Abstract
Background
Calcium phosphate cement (CPC) has been applied as a biodegradable antibiotic carrier in osteomyelitis. However, the drug delivery, antibacterial efficacy, and degradation rate of CPC are insufficient and require further improvement in clinical application.
Material/Methods
Vancomycin-loaded CPC columns were prepared, and eluted in simulated body fluid. The drug delivery was assessed in the ultrasound group and control group by fluorescence polarization immunoassay. The antibacterial efficacy of vancomycin in the ultrasound group and control groups was investigated by standard plate count method. Low-frequency pulsed ultrasound (46.5 kHz, 900 mW/cm2) was used to produce a sinusoidal wave in the ultrasound groups. The percentage of residual weight was evaluated to assess the degradation of CPC.
Results
The concentration and cumulatively released percentage of vancomycin in the ultrasound group were higher than that in the control group at each time point (p<0.05). The duration of vancomycin concentration over the level of minimum inhibitory concentration was significantly prolonged in the ultrasound group (p<0.05). Antibacterial efficacy of vancomycin in the ultrasound group was significantly greater than that in the control group with same concentration of vancomycin (p<0.05). The percentage of residual weight in the ultrasound group was significantly less than that in the control group (p<0.05).
Conclusions
Low-frequency pulsed ultrasound can enhance vancomycin release, prolong the duration of vancomycin concentration at high levels, and accelerate the degradation rate of vancomycin-loaded CPC.
MeSH Keywords: Calcium Phosphates, Sound, Vancomycin
Background
Bone defects and persistent infection are the 2 main challenges in the treatment of chronic osteomyelitis [1]. Antibiotics-loaded bone cements, for example polymethyl methacrylate (PMMA), were developed to solve these problems. However, these bone cements need a second removal surgery because of their non-biodegradability [2]. Thus, calcium phosphate cement (CPC), which has been proved to be a biodegradable antibiotic carrier, has been widely used as filling material in bone defects in osteomyelitis [3–5]. It fills the space left by debridement, releases their loaded antibiotics during degradation, and leaves no medium and substratum for bacterial colonization [6,7]. However, it was reported that the duration of antibiotics release of CPC was less than 1 month in vitro [8].
Methods to prolong the release of the antibiotics have been investigated and reported. Recently, ultrasound has been found to enhance antibiotic release from bone cement, and this controlled release of antibiotics may help reduce local infection [9]. In our previous study, we reported on low-intensity continuous-wave ultrasound increased drug elution from vancomycin-loaded polymethyl methacrylate (PMMA) in vitro [10]. In another previous study, we determined that low-frequency pulsed ultrasound (LFPUS) could increase drug release from PMMA more effectively than continuous-wave ultrasound [11]. One possible mechanism by which ultrasound enhances antibacterial effects is described as “sonoporation”, which means that the permeability of bacterial cell membranes was increased by ultrasound [12]. Another possible mechanism is that ultrasound can enhance the antibiotic effect by damaging the bacterial biofilms that protect them from bactericidal action [13].
The degradation duration of CPC from 0% to 80%~100% remains for between 6 months to 2 years, and the prolonged duration of degradation may affect the refilling of bone defects and increase the possibility of infection, non-union, or malunion [12,13]. To date, there has been no published study reporting the effect of ultrasound on antibiotics-loaded CPC.
The present study was conducted to investigate 3 hypotheses: (1) whether low-frequency pulsed ultrasound could enhance vancomycin release and prolong the duration of effective antimicrobial activity, which was defined as the longest time period during which the vancomycin concentration was greater than MIC (minimum inhibitory concentration) in vancomycin-loaded CPC; (2) whether low-frequency pulsed ultrasound could enhance antibacterial efficacy of vancomycin in vancomycin-loaded CPC, which has been proved in vancomycin-loaded PMMA; and (3) whether low-frequency pulsed ultrasound could accelerate the degradation rate of CPC.
Material and Methods
Bone cement
Twenty grams of CPC (Shanghai Rebone Biomaterials, China) and 1 gram of vancomycin (Ameresco, U.S.A) were mixed with distilled water at a powder/liquid ratio of 3: 1 to form a paste. Then, the mixture was placed in stainless steel molds, and solidified into columns 3 mm in diameter and 8 mm in height. Finally, the solidified mixture was vacuum-dried and sterilized in irradiation of 25 kGy 60Co to obtain vancomycin-loaded CPC columns. Non-vancomycin CPC columns were obtained by the same procedures without vancomycin (Figure 1).
Figure 1.
