Abstract
Background
Migraine is considered as a risk factor for subclinical brain ischemic lesions, and right-to-left shunt (RLS) is more common among migraineurs. This cross-sectional study assessed the association of RLS with the increased prevalence of subclinical ischemic brain lesions in migraineurs.
Methods
We enrolled 334 migraineurs from a multicentre study from June 2015 to August 2016. Participants were all evaluated using contrast-enhanced transcranial Doppler, magnetic resonance imaging (MRI), and completed a questionnaire covering demographics, the main risk factors of vascular disease, and migraine status. RLS was classified into four grades (Grade 0 = Negative; Grade I = 1 ≤ microbubbles (MBs) ≤ 10; Grade II = MBs > 10 and no curtain; Grade III = curtain). Silent brain ischemic infarctions (SBI) and white matter hyperintensities (WMHs) were evaluated on MRI.
Results
We found no significant differences between migraineurs with RLS and migraineurs without RLS in subclinical ischemic brain lesions.SBI and WMHs did not increase with the size of the RLS(p for trend for SBI = 0.066, p for trend for WMHs = 0.543). Furthermore, curtain RLS in migraineurs was a risk factor for the presence of SBI (p = 0.032, OR = 3.47; 95%CI: 1.12−10.76). There was no association between RLS and the presence of WMHs.
Conclusion
Overall, RLS is not associated with increased SBI or WMHs in migraineurs. However, when RLS is present as a curtain pattern, it is likely to be a risk factor for SBIs in migraineurs.
Trial registration
No. NCT02425696; registered on April 21, 2015.
Electronic supplementary material
The online version of this article (10.1186/s12883-018-1022-7) contains supplementary material, which is available to authorized users.
Keywords: Infarction, Magnetic resonance imaging, Migraine, Patent foramen ovale, Transcranial Doppler ultrasonography, White matter
Background
Migraine, which is the most common type of primary headache encountered in the clinic, affects daily life and even causes ischemic events in sufferers. The relationship between migraine and subclinical brain ischemic lesions, including silent brain infarctions (SBI) and white matter hyperintensities (WMHs) on magnetic resonance imaging (MRI), is complicated and disputed [1–5]. Several studies have reported that SBI and WMHs were also more prevalent in subjects with migraine [1, 3, 6], especially migraine with aura (MA) [2, 7].
Furthermore, right-to-left shunt (RLS) may be a shared risk factor in migraine and subclinical brain ischemic lesions. The relationship between RLS and migraine has been widely investigated [8–10]. Several case−control analyses have indicated that RLS is more common in patients who suffer MA than normals [10, 11]. RLS, and particularly large RLS, caused mainly by a patent foramen ovale (PFO) [12], is also considered to be a cause of cryptogenic stroke (CS) in young patients [13].
Nevertheless, the role of RLS in migraineurs with subclinical brain ischemic lesions remains uncertain. To investigate whether RLS is an etiological factor for subclinical brain ischemic lesions in migraineurs, we cooperated with eight other centres to collect subjects for uniform assessment of subclinical ischemic brain lesions in migraineurs with and without RLS by MRI. In the present study, we evaluated whether, in migraineurs, (1) RLS per se, or a particular size/subtype of RLS, is associated with a higher incidence of SBI and WMHs; (2) RLS is associated with increased SBI located in the posterior circulation; (3) deep WMHs (dWMHs) is more commonly present in subjects with RLS than those without RLS.
Methods
Study population
The study procedures were approved by the Ethics Committee of the First Hospital of Jilin University (clinical trial no. NCT02425696; registered on April 21, 2015).All patients provided written informed consent prior to participation. All methods were carried out in accordance with the approved guidelines.
From June 2015 to August 2016, we consecutively enrolled patients from nine medical centres in China; the patients were aged between 18 and 70 years, and were diagnosed with migraine through a questionnaire based on the International Classification of Headache Disorders, 3rd edition beta version (ICHD-3beta; Headache Classification Committee of the International Headache Society, 2013). All subjects have underwent neurological examination and were screened by transcranial Doppler (TCD), contrast-enhanced TCD (c-TCD), MRI, and a questionnaire to obtain information on demographic characteristics, the main risk factors of vascular disease, and migraine status. The main risk factors of vascular disease include body mass index (BMI), hypertension, diabetes, heart disease (including atrial fibrillation and coronary heart disease), and smoking status.
