Abstract
BACKGROUND
Health‐related quality of life (HRQOL) in patients with chronic‐phase chronic myeloid leukemia (CML) is important because of the requirement for long‐term treatment. This study assessed HRQOL in bosutinib‐treated patients with Philadelphia chromosome–positive CML and resistance or intolerance to 1 (chronic‐phase second‐line [CP2L]) or more (chronic‐phase third‐line [CP3L]) tyrosine kinase inhibitors who had 264 weeks or more of follow‐up (ClinicalTrials.gov identifier NCT00261846).
METHODS
Patient‐reported HRQOL was assessed with the EuroQol 5‐Dimensions Questionnaire (EQ‐5D) and the Functional Assessment of Cancer Therapy–Leukemia (FACT‐Leu).
RESULTS
In total, 284 and 119 patients composed the CP2L and CP3L cohorts, respectively. At treatment completion, more than 50% of the patients in the CP2L and CP3L cohorts completed the EQ‐5D and FACT‐Leu assessments. The EQ‐5D and EQ‐5D visual analog scale scores were stable in both cohorts throughout treatment. The mean FACT‐Leu scores were generally stable over time but were lower in magnitude in the CP3L cohort versus the CP2L cohort. The FACT‐Leu scale scores of a subset of patients with chronic diarrhea (CP2L, n = 101; CP3L, n = 30) were similar to the scores of the larger cohorts. Minimally important differences (MIDs) from baseline for the FACT‐Leu scale scores were observed for the following: emotional well‐being (EWB), Functional Assessment of Cancer Therapy–General (FACT‐G) Total, FACT‐Leu Total, and Functional Assessment of Cancer Therapy Trial Outcome Index (FACT‐TOI) in the CP2L cohort and FACT‐Leu Total in the CP3L cohort. Among patients with chronic diarrhea, MIDs were observed for EWB, FACT‐G Total, FACT‐Leu Total, and FACT‐TOI in the CP2L cohort and for EWB, FACT‐G Total, and FACT‐Leu Total in the CP3L cohort.
CONCLUSIONS
HRQOL was maintained with long‐term bosutinib treatment for patients with CP2L and CP3L CML. Cancer 2018;124:587‐95. © 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society. This is an open access article under the terms of the Creative Commons Attribution‐NonCommercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Keywords: bosutinib, chronic myeloid leukemia, patient‐reported outcomes, quality of life, tyrosine kinase inhibitors
Short abstract
Health‐related quality of life (assessed with the Functional Assessment of Cancer Therapy–Leukemia) and the general health status (assessed with the EuroQol 5‐Dimensions Questionnaire) were largely maintained in chronic‐phase chronic myeloid leukemia patients receiving bosutinib for 264 weeks or longer. Health‐related quality of life was maintained by patients who receive bosutinib as a second‐ or third‐line treatment for chronic‐phase chronic myeloid leukemia.
INTRODUCTION
The treatment of Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia (CML) consists of tyrosine kinase inhibitors (TKIs) in the first‐ and subsequent‐line treatment settings. TKIs are highly effective, but tolerability is a concern for many patients. Studies have shown that 2% to 14% of patients receiving first‐line treatment1, 2, 3, 4 and 9% to 25% receiving second‐line treatment5, 6, 7, 8, 9 discontinue them because of adverse events (AEs). In addition to disrupting treatment continuity, AEs are associated with poor medication adherence rates, which have been shown to negatively affect treatment responses.10, 11 In a multivariate analysis, medication adherence (>90% or ≤ 90%) was an independent predictor of a major molecular response, a 4‐log reduction in Bcr‐Abl transcript levels, and a complete molecular response in patients taking imatinib.10
TKI tolerability has also been shown to negatively affect health‐related quality of life (HRQOL) in patients with CML. HRQOL in patients treated with imatinib, dasatinib, or nilotinib can be poor; symptoms such as itching, edema, cough, dizziness, and diarrhea have been shown to cause greater distress in patients treated with TKIs in comparison with age‐ and sex‐matched controls without cancer.12 A “negative medication experience” resulting from treatment or disease symptoms is associated with lower treatment satisfaction, greater limitations in physical activity, and worse HRQOL outcomes.13 Thus, TKI tolerability affects 2 major components of patient care: treatment adherence, which affects therapeutic efficacy, and HRQOL.
