Abstract
α-Aminomethyl tetrazoles, recently made accessible by an Ugi multicomponent reaction (MCR), were shown to be excellent starting materials for a further Ugi MCR, yielding substituted N-methyl-2-(((1-methyl-1H-tetrazol-5-yl)methyl)amino)acetamides having four points of diversity in a library-to-library approach. The scope and limitations of the two-step sequence was explored by conducting more than 50 reactions. Irrespective of electron-rich and electron-deficient oxo-components and the nature of the isocyanide component, the reactions give excellent yields. Sterically less hindered α-aminomethyl tetrazoles give better yields of in further Ugi MCR. The target scaffold has four points of diversity and is finding applications to fill screening decks for high-throughput screening (HTS) in the European Lead Factory and in structure-based drug design.
Keywords: Ugi reaction, library-to-library approach, high-throughput screening, structure-based drug design, European Lead factory
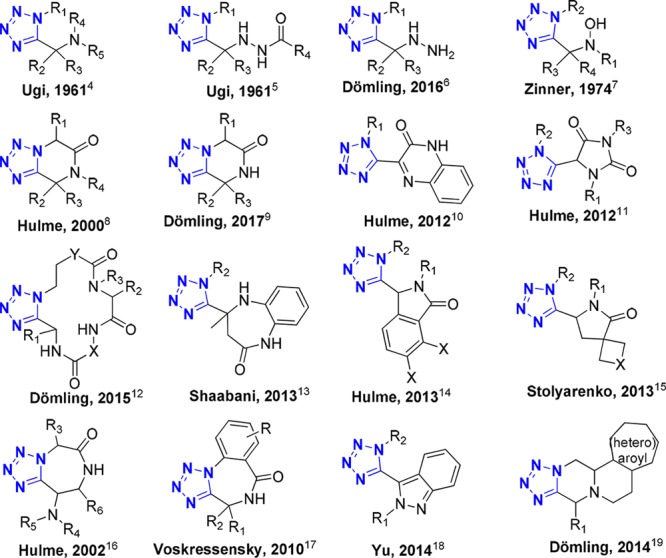
High-throughput screening (HTS) often yields poor or no results for difficult post-genomic targets, such protein–protein interactions. One potential reason is the overpopulation of certain types of molecular shapes in many pharmaceutical screening libraries, which are often based on the preferential use of certain reactions, such as Suzuki–Miyaura and Buchwald–Hartwig coupling processes. In other words, libraries are often designed with synthetic chemistry in mind rather than oriented toward targets and properties.1 Library generation employs familiar steps incorporating easy-to-functionalize groups (e.g., amine, OH, −CHO) addressed with standard commercial reagents (e.g., acid chlorides, boronic acids, sulfonyl chlorides). Multicomponent reaction chemistry different from this standard library approach in that MCRs build complex scaffolds in one step after which no further functionalization is needed or performed.2 We focus here on the tetrazole functional group, a metabolically stable and drug-like fragment accessible by MCR but largely underrepresented in screening libraries. Some MCR-prepared tetrazole scaffolds are shown in Scheme 1 and have been recently reviewed.3−19
Scheme 1. Sixteen Recently Disclosed Mono-, Bi-, Tri-, and Macrocyclic Tetrazole Scaffolds Accessible via Multicomponent Reactions.
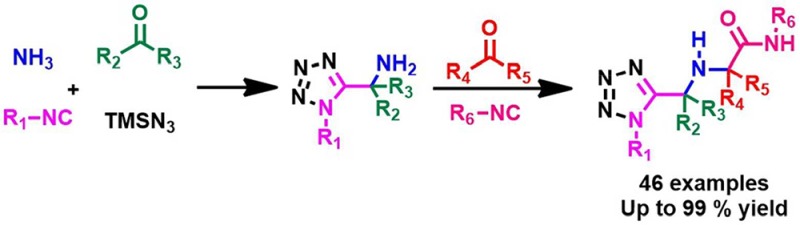
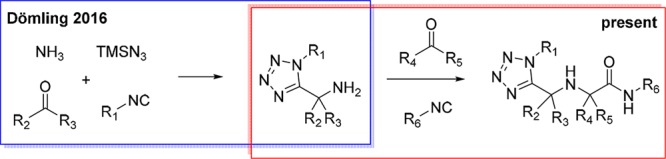
We have recently introduced a Ugi tetrazole variation in which ammonia can be used as an amine component and α-aminomethyl tetrazoles are formed in good yields and diversity.20 To take advantage of the large scope of the reaction, we decided to use the products of the Ugi tetrazole reaction as educts in another Ugi-3CR (Scheme 2), thus perusing a library-to-library approach.
Scheme 2. Ugi-3CR Reaction Presented Herein.
