Abstract
Evidence from in vivo, in vitro and ecological studies are suggestive of a protective effect of vitamin D against pancreatic cancer (PC). However, this has not been confirmed by analytical epidemiological studies. We aimed to examine the association between pre‐diagnostic circulating vitamin D concentrations and PC incidence in European populations. We conducted a pooled nested case‐control study within the European Prospective Investigation into Cancer and Nutrition (EPIC) and the Nord‐Trøndelag Health Study's second survey (HUNT2) cohorts. In total, 738 primary incident PC cases (EPIC n = 626; HUNT2 n = 112; median follow‐up = 6.9 years) were matched to 738 controls. Vitamin D [25(OH)D2 and 25(OH)D3 combined] concentrations were determined using isotope‐dilution liquid chromatography‐tandem mass spectrometry. Conditional logistic regression models with adjustments for body mass index and smoking habits were used to estimate incidence rate ratios (IRRs) and 95% confidence intervals (95%CI). Compared with a reference category of >50 to 75 nmol/L vitamin D, the IRRs (95% CIs) were 0.71 (0.42–1.20); 0.94 (0.72–1.22); 1.12 (0.82–1.53) and 1.26 (0.79–2.01) for clinically pre‐defined categories of ≤25; >25 to 50; >75 to 100; and >100 nmol/L vitamin D, respectively (p for trend = 0.09). Corresponding analyses by quintiles of season‐standardized vitamin D concentrations also did not reveal associations with PC risk (p for trend = 0.23). Although these findings among participants from the largest combination of European cohort studies to date show increasing effect estimates of PC risk with increasing pre‐diagnostic concentrations of vitamin D, they are not statistically significant.
Keywords: vitamin D, pancreatic cancer, nested case–control study, cancer epidemiology
Short abstract
What's new?
Living at lower latitude and increased ultraviolet light exposure are inversely correlated with pancreatic cancer (PC) risk, supporting a model where vitamin D may protect from this devastating cancer. Here, the authors performed the largest combination of European studies to date and find that higher vitamin D concentrations are not associated with a lower risk of PC. They recommend caution before guidelines to increase vitamin D concentrations for the prevention of cancer can be recommended.
Abbreviations
- ATBC
alpha‐tocopherol, beta‐carotene
- BMI
body mass index
- CI
confidence interval
- DBP
vitamin D binding protein
- EPIC
European Prospective Investigation into Cancer and Nutrition
- HUNT2
the Nord‐Trøndelag Health Study's second survey
- IRR
incidence rate ratio
- LC‐MS/MS
liquid chromatography tandem‐mass spectrometry
- PLCO
Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial
- VDPP
Cohort Consortium Vitamin D Pooling Project of Rarer Cancers
Pancreatic cancer (PC) is a relatively rare form of cancer in Europe, with annual incidence rates of 8.3/100,000 in men and 5.5/100,000 in women.1 However, it is an aggressive and devastating malignancy, which is characterised by invasiveness, rapid progression and resistance to treatment. As a result, 5‐year survival rates in Europe are only 7%.2 Prevention is, therefore, key, but with the exception of family history, chronic pancreatitis, diabetes mellitus, smoking, alcohol and obesity as established risk factors,3, 4 a large part of the etiology of PC remains unknown. The identification of (other) modifiable risk factors is, therefore, warranted.
A potentially interesting factor in this respect is vitamin D. In general, vitamin D and its derivatives have been shown to have significant anti‐carcinogenic properties.5, 6 The expression of the enzyme 25‐hydroxyvitamin D‐1 α‐hydroxylase that catalyses the established biomarker of vitamin D status, 25(OH)D,7 to the active vitamin D form, 1α,25(OH)2 D, has been observed in pancreatic duct cells, and in normal and adenocarcinomatous tissues.8, 9 Furthermore, vitamin D analogs inhibit PC cell proliferation, induce differentiation, promote apoptosis and repress metastasis in vitro 10, 11, 12, 13, 14, 15, 16, 17, 18 and inhibit pancreatic tumour growth in vivo.12, 13, 16, 18
Ecological studies have shown that lower latitude and increased ultraviolet B (UVB) radiation are inversely related to PC risk and mortality19, 20, 21 and a preventive role of vitamin D has been suggested. However, an ecological study design has several weaknesses and the validity of associations might be questioned. Analytical epidemiologic studies on vitamin D in relation to PC risk have been conducted, with conflicting results.
In prospective nested case–control studies, blood concentrations of vitamin D have been investigated, which better reflects total vitamin D status. In the Alpha‐Tocopherol, Beta‐Carotene (ATBC) Cancer Prevention Study of male smokers from Finland,22 higher vitamin D concentrations were associated with an increased risk of PC, whereas no overall association was observed in a first report, but an increased risk was shown in a second report of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial from the United States (US).23, 24 When the ATBC and PLCO studies were combined with four other studies from the US and two from China in the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers (VDPP), including 952 PC cases and 1333 controls, an increased risk with higher vitamin D concentrations was observed.25 However, a pooled analysis of 451 PC cases and 1167 controls from five US studies, different from those in the VDPP, showed an inverse association.26
Except for the single study from Finland,22 which was based on male smokers only, no studies on vitamin D concentrations in relation to PC risk have been performed in European populations. Given the paucity of information from European populations, particularly from prospective cohort studies where biological samples are collected before cancer onset, we conducted a pooled nested case–control study within the European Prospective Investigation into Cancer and Nutrition (EPIC) and the Nord‐Trøndelag Health Study's second survey (HUNT2) cohorts to examine the association between pre‐diagnostic circulating concentrations of vitamin D and the incidence of PC.
Material and Methods
Study population
Both the EPIC and HUNT2 cohorts have previously been described in detail.27, 28 In brief, EPIC is a multicentre prospective cohort study designed to investigate the association between diet, various lifestyle and environmental factors and the incidence of different forms of cancer and other chronic diseases. It consists of cohorts in 23 centres from 10 European countries: Denmark, France, Germany, Greece, Italy, The Netherlands, Norway, Spain, Sweden and the United Kingdom. A total of 521,448 subjects joined the study between 1992 and 2000. Habitual dietary intake for the past 12 months was assessed using validated country‐specific food frequency questionnaires29, 30 and country‐specific food composition tables. Participants also completed a lifestyle questionnaire, had their anthropometric measurements recorded (self‐reported in France, Norway and Oxford) and donated a blood sample (in approximately 80% of cohort participants). These blood samples were processed, aliquoted and stored in heat‐sealed straws at −196°C under liquid nitrogen at the International Agency for Research on Cancer (IARC) for all countries except Denmark and Sweden, where tubes were stored at −150°C under nitrogen vapour or at −80°C in freezers, respectively.
Incident PC cases were identified through record linkage with regional cancer registries in Denmark, Norway, The Netherlands, Spain, Sweden, the United Kingdom and in most of the Italian centres. In France, Germany, Greece and Naples (Italy), follow‐up was based on a combination of methods, including health insurance records, cancer and pathology registries and active follow‐up through study participants and their next‐of‐kin. Closure dates for our study were defined as the latest date of complete follow‐up and ranged from December 2007 to December 2008 for centres using registry data and from June 2005 to December 2009 for centres using active follow‐up procedures.
All participants gave written informed consent, and the study was approved by the Ethics Review Committee of IARC and by the local ethical committee of individual EPIC centres.