Patients with the following characteristics were excluded: (1) intracranial or extracranial artery stenosis or occlusion; (2) incomplete MRI or c-TCD, an insufficient temporal window, or who could not perform the Valsalva manoeuvre (VM) due to cognitive disorder, or severe heart or lung disease.
c-TCD protocol
c-TCD examinations were performed by using a hand-held 2-MHz probe connected to the TCD detector (EMS-9A or 9 PB, Delica, China). The procedure was performed by experienced ultrasound technologists blinded to migraine diagnosis and MRI findings. Before the test, patients were asked to practice a standardized VM. Briefly, an 18-gauge catheter was inserted into the patient’s right antecubital vein. Contrast agent was prepared, using 9 ml isotonic saline solution, 1 ml of air, and a drop of the patient’s blood, which was mixed vigorously between two 10-ml syringes through a three-way stopcock, and was injected with the participant in the supine position [14]. After 30 mixing cycles, the contrast agent was injected as a rapid bolus while insonating the left middle cerebral artery (MCA) through the temporal bone window. The insonation lasted 20 s from the injection. The procedure was carried out three times: in the first measurement, injection was performed during normal respiration to detect any permanent RLS. The second and third injections were performed 5 s prior to the start of a 10-s VM. The time interval between injections was at least 5 min.
The maximum number of microbubbles (MBs) was taken as the estimate of the maximum degree of shunt, which was recorded separately from the MCA during rest and after the VM [15]. On the basis of the standards reported by Serena et al., Jauss et al., and Yang et al. [15–17], a four-level RLS categorization, based on the MB count, was applied as follows: Grade 0 = Negative; Grade I = 1 ≤ MBs ≤ 10; Grade II = MBs > 10 and no curtain; Grade III = curtain (Fig. 1). RLS was considered permanent if it occurred during rest, and latent if it only occurred after a VM.
Fig. 1.
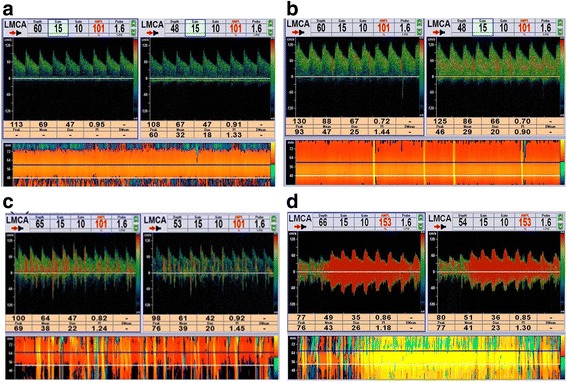
The four-level RLS categorization based on the microbubbles count. a Grade 0 = Negative; b Grade I = 1 ≤ MBs ≤ 10; c Grade II = MBs > 10 and no curtain; d Grade III = curtain. Abbreviations: RLS (right-to-left shunt); MBs (microbubbles)
MRI
We used 1.5-T scanners for whole-brain MRI, consisting of T1-weighted images (T1WI), T2-weighted images (T2WI), and fluid attenuated inversion recovery (FLAIR) images. SBIs were defined as non-mass parenchymal defects with a vascular distribution, isointense to cerebrospinal fluid signal on all sequences, and when supratentorial, surrounded by a hyperintense rim on FLAIR images, as shown in Fig. 2a. In the basal ganglia, only parenchymal defects larger than 3 mm in diameter were considered in order to exclude nonspecific lesions [5]. The SBI should be distinct from dilated vascular space (Virchow−Robin space) based on location, shape, size, and absence of a hyperintense border [6]. The location and number of the infarctions were recorded.
Fig. 2.
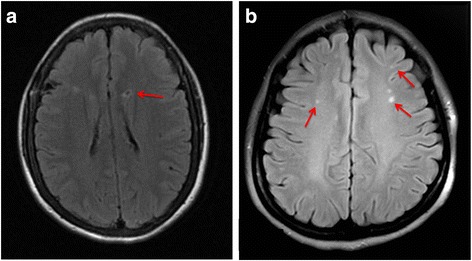
a A subclinical brain infarction in anterior circulation. b The white matter hyperintensity in deep white matter
WMHs were defined as clearly hyperintense areas relative to surrounding white matter on both FLAIR and T2WI and were identified by simultaneous inspection of both aligned images (Fig. 2b) [18]. T2 hyperintense lesions that were not hyperintense on FLAIR images were considered as enlarged Virchow–Robin spaces. Periventricular WMHs (pvWMHs) were assessed in three regions (frontal and posterior horns and bands); dWMHs were located in deep white-matter tracts and were not attached to lateral ventricle lesions [5, 6].
Using the above-mentioned criteria, all of the neuroimages from a multicentre database were read by two trained neurologists, who were blinded to any clinical data.
