ABSTRACT
Background and objective
Intravenous (i.v.) antibiotics are needed for rescue when preventative therapy fails to achieve stability among adults with cystic fibrosis (CF). Understanding the distribution of i.v. days can provide insight into the care that adults with CF need. We aim to determine the baseline characteristics that are associated with higher i.v. use, in particular to test the hypothesis that prior‐year i.v. use is associated with future‐year i.v. use.
Methods
This is a cross‐sectional analysis of the 2013–2014 UK CF registry data. Stepwise logistic regression was performed using current‐year i.v. days as the dependent variable, and demographic variables including prior‐year i.v. days as the covariates. Based on these results, study sample was divided into clinically meaningful subgroups using analysis similar to tree‐based method.
Results
Data were available for 4269 adults in 2013 and 4644 adults in 2014. Prior‐year i.v. use was the strongest predictor for current‐year i.v. use followed by forced expiratory volume in 1 s (FEV1). Adults with high prior‐year i.v. use (>14 days) continued to require high levels of i.v., regardless of FEV1. Those with high prior‐year i.v. use and FEV1 ≥70% had higher current‐year i.v. days compared to adults with low prior‐year i.v. use and FEV1 <40% (28 days, interquartile range (IQR): 11–41 days vs 14 days, IQR: 0–28 days; Mann–Whitney P‐value <0.001 in 2013).
Conclusion
CF people with prior high levels of rescue often continue to need high levels of rescue even if they have good FEV1. The reasons for this require further investigations.
Keywords: cystic fibrosis, data interpretation, intravenous antibiotic, registry analysis, pulmonary exacerbation
Short abstract
Intravenous (i.v.) antibiotic is an important treatment option in cystic fibrosis and is also a marker of pulmonary exacerbations. Our study showed that previous‐year i.v. use is a strong predictor of current‐year i.v. use. This finding could help clinicians to identify people most at risk of future exacerbation.
Abbreviations
- CF
cystic fibrosis
- CFRD
CF‐related diabetes
- CFTR
CF transmembrane regulator
- FEV1
forced expiratory volume in 1 s
- IQR
interquartile range
- NHS
National Health Service
- PERT
pancreatic replacement therapy
- US CFFPR
US CF Foundation Patient Registry
INTRODUCTION
Cystic fibrosis (CF) is an autosomal recessive genetic condition caused by mutations in the gene encoding the CF transmembrane regulator (CFTR) that affects around 10 000 people in the UK.1, 2 CF is a multisystem condition1 but lungs are the main organ affected, with CFTR dysfunction causing abnormal airway surface liquid.1, 3 People with CF are particularly susceptible to infection causing acute deterioration in lung health (i.e. pulmonary exacerbations), which leads to progressive lung damage and eventually respiratory failure.4
An important treatment option in CF is preventative inhaled therapies consisting of mucolytics and antibiotics, which have proven efficacy in reducing the frequency of exacerbations.5, 6 The effectiveness of these treatments is however limited by a real‐world medication adherence rates of 35–50%.7, 8 Acute treatment of pulmonary exacerbations has relatively limited research evidence.9 However, the importance of intravenous (i.v.) antibiotics in managing CF is indisputable—CF centres that use less antibiotics or have higher threshold for initiating i.v. in the face of an exacerbation were associated with people having lower lung function.10, 11 Indeed, i.v. antibiotics are recommended in all the major CF guidelines to treat exacerbations.12, 13, 14, 15
Understanding the clinical characteristics associated with i.v. antibiotics use is clinically important as it can provide insight into the care that people with CF need and the resources that are required for CF care. Exacerbation is also an important end point in CF clinical trials,16 and clinical factors associated with exacerbation could have implications for trial design.
A recent US CF Foundation Patient Registry (US CFFPR) data analysis has found that the frequency of prior‐year i.v.‐treated exacerbations is strongly associated with the frequency of future‐year i.v.‐treated exacerbations,17 even after adjustment for the other case‐mix factors, for example lung function.17, 18, 19 We analysed the 2013–2014 UK CF registry data to test whether the hypothesis that prior‐year i.v. use is associated with future‐year i.v. use, is also generalizable to the UK CF population.
METHODS
This is a cross‐sectional analysis using the UK CF registry data for 2013–2014 from 28 UK adult CF centres. National Health Service (NHS) research ethics approval (Huntingdon Research Ethics Committee 07/Q0104/2) was granted for the UK CF Registry. Under the terms of the NHS ethics approval, the UK CF Trust steering committee approved this study.
People with lung transplantation and those on ivacaftor were excluded as both treatments have transformative effects on health outcomes,20, 21 such that their exacerbation rates and forced expiratory volume in 1 s (FEV1) no longer represent that of a typical adult with CF.
