Abstract
GVHD remains a major source of morbidity and mortality after allogeneic BMT. GVHD is mediated by alloreactive T cells derived from the hematopoietic graft that target host tissues. Pre-clinical models have shown that presentation of alloantigens by host DCs results in the activation of donor-derived T cells that mediate GVHD. Strategies that interfere with the Ag-presenting capacity of DCs after allogeneic transplantation may decrease the risk of developing GVHD. Vitamin D is a hormone essential for calcium metabolism that shows immunomodulatory properties. We showed that correction of vitamin D deficiency appeared to mitigate manifestations of GVHD. In preclinical studies, we have shown that vitamin D inhibits DC maturation, polarizes T-cell populations toward the expression of Th2 as compared with Th1 cytokines, and blunts allogeneic T-cell proliferation in response to DC stimulation. Exposure to vitamin D resulted in increased expression of IDO, an enzyme responsible for tryptophan metabolism that is upregulated in tolerizing DCs. These data suggest that exposure to vitamin D results in immature DC populations that bias toward tolerizing rather than stimulatory T-cell populations. Vitamin D may therefore have a role in the prevention of GVHD.
Keywords: vitamin D, GVHD, DC
Introduction
Allogeneic BMT is a uniquely curative therapy for patients with hematologic malignancies.1,2 The graft vs tumor effect mediated by donor lymphocytes is essential in eliminating residual disease post transplant and preventing relapse, and can result in long-term disease control in chemotherapy refractory disease. However, the expansion and activation of alloreactive T cells after transplantation results in the development of GVHD, which remains a major source of morbidity and mortality. A focus of research interest lies in identifying strategies to minimize the adverse effects of GVHD, while maintaining graft vs tumor effect.
DCs are the most potent APCs that constitutively express costimulatory molecules and stimulatory cytokines resulting in their unique capacity for inducing primary immunity. The phenotypic characteristics of DC evolve with stage of maturation. Immature DCs excel at Ag uptake and processing but are poor stimulators because of the relative lack of expression of costimulatory molecules. Stimulation with immature DCs may be associated with tolerization of reactive T-cell populations. In contrast, DC maturation is associated with increased expression of costimulatory molecules and the potent capacity to stimulate T-cell proliferation.
The persistence of host DCs in the early post transplant period has a role in the activation and expansion of alloreactive lymphocytes and the concomitant risk of GVHD.3 Donor-derived DCs have also been shown to contribute to GVHD through the cross presentation of alloantigens. A major focus of research involves manipulating DC recovery post transplant to minimize the activation of alloreactive lymphocytes to prevent GVHD. Treatment that can interfere with the allo-stimulatory capacity of host DCs and polarize toward tolerizing DC populations may be effective in the prevention and treatment of GVHD.
Vitamin D is a hormone involved in bone metabolism and calcium homeostasis that has been shown to have a significant role in the regulation of host immune responses and the prevention of autoimmunity. In animal models, vitamin D has been shown to reduce airway inflammation in a rat asthma model, and suppress or prevent autoimmune encephalomyelitis, arthritis, inflammatory bowel disease and diabetes. Importantly, vitamin D and its analogs prolonged survival of heart and small bowel allografts.4–6
In this study, we sought to assess the effect of vitamin D exposure on the phenotypic and functional characteristics of DC and T-cell populations and the ability of DCs to stimulate the expansion of alloreactive responses. We examined the effect of vitamin D on measures of DC maturation as manifested by expression of costimulatory and maturation markers. We also assessed effect of vitamin D on the expression of Th1 and Th2 cytokines and allogeneic T-cell proliferation after DC-mediated T-cell stimulation. We subsequently examined the effect of vitamin D on inhibitory signaling pathways in the DC and T-cell populations.
Methods
Generation of monocyte-derived DCs
PBMCs were isolated from leukopaks obtained from normal donors by ficoll density centrifugation. PBMCs were incubated in RPMI 1640 complete medium containing 2 mm L-glutamine, heat inactivated 10% human AB male serum, 100 U/ml penicillin and 100 µg/ml streptomycin for 2 h at 37 °C in a humidified 5% CO2 incubator. The monocyte-enriched adherent fraction was cultured in complete medium containing GM-CSF (1000 U/ml) and IL-4 (1000 U/ml) for 5 days to generate immature DCs, and matured in the presence of TNFα (25 ng/ml) for 48 h. To assess the effect of vitamin D on DC maturation, 10 nm vitamin D was added to the culture medium on day 1.
