Abstract
Irritable bowel syndrome (IBS) is a common disorder in gastrointestinal system and impairs the quality of life of the patients. Clostridium butyricum (CB) is a probiotics that has been used in several gastrointestinal diseases. The efficacy of CB in treating IBS is still unknown. This prospective, multi-centre, randomized, double-blind, placebo-controlled trial aimed to assess the efficacy and safety of CB in treating diarrhea-predominant IBS (IBS-D) and analyze the fecal microbiota after treatment. Two hundred patients with IBS-D were recruited and were given CB or placebo for 4 weeks. End points included change from baseline in IBS symptoms, quality of life, stool consistency and frequency. Compared with placebo, CB is effective in improving the overall IBS-D symptoms (−62.12 ± 74.00 vs. −40.74 ± 63.67, P = 0.038) as well as quality of life (7.232 ± 14.06 vs. 3.159 ± 11.73, P = 0.032) and stool frequency (−1.602 ± 1.416 vs. −1.086 ± 1.644, P = 0.035). The responder rates are found higher in CB compared with the placebo (44.76% vs. 30.53%, P = 0.042). The change in fecal microbiota was analyzed and function pathways of CB in treating IBS-D were predicted. In conclusion, CB improves overall symptoms, quality of life and stool frequency in IBS-D patients and is considered to be used as a probiotics in treating IBS-D clinically.
Introduction
Irritable bowel syndrome (IBS) is one of the most common gastrointestinal disorders characterized by abdominal pain or discomfort associated with defecation and change in bowel habit1. IBS effects 15–20% of the population especially in industrial nations and may impair social and personal functions and effect the quality of life of the patients2,3. IBS is classified into 4 subtypes based on the symptoms and diarrhea-predominant IBS (IBS-D) is more prevalent in a community-based data4. The concise etiology and pathophysiology of IBS remain unknown, while some factors involve in this process such as gastrointestinal motility, visceral hypersensitivity, psychosocial factors, immune activation and also intestinal microbiota alteration5,6. An increase of Firmicutes-associated taxa, a depletion of Bacteroidetes-related taxa and a significantly lower biodiversity of microbes happen in the intestinal microbiota of IBS patient7,8. In this way, the improvement of the composition of the intestinal microbiota becomes to the target of treating IBS.
Probiotics are medications that can supplement the intestinal microbiota and improve microbiota characteristics9. Several researches prove that probiotics can promote IBS symptoms and quality of life10–12. The effects of probiotics include improvement of mucosal barrier function, promoting visceral hypersensitivity, effect on gastrointestinal motility and regulation of immune responses13–16. Clostridium butyricum (CB) is a butyric acid-producing Gram-positive anaerobe which exists in the intestine of humans and has been clinically used in several diseases such as inflammatory bowel disease (IBD) and antimicrobial-associated diarrhea17–19. We propose that the protective function of CB may exist in treating IBS-D. In this study, we aimed to assess the efficacy and safety of CB in IBS-D patients in a multi-centre, randomized, double-blind, placebo-controlled trial and the potential function of CB based on intestinal microbiota.
Methods
Participants
Male and female outpatients aged 18–65 years who were diagnosed with IBS-D were recruited in three centers in Shandong Province, China. The aim of setting upper age limit was to minimize the number of patients with age-related organic diseases that can lead to IBS-like symptoms. The diagnosis of IBS-D was according to Rome III criteria. Examinations within 3 months were negative including whole blood count, blood chemistry, stool routine, colonoscopy and barium enema examination. Exclusion criteria included other organic gastrointestinal diseases (inflammatory bowel diseases, celiac disease, gastrointestinal infection, gastrointestinal tumor, lactose intolerance, etc); organic diseases (hepatic, renal or cardiac dysfunction, diabetes mellitus, tumor, etc); long-term use of antipsychotics or systemic corticosteroids; the use of antibiotics, probiotics, laxative and other medications that may influence bowel movement for the 4 weeks prior to the study; the examination of colonoscopy and barium enema or the history of acute gastroenteritis in 2 weeks prior to the study; pregnancy or lactation.
Study design
This was a prospective, multi-centre, randomized, double-blind, placebo-controlled trial designed to investigate the efficacy and safety of the CB in the treatment of IBS-D. The trial took place at Qilu Hospital of Shandong University, Taian City Central Hospital and Linyi People’s Hospital between December 2015 and November 2016. The study was carried out in accordance with the Declaration of Helsinki, registered on clinicaltrials.gov (NCT02614963) and was approved by the ethics committees in Shandong University affiliated Qilu Hospital. The trial design was performed according to the CONSORT statement.
All of the patients who fulfilled the inclusion received informed consent before the study. The patients recorded their basic information, symptoms, IBS symptom severity scale (IBS-SSS)20, IBS quality of life (IBS-QOL)21 score, stool consistency and frequency from questionnaires and provided a stool sample before divided randomly into CB and placebo groups. The CB capsules (ATaiNing, Qingdao Eastsea Pharmaceutical Co., Ltd., 420 mg per capsule, 1.5 × 107 colony forming units (CFU)/g) were provided in treatment group. The placebo capsule had a same shape, taste and packaging with the CB capsule. The medications were labeled by random numbers based on CB or placebo and were allotted to patients randomly. All patients and researchers were blinded for CB or placebo during the trial. All of the patients in CB and placebo groups took 3 capsules 3 times a day for 4 weeks. At the time of discontinuation, the patients visited the clinic at the end of week 4 and recorded their symptoms, IBS-SSS, IBS-QOL score, stool consistency and frequency and adverse events from questionnaires and provided a stool sample. All stool samples were collected for a further analysis using 16 s rRNA pyrosequencing and metagenome sequencing analysis. Other probiotics and medications that might influence the results of the study were not allowed during the whole trial. All patients and investigators were blinded until the study finish.