Data
The following data were obtained
Demographics: age, gender, CF centre identifier;
Pancreatic status: people on pancreatic replacement therapy (PERT) were considered ‘pancreatic insufficient’ while those not on PERT were considered ‘pancreatic sufficient’;
CF‐related diabetes: present (as defined by the UK CF Trust guideline),22 or not present;
Pseudomonas aeruginosa status: no P. aeruginosa (negative cultures over a year), intermittent (positive cultures not fulfilling definition of chronic) or chronic (≥2 samples positive in one year);23
Body mass index, BMI, in kg/m2;
FEV1 during annual review (in % predicted, calculated with Knudson equation);24
Annual total i.v. antibiotic days (in number of days).
Data were collected from people aged ≥16 years during annual reviews from January 2013 to December 2014. Since prior‐year i.v. use was a covariate in the analysis, i.v. use data for 2012 were also obtained.
Number of days on i.v. antibiotic, instead of number of i.v. courses, was chosen for analysis since it captures information on the cumulative i.v. antibiotic exposure to treat pulmonary exacerbations over a 1‐year period (not all i.v. courses are of the same duration).25
Statistical analysis
Analyses were performed using SPSS v22 (IBM Corp, Armonk, NY, USA). Data for 2013 and 2014 were analysed separately to determine the consistency of any observations. The analysis consisted of two stages. First, logistic regression was performed to identify the order of the strength of association between the demographic and clinical variables with the current ‐ year i.v. use. Based on these results, the study sample was divided into clinically meaningful subgroups using analysis similar to tree‐based method26 for comparison of current‐year i.v. use between the subgroups.
Logistic regression was performed using current‐year i.v. days (≤14 days vs >14 days) as the dependent variable. 14‐Day was selected as the cut‐off since mean and median duration of each i.v. course in 2013 and 2014 was 14 days. The duration of a ‘standard’ i.v. course to treat an exacerbation is usually 14 days,12, 15 and people with ≥2 exacerbations per year (i.e. time between exacerbation <6 months17) have the most rapid FEV1 decline.27 Age, gender, CF centre (as a categorical variable), pancreatic status, CF‐related diabetes, intermittent P. aeruginosa (yes/no), chronic P. aeruginosa (yes/no), BMI, %FEV1 and prior‐year i.v. days (as a continuous variable) were the covariates. For this procedure, forward stepwise conditional analysis (probability for entry 0.05; probability for removal 0.10) was performed in a binary logistic regression model. The procedure began by identifying the covariate that was most strongly associated with current‐year i.v. use. The next strongest associated covariate was then selected after controlling for the first covariate. This continued until no further statistically significant covariates can be added to the model. Stepwise regression allows a relatively parsimonious model to be built even when several correlated covariates are present,28, 29 but it is essentially an exploratory analysis because it can lead to inflated Type I errors.30, 31
Therefore, the results from the stepwise logistic regression were subjected to a further analysis similar to tree‐based method to test the hypothesis that prior‐year i.v. use is associated with future‐year i.v. use. Tree‐based method is an efficient approach to understand the impact of risk factors for a condition where many potential confounders exist.24 For this analysis, the covariate most strongly associated with current‐year i.v. use as identified by stepwise logistic regression was used for the first ‘layer’ division of the study sample. Then, a further ‘layer’ of division for each generated subgroup was carried out using the next strongest associated covariate, and so on. For continuous covariates, clinical meaningful cut‐off points were used to divide the cohort instead of data‐driven optimal cut‐off points to avoid overfitting.32 For %FEV1, internationally accepted categories (<40%, 40–69.9% and ≥70%) were used as these categories are also applicable to the UK CF registry data.33 Prior‐year i.v. days were categorized into ≤14 days versus >14 days, similar to current‐year i.v. days. The current‐year i.v. days, as a continuous variable, were compared between subgroups at each ‘layer’ of the division using Mann–Whitney test or Jonckheere–Terpstra test depending on the number of subgroups, due to the non‐normal distribution of i.v. days. Jonckheere–Terpstra test is a generalization of the Mann–Whitney test for more than two ordered groups.
The analyses included those without any pulmonary exacerbations needing i.v. antibiotics (n = 1847, 43.3% of the sample for 2013; n = 2037, 43.9% of the sample for 2014) to obtain a more representative understanding of the adult CF population in the UK. P‐value <0.05 was considered to be statistically significant. Complete case approach was used as the extent of missing data (summarized in Fig. 1) was small. There were >1500 people with >14 days i.v. days for each year, which is more than adequate power for nine covariates in a binary logistic regression model.34 The study sample was also considerably larger than previous studies looking at factors associated with exacerbations18, 19 (except the recent US CFFPR analysis), which should allow adequate power for hypothesis testing between the different subgroups.
Figure 1.