Statistical analysis
We used Pearson’s χ2 test to analyse discontinuous variables. For analysis of continuous variables, unpaired t-tests were used. The p-values were two-tailed, and p ≤ 0.05 was considered statistical significant. We used binary logistic regression models (odds ratio [OR], 95% confidence interval [CI]) to control age, sex, the main risk factors of vascular disease and aura for MRI outcomes among different groups, which were divided by the grade or type of RLS. All analyses were performed using IBM SPSS Statistics 20.0.
Results
This study included 412 migraineurs; 78 subjects were excluded (due to artery stenosis in 6, incomplete MRI in 7, incomplete c-TCD in 5, insufficient temporal window in 49, and improper VM execution in 11) (Fig. 3). Among the 334 subjects enrolled in our study, 224 subjects had a RLS (mean age 42.49 ± 10.97 years; 160 females) and 110 had no RLS (mean age 44.03 ± 10.12 years, 81 females). Demographic and clinical data of the subjects in groups with and without RLS are summarized in Table 1. Except that the prevalence of aura was higher in patients with RLS than in those without RLS (29.9% vs. 17.3%, p = 0.013), there were no significant differences between the two groups.
Fig. 3.
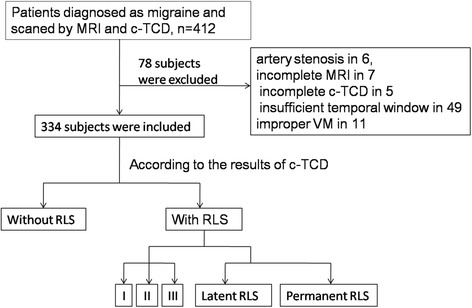
Flow chart of patient enrolment
Table 1.
Characteristics of the patients
| Characteristics | RLS | No RLS | P value |
|---|---|---|---|
| (n = 224) | (n = 110) | ||
| Demographics | |||
| Age (mean ± SD, years) | 42.49 ± 10.97 | 44.03 ± 10.12 | 0.147♯ |
| Female | 160 (71.4) | 81 (73.6) | 0.672* |
| The main risk factors of vascular disease | |||
| BMI (mean ± SD) | 22.98 ± 3.32 | 20.03 ± 3.61 | 0.281♯ |
| Hypertension | 25 (11.2) | 15 (13.6) | 0.513* |
| Diabetes | 5 (2.2) | 5 (4.5) | 0.244* |
| Heart disease | 6 (2.7) | 4 (3.6) | 0.629* |
| Smoking | 0.627* | ||
| Never | 189 (84.4) | 97 (88.2) | |
| Current | 23 (10.3) | 8 (7.3) | |
| Former | 12 (5.4) | 5 (4.5) | |
| Headache characteristics | |||
| Aura | 67 (29.9) | 19 (17.3) | 0.013* |
| Family history of headache | 65 (29.0) | 26 (23.6) | 0.299* |
| Age of onset | 31.01 ± 11.85 | 32.65 ± 11.92 | 0.776♯ |
| Years from the first attack | 11.84 ± 11.16 | 11.39 ± 10.45 | 0.731♯ |
| MRI Findings | |||
| 0.265* | |||
| SBI | 20 (8.9) | 6 (5.5) | |
| 0.066※ | |||
| Posterior circulation only | 10 (4.5) | 1 (0.9) | 0.166* |
| Single silent brain infarct | 12 (5.4) | 4 (3.6) | |
| 0.355* | |||
| WMHs | 122 (54.5) | 54 (49.1) | |
| 0.543※ | |||
| pvWMHs | 7 (3.1) | 2 (1.8) | |
| dWMHs | 94 (42.0) | 46 (41.8) | 0.980* |
| dWMHs and pvWMHs | 21 (9.4) | 6 (5.5) | |
Abbreviations: BMI body mass index, MRI magnetic resonance imaging, SBI silent brain infarctions, WMHs white matter hyperintensities, pvWMHs periventricular WMHs, dWMHs deep WMHs
Data are presented as n (%) unless otherwise specified
*Pearson’s χ2, unadjusted for aura;
♯unpaired t-test
※P for trend computed by binary logistic regression
We found 26 subjects with SBI among the migraineurs in this study; in 61.5% (16/26), the SBI was a single lesion. Compared with the no-RLS group, SBI (8.9% vs. 5.5%, p = 0.265) and WMHs (54.5% vs. 49.1%, p = 0.355) did not increase with RLS in migraineurs (Table 1). These results were still not statistically significant after adjustment for aura.