Clinical Outcome Assessments
The primary endpoint of our study was the difference in change of IBS symptoms between the two groups as measured by the IBS-SSS20 from baseline to week 4. The IBS-SSS contained 5 questions to assess the IBS symptom from 4 aspects: abdominal pain (degree and frequency), bloating, satisfaction with bowel habit and overall interference with QOL. The IBS-SSS total score ranged from 0 to 500 points with 100 points each question. A higher score indicated a more severe condition. Total IBS-SSS score below 175 points represented mild IBS, 175–300 points represented moderate severity and score above 300 points represented severe IBS. A reduction of ≥50 points of total IBS-SSS score was defined as response to treatment20.
Secondary endpoints included the difference in changes of IBS-QOL21 scores, Bristol stool scale22 and stool frequency from baseline to week 4. The IBS-QOL score ranged from 0 to 100 points and a higher score indicated a better QOL. The score contained 34 questions from 8 aspects: dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual concern and relationship. Stool consistency was assessed by the 7-point Bristol stool scale, a higher score indicating a softer stool. Stool frequency was defined as stools per day.
Stool sample storage and DNA extraction
Stool samples were collected from IBS-D patients in CB and placebo groups before (baseline) and after (week 4) treatment. All of the stool samples were immediately stored at −80 °C and were transferred to Majorbio (Shanghai, China) where the total DNA was extracted and tested according to the standardized protocol23 for further analysis.
16 s rRNA pyrosequencing and metagenome sequencing analysis
16 s rRNA pyrosequencing was processed at Majorbio (Shanghai, China) by using Illumina Miseq system. Similar sequences were clustered into the same operational taxonomy unit (OTU) with a 97% sequence identity. The Chao index, Sobs index and Shannon index were calculated to assess the alpha-diversity in each sample. The cluster analysis based on the Euclidean distance was conducted based on the relative abundances of all OTUs. The principal co-ordinates analysis (PCoA) based on the Braycurtis distance was performed to assess the beta-diversity.
The metagenome sequencing was performed and analyzed at Majorbio (Shanghai, China) by using Illumina Hiseq system. The raw sequences were decoded, denoised, trimmed and then assembled for gene prediction by MetaGene (http://metagene.cb.k.u-tokyo.ac.jp/). The predicted genes were clustered (95% identity, 90% coverage) by CD-HIT (http://www.bioinformatics.org/cd-hit/) into a non-redundant gene catalog. EggNOG (evolutionary genealogy of genes: Non-supervised Orthologous Groups, http://eggnog.embl.de/) and KEGG (Kyoto Encyclopedia of Genes and Genomes, http://www.genome.jp/kegg/) databases were used to predict gene function. The LEfSe (Linear discriminant analysis Effect Size) was conducted to indicate the significant factors between the groups. The STAMP analysis was used to assess function richness between groups. The whole metabolic pathways were mapped by iPath2.0 (http://pathways.embl.de).
Statistical analysis
The sample size calculation was based on the response rate defined as a reduction of ≥50 points of total IBS-SSS score (35% in the placebo group, 60% in the CB group). The size of the sample was 79 per group with the power of 90%, α = 5%. The sample size was also calculated by the change of IBS-SSS score with the power of 80% and α = 5% and the result was smaller than prior calculation. As a result, we planned to enroll a total of 190 patients to allow for a 20% of drop-out.
Clinical data analyses were performed by GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA) and R 3.1.1 was used in microbiota analysis. The clinical data was analyzed by one researcher (Yi-Yuan Sun) and the analysis of sequencing data was performed by four researchers (Yi-Yuan Sun, Li-Xiang Li, Zhen Li and Ming Li).Missing data were not imputed in the end points. For responder rate, patients with missing data in IBS symptoms were considered to be non-responders. An intention-to-treat analysis was used in responder rate calculation and per-protocol analysis in symptom analysis. The chi-squared analysis was used in proportion data. Symptom differences between groups were tested by nonparametric Wilcoxon test. Data with normal distribution was determined by the Student’s t-test. For microbiota analysis, Wilcoxon test was used in diversity index calculation. Fisher’s exact test was performed in cluster analysis. Different OTUs and PCoA analysis between groups was determined by Kruskal-Wallis test. Gene function differences were tested by ANOSIM analysis. Welch’s t-test was used in STAMP analysis. All P-values were two-sided and were considered as statistical significance below 0.05.
Results
Subjects and baseline characteristics
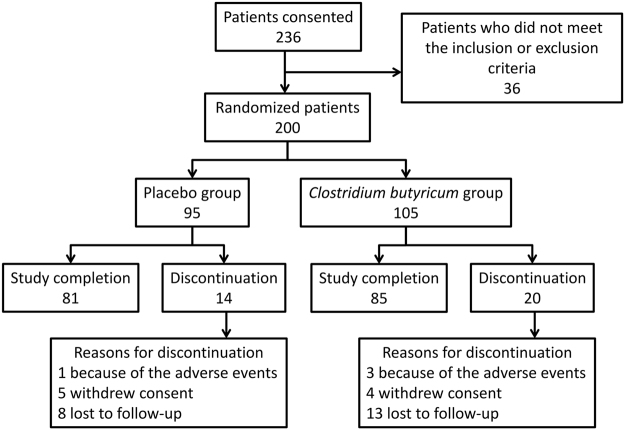
A total of 236 patients were screened in the study and 200 patients met inclusion criteria and were randomized to receive either placebo (N = 95) or CB (N = 105). Thirty-four (17.0%) patients did not complete the study: 4 (1 in the placebo group, 3 in the CB group) because of the adverse events, 9 (5 in the placebo group, 4 in the CB group) who withdrew consent and 21 (8 in the placebo group, 13 in the CB group) who were lost to follow-up. A total of 166 patients finished the study, with 81 in placebo group and 85 in CB group, respectively (Fig. 1).
Figure 1.
Flow chart of this study. Reasons for discontinuation are shown.
In terms of baseline characteristics, demographics were well balanced between the placebo group and the CB group. IBS-SSS (232.4 ± 66.09 vs. 243.8 ± 87.93) and IBS-QOL scores (82.66 ± 16.36 vs. 78.90 ± 19.47) showed no significant differences between the placebo group and the CB group in baseline (Table 1).
