Abstract
Aims
Therapeutic inertia, defined as the failure to initiate or intensify therapy in a timely manner according to evidence‐based clinical guidelines, is a key reason for uncontrolled hyperglycaemia in patients with type 2 diabetes. The aims of this systematic review were to identify how therapeutic inertia in the management of hyperglycaemia was measured and to assess its extent over the past decade.
Materials and Methods
Systematic searches for articles published from January 1, 2004 to August 1, 2016 were conducted in MEDLINE and Embase. Two researchers independently screened all of the titles and abstracts, and the full texts of publications deemed relevant. Data were extracted by a single researcher using a standardized data extraction form.
Results
The final selection for the review included 53 articles. Measurements used to assess therapeutic inertia varied across studies, making comparisons difficult. Data from low‐ to middle‐income countries were scarce. In most studies, the median time to treatment intensification after a glycated haemoglobin (HbA1c) measurement above target was more than 1 year (range 0.3 to >7.2 years). Therapeutic inertia increased as the number of antidiabetic drugs rose and decreased with increasing HbA1c levels. Data were mainly available from Western countries. Diversity of inertia measures precluded meta‐analysis.
Conclusions
Therapeutic inertia in the management of hyperglycaemia in patients with type 2 diabetes is a major concern. This is well documented in Western countries, but corresponding data are urgently needed in low‐ and middle‐income countries, in view of their high prevalence of type 2 diabetes.
Keywords: antidiabetic drug, glycaemic control, systematic review, type 2 diabetes
1. INTRODUCTION
The importance of glycaemic control in patients with type 2 diabetes to reduce the risk of microvascular and macrovascular complications is well established1, 2, 3, 4, 5 and widely recognized by current clinical guidelines.6, 7, 8, 9, 10 For example, the joint position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) advocates a change of therapy if glycated haemoglobin (HbA1c) targets are not achieved after 3 months.6
Despite the introduction of many glucose‐lowering therapies that have proved to be efficacious in clinical trials, glycaemic control remains suboptimal in many patients globally. For example, in European countries with broad access to glucose‐lowering therapies, the GUIDANCE (N = 7597) and PANORAMA (N = 5817) studies showed that only 53.6% and 62.6% of patients, respectively, achieved the recommended HbA1c target of ≤7% (53 mmol/mol).11, 12
Several studies have identified 2 main reasons for suboptimal glycaemic control in clinical practice: (1) patient non‐adherence to prescribed treatment and (2) clinical or therapeutic inertia, defined as the failure to initiate or intensify therapy in a timely manner according to evidence‐based clinical guidelines in individuals who are likely to benefit from such intensification.13, 14 The reasons for clinical or therapeutic inertia are multiple and complex, and include patient‐, physician‐ and system‐level barriers.15
The primary objective of this systematic review was to identify studies assessing the extent of therapeutic inertia in the treatment of hyperglycaemia in different populations of patients with type 2 diabetes. The secondary objective was to provide an overview of how therapeutic inertia was defined and assessed in different studies. Assessing the extent of therapeutic inertia is key to implementing interventions to reduce its occurrence, which will contribute to improving glycaemic control and ultimately patient outcomes.
2. MATERIALS AND METHODS
This systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) on April 13, 2016 (registration number CRD42016036483) and followed Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.
2.1. Data sources and searches
Systematic searches for articles published from January 1, 2004 to August 1, 2016 were conducted in MEDLINE and Embase using the OvidSP database search interface. A start date of January 1, 2004 was chosen, to include the seminal article on therapeutic inertia in the management of patients with type 2 diabetes by Brown et al. published in 2004.16 This period also covers the publication of results from several outcome studies such as the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) study,5 the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study,17 and the 10‐year follow‐up of the UK Prospective Diabetes Study (UKPDS),2 which may have had an impact on the management of patients with type 2 diabetes in clinical practice.
Medical Subject Headings were used when available. A Medical Subject Heading for clinical or therapeutic inertia does not exist. Therefore, related terms were used instead (eg, “clinical competence,” “health care delivery” and “guideline adherence”). Detailed search strings used for both MEDLINE and Embase, and the corresponding numbers of identified publications are shown in Tables S1 and S2, respectively.
2.2. Study selection
Broad inclusion criteria were used to minimize the risk of excluding relevant studies. All publications involving studies of patients with type 2 diabetes that reported a quantitative measure of therapeutic inertia were included. Conversely, articles covering studies with insufficient data (eg, those without a description of the intensification step and those not reporting the glycaemic level threshold used to determine whether treatment intensification was required) were excluded. No language restrictions were imposed, to increase the likelihood of finding data from as many countries as possible. Congress abstracts were excluded from this systematic review because they do not provide sufficient data for effective analysis. Non‐original research articles (eg, editorials, letters, comments, guidelines and reviews) were also excluded. No other quality criteria were used to exclude studies from the systematic review.
Two researchers, S. Pi. and Andrew Mayhook (Oxford PharmaGenesis, Oxford, UK), screened all titles and abstracts independently, in accordance with the inclusion and exclusion criteria described above. Full texts were retrieved for publications that met the inclusion criteria and for those that could not be adequately assessed for inclusion with the information provided in the abstract. The 2 researchers independently assessed the full texts for inclusion and discussed their decisions before reaching a consensus on the final list of articles to be included in the review.
2.3. Data extraction
Data were extracted by a single researcher (S. Pi.). A standardized form was used to collect the following items when available: authors, year of publication, location, study design, period, sample size, patient and physician characteristics, definition of treatment intensification, glucose‐lowering agents used before and after treatment intensification, and measure(s) of therapeutic inertia (including the HbA1c threshold used to identify patients who required treatment intensification).
3. RESULTS
Out of 7698 combined search results, 53 articles were identified that reported at least 1 measure of therapeutic inertia in the management of hyperglycaemia in individuals with type 2 diabetes.16, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 The main reasons for exclusion of publications other than duplicates and those covering irrelevant topics were that they reported non‐original research (eg, editorials, letters, comments and guidelines) or they were congress abstracts (Figure 1). In addition, articles reporting the time to treatment intensification without reporting HbA1c results (eg, the time from type 2 diagnosis to insulin therapy initiation) were excluded.
Figure 1.
