Abstract
Objective
The ketones d‐β‐hydroxybutyrate (BHB) and acetoacetate are elevated during prolonged fasting or during a “ketogenic” diet. Although weight loss on a ketogenic diet may be associated with decreased appetite and altered gut hormone levels, it is unknown whether such changes are caused by elevated blood ketones. This study investigated the effects of an exogenous ketone ester (KE) on appetite.
Methods
Following an overnight fast, subjects with normal weight (n = 15) consumed 1.9 kcal/kg of KE, or isocaloric dextrose (DEXT), in drinks matched for volume, taste, tonicity, and color. Blood samples were analyzed for BHB, glucose, insulin, ghrelin, glucagon‐like peptide 1 (GLP‐1), and peptide tyrosine tyrosine (PYY), and a three‐measure visual analogue scale was used to measure hunger, fullness, and desire to eat.
Results
KE consumption increased blood BHB levels from 0.2 to 3.3 mM after 60 minutes. DEXT consumption increased plasma glucose levels between 30 and 60 minutes. Postprandial plasma insulin, ghrelin, GLP‐1, and PYY levels were significantly lower 2 to 4 hours after KE consumption, compared with DEXT consumption. Temporally related to the observed suppression of ghrelin, reported hunger and desire to eat were also significantly suppressed 1.5 hours after consumption of KE, compared with consumption of DEXT.
Conclusions
Increased blood ketone levels may directly suppress appetite, as KE drinks lowered plasma ghrelin levels, perceived hunger, and desire to eat.
Introduction
Successful weight loss requires prolonged maintenance of a dietary calorie deficit. Perceived hunger is a significant barrier to long‐term weight loss 12. Diets that are “ketogenic” (low carbohydrate, high fat) are an effective strategy for weight loss and have been experimentally and anecdotally linked to decreased appetite 1, 2, 3. During a ketogenic diet, the body produces ketone bodies from lipid stored in adipose tissue. Ketones are then oxidized throughout the body as an alternative energy source during low dietary carbohydrate intake, when falling blood glucose levels threaten cerebral function 4. Decreased appetite during a ketogenic diet may be linked to elevated plasma ketone levels 5.
The mechanism whereby ketones could decrease appetite may be via central actions in the brain or by changes to peripheral hormone secretion 5. To support a central effect, intracerebral infusions of the ketone body d‐β‐hydroxybutyrate (BHB) decrease food intake in rodents 6, and BHB increases expression of orexigenic neuropeptides (i.e., agouti‐related peptide) in hypothalamic cells in vitro 7.
Evidence for peripheral effects can be seen in patients following a ketogenic diet, who have altered fasting and postprandial levels of some gut hormones, including the “hunger hormone,” ghrelin 2, 8. Ghrelin is produced by oxyntic cells of the stomach, and circulating plasma levels are highest during periods of starvation, whereas levels are rapidly downregulated following a meal 9. Ghrelin acts on the hypothalamus and vagus nerve to stimulate feeding 10, and basal circulating plasma levels are raised following periods of restricted food intake and weight loss. Importantly, increased basal ghrelin is implicated in overeating, and weight regain, following a diet 9.
We have developed a ketone ester (KE) drink that rapidly raises levels of blood BHB without dietary manipulation 11, 13, thereby allowing any direct satiating effect of ketones, per se, to be separated from other dietary and adaptive changes that accompany a ketogenic diet. In this study, we sought to determine whether KE drinks suppressed appetite via changes in plasma ghrelin levels.
Methods
Study design and participants
A randomized, single‐blinded, crossover study examined the effects of isocaloric KE and dextrose (DEXT) drinks on appetite in healthy participants with normal weight (n = 15) (Figure 1A). An external Research Ethics Committee (NHS Queen's Square 14/LO/0288) approved the study, which was conducted at the University of Oxford in accordance with the Declaration of Helsinki. The study was approved as a “basic science study” according to the definition from the UK Health Research Authority. Participants were healthy, aged 21 to 42, and had no history of major illness (anthropometric characteristics are provided in Table 1). Participants provided written informed consent prior to inclusion. Drink order was randomized prior to commencement.