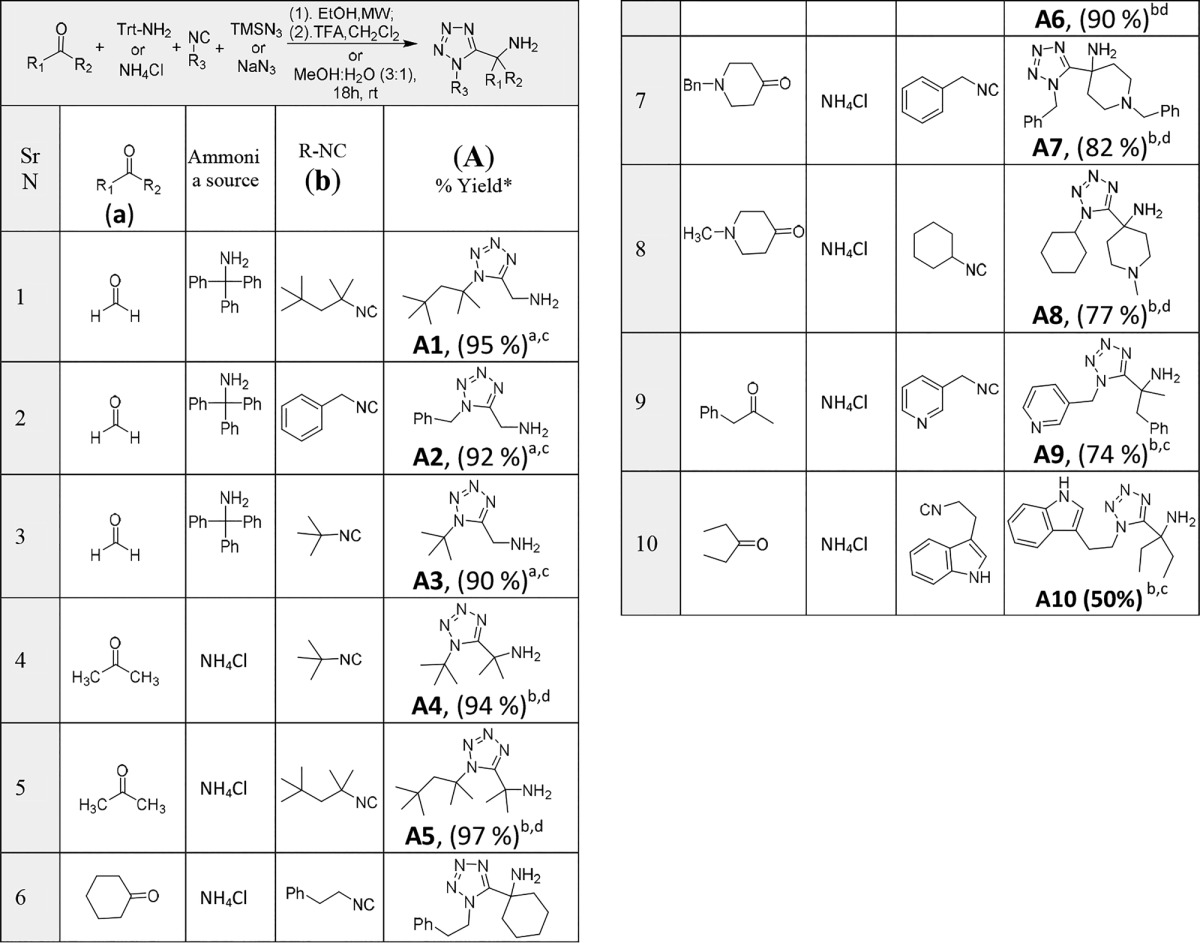
α-Amino monosubstituted methyl tetrazoles can be obtained from aldehydes, whereas α-amino disubstituted methyl tetrazoles are derived from ketones.20,21 To initiate the study, we scaled up few α-amino mono or disubstituted methyl tetrazoles with selected aldehydes and ketone (Table 1). These reactions proceeded at 10–25 mmol scale in the same manner as the previously reported 1–2 mmol scale under identical reaction conditions (entry 1–10, Table 1).
Table 1. Scale-up Synthesis of α-Aminomethyl Tetrazoles.