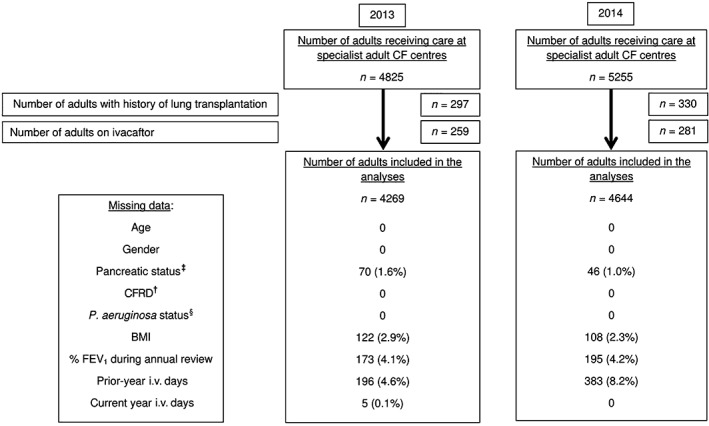
Summary of the number of adults included in the analyses and details regarding missing data. †Cystic fibrosis (CF)‐related diabetes (CFRD) data are collected by the UK CF registry with only a check‐box for ‘CFRD present’. Therefore, if data are not available, it is assumed the person has no CFRD. It is therefore difficult to distinguish missing data from ‘no CFRD’ and hence missing data = 0. ‡Data for pancreatic replacement therapy (PERT) use were obtained. People on PERT were considered ‘pancreatic insufficient’. People not on PERT were considered ‘pancreatic sufficient’. PERT use documented as ‘unknown’ is considered as missing data. § P. aeruginosa status is collected by the UK CF registry with check‐boxes for ‘chronic’ and ‘intermittent’. Therefore, if data are not available, it is assumed the person has no P. aeruginosa. It is therefore difficult to distinguish missing data from ‘no P. aeruginosa’ and hence missing data = 0.
RESULTS
A total of 4269 adults were included in the analysis for 2013 and 4644 for 2014, with 4874 study subjects in total across both years. In total, 103 453 i.v. days were used in 2013 and 111 981 i.v. days were used in 2014. Figure 1 summarizes the numbers of adults excluded and missing data. Baseline demographics stratified according to previous i.v. days was summarized in Table 1. Those with previous ‐ year i.v. use >14 days had lower FEV1 and higher current‐year i.v. use for both 2013 and 2014.
Table 1.
Current year demographic and clinical characteristics stratified by prior‐year i.v. days
| Demographics and clinical characteristics | 2013 | 2014 | ||||
|---|---|---|---|---|---|---|
| Prior‐year i.v. days ≤ 14† n = 2433 | Prior‐year i.v. days > 14† n = 1640 | Overall n = 4269 | Prior‐year i.v. days ≤ 14‡ n = 2586 | Prior‐year i.v. days > 14‡ n = 1675 | Overall n = 4644 | |
| Age (years) median (IQR) | 28 (22–37) | 27 (22–33) | 28 (22–35) | 28 (22–37) | 27 (23–34) | 28 (22–36) |
| Female (%) | 995 (40.9) | 849 (51.8) | 1936 (45.4) | 1066 (41.2) | 877 (52.4) | 2096 (45.1) |
| Pancreatic insufficient (%) | 1858 (77.5) | 1487 (92.0) | 3449 (82.1) | 1967 (77.0) | 1526 (91.8) | 3756 (81.7) |
| CF‐related diabetes (%) | 568 (23.3) | 760 (46.3) | 1356 (31.8) | 620 (24.0) | 810 (48.4) | 1525 (32.8) |
| P. aeruginosa status | ||||||
| Chronic P. aeruginosa (%) | 1068 (43.9) | 1154 (70.4) | 2276 (53.3) | 1033 (39.9) | 1167 (69.7) | 2340 (50.4) |
| Intermittent P. aeruginosa (%) | 350 (14.4) | 201 (12.3) | 576 (13.5) | 422 (16.3) | 183 (10.9) | 689 (14.8) |
| BMI in kg/m2, mean (SD) | 23.2 (3.9) | 21.5 (3.6) | 22.6 (3.9) | 23.3 (3.9) | 21.5 (3.5) | 22.6 (3.9) |
| % predicted FEV1, mean (SD) | 73.0 (23.4) | 51.8 (21.1) | 65.0 (24.8) | 73.4 (23.6) | 52.7 (21.4) | 65.8 (25.0) |
| Current‐year i.v. days, median (IQR) | 0 (0–14) | 42 (16–67) | 14 (0–35) | 0 (0–14) | 40 (15–66) | 14 (0–34) |
Graphs displaying the relationships between continuous covariates (age, BMI, %FEV1 and prior‐year i.v. days) with current‐year i.v. days are available in Appendix S1 (Supplementary Information). Contingency tables for all covariates are available in Appendix S2 (Supplementary Information).
For both 2013 and 2014, prior‐year i.v. use was the strongest predictor for current‐year i.v. use, followed by FEV1. Other covariates such as CF centre and pancreatic status were also associated with i.v. use, but the relationship was weaker and less consistent (Wald statistic <100) once prior‐year i.v. use and FEV1 had been taken into account (final model summarized in Table 2). Prior‐year i.v. use and FEV1 remained the strongest predictors for current‐year i.v. use in various sensitivity analyses (Appendix S3, Supplementary Information).
Table 2.