Figure 1.
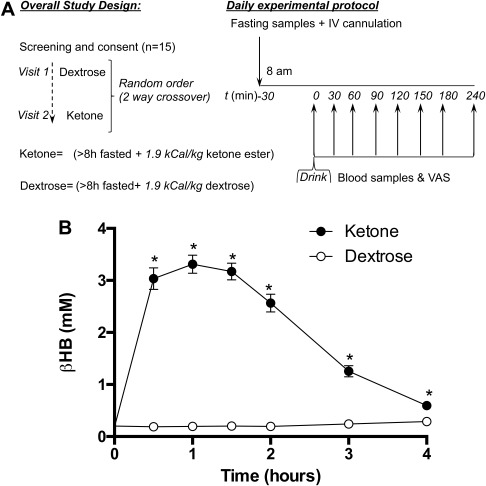
(A) Schematic illustrating the study protocol. (B) Blood BHB kinetics following isocaloric KE and DEXT drinks in 15 subjects at rest. Values are means ± SEM. *P < 0.05 difference between KE and DEXT.
Table 1.
Physical characteristics of subjects (n = 15)
| Age, y, mean (range) | 28 (21‐42) |
| Height, m, mean (range) | 1.8 (1.5‐2.1) |
| Weight, kg, mean (range) | 73 (54‐111) |
| BMI, kg/m2, mean (range) | 22 (19‐28) |
| Sex, n | |
| Male | 10 |
| Female | 5 |
Visit protocol
Participants refrained from alcohol and caffeine for 24 hours prior to each visit and consumed an identical evening meal at the same time before each visit. Testing started at 0800 hours following an overnight (> 8 h) fast, with a minimum of 72 hours between visits. Venous blood samples (2 mL) were obtained by using a 22‐gauge catheter inserted into an antecubital vein. Fasting blood samples were collected prior to all interventions and then at regular intervals for 4 hours following the study drink. At identical time points, participants completed a validated three‐measure visual analogue scale (VAS) to assess “hunger,” “desire to eat,” and “fullness” 14. Each drink contained 1.9 kcal/kg of BHB (from KE) or DEXT. Drinks were diluted to 500 mL with a commercially available citrus‐flavored drink containing 65 kcal (5 g of carbohydrate) (Glaceau; Coca‐Cola Great Britain, London, UK). The DEXT drink was taste‐matched by using a bitterness additive (Symrise, Holzminden, Germany).
Analysis
Blood BHB was measured by using a handheld monitor and reagent strips (Precision Xtra, Abbott Diabetes Care, Maidenhead, UK). Blood samples were stored on ice and centrifuged; duplicate plasma aliquots were stored at −80°C and analyzed within 6 weeks. Plasma glucose was assayed by using a commercial analyzer (ABX Pentra; HORIBA ABX SAS, Montpellier, France), while insulin and total ghrelin were measured by using commercially available enzyme‐linked immunosorbent assays (ELISA) (Mercodia AB, Uppsala, Sweden, and Merck, Millipore, Germany). Total glucagon‐like peptide 1 (GLP‐1) and peptide tyrosine tyrosine (PYY) assays were performed by the Core Biochemical Assay Laboratory of the National Institute for Health Research Cambridge Biomedical Research Centre (http://www.cuh.org.uk/core-biochemical-assay-laboratory).
Statistical methods
Prism 6 software (GraphPad Software, Inc.) was used for statistical analysis. VAS scores were measured as a distance (mm) and normalized by taking the baseline distance for each visit as zero. Values are means ± standard error of the mean (SEM) with significance at P < 0.05. Following initial tests to ensure that normality and sphericity assumptions were not violated, two‐way repeated‐measures analysis of variance (ANOVA) or a Mann‐Whitney U test with post hoc correction was performed, as appropriate. Correlations were calculated between blood BHB and VAS score for each individual during the KE visit at each time point post baseline (total points = 105) by using a two‐tailed Pearson test (with Pearson r reported as r and significance as P) with a 95% confidence interval.