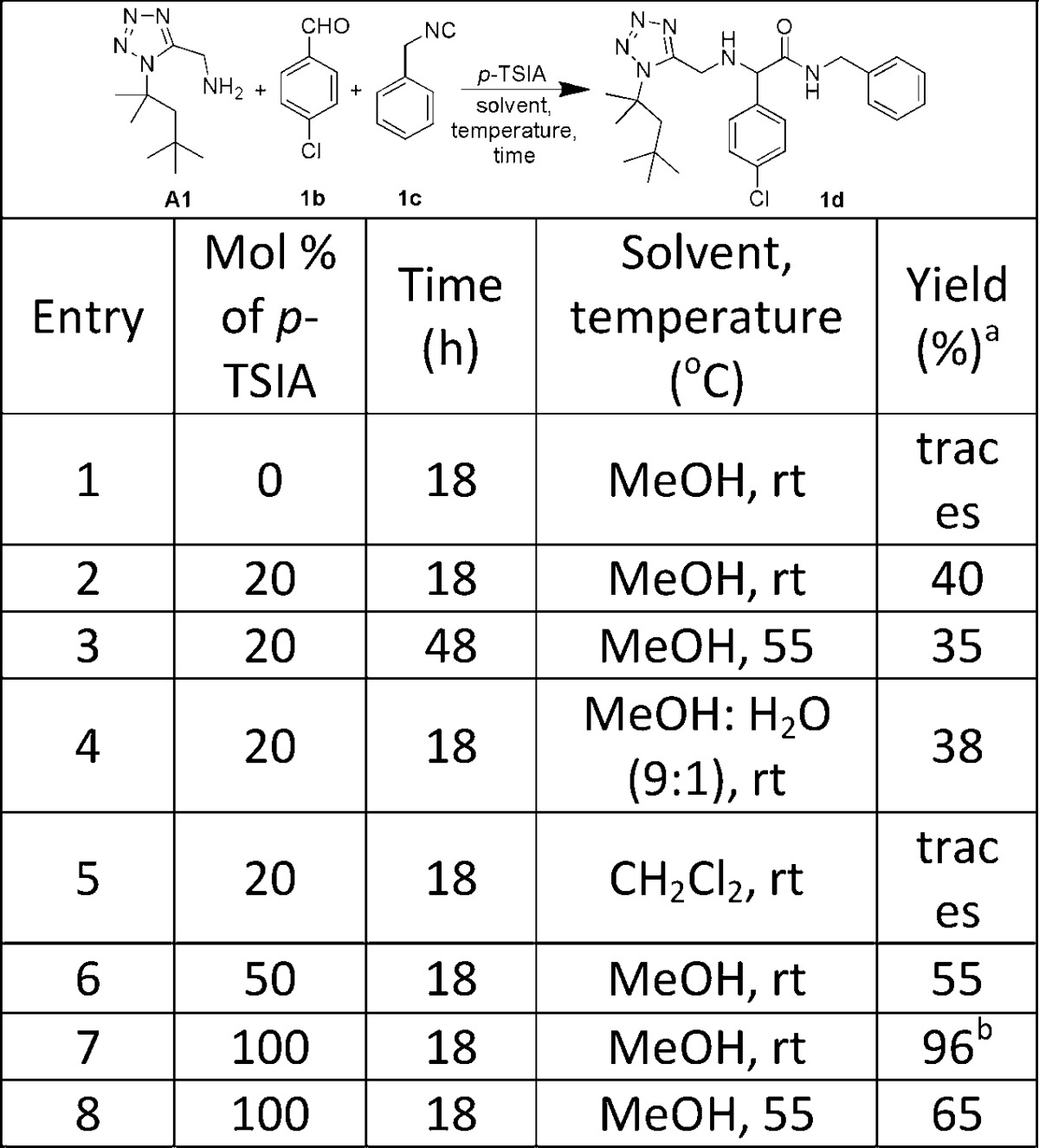
For the optimization of the reaction conditions, we tested the Ugi three-component reaction (U-3CR) of tert-octyltetrazolo-5-methylamine (A1), p-chlorobenzaldehyde (1b), and benzyl isocyanide (1c) with various Lewis acids, such as Sc(OTf)3, Al(OTf)3, Cu(OTf)3, Zn(OTf)3, ZnCl2, HClO4, TiCl4, ZrCl4, BCl3, B(OH)3, CH3SO3H, p-TSA in 10 to 20 mol % and HCl in methanol (1 equiv), in solvents, such as toluene, dichloromethane, and methanol. Disappointingly, all initial attempt failed to provide good yield of product 1d. Then, we increasing the reaction time with various temperature combinations from room temperature to 55 °C, but again we did not obtain satisfactory product 1d formation. Next, we following the procedures of List22 and Li23 and we tested this reaction with 10% phenyl phosphinic acid22 in toluene and 20% p-toluenesulfinic acid (p-TSIA)23 in methanol. Encouragingly, p-toluenesulfinic acid (20 mol %) in methanol stand out giving the desired product in moderate yield (1d, 40%). Thus, we selected p-TSIA to optimize the reaction conditions further with respect to solvent, temperature, reaction time and ratio of p-TSIA (Table 2).
Table 2. Optimize the Reaction Conditions with p-TSIA.
% yield confirmed by SFC-MS.
Isolated yield.