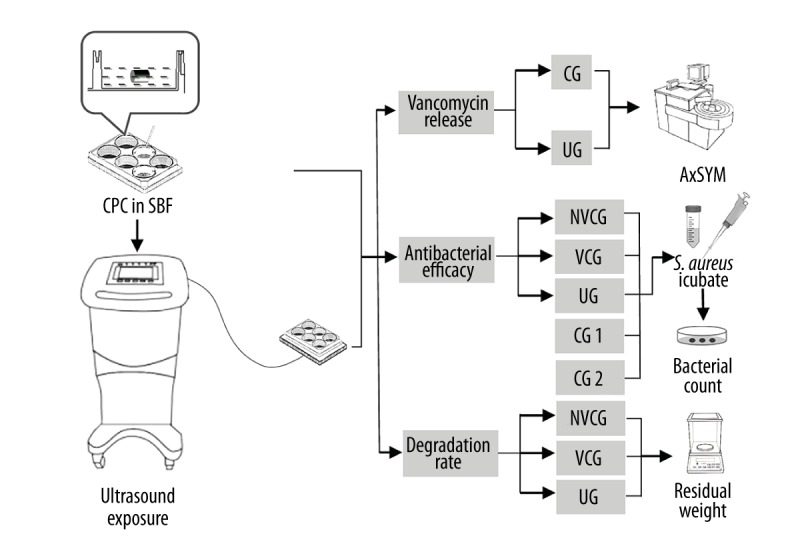
Flow chart of the study. Vancomycin-loaded CPC columns and SBF were placed together and exposed to the ultrasound system. There were different groups in the 3 studies: the study of vancomycin release, the study of antibacterial efficacy, and the study of CPC degradation.
Microorganisms
Staphylococcus aureus ATCC 13565 (S. aureus; Biological Authentication Research Institute, China), which was a standard strain of gram-positive bacteria, was incubated in Mueller-Hinton broth (Oxoid, Basingstoke, UK) for 24 h, and then diluted to produce a suspension of 108 CFU/ml of exponential-phase bacteria. The MIC of vancomycin for this strain was determined to be 2 μg/ml (Figure 1).
Ultrasound exposure system
The ultrasonic generator (Nexus, Hexin Biomedical Devices, China), for which 3.0-cm-diameter unfocused transducers were specifically designed for use in a 6-well culture plate, produced a sinusoidal wave with a frequency of 46.5 kHz, intensity of 900 mW/cm2, and a 1: 3 duty cycle of pulse. All experiments were carried out at 37°C (Figure 1).
Stimulated body fluid (SBF)
SBF solution was prepared by dissolving 7.9946 g NaCl, 0.3529 g Na2HPO4, 0.2237 g KCl, 0.3050 g MgC12•H2O, 0.7102 g Na2SO4, and 0.1742 g K2HPO4 in deionized water to 1000 ml (Figure 1).
Study of vancomycin release
There were 2 groups in the study of vancomycin release: the control group (CG) and the ultrasound group (UG). We placed 24 vancomycin-loaded CPC columns and SBF in the 6-well cell culture plates (1 column and 3 ml SBF for each well) and randomly divided them into 2 groups. In the CG, culture plates were positioned in a normal incubator at 37°C. In the UG, the ultrasound exposure system was set up and culture plates were positioned on the transducers. The CPC columns in the ultrasound group were insonated for 2 h daily. SBF was replaced daily. The eluted SBF samples were obtained on days 1, 3, 7, and 14, and weeks 4, 8, and 12. The vancomycin concentrations in the eluted samples were measured by fluorescence polarization immunoassay (FPIA, AxSYM, Abbott Laboratories) (Figure 1).
Study of antibacterial efficacy
There were 24 vancomycin-loaded CPC columns and 12 non-vancomycin CPC columns, which were placed in the 6-well cell culture plates with SBF (1 column and 3 ml SBF for each well), randomly divided into 3 groups: the non-vancomycin control group (NVCG, n=12), the vancomycin-load control group (VCG, n=12), and the ultrasound group (UG, n=12). In NVCG, culture plates with non-vancomycin CPC columns were positioned in a normal incubator at 37°C. In the VCG, culture plates with vancomycin-loaded CPC columns were positioned in a normal incubator at 37°C. In the UG, culture plates with vancomycin-loaded CPC columns were positioned on the ultrasound transducers and insonated for 2 h daily. SBF was replaced daily. The eluted SBF samples in each group were changed to a suspension of 3 ml Mueller-Hinton broth with 108 CFU/ml of exponential-phase S. aureus in months 1, 2, and 3. The number of bacteria in each well was determined by standard plate count method.