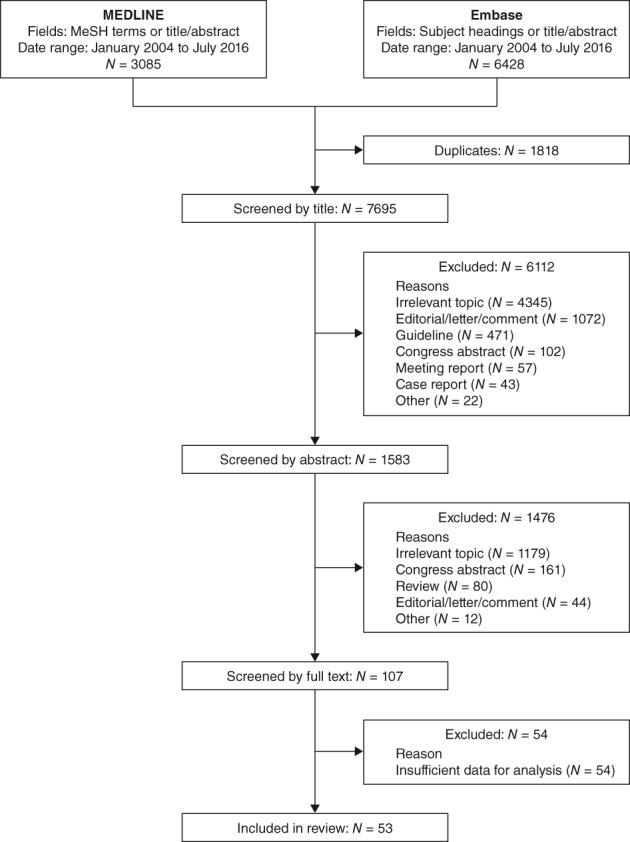
Flow diagram of study selection process. Titles, abstracts and full texts were screened independently by 2 researchers
3.1. Study characteristics
Study characteristics are summarized in Table S3. The majority of studies were conducted in North America (29 studies) and Europe (20 studies). Three studies were carried out in Asia,35, 47, 53 and a single study was conducted in Israel.62 Articles mainly reported data from cohort studies, using data from medical records or chart reviews,20, 21, 22, 26, 28, 30, 32, 44, 46, 47, 51, 52, 57, 60, 62, 64 or from claims, clinical research or administrative databases. 16, 18, 19, 23, 24, 25, 27, 29, 35, 36, 37, 38, 39, 40, 42, 43, 46, 50, 54, 55, 56, 57, 58, 59, 61, 65, 66, 69 Four articles reported results from cross‐sectional studies, and the data were collected using provider questionnaires or surveys.41, 45, 48, 49 A single publication reported results from a randomized clinical trial that evaluated the impact of physician education on the management of individuals with type 2 diabetes,53 and another provided results from a post hoc analysis of a randomized controlled trial.63
Patients were managed by primary care providers in 21 studies,19, 21, 25, 34, 37, 38, 39, 41, 44, 45, 48, 50, 53, 54, 59, 60, 61, 63, 64, 65, 67 by both primary care providers and secondary care specialists in 6 studies,23, 30, 35, 47, 58, 68 and by secondary care specialists alone in 1 study.69 The healthcare providers responsible for patient care were not described in 25 studies.16, 18, 20, 22, 24, 26, 27, 28, 29, 31, 32, 33, 36, 40, 42, 43, 46, 49, 51, 52, 55, 56, 57, 62, 66 Treatment characteristics varied across studies. Among articles that described therapy before treatment intensification, patients were managed exclusively with oral antidiabetic drugs (OADs) in most cases.16, 18, 19, 23, 28, 29, 30, 31, 35, 39, 43, 47, 50, 51, 54, 58, 62, 65, 66 The study by Brown et al. also included a group of patients managed with non‐pharmacological treatment (ie, diet and exercise exclusively).16 Two studies included patients managed with OADs or diet and exercise alone.22, 46 The study by Kristensen et al. included only patients managed with non‐pharmacological treatment.40 A single study considered 3 different treatment groups (OADs alone, insulin alone, and diet and exercise alone),68 and 2 others investigated patients treated with OADs and/or injectable drugs.25, 26 Another group of studies included only patients who were not treated with insulin but did not describe their therapies in more detail (ie, glucagon‐like peptide‐1 [GLP‐1] receptor agonists were not explicitly excluded).24, 36, 37, 41, 52, 55, 60, 61, 64, 69 By contrast, a study by Khunti et al. assessed treatment intensification in patients whose therapies included basal insulin.38 Fifteen publications did not describe the treatments used before intensification.21, 26, 27, 32, 33, 42, 44, 45, 48, 49, 53, 57, 59, 63, 67
3.2. Measures of therapeutic inertia
There is no accepted measure to describe clinical or therapeutic inertia. For the purpose of this systematic review, studies were classified into 4 categories based on the measurement(s) used to quantify clinical/therapeutic inertia: (1) the mean or median length of time between at least one HbA1c measurement above a certain threshold and treatment intensification18, 23, 28, 38, 43, 50, 54, 56, 62, 66; (2) the proportion of patients with at least 1 HbA1c measurement above a certain threshold who received treatment intensification within a given time frame18, 19, 21, 22, 24, 25, 27, 28, 29, 31, 32, 35, 37, 40, 42, 43, 46, 47, 50, 51, 52, 53, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66; (3) the glycaemic burden, defined as the length of time during which a patient had an HbA1c level above a certain threshold during a given period of time16, 34, 36, 39, 69; and (4) all other measurements.20, 26, 30, 33, 34, 41, 44, 45, 48, 49, 67, 68 HbA1c thresholds and the lengths of time to assess therapeutic inertia varied widely across the 53 studies, making comparisons difficult.
3.3. Time to treatment intensification
Results from the 10 publications that reported the median time to treatment intensification are shown in Table S3 and Figure 2. For patients who received a single OAD,23, 28, 29, 50, 62, 66 the median time to treatment intensification with any drug (ie, by addition of 1 OAD or insulin/other injectable drug) was 0.3 to 2.7 years after at least 1 HbA1c measurement above target. The time to treatment intensification was generally longer in studies that included patients treated with more than one OAD and ranged from 1.3 to 4.9 years.18, 43, 54, 56 In most of these studies, less than 50% of the patients received treatment intensification before the end of the follow‐up period. The study by Rubino et al. specifically reported treatment intensification with insulin in patients using 2 or more OADs.54 The time to treatment intensification estimated by Kaplan–Meier survival analysis was 4.9 and 4.2 years for patients with HbA1c levels of ≥8.0% and ≥9.0%, respectively. A single study assessed therapeutic inertia in patients using basal insulin.38 The time to treatment intensification (addition of bolus insulin, premix insulin or a GLP‐1 receptor agonist) was estimated by Kaplan–Meier survival analysis to be 3.7 and 3.2 years for patients with HbA1c levels of ≥7.5% and ≥8.0%, respectively. For each of the 5 studies that considered different HbA1c targets,23, 28, 38, 50, 54 the median time to treatment intensification decreased with increasing HbA1c targets regardless of the index treatment.