Summary of the output from the final binary logistic regression model which include all nine covariates listed
| 2013 (3843 study subjects included in the analysis) | 2014 (4040 study subjects included in the analysis) | ||||||
|---|---|---|---|---|---|---|---|
| Covariates | Wald statistic | P‐value | Adjusted odds ratio (95% CI) | Covariates | Wald statistic | P‐value | Adjusted odds ratio (95% CI) |
| Prior‐year i.v. days | 449.5 | <0.001 | 1.06 (1.05–1.06) | Prior‐year i.v. days | 458.1 | <0.001 | 1.06 (1.05–1.06) |
| % Predicted FEV1 | 140.1 | <0.001 | 0.97 (0.97–0.98) | % Predicted FEV1 | 172.3 | <0.001 | 0.97 (0.97–0.98) |
| CF centre | 90.3 | <0.001 | Pancreatic insufficient | 32.5 | <0.001 | 2.17 (1.66–2.83) | |
| Pancreatic insufficient | 9.6 | <0.001 | 1.59 (1.19–2.13) | Female | 32.4 | <0.001 | 1.64 (1.38–1.95) |
| Chronic P. aeruginosa | 21.9 | <0.001 | 1.57 (1.30–1.89) | Chronic P. aeruginosa | 30.9 | <0.001 | 1.78 (1.45–2.18) |
| Female | 15.9 | <0.001 | 1.44 (1.20–1.71) | CF centre | 67.6 | <0.001 | |
| Age (years) | 17.6 | <0.001 | 0.98 (0.97–0.99) | Intermittent P. aeruginosa | 4.2 | 0.041 | 1.33 (1.01–1.75) |
| CF‐related diabetes | 13.6 | <0.001 | 1.44 (1.19–1.75) | ||||
CF, cystic fibrosis; FEV1, forced expiratory volume in 1 s.
Therefore, the cohort was divided into two groups based on prior‐year i.v. use (≤14 days and >14 days) and then each group is further divided into three subgroups based on %FEV1 (<40%, 40–69.9% and ≥70%) to generate six subgroups in total. The results of this analysis are summarized in Figures 2 and 3.
Figure 2.
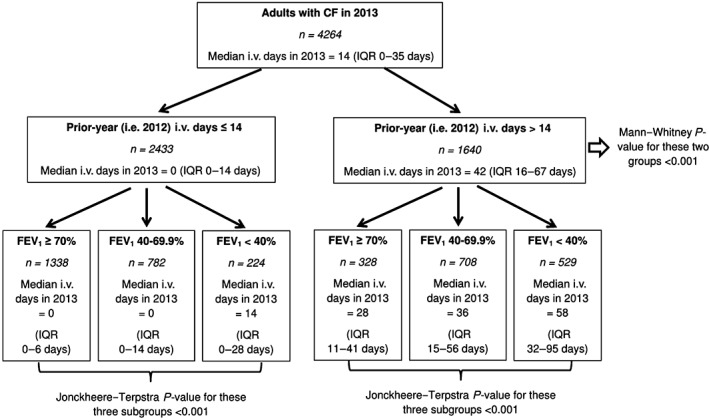
Tree‐based diagram for 2013 to summarize current‐year i.v. days according to the different clinical subgroups. CF, cystic fibrosis; FEV1, forced expiratory volume in 1 s; IQR, interquartile range.
Figure 3.
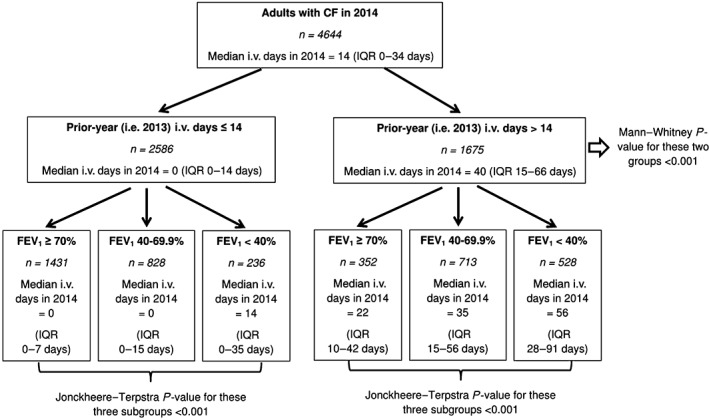
Tree‐based diagram for 2014 to summarize current‐year i.v. days according to the different clinical subgroups. CF, cystic fibrosis; FEV1, forced expiratory volume in 1 s; IQR, interquartile range.
Current‐year i.v. use was clearly different between all the subgroups and the results were consistent for both 2013 and 2014. Adults with high prior‐year i.v. use (i.e. >14 days) continued to require high levels of i.v., even if they have good FEV1. Indeed, adults with high prior‐year i.v. use and FEV1 ≥ 70% had higher current‐year i.v. days compared to adults with low prior‐year i.v. use and FEV1 < 40% for both 2013 (28 days, interquartile range (IQR): 11–41 days vs 14 days, IQR: 0–28 days; P < 0.001) and 2014 (22 days, IQR: 10–42 days vs 14 days, IQR: 0–35 days; P = 0.003).
