Abstract
A recent study reveals sexually dimorphic disease-associated gene-expression modules and hub genes in postmortem brains from female and male individuals with depression. These modules are conserved in mouse models of depression.
Depression is a widespread, debilitating illness that is the leading cause of disability worldwide1. Rates of depression are two- to three-fold greater in women than in men, and previous studies conducted over past decades have reported differences in neurotransmitter, endocrine, and metabolic systems that could contribute to sex differences in mood susceptibility2–5. Despite these efforts, the heterogeneity of depression and the problems inherent in identifying illness-related gene variants have made it difficult to identify the molecular and neurobiological bases of depression, particularly, the greater incidence of the condition in women. In this issue, Labonté et al.6 use a bioinformatics approach7,8 to demonstrate that depression is characterized by different disease-associated gene modules in the postmortem brains of women relative to those in men, and that these modules are conserved in mice. The findings define for the first time the notable sexually dimorphic gene-expression networks in key brain regions that are implicated in depression, and so will help to elucidate the pathophysiology of and potential avenues for treating depression, particularly in women.
The authors analyzed gene-expression patterns in postmortem tissue from a total of 48 people in an initial cohort of female and male control individuals and those affected by major depressive disorder (MDD). Their study included analysis of tissue from six different brain regions implicated in depression: prefrontal cortical (PFC) subregions (ventromedial PFC (vmPFC), dorsolateral PFC, and orbital frontal PFC), anterior insula, nucleus accumbens, and ventral subiculum. They found sexual dimorphism in the levels of differentially expressed genes (DEGs) in brains from individuals with depression as compared to those from controls (with only a 5–10% overlap in differentially expressed genes between females and males with depression). Furthermore, there were sex differences in the regulation of interconnected gene networks that were conserved across brain regions in females and males with MDD. These findings were largely confirmed in a second cohort (50 additional female and male individuals, both control and with MDD), a major strength of this work. Further analysis of gene networks across brain regions identified sex- and depression-specific disease modules, with low levels of homology between brain regions in females and males. For example, of the 55 male-specific depression-associated gene modules, only ten showed conserved connectivity in females. Women and men also showed differential enrichment of depression-associated modules that are specific to different cell types and cell-specific functions.
Network analysis was also used to identify key sex-specific hub genes that are highly connected to multiple DEGs in and between disease modules and across different brain regions. In females with depression, one of the most highly ranked modules, on the basis of the number of interconnecting DEGs across brain regions, is enriched for MAPK activity and is composed of 18 genes, four of which are hubs with multiple down-regulated genes, as compared to controls, in multiple brain regions. In males with depression, one of the most highly ranked modules is enriched in genes associated with synaptic transmission; this module is composed of 311 genes, of which 43 are hubs with many upregulated transcripts, particularly in the vmPFC. The results suggest that these modules are subjected to sex-specific gene regulation that is distinctly associated with depression in each sex.
To study the functional impact of these gender-specific depression modules and hubs, the authors turned to a mouse model of depression9,10, in which chronic variable stress (CVS) exposure for 21 d produces depression-like behavior, including increased anxiety and anhedonia, both of which are core symptoms of depression. The fact that CVS produces similar levels of depressive behaviors in both males and females demonstrates a limitation of this model. Nevertheless, transcript analysis in the Labonté et al.6 study shows very low overlap of DEGs (20–25%) between female and male mice exposed to CVS in the two brain regions examined (the vmPFC and nucleus accumbens). Importantly, the results also show substantial overlap of DEGs, modules, and hub genes in female mice exposed to CVS as compared to women with depression, as well as in male mice exposed to CVS relative to men with depression (for example, in the vmPFC, there are 251 up- or downregulated transcripts conserved in females, and 152 in males), demonstrating that the CVS-exposed mouse is a valid model system.
The authors’ findings in humans show that DUSP6, a protein phosphatase that inactivates ERK signaling, is a highly interconnected hub gene that is decreased in the vmPFC of females with depression, as is Dusp6 in mice exposed to CVS. In men with depression, the authors identified a different module and highly connected hub gene, the transcription factor EMX1, which regulates the development of cortical neurons, that is increased in vmPFC of males with depression and CVS mice. In female mice, a reduction in Dusp6 expression in the vmPFC was sufficient to increase susceptibility to depressive behaviors, such as increased latency before feeding and decreased sucrose preference, but male mice with reduced expression of this gene in the vmPFC had no discernible changes in behavior. Furthermore, these mice also had gene-transcript alterations that overlapped with the changes caused by CVS, which provides evidence that this hub gene is a key regulator of multiple, connected genes and depression-associated modules. Conversely, overexpression of EMX1 in the vmPFC reproduced some, but not all, of the depressive behaviors in males, such as increased anxiety but not anhedonia, which indicates that other hub genes are involved in the development of depressive behaviors in males. Importantly, overexpression of Emx1 in the vmPFC of female mice had no effect on their behavior. Finally, decreased DUSP6 or increased EMX1 expression resulted in elevated activity of vmPFC pyramidal neurons in females and males, respectively, demonstrating a functional convergence of these two hub genes that could underlie the similar behavioral deficits observed in both sexes.