Characterization of the phenotypic properties of DCs generated in the presence of vitamin D
DC preparations generated in the presence and absence of vitamin D were incubated with primary mouse anti-human monoclonal antibodies: CD80, CD83, CD86, and matching isotype controls. After washing twice, the bound primary monoclonal antibodies were detected by labeling the cells with FITC-conjugated goat anti-mouse IgG1 for 30 min at 4 °C, washed and fixed in 2% paraformaldehyde.
Allogeneic MLRs
To assess the capacity of DCs to stimulate allogeneic T-cell proliferation in the presence of vitamin D, DCs were cocultured with allogeneic T cells in the presence of 10 nm vitamin D. T cells and DCs were cocultured at a ratio of 1:10 in 96-well U-bottom culture plates for 5 days. T-cell proliferation was determined by quantifying uptake of (3H)-thymidine (1 µCi/well) after overnight pulsing. The stimulation index was calculated according to formula: (3H)-thymidine uptake after coculture of DC and T cells/(3H)-thymidine uptake of unstimulated T cells.
Effect of vitamin D on DC polarization of T cells
DCs were cocultured with allogeneic T cells for 5 days at a ratio of 1:10 in the presence and absence of 10 nm of vitamin D. Cocultures were pulsed with GolgiPlug (BD Bioscience, San Jose, CA, USA) for 3–4 h at 37 °C before analysis. Cells were harvested and cultured with murine anti-human FITC-conjugated anti-CD4 or anti-CD8. Cells were then permeabilized by incubation in Cytofix/Cytoperm plus (containing formaldehyde and saponin) (BD Pharmingen, San Jose, CA, USA) for 30 min at 4 °C, washed twice in Perm/Wash solution and incubated with PE-conjugated IFN gamma, IL-10 or a matched isotype control Ab for 30 min. Labeled cells were analyzed by flow cytometry using FACScan and CellQuest Program (BD FACScomp Software, San Jose, CA, USA).
Capacity of DCs to stimulate regulatory and activated T-cell populations in the presence of vitamin D
Allogeneic T-cell preparations were cocultured with DCs for 5–7 days at a 10:1 ratio in the presence and absence of 10 ηm vitamin D added on day 1. The cell preparations were incubated with FITC-conjugated anti-CD4, Cychrome-conjugated anti-CD25 and PE-conjugated anti-CD69. Alternatively, cells were permeabilized and cultured with PE-conjugated Ab directed against Foxp3. Cells were subsequently analyzed by multichannel flow cytometry. In some studies, CD4 T cells were isolated by magnetic bead isolation, and the resultant population underwent bidimensional staining for CD25 and the indicated marker.
Evaluation of STAT-1 pathway signaling and IDO expression
Cells were lysed in the presence lysis buffer containing 50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 10 mm NaF, 1 mm NaV, 1 mm dithiothreitol, 1.0% NP40, 1 mm PMSF and protease inhibitor mixture. Soluble proteins were incubated with anti-STAT1, anti-STAT3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-IDO. The reactivity was detected with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (GE Healthcare, Piscataway, NJ, USA).
Statistical analysis
Assessment of statistical significance was determined using a paired Wilcoxon signed-rank test.