The HUNT study was initiated in 1984, inviting the total adult population of over 20 years of age in the county of Nord‐Trøndelag in Norway for a general population‐based health screening. The main emphasis was initially on hypertension and diabetes, but this was later extended to include a large number of health problems and disease categories. For our analyses, the 65,237 participants of the second HUNT survey (HUNT2) were included. Between 1995 and 1997, these participants filled out questionnaires on a wide range of topics (e.g., use of alcohol and tobacco, physical activity and medical history), had a clinical examination and donated a blood sample. These samples were stored in a biobank at −80°C.
Based on the unique national identity number, assigned to all Norwegian residents, the participants in HUNT are linked to different national registries to access migration, emigration, cancer incidence and mortality data. The last record linkages for our study with the Norwegian Cancer Registry identified cancer cases diagnosed until September 2007.
All participants gave written informed consent at baseline, including future linkage to national registries, and the study was recommended by the Regional Committee for Medical Research Ethics and approved by the Data Inspectorate of Norway.
Nested case–control design
Cases in our study included primary incident pancreatic adenocarcinomas (International Classification of Diseases for Oncology, Third Edition, codes C250–C259 or C25.0–C25.3 and C25.7–C25.9). Endocrine pancreatic tumours (code C25.4; histology types 8150, 8151, 8153, 8155, 8240 and 8246) were excluded, because the aetiology of these cancers may be different.
During the follow‐up period, 1,013 PC cases were identified in the EPIC cohort. Of these, 33 endocrine cases were excluded. After further exclusions (283 cases who did not have blood sample available, two cases who had in situ tumours or tumours of non‐malignant morphology, 65 cases who had a secondary tumour and four cases who did not have lifestyle data available), a total of 626 incident PC cases with available questionnaire data and blood samples were identified for our study.
In the HUNT2 cohort, 117 PC cases were identified, of which 5 endocrine tumours were excluded, leaving 112 incident cases for our study.
Among this total of 738 cases, 493 (67%) were microscopically confirmed, based on histology of the primary tumour (N = 251), histology of the metastasis (N = 82), cytology (N = 117) or autopsy (N = 43).
Control subjects were selected by incidence density sampling from all cohort members alive and free of cancer (except non‐melanoma skin cancer) at the time of diagnosis of the matching case and were matched to cases by study centre, sex, duration of follow‐up, age at blood collection (± 1 month to ± 5 years) and fasting status at the time of blood collection (< 3 hrs (not fasting), 3–6 hrs (in between) or > 6 hrs (fasting)). For the EPIC study, participants were also matched on date of blood collection (± 1 month to ± 1 year) and time of blood collection (± 1 hrs to ± 4 hrs). For every case, one matched control was identified.
Laboratory measurements
Concentrations of both forms of vitamin D status [25(OH)D2 and 25(OH)D3] were measured in blood serum (plasma for the samples of Umea [Sweden]), using isotope‐dilution liquid chromatography (LC) tandem‐mass spectrometry (MS/MS),31 at the department of clinical chemistry, Canisius Wilhelmina Hospital in Nijmegen, The Netherlands. The inter‐assay coefficients of variation were 5.3%, 3.1% and 2.9% at 25(OH)D3 concentrations of 39.0, 92.5 and 127.0, nmol/L, respectively, and 9.5%, 5.5% and 5.6% at 25(OH)D2 concentrations of 32.9, 57.3 and 111.0 nmol/L, respectively. For technical reasons, EPIC and HUNT2 samples were measured sequentially. In addition, 11% of case–control sets were not measured in the same analytical batch. However, batch to batch differences are considered to be minor: no significant between‐day drift, time shifts or other trends were observed. For all analyses, laboratory technicians were blinded to case–control status of the samples.
Concentrations of 25(OH)D2 were only observed in 24 persons (1.6%) of the population, of which 3 came from Denmark, 4 from Spain, 13 from Sweden and 4 from the HUNT2 cohort in Norway. For our analyses, total vitamin D status was evaluated by adding 25(OH)D2 to 25(OH)D3 concentrations.
Data analysis
Means with standard deviations, medians with interquartile ranges or frequencies (where appropriate) of baseline characteristics were computed and compared between cases and controls of the EPIC and HUNT2 cohorts separately. Differences between cases and controls were tested by paired t test or by conditional logistic regression.
An incidence rate ratio (IRR), which is the interpretation of an odds ratio in an incidence density sampling design,32 and 95% confidence interval (95% CI) for the association between vitamin D status and PC was estimated by conditional logistic regression analysis.
To compare our findings with results from literature, vitamin D concentrations were divided into five categories (≤25; >25 to 50; >50 to 75; >75 to 100 and >100 nmol/L) according to clinically defined cut‐points, which are based on the proposed levels of vitamin D deficiency, insufficiency and sufficiency.33, 34, 35, 36 The middle category was used as reference to provide stability in the statistical analyses. To test for trend across categories, the categories of vitamin D were modelled as continuous variables, in which each category was assigned the median value of controls in that category.
In addition, vitamin D concentrations were divided into overall quintiles as well as cohort‐specific quintiles, defined by the distribution in control subjects. Vitamin D concentrations were also log2‐transformed. The IRR for a log2‐transformed variable corresponds to the change in PC risk by doubling the blood concentrations.
Since season of blood collection may affect vitamin D levels, two approaches were used to take this into account: (i) adjustment for month of blood collection; (ii) standardization of vitamin D levels by adding the overall mean of vitamin D for all subjects to the residuals derived from (iia) a simple regression model fitted to vitamin D concentration by month of blood collection, (iib) a regression of vitamin D levels on the periodic function – sin(2πX/12) – cos (2πX/12), where X is the month of blood collection; and (iic) a non‐parametric local regression (PROC LOESS; SAS Institute, Cary, NC) with vitamin D status as the dependent variable and week of the year of blood donation as the independent variable.37, 38 Since the results were similar for all different approaches to take seasonal variation into account, adjustment by LOESS residuals was used in all final models on quintiles and a doubling of vitamin D concentrations.
IRR estimates were computed both in a crude model, which was conditioned on the matching factors and in a multivariable model, which was developed by individually adding variables to the model. Variables examined as potential confounders were body mass index (BMI; weight (kg)/height(m)2), waist‐to‐hip ratio, waist circumference (cm), hip circumference (cm), alcohol consumption (g/d), physical activity (inactive, active), smoking habits (never smokers, former smokers who quitted ≥15 years earlier, former smokers who quitted between 0 and 15 years earlier, currents smokers who smoke <15 cigarettes/day, current smokers who smoke ≥15 cigarettes/day, former/current smokers with years since quitting/dose unknown), smoking duration, educational level (primary school or less, secondary school lower level, secondary school higher level, college/university degree), diabetes (yes, no), any vitamin use (yes, no) and season of blood collection (winter: December–February; spring: March–May; summer: June–August; autumn: September–November). The final multivariable model included BMI and smoking habits as these were associated with both the disease and the risk factor and changed the risk estimate by 10% or more. The dietary variables red meat, processed meat and fruit and vegetable intake were also investigated as potential confounders for cases and controls from the EPIC study, but they did not change the point estimates appreciably and were therefore not included in any model.
