Abstract
Aim
We compared neonatal deaths and end‐of‐life decisions in a neonatal intensive care unit (NICU) and paediatric intensive care unit (PICU) in a Dutch tertiary children's hospital.
Subjects
All 235 full‐term infants who died within 28 days of life between 2003 and 2013 in the NICU (n = 199) and PICU (n = 36) were retrospectively studied.
Results
The median length of stay was three days in the NICU and seven days in the PICU (p = 0.003). The main reasons for NICU stays were asphyxia (52.8%) and congenital malformations (42.2%), and in the PICU, they were congenital malformations (97.2%) and primarily cardiac problems (83.3%, p < 0.001). The median age of death was three days in the NICU and eight days in the PICU (p < 0.001), and mortality despite full intensive care treatment was 4.0% and 25.0%, respectively. Intensive treatment was discontinued because of poor survival chances in 25.1% of NICU and 52.8% of PICU cases (p < 0.001), and care was redirected because of expected poor quality of life in 70.9% and 22.2%, respectively.
Conclusion
Differences between the age at death and end‐of‐life decisions were found between full‐term infants in the NICU and PICU in the same children's hospital. Underlying disorders and doctors’ attitudes may have played a role.
Keywords: End‐of‐life decisions, Ethics, Mortality, Neonatal intensive care unit, Paediatric intensive care unit
Abbreviations
- NICU
Neonatal intensive care unit
- PICU
Paediatric intensive care unit
Key notes.
We compared neonatal deaths and end‐of‐life decisions in the neonatal (n = 199) and paediatric intensive care units (n = 36) of a Dutch tertiary children's hospital.
All 235 full‐term infants who died within 28 days of life in the units from 2003 to 2013 were retrospectively studied.
Differences were found in the median length of stay and age at death and discontinued and redirected care because of poor survival and quality of life, respectively.
Introduction
Most of the studies on neonatal deaths in the Western world have focused on preterm infants and have identified four major causes of mortality: prematurity, congenital malformations, perinatal asphyxia, and sepsis 1, 2, 3, 4, 5, 6, 7. In the Netherlands, full‐term neonates who suffer from critical illnesses are commonly admitted to a neonatal intensive care unit (NICU) or paediatric intensive care unit (PICU) depending on the nature of their illness 8. Although the major causes of neonatal deaths are known, there have been no studies that have compared the mortality and causes and timing of neonatal deaths in NICUs and PICUs. There are different factors that can have an impact on the neonatal mortality rate, including birthweight and gestational age 9, 10, 11, 12, 13. The postnatal age at death and length of intensive care stay may be simultaneously influenced by the underlying illness, but this has not been studied in much detail. In addition, neonatologists who work in a NICU may have different attitudes towards end‐of‐life decisions than the paediatric intensivists or paediatric anaesthesiologists who work in a PICU. End‐of‐life decisions can be classified using different systems 4, 5, 14, 15, 16, 17, 18, 19, 20, 21, 22 and have been widely studied in NICUs. However, little is known about the process of end‐of‐life decision‐making for neonates in PICUs.
The aim of this study was threefold. Firstly, we wanted to determine whether there was a difference between the causes of neonatal deaths in full‐term infants in the NICU and PICU in the same tertiary children's hospital and the percentage of autopsies that were performed. Secondly, we wanted to investigate which factors predisposed the infants to death. Thirdly, we wanted to investigate whether there was a difference in end‐of‐life decision‐making between the NICU and PICU in the same hospital.
Methods
We performed a retrospective, observational study at the Wilhelmina Children's Hospital, University Medical Center Utrecht, the Netherlands, which is a university teaching hospital with a level 3 NICU and a level 3 PICU. The hospital is one of the four paediatric cardiac centres in the Netherlands, and it has a specific strategy for admitting neonates to either the NICU or PICU. Neonates with congenital heart diseases that need cardiac surgery are usually admitted to the PICU, whereas all other neonates, including those with noncardiac congenital malformations, are admitted to the NICU. Exceptions can be made if there is a shortage of beds at one of the units.