Bosutinib, a dual Src‐Abl TKI, is indicated for the treatment of Ph+ chronic‐phase (CP), accelerated‐phase (AP), or blast‐phase (BP) CML in adults with resistance or intolerance to prior therapy.14 In an open‐label phase 1/2 study, efficacy and safety were demonstrated for bosutinib at 500 mg/d in adults with Ph+ CML resistant or intolerant to imatinib (ClinicalTrials.gov identifier NCT00261846; n = 288).15 After a median follow‐up of 24 months, 53% of the patients (54% who were imatinib‐resistant and 49% who were imatinib‐intolerant) achieved a cumulative major cytogenetic response, and 41% achieved a cumulative complete cytogenetic response (41% who were imatinib‐resistant and 41% who were imatinib‐intolerant).15 The most common nonhematologic AEs were diarrhea, nausea, rash, and vomiting; grade 3/4 rash and diarrhea each occurred in 9% of patients. Longer‐term follow‐up (≤5 years) showed a durable response and no new safety concerns.16, 17, 18 HRQOL for patients enrolled in this study has been previously reported for patients with CP,19 AP,20 and BP CML20 on the basis of 96 weeks of follow‐up. Here, we provide an update on long‐term HRQOL for the chronic‐phase second‐line (CP2L) and chronic‐phase third‐line (CP3L) cohorts from this study, for whom data for 264 weeks or more of follow‐up are available. HRQOL was also assessed for the subset of patients with chronic diarrhea to examine how this AE affects patient HRQOL. Patients with CP CML require treatment for many years; therefore, an understanding of how therapy affects long‐term HRQOL is of high importance.
MATERIALS AND METHODS
Full details of this trial have previously been published.15, 17, 18 In brief, eligible patients were 18 years old or older, had a confirmed diagnosis of any phase of Ph+ CML or Ph+ acute lymphocytic leukemia with resistance (full dose of 600 mg/d or higher) or intolerance (any dose) to imatinib, had an Eastern Cooperative Oncology Group performance status of 0 or 1 for CP CML or 0 to 2 for advanced‐phase CML, had adequate bone marrow function (CP CML with a history of imatinib resistance only), and had adequate hepatic and renal function. For the evaluation of HRQOL, the following 2 cohorts were assessed: 1) CP CML after resistance or intolerance to imatinib (CP2L) and 2) CP CML after resistance or intolerance to imatinib followed by resistance to dasatinib, intolerance to dasatinib, or resistance to nilotinib (CP3L). The results for advanced CML, AP/BP CML, and acute lymphocytic leukemia with resistance or intolerance to imatinib with or without prior TKI exposure are not presented here. Inclusion and exclusion criteria have been previously published.15, 17, 18 The study protocol was approved by each site's ethics board and conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
HRQOL Assessments
HRQOL assessments from the patients' perspectives were evaluated as exploratory endpoints and included the EuroQol 5‐Dimensions Questionnaire (EQ‐5D) and the Functional Assessment of Cancer Therapy–Leukemia (FACT‐Leu). EQ‐5D is a 5‐item assessment of a patient's general health status across 5 dimensions (mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression) and has an overall health visual analog scale (VAS).21 The potential responses for each dimension of the 3‐level version of EQ‐5D include “no problems,” “some problems,” and “extreme problems.” FACT‐Leu, a 44‐item self‐reported assessment, measures general (Functional Assessment of Cancer Therapy–General [FACT‐G]) and leukemia‐specific HRQOL outcomes as well as additional concerns.19, 22 FACT‐Leu summary scale measures include FACT‐G (physical well‐being [PWB], social/family well‐being [SWB], emotional well‐being [EWB], and functional well‐being [FWB]), FACT‐Leu Total (FACT‐G and leukemia subscale), and the FACT‐Leu Trial Outcome Index (TOI; PWB, FWB, and leukemia subscale). Minimally important differences (MIDs) for each FACT‐Leu scale have been identified: PWB (2 or 3 points), SWB (not available), EWB (2 points), FWB (2 or 3 points), FACT‐G Total (3‐7 points), FACT‐TOI (5 or 6 points), and FACT‐Leu Total (6‐12 points).
The EQ‐5D and FACT‐Leu assessments were administered at baseline (ie, screening); at weeks 4, 8, and 12; every 12 weeks thereafter; and at treatment completion. Assessments were also administered at the times of disease progression, grade 3 or 4 toxicity, and early withdrawal. To determine the impact of chronic diarrhea on HRQOL, a post hoc assessment of the subset of patients with chronic diarrhea (defined as an individual episode of diarrhea lasting 4 weeks or longer) was completed. Descriptive statistics were obtained for the scale scores and the mean change from baseline score at each time point. Within‐cohort comparisons were assessed with paired t tests. There were no adjustments for multiplicity of testing, so overall trends in the data, not P values, are reported. EQ‐5D scores were derived with the UK value set.