Table 1.
Demographics and Baseline Characteristics of the patients.
| Placebo group (n = 95) | Clostridium butyricum group (n = 105) | |
|---|---|---|
| Age [mean (SD)] | 44.91 (13.01) | 43.00 (12.45) |
| Sex | ||
| Female (%) | 42 (44.21%) | 42 (40.00%) |
| Male (%) | 53 (55.79%) | 63 (60.00%) |
| Baseline IBS-SSS [mean (SD)] | 232.4 (66.09) | 243.8 (87.93) |
| Baseline IBS-QOL score [mean (SD)] | 82.66 (16.36) | 78.90 (19.47) |
Assessment of IBS symptoms
The primary endpoint was the reduction of IBS-SSS from baseline to week 4. There was a significant reduction of IBS-SSS from baseline to week 4 in the CB group compared with that in the placebo group (−62.12 ± 74.00 vs. −40.74 ± 63.67, P = 0.038) (Table 2). This change in IBS-SSS indicated the improvement of IBS symptoms in the CB group compared with the placebo group. The changes in individual component scores for IBS-SSS were also analyzed. There was a significant reduction in the component scores for bowel habit (−20.71 ± 22.10 vs. −12.84 ± 21.48, P = 0.014) and QOL satisfaction (−13.18 ± 19.41 vs. −6.667 ± 13.78, P = 0.018) in the CB group compared with that in the placebo group. However, there were no significant differences in the component scores for pain (−22.59 ± 36.62 vs. −16.54 ± 37.12, P = 0.276) and bloating (−5.647 ± 19.18 vs. −4.691 ± 16.21, P = 0.485) between patients taking CB and placebo. The responder rate was assessed by a reduction of IBS-SSS ≥50 points. A higher overall responder rate was found in the CB group compared with that in the placebo group (44.76% vs. 30.53%, P = 0.042) by an ITT analysis (Fig. 2). For the patients with moderate to severe symptoms (IBS-SSS >175 points), the responder rate in the CB group was significantly higher than that in the placebo group (54.22% vs. 32.91%, P = 0.007). The result showed a significant efficacy of CB in treating IBS-D especially for the patients with moderate to severe symptoms.
Table 2.
IBS-SSS scores: baseline to week 4.
| IBS-SSS [Mean (SD)] and change from baseline [Mean (SD)] | |||||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 4 | Change from baseline | |||||
| Placebo | CB | Placebo | CB | Placebo | CB | P value | |
| IBS-SSS total | 230.4 (67.16) | 228.5 (83.63) | 189.6 (70.36) | 166.4 (66.56) | −40.74 (63.67) | −62.12 (74.00) | 0.038 |
| Pain | 95.31 (37.98) | 92.00 (41.31) | 78.77 (37.86) | 69.41 (35.13) | −16.54 (37.12) | −22.59 (36.62) | 0.276 |
| Bloating | 35.31 (13.57) | 35.53 (19.35) | 30.62 (14.17) | 29.88 (15.62) | −4.691 (16.21) | −5.647 (19.18) | 0.485 |
| Bowel habit | 55.56 (19.49) | 53.88 (20.94) | 42.73 (19.43) | 33.18 (18.52) | −12.84 (21.48) | −20.71 (22.10) | 0.014 |
| QOL satisfaction | 44.20 (16.95) | 47.06 (19.93) | 37.53 (15.29) | 33.88 (16.04) | −6.667 (13.78) | −13.18 (19.41) | 0.018 |
Figure 2.
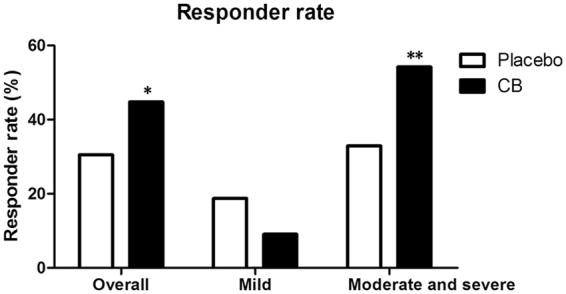
Responder rate after treatment in placebo and Clostridium butyricum (CB) groups. The overall responder rate was higher in the CB group compared with that in the placebo group (P = 0.042). The responder rate of patients with moderate to severe symptoms in the CB group was significantly higher than that in the placebo group (P = 0.007). *P < 0.05, **P < 0.01.
Secondary endpoints
Secondary endpoints included the changes of IBS-QOL scores, Bristol stool scale and stool frequency from baseline to week 4. Significant improvement in change of overall IBS-QOL score was observed in the CB group compared with the placebo group (7.232 ± 14.06 vs. 3.159 ± 11.73, P = 0.032) (Table 3). Furthermore, there was a significant improvement in change of interference with activity (8.866 ± 15.81 vs. 2.822 ± 13.34, P = 0.003) and health worry (12.25 ± 18.03 vs. 2.469 ± 16.43, P < 0.001), but not dysphoria, body image, food avoidance, social reaction, sexual concerns, relationship (8.934 ± 17.67 vs. 5.980 ± 16.22, P = 0.135; 3.603 ± 14.12 vs. 0.7716 ± 14.02, P = 0.072; 7.451 ± 22.49 vs. 4.630 ± 20.28, P = 0.381; 4.853 ± 16.38 vs. 0.6173 ± 10.44, P = 0.074; 5.147 ± 17.07 vs. 1.080 ± 13.29, P = 0.119; 3.039 ± 13.96 vs. 3.601 ± 13.88, P = 0.751, respectively) in the CB group compared with the placebo group.
Table 3.