Full‐term neonates with a gestational age of 37 weeks or more who died within 28 days of life in the NICU or PICU from 2003 to 2013 were included in this study.
NICU and PICU mortality data were collected from the joint patient data management system: total admissions, birthweight, gestational age, postnatal age at admission and death, length of stay, where they were admitted from – for example home or another hospital – the disorders that led to death, end‐of‐life decisions and whether an autopsy was performed.
End‐of‐life decisions were categorised by a modified classification that is widely used in the Netherlands 23. Three groups were distinguished: full intensive care treatment until death, discontinued treatment because of a poor chance of survival and discontinued treatment because of an expected poor quality of life. Patients were classified by evaluating the medical records.
The ethics committee of the University Medical Centre Utrecht waived the requirement to obtain informed consent for this retrospective study with anonymised data.
Student's t‐tests, chi‐square tests and ANOVA were used where appropriate. An alpha of 0.05 was considered significant.
Results
During the study period from 2003 to 2013, 3175 neonates were admitted to the NICU and the PICU. Of these patients, 96 stayed in both the NICU and the PICU during the first 28 days of life and were excluded from the analysis. This means that 2373 neonates were exclusively admitted to the NICU and 706 were exclusively admitted to the PICU (Fig. 1). The main reasons for NICU stays were asphyxia (52.8%) and congenital malformations (42.2%), and in the PICU, they were congenital malformations (97.2%) and primarily cardiac problems (83.3%, p < 0.001).
Figure 1.
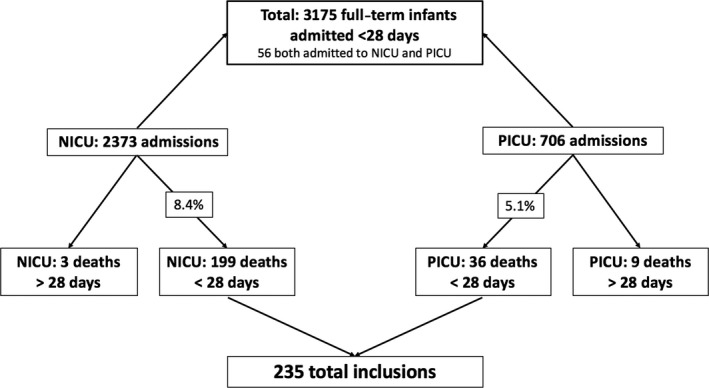
Flow chart of included patients.
In the NICU, 199 full‐term patients died within 28 days of life (8.4%) and the number of deaths in the PICU was 36 (5.1%). The patient characteristics are shown in Table 1. Birthweight and gestational age were not significantly different between the two units. However, the postnatal age of death showed a significant difference of five days: the median age of death was three days in the NICU and eight days in the PICU (p < 0.001). In addition, the length of stay in the PICU was significantly longer than the NICU (seven days versus three days, p = 0.003). NICU patients mainly came from a level 2 hospital (71.9%) or were born in the Wilhelmina Children's Hospital (21.6%). Of the PICU patients, 50% came from a level 2 hospital, 33.3% were born in the Wilhelmina Children's hospital, and 16.7% were transferred from another level 3 university hospital.
Table 1.
Patient characteristics
| Total number of patients who died | All patients n = 235 | NICU patients n = 199 | PICU patients n = 36 | p‐Value |
|---|---|---|---|---|
| Total admissions (n, mortality in %) | 3079 (7.6) | 2373 (8.4) | 706 (5.1) | 0.005 |
| Birthweight in grams (median, IQR) | 3350 (790) | 3380 (815) | 3312 (708) | N.S. |
| Gestational age in days (median, IQR) | 278 (16) | 278 (18) | 276 (13) | N.S. |
| Postnatal age of death (median, IQR) | 4 (5) | 3 (3) | 8 (11) | <0.001 |
| Postnatal age of death (n, %) | ||||
| Less than seven days | 173 (73.6) | 160 (80.4) | 13 (36.1) | <0.001 |
| 7–28 days | 62 (26.4) | 39 (19.6) | 23 (63.9) | |
| Length of stay (LOS) (median, IQR) | 3 (4) | 3 (4) | 7 (8) | 0.003 |
| Place of origin (n, %) | ||||
| At home | 6 (2.6) | 6 (3.0) | 0 (0) | 0.003 |
| Born in study hospital | 55 (23.4) | 43 (21.6) | 12 (33.3) | |
| Level 2 hospital | 161 (68.5) | 143 (71.9) | 18 (50.0) | |
| Other level 3 hospital | 12 (5.1) | 6 (3.0) | 6 (16.7) | |
| Abroad | 1 (0.4) | 1 (0.5) | 0 (0) | |
| Autopsy performed (n, %) | 94 (40.0) | 90 (45.2) | 4 (11.1) | <0.001 |
N.S.: not significant.
