Summary
Previously in the SCALE Obesity and Prediabetes trial, at 1 year, participants with obesity (or overweight with comorbidities) and prediabetes receiving liraglutide 3.0 mg experienced greater improvements in health‐related quality of life (HRQoL) than those receiving placebo. The current study extends these findings by examining 3‐year changes in HRQoL. HRQoL was assessed using the obesity‐specific Impact of Weight on Quality of Life‐Lite (IWQOL‐Lite) questionnaire, as well as the Short‐Form 36 v2 (SF‐36) health survey. At 3 years, mean change (±standard deviation) in IWQOL‐Lite total score from baseline for liraglutide (n = 1472) was 11.0 ± 14.2, vs. 8.1 ± 14.7 for placebo (n = 738) (estimated treatment difference [ETD] 3.4 [95% confidence interval (CI): 2.0, 4.7], P < 0.0001). Mean change in SF‐36 physical component summary (PCS) score from baseline for liraglutide was 3.1 ± 7.3, vs. 2.6 ± 7.6 for placebo (ETD 0.87 [95% CI: 0.17, 1.6], P = 0.0156). Mean change in SF‐36 mental component summary score did not significantly differ between groups. Both IWQOL‐Lite total score and PCS score demonstrated an association between greater HRQoL improvement with higher weight loss. Liraglutide 3.0 mg was also associated with improved health utility (Short‐Form‐6D and EuroQol‐5D, mapped from IWQOL‐Lite and/or SF‐36) vs. placebo. Liraglutide 3.0 mg, plus diet and exercise, is associated with long‐term improvements in HRQoL with obesity or overweight with comorbidity vs. placebo.
Keywords: IWQOL‐Lite, liraglutide 3.0 mg, SF‐36 v2, weight loss
What is already known about this subject
Weight loss can improve health‐related quality of life (HRQoL) in persons with obesity, but results vary considerably across studies and few studies assess long‐term follow‐up.
Liraglutide 3.0 mg is a glucagon‐like peptide‐1 receptor agonist approved for weight management in people with obesity or overweight with weight‐related complications.
A 1‐year analysis of the SCALE Obesity and Prediabetes trial showed that more participants on liraglutide 3.0 mg experienced meaningful improvements in HRQoL scores than those receiving placebo.
What this study adds
Durability of changes in HRQoL is of importance in chronic diseases, whereby improvements in HRQoL evident in the short term may not be maintained over longer periods.
This 3‐year evaluation of the SCALE trial shows that improvements in HRQoL at Year 1 were generally maintained at Year 3, with scores favouring liraglutide 3.0 mg over placebo.
Consistent with the 1‐year analysis, the greatest sustained improvements were observed in the physical aspects of HRQoL, although there were some improvements in certain aspects of psychosocial functioning.
Introduction
In addition to the health risks of obesity 1, numerous studies have shown that obesity is associated with reduced health‐related quality of life (HRQoL) 2, affecting physical, psychological and social functioning and well‐being 3. There is growing support for the importance of measuring patient impacts on feeling and function in clinical trials 4, 5. On a patient level, improved functioning and wellbeing for persons with obesity may correspond to greater ease in their daily lives, such as experiencing increased energy, improved self‐esteem, and/or greater mobility. Although weight loss in persons with obesity may improve HRQoL, statistically significant improvements in HRQoL in weight loss trials are not consistently observed 6, 7.
Liraglutide, a glucagon‐like peptide‐1 (GLP‐1) receptor agonist, is approved for weight management in people with obesity (body mass index [BMI] ≥30 kg m−2) or overweight (BMI ≥27 kg m−2) with weight‐related complications 8, 9. The effects of liraglutide 3.0 mg on weight loss and HRQoL at 1 year, in conjunction with a diet and exercise intervention, have been established previously 10, 11. Participants receiving liraglutide 3.0 mg experienced significantly greater improvements vs. placebo on both an obesity‐specific measure of HRQoL (Impact of Weight on Quality of Life‐Lite [IWQOL‐Lite]) 12, 13 and a general health measure (Short‐Form 36 v2 [SF‐36]) 14 across all subscales measured 11. Moreover, more individuals taking liraglutide 3.0 mg experienced clinically meaningful improvements on both these measures (i.e. total IWQOL‐Lite score and Physical Component Summary (PCS) of the SF‐36) compared with those on placebo.
To determine the durability of HRQoL improvements, we present 3‐year HRQoL data from the SCALE Obesity and Prediabetes trial.
Materials and methods
The design and primary outcomes of the SCALE Obesity and Prediabetes trial have been reported previously 10, 15. Briefly, the study included participants with prediabetes 16 and either a BMI of ≥30 kg m−2 or a BMI of ≥27 kg m−2 with the weight‐related conditions of hypertension or dyslipidaemia. All participants were advised to consume a 500 kcal d−1 deficit diet and engage in physical activity for a minimum of 150 min week−1. Enrolled participants were randomized to once‐daily subcutaneous liraglutide 3.0 mg (n = 1505) or placebo (n = 749) for 160 weeks 10.
Health‐related quality of life measures
HRQoL was assessed using both the IWQOL‐Lite and the SF‐36 v2 health survey. The 31‐item IWQOL‐Lite assesses an individual's perception of the impact of weight on quality of life in five domains (physical function, self‐esteem, sexual life, public distress and work), as well as a total score 12, 13. The IWQOL‐Lite is an obesity‐specific measure and as such is sensitive to both the degree of obesity and changes in weight 17, 18. Scores range from 0 to 100, with lower scores indicating greater impairment. The 36‐item SF‐36v2 questionnaire assesses an individual's general health status across eight subscales (physical functioning, role‐physical, bodily pain, general health, vitality, social functioning, role‐emotional and mental health). Two summary measures – the PCS and the mental component summary (MCS) – are calculated from the eight scales using different weightings 14. Lower scores represent impaired health status, with a score of 50 being the mean for the US general population.