We observed that rising the reaction temperature (entry 3, Table2) and using methanol–water as 9:1 mixture to promote this reaction (entry 4, Table 2), also did not improve the yield. By changing the solvent from methanol to dichloromethane we found only trace product formation (entry 5, Table 2). Finally, we decided to use p-TSIA in an (semi)stochiometric amounts (entry 6–8, Table 2). Surprisingly, we observed the stochiometric use of p-TSIA at room temperature gave the product 1d in excellent 96% yield, while rising the temperature again resulted in lower yields.
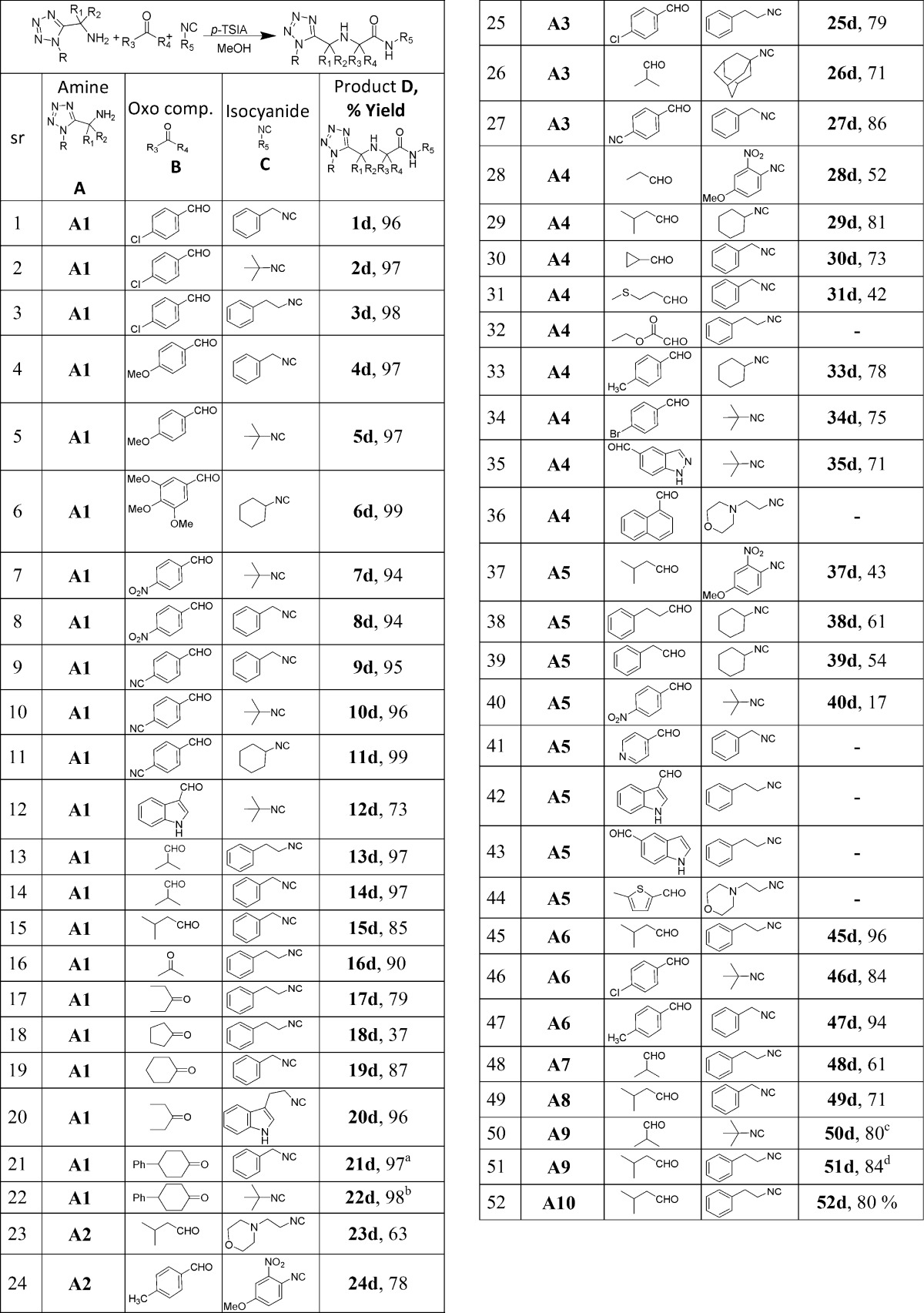
With these optimized reaction conditions in hand we initiated our study to explore the scope and limitations of the N-alkyl tetrazolo-5-methylamines (A), oxo components (B), and isocyanides (C) (Table 3).
Table 3. Ugi-3CR of Different Amino Methyl Tetrazoles with Different Oxo Components and Isocyanides.
Isolated yields.
Cis/trans ratio 4:3.
Cis/trans ratio 19:1.
Cis/trans ratio 3:2.
Cis/trans ratio 5:1.