Table 2a shows the major causes of death, with a significant difference between the NICU and PICU with regard to congenital malformations (42.2% versus 97.2%, p < 0.001) and asphyxia (52.8% and 0%, p < 0.001). In Table 2b, the separate causes are described in more detail. The diagnoses of sepsis, infections, congenital pulmonary disease and secondary pulmonary disease were not significantly different between the two units. However, circulatory insufficiency was significantly more common in the PICU than NICU population (94.4% versus 60.8%) and secondary heart failure was significantly more common in the NICU than PICU population (40.7% versus 8.3%). No patients with asphyxia were admitted to the PICU.
Table 2.
(a) Causes of death at the NICU and the PICU – major groups. (b) Causes of death at the NICU and the PICU in detaila
| Total number of patients who died | All patients n = 235 | NICU patients n = 199 | PICU patients n = 36 | p‐Value |
|---|---|---|---|---|
| (a) | ||||
| Congenital malformation (n, %) | 119 (50.6) | 84 (42.2) | 35 (97.2) | <0.001 |
| Asphyxia (n, %) | 105 (44.7) | 105 (52.8) | 0 (0) | <0.001 |
| Other/unknown condition (n, %) | 11 (4.7) | 10 (5.0) | 1 (2.8) | N.S. |
| (b) | ||||
| Sepsis (n, %) | 12 (5.1) | 9 (4.5) | 3 (8.3) | N.S. |
| Infection (n, %) | 20 (8.5) | 18 (9.0) | 2 (5.6) | N.S. |
| Congenital pulmonary disease (n, %) | 19 (8.1) | 16 (8.0) | 3 (8.3) | N.S. |
| Secondary pulmonary dysfunction (n, %) | 170 (72.3) | 146 (73.4) | 24 (66.7) | N.S. |
| Circulatory insufficiency (n, %) | 155 (66.0) | 121 (60.8) | 34 (94.4) | <0.001 |
| Congenital heart disease (n, %) | 63 (26.8) | 33 (16.6) | 30 (83.3) | <0.001 |
| Secondary heart dysfunction (n, %) | 84 (35.7) | 81 (40.7) | 3 (8.3) | <0.001 |
| Congenital cerebral disease (n, %) | 43 (18.3) | 40 (20.1) | 3 (8.3) | N.S. |
| Secondary cerebral dysfunction (n, %) | 129 (54.9) | 122 (61.3) | 7 (19.4) | <0.001 |
| Genetic disorder (n, %) | 47 (20.0) | 40 (20.1) | 7 (19.4) | N.S. |
Total of percentages is more than 100 as several conditions may have been assigned for one patient.
N.S.: not significant.
There were significant differences in clinical characteristics between the two groups. Birthweight was significantly higher in patients with asphyxia than in patients with a congenital malformation. Children with asphyxia also had a significant higher gestational age, with a median of 280 days, than children with a congenital malformation: 273 days in the NICU and 276 days in the PICU. Other significant differences between the groups were the postnatal age at death, the length of stay and where they had been admitted from, for example home or another hospital (Table 3).
Table 3.
