% vs 8.52% [P < .001]; 6‐month HbA1c change, −1.10% vs −0.98% [P < .01]; Table 3).
3.6. Multivariable regression analysis
When simultaneously modelling the effects of all previously examined variables on 6‐month change in HbA1c, we found that the following were significantly associated with greater baseline‐adjusted 6‐month reductions in HbA1c: residence in Germany (vs the UK); <6 months between T2DM diagnosis and second‐line treatment initiation; older age; lower BMI and male sex (vs female) (Table 4). HbA1c reductions were higher with MET + SU compared with other second‐line treatment options, but the differences between groups were small. Thus, overall, the findings from this multivariate regression analysis were consistent with the results of univariate anaysis.
Table 4.
Results from a multivariable regression analysis for 6‐month change in HbA1c
| Number of patients | Mean adjusted difference (95% CI) in 6‐month change in HbA1c (%) compared with reference groupa | P value | |
|---|---|---|---|
| Country | |||
| Germany | 3120 | −0.17 (−0.22, −0.12) | <.001 |
| UK | 7136 | – | – |
| Second‐line treatment | |||
| MET + SU | 4191 | – | – |
| MET + DPP‐4i | 3147 | 0.09 (0.04, 0.14) | <.001 |
| SU monotherapy | 1278 | 0.18 (0.12, 0.24) | <.001 |
| DPP‐4i monotherapy | 1031 | 0.28 (0.21, 0.35) | <.001 |
| Insulin (monotherapy or in combination) | 178 | 0.24 (0.08, 0.40) | .003 |
| Other monotherapies or combination therapies | 431 | 0.11 (0.01, 0.20) | .027 |
| Time since diagnosis to second‐line treatment initiation (years) | |||
| <0.5 | 1313 | −0.39 (−0.45, −0.32) | <.001 |
| 0.5 to <1 | 684 | 0.01 (−0.06, 0.09) | .768 |
| ≥1 | 7491 | – | – |
| Unknown | 768 | −0.01 (−0.07, 0.06) | .856 |
| Age (years) | |||
| <50 | 1540 | 0.34 (0.27, 0.40) | <.001 |
| 50 to <60 | 2657 | 0.12 (0.06, 0.17) | <.001 |
| 60 to <70 | 2982 | 0.03 (−0.01, 0.08) | .167 |
| ≥70 | 3077 | – | – |
| Body mass index (kg/m2) | |||
| <25 | 663 | −0.16 (−0.25, −0.07) | <.001 |
| 25 to <30 | 2089 | −0.10 (−0.16, −0.03) | .002 |
| 30 to <35 | 2240 | −0.04 (−0.10, 0.02) | .182 |
| ≥35 | 2068 | – | – |
| Unknown | 3196 | −0.06 (−0.12, 0.00) | .036 |
| Sex | |||
| Female | 4335 | 0.12 (0.08, 0.15) | <.001 |
| Male | 5921 | – | – |
Abbreviations: CI, confidence interval; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; HbA1c, glycated haemoglobin; MET, metformin; SU, sulfonylurea.
Data are reported as mean (95% CI).
Estimates are from a multivariable regression model, adjusted for baseline HbA1c and all other variables in the table, for those with mean baseline HbA1c (8.68%).
4. DISCUSSION
4.1. Overview of study findings
The aim of this study was to elucidate determinants of change in HbA1c following initiation of second‐line glucose‐lowering therapies, using data from 2 nationally representative databases. After 6 months of second‐line treatment, overall mean baseline‐adjusted HbA1c levels decreased by approximately 1.1%, regardless of the second‐line therapy. The 6‐month change in HbA1c was influenced by several patient characteristics: baseline HbA1c, country, time since T2DM diagnosis, age, BMI and sex.