To evaluate whether preclinical disease may have influenced the results, additional analyses were conducted after exclusion of cases that were diagnosed within 2 years after recruitment and their matched controls (leaving approximately 87% of the population). In addition, the association between vitamin D and PC was examined by tertiles of follow‐up time. Further sensitivity analyses were performed in which only microscopically confirmed PC cases (67%) and their matched controls were included.
Possible heterogeneity of effects by log2 transformed values of vitamin D levels between categories of matching factors (age groups [median split], sex, season of blood collection, region [North: Norway, Sweden, Denmark, The Netherlands, Germany and United Kingdom; South: France, Italy, Spain and Greece], latitude [30–50 and 50–70°N] and country) was tested using the heterogeneity statistic derived from the inverse variance method.
Joint effects of several factors (in median split or pre‐defined categories) with season‐standardized vitamin D concentrations (in quartiles) were calculated, for which a combined reference category of the lowest category of these factors with a low vitamin D concentration was used. These factors are BMI, physical activity, smoking status, alcohol consumption, multivitamin use, diabetes at baseline, calcium intake (only available for the EPIC cohort) and retinol intake (only available for the EPIC cohort). Interaction (on the multiplicative scale) was tested by including a product term of the above‐mentioned factors with season‐standardized vitamin D status into the model. In addition, heterogeneity of effects by log2 transformed values of vitamin D levels by strata of the above‐mentioned factors were tested using the heterogeneity statistic derived from the inverse variance method.
All analyses were performed using SAS Software (version 9.3, SAS Institute Inc, Cary, NC, USA). For all analyses two‐sided p < 0.05 were considered statistically significant.
Results
In the EPIC cohort, the mean age of PC cases was 57.7 years at recruitment and they were followed for 7.0 years on average (Table 1). PC cases from EPIC were heavier, had a larger waist circumference and waist–hip ratio, were more likely to be current smokers and to have diabetes than controls.
Table 1.
Description of PC cases and matched controls for the EPIC and HUNT2 studies separately
| EPIC | HUNT2 | |||||
|---|---|---|---|---|---|---|
| Cases (n = 626) | Matched controls (n = 626) | p valuea | Cases (n = 112) | Matched controls (n = 112) | p valuea | |
| Matched variables | ||||||
| Years of follow‐up, mean (sd) | 7.0 (3.7) | – | – | 5.8 (3.2) | – | – |
| Age at recruitment (years), mean (sd) | 57.7 (7.8) | 57.7 (7.8) | – | 68.0 (10.7) | 68.0 (10.6) | – |
| Women, n (%) | 337 (53.9) | 337 (53.9) | – | 59 (52.7) | 59 (52.7) | – |
| Residential region, n (%) | – | – | ||||
| North (UK, NL, Germany, Denmark, Sweden, Norway) | 457 (73.0) | 457 (73.0) | 112 (100.0) | 112 (100.0) | ||
| South (France, Italy, Spain, Greece) | 169 (27.0) | 169 (27.0) | – | – | ||
| Country, n (%) | ||||||
| HUNT2 cohort | – | |||||
| Norway | – | – | 112 (100.0) | 112 (100.0) | ||
| EPIC cohort | – | |||||
| Denmark | 79 (12.6) | 79 (12.6) | – | – | ||
| France | 12 (1.9) | 12 (1.9) | – | – | ||
| Germany | 86 (13.7) | 86 (13.7) | – | – | ||
| Greece | 36 (5.8) | 36 (5.8) | – | – | ||
| Italy | 66 (10.5) | 66 (10.5) | – | – | ||
| The Netherlands | 62 (9.9) | 62 (9.9) | – | – | ||
| Norway | 5 (0.8) | 5 (0.8) | – | – | ||
| Spain | 55 (8.8) | 55 (8.8) | – | – | ||
| Sweden | 145 (23.2) | 145 (23.2) | – | – | ||
| United Kingdom | 80 (12.8) | 80 (12.8) | – | – | ||
| Season of blood collection, n (%) | – | – | ||||
| Winter (Dec, Jan, Feb) | 134 (21.4) | 135 (21.6) | 24 (21.4) | 25 (22.3) | ||
| Spring (Mar, Apr, May) | 190 (30.4) | 197 (31.5) | 26 (23.2) | 25 (22.3) | ||
| Summer (Jun, Jul, Aug) | 125 (20.0) | 123 (19.7) | 19 (17.0) | 19 (17.0) | ||
| Autumn (Sep, Oct, Nov) | 177 (28.3) | 171 (27.3) | 43 (38.4) | 43 (38.4) | ||
| Fasting status, n (%) | – | – | ||||
| <3 hrs | 253 (40.4) | 263 (42.0) | 78 (69.6) | 80 (71.4) | ||
| 3–6 hrs | 100 (16.0) | 97 (15.5) | 32 (28.6) | 31 (27.7) | ||
| >6 hrs | 171 (27.3) | 165 (26.4) | 1 (0.9) | 1 (0.9) | ||
| Use of pill/HRT/ERT at blood collection, yes, n (%) | 56 (9.0) | 56 (9.0) | – | 6 (5.4) | 6 (5.4) | – |
| Characteristics | ||||||
| Height (cm), mean (sd) | 167.7 (9.4) | 167.2 (9.7) | 0.20 | 166.5 (9.6) | 166.3 (8.9) | 0.87 |
| Weight (kg), mean (sd) | 74.7 (13.6) | 73.4 (13.9) | 0.06 | 75.1 (13.6) | 74.3 (12.6) | 0.55 |
| BMI (kg/m2), mean (sd) | 26.6 (4.2) | 26.2 (4.1) | 0.13 | 27.0 (3.9) | 26.8 (3.8) | 0.61 |
| Waist circumference (cm), mean (sd) | 89.8 (12.8) | 88.3 (12.9) | 0.02 | 90.4 (11.5) | 89.3 (10.8) | 0.38 |
| Hip circumference (cm), mean (sd) | 101.8 (8.6) | 101.1 (8.3) | 0.2 | 103.5 (8.8) | 102.6 (6.9) | 0.33 |
| Waist–hip ratio, mean (sd) | 0.9 (0.1) | 0.9 (0.1) | 0.01 | 0.9 (0.08) | 0.9 (0.09) | 0.79 |
| Education level, n (%) | 0.20 | 0.45 | ||||
| Primary school or less | 278 (44.4) | 248 (39.6) | 53 (47.3) | 57 (50.9) | ||
| Secondary school lower level | 142 (22.7) | 166 (26.5) | 33 (29.5) | 28 (25.0) | ||
| Secondary school higher level | 73 (11.7) | 79 (12.6) | 1 (0.9) | 5 (4.5) | ||
| College/University degree | 113 (18.1) | 116 (18.5) | 6 (5.4) | 13 (11.6) | ||
| Smoking status, n (%) | <0.01 | 0.08 | ||||
| Never | 233 (37.2) | 287 (45.9) | 40 (35.7) | 48 (42.9) | ||
| Past | 183 (29.2) | 201 (32.1) | 36 (32.1) | 43 (38.4) | ||
| Current | 201 (32.1) | 133 (21.3) | 26 (32.1) | 21 (18.8) | ||
| Age start smoking (years), mean (sd) | 20.1 (6.5) | 20.3 (6.4) | 0.43 | 22.8 (8.5) | 21.0 (8.1) | 0.79 |
| Duration of smoking (years), mean (sd) | 29.4 (12.0) | 27.4 (12.9) | 0.19 | 32.5 (14.7) | 29.6 (14.8) | 0.09 |
| Time since quitting (years), mean (sd) | 16.0 (11.5) | 16.0 (10.3) | 0.16 | 18.1 (11.6) | 21.8 (13.2) | 0.74 |