IBS-QOL scores: baseline to week 4.
| IBS-QOL [Mean (SD)] and change from baseline [Mean (SD)] | |||||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 4 | Change from baseline | |||||
| Placebo | CB | Placebo | CB | Placebo | CB | P value | |
| QOL overall | 82.88 (16.14) | 81.28 (18.85) | 86.04 (13.74) | 88.51 (14.40) | 3.159 (11.73) | 7.232 (14.06) | 0.032 |
| Dysphoria | 77.85 (23.08) | 79.34 (23.88) | 83.83 (18.64) | 88.27 (19.06) | 5.980 (16.22) | 8.934 (17.67) | 0.135 |
| Activity interference | 85.14 (17.83) | 80.71 (20.86) | 87.96 (13.81) | 89.58 (14.86) | 2.822 (13.34) | 8.866 (15.81) | 0.003 |
| Body image | 91.20 (15.99) | 90.00 (16.49) | 91.98 (13.08) | 93.60 (11.33) | 0.7716 (14.02) | 3.603 (14.12) | 0.072 |
| Health worry | 77.26 (20.83) | 72.16 (23.34) | 79.73 (20.49) | 84.41 (20.32) | 2.469 (16.43) | 12.25 (18.03) | 0.000 |
| Food avoidance | 68.31 (22.65) | 65.39 (22.14) | 72.94 (21.02) | 72.84 (19.89) | 4.630 (20.28) | 7.451 (22.49) | 0.381 |
| Social reaction | 87.35 (17.76) | 86.84 (20.00) | 87.96 (17.22) | 91.69 (14.61) | 0.6173 (10.44) | 4.853 (16.38) | 0.074 |
| Sexual | 90.28 (21.01) | 86.62 (24.15) | 91.36 (18.50) | 91.76 (15.97) | 1.080 (13.29) | 5.147 (17.07) | 0.119 |
| Relationship | 89.20 (19.38) | 90.20 (16.82) | 92.80 (15.91) | 93.24 (12.76) | 3.601 (13.88) | 3.039 (13.96) | 0.751 |
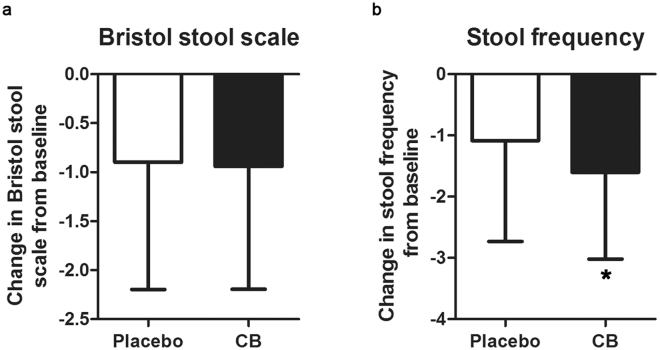
A total of 164 patients (80 in the placebo group, 84 in the CB group) assessed Bristol stool scale and 124 patients (58 in the placebo group, 66 in the CB group) assessed stool frequency (stools per day) at baseline and week 4 (Fig. 3). No significant difference was found in change of Bristol stool scale from baseline to week 4 between the CB group and the placebo group (−1.012 ± 1.078 vs. −0.9000 ± 1.296, P = 0.259). However, the improvement of stool frequency from baseline to week 4 was significantly superior in the CB group than in the placebo group (−1.602 ± 1.416 vs. −1.086 ± 1.644, P = 0.035).
Figure 3.
The improvement of stool consistency and frequency in placebo and Clostridium butyricum (CB) groups. (a) No significant difference was found in change of Bristol stool scale from baseline to week 4 between the CB group and the placebo group (P = 0.259). (b) The reduction of stool frequency in CB group was significantly superior than that in the placebo group (P = 0.035). *P < 0.05.
Safety
The patients took medications as we had informed. Only 8 adverse events were reported in this study, 2 (2.11%) in the placebo group and 6 (5.71%) in the CB group. Among these adverse events, 6 were worse abdominal pain (2 in the placebo group, 4 in the CB group), 1 was bloating in the CB group and 1 was hyperactive bowel sound in the CB group. No severe adverse events have been recorded in either group.
16 s rRNA pyrosequencing analysis of stool samples
A total of 200 stool samples from baseline and week 4 of 100 patients (42 in the placebo group, 58 in the CB group) were analyzed in this study using 16 s rRNA pyrosequencing. The demographics of the placebo group and the CB group were shown in Supplementary Table 1. No significant differences were found between two groups.
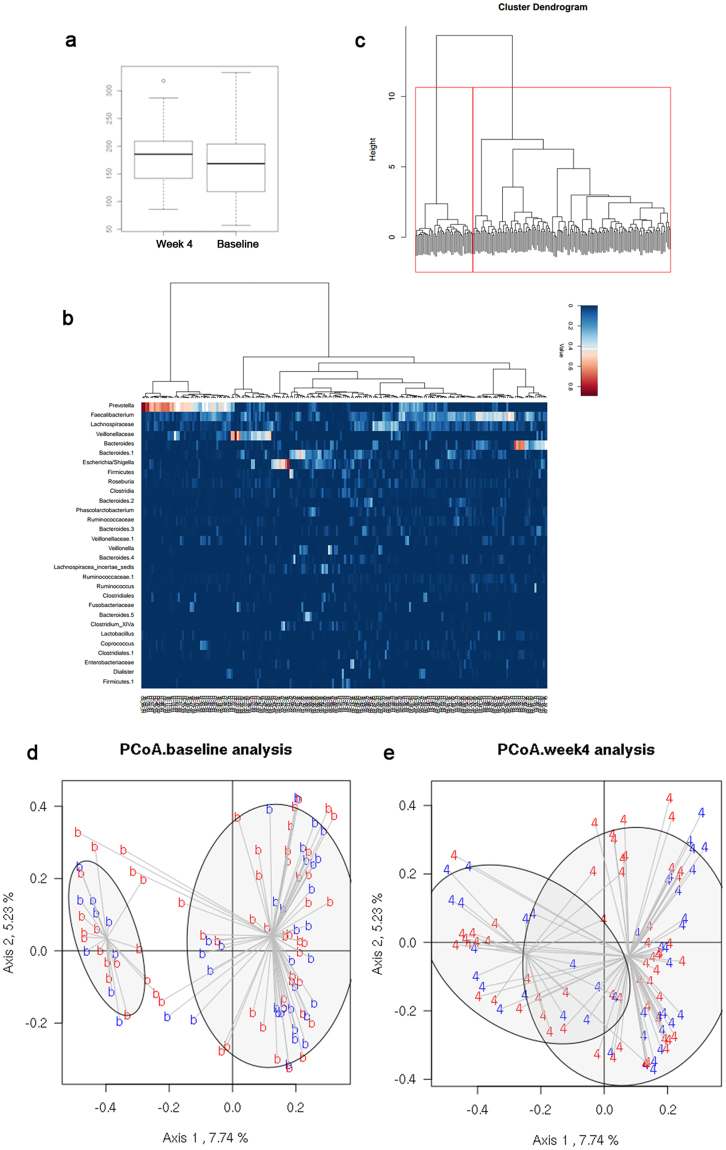
A total of 7,406,811 sequences were finally analyzed and were clustered into 797 OTUs. We compared microbial diversity between baseline and week 4 levels with Chao, Sobs and Shannon index. No significant differences were found in both two groups. Sobs index showed an increased tendency after treating with CB (P = 0.063), indicating a potential function of CB in increasing microbial community richness (Fig. 4a).