Figure 2.
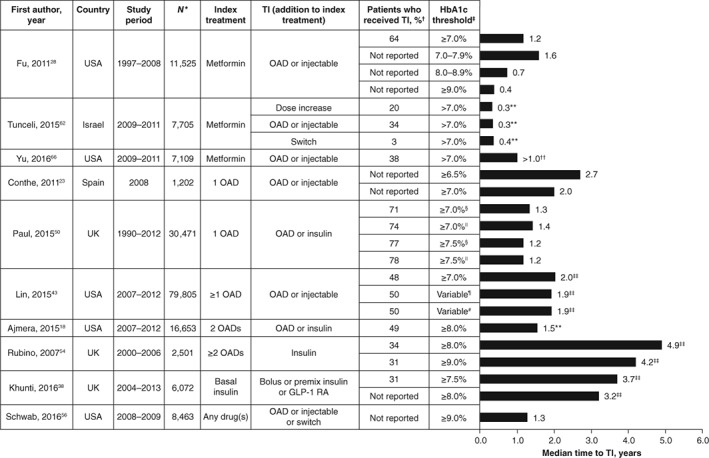
Median time to treatment intensification. Data are given as median times to treatment intensification from the time HbA1c level was above the threshold shown in the table, unless otherwise stated. *Total number of patients for whom treatment intensification was required in each study. †Proportion of patients who received treatment intensification by the end of the study period. ‡HbA1c target used to define inadequate glycaemic control in patients who required treatment intensification. §Consistently above HbA1c target for 1 year post diagnosis. ||Consistently above HbA1c target for 2 years post diagnosis. ¶Modified HbA1c target defined by Ismail‐Beigi et al. that was based on patient age and the presence or absence of macrovascular and microvascular complications, resulting in an individualized HbA1c level between ≤6.5% and <8.0%.70 #Modified Healthcare Effectiveness Data and Information Set (HEDIS) target of HbA1c <7.0% for patients aged <65 years without evidence of significant morbidities and HbA1c <8.0% for all other patients (set by the National Committee for Quality Assurance Healthcare in 2013). **Median time to treatment intensification calculated only for patients who received treatment intensification during the study period. ††Fewer than 50% of patients had received treatment intensification by the end of the study period. ‡‡Estimated by Kaplan–Meier analysis. GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated haemoglobin; OAD, oral antidiabetic drug; TI, treatment intensification
3.4. Proportion of patients who received treatment intensification
A total of 34 studies reported the proportions of patients who received treatment intensification within a given a period of time (Table S3).18, 19, 21, 22, 24, 25, 27, 28, 29, 31, 32, 35, 37, 40, 42, 43, 46, 47, 50, 51, 52, 53, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66 Results from studies that included a single treatment intensification step (eg, a specific number of OADs at baseline) and those combining several baseline treatments (eg, baseline treatment described as other than insulin) are summarized in Figures 3 and 4, respectively.
Figure 3.
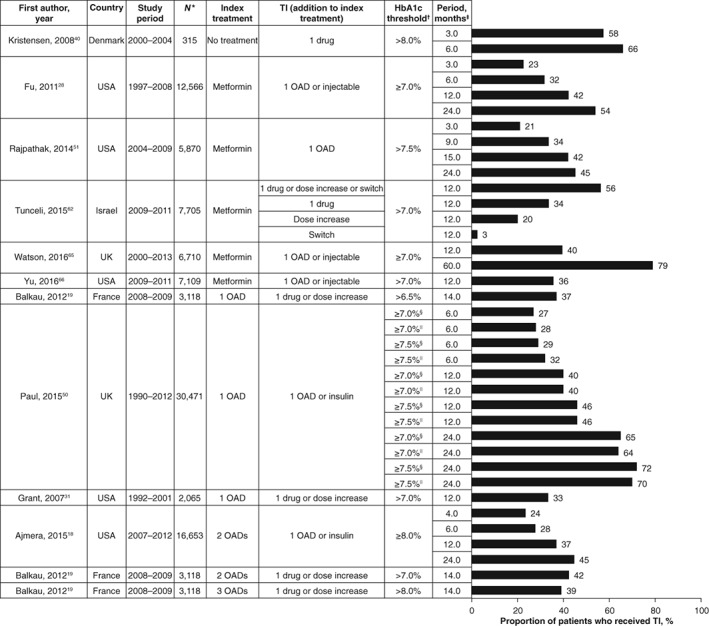
Proportion of patients who received treatment intensification after a given period of time (patients managed with a defined number of OADs). *Total number of patients for whom treatment intensification was required. †HbA1c target used to define suboptimal glycaemic control in patients who required treatment intensification. ‡Length of time to assess treatment intensification after HbA1c level was above target. §Consistently above HbA1c target for 1 year post diagnosis. ||Consistently above HbA1c target for 2 years post diagnosis. HbA1c, glycated haemoglobin; OAD, oral antidiabetic drug; TI, treatment intensification
Figure 4.