4.2. Treatment patterns in patients initiating second‐line therapy
A key finding from this study was that most (89%) patients had inadequate glycaemic control (baseline HbA1c ≥7.0%) and almost one‐third of patients had baseline HbA1c ≥9.0%. This is suggestive of a delay in treatment intensification, as has been reported in several real‐world studies in the UK and elsewhere.15, 16, 17 Major guidelines stipulate the need for treatment intensification if patients receiving MET monotherapy do not achieve HbA1c targets within 3 months; however, in a recent retrospective cohort analysis of >80 000 patients with T2DM in the UK, Khunti et al. reported delays in treatment intensification of >7 years, which resulted in prolonged periods of poor glycaemic control. These findings are significant, given the substantial body of evidence of a beneficial effect of timely establishment of glycaemic control on HbA1c reductions and diabetes complications.6, 18, 19 Moreover, there is evidence of a sustained legacy effect of early intensive glucose control; in a 10‐year follow‐up of the UK Prospective Diabetes Study, patients who received early intensive glucose‐lowering therapy experienced a continued reduction in microvascular risks, as well as emergent risk reductions for cardiovascular events, compared with patients who received conventional therapy.2
A second important finding was that, despite guideline recommendations to add additional glucose‐lowering agents to MET as second‐line therapy, almost 25% of patients in the study cohort discontinued MET and switched to another monotherapy or insulin (either as add‐on or as a single agent). However, it is important to acknowledge that an early change in therapy is often attributable to poor medication tolerability, rather than a lack of glycaemic control. MET is associated with gastrointestinal side effects in approximately 25% of patients and is also contraindicated in patients with renal impairment.20, 21 Furthermore, it is noteworthy that the 11% of patients in the present analysis with HbA1c <7.0% are more likely than others to have switched therapies because of poor MET tolerance. Unfortunately, data on patients' reasons for switching from MET therapy were not documented in the databases used in this study.
Most patients in the study cohort (71.6%) initiated add‐on therapy with an additional glucose‐lowering agent, consistent with guideline recommendations.5, 6, 7, 8 The most commonly prescribed add‐on therapies were SUs and DPP‐4is. Interestingly, while MET + SU was the most popular choice of second‐line treatment in the UK (56.8%), it was rarely chosen in Germany (4.4%). This may be because, until 2015, the UK National Institute for Health and Care Excellence guidelines recommended SUs as the add‐on therapy of choice when intensifying treatment in patients with T2DM.22 Moreover, while a treatment algorithm proposed by the German Diabetes Society presents MET + SU and MET + DPP‐4i as equivalent treatment options,23 MET + DPP‐4i may have recently replaced MET + SU in Germany, because of the lower risk of weight gain and hypoglycaemia with DPP‐4i than with SU therapy.6, 9, 24, 25
4.3. Changes in 6‐month HbA1c with second‐line diabetes treatments
After 6 months of second‐line treatment, HbA1c decreased in all treatment groups and there was a non‐linear relationship between baseline HbA1c and 6‐month HbA1c change. There were some small differences between treatment groups, with combination therapies associated with larger HbA1c reductions than monotherapies, and SUs associated with slightly greater HbA1c reductions than DPP‐4is. However, these findings should be considered carefully, given that this was a descriptive study and, therefore, not designed to test hypotheses or to formally compare the effectiveness of treatments. Therefore, it is our view that the choice of second‐line treatment had only a modest impact on 6‐month baseline‐adjusted HbA1c reductions, even after controlling for covariates in a multivariable analysis. Moreover, these findings were broadly consistent across all baseline HbA1c values. These real‐world findings were consistent with a recent meta‐analysis, in which all investigated drug classes lowered HbA1c to a similar extent when combined with MET,26 as well as with findings from a primary care database study in Germany.27 Given the similar efficacy of all second‐line treatments, their associated side effects (eg, risks of weight gain, hypoglycaemia) and additional benefits (eg, weight reduction, blood pressure reduction)6, 9 may be the most important considerations when selecting second‐line treatment options for patients with T2DM.