Figure 4.
16 s rRNA pyrosequencing analysis of stool samples. (a) Wilcoxon test of Sob index showed an increased tendency after treating with Clostridium butyricum (CB) (P = 0.063). (b) Heat-map plot with 30 most abundant OTUs in all stool samples. (c) Two clusters were observed using Fisher’s exact test. (d,e) The PCoA plot of placebo and CB groups in baseline and week 4.
To evaluate the whole microbial community in all stool samples, we plotted the 30 most abundant OTUs in a heat-map plot (Fig. 4b). Prevotella, Faecalibacterium, Lachnospiraceae, Veillonellaceae and Bacteroides were the most abundant OTUs in most stool samples. A cluster analysis was performed to determine whether the microbiota was clustered and two clusters were found based on the abundance of all of the variable taxa (Fig. 4c). There were no significant differences between two clusters in both baseline (P = 0.644) and week 4 (P = 0.805) level. We then evaluated the microbial community in placebo group and CB group at baseline and week 4. Parsimony test showed a significant difference between two groups at week 4 (P = 0.016). To confirm this result, a PcoA analysis was performed (Fig. 4d,e). The microbial community was similar in two groups at baseline but a significant difference was found at week 4 (χ2 = 7.006, df = 1, P = 0.008).
We further analyzed the microbiota differences between placebo group and CB group in OTU level. 187 and 137 discriminating OTUS were found between two groups at baseline and week 4 respectively. 45 significantly changed OTUs from baseline to week 4 were found between two groups (Supplementary Table 2). These OTUs might indicate the change of intestinal microbiota during the treatment of CB. Clostridium sensu stricto was one of the most significantly changed OTU between two groups. No significance was observed at baseline between placebo group and CB group (0.0006118 ± 0.001396 vs. 0.001860 ± 0.004609, P = 0.572) but a significant reduction of Clostridium sensu stricto was existed in CB group (−0.0007532 ± 0.005059 vs. 0.01950 ± 0.007487, P = 0.023). We then analyzed different OTUs between responder and non-responder in CB group. There was a tendency of more Clostridium sensu stricto in responder at baseline (0.003260 ± 0.005983 vs. 0.0007221 ± 0.002685, P = 0.057) and a significant reduction of Clostridium sensu stricto after treating with CB in responder compared with non-responder (−0.002445 ± 0.006260 vs. 0.0006213 ± 0.003333, P < 0.001).
Function prediction of CB in treating IBS-D by metagenomic analysis
Fifty-two stool samples from baseline and week 4 of 26 patients (13 per group) were analyzed via metagenome sequencing. The samples of two groups were well paired. The demographics of the placebo group and the CB group were shown in Supplementary Table 3 and no significant differences were found between two groups.
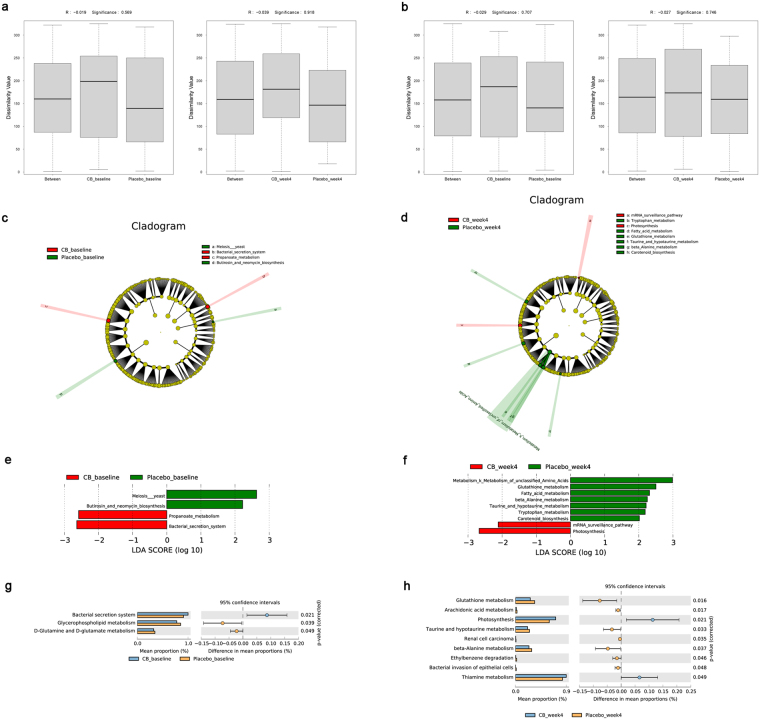
A total of 13,716,343 genes were tested and finally 2,696,668 genes were cataloged into a non-redundant gene catalog for further analysis. The eggNOG and KEGG annotation were used for function prediction of CB. We identified 17,454 eggNOG orthologues and 5,971 KEGG orthologues totally. However, no significant differences were observed between placebo group and CB groups in baseline and week 4 for eggNOG (P = 0.569 in baseline, P = 0.918 in week 4) and KEGG (P = 0.707 in baseline, P = 0.746 in week 4) analysis (Fig. 5a,b).