Results
Following KE consumption, blood BHB levels rapidly increased to 3.3 ± 0.2 mM after 1 hour and gradually fell over the remaining 3 hours, whereas DEXT administration had no effect on BHB levels (Figure 1B). The perception of hunger and desire to eat fell by a similar extent after both drinks, but KE lowered both parameters by ∼50% for 1.5 to 4 hours compared with DEXT drinks (Figure 2A‐2B). Perceived fullness was the same following both DEXT and KE drinks (Figure 2C). Increasing ketonemia was significantly correlated to decreased hunger, desire to eat, and increased fullness (Figure 2D‐2F).
Figure 2.
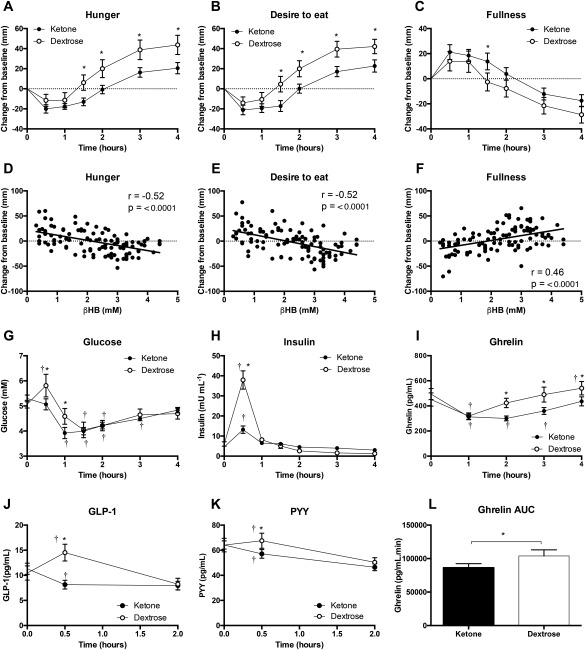
Changes in VAS responses from baseline, correlation between BHB and VAS score, and levels of plasma glucose, insulin, ghrelin, GLP‐1, and PYY following isocaloric KE and DEXT drinks in 15 subjects at rest. Panels 2D‐2F show data from the KE visit only: each point is one of the time series points between 30 and 240 minutes (seven time points) for each participant (n = 15), giving a total of 105 points. Values are means ± SEM. r = Pearson's r. *P < 0.05 difference between KE and DEXT; † P < 0.05 difference from baseline value.
To explore possible mechanisms for these observations, we compared plasma glucose, insulin, ghrelin, GLP‐1, and PYY levels after KE and DEXT drinks. Plasma glucose levels were significantly higher 30 minutes after DEXT consumption than after KE consumption (5.8 ± 0.5 mM vs. 5.1 ± 0.2 mM, P = 0.02) (Figure 2G). Plasma insulin levels rose after both KE and DEXT consumption, but levels were threefold higher 30 minutes after DEXT administration, compared with KE administration (38 ± 5 mU/L vs. 13 ± 2 mU/L, P < 0.001) (Figure 2H). After 90 minutes, there were no significant differences between plasma glucose and insulin levels following KE and DEXT drinks. Plasma ghrelin fell to ≈ 320 pg/mL 1 hour following both drinks, but the postprandial rise in ghrelin was significantly attenuated following KE administration, remaining > 100 pg/mL lower between 2 to 4 hours post drink than after DEXT. Total ghrelin area under the curve (AUC) was significantly lower with KE than with DEXT (86,294 ± 6,193 pg·min/mL vs. 103,780 ± 9,136 pg·min/mL, P < 0.05) (Figure 2I‐2L). As expected, GLP‐1 and PYY were elevated following DEXT administration. However, plasma GLP‐1 and PYY levels were not raised in response to KE drinks after 30 minutes and were lower than DEXT (GLP‐1: 15 pg/mL vs. 8 pg/mL, P < 0.001; PYY: 68 pg/mL vs. 57 pg/mL, P = 0.001) (Figure 2J‐2K).