| Smoking dose (cig/day), mean (sd) | 15.7 (8.4) | 16.3 (8.8) | 0.35 | 10.9 (6.4) | 10.2 (6.2) | 0.16 |
| Smoking habits combined, n (%) | <0.01 | 0.15 | ||||
| Never | 233 (37.2) | 287 (45.9) | 40 (35.7) | 48 (42.9) | ||
| Former, time since quitting >15 yrs | 87 (13.9) | 89 (14.2) | 18 (16.1) | 27 (24.1) | ||
| Former, time since quitting 0–15 yrs | 90 (14.4) | 103 (16.5) | 15 (13.4) | 14 (12.5) | ||
| Current, 0–15 cig/day | 97 (15.5) | 60 (9.6) | 27 (24.1) | 17 (15.2) | ||
| Current, >15 cig/day | 80 (12.8) | 52 (8.3) | 7 (6.3) | 1 (0.9) | ||
| Former/current, quitting/dose unknown | 30 (4.8) | 30 (4.8) | 5 (4.5) | 5 (4.5) | ||
| Physical activity, n (%) | 0.53 | 0.59 | ||||
| Inactive | 174 (27.8) | 184 (29.4) | 12 (10.7) | 12 (10.7) | ||
| Active | 432 (69.0) | 426 (68.1) | 73 (65.2) | 80 (71.4) | ||
| Diabetes, yes, n (%) | 45 (7.2) | 28 (4.5) | 0.03 | 4 (3.6) | 7 (6.3) | 0.37 |
| Vitamin D status | ||||||
| Serum 25(OH)D (nmol/L), mean (sd) | 60.0 (27.3) | 59.4 (27.1) | 0.57 | 70.7 (23.8) | 64.7 (20.3) | 0.05 |
| Quintiles of serum 25(OH)D | 0.35 | 0.18 | ||||
| Q1, n (%) | 118 (18.9) | 139 (22.2) | 9 (8.0) | 9 (8.0) | ||
| Q2, n (%) | 144 (23.0) | 126 (20.1) | 13 (11.6) | 21 (18.8) | ||
| Q3, n (%) | 131 (21.0) | 121 (19.3) | 26 (23.2) | 26 (23.2) | ||
| Q4, n (%) | 105 (16.8) | 118 (18.9) | 23 (20.5) | 31 (27.7) | ||
| Q5, n (%) | 128 (20.5) | 122 (19.5) | 41 (36.6) | 25 (22.3) | ||
| Predefined cut‐points of serum 25(OH)D | 0.90 | 0.26 | ||||
| ≤25 nmol/L, n(%) | 33 (5.3) | 39 (6.2) | 1 (0.9) | 4 (3.6) | ||
| >25 to 50 nmol/L, n (%) | 214 (34.2) | 216 (34.5) | 20 (17.9) | 21 (18.8) | ||
| >50 to 75 nmol/L, n (%) | 233 (37.2) | 232 (37.1) | 46 (41.1) | 56 (50.0) | ||
| >75 to 100 nmol/L, n (%) | 92 (14.7) | 90 (14.4) | 33 (29.5) | 25 (22.3) | ||
| > 100 nmol/L, n (%) | 54 (8.6) | 49 (7.8) | 12 (10.7) | 6 (5.4) | ||
| Dietary variables | ||||||
| Alcohol (g/day)b | 6.0 (0.9–19.3) | 5.7 (1.1–17.9) | 0.64 | 1.4 (0.0–4.3) | 0.7 (0.0–2.9) | 0.13 |
| Any vitamin use, n (%) | 0.36 | 0.22 | ||||
| Yes | 211 (33.7) | 222 (35.5) | 12 (10.7) | 11 (9.8) | ||
| No | 337 (53.8) | 323 (51.6) | 11 (9.8) | 17 (15.2) | ||
p Values for differences in means between cases and controls were determined by paired t test, whereas differences in categorical variables were determined by conditional logistic regression. No p values were determined for years of follow‐up, age at recruitment, sex, residential region, country, season of blood collection, fasting status, and use of pill/HRT/ERT at blood collection, because these variables were used for matching.
Median (p25–p75).
In the HUNT2 cohort, the mean age of PC cases was 68.0 years at recruitment and they were followed for 5.8 years on average. PC cases from HUNT2 were more likely to be current smokers and tended to have a longer duration of smoking than controls.
When pre‐defined cut‐points of vitamin D concentrations were investigated in relation to PC risk, a trend was observed, which was not statistically significant (p for trend = 0.09; Table 2). Compared with the reference (> 50 to 75 nmol/L), lower vitamin D levels showed decreased effect estimates (≤25.0 nmol/L: IRR (95% CI) = 0.71 (0.42–1.20); >25 to 50 nmol/L: 0.94 (0.72–1.22)), whereas higher levels showed increased effect estimates (>75 to 100 nmol/L = 1.12 (0.82–1.53); >100 nmol/L = 1.26 (0.79–2.01)) in the adjusted model.
Table 2.
Incidence rate ratios (IRR) of PC according to predefined cut‐points and standardized circulating concentrations of 25‐hydroxy vitamin D
| Pre‐defined cut‐points | p for trend | ||||||
|---|---|---|---|---|---|---|---|
| Vitamin D (nmol/L) | ≤ 25 | >25 to 50 | >50 to 75 | >75 to 100 | >100 | ||
| N cases/controls | 34/43 | 234/237 | 279/288 | 125/115 | 66/55 | ||
| Crude IRR1 | 0.78 (0.47–1.29) | 1.0 (0.77–1.28) | Ref | 1.15 (0.85–1.56) | 1.36 (0.87–2.13) | 0.11 | |
| N cases/controls | 34/43 | 231/233 | 275/281 | 123/115 | 64/55 | ||
| Adjusted IRR2 | 0.71 (0.42–1.20) | 0.94 (0.72–1.22) | Ref | 1.12 (0.82–1.53) | 1.26 (0.79–2.01) | 0.09 | |
| Overall quintiles | |||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | Doubling of concentration | |
| Vitamin D (nmol/L)3 | ≤ 39.5 | 39.6–51.7 | 51.8–63.5 | 63.6–77.9 | >78.0 | ||
| N cases/controls | 133/148 | 159/148 | 138/146 | 143/148 | 165/148 | 738/738 | |
| Crude IRR1 | Ref | 1.20 (0.87–1.65) | 1.08 (0.77–1.50) | 1.10 (0.78–1.55) | 1.31 (0.91–1.89) | 0.27 | 1.11 (0.92–1.34) |
| N cases/controls | 131/146 | 158/143 | 134/144 | 141/146 | 163/148 | 727/727 | |
| Adjusted IRR2 | Ref | 1.32 (0.95–1.85) | 1.14 (0.81–1.62) | 1.18 (0.83–1.69) | 1.38 (0.94–2.01) | 0.23 | 1.16 (0.95–1.41) |
| Cohort‐specific quintiles | |||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | Doubling of concentration | |
| Vitamin D (nmol/L)3 | |||||||
| EPIC | ≤ 39.1 | 39.1–49.8 | 49.8–63.3 | 63.3–77.2 | >77.2 | ||
| HUNT | ≤ 45.1 | 45.1–57.7 | 57.7–66.4 | 66.4–78.5 | >78.5 | ||
| N cases/controls | 136/148 | 150/147 | 146/148 | 138/147 | 168/148 | 738/738 | |
| EPIC | 119/125 | 127/125 | 132/125 | 122/125 | 126/126 | 626/626 | |
| HUNT | 17/23 | 23/22 | 14/23 | 16/22 | 42/22 | 112/112 | |
| Crude IRR1 | Ref | 1.12 (0.81–1.55) | 1.09 (0.79–1.51) | 1.04 (0.74–1.47) | 1.31 (0.91–1.88) | 0.29 | 1.11 (0.92–1.34) |
| N cases/controls | 134/146 | 149/143 | 142/145 | 136/145 | 166/148 | 727/727 | |
| EPIC | 118/124 | 126/121 | 128/122 | 120/123 | 124/126 | 616/616 | |
| HUNT | 16/22 | 23/22 | 14/23 | 16/22 | 42/22 | 111/111 | |
| Adjusted IRR2 | Ref | 1.23 (0.88–1.73) | 1.20 (0.85–1.69) | 1.12 (0.79–1.59) | 1.40 (0.96–2.04) | 0.20 | 1.16 (0.95–1.41) |
Conditioned on matching factors (study centre, sex, duration of follow‐up, age and fasting status at time of blood collection, use of oral contraceptives and/or hormone replacement therapy [only women] and date and time of blood collection [only EPIC]).