Figure 5.
Metagenomic analysis of stool samples. (a) ANOSIM analysis of eggNOG between placebo and Clostridium butyricum (CB) groups at baseline and week 4. (b) ANOSIM analysis of KEGG between placebo and CB groups at baseline and week 4. No significant differences were observed between two groups in baseline and week 4 for eggNOG (P = 0.569 in baseline, P = 0.918 in week 4) and KEGG (P = 0.707 in baseline, P = 0.746 in week 4) analysis. (c,d) LEfSe analysis of KEGG pathways between placebo and CB groups at baseline and week 4. (e,f) LDA score of LEfSe analysis at baseline and week 4. (g,h) STAMP analysis of KEGG pathways between placebo and CB groups at baseline and week 4.
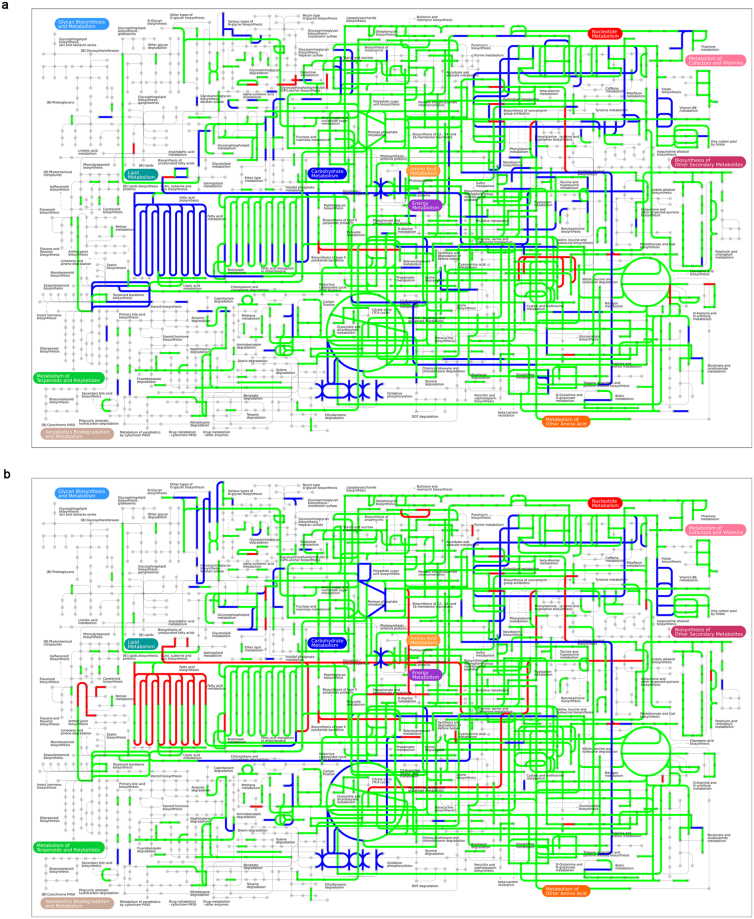
Further we focused on the specific eggNOG orthologues and KEGG pathways between placebo and CB groups. No significant differences were shown between two groups. However, several KEGG pathways were included to distinguish two groups after treatment using the LEfSe method (Fig. 5c–f). The CB group was associated with mRNA surveillance pathway and photosynthesis and the placebo group was more correlated with pathways such as amino acid metabolism, glutathione metabolism, fatty acid metabolism, beta-alanine metabolism, taurine and hypotaurine metabolism, tryptophan metabolism and carotenoid biosynthesis. Function richness was assessed by STAMP analysis (Fig. 5g,h). The CB group was enriched in photosynthesis and thiamine metabolism; however the placebo group was enriched in pathways including glutathione metabolism, arachidonic acid metabolism, taurine and hypotaurine metabolism, beta-alanine metabolism, bacterial invasion of epithelial cells, etc. The whole metabolic pathways of CB and placebo were shown in Fig. 6. All above analysis may demonstrate the potential mechanisms of CB in treating IBS-D.
Figure 6.
The whole metabolic pathways of Clostridium butyricum (CB) and placebo via iPath. The blue lines were metabolic pathways dominant in CB group. The red lines were metabolic pathways dominant in placebo group. The green lines were co-pathways in both groups. (a) The metabolic pathways of two groups at baseline. (b) The metabolic pathways of two groups at week 4.
Discussion
This prospective, multi-centre, randomized, double-blind, placebo-controlled study indicates that CB has advantages in treating IBS-D. In this study, CB promotes the overall IBS symptoms based on bowel habit and quality of life compared with placebo. Interference with activity, health worry and stool frequency are main improvements in bowel habit and quality of life. The responder rates are found higher in CB group compared with the placebo group especially in moderate and severe patients. In the fecal microbiota analysis, we demonstrate no significant differences in microbial diversity before and after treatment between CB and placebo groups. The microbial community is similar in two groups at baseline but a significant difference is found at week 4. The different OTUs are analyzed and Clostridium sensu stricto is one of the most significantly changed OTU between two groups. Further analysis shows a significant reduction of Clostridium sensu stricto in responder compared with non-responder after treating with CB. Function prediction of CB is assessed by metagenomic analysis. Several pathways such as amino acid metabolism, fatty acid metabolism and tryptophan metabolism may play an essential role in treating IBS-D.
Several studies have researched on the effects of probiotics on IBS3,11,12. While some studies show a benefit in IBS symptoms with probiotics, others report limited benefits24. A recent meta-analyse suggested that probiotic therapy improves the overall symptoms and quality of life, but not individual IBS symptoms such as abdominal pain and bloating10. In this study, we demonstrate a similar result. Probiotic CB improves overall IBS symptoms in IBS-D patients compared with placebo. Further analysis shows that the improvement in IBS symptom is related to the change in quality of life and bowel habit, but not the abdominal pain and bloating. The overall responder rate (44.76%) of CB is higher than that of placebo but lower than that in some other studies2. We explain that a higher drop-out rate (19.05%) and a limitation in the reduction of IBS-SSS ≥50 points in mild IBS patients lead to this result, and also this is why the responder rate in moderate to severe patients is higher (54.22%).