When comparing grade by grade, we found a p for trend for SBI = 0.066 (OR = 1.43; 95%CI: 0.98−2.09) and a p for trend for WMHs = 0.543, (OR = 1.07; 95%CI:0.86−1.32). The incidence of SBI in the posterior circulation of migraineurs with RLS was not different from that in those without RLS (p = 0.166). The presence of dWMHs did not increase with the appearance of RLS (42.0% vs. 41.8%, p = 0.980).
However, there was a statistically significant difference in the prevalence of SBI when the subjects were divided by Grade (0−III) (Table 2). The presence of SBI in the Grade III group (curtain RLS) was higher than that in the Grade 0 group (16.7% vs. 5.5%, p = 0.032, OR = 3.47; 95%CI: 1.12−10.76), when controlling for age, sex, aura, and the main vascular disease risk factors in binary logistic regression analysis (Additional file 1). We identified curtain RLS (Grade III) as a risk factor for SBI in migraineurs.
Table 2.
Prevalence of MRI findings by size of RLS
| SBI | WMHs | |||
|---|---|---|---|---|
| n (%) | OR(95%CI) | n (%) | OR(95%CI) | |
| Grade 0 n = 110 |
6 (5.5) | – | 54 (49.1) | – |
| Grade I n = 95 |
7 (7.4) | 1.60 (0.48−5.31) P = 0.447 |
53 (55.8) | 1.63(0.89−3.00) P = 0.114 |
| GradeII n = 63 |
2 (3.2) | 0.38(0.07−2.20) P = 0.281 |
34 (54.0) | 1.28 (0.63−2.57) P = 0.497 |
| Grade III n = 66 |
11 (16.7) | 3.47(1.12−10.76) P = 0.032 |
35 (53.0) | 1.22 (0.62−2.41) P = 0.563 |
Abbreviations: MRI magnetic resonance imaging, RLS right-to-left shunt, SBI silent brain infarctions, WMHs white matter hyperintensities
Odds ratio (OR) is computed by binary logistic regression, as compared to grade 0
When comparing permanent RLS and latent RLS with the group without RLS (Table 3), the incidence of SBI and WMHs were not statistically significantly different.
Table 3.
Prevalence of MRI findings by subtype of RLS
| SBI | WMHs | |||
|---|---|---|---|---|
| n (%) | OR(95%CI) | n (%) | OR(95%CI) | |
| No RLS n = 110 |
6 (5.5) | – | 54 (49.1) | – |
| Latent RLS n = 106 |
6 (4.7) | 2.53(0.87−7.40) P = 0.090 |
60 (56.6) | 1.26(0.70−2.24) P = 0.440 |
| Permanent RLS n = 118 |
14 (11.9) | 0.89(0.25−3.14) P = 0.851 |
62 (52.5) | 1.58(0.88−2.84) P = 0.129 |
Abbreviations: MRI magnetic resonance imaging, RLS right-to-left shunt, SBI silent brain infarctions, WMHs white matter hyperintensities
OR is computed by binary logistic regression compared to no RLS
Discussion
In our study, SBI or WMHs did not increase with the size of RLS, but when the RLS was presented as a curtain pattern, it appeared to be a risk factor for SBI in migraineurs in our study. There was no association between the presence of a RLS and the presence of WMHs in migraineurs. The presence of RLS per se did not increase either SBI or posterior cerebral circulation in migraineurs. The subtype of RLS (permanent and latent) had no effect on SBI or WMHs.
Curtain RLS and SBI in migraineurs
In order to determine the underlying relationship between the size of the RLS and SBI, we classified RLS into four grades according to the number of MBs. In total, of the 19.8% (66/334) individuals with curtain RLS, 16.7% (11/66) showed SBI. In the group without RLS, SBI was found in 5.5% (6/110) of the subjects. Compared to the no-RLS group, the incidence of SBI in the curtain pattern group was 2.4 times higher.
The source of the infarctions in patients with migraine is uncertain. There are several mechanisms underlying migraine-stroke, such as cortical spreading depression (CSD) [19], shared genetic risk factors for migraine and stroke, vasoconstrictor medications taken to treat headache [20], repeated or prolonged reduction of perfusion pressure, and reduced blood flow [21],which may produce thrombosis, embolism, or ischemic events. RLS, such as PFO, was closely associated with migraine [9, 15], and was also likely to increase ischemic brain lesions through paradoxical embolism, potentially leading to migraine attack or stroke. A case−control analysis denied the role of PFO in migraine-associated silent infarctions and ischemic stroke [22]. However, due to the relatively small sample size, they did not perform subgroup analysis based on the size of the PFO. In 1998, Serena et al. conducted a case−control study in which the prevalence of RLS in a nonselected group of patients consecutively admitted for cerebral infarction and TIA was compared with that in healthy control subjects, in order to study the relationship between the magnitude of RLS and stroke subtypes. Surprisingly they found that the curtain pattern was detected only in those with CS, but found no significant association between small RLS and the risk of stroke [16].