Discussion
Ketogenic diets can decrease appetite and reduce calorie intake, but the exact mechanism for the suppressed appetite is unclear 1, 2, 8. Elevated blood BHB occurs during both exogenous and endogenous ketosis; therefore, BHB may be a direct mediator of the lower appetite and altered gut hormone levels seen in both settings. Here, we found that KE drinks delayed the onset of hunger and lowered the desire to eat, in conjunction with a delayed rise in plasma ghrelin levels. Appetite suppression observed following KE drinks was not attributable to higher plasma levels of insulin, glucose, GLP‐1, or PYY, which are conventionally believed to be signals that decrease appetite 15, 16, 17.
Hunger and satiety are signaled by two opposing neural pathways in the arcuate nucleus of the hypothalamus: neuropeptide Y (NPY)/agouti‐related peptide neurons and pro‐opiomelanocortin (POMC) neurons, respectively. Ghrelin, commonly known as the hunger hormone, activates orexigenic NPY neurons. NPY‐expressing neurons inhibit anorexigenic POMC neurons at both their site of origin in the arcuate nucleus of the hypothalamus (via GABAergic inhibition) and in the paraventricular nucleus of the hypothalamus by antagonism of MC4 receptor activation 12. Damping the activity of NPY‐expressing “hunger” neurons by decreasing ghrelin levels may therefore be highly effective in reducing overall food intake.
A putative mechanism whereby BHB could alter gut hormone secretion is through receptor binding to gut enteroendocrine cells; for example, BHB antagonizes GPCR‐41 18, a Gi/Go protein‐coupled receptor expressed throughout the small bowel. Lower levels of fasting plasma ghrelin were observed in patients on a ketogenic diet who achieved ketosis during weight loss 2, 8, with the fall in appetite being correlated to the level of ketosis. However, the effect of BHB alone in such studies is difficult to ascertain in conjunction with increased dietary fat and protein. It is prudent to note that Hall et al. showed that diets low in fat resulted in greater body fat loss than carbohydrate‐restricted diets, highlighting that metabolic adaptation during caloric restriction is complex 18.
It remains unclear to what extent BHB acts centrally in the brain to directly modulate appetite, although such effects are likely, given the evolutionary role of BHB as a cerebral metabolic fuel 4, 5. Furthermore, the neuroprotective effect of calorie restriction in Parkinson's disease is directly linked to des‐acyl ghrelin signaling through AMP‐activated protein kinase, which plays a pivotal role in the regulation of POMC and NPY neuron activity, and the effect of BHB may therefore also be mediated centrally 19. In the future, KE drinks could be used in studies that combine neuroimaging with blood analysis to clarify the interaction between the central and peripheral effects of ketosis on appetite in the absence of the confounding contributions of the ketogenic diet.
Finally, it is important to emphasize that the levels of GLP‐1 and PYY, both satiety signals, were also downregulated by acute KE administration. Given the acute effects of KE consumption on the endocrine system shown here, the potential for adaptations over time, and the mild effects of KE drinks on acid‐base homeostasis (unpublished data), it is unclear whether KE consumption could be used for long‐term appetite control. Therefore, until any chronic effects are fully characterized, ketone supplements should not be used to replace dietary strategies for weight control.
Conclusion
Exogenous ketosis following KE drinks reduced two measures of appetite, hunger and desire to eat, compared with DEXT drinks. This occurred in conjunction with decreased levels of the hunger hormone, ghrelin. Therefore, KE drinks offer a unique opportunity to isolate and exploit the effects of ketosis on appetite without other dietary interventions.
See Commentary, pg. 252.
Funding agencies: This work was undertaken as part of an Industrial DPhil Fellowship to BJS from the Royal Commission for the Exhibition of 1851. TΔS Ltd. provided the ketone ester, ΔG.
Disclosure: The intellectual property covering the uses of ketones and ketone esters is owned by BTG Ltd., the University of Oxford, the National Institutes of Health, and TΔS Ltd. Should royalties ever accrue from these patents, Professor Kieran Clarke and Dr. Pete Cox, as inventors, will receive a share of the royalties under the terms proscribed by Oxford University. Professor Kieran Clarke is a director of TΔS Ltd., a company spun out of the University of Oxford to develop and commercialize products based on the science of ketone bodies in human nutrition. BJS was an employee of TΔS Ltd.