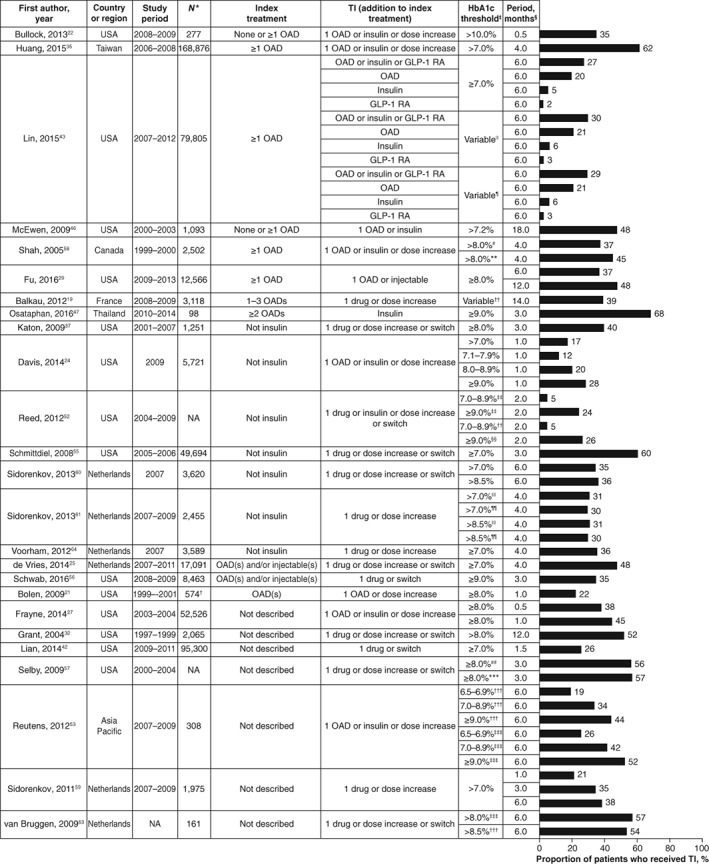
Proportion of patients who received treatment intensification after a given period of time (number of drugs before treatment intensification not clearly defined). *Total number of patients for whom treatment intensification was required. †Total number of clinical encounters that required treatment intensification. ‡HbA1c target used to define suboptimal glycaemic control in patients who required treatment intensification. §Length of time to assess treatment intensification after HbA1c level was above target. ||Modified HbA1c target defined by Ismail‐Beigi et al., which was based on patient age and the presence or absence of macrovascular and microvascular complications, resulting in individualized HbA1c levels between ≤6.5% and <8.0%.70 ¶Modified Healthcare Effectiveness Data and Information Set (HEDIS) target of <7.0% for patients aged <65 years without evidence of significant morbidities and <8.0% for all other patients (set by the National Committee for Quality Assurance Healthcare in 2013). #Primary care. **Specialist care. ††HbA1c level >6.5% for 1 OAD, >7.0% for 2 OADs and >8.0% for 3 OADs. ‡‡Before implementation of electronic health record system. §§After implementation of electronic health record system. ||||One HbA1c measurement above target. ¶¶Two consecutive HbA1c measurements above target. ##In 2011. ***In 2013. †††Control group. ‡‡‡Intervention group (healthcare professional training on clinical guidelines). Abbreviations: GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated haemoglobin; NA, not available (not reported); OAD, oral antidiabetic drug; TI, treatment intensification
In most of these studies, less than 50% of patients received treatment intensification for follow‐up periods of less than 12 months. Exceptions were observed for patients managed with diet and exercise only at baseline40 and for patients with HbA1c levels ≥9.0%.47, 53 Four other studies found treatment intensification in more than 50% of patients within 6 months or less of having an HbA1c level above target.35, 55, 57, 63 In 3 of these studies, patients were managed by physicians taking part in a pay‐per‐performance programme,35 or they were members of a large, integrated managed care consortium (Kaiser Permanente Northern California).55, 57 The fourth study was a post hoc analysis of a randomized controlled trial concerning the implementation of locally adapted guidelines.63
Unsurprisingly, for studies that considered several follow‐up periods, the proportion of patients who received treatment intensification rose with increasing lengths of follow‐up. Nevertheless, even after periods longer than 12 months following an HbA1c measurement above target, the proportion of patients who had received treatment intensification was only 37% to 79%.18, 19, 28, 51, 65 In 4 studies in which different HbA1c thresholds were analysed, the proportion of patients who received treatment intensification rose with increasing HbA1c values.24, 50, 52, 53 By contrast, in 2 studies by Sidorenkov et al.60, 61 the proportions of patients who received treatment intensification were similar for those with HbA1c >7.0% and those with HbA1c >8.5%.
In the single study that reported proportions of patients receiving treatment intensification within 6 months for different treatment regimens,43 proportions were lower for insulin (5% to 6%) and GLP‐1 receptor agonists (2% to 3%) than for addition of an OAD (20% to 21%).
3.5. Glycaemic burden
Five publications reported glycaemic burden (ie, the length of time with HbA1c above target during a given period).16, 34, 36, 39, 69 Results of these studies are summarized in Figure 5 and Table S3. The studies by Brown et al. and Khunti et al. identified patients who required treatment intensification, and they assessed the glycaemic burden until treatment intensification.16, 39 By contrast, 2 other studies identified a cohort of patients who initiated insulin and assessed glycaemic burden retrospectively.36, 69 In the study by Halimi et al.34 patients with poor glycaemic control were identified during a routine visit, and the length of time their HbA1c level had been above target was calculated using medical records.
Figure 5.
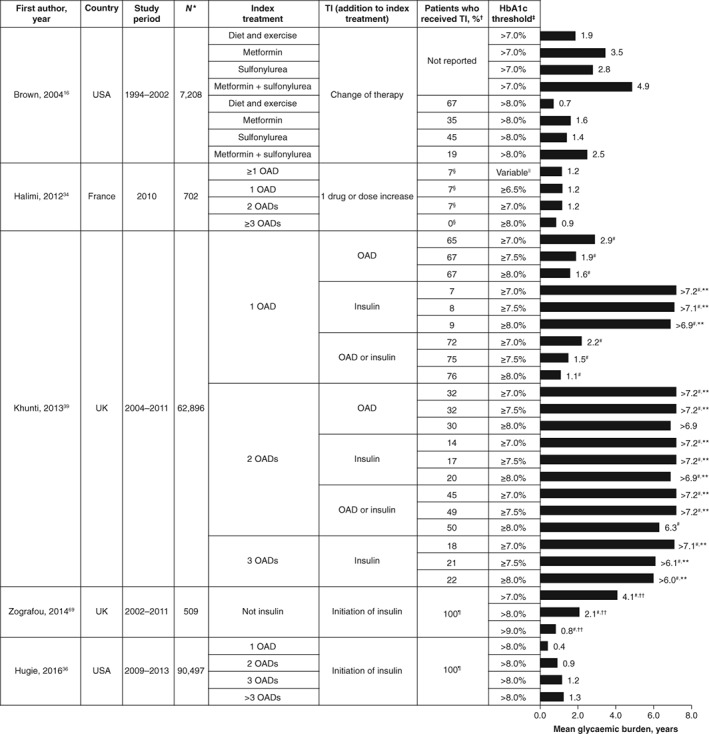
Glycaemic burden (defined as the length of time with HbA1c level above target during a given period of time). Data are shown as means unless otherwise stated. *Total number of patients for whom treatment intensification was required. †Proportion of patients who received treatment intensification by the end of the study period. ‡HbA1c target used to define glycaemic burden. §Proportion of patients who received treatment intensification at the inclusion visit. ||HbA1c ≥6.5% for 1 OAD, HbA1c ≥7.0% for 2 OADs and HbA1c ≥8.0% for 3 OADs. ¶Only patients in whom insulin treatment was initiated were included in the study. #Median glycaemic burden. **Fewer than 50% of patients had received treatment intensification by the end of the study period. ††Glycaemic burden was calculated from type 2 diabetes diagnosis to initiation of insulin therapy. HbA1c, glycated haemoglobin; OAD, oral antidiabetic drug; TI, treatment intensification
In the study by Brown et al.16 the mean glycaemic burden ranged from 0.7 to 4.9 years, depending on therapy and HbA1c threshold. Glycaemic burden increased with the rising number of OADs used and was lower for patients with HbA1c levels >8.0% than for those with HbA1c levels >7.0%. The proportion of patients with HbA1c levels >8.0% who received treatment intensification by the end of the study decreased with the increasing number of OADs (19% to 67%). The study by Khunti et al. reported a median time to treatment intensification from 1.1 years to more than 7.2 years,39 the median glycaemic burden rising with the increasing number of OADs and decreasing with increasing HbA1c values. The proportions of patients who received treatment intensification by the end of the study period were similar for patients with HbA1c levels ≥7.0%, 7.5% and 8.0%, and decreased with the increasing number of OADs; these proportions were lower when treatment was intensified with insulin (7% to 22%) than when it was intensified with an OAD (30% to 67%). Halimi et al. identified patients who received OADs and whose HbA1c was inadequately controlled (2 consecutive HbA1c measurements ≥6.5%, ≥7.0% and ≥8.0% for patients treated with 1, 2 and 3 OADs, respectively).34 Although the HbA1c level had been over target for 0.9 to 1.2 years in these patients, few individuals (0% to 7%) received treatment intensification during the inclusion visit. In the study by Zografou et al.69 glycaemic burden was assessed from diagnosis to insulin treatment initiation; the median time above target was 0.8 to 4.2 years and decreased with increasing HbA1c values. In the study by Hugie et al.,36 glycaemic burden (HbA1c >8.0%) before insulin treatment initiation was 0.4 to 1.3 years and rose with the increasing number of OADs.