RESULTS
Patients
A total of 403 patients composed the safety population (ie, they had received 1 or more doses of bosutinib); 284 and 119 patients were in the CP2L and CP3L cohorts, respectively. Demographic and baseline characteristics are summarized in Table 1. In total, 24% of the patients in the CP2L cohort and 28% of the patients in the CP3L cohort discontinued therapy because of an AE. At baseline, 87% of EQ‐5D assessments were collected in both the CP2L and CP3L cohorts. Similarly, 86% to 87% of FACT‐Leu assessments were collected in both cohorts at baseline. At most time points throughout the study, 43% or more of the EQ‐5D and FACT‐Leu assessments were collected in the CP2L cohort, and 22% or more were collected in the CP3L cohort; however, at weeks 108, 132, and 156, substantially fewer assessments (<10%) were collected in both cohorts. At treatment completion, 68% and 66% of EQ‐5D assessments and 66% and 65% of FACT‐Leu assessments were collected in the CP2L and CP3L cohorts, respectively. Among patients with chronic diarrhea, 101 and 30 patients were in the CP2L and CP3L cohorts, respectively.
Table 1.
Demographics and Baseline Characteristics (Safety Population)
| Characteristic | CP2L (n = 284) | CP3L (n = 119) | Total (n = 403) |
|---|---|---|---|
| Male, No. (%) | 149 (52.5) | 53 (44.5) | 202 (50.1) |
| Race, No. (%) | |||
| Asian | 62 (21.8) | 15 (12.6) | 77 (19.1) |
| Black | 16 (5.6) | 6 (5.0) | 22 (5.5) |
| Other | 20 (7.0) | 11 (9.2) | 31 (7.7) |
| White | 186 (65.5) | 87 (73.1) | 273 (67.7) |
| Age | |||
| Mean (SD), y | 51.4 (15.1) | 54.6 (13.0) | 52.4 (14.6) |
| ≥65 y, No. (%) | 63 (22.2) | 27 (22.7) | 90 (22.3) |
| ECOG performance status, No. (%) | |||
| 0 | 217 (76.4) | 85 (71.4) | 302 (74.9) |
| 1 | 65 (22.9) | 33 (27.7) | 98 (24.3) |
| 2 | 1 (0.4) | 0 | 1 (0.2) |
| Not collected | 1 (0.4) | 1 (0.8) | 2 (0.5) |
| Prior therapies, No. (%)a | |||
| 1 | 184 (64.8) | 0 | 184 (45.7) |
| 2 | 100 (35.2) | 52 (43.7) | 152 (37.7) |
| 3 | 0 | 65 (54.6) | 65 (16.1) |
| 4 | 0 | 2 (1.7) | 2 (0.5) |
| Prior interferon therapy, No. (%) | 100 (35.2) | 65 (54.6) | 165 (40.9) |
| Prior imatinib therapy, No. (%) | |||
| Intolerant | 89 (31.3) | 36 (30.3) | 125 (31.0) |
| Resistant | 195 (68.7) | 83 (69.7) | 278 (69.0) |
| Prior dasatinib therapy, No. (%) | 0 | 92 (77.3) | 92 (22.8) |
| Prior nilotinib therapy, No. (%) | 0 | 31 (26.1) | 31 (7.7) |
| Prior stem cell transplant, No. (%) | 8 (2.8) | 9 (7.6) | 17 (4.2) |
Abbreviations: CP2L, chronic‐phase second‐line; CP3L, chronic‐phase third‐line; ECOG, Eastern Cooperative Oncology Group; SD, standard deviation.
If a subject had received 1 or more treatment regimens with imatinib, dasatinib, nilotinib, or interferon, the subject was counted only once for the respective treatment.
HRQOL Assessments
EQ‐5D scores
At baseline, the mean EQ‐5D scores were 0.83 (95% confidence interval [CI], 0.80‐0.85) and 0.80 (95% CI, 0.76‐0.85) in the CP2L and CP3L cohorts, respectively. The EQ‐5D scores appeared to be relatively stable throughout 384 weeks of bosutinib treatment in both cohorts despite a notable decrease in the number of patients in each cohort at later time points (Fig. 1). Improvements from baseline were observed at weeks 36, 96, 192, and 360 in the CP2L cohort.
Figure 1.
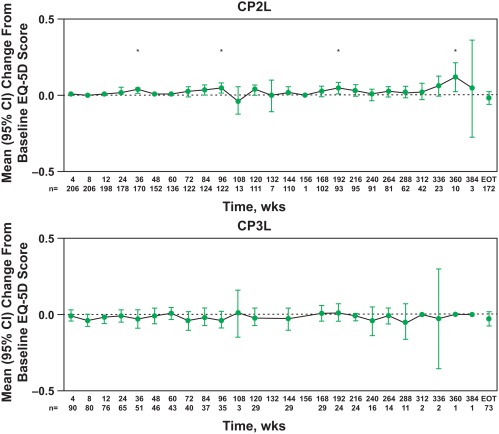
EQ‐5D score changes from baseline by visit according to chronic myeloid leukemia phase. *P ≤ .05 for a within‐group comparison between the specified time point and baseline using the paired t test. CI indicates confidence interval; CP2L, chronic‐phase second‐line; CP3L, chronic‐phase third‐line; EOT, end of treatment/treatment completion; EQ‐5D, EuroQol 5‐Dimensions Questionnaire.