This study emphasized the importance of paying attention to curtain RLS. In a previous study about RLS subtypes in Chinese patients with CS, the results suggested that a large RLS was a pathological condition in CS [13]. This is not unexpected if we assume that a larger RLS would increase the risk of paradoxical embolism. Moreover, it has been found that dynamic cerebral autoregulation is impaired in migraineurs with a large RLS, and this may represent a potential mechanism linking RLS, migraine, and CS [23].
Permanent RLS and SBI in migraineurs
Permanent RLS, which was regarded as dangerous RLS, was reported to increase ischemic cerebral lesions and previous recurrent stroke [24].Given that permanent RLS could occur during rest and without the induction of VM or similar actions, we could hypothesize that permanent RLS may pose a higher risk of paradoxical embolism. In our study, the frequency of SBI was in fact higher in the permanent RLS group than in the latent RLS and no-RLS group (11.9% vs. 4.7% vs. 5.5%), although the differences were not statistically significant. A larger sample should be established to confirm the role of permanent RLS in the occurrence of SBI.
RLS and SBI located in the posterior circulation in migraineurs
Kruit et al. found that the prevalence of SBI located in the posterior circulation was higher in migraineurs, particularly when located in the cerebellum, in a population-based MRI CAMERA study [25]. However, RLS is reportedly not associated with specific ischemic patterns [26]. Our study did not find a higher frequency of SBI in the posterior circulation in the RLS group than in the no-RLS group. Due to the limited number of samples with SBI, we did not investigate the relationship between different RLS sizes and SBI in the posterior circulation further, and therefore, we cannot exclude an underlying connection.
RLS and WMHs in migraineurs
It has been reported that migraine patients have a 4-fold higher risk of having white matter lesions than no-migraine subjects [27], and dWMHs have attracted much attention [1]. However, the mechanisms underlying white matter lesions are not completely understood and they might be associated with ischemic complications of various microvascular processes, such as ischemia, oxidative stress, energy deprivation, hypoglycaemia, or platelet hypercoagulability [6].In terms of RLS and WMHs, Del Sette et al. have reported that RLS does not increase the number and volume of WMHs in migraineurs [28]. In our study, we only focused on the presence and location of WMHs and did not measure the number and the load of lesions. We found no relationship between RLS and the presence of WMHs or dWMHs in migraineurs, which is in line with the findings of other studies [5]. However, a study in South Korea found that small dWMHs were associated with RLS in migraineurs [29]. It might be inferred that a stronger relationship is likely to exist between WMHs and RLS.
The effect of RLS closure
The effect of RLS closure as a treatment has not been clear. RLS closure may reduce stroke recurrence and migraine attacks [30–32]. However, numerous randomized clinical trials have reported that, as a secondary prevention of cryptogenic embolism, PFO closure did not significantly reduce the risk of recurrent embolic events or death, as compared with medical therapy [33, 34]. In a study published in the New England Journal of Medicine in 2013 [33], the primary end-point, such as death, nonfatal stroke, TIA, or peripheral embolism, occurred at a frequency of 3.4% (7/204) in the PFO-closure group; this low-probability event requires validation in a sizeable sample. Given the results of our study, it could be considered whether the selection of patients with curtain RLS for PFO-closure treatment may improve the preventative effect. Randomized controlled trials may clarify this, and may better reflect the role of curtain RLS in mediating migraine and silent brain lesions.
Strengths and limitations
In the past, in similar studies about the relationship between RLS and subclinical cerebral lesions, investigators generally classified the size of RLS as absent, small, medium, or large, according to the number of MBs observed. However, we singled out curtain RLS alone and identified it as a risk factor for SBI. In addition, a strength of our study is its unified multicentre design. We included well-defined migraine patients with or without aura, and excluded individuals with other types of headaches, e.g., chronic daily headache, tension headache, or cluster headache. We considered the subjects’ demographics, medical history, and migraine status by means of a questionnaire, and the complete c-TCD and MRI evaluation data of all patients.
Yet, our study also had some potential limitations. Overall, there were only 26 patients with SBI; the incidence of SBI in subjects with RLS was only 8.9%, which was a low-probability event and might result in inadequate statistical power. The number of subjects with the different grades of RLS was not sufficiently large, and the sample size of our study (n = 334) was not quite adequate to draw concrete conclusions. Secondly, c-TCD tests and MRI scans for subjects were performed in different centres, which led to unavoidable differences in sensitivity. In addition, the data were obtained in patients from hospital departments, who may have been more severely affected than the average migraineur. Thus, it is uncertain whether and to what degree these conclusions can be applied to all migraineurs. Moreover, our study was based on real-world observations, and thus we cannot discriminate the real causes of embolism. Further studies are needed to expand the sample size to verify that curtain RLS is indeed a risk factor for SBI in migraine. Furthermore, it should be investigated whether the prevalence of SBI in migraineurs with curtain RLS is associated with aura, and whether curtain RLS closure has a protective effect.