Congenital malformations and asphyxia: clinical characteristics
| Asphyxia n = 105 | Congenital malformations NICU n = 84 | Congenital malformations PICU n = 35 | p‐Value | |
|---|---|---|---|---|
| Birthweight in grams (median, IQR) | 3524 (728) | 3051 (981) | 3212 (705) | <0.001 |
| Gestational age in days (median, IQR) | 280 (14) | 273 (14) | 276 (12) | <0.001 |
| Postnatal age of death (median, IQR) | 3 (3) | 6 (7) | 10 (11) | <0.001 |
| Postnatal age of death (n, %) | ||||
| Less than seven days | 102 (97.1) | 55 (65.5) | 12 (34.3) | <0.001 |
| 7–28 days | 3 (2.9) | 29 (34.5) | 23 (65.7) | |
| Length of stay (median, IQR) | 3 (3) | 6 (7) | 7 (8) | <0.001 |
| Place of origin (n, %) | ||||
| At home | 4 (3.8) | 2 (2.4) | 0 (0) | 0.001 |
| Born in study hospital | 16 (15.2) | 27 (32.1) | 12 (34.3) | |
| Level 2 hospital | 84 (80.0) | 50 (59.5) | 17 (48.6) | |
| Other level 3 hospital | 1 (1.0) | 4 (4.8) | 6 (17.1) | |
| Abroad | 0 (0) | 1 (1.2) | 0 (0) | |
| Autopsy performed (n, %) | 52 (49.5) | 32 (38.1) | 4 (11.4) | 0.001 |
Table 1 shows that four times as many autopsies were performed after deaths in the NICU (45.0%) than PICU (11.1%). When we focused on patients with congenital malformations, we also found a significant difference between the autopsies performed in the NICU (38.1%) and PICU (11.4%, p = 0.003). Patients with asphyxia had significantly more autopsies (49.5%) (Table 3). No significant difference was found between the percentages of autopsies performed and the different end‐of‐life decisions.
There was a significant difference in end‐of‐life decisions between the groups (Table 4a) and for the different medical disorders (Table 4b). A quarter (25.0%) of all PICU patients died despite full intensive care treatment, while almost none (4%) of the NICU patients died without end‐of‐life decision‐making (Table 4a). More than half (52.8%) of the PICU patients died because treatment was discontinued because of poor chances of survival, while this decision was made in 25.1% of the NICU patients (Table 4a). In 70.9% of the NICU patients, treatment was discontinued because of poor quality of life, while this figure was only 22.2% of the PICU patients. Most of the patients with asphyxia died after treatment was discontinued, while only 45.2% of the patients with a congenital malformation in the NICU and 22.9% in the PICU did.
Table 4.
(a) End‐of‐life decisions at NICU and PICU. (b) End‐of‐life decisions and disorders
| Total number of patients who died | All patients n = 235 | NICU patients n = 199 | PICU patients n = 36 | p‐Value |
|---|---|---|---|---|
| (a) | ||||
| End‐of‐life decision (n, %) | ||||
| No end‐of‐life decision | 17 (7.2) | 8 (4.0) | 9 (25.0) | <0.001 |
| Expected poor chances of survival | 69 (29.4) | 50 (25.1) | 19 (52.8) | |
| Expected poor quality of life | 149 (63.4) | 141 (70.9) | 8 (22.2) | |
| Asphyxia n = 105 | Congenital malformations NICU n = 84 | Congenital malformations PICU n = 35 | p‐Value | |
|---|---|---|---|---|
| (b) | ||||
| End‐of‐life decision (n, %) | ||||
| No end‐of‐life decision | 3 (2.9) | 4 (4.8) | 8 (22.9) | <0.001 |
| Expected poor chances of survival | 8 (7.6) | 42 (50.0) | 19 (54.3) | |
| Expected poor quality of life | 94 (89.5) | 38 (45.2) | 8 (22.9) | |
Of the patients with a congenital malformation, 4.8% died despite full intensive care treatment in the NICU and 22.9% in the PICU (Table 4b), whereas 45.2% died despite of poor quality of life in the NICU and 22.9% in the PICU.