At treatment completion, for patients with both baseline and treatment completion values, most patients in the CP2L (59%‐91%) and CP3L cohorts (55%‐92%) reported “no problems” on all 5 dimensions of the EQ‐5D (Table 2). A minority of patients reported “some problems” in the CP2L (≤37%) and CP3L cohorts (≤41%) on any 1 of the 5 dimensions of the EQ‐5D; “extreme problems” were reported by 5% or fewer of the patients in each cohort.
Table 2.
Percentages of Patients Taking Bosutinib in the CP2L and CP3L Cohorts Who Reported No, Some, or Extreme Problems on the EuroQol 5‐Dimensions Questionnaire at Baseline and Treatment Completion
| No Problems | Some Problems | Extreme Problems | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline, % | Treatment Completion, % | Difference | Baseline, % | Treatment Completion, % | Difference | Baseline, % | Treatment Completion, % | Difference | |
| CP2L cohort (n = 173) | |||||||||
| Mobilitya | 75.0 | 77.3 | 2.3 | 25.0 | 22.1 | −2.9 | 0 | 0.6 | 0.6 |
| Self‐care | 96.5 | 90.8 | −5.7 | 3.5 | 9.2 | 5.7 | 0 | 0 | 0 |
| Usual activities | 76.9 | 71.1 | −5.8 | 23.1 | 26.6 | 3.5 | 0 | 2.3 | 2.3 |
| Pain/discomforta | 59.3 | 58.7 | −0.6 | 37.2 | 36.6 | −0.6 | 3.5 | 4.7 | 1.2 |
| Anxiety/depression | 62.4 | 64.7 | 2.3 | 37.0 | 33.5 | −3.5 | 0.6 | 1.7 | 1.1 |
| CP3L cohort (n = 73) | |||||||||
| Mobility | 79.5 | 76.7 | −2.8 | 20.5 | 23.3 | 2.8 | 0 | 0 | 0 |
| Self‐care | 95.9 | 91.8 | −4.1 | 4.1 | 6.8 | 2.7 | 0 | 1.4 | 1.4 |
| Usual activities | 69.9 | 67.1 | −2.8 | 28.8 | 31.5 | 2.7 | 1.4 | 1.4 | 0 |
| Pain/discomfort | 58.9 | 56.2 | −2.7 | 35.6 | 39.7 | 4.1 | 5.5 | 4.1 | −1.4 |
| Anxiety/depression | 58.9 | 54.8 | −4.1 | 41.1 | 41.1 | 0 | 0 | 4.1 | 4.1 |
Abbreviations: CP2L, chronic‐phase second‐line; CP3L, chronic‐phase third‐line.
n = 172.
EQ‐5D VAS scores
At baseline, the mean EQ‐5D VAS scores were 71.31 (95% CI, 68.32‐74.29) and 72.56 (95% CI, 67.92‐77.21) in the CP2L and CP3L cohorts, respectively. Similar to the EQ‐5D scores, the EQ‐5D VAS scores were generally stable throughout 384 weeks of bosutinib treatment in both cohorts (Fig. 2). Improvements from baseline were observed at weeks 8 to 96, 120, 144, and 168 to 264 in the CP2L cohort and at weeks 48 to 96, 120, 168, and 240 in the CP3L cohort.
Figure 2.
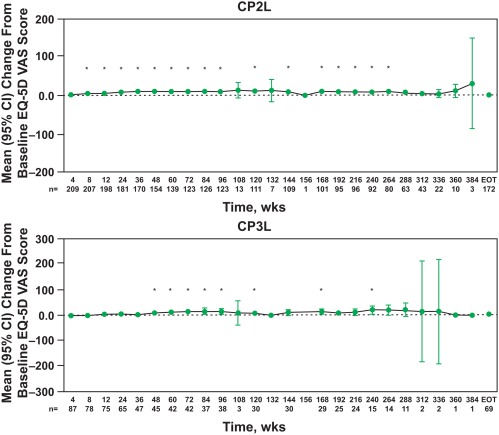
EQ‐5D VAS score changes from baseline by visit according to chronic myeloid leukemia phase. *P ≤ .05 for a within‐group comparison between the specified time point and baseline using the paired t test. CI indicates confidence interval; CP2L, chronic‐phase second‐line; CP3L, chronic‐phase third‐line; EOT, end of treatment/treatment completion; EQ‐5D, EuroQol 5‐Dimensions Questionnaire; VAS, visual analog scale.