The CB has been clinically used for several years but the effect and mechanism of the CB on IBS is unclear. Asuka Yasueda et al.18 have found that CB has benefit in the prevention of pouchitis and analyzed the characteristic intestinal flora after treating with CB or placebo. In an experimental colitis model in mice, CB suppresses symptoms via immune pathways25,26 but does not alter the composition of gut microbiota26. Since IBS is different from the organic gastrointestinal diseases, we focus on the change of microbiota during the use of CB. However, no significant differences are found in week and change of Bristol stool scale in both clinic symptoms and fecal samples and we suppose that this difference in baseline is permitted. As a result, the diversity analysis shows no differences before and after treatment in two groups but the microbial communities are different after treating with CB or placebo. A typical genus, Clostridium sensu stricto, is confirmed as a potential factor of CB in IBS-D treatment.
Several studies have shown an increase of short-chain fatty acids (SCFAs) level in IBS27,28. SCFAs, such as butyrate and acetate, have many benefits and play an important role in intestine function: enhancing the intestinal barrier, improving intestinal microbiota, regulating immune system and promoting gastrointestinal motility29–32. However, butyrate may promote visceral hypersensitivity33,34, which is a pathophysiological mechanism in IBS. Clostridium sensu stricto is a representative cluster of the genus Clostridium and exhibits SCFAs35 and it is reported that there is an increase of Clostridium in fecal samples of IBS patients36,37. In this study, we find a decrease of Clostridium sensu stricto after treating with CB and also a significant reduction of Clostridium sensu stricto in responder compared with non-responder. It is supposed to be a potential mechanism in treating IBS-D by CB.
In this study, we demonstrate several significantly different pathways between the placebo group and the CB group. Compared with placebo, treating with CB may downregulate pathways including fatty acid metabolism, beta-alanine metabolism, tryptophan metabolism, etc. Increased level of SCFAs is observed in IBS27,28. In the present study, we declare a decrease of SCFA-produced Clostridium sensu stricto after treating with CB, which may improve fatty acid metabolism in IBS patients. Increased level of alanine is reported in stool samples of IBS patients38 and the metabolite of tryptophan, serotonin, play an important role in gastrointestinal motility and IBS39,40. The improvement of alanine and tryptophan metabolism may reflect the alanine and serotonin in bowel and ameliorate the IBS symptoms.
In general, the analysis of stool samples between the placebo group and the CB group is not as significant as we expected. Associated with the clinical symptoms, the improvement of IBS symptom is based on quality of life and stool frequency, but not abdominal pain and bloating. These results may indicate a limited change in bowel microbiota or regulated pathways.
There are several limitations of this study. We analyze the IBS symptoms and fecal microbiota only at baseline and week 4 without a long-term surveillance. The continuous change in IBS symptom scores and fecal microbiota and the reaction after discontinuation cannot be proved. Furthermore, as only one dose of CB is used in this study, we cannot confirm the efficacy of CB with different doses in IBS treatment and the optimal dosing regimen. A further study of clinic use of CB is still needed. Another limitation is that lack of diet evaluation. However, we consider that a large sample size and randomization may reduce the difference of diet among patients and the effect of diet on the final results is weak. We focus on the fecal microbiota as a mechanism of CB in treating IBS-D. Since it is reported that CB participates in intestine function with some other mechanisms, such as immune regulation, a more detailed mechanism of CB in basic medicine and clinic is required.
In conclusion, this study has shown that CB is effective in improving the overall IBS-D symptoms, especially in bowel habit and quality of life. The responder rates are found higher in CB compared with the placebo. The fecal microbiota analysis shows a different microbial community after treating with CB and a typical genus, Clostridium sensu stricto, is decreased with the treatment of CB. CB is involved in several metabolic pathways to perform its function in treating IBS-D.
Electronic supplementary material
Acknowledgements
The research is supported by the National Natural Science Foundation of China (No. 81330012) and Natural Science Foundation of Shandong Provence (No. ZR2015H2003).