First, the reaction of various oxo-components (aldehydes and ketones) and isocyanides with N-tert-octyl tetrazolo-5-methylamine (A1) as the amine component was studied (Table 3, entries 1–22). Aromatic, substituted aromatic and heterocyclic aldehydes, for example indole-3-carboxaldehyde (Table 3, 12b, 73%) gave good yields (Table 3, entries 1–12). The electronic properties of aromatic aldehydes did not influence the yields of the reactions (Table 3, entry 4–11). Aliphatic aldehydes and ketones including sterically demanding cyclic ketones, similarly, gave excellent yields (Table 3, entries 13–22). Moreover, the reaction of A3 with bulky 1-adamantyl isocyanide (26c) also gave good yield (26d, 71%). Use of hydrophilic 2-morpholinoethyl isocyanide resulted in lowering of the yield (23d, 63%), presumably due to loss of material during workup.
Furthermore, we extend the scope and limitation analysis toward the amine component using several other N-alkyl tetrazolo-5-α,α-disubstituted methylamines, such as A4–A10 (Table 3, entries 28–52). For example, the gem-dimethyl moiety is frequently used to improve PKPD and target engagement properties of compounds.24 Use of N-tert-butyl tetrazolo-5-α,α-dimethyl methylamine (A4) provided the product in 42–81% yields (Table 3, entry 28–36). Aromatic aldehydes gave excellent yields (Table 3, entry 33–35). When we used bulkier N-tert-octyl tetrazolo-5-α,α-dimethyl methylamine (A5), yields dropped as compared to N-tert-octyl tetrazolo-5-methylamine (A1). In this case, aromatic heterocyclic aldehydes failed to give any products (Table 3, entries 41–44).
Next, we investigated combinations of bulky α,α-disubstituted methylamines with N-tetrazolyl side chains, such as phenylethyl, benzyl, and cyclohexyl groups (Table 3, entry 45–52). Surprisingly, excellent results were also obtained in these cases (Table 3, entries 45–52).
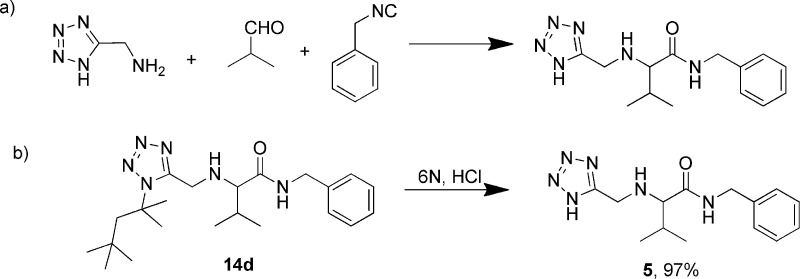
The same reaction strategy was also applied to N-H-tetrazolo-5-methylamine,25,26 as analogously to the report of Ley et al.27 (Scheme 3a) but no product could be isolated. However, we could synthesize a similar product (5) by acidic cleavage of the N-tert-octyl group of the intermediate Ugi adducts (14d). Usage of 6N aqueous hydrochloric acid and stirring overnight accomplished the product 5 in excellent yield (Scheme 3b).
Scheme 3. (a) Ugi-3CR Reaction of N-H-Tetrazolo-5-methylamine and (b) Deprotection of N-tert-Octyl Group.
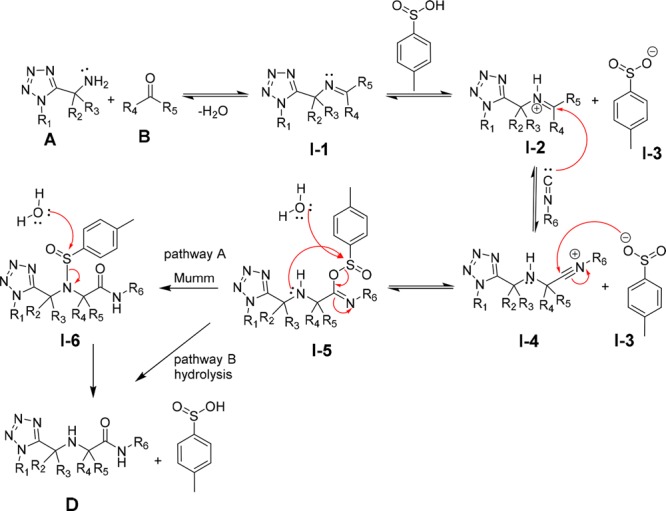
With these overall results, we propose a plausible reaction mechanism (Scheme 4). Accordingly, the reaction proceeds with N-alkyl tetrazolo-5-methylamines to form an imine (I-1), with loss of one equivalent of water. Protonation with p-toluenesulfinic acid activates the imine to yield the iminium ion (I-2), which then undergoes nucleophilic addition to the isocyanide (C) to give the intermediate nitrilium ion species (I-4). The nucleophilic trapping of this intermediate by the p-toluenesulfinate counteranion affords the p-toluenesulfinic imidoyl species (I-5). The final step is a Mumm rearrangement with the transfer of the p-toluenesulfinate group (I-3) from the oxygen atom to the nitrogen atom of the former amine (Scheme 4) to form p-toluenesulfinic amide (I-6, pathway A). Since p-toluenesulfinate is a good leaving group, it is replaced by the nucleophile water which was generated during the imine formation process. Alternatively, water attacks p-toluenesulfinic imidoyl species (I-5) to give product D without Mumm rearrangement (pathway B).