Discussion
This study demonstrated major differences in mortality, age at death, cause of death and end‐of‐life decisions in full‐term neonates admitted to the NICU and PICU at the same children's hospital in the Netherlands. Our study confirmed that most of the deaths in the NICU were as a result of perinatal asphyxia 1, 2, 3, 4, 5, 6, 7, which often leads to circulatory insufficiency and secondary cerebral and pulmonary dysfunction. After asphyxia, noncardiac congenital malformations were also common in the NICU. Only a small percentage of deaths occurred in apparently healthy full‐term neonates without any other disorder who died due to sepsis, while more neonates died due to sepsis in combination with another condition. Morbidity and mortality as a result of sepsis are still high in modern neonatal medicine. However, little has been specifically published about neonates in PICUs. The most common causes of death for children admitted to the PICU were congenital cardiac malformations and infections 6, 17, 24, 25, which may have caused respiratory failure and adverse neurological conditions 17, 26, 27, and this corresponded with our study. Studies have shown that most neonates died in NICUs after active end‐of‐life decisions made by their parents and caregivers 1, 27, 28. Our study confirmed this, as treatment was discontinued in nearly all of the NICU patients, who died. This percentage was significantly lower in our PICU. Fontana et al. found that in a large tertiary hospital in Canada, there was no significant difference between the NICU and the PICU with regard to the number of children that died under full supportive care 28. However, they did include extremely preterm born neonates, whereas we excluded preterm infants as well as all patients who lived beyond 28 days. Their NICU and PICU units were comparable to ours, as children with perinatal asphyxia were admitted to the NICU, children with congenital heart disease were admitted to the PICU and both their NICU and PICU cared for children dying from congenital malformations. Our study echoed their findings in that the reason for discontinuing therapy for NICU patients was poor quality of life 28. In addition, we found that the most common reason for withholding therapy in PICU patients was their poor chance of survival. Fewer end‐of‐life decisions were made in the PICU at our hospital and neonates stayed significantly longer in the PICU than NICU before they died. Wilkinson and Weitz 29 stated that patients that stayed in PICUs for a longer time had more discomfort and underwent more painful procedures. If children who will not survive are identified earlier in their admission, making end‐of‐life decisions can reduce their stay and discomfort in the ICU 6, 18.
There can be a number of explanations for this difference in end‐of‐life decision‐making in the NICU and PICU. Based on the patient populations, the circumstances in the different units required different approaches. This difference was particularly notable when we only focused on the neonates with congenital diseases. The difference in this population may be caused by underlying conditions or by unit policy, as fewer end‐of‐life decisions were made in the overall PICU population and poor expected quality of life was the main consideration for discontinuing treatment in the NICU population. Overall mortality in the full‐term infants in our NICU (8%) was higher than those in our PICU (5%) during the study period. With the strong reduction in sepsis due to meningococci, Haemophilus influenzae and pneumococci in the Netherlands, and the lower gestational ages of patients admitted to NICUs, patient mortality is more common in a NICU than PICU. However, it is unknown whether this has an impact on the decision‐making between neonatologists and paediatric intensivists.
Most Western countries have special NICU departments for critically ill neonates 16, but little is known about how neonates in need of intensive care treatment are allocated to different intensive care units in other countries. As this study confirmed, different units have different patient populations, expertise and different ways of treating critically ill patients 5. Neonatologists and paediatric intensivists follow a different subspecialty training programme in the Netherlands. The level 3 NICUs and PICUs in all the university hospitals in the Netherlands have a different composition of the staff. Paediatric intensivists, as well as paediatric anaesthesiologists, and paediatric cardio‐anaesthesiologists are responsible for the care of patients, including those with cardiac abnormalities, admitted to the PICU. We cannot exclude that educational and ethical differences between neonatologists and other specialists have led to different approaches and attitudes towards end‐of‐life decisions.
The strengths of our study were that we collected a large quantity of data over a 10‐year study period and made a clear distinction between a specific group of patients, namely full‐term neonates, in the NICU and PICU. Our data might be the starting point for discussions on benchmarking procedures.