Author contributions: BJS, KC, and PJC designed the research studies. BJS, PJC, HdW and RDE carried out the studies. BJS, HdW, and MC analyzed the data. BJS wrote the paper with help from MC, HdW, and KC. HdW had primary responsibility for the final content. All authors read and approved the final manuscript.
Clinical trial registration: Research Ethics Committee (NHS Queen's Square 14/LO/0288), www.hra.nhs.uk.
References
- 1. Dietrich MO, Horvath TL. Limitations in anti‐obesity drug development: the critical role of hunger‐promoting neurons. Nat Rev Drug Discov 2012;11:675‐691. [DOI] [PubMed] [Google Scholar]
- 2. Gibson AA, Seimon RV, Lee CM, et al. Do ketogenic diets really suppress appetite? A systematic review and meta‐analysis. Obes Rev 2015;16:64‐76. [DOI] [PubMed] [Google Scholar]
- 3. Sumithran P, Prendergast LA, Delbridge E, et al. Ketosis and appetite‐mediating nutrients and hormones after weight loss. Eur J Clin Nutr 2013;67:759‐764. [DOI] [PubMed] [Google Scholar]
- 4. Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high‐protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr 2008;87:44‐55. [DOI] [PubMed] [Google Scholar]
- 5. Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF. Brain metabolism during fasting. J Clin Invest 1967;46:1589‐1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paoli A, Bosco G, Camporesi EM, Mangar D. Ketosis, ketogenic diet and food intake control: a complex relationship. Front Psychol 2015;6:27. doi:10.3389/fpsyg.2015.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arase K, Fisler JS, Shargill NS, York DA, Bray GA. Intracerebroventricular infusions of 3‐OHB and insulin in a rat model of dietary obesity. Am J Physiol 1988;255:R974‐R981. [DOI] [PubMed] [Google Scholar]
- 8. Laeger T, Pöhland R, Metges CC, Kuhla B. The ketone body β‐hydroxybutyric acid influences agouti‐related peptide expression via AMP‐activated protein kinase in hypothalamic GT1‐7 cells. J Endocrinol 2012;213:193‐203. [DOI] [PubMed] [Google Scholar]
- 9. Chearskul S, Delbridge E, Shulkes A, Proietto J, Kriketos A. Effect of weight loss and ketosis on postprandial cholecystokinin and free fatty acid concentrations. Am J Clin Nutr 2008;87:1238‐1246. [DOI] [PubMed] [Google Scholar]
- 10. Müller TD, Nogueiras R, Andermann ML, et al. Ghrelin. Mol Metab 2015;4:437‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. le Roux CW, Neary NM, Halsey TJ, et al. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J Clin Endocrinol Metab 2005;90:4521‐4524. [DOI] [PubMed] [Google Scholar]
- 12. Cox Pete J, Kirk T, Ashmore T, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab 2016;24:256–268. [DOI] [PubMed] [Google Scholar]
- 13. Clarke K, Tchabanenko K, Pawlosky R, et al. Kinetics, safety and tolerability of (R)‐3‐hydroxybutyl (R)‐3‐hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol 2012;63:401‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stubbs RJ, Hughes DA, Johnstone AM, et al. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand‐held computerized systems for temporal tracking of appetite ratings. Br J Nutr 2000;84:405‐415. [DOI] [PubMed] [Google Scholar]
- 15. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature 2006;443:289‐295. [DOI] [PubMed] [Google Scholar]
- 16. Mayer J. Regulation of energy intake and the body weight: the glucostatic theory and the lipostatic hypothesis. Ann N Y Acad Sci 1955;63:15‐43. [DOI] [PubMed] [Google Scholar]
- 17. Parker HE, Gribble FM, Reimann F. The role of gut endocrine cells in control of metabolism and appetite. Exp Physiol 2014;99:1116‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hall KD, Bemis T, Brychta R, et al. Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell Metab 2015;22:427‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bayliss JA, Lemus MB, Stark R, et al. Ghrelin‐AMPK signaling mediates the neuroprotective effects of calorie restriction in Parkinson's disease. J Neurosci 2016;36:3049‐3063. [DOI] [PMC free article] [PubMed] [Google Scholar]