HRQoL was assessed in countries where linguistically verified translations of the IWQOL‐Lite and SF‐36 were available. This accounted for 14 of the 27 participating countries and ~82% of trial participants. HRQoL was assessed at baseline, Week 28 and Week 56, and approximately once every 6 months thereafter until Week 160.
To ensure broad applicability of the results and ease of comparative interpretation with other studies, HRQoL measures were also mapped to two measures of health utility; the Short‐Form‐6D (SF‐6D) and EuroQoL‐5D (EQ‐5D) 19, 20, 21. Health utility is measured on an interval scale with zero reflecting states of health equivalent to death and one reflecting perfect health. Using a validated mapping algorithm 22, scores from the IWQOL‐Lite were mapped to the Short‐Form‐6D (SF‐6D). Similarly, SF‐36 scores were mapped to the same scale using the appropriate algorithm 20, 21. Additionally, as a supportive analysis, SF‐36 scores were also mapped to the EuroQoL‐5D (EQ‐5D) 19. There is currently no algorithm for mapping the IWQOL‐Lite to EQ‐5D.
Statistical methods
Baseline characteristics were evaluated using descriptive statistics. Differences in absolute changes in HRQoL scores from Week 0 to Week 160 between treatment groups were assessed using analysis of covariance (ancova) with treatment, country, gender, BMI stratification groups (<30, ≥30 kg m−2) as fixed factors, and baseline HRQoL scores (at Week 0) as covariates. Health utility scores were analysed using a similar model.
This post hoc, exploratory analysis was based on the full analysis set (FAS) of all subjects with prediabetes (all randomized participants exposed to at least one dose of the trial product and with at least one post‐baseline weight measurement) from the subset of countries where HRQoL was assessed. Differences between the treatment groups were estimated and presented with two‐sided 95% confidence interval (CI) and P‐value representing test for no treatment difference. No adjustment for multiple testing has been made.
Missing HRQoL and body weight values at 160 weeks were imputed using last observation carried forward (LOCF).
Meaningful change was defined differently for the IWQOL‐Lite and the SF‐36 according to published algorithms 23, 24. Increases of 7.7–12 points on the IWQOL‐Lite total score were considered to signify meaningful improvements, depending on baseline score. Conversely, decreases of 4.4–7.8 points on the IWQOL‐Lite total score were considered to be meaningful deteriorations, depending on baseline score. Changes from baseline of >3.8 points on the SF‐36 PCS score or >4.6 points on the SF‐36 MCS score were considered meaningful. The odds of achieving a meaningful improvement in the HRQoL score were analysed in an ordinal regression using a cumulative logit link. The model included treatment, sex, country and BMI stratification factor as fixed factors and baseline HRQoL score (IWQOL‐Lite total score, SF‐36 PCS score or SF‐36 MCS score, as appropriate) as a covariate. The ordinal odds‐ratio assumes that the odds of improvement relative to no‐change or deterioration is the same as the odds of improvement or no‐change relative to deterioration for liraglutide 3.0 mg relative to placebo.
Changes in HRQoL scores were also evaluated by categories of weight change at 160 weeks according to the following categories: weight gain, weight loss 0–4.9%, weight loss 5–9.9%, weight loss 10–14.9% and weight loss ≥15%.
Results
Participant characteristics
A total of 2254 participants were randomized and included in the prediabetes cohort with a planned duration of 3 years; of these 1791 (n = 1203 liraglutide, n = 588 placebo) participants were from countries where HRQoL was assessed. At Week 160, the FAS includes 1175 liraglutide patients (97.7%) and 580 placebo participants (98.6%) with HRQoL data available. Subject demographics and baseline characteristics were balanced between treatment groups (Table 1). Of the subset of participants who were assessed for HRQoL, 661 on liraglutide (51%) and 249 on placebo (42%) completed 160 weeks of treatment.
Table 1.