There were some limitations of our study. By only including neonates that died during the neonatal period, of up to 28 days, we excluded patients that died after this period. In our study period, nine patients were admitted to the NICU or PICU before 28 days of birth and died in the PICU after the neonatal period. Another three patients were just admitted to the NICU before 28 days after birth and died after 28 days. However, as these numbers were small compared to the other data, it did not affect our conclusions. Finally, the cause of death and end‐of‐life decisions were not recorded prospectively, but were obtained from the medical records. Nevertheless, these data were described in detail in all cases and this enabled us to make a proper classification.
Conclusion
This study, which was carried out in the same children's hospital in the Netherlands from 2003 to 2013, found that full‐term neonates died sooner after being admitted to the NICU than PICU, which may have been due to underlying disease. In the NICU, most full‐term neonates died due to the results of perinatal asphyxia, and in the PICU, neonates mainly died due to congenital cardiac malformations. End‐of‐life decisions were remarkably different between both units and were guided by the patient population rather than their age.
Finance
No external funding was received for this study.
Conflicts of interest
The authors have no conflict of interests to declare.
Acknowledgements
The authors would like to thank Idse Visser, MA, MSc, of the Dutch Paediatric Intensive Care Evaluation for helping to collect data on the PICU patients.
References
- 1. Hellmann J, Knighton R, Lee SK, Shah PS. Neonatal deaths: prospective exploration of the causes and process of end‐of‐life decisions. Arch Dis Child Fetal Neonatal Ed 2016; 2: 106–7. [DOI] [PubMed] [Google Scholar]
- 2. Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: When? Where? Why? Lancet 2005; 365: 891–900. [DOI] [PubMed] [Google Scholar]
- 3. Chan A, King JF, Flenady V, Haslam RH, Tudehope DI. Classification of perinatal deaths: development of the Australian and New Zealand classifications. J Paediatr Child Health 2004; 40: 340–7. [DOI] [PubMed] [Google Scholar]
- 4. Verhagen AA, Dorscheidt JH, Engels B, Hubben JH, Sauer PJ. End‐of‐life decisions in Dutch Neonatal Intensive Care Units. Arch Pediatr Adolesc Med 2009; 163: 895–901. [DOI] [PubMed] [Google Scholar]
- 5. Verhagen AA, Janvier A, Leuthner SR, Andrews B, Lagatta J, Bos AF, et al. Categorizing neonatal deaths: a cross‐cultural study in the United States, Canada and The Netherlands. J Pediatr 2010; 156: 33–7. [DOI] [PubMed] [Google Scholar]
- 6. Chow S, Chow R, Popovic M, Lam M, Popovic M, Merrick J, et al. A selected review of the mortality rates of neonatal intensive care units. Front Public Health 2015; 3: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lam V, Kain N, Joynt C, van Manen MA. A descriptive report of end‐of‐life decisions care practices occurring in two neonatal intensive care units. Palliat Med 2016; 30: 971–8. [DOI] [PubMed] [Google Scholar]
- 8. Biban P, Spaggiari S. “Cohabitation” between NICU and PICU. J Matern Fetal Neonatal Med 2011; 24: 91–3. [DOI] [PubMed] [Google Scholar]
- 9. Melve K, Skjaerven R. Birthweight and perinatal mortality: paradoxes, social class, and sibling dependencies. Int J Epidemiol 2003; 32: 625–32. [DOI] [PubMed] [Google Scholar]
- 10. Gaiva MAM, Fujimori E, Sato APS. Neonatal mortality in infants with low birth weight. Rev Esc Enferm US 2014; 48: 778–85. [DOI] [PubMed] [Google Scholar]
- 11. Kramer H, Trampisch H, Rammos S, Giese A. Birth weight of children with congenital heart disease. Eur J Pediatr 1990; 149: 752–7. [DOI] [PubMed] [Google Scholar]