Results
Effect of vitamin D on the phenotype of ex vivo generated DC
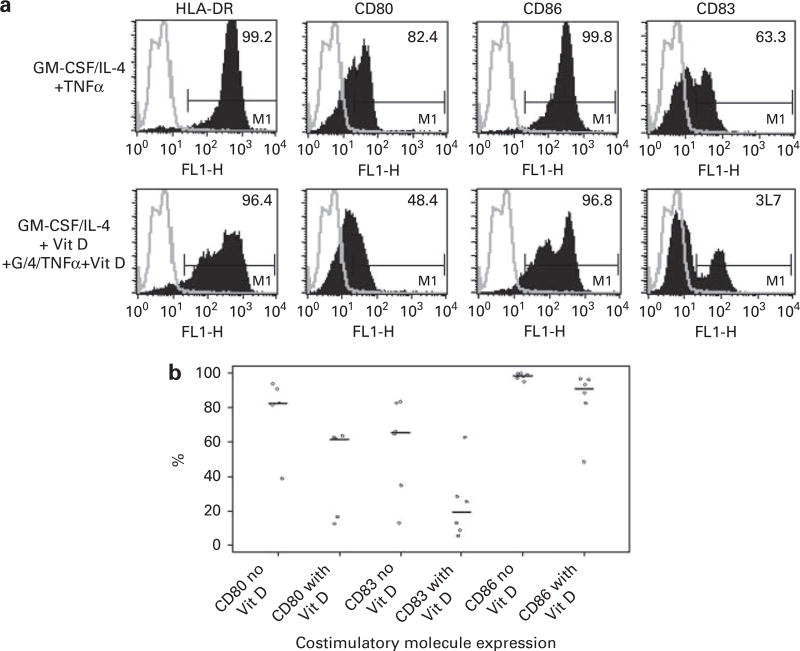
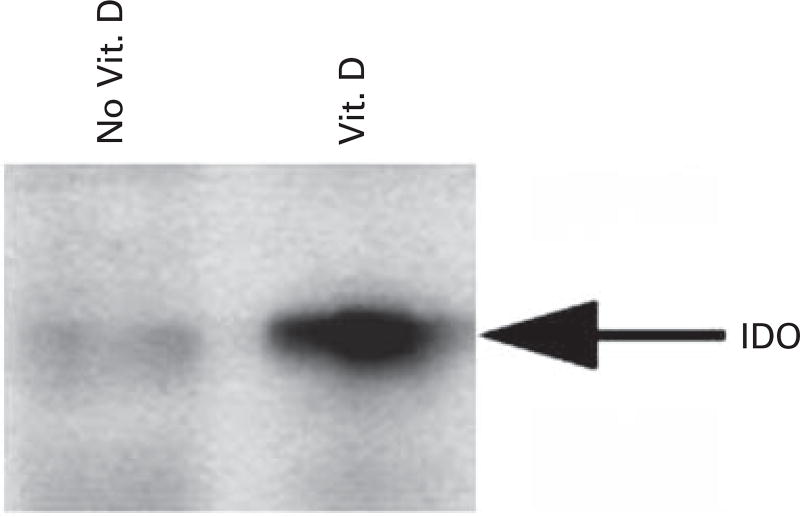
We evaluated the effect of in vitro vitamin D exposure on measures of DC maturation and activation. DCs were generated from peripheral blood progenitor populations cultured with GM-CSF and IL-4 for 1 week followed by maturation with TNFα. Exposure to vitamin D hindered DC maturation as manifested by decreased expression of costimulatory and maturation markers. In the presence of vitamin D, median expression of the maturation marker CD83 decreased from 65 to 19% (N = 6; P = 0.04) (Figures 1a and b). Similarly, median expression of the costimulatory molecule, CD80 decreased from 82 to 61% (N = 5; P = 0.05). Expression of CD86, was modestly decreased from 98 to 91% (N = 6; P = 0.01). These results suggest that vitamin D polarizes DCs toward an immature phenotype. Presentation of Ag in the absence of costimulation results in an inhibitory signal to potentially reactive T cells. IDO is an enzyme involved in tryptophan metabolism, and has been shown to be expressed by immature DCs that exert a tolerizing effect on T cells. Exposure to vitamin D enhances IDO expression, as shown by increased IDO protein expression by western blot (Figure 2).
Figure 1.
(a) A representative example showing the expression of CD80, CD83, CD86 on DCs generated in the presence and absence of 10 ηm vitamin D. Upper panels: Expression of CD80, CD83 and CD86 on DCs generated by culturing adherent mononuclear cells isolated from leukopak collections with GM-CSF, IL-4 and TNFα. Lower panels: Expression of CD80, CD83 and CD86 on DCs generated by culturing adherent mononuclear cells isolated from leukopak collections with GM-CSF, IL-4 and TNFα in the presence of 10 ηm vitamin D. (b) Summary data showing the expression of CD80, CD83 and CD86 on DCs generated in the presence and absence of 10 ηm vitamin D. Phenotypic analysis was performed on paired samples of DCs generated in the presence and absence of vitamin D. Cells were incubated with the appropriate Ab and analyzed by flow cytometry. Each dot represents the percent expression of CD80, CD83 and CD86 in an individual experiment, and the line represents the median percent expression of the indicated surface molecule.