Subject demographics and baseline characteristics (observed means ± SD) for the total trial population and participants in whom HRQoL was assessed
| Total trial population | HRQoL‐assessed population | |||||||
|---|---|---|---|---|---|---|---|---|
| Liraglutide 3.0 mg (n = 1505) | Placebo (n = 749) | Liraglutide 3.0 mg (n = 1203) | Placebo (n = 588) | |||||
| Age, years (SD) | 47.5 | (11.7) | 47.3 | (11.8) | 48.0 | (11.7) | 47.8 | (11.7) |
| Age group, years (%) | ||||||||
| 18–39 | 395 | (26.2) | 202 | (27.0) | 297 | (24.7) | 151 | (25.7) |
| 40–64 | 1005 | (66.8) | 493 | (65.8) | 817 | (67.9) | 393 | (66.8) |
| 65–74 | 99 | (6.6) | 53 | (7.1) | 84 | (7.0) | 43 | (7.3) |
| ≥75 | 6 | (0.4) | 1 | (0.1) | 5 | (0.4) | 1 | (0.2) |
| Ethnicity, n (%) | ||||||||
| Hispanic or Latino | 143 | (9.5) | 70 | (9.3) | 142 | (11.8) | 70 | (11.9) |
| Not Hispanic or Latino | 1362 | (90.5) | 679 | (90.7) | 1061 | (88.2) | 518 | (88.1) |
| Race | ||||||||
| White | 1256 | (83.5) | 628 | (83.8) | 1030 | (85.6) | 503 | (85.5) |
| Black or African‐American | 146 | (9.7) | 71 | (9.5) | 138 | (11.5) | 68 | (11.6) |
| Asian | 75 | (5.0) | 39 | (5.2) | 8 | (0.7) | 6 | (1.0) |
| American Indian or Alaskan native | 5 | (0.3) | 2 | (0.3) | 5 | (0.4) | 2 | (0.3) |
| Native Hawaiian or other Pacific islander | 1 | (<0.1) | 1 | (0.1) | 1 | (<0.1) | 1 | (0.2) |
| Other | 22 | (1.5) | 8 | (1.1) | 21 | (1.7) | 8 | (1.4) |
| Gender (%) | ||||||||
| Female | 1141 | (75.8) | 573 | (76.5) | 929 | (77.2) | 461 | (78.4) |
| Male | 364 | (24.2) | 176 | (23.5) | 274 | (22.8) | 127 | (21.6) |
| BMI, kg m−2 (SD) | 38.8 | (6.4) | 39.0 | (6.3) | 39.0 | (6.5) | 39.4 | (6.6) |
| BMI group kg m−2, n (%) | ||||||||
| 27.0–29.9 | 39 | (2.6) | 23 | (3.1) | 31 | (2.6) | 19 | (3.2) |
| 30.0–34.9 | 427 | (28.4) | 197 | (26.3) | 320 | (26.6) | 144 | (24.5) |
| 35.0–39.9 | 492 | (32.7) | 245 | (32.7) | 402 | (33.4) | 181 | (30.8) |
| >40 | 547 | (36.3) | 284 | (37.9) | 450 | (37.4) | 244 | (41.5) |
| Waist circumference, cm (SD) | 116.6 | (14.4) | 116.7 | (13.9) | 116.7 | (14.6) | 117.3 | (14.2) |
| Body weight, kg (SD) | 107.5 | (21.6) | 107.9 | (21.8) | 107.8 | (21.8) | 109.1 | (22.2) |
| Glycated haemoglobin, % (SD) | 5.8 | (0.3) | 5.7 | (0.3) | 5.7 | (0.3) | 5.7 | (0.3) |
| Fasting glucose, mg dL−1 (SD) | 99.1 | (11.1) | 98.4 | (9.8) | 99.0 | (11.2) | 98.1 | (9.8) |
| History of CVD, yes (%) | 191 | (12.7) | 99 | (13.2) | 159 | (13.2) | 85 | (14.5) |
| Blood pressure, mmHg (SD) | ||||||||
| Systolic | 124.7 | (12.9) | 125.0 | (12.8) | 124.3 | (13.0) | 124.6 | (12.7) |
| Diastolic | 79.4 | (8.4) | 79.8 | (8.3) | 79.3 | (8.5) | 79.7 | (8.3) |
| Cholesterol, mg dL−1 (CVD) | ||||||||
| Total | 192.6 | (19.0) | 196.5 | (19.0) | 193.2 | (19.2) | 196.3 | (19.0) |
| LDL | 110.7 | (27.9) | 114.0 | (28.0) | 110.9 | (28.3) | 113.5 | (28.1) |
| HDL | 50.1 | (26.1) | 50.1 | (26.4) | 50.6 | (26.1) | 50.6 | (25.9) |
n, number of subjects; %, percentages are based on n. Subjects from France did not report race; CVD (by MedDRA search) includes the SMQs ischemic heart disease, cardiac failure, and central nervous system haemorrhages and cerebrovascular conditions and embolic and thrombotic events. Data are for subjects from countries where the HRQoL questionnaires were used. BMI, body mass index; CDV, cardiovascular disease; CI, confidence interval; HDL; high‐density lipoprotein; HRQoL, health‐related quality of life; LDL, low‐density lipoprotein; SD, standard deviation; SMQ, Standardised MedDRA Query.
Change in body weight over time
Among participants with HRQoL data, a greater proportion of participants lost weight over 3 years in the liraglutide 3.0 mg group than in the placebo group. Participants in the ≥15% weight‐loss category included 10.9% of the liraglutide participants (n = 161) and 3.1% of the placebo participants (n = 23). Similarly, greater proportions of participants in the liraglutide group lost 10–14.9% (13.8% [n = 203] vs. 6.8% [n = 50]) or lost 5–9.9% (24.7% [n = 363] vs. 13.7% [n = 101]) of their body weight compared with those receiving placebo. However, in the 0 to <5% weight loss group, the proportions were liraglutide 35.4% (n = 521) and placebo 37.3% (n = 275), respectively. Weight gain over 3 years was evident in 285 participants (38.6%) on placebo and 219 participants (14.9%) receiving liraglutide.
Impact of Weight on Quality of Life‐Lite results
At baseline, observed IWQOL‐Lite scores were similar between treatment groups (Table 2), and indicated an overall population assessment of ‘moderate impairment’ 23. Over the course of 3 years, observed mean IWQOL‐Lite total scores increased initially in both treatment groups and were generally maintained thereafter (Fig. 1a). At 3 years, a greater change from baseline in IWQOL‐Lite score was observed for liraglutide 3.0 mg vs. placebo (Table 2).
Table 2.