Conditioned on matching factors and adjusted for BMI and smoking habits.
Standardized by week of blood collection using LOESS residuals.
Season‐standardized circulating vitamin D concentrations were not associated with risk of PC (Table 2). Compared with the lowest overall quintile (Q1), IRRs with 95% CIs were 1.32 (0.95–1.85) for Q2, 1.14 (0.81–1.62) for Q3, 1.18 (0.83–1.69) for Q4 and 1.38 (0.94–2.01) for Q5 (p for trend = 0.23). Effect estimates for cohort‐specific quintiles were comparable. A doubling of vitamin D concentrations was also not associated with PC risk (IRR (95% CI) = 1.16 (0.95–1.41). A model that, in addition to BMI and smoking habits, was further adjusted for waist–hip ratio, physical activity, alcohol, diabetes, education and vitamin use showed similar effect estimates (e.g., IRRlog2 (95% CI) = 1.20 (0.94–1.54)).
When the first 2 years of follow‐up were excluded (leaving approximately 87% of the population in the analyses), the trend over pre‐defined cut‐points reached statistical significance (p for trend 0.04), whereas the trend over season‐standardized quintiles of vitamin D concentrations did not (p for trend 0.08). When follow‐up time was divided in tertiles, the trend over pre‐defined cut‐points as well as the one over season‐standardized quintiles was only statistically significant in the second tertile (p for trend for increasing tertiles of follow‐up time = 0.48, 0.004 and 0.43 for pre‐defined cut‐points and 0.79, 0.004 and 0.51 for quintiles).
The trends over pre‐defined cut‐points and season‐standardized quintiles were not statistically significant when only confirmed PC cases (67%) were included in the analyses (p for trend 0.22 and 0.72, respectively).
No heterogeneity was observed by age (IRRlog2 (95% CI) = 1.06 (0.79–1.42) for younger age and 1.29 (0.97–1.72) for older age; p heterogeneity = 0.34), sex (IRRlog2 (95% CI) = 1.13 (0.83–1.55) for men and 1.18 (0.90–1.53) for women; p heterogeneity = 0.86), season of blood collection (IRRlog2 (95% CI) = 0.87 (0.48–1.55) for winter, 0.90 (0.51–1.59) for spring, 0.94 (0.51–1.74) for summer and 1.16 (0.78–1.73) for autumn; p heterogeneity = 0.82), region (IRRlog2 (95% CI) = 1.18 (0.94–1.48) for north and 1.14 (0.73–1.76) for south p heterogeneity = 0.88), nor latitude (IRRlog2 (95% CI) = 1.07 (0.71–1.61) for 30–50° N and 1.20 (0.95–1.51) for 50–70° N; p heterogeneity = 0.63).
Although none of the countries within the EPIC cohort separately showed a statistically significantly increased PC risk for every doubling of season‐standardized vitamin D concentrations, all countries except Germany and Greece showed effect estimates above the null value (p for heterogeneity between EPIC countries = 0.97; Fig. 1). The IRR (95% CI) for every doubling in season‐standardized vitamin D concentrations was 1.08 (0.87–1.35) for the EPIC cohort, whereas it was 1.73 (1.00–3.01) for the HUNT2 cohort (p for heterogeneity between EPIC and HUNT2 = 0.12; Fig. 1).
Figure 1.
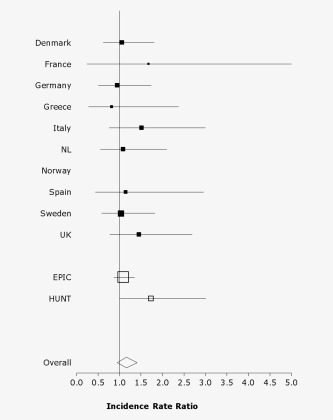
Country‐specific incidence rate ratios (95% CI) of PC according to a doubling of standardized circulating 25‐hydroxy vitamin D concentrations. Conditioned on matching factors and adjusted for BMI and smoking habits. No incidence rate ratios were obtained for EPIC‐Norway due to the small population. p value for heterogeneity between EPIC‐countries was 0.97 and between the EPIC and HUNT2 cohorts was 0.12.
Neither interaction nor heterogeneity of effects was observed for vitamin D and any of the factors tested (Table 3).
Table 3.