- 12. Wogu A, Loffredo C, Bebu I, Luta G. Mediation analysis of gestational age, congenital heart defects, and infant birth‐weight. BMC Res Notes 2014; 7: 926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kramer MS, Demissie K, Yang H, Platt RW, Sauvé R, Liston R. The Contribution of Mild and Moderate Preterm Birth to Infant Mortality. JAMA 2000; 284: 843. [DOI] [PubMed] [Google Scholar]
- 14. Cuttini M, Nadai M, Kaminski M, Hansen G, de Leeuw R, Lenoir S, et al. End‐of‐life decisions in neonatal intensive care: physicians’ self‐reported practices in seven European countries. Lancet 2000; 355: 2112–8. [DOI] [PubMed] [Google Scholar]
- 15. Devictor DJ, Latour JM. Forgoing life support: how the decision is made in European pediatric intensive care units. Intensive Care Med 2011; 37: 1881. [DOI] [PubMed] [Google Scholar]
- 16. Ryan CA, Byrne P, Kuhn S, Tyebkhan J. No resuscitation and withdrawal of therapy in a neonatal and a pediatric intensive care unit in Canada. J Pediatr 1993; 123: 534–8. [DOI] [PubMed] [Google Scholar]
- 17. Vrakking AM, van der Heide A, Onwuteaka‐Philipsen BD, Keij‐Deerenberg IM, van der Maas PJ, van der Wal G. Medical end‐of‐life decisions made for neonates and infants in the Netherlands, 1995–2001. Lancet 2005; 365: 1329–31. [DOI] [PubMed] [Google Scholar]
- 18. Larcher V, Craig F, Bhogal K, Wilkonson D, Brierley J. Making decisions to limit treatment in life‐limiting and life‐threatening conditions in children: a framework for practice. Arch Dis Child 2015; 100: 3–23. [DOI] [PubMed] [Google Scholar]
- 19. Chan LCN, Cheung HM, Poon TCW, Ma TP, Lam HS, Ng PC. End‐of‐life decision‐making for newborns: a 12‐year experience in Hong Kong. Arch Dis Child Fetal Neonatal Ed 2016; 101: 37–42. [DOI] [PubMed] [Google Scholar]
- 20. van Loenhout RB, van der Geest IMM, Vrakking AM, van der Heide A, Pieters R, van den Heuvel‐Eibrink MM. End‐of‐life Decisions in Pediatric Cancer Patients. J Palliat Med 2015; 18: 697–702. [DOI] [PubMed] [Google Scholar]
- 21. Willems DL, Verhagen AA, van Wijlick E. Infants’ Best Interests in End‐of‐life Care for Newborns. Pediatrics 2014; 134: 1163–8. [DOI] [PubMed] [Google Scholar]
- 22. Janvier A, Barringtion K, Farlow B. Communication with parents concerning withholding or withdrawing of life‐sustaining interventions in neonatology. Semin Perinatol 2014; 38: 38–46. [DOI] [PubMed] [Google Scholar]
- 23. Nederlandse Vereniging voor Kindergeneeskunde . Doen of laten? Grenzen van het medisch handelen in de neonatologie. Utrecht: Drukkerij den Daas BV, 1992. [Google Scholar]
- 24. Sands R, Manning JC, Vyas H, Rashid A. Characteristics of deaths in paediatric intensive care: a 10‐year study. Nurs Crit Care 2009; 14: 235–40. [DOI] [PubMed] [Google Scholar]
- 25. Slater A, Shann F, Pearson G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med 2003; 29: 278–85. [DOI] [PubMed] [Google Scholar]
- 26. Burns JP, Sellers DE, Meyer EC, Lewis‐Newby M, Truog RD. Epidemiology of Death in the Pediatric Intensive Care Unit at Five U.S. Teaching Hospitals. Crit Care Med 2001; 42: 2101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weiner J, Sharma J, Lantos J, Kilbride H. How Infants Die in the Neonatal Intensive Care Unit: trends From 1999 Through 2008. Arch Pediatr Adolesc Med 2011; 165: 630–4. [DOI] [PubMed] [Google Scholar]
- 28. Fontana MS, Farrell C, Gauvin F, Lacroix J, Janvier A. Modes of Death in Pediatrics: differences in the Ethical Approach in Neonatal and Pediatric Patients. J Pediatr 2013; 162: 1107–11. [DOI] [PubMed] [Google Scholar]
- 29. Wilkinson D, Weitz J. Dying later, surviving longer. Arch Dis Child 2016; 101: 783–4. [DOI] [PubMed] [Google Scholar]