Figure 2.
Expression of IDO protein by DCs generated in the presence and absence of vitamin D. DCs were generated by culturing adherent mononuclear cells isolated from leukopak collections with GM-CSF, IL-4 and TNFα in the presence or absence of 10 ηm vitamin D. IDO protein expression was assessed by western blot analysis.
Vitamin D suppresses the allogeneic T-cell stimulatory capacity of DCs
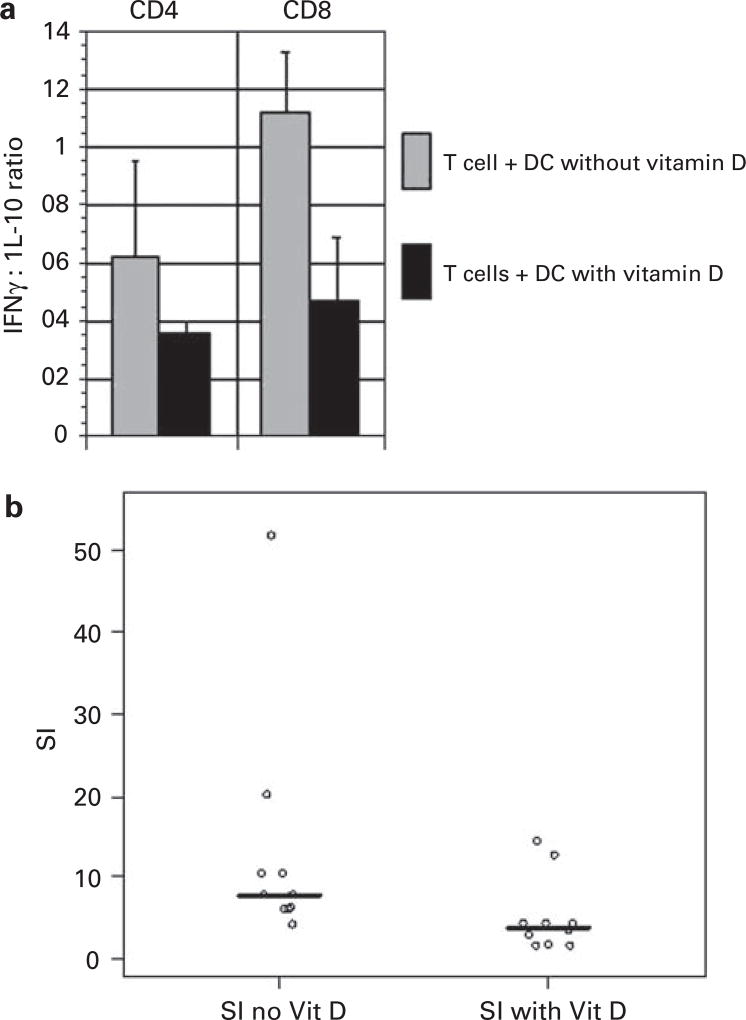
We next examined the phenotypic characteristics of T cells that were stimulated with DCs that had been differentiated in the presence of vitamin D. T cells were cocultured with vitamin D-treated DCs and control DCs for 5 days and the nature of cytokine expression was assessed by intracellular FACS analysis. Vitamin D-treated DCs induced T expression of IL-10 with the relative suppression of IFNγ suggestive of their polarization toward a Th2 phenotype. The ratio of IL-10: IFNγ secretion was increased by twofold in T cells that had been stimulated by vitamin D-treated and untreated DCs, respectively (N = 3) (Figure 3a). It is noteworthy that vitamin D-treated DCs also showed a diminished capacity to stimulate T-cell proliferation. Exposure to vitamin D resulted in a greater than twofold decrease in DC-mediated T-cell proliferative response (N = 10; P = 0.02) (Figure 3b).
Figure 3.
(a) Ratio of IFN gamma/IL-10 expressing CD4 and CD8T cells in the presence and absence of vitamin D. Mean values of three experiments are presented, with associated s.e. of the means. (b) Allogeneic T-cell proliferation after stimulation with mature DCs in the presence or absence of vitamin D. Allogeneic T cells were cocultured with mature DCs for 5 days in the presence or absence of vitamin D. Proliferation was measured by uptake of tritiated thymidine after an overnight pulse. Results are expressed as a stimulation index (T-cell proliferation following coculture/proliferation of unstimulated T cells). Each dot represents the stimulation index (SI) of an individual experiment, and the line represents the median SI.