FACT‐Leu
The baseline mean FACT‐Leu scores (and 95% CIs) in the CP2L and CP3L cohorts were comparable. FACT‐Leu change from baseline scores for general and summary scales are presented in Figure 3; the magnitude of the changes was generally lower in the CP3L cohort versus the CP2L cohort. At various time points, an improvement in PWB was observed in the CP2L cohort (weeks 60, 120, and 168). Consistent improvements from baseline, except at treatment completion, in the EWB, FACT‐Leu Total, FACT‐TOI, and additional concerns scores were observed over time with bosutinib treatment in the CP2L cohort. In addition, MIDs denoting a benefit were observed in the CP2L cohort at weeks 168, 216, and 264 for EWB and at weeks 168 and 216 for FACT‐G Total and FACT‐Leu Total; an MID was observed for FACT‐TOI in the CP2L cohort at week 168 only. A decline in FWB was observed at treatment completion in the CP2L cohort, but there was no evidence of a progressive decline over time; in fact, scale scores showed small improvements over time. There were no clear trends in FACT‐Leu change from baseline scores in the CP3L cohort, with an MID denoting a benefit surpassed at week 264 only for the change from baseline in the FACT‐Leu Total score. An improvement in EWB (week 168) and declines in SWB (week 60), FWB (week 60 and treatment completion), and FACT‐G Total (treatment completion only) were observed in the CP3L cohort.
Figure 3.
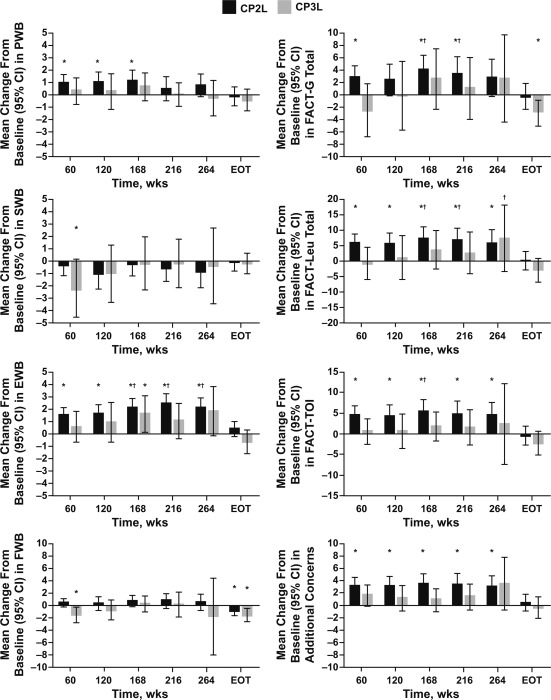
FACT‐Leu scale score changes from baseline by visit and at treatment completion. The ranges of assessments for the listed scale scores within the CP2L cohort (n = 284) were 135 to 138 for week 60, 108 to 112 for week 120, 100 to 103 for week 168, 94 to 95 for week 216, 78 to 80 for week 264, and 171 to 174 at EOT. The ranges of assessments for the listed scale scores within the CP3L cohort (n = 119) were 41 to 43 for week 60, 28 to 30 for week 120, 27 to 29 for week 168, 23 to 24 for week 216, 12 to 14 for week 264, and 74 to 75 at EOT. The baseline values for the CP2L cohort were 22.96 (PWB), 21.17 (SWB), 17.76 (EWB), 19.09 (FWB), 81.04 (FACT‐G Total), 133.22 (FACT‐Leu Total), 94.28 (FACT‐TOI), and 52.16 (additional concerns). The baseline values for the CP3L cohort were 22.61 (PWB), 21.09 (SWB), 17.96 (EWB), 19.31 (FWB), 80.81 (FACT‐G Total), 132.50 (FACT‐Leu Total), 93.75 (FACT‐TOI), and 51.83 (additional concerns). *P ≤ .05 for a within‐group comparison between the specified time point and baseline using the paired t test. †Minimally important differences. Positive values indicate an improvement in HRQOL, and negative scores indicate reduced HRQOL. CI indicates confidence interval; CP2L, chronic‐phase second‐line; CP3L, chronic‐phase third‐line; EOT, end of treatment/treatment completion; EWB, emotional well‐being; FACT‐G, Functional Assessment of Cancer Therapy–General; FACT‐Leu, Functional Assessment of Cancer Therapy–Leukemia; FACT‐TOI, Functional Assessment of Cancer Therapy Trial Outcome Index; FWB, functional well‐being; PWB, physical well‐being; SWB, social/family well‐being.