At the end of the 12th week, the vancomycin concentrations in the eluted samples of UG on were recorded as c1, c2…, c12, and these 12 samples were eluted in 3 ml Mueller-Hinton broth with 108 CFU/ml of exponential-phase S. aureus and insonated by LFPUS for 2 h. Another 24 tubes with 3 ml Mueller-Hinton broth, which also had 108 CFU/ml of exponential-phase S. aureus, were divided into 2 control groups: control group 1 (CG1, n=12), and control group 2 (CG2, n=12). In the CG1, there was no additional treatment of the tubes. In CG2, tubes were resolved with the same concentration of vancomycin as that of UG (concentration of vancomycin=c1, c2…, c12). All the samples in the 3 groups were incubated at 37°C for 24 h. The number of bacteria in each tube was determined by standard plate count method (Figure 1).
Study of CPC degradation
Vancomycin-loaded CPC and non-vancomycin CPC columns were placed in the 6-well cell culture plates with SBF (1 column and 3 ml SBF for each well). There were 36 columns randomly divided into 3 groups: the non-vancomycin control group (NVCG, n=12), the vancomycin-loaded control group (VCG, n=12), and the ultrasound group (UG, n=12). In the 2 control groups, culture plates with vancomycin-loaded CPC and non-vancomycin CPC were positioned in a normal incubator at 37°C. In the UG, the culture plates with vancomycin-loaded CPC were positioned on the ultrasound transducers. The rate of degradation was evaluated by measuring the residual weight of the samples at weeks 1, 2, 4, 8, and 12. At different time points, the samples were separated from SBF and dried. The residual CPC mass was weighed, and then the samples were replaced in refreshed SBF in the NVCG, VCG, and UG. The percentage of residual weight was calculated by the following formula: Wr%=Wn/W0×100%, where W0 is the initial weight of the sample and Wn is the weight of the dried sample at different time points (Figure 1).
Statistical analysis
The results of vancomycin amount, clonal formation unit, and residual mass are expressed as mean ± standard deviations (SD). Comparative studies of the means were performed using one-way analysis of variance (ANOVA) of the factorial design (p<0.05).
Results
LFPUS enhanced vancomycin release
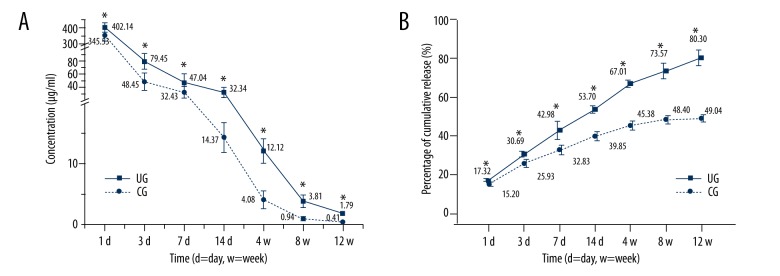
Figure 2A shows the profiles of local drug release for the 2 groups (CG and UG) at 12 weeks. The release curves revealed that the vancomycin concentration in the eluted samples after LFPUS treatment was higher than that of the control group (p<0.05 in each time point). The MIC of vancomycin was 2 μg/ml and the concentration in CG was less than this level during weeks 4–8. The concentration in US was less than this level during weeks 8–12. Figure 2B illustrates the percentage of cumulative released vancomycin for the 2 groups, and the cumulation in UG was significantly more than that in the CG (p<0.05 in each time point).
Figure 2.
LFPUS enhanced vancomycin release. (A) The vancomycin concentration in the eluted samples after LFPUS treatment were higher than that of the control group (p<0.05 in each time point). (B) The percentage of cumulative released vancomycin for the 2 groups and the cumulation in UG was significantly more than that in the CG (p<0.05 in each time point) (* P<0.05).
LFPUS enhanced antibacterial efficacy
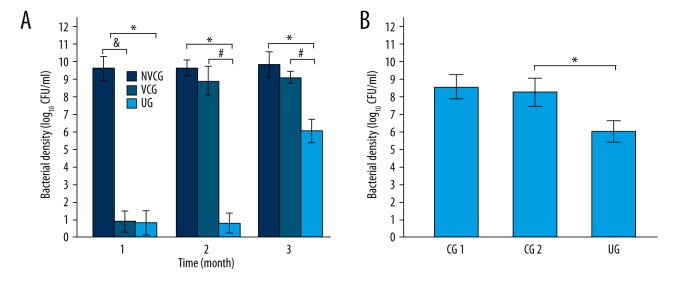
The antibacterial efficacy of vancomycin in the 3 groups (NVCG, VCG, and UG) is illustrated in Figure 3A. The antibacterial effect was similar between the VCG and UG in the first month, and both of them were significantly better than that of NVCG. There were no significant differences between the VCG and NVCG after 2–3 months. The antibacterial effect in the UG was significantly greater than that in the VCG and NVCG after 2–3 months.