The clinical subgroups could be generated using different cut‐off points for prior‐year i.v. use but the results remained similar (see Appendix S4, Supplementary Information, for these sensitivity analyses).
DISCUSSION
This study found that adults with high prior‐year i.v. use (>14 days) and low FEV1 (<40%) required the most i.v. among the six different clinical subgroups. In 2013, this group consisted 13.4% of the population but consumed 38.7% of i.v. days. Prior‐year i.v. use was a much stronger predictor of current‐year i.v. use compared to FEV1, such that those with high prior‐year i.v. use and high FEV1 (≥70%) still used more i.v. therapies compared to those with low prior‐year i.v. use (≤14 days) and low FEV1. The 2013 results were consistent with 2014 results, suggesting that chance is extremely unlikely to explain these results.
The finding that prior‐year i.v. use was the strongest independent predictor of current‐year i.v. use is consistent with the recent US CFFPR analysis.17 Whilst we acknowledge the limitations of retrospective observational registry‐based analyses that have been previously discussed,17, 35 the consistency and magnitude of the finding would suggest a genuine signal. The implications of this finding for clinical trials had also been discussed in detail—stratification of subject randomization by prior‐year i.v. use would be helpful to balance the baseline risk of pulmonary exacerbation. In fact, our randomized control trial to evaluate a complex self‐care intervention used this strategy for randomization.36
This finding also has other important clinical implications and could suggest possible differences in management strategies. Management of CF can be broadly dichotomized into ‘rescue’ therapy with i.v. antibiotics to treat pulmonary exacerbations and ‘prevention’ with inhaled therapies to minimize the risk of exacerbations.37 Whilst exacerbations may be stochastic events, the frequency and severity of exacerbations over a given period in a person with CF will be influenced by their clinical characteristics, environmental exposures and medical treatments.38 Given the efficacy of preventative inhaled therapies in reducing the frequency of exacerbations,5, 6 a possible explanation for high i.v. use would be the reliance on rescue therapies to compensate for insufficient utilization of preventative therapies.
Prior‐year i.v. use could also guide clinical strategy in managing CF. Insufficient utilization of preventative therapy could be due to low adherence, as median adherence with inhaled therapies in the real world is only 35–50%.7, 8, 39 Therefore, it is important to obtain objective adherence data wherever possible when assessing an adult with CF, especially if i.v. use is disproportionately high in relation to FEV1.
If objective nebulizer adherence is satisfactory, another cause for insufficient utilization of preventative therapy to consider is ‘therapeutic inertia’. Therapeutic inertia refers to the under‐prescription of efficacious treatments.40 In CF, the prescription of inhaled therapies have increased since mid 1990s.41, 42, 43 Adherence rates to international prescribing guidelines now exceed 90% for some specialist CF centres.44 If i.v. requirement is still disproportionately high but objective adherence is high and no obvious complications are found, escalation of preventative therapies should be considered. An example would be the initiation of inhaled antibiotic treatment for someone who is only on inhaled mucolytics. The detection of P. aeruginosa infection would typically prompt the prescription of inhaled antibiotics. However, there may be merit in using long‐term inhaled antibiotics, even when P. aeruginosa is not detected, if there is a history of frequent exacerbations.45 This is a strategy that has some evidence of success in non‐CF bronchiectasis.46
Our finding highlights potential limitations of FEV1 as a marker of lung health. Low FEV1 is a known poor prognostic marker in CF and is strongly associated with mortality.47, 48 FEV1 trend is therefore a commonly used primary end point in CF clinical trials and an important parameter measured during annual reviews as a marker of lung health for adults with CF.16, 49 However, as the health of people with CF improved over time, the sensitivity of FEV1 as a marker of lung health decreases.50 This study found a group of people with high i.v. use year on year, yet who maintained relatively good lung function (FEV1 > 70%). It may be possible to initially fend off FEV1 decline despite frequent exacerbations by simply relying on prompt and aggressive rescue i.v. treatments. Such strategy is likely to result in wild fluctuations of FEV1, with high FEV1 post i.v. treatment not sustained due to lack of preventative treatments. A trend of declining FEV1 tends to be predated by increased FEV1 variability51; hence, FEV1 variability is a more sensitive marker of lung health. There is increasing evidence regarding the prognostic impact of FEV1 variability,51, 52 but we were not able to study the correlation between FEV1 variability and i.v. use as the UK CF registry does not routinely collect encounter‐based FEV1 data.
In conclusion, exacerbations as indicated by i.v. use are clinically important events in CF with long‐term impact on morbidity and mortality. Prevention of exacerbations is a priority due to the risk of FEV1 decline and lung damage even with intensive i.v. antibiotics, hence the importance of understanding the predictors of frequent exacerbations. This UK CF registry analysis used i.v. days as a marker of the frequency and severity of exacerbations, and found that current‐year i.v. use is most strongly associated with prior‐year i.v. use. A recent US CFFPR data analysis also found similar results.17 That means those with history of frequent exacerbations continue to remain most at risk for future exacerbations, even with good FEV1. Inadequate utilization of preventative inhaled therapies may be one of the reasons for this reliance on rescue therapies. Therefore, high i.v. use should prompt clinicians to assess both the adherence rate and the prescribed inhaled therapies regime.