Scheme 4. Plausible Reaction Mechanism.
To confirm the structures of the final Ugi 3-CR products we could grow several crystals in ethanol for X-ray structure analysis. The resulting structures of 1d, 2d, 17d, 18d, and 22d are shown in Figure 1 and give some insight into the hydrogen bonding pattern of the α-amino tetrazole moiety.
Figure 1.
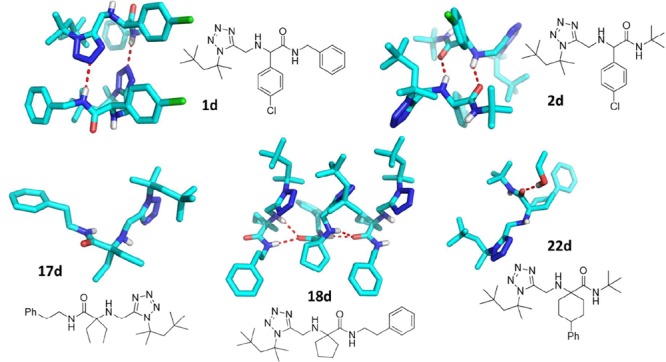
Crystal structure analysis and hydrogen bonding interactions (red dotted lines) of 1d, 2d, 17d, 18d, and 22d. Compound 1d for example is a noncovalent dimer formed by hydrogen bonds between tetrazole-N3 and the amide NH of the adjacent molecule.
In summary, we introduced a powerful library-to-library approach which can potentially span a large chemical space with four elements of diversity introduced by common building blocks, such as isocyanides and oxo components. A detailed analysis of the scope and limitations shows a great diversity of carbonyl components (including electron-rich and electron-deficient aldehydes, cyclic and acyclic ketones) to give mostly good to excellent yields, irrespective of the nature of the isocyanide component. Sterically less hindered N-alkyl tetrazolo-5-α,α-unsubstituted methylamines gave significantly better yields compared to N-alkyl tetrazolo-5-α,α-disubstituted methylamines. The scaffold is currently used in the European Lead Factory to enhance the screening deck.28 Moreover, efforts are ongoing to explore this rich and novel chemical space for islands of biological activity.
Acknowledgments
The work was financially supported by the NIH (NIH 2R01GM097082-05) and by the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115489, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in-kind contribution and was also supported by the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (Contract No. POIG.02.01.00-12-023/08). Moreover, funding has also been received from the European Union’s Horizon 2020 research and innovation program under MSC ITN “Accelerated Early stage drug dIScovery” (AEGIS, grant agreement No 675555), and CoFund ALERT (grant agreement No 665250).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscombsci.7b00137.
Crystallographic information file for compound 1d (CIF)
Crystallographic information file for compound 2d (CIF)
Crystallographic information file for compound 17d (CIF)
Crystallographic information file for compound 18d (CIF)
Crystallographic information file for compound 22d (CIF)
General methods, preparations of compound d, 1H NMR, 13C NMR and SFC-MS data and 1H NMR and 13C NMR spectra for compounds 1d–52d and 5, crystal structure determination, (PDF)
Author Contributions
The manuscript was written through contributions of P.P. and A.D. The crystallographic study contributed by K.K and K.J.
The authors declare no competing financial interest.