Figure 3.
LFPUS enhanced antibacterial efficacy. (A) The antibacterial effect in the UG was significantly better than in the VCG and NVCG after 2–3 months. (B) After 3 months, at the same concentration, the antibacterial efficacy of vancomycin in the ultrasound exposure group (UG) was significantly better than that in the control groups (*, # or & P<0.05).
Figure 3B shows that the antibacterial efficacy of vancomycin in the UG was significantly greater than that in the CG2 with the same concentration of vancomycin at the end of treatment.
LFPUS enhanced CPC degradation
The percentage of residual weight is illustrated in Figure 4. The vancomycin-loaded CPC had a higher degradation rate than non-vancomycin CPC, although the differences were not significant at each time point (p>0.05). The mean percentage of residual weight in NVCG was 93.9% compared with 92.3% in the VCG at the end of treatment. The percentage of residual weight in the UG was significantly higher than that in the VCG and NVCG in each time point (p<0.05), and had decreased to 78.3% by the end of treatment.
Figure 4.
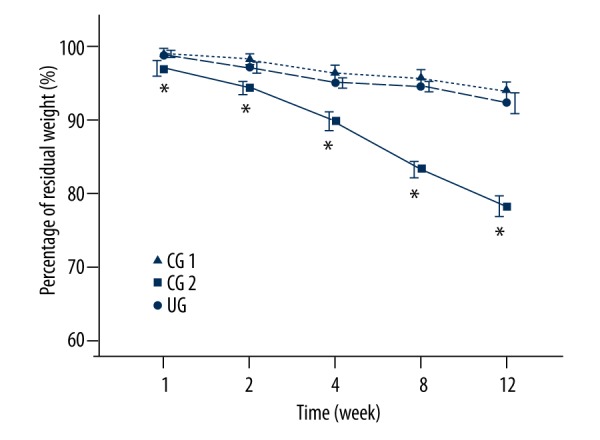
LFPUS enhanced CPC degradation. The percentage of residual weight in UG was significantly lower than that in the VCG and NVCG at each time point (* p<0.05), and had decreased to 78.3% by the end of treatment.
Discussion
Established bone infection and defects (e.g., infected non-union and osteomyelitis) remain a challenge to orthopedic surgeons. It is difficult to achieve a sufficient antibiotic concentration at the local site of infection by intravenous administration because of local bacterial biofilms and damaged vascularization [14]. Another problem is that the formation of sequestra, which are areas of dead infected bone contained by a compromised envelope of soft tissue, can reduce the efficacy of systemic administration of antibiotics [15]. A delivery system that maintains a long-term and stable release of antibiotics is required. Traditionally, PMMA has been used for controlled antibiotic release, but it is non-biodegradable and must be removed by a second surgery. Another disadvantage is that PMMA produces a large amount of heat during its setting reaction, which causes a risk of bone and soft tissue cell death and necrosis [16]. An alternative biodegradable antibiotic carrier is CPC, which has chemical and crystal structures similar to bone. However, the ability of CPC to release antibiotics and to degrade are limited, and require further improvement in clinical application.
There is no previously published study on the effect of ultrasound on release of antibiotics-loaded CPC. The aim of the present study was to evaluate the use of LFPUS for drug release in vancomycin-loaded CPC, and to shorten the duration of degradation to months rather than years. To the best of our knowledge, the present study is the first to investigate the synergism of ultrasound and antibiotics-loaded CPC in killing S. aureus.
We hypothesized that low-frequency pulsed ultrasound could enhance vancomycin release and prolong the duration of vancomycin concentration greater than MIC in vancomycin-loaded CPC. The results indeed confirmed that antibiotic release seems to be significantly faster and is prolonged by ultrasound. It was reported that the duration of antibiotics release of CPC was less than 1 month in vitro [17]. Ultrasound has been proved to be able to enhance the antibiotic release from bone cement in some previous studies. It was also reported that low-frequency ultrasound increased release of gentamicin from antibiotic-loaded acrylic bone cements [18,19]. In our previous study, low-intensity continuous-wave ultrasound increased vancomycin elution from PMMA in vitro [11]. One interpretation of the increased antibiotic release of CPC is that it is linked with the increasing degradation rate, and another interpretation is that ultrasound induces microstreaming, which seems to be essential for enhanced antibiotic release by ultrasound [20].