Observed mean change from baseline to Week 160 in total and subscale HRQoL scores by treatment arm for IWQOL‐Lite and SF‐36
| Summary/subscale score | Liraglutide 3.0 mg | Placebo | ||||
|---|---|---|---|---|---|---|
| n | Mean score at baseline | Change from baseline at Week 160 | n | Mean score at baseline | Change from baseline at Week 160 | |
| IWQOL‐Lite total | 1117 | 72.13 | 10.96 | 517 | 70.71 | 8.11 |
| Physical function | 1117 | 66.72 | 13.19 | 518 | 65.20 | 9.59 |
| Self‐esteem | 1119 | 62.18 | 14.78 | 518 | 59.43 | 11.82 |
| Sexual life | 1092 | 76.64 | 9.29 | 508 | 76.30 | 6.16 |
| Public distress | 1119 | 83.44 | 5.99 | 518 | 82.43 | 4.34 |
| Work | 1115 | 85.91 | 5.87 | 516 | 85.55 | 4.42 |
| SF‐36 PCS | 993 | 47.28 | 3.10 | 469 | 46.57 | 2.61 |
| SF‐36 MCS | 993 | 53.90 | −0.46 | 469 | 54.00 | −1.40 |
| Physical functioning | 996 | 47.07 | 3.50 | 470 | 46.42 | 2.48 |
| Role physical | 996 | 49.14 | 2.43 | 470 | 48.72 | 2.12 |
| Bodily pain | 996 | 50.16 | 0.82 | 470 | 49.51 | 0.51 |
| General health | 995 | 48.74 | 2.25 | 469 | 48.11 | 1.39 |
| Vitality | 995 | 52.35 | 1.84 | 470 | 51.80 | 1.07 |
| Social functioning | 995 | 51.55 | 0.25 | 470 | 51.94 | −0.51 |
| Role emotional | 996 | 51.28 | 0.38 | 470 | 51.50 | −0.60 |
| Mental health | 995 | 53.28 | 0.19 | 470 | 52.78 | −0.81 |
n, number of subjects; %, percentages are based on n; missing data are imputed using last observation carried forward; scores are on a scale 0–100 whereby 0 = worst and 100 = best. HRQoL, health‐related quality of life; IWQOL‐Lite, Impact of Weight on Quality of Life‐Lite; MCS, mental component summary; PCS, physical component summary; SF‐36, Short‐Form 36 v2.
Figure 1.
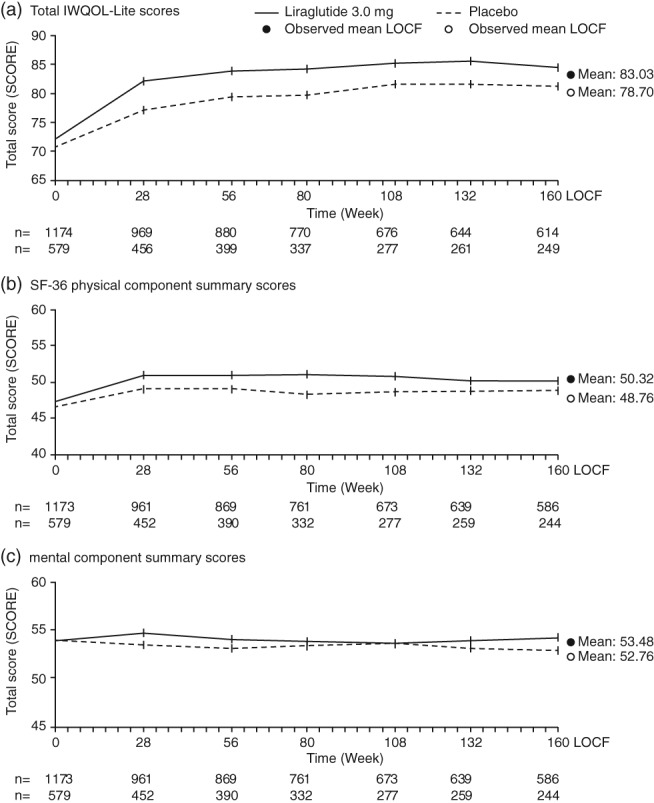
Total IWQOL‐Lite (a) and SF‐36 PCS (b) and MCS (c) scores over time. Full analysis set, observed values for participants with HRQoL data; values are means ± SE. HRQoL, health‐related quality of life; IWQOL‐Lite, Impact of Weight on Quality of Life‐Lite; MCS, mental component summary; PCS, physical component summary; SE, standard error; SF‐36, Short‐Form 36 v2.
At 3 years, the estimated mean change from baseline in IWQOL‐Lite total score and each of its five subscales was statistically significantly greater for liraglutide 3.0 mg compared with placebo. At 3 years, the estimated mean change in the IWQOL‐Lite total score from baseline for liraglutide was 11.1, compared with 7.8 for placebo; ETD 3.35 (95% CI 2.04; 4.66), P < 0.0001 (Fig. 2). A greater proportion of participants in the liraglutide 3.0 mg group (9.1%) achieved a maximum total score on the IWQOL‐Lite compared with participants in the placebo group (4.6%). At baseline only 1.2% of liraglutide 3.0 mg group had a maximum score, compared with 0.3% of participants on placebo. The greatest difference between liraglutide 3.0 mg and placebo was in the physical function subscale (ETD 4.5 [95% CI: 2.9, 6.1], P < 0.0001). Significant improvements were also present in the subscales self‐esteem, sexual life, public distress and work, with liraglutide 3.0 mg vs. placebo (Fig. 2).
Figure 2.