Conclusions
In the present study, we concluded that subclinical ischemic lesions were neither more prevalent in migraineurs with RLS than in migraineurs without RLS, nor increased with the size of the RLS. Nevertheless, curtain RLS may be a risk factor for silent brain infarctions.
Additional file
Binary logistic regression test for possible factors of SBI. (DOCX 16 kb)
Acknowledgments
We thank all of the subjects and medical staff for their assistance with this study.
Funding
No Funding.
Availability of data and materials
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- CS
Cryptogenic stroke
- c-TCD
contrast-enhanced TCD
- dWMHs
deep WMHs
- FLAIR
Fluid attenuated inversion recovery
- MA
Migraine with aura
- MBs
Microbubbles
- MCA
Middle cerebral artery
- MRI
Magnetic resonance imaging
- OR
Odds ratio
- PFO
Patent foramen ovale
- pvWMHs
periventricular WMHs
- RLS
Right-to-left shunt
- SBI
Silent brain infarctions
- T1WI
T1-weighted image
- T2WI
T2-weighted image
- TCD
Transcranial Doppler
- VM
Valsalva manoeuvre
- WMHs
White matter hyperintensities
Authors’ contributions
Y-qX designed the study. S-bW, Q-T, C-Z, G-lZ, Y-jL, P-L, Y-You, R-G and Y-hC enrolled the participants, recorded and collection the data, and revised the study design and the manuscript. X-hJ wrote the main manuscript text and analysed the data. All authors reviewed the manuscript, while Y-qX revised the text. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study procedures were approved by the Ethics Committee of the First Hospital of Jilin University. The number of approval letter was 2015–180. All patients provided written informed consent prior to participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12883-018-1022-7) contains supplementary material, which is available to authorized users.
Contributor Information
Xiao-han Jiang, Email: xiaohan.jiang1213@foxmail.com.
Si-bo Wang, Email: wangsibo92@163.com.
Qian Tian, Email: 13969927563@163.com.
Chi Zhong, Email: zhong7376_cn@sina.com.
Guan-ling Zhang, Email: 1627990831@qq.com.
Ya-jie Li, Email: 404685136@qq.com.
Pan Lin, Email: zy277299@126.com.
Yong You, Email: youy_usc@qq.com.
Rong Guo, Email: 2451325619@qq.com.
Ying-hua Cui, Email: cuiyinghua196899@126.com.
Ying-qi Xing, Phone: 86-15844047846, Email: xingyq2009@sina.com.
References
- 1.Kruit MC, van Buchem MA, Launer LJ, Terwindt GM, Ferrari MD. Migraine is associated with an increased risk of deep white matter lesions, subclinical posterior circulation infarcts and brain iron accumulation: the population-based MRI CAMERA study. Cephalalgia. 2010;30(2):129–136. doi: 10.1111/j.1468-2982.2009.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurth T, Mohamed S, Maillard P, Zhu YC, Chabriat H, Mazoyer B, Bousser MG, Dufouil C, Tzourio C. Headache, migraine, and structural brain lesions and function: population based epidemiology of vascular ageing-MRI study. BMJ. 2011;342:c7357. doi: 10.1136/bmj.c7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruchalla E. Migraine: structural brain changes in MRI. JAMA. 2013;185(12):1130. doi: 10.1055/s-0033-1346802. [DOI] [PubMed] [Google Scholar]
- 4.Monteith T, Gardener H, Rundek T, Dong C, Yoshita M, Elkind MS, DeCarli C, Sacco RL, Wright CB. Migraine, white matter hyperintensities, and subclinical brain infarction in a diverse community: the northern Manhattan study. Stroke. 2014;45(6):1830–1832. doi: 10.1161/STROKEAHA.114.005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koppen H, Palm-Meinders IH, Mess WH, Keunen RW, Terwindt GM, Launer LJ, van Buchem MA, Kruit MC, Ferrari MD. Systemic right-to-left shunts, ischemic brain lesions, and persistent migraine activity. Neurology. 2016;86(18):1668–1675. doi: 10.1212/WNL.0000000000002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kruit MC, van Buchem MA, Hofman PA, Bakkers JT, Terwindt GM, Ferrari MD, Launer LJ. Migraine as a risk factor for subclinical brain lesions. JAMA. 2004;291(4):427–434. doi: 10.1001/jama.291.4.427. [DOI] [PubMed] [Google Scholar]