Effect of vitamin D on T-cell response to mitogens and recall Ags
We examined the effect of vitamin D exposure on direct stimulation of T cells in the absence of APCs. We assessed T-cell response to mitogenic stimulation with phytohemagglutinin or ligation of the CD3/CD28 complex using beads coated with anti-CD3/CD28 Ab. Vitamin D did not effect T-cell response to stimulation with anti-CD3/CD28 (stimulation index 22 and 26 in the presence and absence of vitamin D respectively, P = NS) or phytohemagglutinin (stimulation index 24 and 25 in the presence and absence of vitamin D respectively, P = NS). We subsequently examined the effect of vitamin D on response to a recall Ag by measuring proliferation of PBMCs pulsed with tetanus toxoid. Exposure to vitamin D did not inhibit the proliferative response (stimulation index 4.1 and 3.8 in the presence and absence of vitamin D respectively). This data suggest that the suppressive effect of vitamin D is primarily mediated by its effect on DC-mediated stimulation of allogeneic T-cell populations. In contrast, no effect was observed on Ag-specific responses. It is noteworthy that the percentage of FOXP3 expressing regulatory T cells remained unchanged, accounting for 8 and 7%of the T-cell population respectively in the presence and absence of vitamin D.
Mechanism of vitamin D-mediated effect on DCs
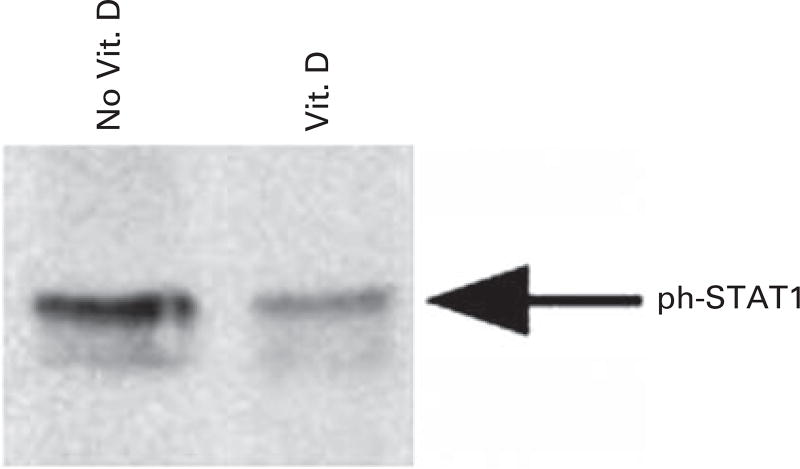
We next examined the effect of vitamin D on signaling pathways of DCs and T cells that are central for immune modulation. The STAT signaling pathways are associated with cytokine stimulation. STAT1 is expressed as a part of IFN signaling and is expressed in the context of DC maturation. As such, STAT1 signaling is upregulated in settings promoting DC-mediated stimulation of Th1 T-cell responses. We have shown that exposure to vitamin D resulted in reduced expression of STAT1 and its activated phosphorylated form in DC populations (Figure 4). These findings are consistent with the observation that vitamin D disrupts DC maturation. In contrast, STAT3 expression is induced by IL-6 and is an essential pathway mediating immune suppression. STAT3 is associated with inhibition of Th1-mediated inflammation and activation of genes associated with immune suppression. We examined the effect of vitamin D exposure on STAT3 expression in T-cell populations. The presence of vitamin D resulted in an increase in STAT3 in T-cell populations.
Figure 4.
Analysis of DCs generated in the presence and absence of vitamin D for P-STAT1 levels. DCs were generated from adherent mononuclear cells in the presence and absence of 10 ηm vitamin D, and the level of P-STAT1 was assessed by western blot analysis.