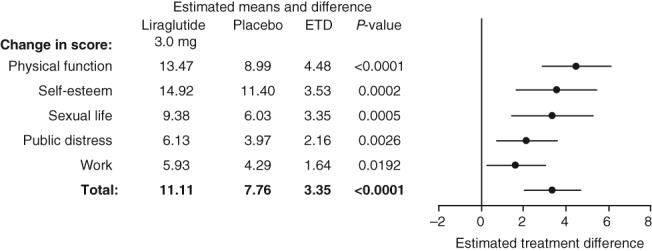
IWQOL‐Lite ETD for total and subscale scores at 3 years. Change in score is the estimated mean change from baseline to Week 160; ETD, ; right panel shows estimated treatment difference ± 95% confidence intervals; missing data are imputed using last observation carried forward. The data are analysed using analysis of covariance (ancova) with treatment, country, gender, BMI stratification groups (<30, ≥30 kg m−2) as fixed factors, and baseline HRQoL scores (at Week 0) as covariates. BMI, body mass index; ETD, estimated treatment difference; HRQoL, health‐related quality of life; IWQOL‐Lite, Impact of Weight on Quality of Life‐Lite.
Using the meaningful change algorithm for the IWQOL‐Lite, the odds of achieving a meaningful improvement for the IWQOL‐Lite total score were higher with liraglutide 3.0 mg than with placebo [OR 1.6 (95 %CI: 1.3; 2.0), P < 0.0001] (Fig. 3).
Figure 3.
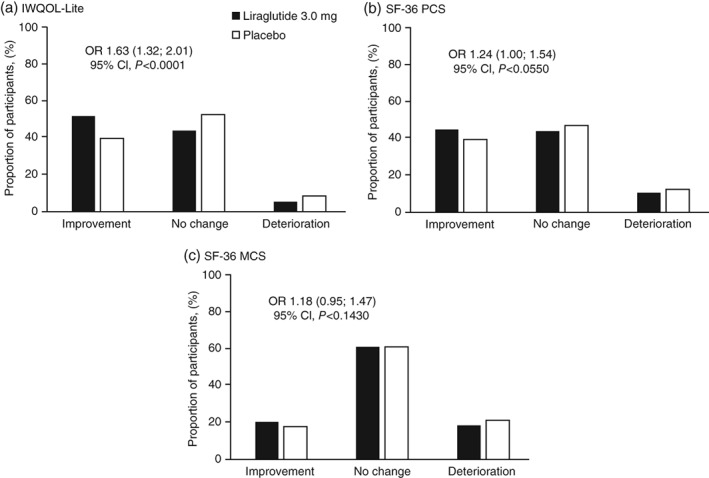
Proportion of subjects with meaningful estimated mean change from baseline to 3 years in HRQoL for IWQOL‐Lite total score and SF‐36 summary scores. Meaningful estimated mean change from baseline to Week 160 (LOCF) was defined differently for the IWQOL‐Lite and the SF‐36 according to published algorithms. The data was analysed in an ordinal regression using a cumulative logit link. The model includes treatment, sex, country, BMI stratification factor as fixed factors and the baseline HRQoL score (IWQOL‐Lite total score, SF‐36 PCS score or SF‐36 MCS score, as appropriate) as a covariate. Graphs are estimated proportions. OR for response classes was calculated as the ratio of results from participants receiving liraglutide 3.0 mg and placebo. BMI, body mass index, CI, confidence interval; HRQoL, health‐related quality of life; IWQOL‐Lite, Impact of Weight on Quality of Life‐Lite, LOCF, last observation carried forward; MCS, mental component summary; OR, odds ratio; PCS, physical component summary; SF‐36, Short‐Form 36 v2.
Short‐Form 36 results
At baseline, SF‐36 scores were similar between treatment groups (Table 2) which further supported the observation of HRQoL being moderately reduced in the assessed population. In the subsequent 3‐year study period, PCS increased in both treatment groups during the first 28 weeks and remained stable thereafter (Fig. 1b). MCS was largely unchanged from baseline levels throughout the study for both treatment groups (Fig. 1c). Mean PCS scores increased initially in both treatment groups and were generally maintained for up to 3 years (Fig. 1b), while MCS scores remained relatively unchanged over the 3‐year period (Fig. 1c).
At 3 years, the observed mean change from baseline (±standard deviation [SD]) in PCS score for liraglutide was statistically significantly greater (3.1 ± 7.3), compared with placebo (2.6 ± 7.6) (Table 2). A slight decline in MCS scores was noted in both groups at 3 years relative to baseline (Table 2); liraglutide −0.46 ± 8.7 and placebo −1.4 ± 9.2. ETDs for PCS and MCS scores were 0.87 ([95% CI: 0.17, 1.58], P = 0.0156) and 0.77 ([95% CI: −0.09, 1.63], P = 0.0778), respectively (Fig. 4).
Figure 4.
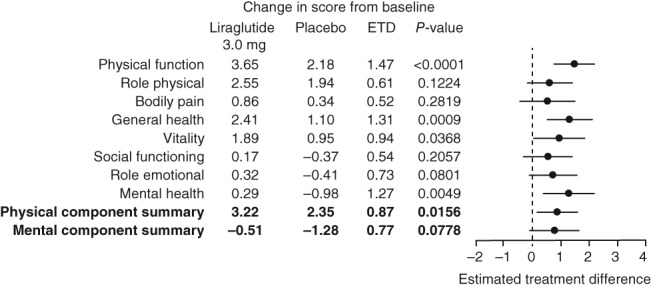
SF‐36 ETD for physical component summary, mental component summary and subscale scores at 3 years. Change in score is the estimated mean change from baseline to Week 160; ETD; right panel shows estimated treatment difference ± 95% confidence intervals; missing data are imputed using last observation carried forward. The data are analysed using analysis of covariance (ancova) with treatment, country, gender, BMI stratification groups (<30, ≥30 kg m−2) as fixed factors, and baseline HRQoL scores (at Week 0) as covariates. BMI, body mass index; ETD, estimated treatment difference; SF‐36, Short‐Form 36 v2.