4.4. Additional determinants of change in HbA1c following initiation of second‐line glucose‐lowering therapies
As seen previously, there was a positive relationship between baseline HbA1c and the magnitude of 6‐month HbA1c reductions.28 Our multivariable analysis revealed 5 additional patient characteristics that affected baseline‐adjusted 6‐month HbA1c reductions following initiation of second‐line glucose‐lowering therapy. These included country, age and sex, as well as the “modifiable” variables: time since T2DM diagnosis and BMI. While the magnitude of the effects of country, BMI and sex were small and, therefore, probably insignificant from a clinical perspective, it is worth noting that small differences may have a cumulative effect when considered from a public health perspective. For example, the proportion of patients achieving HbA1c <7.0% after 6 months of continuous second‐line treatment was higher in Germany than in the UK (46.9% vs 34.7%), an effect probably attributable to the lower mean baseline HbA1c and the slightly higher baseline‐adjusted reduction in HbA1c in Germany compared with that in the UK (baseline HbA1c: 7.91% vs 9.02%; baseline‐adjusted HbA1c reduction: 1.11% vs 1.04%). Thus, it may be pertinent for clinicians to consider additional patient characteristics, particularly age and time since T2DM diagnosis, when making treatment decisions, in accordance with recommendations from a recent joint statement from the ADA and the EASD.6
4.5. Study strengths and limitations
Particular strengths of this study were the use of large representative cohorts, the use of data from 2 countries and the ability to adjust 6‐month HbA1c changes for baseline HbA1c values. Furthermore, the use of multivariable analysis enabled the effects of individual patient characteristics on 6‐month HbA1c changes to be assessed in isolation.
Limitations of the present study include the fact that patient medical records show prescriptions rather than medication use, so analyses assume patient adherence to study medication.29, 30 Additionally, the reasons for changing treatment or initiating a particular second‐line therapy are not routinely captured in EMRs. Patients who switch therapy might be more likely to do so as a consequence of adverse events than of poor glycaemic control; the opposite may be true of patients who initiate add‐on therapy to MET. EMR studies depend on recording of patient data by phycisians, which presents the possibility of some information being omitted; however, the frequency of this is expected to be low.
In this analysis, HbA1c data were not always available for the exact time points of interest, an issue that necessitated the use of time windows, and which may have impacted the precision of the analysis. There was also inconsistency in the accuracy and completeness of the variables of interest, and no data were included on prescription of diet or exercise or on the socio‐economic status of patients. Furthermore, the present analysis does not include data from patients taking sodium–glucose linked transporter 2 inhibitors, an important new class of glucose‐lowering drugs, because these medicines were not approved by the European Medicines Agency at the start of the study. There are also no data on GLP‐1RAs.
4.6. Conclusions
Key findings from this study were, first, that second‐line glucose‐lowering therapies are frequently initiated far later and at higher HbA1c levels than those recommended by guidelines. Second, almost one‐quarter of patients in the study discontinued MET therapy, which is more than expected if guideline recommendations were applied. While the 6‐month change in HbA1c did not differ much according to choice of second‐line therapy, there was a non‐linear relationship between baseline HbA1c and 6‐month HbA1c changes. Moreover, certain additional patient characteristics, including time since T2DM diagnosis and age, merit consideration when making treatment decisions. These data will complement future results from the prospective DISCOVER studies.
ORCID
Kamlesh Khunti http://orcid.org/0000-0003-2343-7099
Linong Ji http://orcid.org/0000-0003-1305-1598
Supporting information
Appendix S1 Mean baseline‐adjusted decrease in HbA1c, by second‐line treatment (test for heterogeneity P < 0.001)
Figure S1. For those with mean baseline HbA1c (8.68%). CI, confidence interval; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; HbA1c, glycated haemoglobin; MET, metformin; SU, sulfonylurea.
ACKNOWLEDGMENTS
Medical writing support was provided by Lucy Ambrose, DPhil, of Oxford PharmaGenesis, Oxford, UK, and was funded by AstraZeneca.