FACT‐Leu in patients with chronic diarrhea
In the subset of patients with chronic diarrhea, the baseline FACT‐Leu general and summary scales were generally similar across the CP2L and CP3L cohorts. FACT‐Leu change from baseline scores for the general and summary scales were generally lower for patients with chronic diarrhea in the CP3L cohort versus the CP2L cohort (Supporting Table 1 [see online supporting information]). In the CP2L cohort, improvements in PWB were observed at weeks 60 and 120. Consistent improvements from baseline over time in EWB, FACT‐Leu Total (except at week 120 and at treatment completion), FACT‐TOI (except at treatment completion), and additional concerns were observed in patients with chronic diarrhea in the CP2L cohort. Changes from baseline achieving an MID (ie, improved HRQOL) were observed at weeks 168, 216, and 264 for EWB, FACT‐G Total, FACT‐Leu Total, and FACT‐TOI (except week 216). In patients with chronic diarrhea in the CP3L cohort, FACT‐Leu scores were generally stable. Changes from baseline surpassing MIDs were observed at week 168 for EWB, at weeks 60 and 168 for FACT‐G Total, and at week 168 for FACT‐Leu Total. Although declines from baseline in scores and MIDs denoting impaired HRQOL were observed at treatment completion for FWB (and week 264), FACT‐G Total, and FACT‐TOI, scores were generally stable throughout treatment and did not show a consistent decline.
In comparison with the larger CP2L and CP3L cohorts, FACT‐Leu scores for patients with chronic diarrhea were generally similar and in several instances higher. At treatment completion, patients with chronic diarrhea in the CP2L cohort had higher FACT‐Leu scores than the total study population. In the CP3L cohort, patients with chronic diarrhea had similar or lower FACT‐Leu scores at treatment completion (except for SWB) in comparison with the total study population.
DISCUSSION
Patients with CP CML are living much longer because of the advent of TKIs. Therefore, these patients require long‐term treatment, and the corresponding impact on HRQOL is an important consideration in treatment decisions. This analysis showed that HRQOL is largely maintained over the long term in patients who received bosutinib for CP2L and CP3L CML, indicating that bosutinib is able to preserve HRQOL in patients who are treated after frontline therapy and in more heavily treated patients such as those in later lines of therapy.
Our results are consistent with a previous HRQOL analysis from this same study that demonstrated stability and/or improvement in FACT‐Leu general and summary scale scores in the CP2L cohort of imatinib‐resistant and ‐intolerant patients over 96 weeks of treatment.19 In the current analysis, we focused on both the CP2L and CP3L cohorts, regardless of imatinib resistance or intolerance, with 264 weeks or more of follow‐up. In CP2L patients, changes from baseline scores surpassing MIDs were observed at weeks 168 to 264 for EWB and at weeks 168 and 216 for the FACT‐G, FACT‐Leu Total, and FACT‐TOI scales (week 168 only). In the CP3L cohort, changes from baseline scores surpassing the MIDs were reached at week 264 for the FACT‐Leu Total scale but not for any of the other summary scale scores for this cohort of patients. Greater impairment in HRQOL was observed in the CP3L cohort versus the CP2L cohort, as would be expected for patients with more advanced disease or previous TKI tolerability issues. Taken together, the results show that bosutinib is associated with clinically meaningful improvements in EWB and FACT‐Leu summary scale HRQOL measures, particularly for patients receiving second‐line treatment.
Because of the impact of AEs on medication adherence, therapeutic efficacy, and HRQOL, differences between the safety profiles of TKIs should be core components of treatment selection to ensure optimal outcomes. Bosutinib is associated with a unique safety profile; AEs are typically transient and manageable.23 The most common treatment‐emergent AEs associated with bosutinib are diarrhea, nausea, vomiting, and thrombocytopenia.23 Diarrhea, the most common AE, affects approximately 83% to 86% of CP2L and CP3L patients receiving bosutinib.23 Although most patients experience diarrhea, the vast majority of events are grade 1/2 in intensity, and only approximately 9% to 10% of patients experience grade 3/4 diarrhea.15, 18, 23 Diarrhea AEs typically occur early during treatment (median onset, 2 days), are transient (median duration, 2 days), and typically are resolved in almost all patients.23 We examined the impact of chronic diarrhea on HRQOL because it is the most common AE associated with bosutinib. In this study, we demonstrated that the FACT‐Leu HRQOL for patients with chronic diarrhea was not appreciably different from that seen in the total cohort of patients. In some instances, greater HRQOL improvements were observed in patients with chronic diarrhea, especially for the CP2L cohort. Therefore, even though diarrhea is a common AE with bosutinib, it appears to have minimal impact on long‐term HRQOL.
Although our study did not specifically examine HRQOL outcomes in patients experiencing any AE, the PWB component of FACT‐Leu addresses the degree to which patients are bothered by AEs in general. PWB remained stable in the CP2L and CP3L cohorts over time except at week 264 (CP3L only) and at treatment completion. In addition, 24% of the patients in the CP2L cohort and 28% of the patients in the CP3L cohort discontinued treatment because of AEs. Most patients who discontinued treatment because of AEs did so early during study treatment (CP2L, weeks 1‐24; CP3L, weeks 1‐36). Therefore, our results are most applicable to those patients who were able to tolerate bosutinib and remain on therapy. We conducted a post hoc analysis to determine whether HRQOL outcomes were different between patients who discontinued treatment because of an AE and the overall cohort of patients (ie, patients remaining on treatment plus those who discontinued treatment). This analysis demonstrated that FACT scores were generally similar between the 2 cohorts of patients and in some instances were higher for patients who discontinued treatment because of an AE. Future studies assessing HRQOL in patients experiencing AEs leading to discontinuation could provide insight into the degree to which AEs affect HRQOL outcomes. Future research assessing HRQOL outcomes after treatment cessation in individuals who discontinue because of AEs would help to further elucidate how AEs, symptoms associated with CML, or other factors affect HRQOL in these patients.