The greatest difference between liraglutide 3.0 mg and placebo was found in the physical functioning subscale (ETD 1.47 [95% CI: 0.78, 2.16], P < 0.0001; Fig. 4). Significant improvements were also observed in general health, vitality and mental health with liraglutide 3.0 mg vs. placebo. Between‐group differences in physical role functioning, bodily pain, social functioning and emotional role functioning did not reach statistical significance (Fig. 4).
Using the meaningful change algorithm for the SF‐36, the odds of participants achieving meaningful improvement for the PCS score were not significantly different between the treatment groups (Fig. 3).
Health utility
For health utility scores, the mean IWQOL‐Lite‐derived SF‐6D scores (±SD) for liraglutide 3.0 mg and placebo were 0.75 ± 0.09 and 0.74 ± 0.09 at baseline, and 0.80 ± 0.08 and 0.78 ± 0.09 at 3 years, respectively (Table 3). At 3 years, the ETD was 0.014 (95% CI: 0.008, 0.021), P < 0.0001.
Table 3.
Health utility scores at baseline and 3 years plus estimated treatment difference for liraglutide 3.0 mg and placebo
| Source data | n | Estimate |
|---|---|---|
| IWQOL‐Lite‐derived data | ||
| Mean SF‐6D estimate at baseline | ||
| Liraglutide 3.0 mg | 1149 | 0.75 |
| Placebo | 572 | 0.74 |
| Mean SF‐6D estimate at 3 years | ||
| Liraglutide 3.0 mg | 1114 | 0.80 |
| Placebo | 517 | 0.78 |
| ETD (95% CI) P‐value | 1090/509 | 0.014 (0.008; 0.021) <0.0001 |
| SF‐36‐derived data | ||
| Mean SF‐6D estimate at baseline | ||
| Liraglutide 3.0 mg | 1162 | 0.76 |
| Placebo | 576 | 0.75 |
| Mean SF‐6D estimate at 3 years | ||
| Liraglutide 3.0 mg | 1117 | 0.78 |
| Placebo | 517 | 0.76 |
| ETD (95% CI) P‐value | 1104/514 | 0.014 (0.002; 0.025) <0.0182 |
| Mean EQ‐5D estimate at baseline | ||
| Liraglutide 3.0 mg | 1173 | 0.93 |
| Placebo | 579 | 0.92 |
| Mean EQ‐5D estimate at 3 years | ||
| Liraglutide 3.0 mg | 995 | 0.94 |
| Placebo | 470 | 0.93 |
| ETD (95% CI) P‐value | 993/469 | 0.007 (0.002; 0.013) <0.0116 |
Health utility scores were mapped from IWQOL‐Lite and SF‐36 data using established algorithms; Missing values post‐baseline were imputed using last observation carried forward. CI, confidence interval; EQ‐5D, European Quality of Life 5 Dimensions; ETD, estimated treatment difference (liraglutide 3.0 mg‐placebo); IWQOL, Impact of Weight on Quality of Life; SD, standard deviation; SF‐36, Short Form‐36 v2; SF‐6D, Short Form‐6D.
The corresponding baseline values for SF‐6D scores derived from the SF‐36 were 0.76 ± 0.11 and 0.75 ± 0.11. Change from baseline to 3 years was 0.02 ± 0.12 for liraglutide 3.0 mg and 0.01 ± 0.12 for placebo. At 3 years the ETD for SF‐36‐derived SF‐6D was 0.014 (95% CI 0.002, 0.025), P = 0.0182. SF‐36‐derived EQ‐5D scores were higher for liraglutide 3.0 mg vs. placebo: estimated difference 0.007 (95% CI: 0.002, 0.013), P = 0.0116 (Table 3).
Categorical weight loss and health‐related quality of life
Changes in HRQoL summary scores by weight loss category are shown in Table 4. For the IWQOL‐Lite total score and PCS there was an association between greater improvement in scores and higher weight loss; however, there was no clear relationship between MCS scores and the percentage of weight lost. An improvement in MCS was only noted in the highest weight loss category (≥15%), and all other categories showed some degree of decline in score.
Table 4.
Change in HRQoL summary scores by weight loss category from baseline to 3 years
| Summary score | Estimated mean change by weight loss category | ||||
|---|---|---|---|---|---|
| ≥15% | ≥10%, <15% | ≥5%, <10% | 0–5% | Gain | |
| IWQOL‐Lite total score | 18.72 | 14.91 | 12.01 | 8.33 | 5.23 |
| SF‐36 PCS | 6.15 | 3.99 | 4.02 | 2.50 | 0.55 |
| SF‐36 MCS | 0.48 | −0.30 | −1.08 | −0.79 | −1.03 |
Data analysed using a linear regression model. Missing values post‐baseline were imputed using last observation carried forward. HRQoL, health‐related quality of life; IWQOL‐Lite, Impact of Weight on Quality of Life‐Lite; SF‐36, Short Form‐36 v2; PCS, physical component score; MCS, mental component score.