Conflicts of interest
K. K., J. M., M. B. G., J. C.‐R., B. C., P. F., N. H., K. H., M. K., A. N., M. V. S., L. J. and S. P. were or are members of the DISCOVER Scientific Committee. J. M., P. F., N. H. and K. H. are employees of AstraZeneca. L. G.‐A. and J. H. are employees of QuintilesIMS Health and provide consulting services. J. C.‐R. is an employee of Evidera. In addition, K. K. has received honoraria and research support from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche and Sanofi, and acknowledges support from the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care – East Midlands (CLAHRC – EM), and the NIHR Leicester‐Loughborough Diet, Lifestyle and Physical Activity Biomedical Research Unit, which is a partnership between University Hospitals of Leicester NHS Trust, Loughborough University and the University of Leicester. M. B. G. previously received honoraria from Merck Serono. B. C. has received honoraria from AstraZeneca, Boehringer Ingelheim, Lilly, Merck Sharpe & Dohme, Novartis, Novo Nordisk, Sanofi and Takeda; M. K. has received honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Glytec Systems, Merck (Diabetes), Novo Nordisk, Sanofi, Janssen, Eisai and ZS Pharma, and research support from AstraZeneca and Boehringer Ingelheim. A. N. has received honoraria from AstraZeneca, Eli Lilly, Medtronic and Novo Nordisk, and research support from Artsana, Dexcom, Novo Nordisk and Sanofi‐Aventis. M. V. S. has received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharpe & Dohme, Novo Nordisk and Sanofi, and research support from Sanofi. L. J. has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, Merck, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche, Sanofi and Takeda, and research support from AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novartis, Roche and Sanofi. S. P. has received honoraria from AstraZeneca. T. R. G. has no disclosures.
Author contributions
K. K., T. G. and S. P. contributed to study design. L. G.‐A. and J. H. were involved in data collection. K. K., T. G. and S. P. wrote the first draft of the manuscript. All authors contributed to the analysis and interpretation of the data, provided critical input during the development of the manuscript and approved the final version for submission.
Khunti K, Godec TR, Medina J, et al. Patterns of glycaemic control in patients with type 2 diabetes mellitus initiating second‐line therapy after metformin monotherapy: Retrospective data for 10 256 individuals from the United Kingdom and Germany. Diabetes Obes Metab. 2018;20:389–399. https://doi.org/10.1111/dom.13083
Funding information AstraZeneca.
REFERENCES
- 1. International Diabetes Federation . IDF Diabetes Atlas. 7th ed.; 2015. http://www.diabetesatlas.org/. Accessed November 30, 2016. [Google Scholar]
- 2. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. [DOI] [PubMed] [Google Scholar]
- 3. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. [DOI] [PubMed] [Google Scholar]
- 4. UK Prospective Diabetes Study Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 5. Qaseem A, Humphrey LL, Sweet DE, Starkey M, Shekelle P. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2012;156:218–231. [DOI] [PubMed] [Google Scholar]
- 6. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429–442. [DOI] [PubMed] [Google Scholar]
- 7. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract. 2015;21:438–447. [DOI] [PubMed] [Google Scholar]
- 8. International Diabetes Federation . Global guideline for type 2 diabetes. 2012. https://www.idf.org/e-library/guidelines/79-global-guideline-for-type-2-diabetes.html. Accessed April 24, 2017.
- 9. American Diabetes Association . Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(suppl 1):S1–S132. [DOI] [PubMed] [Google Scholar]
- 10. Ji L, Bonnet F, Charbonnel B, et al. Towards an improved global understanding of treatment and outcomes in people with type 2 diabetes: rationale and methods of the DISCOVER observational study program. J Diabetes Complications. 2017;31(7):1188–1196. [DOI] [PubMed] [Google Scholar]
- 11. Becher H, Kostev K, Schröder‐Bernhardi D. Validity and representativeness of the “Disease Analyzer” patient database for use in pharmaco‐epidemiological and pharmacoeconomic studies. Int J Clin Pharmacol Ther. 2009;47:617–626. [DOI] [PubMed] [Google Scholar]
- 12. The Health Improvement Network Research Team . THIN Database. https://www.ucl.ac.uk/pcph/research-groups-themes/thin-pub/database. Accessed April 24, 2017.