Disclosure statement
Part of the analysis in this paper has been presented as an oral presentation in the 2016 European Cystic Fibrosis Society Conference. The abstract is published in the Journal of Cystic Fibrosis 2016, Vol 15, Suppl 1, entitled Rescue therapy within the UK CF registry: an exploration of the predictors of i.v. antibiotic use amongst adults with CF.
Supporting information
Appendix S1 The relationships between age, BMI, % forced expiratory volume in 1 s and prior‐year i.v. days with current‐year i.v. days for 2013 and 2014.
Appendix S2 A contingency table showing the distribution of covariates according to current‐year i.v. days.
Appendix S3 Sensitivity analyses using number of i.v. courses (instead of i.v. days) as the dependent variable in a logistic regression model and using ordinal regression models to explore different cut‐off points for i.v. courses and i.v. days.
Appendix S4 Sensitivity analyses using number of i.v. courses (instead of i.v. days) and different cut‐off points to generate the clinical subgroups.
Acknowledgements
This report is independent research arising from a Doctoral Research Fellowship, Z.H.H., DRF‐2014‐07‐092) supported by the National Institute for Health Research. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. We would like to thank the UK CF Trust for supplying the data that made this analysis possible. We would also like to acknowledge all the people with CF in the UK who consent to be part of the UK CF registry.
Hoo, Z.H. , Wildman, M.J. , Curley, R. , Walters, S.J. and Campbell, M.J. (2017) Rescue therapy within the UK Cystic Fibrosis Registry: An exploration of predictors of intravenous antibiotic use amongst adults with CF. Respirology, 23: 190–197., doi: 10.1111/resp.13174.
(Associate Editor: Claire Wainwright)
REFERENCES
- 1. Elborn JS. Cystic fibrosis. Lancet 2016; 388: 2519–31. [DOI] [PubMed] [Google Scholar]
- 2. The UK CF Registry Steering Committee . UK Cystic Fibrosis Registry 2015 Annual Data Report, 2016. [Accessed 31 Jan 2017.] Available from URL:https://www.cysticfibrosis.org.uk/the-work-we-do/uk-cf-registry/reporting-and-resources
- 3. Haq IJ, Gray MA, Garnett JP, Ward C, Brodlie M. Airway surface liquid homeostasis in cystic fibrosis: pathophysiology and therapeutic targets. Thorax 2016; 71: 284–7. [DOI] [PubMed] [Google Scholar]
- 4. Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: epidemiology and pathogenesis. Thorax 2007; 62: 360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ryan G, Singh M, Dwan K. Inhaled antibiotics for long‐term therapy in cystic fibrosis. Cochrane Database Syst. Rev. 2011; 3: CD001021. [DOI] [PubMed] [Google Scholar]
- 6. Yang C, Chilvers M, Montgomery M, Nolan SJ. Dornase alfa for cystic fibrosis. Cochrane Database Syst. Rev. 2016; 4: CD001127. [DOI] [PubMed] [Google Scholar]
- 7. Daniels T, Goodacre L, Sutton C, Pollard K, Conway S, Peckham D. Accurate assessment of adherence: self‐report and clinician report vs electronic monitoring of nebulizers. Chest 2011; 140: 425–32. [DOI] [PubMed] [Google Scholar]
- 8. Quittner AL, Zhang J, Marynchenko M, Chopra PA, Signorovitch J, Yushkina Y, Riekert KA. Pulmonary medication adherence and health‐care use in cystic fibrosis. Chest 2014; 146: 142–51. [DOI] [PubMed] [Google Scholar]
- 9. Hurley MN, Prayle AP, Flume P. Intravenous antibiotics for pulmonary exacerbations in people with cystic fibrosis. Cochrane Database Syst. Rev. 2015; 7: CD009730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson C, Butler SM, Konstan MW, Morgan W, Wohl ME. Factors influencing outcomes in cystic fibrosis: a center‐based analysis. Chest 2003; 123: 20–7. [DOI] [PubMed] [Google Scholar]
- 11. Schechter MS, Regelmann WE, Sawicki GS, Rasouliyan L, VanDevanter DR, Rosenfeld M, Pasta D, Morgan W, Konstan MW. Antibiotic treatment of signs and symptoms of pulmonary exacerbations: a comparison by care site. Pediatr. Pulmonol. 2015; 50: 431–40. [DOI] [PubMed] [Google Scholar]
- 12. UK Cystic Fibrosis Trust Antibiotic Working Group . Antibiotic treatment for cystic fibrosis. 2009. [Accessed 31 Jan 2017.] Available from URL: https://www.cysticfibrosis.org.uk/the-work-we-do/clinical-care/consensus-documents
- 13. Doring G, Flume P, Heijerman H, Elborn JS; Consensus Study Group . Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J. Cyst. Fibros. 2012; 11: 461–79. [DOI] [PubMed] [Google Scholar]
- 14. Flume PA, Mogayzel PJ Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, Marshall BC; Clinical Practice Guidelines for Pulmonary Therapies Committee . Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am. J. Respir. Crit. Care Med. 2009; 180: 802–8. [DOI] [PubMed] [Google Scholar]