3.6. Other measures of therapeutic inertia
In addition to the studies above, 12 others determined the proportions of patients who received treatment intensification without specifying a time frame30, 33, 34, 44 or measured therapeutic inertia by assessing the proportion of clinical encounters for which treatment intensification was recommended by guidelines and did not occur (Table S3).20, 26, 41, 45, 48, 49, 67, 68 In 4 of these studies, treatment intensification was assessed by questionnaires completed by physicians41, 45, 49 or patients.48 The different and insufficiently described methodologies precluded any comparisons among these studies. In the study by Ziemer et al.,68 treatment intensification rates increased with rising plasma glucose levels and were higher in specialist care than in primary care settings. Similarly, in the studies by Parchman et al.48 and Parnes et al.,49 rates of treatment intensification increased with rising HbA1c values. An opposite trend was observed in the study by Lang et al.,41 in which the proportions of patients who received treatment intensification decreased with increasing HbA1c values.
4. DISCUSSION
To our knowledge, this is the first systematic review to analyse the global extent of therapeutic inertia in the management of hyperglycaemia in patients with type 2 diabetes within our search timeframe. The results clearly demonstrate that delays in treatment intensification are widespread in both primary and specialist care, and occur at all stages of the treatment pathway, from initiation of oral therapy after failure of non‐pharmacological treatment (diet and exercise), through addition of OAD(s), to initiation and intensification of insulin therapy. In studies that considered several treatments, delay in intensification was found to increase with rising numbers of OADs. The longest delays were reported for initiation of insulin, which reflects reticence on the part of both patients and healthcare professionals to initiate and intensify insulin therapy, for reasons that include fear of injection pain, potential side effects (hypoglycaemia and weight gain) and reduced quality of life, alongside concerns about adherence to treatment.71, 72, 73, 74
Although the ADA/EASD joint position statement recommends a change of therapy if HbA1c targets are not achieved after 3 months,6 the reported times to treatment intensification were generally much higher than 3 months, and the proportion of patients who received treatment intensification after this period was low. In all studies that compared several HbA1c thresholds, higher HbA1c values were associated with shorter times to treatment intensification and/or a higher proportion of patients who underwent treatment change within a given follow‐up period. Although the heterogeneity of the included studies precluded identification of secular trends in the evolution of therapeutic inertia, the results suggest that therapeutic inertia has been a persistent issue over the past decade. As mentioned previously, inertia does not have an associated Medical Subject Heading and may have diverse definitions, making the design of the search string difficult. Although we used a comprehensive search strategy and identified a large number of studies, some relevant publications may have been missed. The diversity of inertia measures, patient populations, treatments and HbA1c targets used to assess glycaemic control made comparisons among studies difficult and precluded any meta‐analysis of the results. Indeed, the extent of therapeutic inertia depends on the definitions of treatment goals (based on different clinical guidelines), therapies and time windows selected to assess treatment intensification in individual studies. Despite these limitations, some useful inferences can be drawn from the data.
First, our systematic review highlighted a lack of data on treatment intensification outside North America and Western Europe. Although searches were not restricted to specific countries or regions and languages, only 3 studies were conducted in Asia, only 1 in Eastern Europe (Croatia) and only 1 in Israel. Given the high prevalence of type 2 diabetes in many low‐ and medium‐income countries,75 studies to quantify and address therapeutic inertia in those countries may be a valuable opportunity to improve glycaemic control and patient outcomes.
Whether the delays in treatment intensification identified in this review represent true therapeutic inertia may be contentious. Most of the reviewed studies used generic targets (eg HbA1c level >7.0% for all patients as opposed to individualized targets) to assess glycaemic control and thus therapeutic inertia. Some studies may, therefore, overestimate the prevalence of therapeutic inertia because treatment intensification may not be warranted in certain patients (eg, in elderly individuals). It should be noted, however, that the study by Lin et al. found similar results for a generic HbA1c target of 7.0% and 2 alternative individualized HbA1c thresholds.43
Other methodological aspects of some of the studies should be carefully considered when interpreting the results and the degree to which they represent therapeutic inertia. Several studies quantified therapeutic inertia by calculating the number of visits during which treatment intensification was indicated by guidelines but did not occur.41, 45, 68 This approach may not provide a representative picture of therapeutic inertia. At the level of a visit, competing demands may prevent treatment intensification, particularly in primary care. As visits are time‐constrained, physicians and patients may prioritize more pressing issues (eg, symptomatic comorbidity or counselling for smoking‐cessation) and thus delay treatment intensification to another visit.48 Competing demands were one of the main reasons for inaction cited by healthcare providers in the study by Parnes et al.49 In this context, there is an opportunity for pharmacists to play an important role in timely treatment intensification. However, none of the articles included in this review reported data on the management of patients by pharmacists. Some studies may also overestimate the prevalence of therapeutic inertia because they assess treatment intensification after a single HbA1c measurement above target. Some physicians may wait for confirmation of suboptimal glycaemic control (ie, a second consecutive HbA1c measurement above target) before intensifying treatment, particularly for patients who are close to their glycaemic target. In that case, assessing treatment intensification after 2 consecutive measurements above target or using glycaemic burden is likely to provide a more accurate estimate of true therapeutic inertia. Nevertheless, a study by Sidorenkov et al. found very similar proportions of patients receiving treatment intensification after a single HbA1c measurement or after 2 consecutive HbA1c measurements above target.60 These variations in methodology across the included studies highlight the need for accepted definitions of therapeutic inertia for use in clinical research, to ensure that therapeutic inertia is accurately measured and reported.