Joint effectsa of potential effect modifiers with quartiles, and strata of potential effect modifiers by doubling of concentrations, of standardized circulating 25‐hydroxy vitamin D in relation to PC risk
| Vitamin D status (nmol/L) | Q1 | Q2 | Q3 | Q4 | p interactionb | Doubling of concentration | p heterogeneityc | |
|---|---|---|---|---|---|---|---|---|
| BMI | N (cases/controls) | 163/183 | 194/179 | 173/182 | 197/183 | |||
| <25.0 kg/m2 | 283/298 | Ref | 1.18 (0.70–2.00) | 1.40 (0.83–2.36) | 1.30 (0.78–2.20) | 0.47 | 1.27 (0.75–2.18) | 0.97 |
| ≥25.0 kg/m2 | 444/429 | 0.95 (0.55–1.64) | 1.32 (0.78–2.24) | 0.97 (0.57–1.66) | 1.31 (0.75–2.26) | 1.26 (0.91–1.74) | ||
| Physical activity | 151/172 | 173/165 | 162/162 | 184/171 | ||||
| Inactive | 181/186 | Ref | 1.29 (0.73–2.26) | 0.85 (0.46–1.57) | 0.90 (0.47–1.72) | 0.30 | 1.08 (0.52–2.24) | 0.88 |
| Active | 489/484 | 0.86 (0.52–1.41) | 1.14 (0.70–1.85) | 1.21 (0.75–1.97) | 1.34 (0.81–2.21) | 1.02 (0.77–1.35) | ||
| Smoking status | 163/183 | 194/179 | 173/182 | 197/183 | ||||
| Never | 272/330 | Ref | 1.29 (0.81–2.07) | 1.13 (0.71–1.78) | 1.55 (0.93–2.59) | 0.52 | 0.94 (0.60–1.48) | 0.96 |
| Former | 218/243 | 0.94 (0.52–1.70) | 1.38 (0.83–2.28) | 1.59 (0.95–2.65) | 1.58 (0.93–2.66) | 1.13 (0.58–2.21) | ||
| Current | 237/154 | 2.66 (1.56–4.56) | 3.46 (1.94–6.18) | 2.18 (1.21–3.92) | 2.45 (1.41–4.27) | 0.90 (0.47–1.72) | ||
| Alcohol | 153/175 | 179/164 | 157/159 | 171/162 | ||||
| <5.0 g/day (median) | 315/322 | Ref | 1.27 (0.81–1.98) | 1.25 (0.78–1.99) | 1.22 (0.76–1.97) | 0.90 | 1.24 (0.83–1.85) | 0.98 |
| ≥5.0 g/day | 345/338 | 0.98 (0.62–1.54) | 1.41 (0.90–2.22) | 1.18 (0.74–1.90) | 1.43 (0.87–2.34) | 1.23 (0.85–1.78) | ||
| Multivitamin use | 123/143 | 145/130 | 137/136 | 144/140 | ||||
| No | 338/327 | Ref | 1.40 (0.91–2.15) | 1.24 (0.79–1.94) | 1.50 (0.93–2.42) | 0.80 | 1.12 (0.78–1.62) | 0.60 |
| Yes | 211/222 | 0.96 (0.52–1.75) | 1.27 (0.77–2.11) | 1.24 (0.75–2.05) | 1.09 (0.65–1.81) | 1.33 (0.75–2.36) | ||
| Diabetes | 160/180 | 190/173 | 167/175 | 186/175 | ||||
| No | 657/669 | Ref | 1.30 (0.95–1.78) | 1.10 (0.79–1.53) | 1.27 (0.89–1.81) | 0.67 | 1.18 (0.95–1.45) | – |
| Yes | 46/34 | 0.94 (0.36–2.43) | 1.60 (0.65–3.94) | 2.23 (0.83–5.99) | 2.20 (0.72–6.70) | Sample too small | ||
| Calcium (only EPIC) | 149/165 | 169/152 | 144/146 | 150/149 | ||||
| <959 mg/day (median) | 303/313 | Ref | 1.50 (0.94–2.39) | 1.25 (0.79–1.98) | 1.43 (0.86–2.37) | 0.73 | 1.15 (0.70–1.89) | 0.85 |
| ≥959 mg/day | 309/299 | 1.36 (0.85–2.19) | 1.56 (0.99–2.46) | 1.45 (0.90–2.33) | 1.34 (0.90–2.33) | 1.08 (0.72–1.62) | ||
| Retinol (only EPIC) | 149/165 | 169/152 | 144/146 | 150/149 | ||||
| <700 μg/day (median) | 296/315 | Ref | 1.26 (0.83–1.91) | 1.31 (0.82–2.09) | 1.32 (0.79–2.19) | 0.68 | 1.44 (0.97–2.15) | 0.10 |
| ≥700 μg /day | 316/297 | 1.23 (0.74–2.04) | 1.67 (1.04–2.67) | 1.22 (0.77–1.93) | 1.27 (0.78–2.05) | 0.96 (0.62–1.48) |
Conditioned on matching factors and adjusted for BMI and smoking habits.
Interaction (on the multiplicative scale) was tested by including a product‐term of characteristics with quartiles of vitamin D in the model.
Possible heterogeneity of effects by log2 transformed values of vitamin D levels between strata of potential effect modifiers was tested using the heterogeneity statistic derived from the inverse variance method.
Discussion
In our study, the largest combination of European studies to date on this topic, higher vitamin D concentrations are not inversely associated with PC risk. In fact, increasing effect estimates of PC risk with a borderline statistically significant trend were observed with increasing pre‐defined cut‐points of vitamin D status, whereas season‐standardized quintiles did not show an association with risk of PC.
Our findings are fairly consistent with observations from other studies as hardly any of them showed evidence of an inverse association. Although optimal levels of 25(OH)D have not been definitively determined, a classification of clinically relevant cut‐points has been used before. The VDPP25 first used these cut‐points, where a low vitamin D concentration (< 50 nmol/L) compared with a reference category of 50 to <75 nmol was not associated with PC risk, while a high vitamin D concentration (≥ 100 nmol/L) was associated with a statistically significant twofold increase in PC risk (OR (95% CI) = 2.12 (1.23–3.64)).25 The pooling project included participants from eight cohorts, among which were the ATBC study22 and the PLCO cohort.23 Both these studies already published results on vitamin D status and PC risk, but divided vitamin D in quintiles instead of clinically defined cut‐points. Using these quintiles, the ATBC study revealed a nearly threefold increase in PC risk for the highest quintile in comparison with the lowest quintile (OR (95% CI) = 2.92 (1.56–5.48; p for trend 0.001).22 In the PLCO, no association was observed in the overall analysis, but a nearly fourfold increase in PC risk for the highest versus the lowest quintile ((OR (95% CI) = 3.91 (1.19–12.85; p for trend 0.10)) was shown in a subgroup of participants living at northern latitudes.23 In a subsequent analysis, using clinically defined cut‐points, an increase in PC risk was observed for a high vitamin D concentration (≥ 100 nmol/L) compared with a reference category of 50 to <75 nmol ((OR (95% CI) = 3.23 (1.24–8.44)) in the overall group of the PLCO study.24 The only study that did observe an inverse association between vitamin D concentrations and PC risk is a pooled analysis of participants from five cohorts.26 Here, the odds ratio for the highest quintile of vitamin D concentrations compared with the lowest quintile was 0.67 (95% CI 0.46–0.97; p for trend 0.03). The inverse linear association observed for quintiles was not observed when Wolpin et al. divided vitamin D concentrations according to clinically relevant cut‐points as defined in the VDPP study.26 However, they also did not observe an increased PC risk for high vitamin D concentrations of ≥100 nmol/L. Although we did not detect a direct association between high vitamin D concentrations and PC either, effect estimates seemed to increase with increasing concentrations of vitamin D. In light of these results, we cannot state that higher vitamin D concentrations are related to a higher PC risk, but it seems reasonable to conclude that higher vitamin D concentrations are not related to a lower PC risk in this population.
Except for the ATBC study from Finland,22 this is the first study on vitamin D concentrations in relation to PC risk among European populations. One may hypothesize that this relation may differ with the associations observed in populations from the US, due to differences in latitude and fortified foods. Most of Europe lies above 37° N latitude, whereas this is only true for the northern half of the US. Since UVB is efficiently absorbed by the ozone layer from November through February above 37° N latitude,39, 40 nearly all European residents have low, if any, endogenous vitamin D production during these months and are thus more dependent on vitamin D intake from foods and supplements than residents from the US. In addition, vitamin D fortification of foods differs between Europe and the US, where fortification of milk, for example, is the exception in Europe rather than the rule in the US.41 As the amount of vitamin D that was added to milk was not very consistent in the 1990s,42, 43 it is less likely that hypothesized differences in associations between populations from the US and Europe are due to differences in food fortification than to differences in latitude. Even though there may be a difference in the sources of vitamin D concentrations between populations from Europe and the US, the vitamin D concentrations from our European study are comparable to those from US studies in the 1990s, and no large differences were observed for the association between vitamin D concentrations and PC risk.