Supplementary Material
References
- a Brown D. G.; Boström J. Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone?. J. Med. Chem. 2016, 59, 4443–4458. 10.1021/acs.jmedchem.5b01409. [DOI] [PubMed] [Google Scholar]; b Roughley S. D.; Jordan A. M. The medicinal chemists toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 2011, 54, 3451. 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- a Dömling A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]; b Dömling A.; Wang W.; Wang K. Chemistry and Biology Of Multicomponent Reactions. Chem. Rev. 2012, 112, 3083–3135. 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kaur N. Synthesis of Five-Membered N,N,N- and N,N,N,N-Heterocyclic Compounds: Applications of Microwaves. Synth. Commun. 2015, 45, 1711–1742. 10.1080/00397911.2013.828756. [DOI] [Google Scholar]; b Maleki A.; Sarvary A. Synthesis of tetrazoles via isocyanide-based reactions. RSC Adv. 2015, 5, 60938–60955. 10.1039/C5RA11531K. [DOI] [Google Scholar]; c Sarvary A.; Maleki A. A review of syntheses of 1,5-disubstituted tetrazole derivatives. Mol. Diversity 2015, 19, 189–212. 10.1007/s11030-014-9553-3. [DOI] [PubMed] [Google Scholar]
- Ugi I.; Steinbrueckner C. Isonitrile, II. Reaktion von Isonitrilen mit carbonylverbindungen, Aminen und Stickstoffwasserstoffsäure. Chem. Ber. 1961, 94, 734–742. 10.1002/cber.19610940323. [DOI] [Google Scholar]
- Ugi I.; Bodesheim F. Isonitrile, VIII. Umsetzung von Isonitrilen mit Hydrazonen und Stickstoffwasserstoffsäure. Chem. Ber. 1961, 94, 2797–2801. 10.1002/cber.19610941031. [DOI] [Google Scholar]
- Patil P.; Zhang J.; Kurpiewska K.; Kalinowska-Tłuścik J.; Dömling A. Hydrazine in the Ugi Tetrazole Reaction. Synthesis 2016, 48, 1122–1130. 10.1055/s-0035-1561353. [DOI] [Google Scholar]
- Zinner G.; Moderhack D.; Hantelmann O.; Bock O. Hydroxylamine in der Vierkomponenten-Kondensation nach Ugi, II. Chem. Ber. 1974, 107, 2947–2955. 10.1002/cber.19741070918. [DOI] [Google Scholar]
- Nixey T.; Kelly M.; Hulme C. The one-pot solution phase preparation of fused tetrazole-ketopiperazines. Tetrahedron Lett. 2000, 41, 8729–8733. 10.1016/S0040-4039(00)01563-X. [DOI] [Google Scholar]
- Patil P.; Zhang J.; Kurpiewska K.; Kalinowska-Tłuścik J.; Dömling A. Ammonia-Promoted One-Pot Tetrazolopiperidinone Synthesis by Ugi Reaction. ACS Comb. Sci. 2017, 19, 343–350. 10.1021/acscombsci.7b00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawan S.; Nichol G.; Hulme C. Concise route to a series of novel 3-(tetrazol-5-yl)quinoxalin-2(1H)-ones. Tetrahedron Lett. 2012, 53, 1664–1667. 10.1016/j.tetlet.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medda F.; Hulme C. A facile and rapid route for the synthesis of novel 1,5-substituted tetrazole hydantoins and thiohydantoins via a TMSN 3-Ugi/RNCX cyclization. Tetrahedron Lett. 2012, 53, 5593–5596. 10.1016/j.tetlet.2012.07.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G.; Abdelraheem E.; Neochoritis C.; Kurpiewska K.; Kalinowska-Tłuścik J.; McGowan D.; Dömling A. Versatile Multicomponent Reaction Macrocycle Synthesis Using α-Isocyano-ω-carboxylic Acids. Org. Lett. 2015, 17, 4980–4983. 10.1021/acs.orglett.5b02419. [DOI] [PubMed] [Google Scholar]
- Shaabani A.; Hezarkhani Z.; Mofakham H.; Ng S. Synthesis of highly regioselective bifunctional tricyclic tetrazole-1 H -benzo[ b ][1,4]diazepins. Synlett 2013, 24, 1485–1492. 10.1055/s-0033-1338953. [DOI] [Google Scholar]
- Gunawan S.; Hulme C. Bifunctional building blocks in the Ugi-azide condensation reaction: a general strategy toward exploration of new molecular diversity. Org. Biomol. Chem. 2013, 11, 6036–6046. 10.1039/c3ob40900g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolyarenko V.; Evdokimov A.; Shishkin V. Synthesis of Tetrazole-substituted Spirocyclic γ-lactams by One-pot Azido-Ugi Reaction–cyclization. Mendeleev Commun. 2013, 23, 108–109. 10.1016/j.mencom.2013.03.020. [DOI] [Google Scholar]
- Nixey T.; Kelly M.; Semin D.; Hulme C. Short solution phase preparation of fused azepine-tetrazoles via a UDC (Ugi/de-Boc/cyclize) strategy. Tetrahedron Lett. 2002, 43, 3681–3684. 10.1016/S0040-4039(02)00636-6. [DOI] [Google Scholar]