Discussion
The purpose of these analyses was to examine the durability of improvements in HRQoL, as assessed at 3 years, in participants in the SCALE Obesity and Prediabetes trial (i.e. individuals with obesity, or overweight with comorbidities, and prediabetes). Scores on obesity‐specific HRQoL, the physical aspects of general HRQoL, and health utility were improved at 3 years in participants receiving liraglutide 3.0 mg vs. those receiving placebo, plus diet and exercise. Reinforcing data from the 1‐year analysis 11, increasing weight loss was associated with greater improvements in both obesity‐specific and physical HRQoL, regardless of treatment arm. Improvement in the mental aspects of HRQoL, as assessed by the SF‐36, was seen only in individuals whose weight was reduced by at least 15%. Similar to 1‐year results, the greatest sustained improvements were observed in the physical aspects of HRQoL (on both instruments), although significant improvements were also found on other subscales of the IWQOL‐Lite. While treatment with liraglutide 3.0 mg is associated with significantly higher total IWQOL‐Lite scores vs. placebo, the observed mean increase for both treatments fall within the established interval for meaningful improvements, suggesting that weight loss from diet and exercise can lead to meaningful improvements in HRQoL.
The data showed little effect of liraglutide 3.0 mg as compared with placebo on the mental component of the SF‐36. These findings are in line with data from other studies, which have shown muted effects of weight loss on mental HRQoL, but consistent positive effects on physical HRQoL. A recent systematic review of the impact of weight change on quality of life in adults with overweight/obesity in the United States reported that improvements in physical HRQoL were more commonly statistically significant than improvements in mental HRQoL 25.
The BMI threshold at which weight loss‐associated mean changes in mental HRQoL scores are evident from baseline to 3 years may be higher than in our investigation. Warkentin et al. showed that HRQoL instruments were relatively insensitive to weight loss in a population of patients with a higher mean BMI than in the present study (48 kg m−2). In these patients, weight loss of at least 20% was required to achieve clinically important improvements in HRQoL 26. The same group conducted a meta‐analysis of weight loss trials, and found that weight loss improved HRQoL in physical domains, but less so in mental health domains 6. In the SCALE programme, a hypothesis‐generating model was applied to determine to what degree the benefits of liraglutide 3.0 mg could be assigned to the drug itself, or to the effect of the weight loss a person may achieve while taking liraglutide 3.0 mg. The analysis indicated that weight loss has a positive impact on a number of cardiometabolic risk factors, including high blood pressure and cholesterol; and that liraglutide 3.0 mg had independent effects on glycaemic control among other endpoints 27. Weight loss, therefore, was a significant contributor to overall treatment effect. However, we cannot exclude the possibility that differences between physical and mental subscales and summary scores over time are affected either in part, or fully, by subject withdrawal patterns.
Results of health utility mapping provided additional support for differences between liraglutide 3.0 mg vs. placebo at 3 years. Although the values obtained appear numerically small, they are in line with estimates of minimally important differences (MIDs) in EQ‐5D and SF‐6D in stroke (MID estimates for EQ‐5D range from 0.08 to 0.12 and those for SF‐6D range from 0.04 to 0.14 in stroke patients) 28.
When SF‐36 subscales are examined, it is evident that there were no changes in bodily pain from Weeks 0 to 162 in either the liraglutide 3.0 mg or placebo groups, but physical functioning did improve in both groups.
The strengths of our study are as follows: a double‐blinded, randomized placebo‐controlled trial design with a large population conducted over 3 years, inclusion of both a general health survey measure and an obesity‐specific questionnaire, and mapping results to health utility scales. However, our study has some limitations that need to be considered. Because the SCALE Obesity and Prediabetes trial was not designed with HRQoL as the primary endpoint of study, the HRQoL endpoints described in this report are exploratory only. Additionally, the patient population was also affected by a meaningful dropout rate over 3 years of approximately 50%.
Nevertheless, our data add to the growing body of evidence that links weight loss in obesity with improved HRQoL. This provides physicians with confidence to coach patients on the benefits of weight loss in terms of their daily life and physical function, and may assist in managing the expectations of those who may need to lose relatively little weight (e.g. 5–10%). However, more data are needed in large patient populations; therefore, in clinical trials of weight loss in obesity, we hope to see inclusion of both disease‐specific and general HRQoL instruments as a matter of course.
Conflict of Interest Statement
RLK has served as a consultant to Novo Nordisk, Eisai, and Janssen; served on an advisory board and Steering Committee for Novo Nordisk; and receives royalties from Duke University for the IWQOL‐Lite. BGS is a full‐time employee of Novo Nordisk Inc. and stockholder of Novo Nordisk A/S. HHM is a full‐time employee and stockholder of Novo Nordisk A/S. KF has served on the scientific advisory board for Novo Nordisk and received honoraria as a speaker.
Acknowledgements
KF was involved in the study design and implementation. RLK contributed to the statistical analyses. All authors were involved in the data interpretation, writing the manuscript and had final approval of the submitted and published versions. We thank all investigators, trial staff and participants. We also thank Arne Haahr Andreasen, MSc (Novo Nordisk) for statistical support, Paul Tisdale, PhD, of Watermeadow Medical, an Ashfield Company, funded by Novo Nordisk, for providing medical writing and editorial support. The study was sponsored by Novo Nordisk. Novo Nordisk contributed to study design and conduct, data collection, analysis and interpretation (NCT01272219).
References
- 1. Guh DP, Zhang W, Bansback N et al The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health 2009; 9: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ul‐Haq Z, Mackay DF, Fenwick E, Pell JP. Meta‐analysis of the association between body mass index and health‐related quality of life among adults, assessed by the SF‐36. Obesity (Silver Spring) 2013; 21: E322–E327. [DOI] [PubMed] [Google Scholar]
- 3. Kolotkin RL, Andersen JR. A systematic review of reviews: exploring the relationship between obesity, weight loss and health‐related quality of life. Clinical Obesity 2017. https://doi.org/10.1111/cob.12203. [DOI] [PMC free article] [PubMed]
- 4. Kahan S, Ferguson C, David S, Devine L. Obesity drug outcome measures: results of a multi‐stakeholder critical dialogue. Curr Obes Rep 2013; 2: 128–133. [Google Scholar]
- 5. The George Washington University. Obesity drug outcome measures: a consensus report of considerations regarding pharmacologic intervention. [WWW document]. Accessed 4‐11‐17.