Discussion
GVHD is a major cause of morbidity and mortality in patients undergoing allogeneic transplantation. GVHD is mediated by the presence of alloreactive lymphocytes that undergo activation and expansion in the post transplant period. In a murine model, Shlomchik et al.3 showed that persistence of host APCs post transplant was associated with the development of GVHD. In this model, host DCs capable of presenting mHAs to infused donor T cells potentially initiate T-cell activation and an associated Th-1 cytokine cascade implicated in the pathogenesis of acute GVHD. In another model, MHC class II-deficient mice that were resistant to CD4+ dependent GVHD were injected with either host or donor-derived MHC class II expressing DC or B cells. It was shown that only injection of host DCs breaks GVHD resistance and induces lethal acute GVHD.7 Efforts to modulate DC-mediated stimulation of allogeneic T cells have been examined to reduce the risk of GVHD.
Vitamin D has recently been shown to exhibit potent immunomodulatory effects in models of asthma, inflammatory bowel disease and autoimmune encephalitis.
In this study, we show that exposure to vitamin D hinders the maturation of DCs resulting in diminished expression of costimulatory and maturation markers necessary for the primary activation of T cells. A statistically significant decrease in the mean expression of CD80, CD86 and CD83 was observed after exposure to vitamin D in vitro. DCs treated with vitamin D also showed increased IDO expression. IDO is an enzyme involved in tryptophan degradation, and has a critical in the maintenance of immune tolerance. DCs expressing IDO inhibit T-cell proliferation and induce T-cell apoptosis.8,9 The increased expression of IDO on DCs generated in the presence of vitamin D is consistent with their tolerizing properties. Vitamin D-mediated inhibition of DC differentiation results in the preservation of an immature DC phenotype. As such, vitamin D increases the presence of immature DCs that exert a tolerizing influence on potentially reactive T-cell populations.
Consistent with these findings, vitamin D has been shown in other pre-clinical models to inhibit the maturation of DCs resulting in the accumulation of immature DCs with a potentially immunosuppressive phenotype.10–13 In a murine model, addition of 1α25 hydroxyvitamin D3 to BM cells cultured in the presence of GM-CSF, and IL-4 resulted in lower levels of CD80 and CD86 expression, and decreased IL-12 secretion. In this model, pretreatment of female mice with male DCs generated in the presence of vitamin D enabled the engraftment of male skin grafts on female hosts.14 Consistent with our findings, Piemonti et al.13 showed that vitamin D inhibits DC differentiation from monocytic precursors in vitro. To further evaluate the effect of vitamin D on DC maturation, Griffin et al.11 studied a vitamin D receptor knockout mouse model, and showed that vitamin D inhibits DC maturation from wild type, but not vitamin D receptor knockout mice. In addition, it has been shown that DCs generated in the presence of vitamin D show impaired secretion of IL-12 and induce hyopresponsiveness of allogeneic T cells.13,15 Vitamin D has also been shown to enhance apoptosis of mature DCs resulting in inhibition of T-cell alloreactivity.12 In contrast, in a vitamin D receptor knockout model, allogeneic T-cell proliferation was not inhibited by exposure to vitamin D.
We have shown that T cells stimulated by DCs in the presence of vitamin D show decreased proliferative capacity and skewing toward a Th2 phenotype. A relative increase in T-cell expression of IL-10 as compared with IFNγ was observed. It is noteworthy that in one study, vitamin D was shown to exert an immunomodulatory effect on myeloid DCs, suppressing Th1 responses, whereas the capacity of plasmacytoid DCs to induce suppressor T-cell populations was not affected by vitamin D.12 These data suggest that vitamin D may alter Ag presentation in a manner that inhibits DC-mediated expansion of alloreactive T cells after allogeneic transplantation. In contrast, exposure to vitamin D did not have an effect on T-cell response to mitogens or Ab ligation of the CD3/CD28 complex. These findings suggest that vitamin D uniquely affects DC-mediated stimulation of T cells through its effect on DC phenotype.
The mechanism by which DC maturation is inhibited by vitamin D has not been described. We examined the effect of vitamin D on signaling through the STAT pathways that have a crucial role in immune activation and suppression. DC maturation requires STAT1,16 and activation of STAT1 has been shown to be associated with the differentiation of IL-12 producing DCs.17 Experiments in STAT1 knockout mice have shown that STAT1 signaling mediates Th1 responses.18,19 We show a reduction in STAT1 phosphorylation in DCs generated in the presence of vitamin D, consistent with an inhibition of DC maturation. In a murine model, induction of GVHD is associated with activation of the STAT1 signaling pathway. In this study, we show that vitamin D interferes with STAT1 signaling and may therefore be effective in preventing GVHD.