- 13. Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of The Health Improvement Network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16:393–401. [DOI] [PubMed] [Google Scholar]
- 14. Denburg MR, Haynes K, Shults J, Lewis JD, Leonard MB. Validation of The Health Improvement Network (THIN) database for epidemiologic studies of chronic kidney disease. Pharmacoepidemiol Drug Saf. 2011;20:1138–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80 000 people. Diabetes Care. 2013;36:3411–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah BR, Hux JE, Laupacis A, Zinman B, van Walraven C. Clinical inertia in response to inadequate glycemic control: do specialists differ from primary care physicians? Diabetes Care. 2005;28:600–606. [DOI] [PubMed] [Google Scholar]
- 17. Fu AZ, Qiu Y, Davies MJ, Radican L, Engel SS. Treatment intensification in patients with type 2 diabetes who failed metformin monotherapy. Diabetes Obes Metab. 2011;13:765–769. [DOI] [PubMed] [Google Scholar]
- 18. Fu AZ, Sheehan JJ. Change in HbA1c associated with treatment intensification among patients with type 2 diabetes and poor glycemic control. Curr Med Res Opin. 2017;33:853–858. [DOI] [PubMed] [Google Scholar]
- 19. Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dujic T, Zhou K, Donnelly LA, Tavendale R, Palmer CN, Pearson ER. Association of organic cation transporter 1 with intolerance to metformin in type 2 diabetes: a GoDARTS Study. Diabetes. 2015;64:1786–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pongwecharak J, Tengmeesri N, Malanusorn N, Panthong M, Pawangkapin N. Prescribing metformin in type 2 diabetes with a contraindication: prevalence and outcome. Pharm World Sci. 2009;31:481–486. [DOI] [PubMed] [Google Scholar]
- 22. NICE Clinical Guidelines . The management of type 2 diabetes. 2009. https://www.nice.org.uk/guidance/ta203/resources/nice-recommends-liraglutide-for-type-2-diabetes-mellitus4. Accessed January 20, 2017.
- 23. Pfeiffer AF, Klein HH. The treatment of type 2 diabetes. Dtsch Arztebl Int. 2014;111:69–81; quiz 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta V, Kalra S. Choosing a gliptin. Indian J Endocrinol Metab. 2011;15:298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410–1418. [DOI] [PubMed] [Google Scholar]
- 26. Palmer SC, Mavridis D, Nicolucci A, et al. Comparison of clinical outcomes and adverse events associated with glucose‐lowering drugs in patients with type 2 diabetes: a meta‐analysis. JAMA. 2016;316:313–324. [DOI] [PubMed] [Google Scholar]
- 27. Rathmann W, Bongaerts B, Kostev K. Change in glycated haemoglobin levels after initiating second‐line therapy in type 2 diabetes: a primary care database study. Diabetes Obes Metab. 2016;18:840–843. [DOI] [PubMed] [Google Scholar]
- 28. Wilding JP, Blonde L, Leiter LA, et al. Efficacy and safety of canagliflozin by baseline HbA1c and known duration of type 2 diabetes mellitus. J Diabetes Complications. 2015;29:438–444. [DOI] [PubMed] [Google Scholar]
- 29. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27:1218–1224. [DOI] [PubMed] [Google Scholar]
- 30. Garcia‐Perez LE, Alvarez M, Dilla T, Gil‐Guillen V, Orozco‐Beltran D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4:175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Mean baseline‐adjusted decrease in HbA1c, by second‐line treatment (test for heterogeneity P < 0.001)
Figure S1. For those with mean baseline HbA1c (8.68%). CI, confidence interval; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; HbA1c, glycated haemoglobin; MET, metformin; SU, sulfonylurea.