- 15. Kerem E, Conway S, Elborn S, Heijerman H; Consensus Committee . Standards of care for patients with cystic fibrosis: a European consensus. J. Cyst. Fibros. 2005; 4: 7–26. [DOI] [PubMed] [Google Scholar]
- 16. VanDevanter DR, Konstan MW. Outcome measures for clinical trials assessing treatment of cystic fibrosis lung disease. Clin. Investig. (Lond.) 2012; 2: 163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. VanDevanter DR, Morris NJ, Konstan MW. i.v.‐treated pulmonary exacerbations in the prior year: an important independent risk factor for future pulmonary exacerbation in cystic fibrosis. J. Cyst. Fibros. 2016; 15: 372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Block JK, Vandemheen KL, Tullis E, Fergusson D, Doucette S, Haase D, Berthiaume Y, Brown N, Wilcox P, Bye P et al. Predictors of pulmonary exacerbations in patients with cystic fibrosis infected with multi‐resistant bacteria. Thorax 2006; 61: 969–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jarad NA, Giles K. Risk factors for increased need for intravenous antibiotics for pulmonary exacerbations in adult patients with cystic fibrosis. Chron. Respir. Dis. 2008; 5: 29–33. [DOI] [PubMed] [Google Scholar]
- 20. Pego‐Fernandes PM, Abrao FC, Fernandes FL, Caramori ML, Samano MN, Jatene FB. Spirometric assessment of lung transplant patients: one year follow‐up. Clinics (Sao Paulo) 2009; 64: 519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW et al; VX08‐770‐102 Study Group . A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011; 365: 1663–72. [DOI] [PMC free article] [PubMed]
- 22. The UK Cystic Fibrosis Trust Diabetes Working Group . Management of cystic fibrosis related diabetes mellitus, 2004. [Accessed 30 May 2017.] Available from URL: https://www.cysticfibrosis.org.uk/the-work-we-do/clinical-care/consensus-documents
- 23. Goss CH, MacNeill SJ, Quinton HB, Marshall BC, Elbert A, Knapp EA, Petren K, Gunn E, Osmond J, Bilton D. Children and young adults with CF in the USA have better lung function compared with the UK. Thorax 2015; 70: 229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow‐volume curve with growth and aging. Am. Rev. Respir. Dis. 1983; 127: 725–34. [DOI] [PubMed] [Google Scholar]
- 25. Sequeiros IM, Jarad NA. Extending the course of intravenous antibiotics in adult patients with cystic fibrosis with acute pulmonary exacerbations. Chron. Respir. Dis. 2012; 9: 213–20. [DOI] [PubMed] [Google Scholar]
- 26. Zhang H, Bracken MB. Tree‐based, two‐stage risk factor analysis for spontaneous abortion. Am. J. Epidemiol. 1996; 144: 989–96. [DOI] [PubMed] [Google Scholar]
- 27. Waters V, Stanojevic S, Atenafu EG, Lu A, Yau Y, Tullis E, Ratjen F. Effect of pulmonary exacerbations on long‐term lung function decline in cystic fibrosis. Eur. Respir. J. 2012; 40: 61–6. [DOI] [PubMed] [Google Scholar]
- 28. Uh HW, Mertens BJ, Jan van der Wijk H, Putter H, van Houwelingen HC, Houwing‐Duistermaat JJ. Model selection based on logistic regression in a highly correlated candidate gene region. BMC Proc. 2007; 1: S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Z. Variable selection with stepwise and best subset approaches. Ann. Transl. Med. 2016; 4: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Braun MT, Oswald FL. Exploratory regression analysis: a tool for selecting models and determining predictor importance. Behav. Res. Methods 2011; 43: 331–9. [DOI] [PubMed] [Google Scholar]
- 31. Mundry R, Nunn CL. Stepwise model fitting and statistical inference: turning noise into signal pollution. Am. Nat. 2009; 173: 119–23. [DOI] [PubMed] [Google Scholar]
- 32. Leeflang MM, Moons KG, Reitsma JB, Zwinderman AH. Bias in sensitivity and specificity caused by data‐driven selection of optimal cutoff values: mechanisms, magnitude, and solutions. Clin. Chem. 2008; 54: 729–37. [DOI] [PubMed] [Google Scholar]
- 33. Hoo ZH, Daniels T, Wildman MJ, Teare MD, Bradley JM. Airway clearance techniques used by people with cystic fibrosis in the UK. Physiotherapy 2015; 101: 340–8. [DOI] [PubMed] [Google Scholar]
- 34. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996; 49: 1373–9. [DOI] [PubMed] [Google Scholar]
- 35. Urschel S. Apples, oranges, and statistical magic: limitations of registry studies and need for collaborative studies. J. Heart Lung Transplant. 2015; 34: 1136–8. [DOI] [PubMed] [Google Scholar]
- 36. Wildman MJ. Development and evaluation of an intervention to support Adherence to treatment in adults with Cystic Fibrosis (ACtiF), 2016. [Accessed 31 Jan 2017.] Available from URL: https://www.sheffield.ac.uk/scharr/sections/hsr/mcru/actif.