Signaling through STAT3 is associated with the inhibition of Th1-mediated inflammatory responses. Upregulation of this pathway is observed in both T cells in the setting of malignancy and immune suppression. In this study, increased presence of STAT3 and its phosphorylated activated form was observed in T cells stimulated by vitamin D-treated DCs. STAT3 has been shown to be increased in regulatory T-cell populations in the setting of malignancy. It is noteworthy that in this study, stimulation of T cells with vitamin D-treated DCs did not result in the expansion of FOXP3 + T cells.
The pathophysiology of GVHD involves the presence of host APCs, and is initiated in part by the inflammatory response to tissue damage caused by the conditioning regimen. In murine studies, blocking inflammatory cytokines has been shown to prevent the development of GVHD.20–23 A focus of research interest lies in separating GVHD from the graft vs tumor effect. Traditional immunosuppressant therapy for GVHD primarily targets lymphocytes. However, strategies that interfere with the allostimulatory capacity of DCs in the early post transplant period rather than globally suppressing donor T-cell function may be effective at preventing GVHD while maintaining anti-tumor immunity.
Consistent with its potential role in modulating alloreactive responses, we have observed that correction of vitamin D deficiency was associated with clinical improvement in two patients with steroid refractory GVHD. The first patient was a 26-year-old man with T-cell acute lymphoblastic lymphoma with biopsy proven chronic GVHD of the liver that was only partially responsive to combined immunosuppressive therapy with methylprednisolone, mycophenolate mofetil and extracorporeal photo-pheresis over 4 months. He received vitamin D replacement therapy (vitamin D2) after he was found to have vitamin D deficiency during an evaluation for steroid induced vertebral fractures. Over the subsequent 2 months, his liver function tests normalized and he was successfully tapered completely off steroids without recrudescence of GVHD manifestations.
The second patient was a 47-year-old man who underwent an allogenic transplant from an unrelated donor for refractory acute myelogenous leukemia. His early post transplant course was notable for the development of grade II acute GVHD of the skin and GI tract that progressed to chronic extensive GVHD of the liver and skin despite treatment with multiple immunosuppressive agents. As a part of the workup for refractory hypophosphatemia, he was noted to be vitamin D deficient and was started on vitamin D replacement therapy. Over the subsequent month, his skin and liver GVHD improved and immunosuppressive therapy was tapered off. Although delayed response to immunosuppressive therapy may have had an important role, the initiation of vitamin D appeared to be a potential factor associated with the clinical improvement in GVHD that was observed in both patients. The clinical observation of improvement in steroid refractory GVHD in two patients who were started on vitamin D therapy for treatment of vitamin D deficiency supports our in vitro data and the hypothesis that vitamin D may be effective in the treatment and prevention of GVHD. A clinical study in which vitamin D is given in the early post transplant period for the prevention of GVHD is planned.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Rowe JM. Graft-versus-disease effect following allogeneic transplantation for acute leukaemia. Best Pract Res Clin Haematol. 2008;21:485–502. doi: 10.1016/j.beha.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Bruno B, Giaccone L, Sorasio R, Boccadoro M. Role of allogeneic stem cell transplantation in multiple myeloma. Semin Hematol. 2009;46:158–165. doi: 10.1053/j.seminhematol.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 4.Lemire JM, Archer DC, Khulkarni A, Ince A, Uskokovic MR, Stepkowski S. Prolongation of the survival of murine cardiac allografts by the vitamin D3 analogue 1,25-dihydroxy-delta 16-cholecalciferol. Transplantation. 1992;54:762–763. doi: 10.1097/00007890-199210000-00046. [DOI] [PubMed] [Google Scholar]