The demonstration of sex-specific, functional illness-related gene modules represents a major advance for the field of depression. It will be interesting to confirm these findings in additional postmortem cohorts of females and males with MDD, as well as in other mouse models of depression. These findings also raise many more questions regarding illness modules in other brain regions implicated in depression (such as the hippocampus and amygdala). For example, a previous study reported increased expression of a related gene, DUSP1, in the hippocampus of individuals with depression, and the expression of Dusp1 was necessary and sufficient to cause depression in male rodents11; females were not tested. What is the functional impact of this and other hub genes and modules in vmPFC and other brain regions? Additionally, which of these modules accounts for the higher rates of depression observed in women as compared to men? Importantly, what accounts for the development and the expression of gender-specific gene modules? Are these differences determined early in life by environmental, epigenetic, or X-linked genetic factors; later in life by endocrine and metabolic fluctuations; or by a combination of these factors across the life span? Are there other psychiatric illnesses that are also characterized by sex-specific modules? The current study provides a wealth of information that can be used by others in the field, and that opens the door for future studies to address these and other questions about sexually dimorphic determinants of complex behaviors that could lead to more informed treatment strategies and cures for depression.
Figure 1.
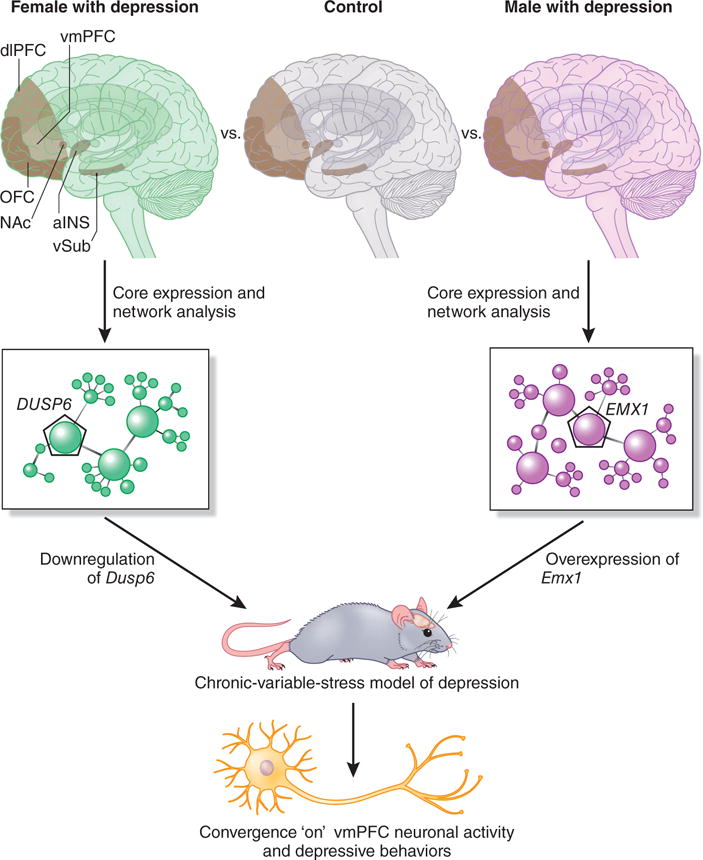
Sex-specific gene-expression modules in depression. Schematic for the analysis of gender-specific depression-related modules in individuals with MDD and stressed mice. RNA-seq analysis was conducted on the indicated regions of the postmortem brains of women and men with depression and those of controls, and on female and male mice exposed to chronic variable stress. Network analysis reveals sex-specific networks across brain regions and identifies disease-related gene modules in women and men that overlap with chronic-stress-related modules in female and male mice. Manipulation of key hub genes in the top-ranked expression modules (decreased Dusp1 in female mice and increased Emx1 in male mice) produces convergent effects on neuronal activity in vmPFC and causes depressive behaviors, but only when these genes are manipulated in the corresponding female or male mice. OFC, orbital frontal cortex; NAc, nucleus accumbens; aINS, anterior insula; vSub, ventral subiculum; dlPFC, dorsolateral prefrontal cortex; vmPFC, ventromedial prefrontal cortex.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Ferrari AJ, et al. PLoS Med. 2013;10:1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seney ML, et al. Front Psychiatry. 2013;4:104. doi: 10.3389/fpsyt.2013.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangasser DA, Valentino RJ. Front Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiller CE, Johnson SL, Abate AC, Schmidt PJ, Rubinow DR. Compr Physiol. 2016;6:1135–1160. doi: 10.1002/cphy.c150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otte C, et al. Nat Rev Dis Primers. 2016;2:16065. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 6.Labonté B, et al. Nat Med. 2017;23:1102–1111. doi: 10.1038/nm.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gustafsson M, et al. Genome Med. 2014;6:82. doi: 10.1186/s13073-014-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowen L, Ideker T, Raphael BJ, Sharan R. Nat Rev Genet. 2017 doi: 10.1038/nrg.2017.38. http://dx.doi.org/10.1038/nrg.2017.38. [DOI] [PubMed]
- 9.Hodes GE, et al. J Neurosci. 2015;35:16362–16376. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willner P. Neurobiol Stress. 2017;6:78–93. doi: 10.1016/j.ynstr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duric V, et al. Nat Med. 2010;16:1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]