- 37. Wildman MJ, Hoo ZH. Moving cystic fibrosis care from rescue to prevention by embedding adherence measurement in routine care. Paediatr. Respir. Rev. 2014; 15(Suppl. 1): 16–8. [DOI] [PubMed] [Google Scholar]
- 38. Wolfenden LL, Schechter MS. Genetic and non‐genetic determinants of outcomes in cystic fibrosis. Paediatr. Respir. Rev. 2009; 10: 32–6. [DOI] [PubMed] [Google Scholar]
- 39. Hoo ZH, Curley R, Walters SJ, Campbell MJ, Wildman MJ. Improving nebuliser adherence in an adult CF centre – differences in unadjusted adherence vs normative adherence [abstract]. Pediatr. Pulmonol. 2016; 51: S449. [Abstract ID – 667]. [Google Scholar]
- 40. Allen JD, Curtiss FR, Fairman KA. Nonadherence, clinical inertia, or therapeutic inertia? J. Manag. Care Pharm. 2009; 15: 690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dasenbrook EC, Konstan MW, VanDevanter DR. Association between the introduction of a new cystic fibrosis inhaled antibiotic class and change in prevalence of patients receiving multiple inhaled antibiotic classes. J. Cyst. Fibros. 2015; 14: 370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Konstan MW, VanDevanter DR, Rasouliyan L, Pasta DJ, Yegin A, Morgan WJ, Wagener JS; Scientific Advisory Group ; Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis . Trends in the use of routine therapies in cystic fibrosis: 1995‐2005. Pediatr. Pulmonol. 2010; 45: 1167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Quinton HB, O'Connor GT. Current issues in quality improvement in cystic fibrosis. Clin. Chest Med. 2007; 28: 459–72. [DOI] [PubMed] [Google Scholar]
- 44. Moore BM, Laguna TA, Liu M, McNamara JJ. Increased adherence to CFF practice guidelines for pulmonary medications correlates with improved FEV1. Pediatr. Pulmonol. 2013; 48: 747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoo ZH, Curley R, Campbell MJ, Walters SJ, Hind D, Wildman MJ. Accurate reporting of adherence to inhaled therapies in adults with cystic fibrosis: methods to calculate “normative adherence”. Patient. Prefer. Adherence 2016; 10: 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nadig TR, Flume PA. Aerosolized antibiotics for patients with bronchiectasis. Am. J. Respir. Crit. Care Med. 2016; 193: 808–10. [DOI] [PubMed] [Google Scholar]
- 47. Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. N. Engl. J. Med. 1992; 326: 1187–91. [DOI] [PubMed] [Google Scholar]
- 48. Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5‐year survivorship model of cystic fibrosis. Am. J. Epidemiol. 2001; 153: 345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Long JM, Fauset‐Jones J, Dixon MJ, Worthington‐Riley D, Sharma V, Patel L, David TJ. Annual review hospital visits for patients with cystic fibrosis. J. R. Soc. Med. 2001; 94(Suppl. 40): 12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stanojevic S, Ratjen F. Physiologic endpoints for clinical studies for cystic fibrosis. J. Cyst. Fibros. 2016; 15: 416–23. [DOI] [PubMed] [Google Scholar]
- 51. Morgan WJ, VanDevanter DR, Pasta DJ, Foreman AJ, Wagener JS, Konstan MW; Scientific Advisory Group ; Investigators and Coordinators of Epidemiologic Study of Cystic Fibrosis . Forced expiratory volume in 1 second variability helps identify patients with cystic fibrosis at risk of greater loss of lung function. J. Pediatr. 2016; 169: 116–21. [DOI] [PubMed] [Google Scholar]
- 52. Adler FR, Liou TG. The dynamics of disease progression in cystic fibrosis. PLoS One 2016; 11: e0156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 The relationships between age, BMI, % forced expiratory volume in 1 s and prior‐year i.v. days with current‐year i.v. days for 2013 and 2014.
Appendix S2 A contingency table showing the distribution of covariates according to current‐year i.v. days.
Appendix S3 Sensitivity analyses using number of i.v. courses (instead of i.v. days) as the dependent variable in a logistic regression model and using ordinal regression models to explore different cut‐off points for i.v. courses and i.v. days.
Appendix S4 Sensitivity analyses using number of i.v. courses (instead of i.v. days) and different cut‐off points to generate the clinical subgroups.