- 5.Hullett DA, Cantorna MT, Redaelli C, Humpal-Winter J, Hayes CE, Sollinger HW, et al. Prolongation of allograft survival by 1,25-dihydroxyvitamin D3. Transplantation. 1998;66:824–828. doi: 10.1097/00007890-199810150-00002. [DOI] [PubMed] [Google Scholar]
- 6.Johnsson C, Tufveson G. MC 1288–a vitamin D analogue with immunosuppressive effects on heart and small bowel grafts. Transpl Int. 1994;7:392–397. doi: 10.1007/BF00346032. [DOI] [PubMed] [Google Scholar]
- 7.Duffner UA, Maeda Y, Cooke KR, Reddy P, Ordemann R, Liu C, et al. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol. 2004;172:7393–7398. doi: 10.4049/jimmunol.172.12.7393. [DOI] [PubMed] [Google Scholar]
- 8.Park MJ, Min SY, Park KS, Cho YG, Cho ML, Jung YO, et al. Indoleamine 2,3-dioxygenase-expressing dendritic cells are involved in the generation of CD4+ CD25+ regulatory T cells in Peyer′s patches in an orally tolerized, collagen-induced arthritis mouse model. Arthritis Res Ther. 2008;10:R11. doi: 10.1186/ar2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fallarino F, Vacca C, Orabona C, Belladonna ML, Bianchi R, Marshall B, et al. Functional expression of indoleamine 2,3-dioxygenase by murine CD8 alpha(+) dendritic cells. Int Immunol. 2002;14:65–68. doi: 10.1093/intimm/14.1.65. [DOI] [PubMed] [Google Scholar]
- 10.Gauzzi MC, Purificato C, Donato K, Jin Y, Wang L, Daniel KC, et al. Suppressive effect of 1alpha,25-dihydroxyvitamin D3 on type I IFN-mediated monocyte differentiation into dendritic cells: impairment of functional activities and chemotaxis. J Immunol. 2005;174:270–276. doi: 10.4049/jimmunol.174.1.270. [DOI] [PubMed] [Google Scholar]
- 11.Griffin MD, Lutz WH, Phan VA, Bachman LA, McKean DJ, Kumar R. Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem Biophys Res Commun. 2000;270:701–708. doi: 10.1006/bbrc.2000.2490. [DOI] [PubMed] [Google Scholar]
- 12.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 13.Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 14.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB down-regulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson SH, Yu CR, Mahdi RM, Ebong S, Egwuagu CE. Dendritic cell maturation requires STAT1 and is under feedback regulation by suppressors of cytokine signaling. J Immunol. 2004;172:2307–2315. doi: 10.4049/jimmunol.172.4.2307. [DOI] [PubMed] [Google Scholar]
- 17.Vakkila J, Demarco RA, Lotze MT. Coordinate NF-kappaB and STAT1 activation promotes development of myeloid type 1 dendritic cells. Scand J Immunol. 2008;67:260–269. doi: 10.1111/j.1365-3083.2007.02068.x. [DOI] [PubMed] [Google Scholar]
- 18.Flores RR, Diggs KA, Tait LM, Morel PA. IFN-gamma negatively regulates CpG-induced IL-10 in bone marrow-derived dendritic cells. J Immunol. 2007;178:211–218. doi: 10.4049/jimmunol.178.1.211. [DOI] [PubMed] [Google Scholar]
- 19.Johnson LM, Scott P. STAT1 expression in dendritic cells, but not T cells, is required for immunity to Leishmania major. J Immunol. 2007;178:7259–7266. doi: 10.4049/jimmunol.178.11.7259. [DOI] [PubMed] [Google Scholar]
- 20.Leng C, Gries M, Ziegler J, Lokshin A, Mascagni P, Lentzsch S, et al. Reduction of graft-versus-host disease by histone deacetylase inhibitor suberonylanilide hydroxamic acid is associated with modulation of inflammatory cytokine milieu and involves inhibition of STAT1. Exp Hematol. 2006;34:776–787. doi: 10.1016/j.exphem.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Hill GR, Teshima T, Gerbitz A, Pan L, Cooke KR, Brinson YS, et al. Differential roles of IL-1 and TNF-alpha on graft-versus-host disease and graft versus leukemia. J Clin Invest. 1999;104:459–467. doi: 10.1172/JCI6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddy P, Teshima T, Kukuruga M, Ordemann R, Liu C, Lowler K, et al. Interleukin-18 regulates acute graft-versus-host disease by enhancing Fas-mediated donor T cell apoptosis. J Exp Med. 2001;194:1433–1440. doi: 10.1084/jem.194.10.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooke KR, Hill GR, Gerbitz A, Kobzik L, Martin TR, Crawford JM, et al. Tumor necrosis factor-alpha neutralization reduces lung injury after experimental allogeneic bone marrow transplantation. Transplantation. 2000;70:272–279. doi: 10.1097/00007890-200007270-00006. [DOI] [PubMed] [Google Scholar]