Although delay in treatment intensification may be justified for some patients, it took longer than recommended by current clinical guidelines for significant proportions of patients to receive treatment intensification. Therapeutic inertia remains a significant barrier to adequate glycaemic control in North America and Europe. In other regions, data are scarce or non‐existent, and studies are warranted to analyse the extent of therapeutic inertia, its causes, and its impact on glycaemic control and patient outcomes globally. Given the risk of microvascular and microvascular complications associated with poor glycaemic control,4, 76, 77, 78, 79 actions such as healthcare quality‐improvement programmes are urgently required to increase adherence to guidelines and to identify patients who may benefit from closer glucose monitoring.
ORCID
Kamlesh Khunti http://orcid.org/0000-0003-2343-7099
Supporting information
Table S1. Search strings used in MEDLINE.
Table S2. Search strings used in Embase.
Table S3. Summary of study characteristics.
ACKNOWLEDGEMENTS
The authors would like to thank Andrew Mayhook of Oxford PharmaGenesis, Oxford, UK, for screening the articles with S. Pi.
Conflict of interest
K. K. has received honoraria and research grants from AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche, Sanofi‐Aventis, Takeda, Bristol‐Myers Squibb and Unilever. K. K. also acknowledges the support of the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care—East Midlands (NIHR CLAHRC—EM) and the National Institute of Health Research (NIHR) Leicester–Loughborough Diet, Lifestyle and Physical Activity Biomedical Research Unit. M. B. G. has received honoraria from AstraZeneca and Merck‐Serono. S. Po. has received honoraria from AstraZeneca. M. V. S. has received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharpe & Dohme, Novartis, Novo Nordisk, Sanofi and Servier, and has received research support from Sanofi. S. Pi. is an employee of Oxford PharmaGenesis, which received funding from AstraZeneca. P. F., N. H. and J. M. are employees of AstraZeneca.
Author contributions
All authors contributed to the design of the search strategy. S. Pi. conducted the searches, screened the hits and extracted the data. K. K. and S. Pi. developed the figures and wrote the first draft of the manuscript. All authors contributed to the analysis and interpretation of the data, and critically reviewed all drafts. All authors approved the final draft for submission. The guarantor of this work is K. K.
Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: A systematic review. Diabetes Obes Metab. 2018;1–11. https://doi.org/10.1111/dom.13088
REFERENCES
- 1. Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta‐analysis of randomised controlled trials. Lancet. 2009;373:1765–1772. [DOI] [PubMed] [Google Scholar]
- 2. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐Year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. [DOI] [PubMed] [Google Scholar]
- 3. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 4. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. [DOI] [PubMed] [Google Scholar]
- 6. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429–442. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association; Standards of medical care in diabetes – 2014. Diabetes Care 2014;37(suppl 1):S14–S80. [DOI] [PubMed] [Google Scholar]
- 8. International Diabetes Federation . Global guideline for type 2 diabetes [article online]. 2012. http://www.idf.org/sites/default/files/IDF-Guideline-for-Type-2-Diabetes.pdf. Accessed June 6, 2017.
- 9. Qaseem A, Humphrey LL, Sweet DE, Starkey M, Shekelle P. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2012;156:218–231. [DOI] [PubMed] [Google Scholar]
- 10. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract. 2015;21:438–447. [DOI] [PubMed] [Google Scholar]
- 11. Stone MA, Charpentier G, Doggen K, et al. Quality of care of people with type 2 diabetes in eight European Countries: findings from the guideline adherence to enhance care (GUIDANCE) study. Diabetes Care. 2013;36:2628–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Pablos‐Velasco P, Parhofer KG, Bradley C, et al. Current level of glycaemic control and its associated factors in patients with type 2 diabetes across Europe: data from the PANORAMA study. Clin Endocrinol (Oxf). 2014;80:47–56. [DOI] [PubMed] [Google Scholar]
- 13. Mateo JF, Gil‐Guillen VF, Mateo E, Orozco D, Carbayo JA, Merino J. Multifactorial approach and adherence to prescribed oral medications in patients with type 2 diabetes. Int J Clin Pract. 2006;60:422–428. [DOI] [PubMed] [Google Scholar]
- 14. Safford MM, Shewchuk R, Qu H, et al. Reasons for not intensifying medications: differentiating “clinical inertia” from appropriate care. J Gen Intern Med. 2007;22:1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zafar A, Stone MA, Davies MJ, Khunti K. Acknowledging and allocating responsibility for clinical inertia in the management of type 2 diabetes in primary care: a qualitative study. Diabet Med. 2015;32:407–413. [DOI] [PubMed] [Google Scholar]
- 16. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535–1540. [DOI] [PubMed] [Google Scholar]
- 17. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ajmera M, Raval A, Zhou S, et al. A real‐world observational study of time to treatment intensification among elderly patients with inadequately controlled type 2 diabetes mellitus. J Manag Care Spec Pharm. 2015;21:1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Balkau B, Bouee S, Avignon A, et al. Type 2 diabetes treatment intensification in general practice in France in 2008–2009: the DIAttitude Study. Diabetes Metab. 2012;38(suppl 3):S29–S35. [DOI] [PubMed] [Google Scholar]
- 20. Berlowitz DR, Ash AS, Glickman M, et al. Developing a quality measure for clinical inertia in diabetes care. Health Serv Res. 2005;40:1836–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bolen SD, Bricker E, Samuels TA, et al. Factors associated with intensification of oral diabetes medications in primary care provider‐patient dyads: a cohort study. Diabetes Care. 2009;32:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bullock KC, Edwards KL, Greene RS, Shah SR, Blaszczyk AT. Race as a factor for intensification of diabetes medications. Diabetes Educ. 2013;39:335–343. [DOI] [PubMed] [Google Scholar]