- 7.Scher AI, Gudmundsson LS, Sigurdsson S, Ghambaryan A, Aspelund T, Eiriksdottir G, van Buchem MA, Gudnason V, Launer LJ. Migraine headache in middle age and late-life brain infarcts. JAMA. 2009;301(24):2563–2570. doi: 10.1001/jama.2009.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilmshurst P, Nightingale S. Relationship between migraine and cardiac and pulmonary right-to-left shunts. Clin Sci (Lond) 2001;100(2):215–220. doi: 10.1042/cs1000215. [DOI] [PubMed] [Google Scholar]
- 9.Domitrz I, Mieszkowski J, Kaminska A. Relationship between migraine and patent foramen ovale: a study of 121 patients with migraine. Headache. 2007;47(9):1311–1318. doi: 10.1111/j.1526-4610.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 10.Wammes-van der Heijden EA, Tijssen CC, Egberts AC. Right-to-left shunt and migraine: the strength of the relationship. Cephalalgia. 2006;26(2):208–213. doi: 10.1111/j.1468-2982.2005.01024.x. [DOI] [PubMed] [Google Scholar]
- 11.Anzola GP, Magoni M, Guindani M, Rozzini L, Dalla Volta G. Potential source of cerebral embolism in migraine with aura: a transcranial Doppler study. Neurology. 1999;52(8):1622–1625. doi: 10.1212/WNL.52.8.1622. [DOI] [PubMed] [Google Scholar]
- 12.Schwerzmann M, Nedeltchev K, Lagger F, Mattle HP, Windecker S, Meier B, Seiler C. Prevalence and size of directly detected patent foramen ovale in migraine with aura. Neurology. 2005;65(9):1415–1418. doi: 10.1212/01.wnl.0000179800.73706.20. [DOI] [PubMed] [Google Scholar]
- 13.Xu WH, Xing YQ, Yan ZR, Jiang JD, Gao S. Cardiac right-to-left shunt subtypes in Chinese patients with cryptogenic strokes: a multicenter case-control study. Eur J Neurol. 2014;21(3):525–528. doi: 10.1111/ene.12351. [DOI] [PubMed] [Google Scholar]
- 14.Hao N, Liu K, Guo ZN, Wu X, Yang Y, Xing Y. Comparison of two contrast agents for right-to-left shunt diagnosis with contrast-enhanced transcranial Doppler. Ultrasound Med Biol. 2014;40(9):2317–2320. doi: 10.1016/j.ultrasmedbio.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Guo ZN, Wu J, Jin H, Wang X, Xu J, Feng J, Xing Y. Prevalence and extent of right-to-left shunt in migraine: a survey of 217 Chinese patients. Eur J Neurol. 2012;19(10):1367–1372. doi: 10.1111/j.1468-1331.2012.03793.x. [DOI] [PubMed] [Google Scholar]
- 16.Serena J, Segura T, Perez-Ayuso MJ, Bassaganyas J, Molins A, Davalos A. The need to quantify right-to-left shunt in acute ischemic stroke: a case-control study. Stroke. 1998;29(7):1322–1328. doi: 10.1161/01.STR.29.7.1322. [DOI] [PubMed] [Google Scholar]
- 17.Jauss M, Zanette E. Detection of right-to-left shunt with ultrasound contrast agent and transcranial Doppler sonography. Cerebrovasc Dis. 2000;10(6):490–496. doi: 10.1159/000016119. [DOI] [PubMed] [Google Scholar]
- 18.Gaist D, Garde E, Blaabjerg M, Nielsen HH, Kroigard T, Ostergaard K, Moller HS, Hjelmborg J, Madsen CG, Iversen P, et al. Migraine with aura and risk of silent brain infarcts and white matter hyperintensities: an MRI study. Brain. 2016;139(Pt 7):2015–2023. doi: 10.1093/brain/aww099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MJ, Lee C, Chung CS. The migraine-stroke connection. J Stroke. 2016;18(2):146–156. doi: 10.5853/jos.2015.01683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Zotto E, Pezzini A, Giossi A, Volonghi I, Padovani A. Migraine and ischemic stroke: a debated question. J Cereb Blood Flow Metab. 2008;28(8):1399–1421. doi: 10.1038/jcbfm.2008.36. [DOI] [PubMed] [Google Scholar]
- 21.Bednarczyk EM, Remler B, Weikart C, Nelson AD, Reed RC. Global cerebral blood flow, blood volume, and oxygen metabolism in patients with migraine headache. Neurology. 1998;50(6):1736–1740. doi: 10.1212/WNL.50.6.1736. [DOI] [PubMed] [Google Scholar]