- Borisov R.; Polyakov A.; Medvedeva L.; Khrustalev V.; Guranova N.; Voskressensky L. Concise Approach toward Tetrazolo[1,5-a][1,4]benzodiazepines via a Novel Multicomponent Isocyanide-Based Condensation. Org. Lett. 2010, 12, 3894–3897. 10.1021/ol101590w. [DOI] [PubMed] [Google Scholar]
- Wu R.; Gao S.; Chen X.; Yang G.; Pan L.; Hu G.; Jia P.; Zhong W.; Yu C. Synthesis of 1-(1H-Tetrazol-5-yl)-2H-isoindole Derivatives through Ugi Four-Component and Silver-Catalyzed Reactions. Eur. J. Org. Chem. 2014, 2014, 3379–3386. 10.1002/ejoc.201402098. [DOI] [Google Scholar]
- Patil P.; Khoury K.; Herdtweck E.; Dömling A. A Universal Isocyanide for Diverse Heterocycle Syntheses. Org. Lett. 2014, 16, 5736–5739. 10.1021/ol5024882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil P.; de Haan M.; Kurpiewska K.; Kalinowska-Tłuścik J.; Dömling A. Versatile Protecting-Group Free Tetrazolomethane Amine Synthesis by Ugi Reaction. ACS Comb. Sci. 2016, 18, 170–175. 10.1021/acscombsci.5b00189. [DOI] [PubMed] [Google Scholar]
- Zhao T.; Boltjes A.; Herdtweck E.; Doemling A. Org. Lett. 2013, 15, 639–641. 10.1021/ol303348m. [DOI] [PubMed] [Google Scholar]
- Pan S. C.; List B. Catalytic Three-Component Ugi Reaction. Angew. Chem., Int. Ed. 2008, 47, 3622–3625. 10.1002/anie.200800494. [DOI] [PubMed] [Google Scholar]
- Saha B.; Frett B.; Wang Y.; Li H. Y. A p-toluenesulfinic acid-catalyzed three-component Ugi-type reaction and its application for the synthesis of α-amino amides and amidines. Tetrahedron Lett. 2013, 54, 2340–2343. 10.1016/j.tetlet.2013.02.055. [DOI] [Google Scholar]
- Talele T. Natural-Products-Inspired Use of the gem-Dimethyl Group in Medicinal Chemistry. J. Med. Chem. 2017, 10.1021/acs.jmedchem.7b00315. [DOI] [PubMed] [Google Scholar]
- Vereshchagin L. I.; Petrov A. V.; Kizhnyaev V. N.; Pokatilov F. A.; Smirnov A. I. Polynuclear nonfused bis(1,3,4-oxadiazole)-containing systems. Russ. Russ. J. Org. Chem. 2006, 42, 1049–1055. 10.1134/S1070428006070219. [DOI] [Google Scholar]
- Matthews H.; Ranson M.; Tyndall J.; Kelso M. Synthesis and preliminary evaluation of amiloride analogs as inhibitors of the urokinase-type plasminogen activator (uPA). Bioorg. Med. Chem. Lett. 2011, 21, 6760–6766. 10.1016/j.bmcl.2011.09.044. [DOI] [PubMed] [Google Scholar]
- Franckevicius V.; Longbottom D.; Turner R.; Ley S. 8,9,10,10a-tetrahydro-6H-tetrazolo[1,5-a] pyrrolo[2,1-c]pyrazines: New heterocyclic frameworks generated by an Ugi-type multicomponent reaction. Synthesis 2006, 2006, 3215–3223. 10.1055/s-2006-950219. [DOI] [Google Scholar]
- a Besnard J.; Jones P. S.; Hopkins A. L.; Pannifer A. D. The joint european compound library: Boosting precompetitive research. Drug Discovery Today 2015, 20, 181–186. 10.1016/j.drudis.2014.08.014. [DOI] [PubMed] [Google Scholar]; b Karawajczyk A.; Giordanetto F.; Benningshof J.; Hamza D.; Kalliokoski T.; Pouwer K.; Morgentin R.; Nelson A.; Müller G.; Piechot D.; Tzalis D. Expansion of chemical space for collaborative lead generation and drug discovery: the European Lead Factory Perspective. Drug Discovery Today 2015, 20, 1310–1316. 10.1016/j.drudis.2015.09.009. [DOI] [PubMed] [Google Scholar]; c Mullard A. European lead factory opens for business. Nat. Rev. Drug Discovery 2013, 12, 173–175. 10.1038/nrd3956. [DOI] [PubMed] [Google Scholar]; d Nelson A.; Roche D. Innovative approaches to the design and synthesis of small molecule libraries. Bioorg. Med. Chem. 2015, 23, 2613. 10.1016/j.bmc.2015.02.046. [DOI] [PubMed] [Google Scholar]; e Paillard G.; Cochrane P.; Jones P. S.; Caracoti A.; van Vlijmen H.; Pannifer A. D.; et al. The ELF Honest Data Broker: informatics enabling public-private collaboration in a precompetitive arena. Drug Discovery Today 2016, 21, 97–102. 10.1016/j.drudis.2015.11.005. [DOI] [PubMed] [Google Scholar]; f The European Lead Factory. https://www.europeanleadfactory.eu/ (accessed on 21 Nov 2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.