- 23. Conthe P, Mata M, Orozco D, et al. Degree of control and delayed intensification of antihyperglycaemic treatment in type 2 diabetes mellitus patients in primary care in Spain. Diabetes Res Clin Pract. 2011;91:108–114. [DOI] [PubMed] [Google Scholar]
- 24. Davis J, Chavez B, Juarez DT. Adjustments to diabetes medications in response to increases in hemoglobin A1c: an epidemiologic study. Ann Pharmacother. 2014;48:41–47. [DOI] [PubMed] [Google Scholar]
- 25. de Vries ST, Voorham J, Haaijer‐Ruskamp FM, Denig P. Potential overtreatment and undertreatment of diabetes in different patient age groups in primary care after the introduction of performance measures. Diabetes Care. 2014;37:1312–1320. [DOI] [PubMed] [Google Scholar]
- 26. Egan BM, Shaftman SR, Wagner CS, Bandyopadhyay D, Szymanski KA. Demographic differences in the treatment and control of glucose in type 2 diabetic patients: implications for health care practice. Ethn Dis. 2012;22:29–37. [PubMed] [Google Scholar]
- 27. Frayne SM, Holmes TH, Berg E, et al. Mental illness and intensification of diabetes medications: an observational cohort study. BMC Health Serv Res. 2014;14:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fu AZ, Qiu Y, Davies MJ, Radican L, Engel SS. Treatment intensification in patients with type 2 diabetes who failed metformin monotherapy. Diabetes Obes Metab. 2011;13:765–769. [DOI] [PubMed] [Google Scholar]
- 29. Fu AZ, Sheehan J. Treatment intensification for patients with type 2 diabetes and poor glycemic control. Diabetes Obes Metab. 2016;10:10. [DOI] [PubMed] [Google Scholar]
- 30. Gonzalez‐Clemente JM, Font B, Lahoz R, Llaurado G, Gambus G. INERTIA study: clinical inertia in non‐insulinized patients on oral hypoglycemic treatment. A study in Spanish primary and specialty care settings. Med Clin (Barc). 2014;142:478–484. [DOI] [PubMed] [Google Scholar]
- 31. Grant R, Adams AS, Trinacty CM, et al. Relationship between patient medication adherence and subsequent clinical inertia in type 2 diabetes glycemic management. Diabetes Care. 2007;30:807–812. [DOI] [PubMed] [Google Scholar]
- 32. Grant RW, Cagliero E, Dubey AK, et al. Clinical inertia in the management of Type 2 diabetes metabolic risk factors. Diabet Med. 2004;21:150–155. [DOI] [PubMed] [Google Scholar]
- 33. Griffith ML, Boord JB, Eden SK, Matheny ME. Clinical inertia of discharge planning among patients with poorly controlled diabetes mellitus. J Clin Endocrinol Metab. 2012;97:2019–2026. [DOI] [PubMed] [Google Scholar]
- 34. Halimi S, Balkau B, Attali C, Detournay B, Amelineau E, Blickle JF. Therapeutic management of orally treated type 2 diabetic patients, by French general practitioners in 2010: the DIAttitude Study. Diabetes Metab. 2012;38(suppl 3):S36–S46. [DOI] [PubMed] [Google Scholar]
- 35. Huang LY, Shau WY, Yeh HL, et al. A model measuring therapeutic inertia and the associated factors among diabetes patients: a nationwide population‐based study in Taiwan. J Clin Pharmacol. 2015;55:17–24. [DOI] [PubMed] [Google Scholar]
- 36. Hugie C, Waterbury NV, Alexander B, Shaw RF, Egge JA. Adding glucose‐lowering agents delays insulin initiation and prolongs hyperglycemia. Am J Manag Care. 2016;22:e134–e140. [PubMed] [Google Scholar]
- 37. Katon W, Russo J, Lin EH, et al. Diabetes and poor disease control: is comorbid depression associated with poor medication adherence or lack of treatment intensification? Psychosom Med. 2009;71:965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kristensen JK, Stoevring H. A follow‐up study of the occurrence and consequences of HbA1c measurements in an unselected cohort of non‐pharmacologically treated patients with type 2 diabetes. Scand J Prim Health Care. 2008;26:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lang VB, Markovic BB, Kranjcevic K. Family physician clinical inertia in glycemic control among patients with type 2 diabetes. Med Sci Monit. 2015;21:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lian J, Liang Y. Diabetes management in the real world and the impact of adherence to guideline recommendations. Curr Med Res Opin. 2014;30:2233–2240. [DOI] [PubMed] [Google Scholar]
- 43. Lin J, Zhou S, Wei W, Pan C, Lingohr‐Smith M, Levin P. Does clinical inertia vary by personalized A1c goal? A study of predictors and prevalence of clinical inertias in a US managed care setting. Endocr Pract. 2015;22:151–161. [DOI] [PubMed] [Google Scholar]
- 44. Lopez‐Simarro F, Brotons C, Moral I, et al. Inertia and treatment compliance in patients with type 2 diabetes in primary care. Med Clin (Barc). 2012;138:377–384. [DOI] [PubMed] [Google Scholar]
- 45. Mata‐Cases M, Benito‐Badorrey B, Roura‐Olmeda P, et al. Clinical inertia in the treatment of hyperglycemia in type 2 diabetes patients in primary care. Curr Med Res Opin. 2013;29:1495–1502. [DOI] [PubMed] [Google Scholar]
- 46. McEwen LN, Bilik D, Johnson SL, et al. Predictors and impact of intensification of antihyperglycemic therapy in type 2 diabetes: translating research into action for diabetes (TRIAD). Diabetes Care. 2009;32:971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Osataphan S, Chalermchai T, Ngaosuwan K. Clinical inertia causing new or progression of diabetic retinopathy in type 2 diabetes: a retrospective cohort study. J Diabetes. 2017;9:267–274. [DOI] [PubMed] [Google Scholar]
- 48. Parchman ML, Pugh JA, Romero RL, Bowers KW. Competing demands or clinical inertia: the case of elevated glycosylated hemoglobin. Ann Fam Med. 2007;5:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parnes BL, Main DS, Dickinson LM, et al. Clinical decisions regarding HbA1c results in primary care: a report from CaReNet and HPRN. Diabetes Care. 2004;27:13–16. [DOI] [PubMed] [Google Scholar]
- 50. Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rajpathak SN, Rajgopalan S, Engel SS. Impact of time to treatment intensification on glycemic goal attainment among patients with type 2 diabetes failing metformin monotherapy. J Diabetes Complications. 2014;28:831–835. [DOI] [PubMed] [Google Scholar]
- 52. Reed M, Huang J, Graetz I, et al. Outpatient electronic health records and the clinical care and outcomes of patients with diabetes mellitus. Ann Intern Med. 2012;157:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reutens AT, Hutchinson R, Van Binh T, et al. The GIANT study, a cluster‐randomised controlled trial of efficacy of education of doctors about type 2 diabetes mellitus management guidelines in primary care practice. Diabetes Res Clin Pract. 2012;98:38–45. [DOI] [PubMed] [Google Scholar]