- 6. Warkentin LM, Das D, Majumdar SR, Johnson JA, Padwal RS. The effect of weight loss on health‐related quality of life: systematic review and meta‐analysis of randomized trials. Obes Rev 2014; 15: 169–182. [DOI] [PubMed] [Google Scholar]
- 7. Maciejewski ML, Patrick DL, Williamson DF. A structured review of randomized controlled trials of weight loss showed little improvement in health‐related quality of life. J Clin Epidemiol 2005; 58: 568–578. [DOI] [PubMed] [Google Scholar]
- 8.Victoza prescribing information. URL: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022341lbl.pdf (accessed 22 Sept 2017)
- 9.Saxenda prescribing information. URL: www.accessdata.fda.gov/drugsatfda_docs/label/2014/206321Orig1s000lbl.pdf (accessed 22 Sept 2017)
- 10. Pi‐Sunyer X, Astrup A, Fujioka K et al A randomized, controlled trial of 3.0 mg of Liraglutide in weight management. N Engl J Med 2015; 373: 11–22. [DOI] [PubMed] [Google Scholar]
- 11. Kolotkin RL, Fujioka K, Wolden ML, Brett JH, Bjorner JB. Improvements in health‐related quality of life with liraglutide 3.0 mg compared with placebo in weight management. Clin Obes 2016; 6: 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res 2001; 9: 102–111. [DOI] [PubMed] [Google Scholar]
- 13. Kolotkin RL, Crosby RD. Psychometric evaluation of the Impact of Weight on Quality of Life‐Lite questionnaire (IWQOL‐Lite) in a community sample. Qual Life Res 2002; 11: 157–171. [DOI] [PubMed] [Google Scholar]
- 14. Optum Inc. SF Health Surveys . 2016. URL: http://campaign.optum.com/content/optum/en/optum-outcomes/what-we-do/health-surveys/sf-36v2-health-survey.html. (accessed 23 Sept 2016).
- 15. le Roux CW, Astrup A, Fujioka K et al SCALE obesity, prediabetes NN8022‐1839 Study Group. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double‐blind trial. Lancet 2017; 389: 1399–1409. [DOI] [PubMed] [Google Scholar]
- 16. American Diabetes Association . Standards of medical care in diabetes‐2012. Diabetes care 2012; 35(Suppl. 1): S11–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Engel SG, Crosby RD, Kolotkin RL. The Impact of weight loss and regain on obesity‐specific quality of life: mirror image or differential effect. Obes Res 2003; 11: 1207–1213. [DOI] [PubMed] [Google Scholar]
- 18. Kolotkin RL, Crosby RD, Williams GR, Hartley GG, Nicol S. The relationship between health‐related quality of life and weight loss. Obes Res 2001; 9: 564–571. [DOI] [PubMed] [Google Scholar]
- 19. Rowen D, Brazier J, Roberts J. Mapping SF‐36 onto the EQ‐5D index: how reliable is the relationship? Health Qual Life Outcomes 2009; 7: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brazier J, Usherwood T, Harper R, Thomas K. Deriving a preference‐based single index measure from the SF‐ 36. J Clin Epidemiol 1998; 51: 1115–1129. [DOI] [PubMed] [Google Scholar]
- 21. Brazier J, Roberts J, Deverill M. The estimation of a preference‐based measure of health from the SF‐36. J Health Econ 2002; 21: 271–292. [DOI] [PubMed] [Google Scholar]
- 22. Brazier JE, Kolotkin RL, Crosby RD, Williams GR. Estimating a preference‐based single index for the Impact of Weight on Quality of Life‐Lite (IWQOL‐Lite) instrument from the SF‐6D. Value Health 2004; 7: 490–498. [DOI] [PubMed] [Google Scholar]
- 23. Crosby RD, Kolotkin RL, Williams GR. An integrated method to determine meaningful changes in health‐related quality of life. J Clin Epidemiol 2004; 57: 1153–1160. [DOI] [PubMed] [Google Scholar]
- 24. Maruish ME. User's Manual for the SF‐36v2 Health Survey, 3rd edn. QualityMetric Inc.: Lincoln, RI, 2011. [Google Scholar]
- 25. Kroes M, Osei‐Assibey G, Baker‐Searle R, Huang J. Impact of weight change on quality of life in adults with overweight/obesity in the United States: a systematic review. Curr Med Res Opin 2016; 32: 485–508. [DOI] [PubMed] [Google Scholar]
- 26. Warkentin LM, Majumdar SR, Johnson JA et al Weight loss required by the severely obese to achieve clinically important differences in health‐related quality of life: two‐year prospective cohort study. BMC Med 2014; 12: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bays HE, Pi‐Sunyer FX, Uddén Hemmingsson J et al Liraglutide 3.0 mg: weight‐loss dependent and independent effects. Endocr Rev 2015; 5(Suppl. 2) Abstract FRI‐551. URL: http://press.endocrine.org/doi/abs/10.1210/endo-meetings.2015.OABA.8.FRI-551 (accessed 2 Sept 2017). [Google Scholar]
- 28. Kim SK, Kim SH, Jo MW, Lee SI. Estimation of minimally important differences in the EQ‐5D and SF‐6D indices and their utility in stroke. Health Qual Life Outcomes 2015; 13: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]