Although several in vitro and in vivo studies have shown that vitamin D has anti‐carcinogenic properties in general,5, 6 few studies have investigated this specifically with respect to PC. Whether vitamin D has anti‐carcinogenic effects on the pancreas is thus largely unclear. The molecular basis by which vitamin D may be involved in pancreatic carcinogenesis should be further investigated. We propose that certain genetic variants affecting vitamin D concentrations may modulate the association between vitamin D and PC risk. Within the vitamin D pathway, genetic variants in the vitamin D binding protein (DBP, corresponding gene GC) are most frequently investigated. It is possible that variants in the DBP gene may affect the vitamin D binding protein concentration in the circulation and therefore may influence the vitamin D bioavailability, the role of which is unknown in pancreatic carcinogenesis. In a recent study of 713 PC cases and 818 controls from five cohorts within the VDPP, the association between vitamin D concentrations and PC risk was not modified by single nucleotide polymorphisms in the DBP gene or 10 other genes in the vitamin D metabolic pathway.44 Nevertheless, it should be kept in mind that in various Genome‐Wide Association Studies on vitamin D concentrations, genetic variants in GC are among the significant findings.45, 46 To unravel the molecular mechanisms by which vitamin D may influence pancreatic carcinogenesis, more studies should investigate vitamin D‐gene interactions with genetic variants in the vitamin D metabolic pathway, but also including the vitamin D receptor (VDR) and its vitamin D‐mediated transcriptionally regulated (VDRE containing) genes and their signalling pathways.47
An important strength of our study is the prospective design with pre‐diagnostic measurements of vitamin D concentrations, which reduces the influence of reversed causation. In addition, pooling two large European studies resulted in a relatively large study population. This population originates from countries from the north to the south of Europe, spanning a wide range of sun exposure, many different lifestyle patterns, dietary habits and PC incidence. A difference in vitamin D status was also observed between the two European studies, where higher concentrations of vitamin D were observed in the HUNT cohort from the North of Norway than in the more centrally located EPIC study. Although this is contrary to what would be expected based on latitude, this may be due to differences in study population, blood sample handling procedures or to a higher use of cod liver oil supplements, which is a long dietary tradition in Norway.48 Finally, another strength of our study is that all samples were transported to the same laboratory for measurement using a single LC‐MS/MS method, which shows close agreement to a reference measurement procedure for 25(OH)D3 and 25(OH)D2 analysis in human serum.31
A limitation of our study is that only a single baseline measurement of vitamin D was used. Vitamin D levels display seasonal variability and a single measurement of vitamin D may not reflect long‐term vitamin D status. However, the concentration of 25(OH)D in samples collected up to 14 years apart was observed to be sufficiently reliable to be used in cohort studies.49 Furthermore, we standardized the vitamin D concentrations by week of blood collection to take season of blood draw into account. While we could not take some risk factors of PC risk, such as family history and chronic pancreatitis, into account due to a lack of information, we did test other established PC risk factors and included BMI and smoking habits into the model to adjust for potential confounding. Although residual confounding by smoking cannot be ruled out, it is not likely, because the findings observed in never smokers were similar to the overall result.
In conclusion, among participants from the largest combination of European studies to date, higher vitamin D concentrations are not associated with a lower risk of PC. More research is needed on the molecular mechanisms by which vitamin D may influence pancreatic carcinogenesis. Until there is a better biological understanding of this mechanism, caution is warranted before guidelines to increase vitamin D concentrations in healthy persons for the prevention of cancer can be recommended.
Acknowledgements
The authors thank H. van Daal and T. Beijers for the 25(OH)D analysis. This work was financially supported by World Cancer Research Fund International and Wereld Kanker Onderzoek Fonds (WKOF) with grant 2010/252 and by the Dutch Cancer Society with grant UW 2010–4872. The coordination of EPIC is financially supported by the European Commission (DG‐SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l'Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), Federal Ministry of Education and Research (BMBF), Deutsche Krebshilfe, Deutsches Krebsforschungszentrum and Federal Ministry of Education and Research (Germany); the Hellenic Health Foundation (Greece); Associazione Italiana per la Ricerca sul Cancro‐AIRC‐Italy and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); ERC‐2009‐AdG 232997 and Nordforsk, Nordic Centre of Excellence programme on Food, Nutrition and Health (Norway); Health Research Fund (FIS), PI13/00061 (to Granada), PI13/01162 (to EPIC‐Murcia), Regional Government of Asturias, Basque Country, Murcia and Navarra, ISCIII RETIC (RD06/0020) (Spain); Swedish Cancer Society, Swedish Research Council and County Councils of Skåne and Västerbotten (Sweden); Cancer Research UK (14136 [to EPIC‐Norfolk]; C570/A16491 and C8221/A19170 [to EPIC‐Oxford]), Medical Research Council (UK) (1000143 [to EPIC‐Norfolk], MR/M012190/1 [to EPIC‐Oxford]). The Nord‐Trøndelag Health Study (The HUNT Study) is a collaboration between HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology NTNU), Nord‐Trøndelag County Council, Central Norway Health Authority, and the Norwegian Institute of Public Health.
Conflict of interest: The authors declare that they have no potential conflicts of interest.
References
- 1. Ferlay J, Soerjamataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer, 2010. Available from: http://globocan.iarc.fr, accessed 7 June 2016.