- 54. Rubino A, McQuay LJ, Gough SC, Kvasz M, Tennis P. Delayed initiation of subcutaneous insulin therapy after failure of oral glucose‐lowering agents in patients with type 2 diabetes: a population‐based analysis in the UK. Diabet Med. 2007;24:1412–1418. [DOI] [PubMed] [Google Scholar]
- 55. Schmittdiel JA, Uratsu CS, Karter AJ, et al. Why don't diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J Gen Intern Med. 2008;23:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schwab P, Saundankar V, Bouchard J, et al. Early treatment revisions by addition or switch for type 2 diabetes: impact on glycemic control, diabetic complications, and healthcare costs. BMJ Open Diabetes Res Care. 2016;4:e000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Selby JV, Uratsu CS, Fireman B, et al. Treatment intensification and risk factor control toward more clinically relevant quality measures. Med Care. 2009;47:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shah BR, Hux JE, Laupacis A, Zinman B, Van Walraven C. Clinical inertia in response to inadequate glycemic control: do specialists differ from primary care physicians? Diabetes Care. 2005;28:600–606. [DOI] [PubMed] [Google Scholar]
- 59. Sidorenkov G, Haaijer‐Ruskamp FM, de Zeeuw D, Denig P. A longitudinal study examining adherence to guidelines in diabetes care according to different definitions of adequacy and timeliness. PLoS One. 2011;6:e24278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sidorenkov G, Voorham J, de Zeeuw D, Haaijer‐Ruskamp FM, Denig P. Do treatment quality indicators predict cardiovascular outcomes in patients with diabetes? PLoS One. 2013;8:e78821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sidorenkov G, Voorham J, Haaijer‐Ruskamp FM, De Zeeuw D, Denig P. Association between performance measures and glycemic control among patients with diabetes in a community‐wide primary care cohort. Med Care. 2013;51:172–179. [DOI] [PubMed] [Google Scholar]
- 62. Tunceli K, Goldshtein I, Yu S, et al. Adherence to treatment guidelines in type 2 diabetes patients failing metformin monotherapy in a real‐world setting. Diabetes Manag. 2015;5:17–24. [Google Scholar]
- 63. van Bruggen R, Gorter K, Stolk R, Klungel O, Rutten G. Clinical inertia in general practice: widespread and related to the outcome of diabetes care. Fam Pract. 2009;26:428–436. [DOI] [PubMed] [Google Scholar]
- 64. Voorham J, Haaijer‐Ruskamp FM, Wolffenbuttel BHR, de Zeeuw D, Stolk RP, Denig P. Differential effects of comorbidity on antihypertensive and glucose‐regulating treatment in diabetes mellitus – a cohort study. PLoS One. 2012;7:e38707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Watson L, Das R, Farquhar R, Langerman H, Barnett AH. Consequences of delaying treatment intensification in type 2 diabetes: evidence from a UK database. Curr Med Res Opin. 2016;32:1465–1475. [DOI] [PubMed] [Google Scholar]
- 66. Yu S, Schwab P, Bian B, Radican L, Tunceli K. Use of add‐on treatment to metformin monotherapy for patients with type 2 diabetes and suboptimal glycemic control: a US database study. J Manag Care Spec Pharm. 2016;22:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ziemer DC, Doyle JP, Barnes CS, et al. An intervention to overcome clinical inertia and improve diabetes mellitus control in a primary care setting: improving primary Care of African Americans with diabetes (IPCAAD) 8. Arch Intern Med. 2006;166:507–513. [DOI] [PubMed] [Google Scholar]
- 68. Ziemer DC, Miller CD, Rhee MK, et al. Clinical inertia contributes to poor diabetes control in a primary care setting. Diabetes Educ. 2005;31:564–571. [DOI] [PubMed] [Google Scholar]
- 69. Zografou I, Strachan M, McKnight J. Delay in starting insulin after failure of other treatments in patients with type 2 diabetes mellitus. Hippokratia. 2014;18:306–309. [PMC free article] [PubMed] [Google Scholar]
- 70. Ismail‐Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011;154:554–559. [DOI] [PubMed] [Google Scholar]
- 71. Karter AJ, Subramanian U, Saha C, et al. Barriers to insulin initiation: the translating research into action for diabetes insulin starts project. Diabetes Care. 2010;33:733–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Escalada J, Orozco‐Beltran D, Morillas C, et al. Attitudes towards insulin initiation in type 2 diabetes patients among healthcare providers: a survey research. Diabetes Res Clin Pract. 2016;122:46–53. [DOI] [PubMed] [Google Scholar]
- 73. Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross‐national diabetes attitudes, wishes, and needs (DAWN) study. Diabetes Care. 2005;28:2673–2679. [DOI] [PubMed] [Google Scholar]
- 74. Nakar S, Yitzhaki G, Rosenberg R, Vinker S. Transition to insulin in type 2 diabetes: family physicians' misconception of patients' fears contributes to existing barriers. J Diabetes Complications. 2007;21:220–226. [DOI] [PubMed] [Google Scholar]
- 75. International Diabetes Federation . IDF Diabetes Atlas – 7th Edition [article online]. 2015. http://www.diabetesatlas.org/. Accessed June 6, 2017.
- 76. Nichols GA, Rosales AG, Perrin NA, Fortmann SP. The association between different A1C‐based measures of glycemia and risk of cardiovascular disease hospitalization. Diabetes Care. 2014;37:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dekker RG II, Qin C, Ho BS, Kadakia AR. The effect of cumulative glycemic burden on the incidence of diabetic foot disease. J Orthop Surg Res. 2016;11(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Low S, Lim SC, Yeoh LY, et al. The effect of long‐term glycemic variability on estimated glomerular filtration rate decline among patients with type 2 diabetes mellitus – insights from the Diabetic Nephropathy Cohort in Singapore. J Diabetes. 2016; Dec 9. https://doi.org/10.1111/1753–0407.12512. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 79. Anjana RM, Shanthirani CS, Unnikrishnan R, et al. Regularity of follow‐up, glycemic burden, and risk of microvascular complications in patients with type 2 diabetes: a 9‐year follow‐up study. Acta Diabetol. 2015;52:601–609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search strings used in MEDLINE.
Table S2. Search strings used in Embase.
Table S3. Summary of study characteristics.