Author Contributions
Y.-Y.S., L.-X.L., X.-L.Z. and Y.-Q.L. designed the study. Y.-Y.S., Y.-Y.L., W.-Z.Z., X.-X.Y., X.G., L.J.S. and X.-L.Z. recruited and screened the patients. Y.-Y.S., L.-X.L., Z.L. and M.L. analyzed 16 s rRNA pyrosequencing and metagenome sequencing data. Y.-Y.S. analyzed the clinical data and wrote the manuscript. All authors read the manuscript and approved the final draft that was submitted.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-21241-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Longstreth GF, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–a double-blind, placebo-controlled study. Alimentary pharmacology & therapeutics. 2011;33:1123–1132. doi: 10.1111/j.1365-2036.2011.04633.x. [DOI] [PubMed] [Google Scholar]
- 3.Ki Cha B, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Journal of clinical gastroenterology. 2012;46:220–227. doi: 10.1097/MCG.0b013e31823712b1. [DOI] [PubMed] [Google Scholar]
- 4.American College of Gastroenterology Task Force on Irritable Bowel, S. et al. An evidence-based position statement on the management of irritable bowel syndrome. The American journal of gastroenterology. 2009;104(Suppl 1):S1–35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 5.Gwee KA, et al. Asian consensus on irritable bowel syndrome. Journal of gastroenterology and hepatology. 2010;25:1189–1205. doi: 10.1111/j.1440-1746.2010.06353.x. [DOI] [PubMed] [Google Scholar]
- 6.Ringel Y, Carroll IM. Alterations in the intestinal microbiota and functional bowel symptoms. Gastrointestinal endoscopy clinics of North America. 2009;19:141–150. doi: 10.1016/j.giec.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Jeffery IB, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 8.Carroll IM, et al. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. American journal of physiology. Gastrointestinal and liver physiology. 2011;301:G799–807. doi: 10.1152/ajpgi.00154.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hod K, Ringel Y. Probiotics in functional bowel disorders. Best practice & research. Clinical gastroenterology. 2016;30:89–97. doi: 10.1016/j.bpg.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, et al. Effects of probiotic type, dose and treatment duration on irritable bowel syndrome diagnosed by Rome III criteria: a meta-analysis. BMC gastroenterology. 2016;16:62. doi: 10.1186/s12876-016-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sisson G, Ayis S, Sherwood RA, Bjarnason I. Randomised clinical trial: A liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome–a 12 week double-blind study. Alimentary pharmacology & therapeutics. 2014;40:51–62. doi: 10.1111/apt.12787. [DOI] [PubMed] [Google Scholar]
- 12.Spiller R, et al. Randomized double blind placebo-controlled trial of Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: improvement in abdominal pain and bloating in those with predominant constipation. United European gastroenterology journal. 2016;4:353–362. doi: 10.1177/2050640615602571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng J, et al. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Alimentary pharmacology & therapeutics. 2008;28:994–1002. doi: 10.1111/j.1365-2036.2008.03818.x. [DOI] [PubMed] [Google Scholar]
- 14.Rousseaux C, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nature medicine. 2007;13:35–37. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- 15.Wang B, et al. Luminal administration ex vivo of a live Lactobacillus species moderates mouse jejunal motility within minutes. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24:4078–4088. doi: 10.1096/fj.09-153841. [DOI] [PubMed] [Google Scholar]
- 16.O’Mahony L, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 17.Mo S, et al. Genome sequencing of Clostridium butyricum DKU-01, isolated from infant feces. Gut pathogens. 2015;7:8. doi: 10.1186/s13099-015-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasueda A, et al. The effect of Clostridium butyricum MIYAIRI on the prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis. Surgery today. 2016;46:939–949. doi: 10.1007/s00595-015-1261-9. [DOI] [PubMed] [Google Scholar]
- 19.Seki H, et al. Prevention of antibiotic-associated diarrhea in children by Clostridium butyricum MIYAIRI. Pediatrics international: official journal of the Japan Pediatric Society. 2003;45:86–90. doi: 10.1046/j.1442-200X.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- 20.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Alimentary pharmacology & therapeutics. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 21.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Digestive diseases and sciences. 1998;43:400–411. doi: 10.1023/A:1018831127942. [DOI] [PubMed] [Google Scholar]
- 22.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scandinavian journal of gastroenterology. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 23.Wang AH, et al. Human colorectal mucosal microbiota correlates with its host niche physiology revealed by endomicroscopy. Scientific reports. 2016;6:21952. doi: 10.1038/srep21952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niv E, Naftali T, Hallak R, Vaisman N. The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome–a double blind, placebo-controlled, randomized study. Clinical nutrition. 2005;24:925–931. doi: 10.1016/j.clnu.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Kashiwagi I, et al. Smad2 and Smad3 Inversely Regulate TGF-beta Autoinduction in Clostridium butyricum-Activated Dendritic Cells. Immunity. 2015;43:65–79. doi: 10.1016/j.immuni.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi A, et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell host & microbe. 2013;13:711–722. doi: 10.1016/j.chom.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Tana, C. et al. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society22, 512–519, e114–515, 10.1111/j.1365-2982.2009.01427.x (2010). [DOI] [PubMed]
- 28.Treem WR, Ahsan N, Kastoff G, Hyams JS. Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. Journal of pediatric gastroenterology and nutrition. 1996;23:280–286. doi: 10.1097/00005176-199610000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. The Journal of nutrition. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guilloteau P, et al. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutrition research reviews. 2010;23:366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- 31.Meijer K, de Vos P, Priebe MG. Butyrate and other short-chain fatty acids as modulators of immunity: what relevance for health? Current opinion in clinical nutrition and metabolic care. 2010;13:715–721. doi: 10.1097/MCO.0b013e32833eebe5. [DOI] [PubMed] [Google Scholar]
- 32.Leonel AJ, Alvarez-Leite JI. Butyrate: implications for intestinal function. Current opinion in clinical nutrition and metabolic care. 2012;15:474–479. doi: 10.1097/MCO.0b013e32835665fa. [DOI] [PubMed] [Google Scholar]
- 33.Bourdu S, et al. Rectal Instillation of Butyrate Provides a Novel Clinically Relevant Model of Noninflammatory Colonic Hypersensitivity in Rats. Gastroenterology. 2005;128:1996–2008. doi: 10.1053/j.gastro.2005.03.082. [DOI] [PubMed] [Google Scholar]
- 34.Xu D, Wu X, Grabauskas G, Owyang C. Butyrate-induced colonic hypersensitivity is mediated by mitogen-activated protein kinase activation in rat dorsal root ganglia. Gut. 2013;62:1466–1474. doi: 10.1136/gutjnl-2012-302260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta RS, Gao B. Phylogenomic analyses of clostridia and identification of novel protein signatures that are specific to the genus Clostridium sensu stricto (cluster I) International journal of systematic and evolutionary microbiology. 2009;59:285–294. doi: 10.1099/ijs.0.001792-0. [DOI] [PubMed] [Google Scholar]
- 36.Rajilic-Stojanovic M, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–1801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 37.Distrutti E, Monaldi L, Ricci P, Fiorucci S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World journal of gastroenterology. 2016;22:2219–2241. doi: 10.3748/wjg.v22.i7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ponnusamy K, Choi JN, Kim J, Lee SY, Lee CH. Microbial community and metabolomic comparison of irritable bowel syndrome faeces. Journal of medical microbiology. 2011;60:817–827. doi: 10.1099/jmm.0.028126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sikander A, Rana SV, Prasad KK. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clinica chimica acta; international journal of clinical chemistry. 2009;403:47–55. doi: 10.1016/j.cca.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 40.Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34–43. doi: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.