Our study has several limitations, including the small number of patients in the cohorts at later time points, the small number of patients with chronic diarrhea (particularly in the CP3L cohort), and the lack of a comparator group. In addition to the small sample sizes at later time points and the low number of assessments collected (specifically at weeks 108, 132, and 156), it is possible that patients remaining on the study treatment represented patients with a more favorable medication experience and would be expected to have better HRQOL. Assessments collected at these time points may have biased the results by overrepresenting patients with favorable HRQOL outcomes because those with poor HRQOL outcomes may have been less likely to complete assessments, as demonstrated by Hahn et al.24 Although assessment collection at these isolated time points was low, more than 50% of the assessments were collected at treatment completion. Additional assessments that specifically address disease and treatment‐related symptoms, such as the MD Anderson Symptom Inventory for CML,25 in conjunction with HRQOL instruments could also be used in future studies of bosutinib to provide greater insight into the cumulative impact of bosutinib‐related AEs on HRQOL. For additional information on quality‐of‐life assessment in patients with CML receiving TKIs, the reader is referred to a comprehensive review by Efficace and Cannella.26
Because CML patients are living longer with TKIs, they may be on treatment for long periods of time. Therefore, it is important to assess the long‐term impact of treatment on patient HRQOL. The findings of this study suggest that patients with CP Ph+ CML and resistance or intolerance to prior therapy who remained in the study largely maintained HRQOL for 264 weeks or more of treatment with bosutinib. These results place the favorable efficacy and distinct safety profile of bosutinib treatment into a context that considers patients' capacity to manage their disease over the long term.
FUNDING SUPPORT
This study was sponsored by Pfizer Inc. Editorial support was provided by Lauren D'Angelo, PhD (Complete Healthcare Communications, LLC), and was funded by Pfizer.
CONFLICT OF INTEREST DISCLOSURES
Hagop M. Kantarjian reports institutional research funding to MD Anderson from Amgen, Ariad, Astex, Bristol‐Myers Squibb, Novartis, and Pfizer and consultant fees from AbbVie, Actinium, Ariad, ImmunoGen, Orsinex, and Pfizer. Carla M. Mamolo is a shareholder and employee of Pfizer. Jorge E. Cortes reports grants from Ariad, Bristol‐Myers Squibb, Novartis, Pfizer, and Teva and personal fees for consulting from Ariad, Bristol‐Myers Squibb, Novartis, and Pfizer. Tim H. Brümmendorf reports grants and other (manuscript preparation) from Pfizer and Novartis during the conduct of the study and has a patent issued for his work on imatinib and hypusination inhibitors. Yun Su is a shareholder and employee of Pfizer. Arlene L. Reisman is a shareholder and employee of Pfizer. Mark Shapiro was a shareholder and employee of Pfizer at the time of the writing of the manuscript. Jeff H. Lipton reports other (manuscript writing) from Pfizer during the conduct of the study and research funding from Pfizer, Bristol‐Myers Squibb, Ariad, and Novartis for work outside the submitted work.
AUTHOR CONTRIBUTIONS
Hagop M. Kantarjian: Conceptualization, formal analysis, investigation, writing–original draft, and writing–review and editing. Carla M. Mamolo: Conceptualization, visualization, writing–original draft, and writing–review and editing. Carlo Gambacorti‐Passerini: Conceptualization, investigation, and writing–review and editing. Jorge E. Cortes: Conceptualization, formal analysis, investigation, visualization, supervision, writing–original draft, and writing–review and editing. Tim H. Brümmendorf: Conceptualization, resources, and writing–review and editing. Yun Su: Validation and writing–review and editing. Arlene L. Reisman: Conceptualization, methodology, validation, formal analysis, data curation, visualization, and writing–review and editing. Mark Shapiro: Conceptualization, validation, supervision, project administration, and writing–review and editing. Jeff H. Lipton: Investigation and writing–review and editing.
Supporting information
Additional supporting information may be found in the online version of this article.