- 2. Lepage C, Capocaccia R, Hackl M, et al. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999–2007: results of EUROCARE‐5. Eur. J Cancer 2015;51:2169–78. [DOI] [PubMed] [Google Scholar]
- 3. Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: a summary review of meta‐analytical studies. Int J Epidemiol 2015;44:186–98. [DOI] [PubMed] [Google Scholar]
- 4. World Cancer Research Fund/American Institute for Cancer Research . Continuous update project report. Food, nutrition, physical activity, and the prevention of pancreatic cancer. 2012. Available at http://www.dietandcancerreport.org [Google Scholar]
- 5. Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 2007;7:684–700. [DOI] [PubMed] [Google Scholar]
- 6. Feldman D, Krishnan AV, Swami S, et al. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014;14:342–57. [DOI] [PubMed] [Google Scholar]
- 7. IARC Working Group on Vitamin D . Vitamin D and cancer. Lyon, France: IARC, 2008. [Google Scholar]
- 8. Schwartz GG, Eads D, Rao A, et al. Pancreatic cancer cells express 25‐hydroxyvitamin D‐1 alpha‐hydroxylase and their proliferation is inhibited by the prohormone 25‐hydroxyvitamin D3. Carcinogenesis 2004;25:1015–26. [DOI] [PubMed] [Google Scholar]
- 9. Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25‐hydroxyvitamin d(3)‐1 alpha‐hydroxylase. J Clin Endocrinol Metab 2001;86:888–94. [DOI] [PubMed] [Google Scholar]
- 10. Zugmaier G, Jager R, Grage B, et al. Growth‐inhibitory effects of vitamin D analogues and retinoids on human pancreatic cancer cells. Br J Cancer 1996;73:1341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiang KC, Yeh CN, Hsu JT, et al. The vitamin D analog, MART‐10, represses metastasis potential via downregulation of epithelial‐mesenchymal transition in pancreatic cancer cells. Cancer Lett 2014;354:235–44. [DOI] [PubMed] [Google Scholar]
- 12. Chiang KC, Yeh CN, Hsu JT, et al. Evaluation of the potential therapeutic role of a new generation of vitamin D analog, MART‐10, in human pancreatic cancer cells in vitro and in vivo. Cell Cycle 2013;12:1316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colston KW, James SY, Ofori‐Kuragu EA, et al. Vitamin D receptors and anti‐proliferative effects of vitamin D derivatives in human pancreatic carcinoma cells in vivo and in vitro. Br J Cancer 1997;76:1017–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanemaru M, Maehara N, Chijiiwa K. Antiproliferative effect of 1alpha,25‐dihydroxyvitamin D3 involves upregulation of cyclin‐dependent kinase inhibitor p21 in human pancreatic cancer cells. Hepatogastroenterology. 2013;60:1199–205. [DOI] [PubMed] [Google Scholar]
- 15. Kawa S, Nikaido T, Aoki Y, et al. Vitamin D analogues up‐regulate p21 and p27 during growth inhibition of pancreatic cancer cell lines. Br J Cancer 1997;76:884–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawa S, Yoshizawa K, Tokoo M, et al. Inhibitory effect of 220‐oxa‐1,25‐dihydroxyvitamin D3 on the proliferation of pancreatic cancer cell lines. Gastroenterology 1996;110:1605–13. [DOI] [PubMed] [Google Scholar]
- 17. Pettersson F, Colston KW, Dalgleish AG. Differential and antagonistic effects of 9‐cis‐retinoic acid and vitamin D analogues on pancreatic cancer cells in vitro. Br J Cancer 2000;83:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwartz GG, Eads D, Naczki C, et al. 19‐nor‐1 alpha,25‐dihydroxyvitamin D2 (paricalcitol) inhibits the proliferation of human pancreatic cancer cells in vitro and in vivo. Cancer Biol Ther 2008;7:430–6. [DOI] [PubMed] [Google Scholar]
- 19. Mohr SB, Garland CF, Gorham ED, et al. Ultraviolet B irradiance and vitamin D status are inversely associated with incidence rates of pancreatic cancer worldwide. Pancreas 2010;39:669–74. [DOI] [PubMed] [Google Scholar]
- 20. Neale RE, Youlden DR, Krnjacki L, et al. Latitude variation in pancreatic cancer mortality in Australia. Pancreas 2009;38:387–90. [DOI] [PubMed] [Google Scholar]
- 21. Garland CF, Cuomo RE, Gorham ED, et al. Cloud cover‐adjusted ultraviolet B irradiance and pancreatic cancer incidence in 172 countries. J Steroid Biochem Mol Biol 2016;155:257–63. [DOI] [PubMed] [Google Scholar]
- 22. Stolzenberg‐Solomon RZ, Vieth R, Azad A, et al. A prospective nested case‐control study of vitamin D status and pancreatic cancer risk in male smokers. Cancer Res 2006;66:10213–9. [DOI] [PubMed] [Google Scholar]
- 23. Stolzenberg‐Solomon RZ, Hayes RB, Horst RL, et al. Serum vitamin D and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian screening trial. Cancer Res 2009;69:1439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piper MR, Freedman DM, Robien K, et al. Vitamin D‐binding protein and pancreatic cancer: a nested case‐control study. Am J Clin Nutr 2015;101:1206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stolzenberg‐Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25‐hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010;172:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wolpin BM, Ng K, Bao Y, et al. Plasma 25‐hydroxyvitamin D and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2012;21:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Riboli E, Kaaks R. The EPIC Project: rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol 1997;26: S6–S14. [DOI] [PubMed] [Google Scholar]
- 28. Holmen J, Midthjell K, Krüger O, et al. The Nord‐Trondelag Health Study 1995‐97 (HUNT 2): objectives, contents, methods and participation. Norsk Epidemiologi 2003;1:19–32. [Google Scholar]
- 29. Margetts BM, Pietinen P. European Prospective Investigation into Cancer and Nutrition: validity studies on dietary assessment methods. Int J Epidemiol 1997;26: S1–5. [DOI] [PubMed] [Google Scholar]
- 30. Slimani N, Bingham S, Runswick S, et al. Group level validation of protein intakes estimated by 24‐hour diet recall and dietary questionnaires against 24‐hour urinary nitrogen in the European Prospective Investigation into Cancer and Nutrition (EPIC) calibration study. Cancer Epidemiol Biomarkers Prev 2003;12:784–95. [PubMed] [Google Scholar]
- 31. van den Ouweland JM, Beijers AM, Demacker PN, et al. Measurement of 25‐OH‐vitamin D in human serum using liquid chromatography tandem‐mass spectrometry with comparison to radioimmunoassay and automated immunoassay. J Chromatogr B Analyt Technol Biomed Life Sci 2010;878:1163–8. [DOI] [PubMed] [Google Scholar]
- 32. Knol MJ, Vandenbroucke JP, Scott P, et al. What do case‐control studies estimate? Survey of methods and assumptions in published case‐control research. Am J Epidemiol 2008;168:1073–81. [DOI] [PubMed] [Google Scholar]
- 33. Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol 2009;19:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- 35. Lips P. Which circulating level of 25‐hydroxyvitamin D is appropriate?. J Steroid Biochem Mol Biol 2004;89–90:611–4. [DOI] [PubMed] [Google Scholar]
- 36. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Borkowf CB, Albert PS, Abnet CC. Using lowess to remove systematic trends over time in predictor variables prior to logistic regression with quantile categories. Statist Med 2003;22:1477–93. [DOI] [PubMed] [Google Scholar]
- 38. Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc 1988;83:596–610. [Google Scholar]
- 39. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 2004;80:1678s–88s. [DOI] [PubMed] [Google Scholar]
- 40. Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 1988;67:373–8. [DOI] [PubMed] [Google Scholar]
- 41. Calvo MS, Whiting SJ, Barton CN. Vitamin D intake: a global perspective of current status. J Nutr 2005;135:310–6. [DOI] [PubMed] [Google Scholar]
- 42. Chen TC, Shao A, Heath H, 3rd , et al. An update on the vitamin D content of fortified milk from the United States and Canada. N Engl J Med 1993;329:1507 [DOI] [PubMed] [Google Scholar]
- 43. Holick MF, Shao Q, Liu WW, et al. The vitamin D content of fortified milk and infant formula. N Engl J Med 1992;326:1178–81. [DOI] [PubMed] [Google Scholar]
- 44. Arem H, Yu K, Xiong X, et al. Vitamin D metabolic pathway genes and pancreatic cancer risk. PLoS One 2015;10:e0117574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ahn J, Yu K, Stolzenberg‐Solomon R, et al. Genome‐wide association study of circulating vitamin D levels. Hum Mol Genet 2010;19:2739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome‐wide association study. Lancet 2010;376:180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ramagopalan SV, Heger A, Berlanga AJ, et al. A ChIP‐seq defined genome‐wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res 2010;20:1352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lamberg‐Allardt C, Brustad M, Meyer HE, et al. Vitamin D—a systematic literature review for the 5th edition of the nordic nutrition recommendations. Food Nutr Res 2013;57:22671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jorde R, Sneve M, Hutchinson M, et al. Tracking of serum 25‐hydroxyvitamin D levels during 14 years in a population‐based study and during 12 months in an intervention study. Am J Epidemiol 2010;171:903–8. [DOI] [PubMed] [Google Scholar]