- 22.Calviere L, Tall P, Massabuau P, Bonneville F, Larrue V. Migraine with aura and silent brain infarcts lack of mediation of patent foramen ovale. Eur J Neurol. 2013;20(12):1560–1565. doi: 10.1111/ene.12240. [DOI] [PubMed] [Google Scholar]
- 23.Guo ZN, Xing Y, Liu J, Wang S, Yan S, Jin H, Yang Y. Compromised dynamic cerebral autoregulation in patients with a right-to-left shunt: a potential mechanism of migraine and cryptogenic stroke. PLoS One. 2014;9(8):e104849. doi: 10.1371/journal.pone.0104849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rigatelli G, Dell'Avvocata F, Cardaioli P, Giordan M, Braggion G, Aggio S, Chinaglia M, Mandapaka S, Kuruvilla J, Chen JP, et al. Permanent right-to-left shunt is the key factor in managing patent foramen ovale. J Am Coll Cardiol. 2011;58(21):2257–2261. doi: 10.1016/j.jacc.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 25.Kruit MC, Launer LJ, Ferrari MD, van Buchem MA. Infarcts in the posterior circulation territory in migraine. The population-based MRI CAMERA study. Brain. 2005;128(Pt 9):2068–2077. doi: 10.1093/brain/awh542. [DOI] [PubMed] [Google Scholar]
- 26.Feurer R, Sadikovic S, Esposito L, Schwarze J, Bockelbrink A, Hemmer B, Sander D, Poppert H. Lesion patterns in patients with cryptogenic stroke with and without right-to-left-shunt. Eur J Neurol. 2009;16(10):1077–1082. doi: 10.1111/j.1468-1331.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- 27.Swartz RH, Kern RZ. Migraine is associated with magnetic resonance imaging white matter abnormalities: a meta-analysis. Arch Neurol. 2004;61(9):1366–1368. doi: 10.1001/archneur.61.9.1366. [DOI] [PubMed] [Google Scholar]
- 28.Del Sette M, Dinia L, Bonzano L, Roccatagliata L, Finocchi C, Parodi RC, Sivori G, Gandolfo C. White matter lesions in migraine and right-to-left shunt: a conventional and diffusion MRI study. Cephalalgia. 2008;28(4):376–382. doi: 10.1111/j.1468-2982.2008.01544.x. [DOI] [PubMed] [Google Scholar]
- 29.Park HK, Lee SY, Kim SE, Yun CH, Kim SH. Small deep white matter lesions are associated with right-to-left shunts in migraineurs. J Neurol. 2011;258(3):427–433. doi: 10.1007/s00415-010-5771-5. [DOI] [PubMed] [Google Scholar]
- 30.Rigatelli G, Cardaioli P, Dell'Avvocata F, Giordan M, Braggion G, Chinaglia M, Roncon L. Transcatheter patent foramen ovale closure is effective in reducing migraine independently from specific interatrial septum anatomy and closure devices design. Cardiovasc Revasc Med. 2010;11(1):29–33. doi: 10.1016/j.carrev.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Wahl A, Kunz M, Moschovitis A, Nageh T, Schwerzmann M, Seiler C, Mattle HP, Windecker S, Meier B. Long-term results after fluoroscopy-guided closure of patent foramen ovale for secondary prevention of paradoxical embolism. Heart. 2008;94(3):336–341. doi: 10.1136/hrt.2007.118505. [DOI] [PubMed] [Google Scholar]
- 32.Wahl A, Praz F, Tai T, Findling O, Walpoth N, Nedeltchev K, Schwerzmann M, Windecker S, Mattle HP, Meier B. Improvement of migraine headaches after percutaneous closure of patent foramen ovale for secondary prevention of paradoxical embolism. Heart. 2010;96(12):967–973. doi: 10.1136/hrt.2009.181156. [DOI] [PubMed] [Google Scholar]
- 33.Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368(12):1083–1091. doi: 10.1056/NEJMoa1211716. [DOI] [PubMed] [Google Scholar]
- 34.Wolfrum M, Froehlich GM, Knapp G, Casaubon LK, DiNicolantonio JJ, Lansky AJ, Meier P. Stroke prevention by percutaneous closure of patent foramen ovale: a systematic review and meta-analysis. Heart. 2014;100(5):389–395. doi: 10.1136/heartjnl-2013-304394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Binary logistic regression test for possible factors of SBI. (DOCX 16 kb)
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.