Supporting Information 1
REFERENCES
- 1. O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low‐dose cytarabine for newly diagnosed chronic‐phase chronic myeloid leukemia. N Engl J Med. 2003;348:994‐1004. [DOI] [PubMed] [Google Scholar]
- 2. Druker BJ, Guilhot F, O'Brien SG, et al. Five‐year follow‐up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408‐2417. [DOI] [PubMed] [Google Scholar]
- 3. Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic‐phase chronic myeloid leukemia: 2‐year follow‐up from a randomized phase 3 trial (DASISION). Blood. 2012;119:1123‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome–positive chronic myeloid leukemia in chronic phase: ENESTnd 3‐year follow‐up. Leukemia. 2012;26:2197‐2203. [DOI] [PubMed] [Google Scholar]
- 5. Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic‐phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109:2303‐2309. [DOI] [PubMed] [Google Scholar]
- 6. Hochhaus A, Baccarani M, Deininger M, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22:1200‐1206. [DOI] [PubMed] [Google Scholar]
- 7. Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR‐ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome–positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110:3540‐3546. [DOI] [PubMed] [Google Scholar]
- 8. Kantarjian HM, Giles FJ, Bhalla KN, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24‐month follow‐up results. Blood. 2011;117:1141‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shah NP, Guilhot F, Cortes JE, et al. Long‐term outcome with dasatinib after imatinib failure in chronic‐phase chronic myeloid leukemia: follow‐up of a phase 3 study. Blood. 2014;123:2317‐2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381‐2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noens L, van Lierde MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113:5401‐5411. [DOI] [PubMed] [Google Scholar]
- 12. Phillips KM, Pinilla‐Ibarz J, Sotomayor E, et al. Quality of life outcomes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: a controlled comparison. Support Care Cancer. 2013;21:1097‐1103. [DOI] [PubMed] [Google Scholar]
- 13. Hirji I, Gupta S, Goren A, et al. Chronic myeloid leukemia (CML): association of treatment satisfaction, negative medication experience and treatment restrictions with health outcomes, from the patient's perspective. Health Qual Life Outcomes. 2013;11:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bosulif [full prescribing information]. New York, NY: Pfizer Labs; 2014. [Google Scholar]
- 15. Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI‐606) in chronic phase Philadelphia chromosome–positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118:4567‐4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lipton JH, Cortes JE, Khoury HJ, et al. Long‐term bosutinib in patients with chronic phase chronic myeloid leukemia after prior imatinib failure. Paper presented at: Annual Meeting of the American Society of Clinical Oncology; May 29 to June 2, 2015; Chicago, IL.
- 17. Gambacorti‐Passerini C, Brummendorf TH, Kim DW, et al. Bosutinib efficacy and safety in chronic phase chronic myeloid leukemia after imatinib resistance or intolerance: minimum 24‐month follow‐up. Am J Hematol. 2014;89:732‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brummendorf TH, Cortes JE, Khoury HJ, et al. Factors influencing long‐term efficacy and tolerability of bosutinib in chronic phase chronic myeloid leukaemia resistant or intolerant to imatinib. Br J Haematol. 2016;172:97‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trask PC, Cella D, Besson N, Kelly V, Masszi T, Kim DW. Health‐related quality of life of bosutinib (SKI‐606) in imatinib‐resistant or imatinib‐intolerant chronic phase chronic myeloid leukemia. Leuk Res. 2012;36:438‐442. [DOI] [PubMed] [Google Scholar]
- 20. Whiteley J, Reisman A, Shapiro M, Cortes J, Cella D. Health‐related quality of life during bosutinib (SKI‐606) therapy in patients with advanced chronic myeloid leukemia after imatinib failure. Curr Med Res Opin. 2016;32:1325‐1334. [DOI] [PubMed] [Google Scholar]
- 21. van Reenan M, Oppe M. EQ‐5D‐3L User Guide (Version 5.1). Rotterdam, the Netherlands: EuroQol Group; 2015. [Google Scholar]
- 22. Cella D, Jensen SE, Webster K, et al. Measuring health‐related quality of life in leukemia: the Functional Assessment of Cancer Therapy–Leukemia (FACT‐Leu) questionnaire. Value Health. 2012;15:1051‐1058. [DOI] [PubMed] [Google Scholar]
- 23. Kantarjian HM, Cortes JE, Kim DW, et al. Bosutinib safety and management of toxicity in leukemia patients with resistance or intolerance to imatinib and other tyrosine kinase inhibitors. Blood. 2014;123:1309‐1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hahn EA, Glendenning GA, Sorensen MV, et al. Quality of life in patients with newly diagnosed chronic phase chronic myeloid leukemia on imatinib versus interferon alfa plus low‐dose cytarabine: results from the IRIS study. J Clin Oncol. 2003;21:2138‐2146. [DOI] [PubMed] [Google Scholar]
- 25. Williams LA, Garcia Gonzalez AG, Ault P, et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood. 2013;122:641‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Efficace F, Cannella L. The value of quality of life assessment in chronic myeloid leukemia patients receiving tyrosine kinase inhibitors. Hematology Am Soc Hematol Educ Program. 2016;2016:170‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supporting Information 1