We also hypothesized that low-frequency pulsed ultrasound enhances antibacterial efficacy of vancomycin, and this is confirmed by the results of the present study. The mechanism by which ultrasound enhances antibacterial effect is complex. It was reported that the permeability of bacterial cell membranes was increased by ultrasound, and this phenomenon was described as “sonoporation” [21]. Some previous studies indicated that ultrasound can enhance antibiotic effects by damaging the bacterial biofilms [22]. The bacteria in biofilms are sequestered in layers on the bone cement, which protect them from bactericidal action. Ultrasonication can significantly increase the transportation of antibiotics throughout the biofilm, thus increasing their drug susceptibility.
Another hypothesis is that LFPUS increases the degradation rate of CPC. The present study showed that the degradation rate of CPC with LFPUS treatment was at least 2 times that of CPC without LFPUS. It was reported that the weight loss of mass profile for the CPC was only 5% in vivo [23]. A plausible interpretation is that the microstreaming caused by ultrasound increases the solubility of CPC. In the present study, the vancomycin-loaded CPC tended to have a higher degradation rate than non-vancomycin CPC. A previous study reported that the degradation rate of strontium-loaded CPC was decreased compared with that of normal CPC [24]. The physicochemical property of CPC might be changed by loading different chemicals.
We acknowledge certain limitations in the present study. Firstly, the results of this in vitro study of bacteria cannot be directly transferred to humans. In the clinical application, the ultrasound producer could be set by local interaction directly, which has been used for accelerating fracture healing [25]. This in vitro study did not find any negative or deleterious effects of LFPUS on cells or materials. It was reported that has ultrasound had adverse effects such as altering the permeability of the cell membrane [26,27]. Secondly, the antimicrobial efficacy of the combination of LFPUS and antibiotics-loaded CPC on bacteria and the involved cellular and molecular mechanisms deserve further investigation. Thirdly, some details could be improved by further studies; for example, the concentration of antibiotics in CPC, the shape of the CPC, and the ultrasound exposure method.
Conclusions
The present study demonstrated significant evidence that LFPUS, when combined with vancomycin-loaded CPC, can enhance vancomycin release and prolong the duration of vancomycin at high concentrations, increase the bactericidal effect, and accelerate the degradation rate of CPC in vitro.
Footnotes
Conflict of interest
None.
Source of support: This research was supported by Zhejiang Province Natural Science Foundation of China under Grant No. LQ16H060002 and Medical and Health Science and Technology Project of Zhejiang Province under Grant No. 2016KYB120
References
- 1.Liu T, Zhang X, Li Z, Peng D. Management of combined bone defect and limb-length discrepancy after tibial chronic osteomyelitis. Orthopedics. 2011;34(8):e363–67. doi: 10.3928/01477447-20110627-12. [DOI] [PubMed] [Google Scholar]
- 2.Kluin OS, van der Mei HC, Busscher HJ, Neut D. Biodegradable vs. non-biodegradable antibiotic delivery devices in the treatment of osteomyelitis. Expert Opin Drug Deliv. 2013;10(3):341–51. doi: 10.1517/17425247.2013.751371. [DOI] [PubMed] [Google Scholar]
- 3.Schnieders J, Gbureck U, Thull R, Kissel T. Controlled release of gentamicin from calcium phosphate-poly(lactic acid-co-glycolic acid) composite bone cement. Biomaterials. 2006;27(23):4239–49. doi: 10.1016/j.biomaterials.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann MP, Mohammed AR, Perrie Y, et al. High-strength resorbable brushite bone cement with controlled drug-releasing capabilities. Acta Biomater. 2009;5(1):43–49. doi: 10.1016/j.actbio.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Mistry S, Roy S, Maitra NJ, et al. A novel, multi-barrier, drug eluting calcium sulfate/biphasic calcium phosphate biodegradable composite bone cement for treatment of experimental MRSA osteomyelitis in rabbit model. J Control Release. 2016;239:169–81. doi: 10.1016/j.jconrel.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Habraken WJ, Liao HB, Zhang Z, et al. In vivo degradation of calcium phosphate cement incorporated into biodegradable microspheres. Acta Biomater. 2010;6(6):2200–11. doi: 10.1016/j.actbio.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Qin T, López A, Öhman C, et al. Enhanced drug delivery of antibiotic-loaded acrylic bone cements using calcium phosphate spheres. J Appl Biomater Funct Mater. 2015;13(3):e241–47. doi: 10.5301/jabfm.5000222. [DOI] [PubMed] [Google Scholar]
- 8.Urabe K, Naruse K, Hattori H, et al. In vitro comparison of elution characteristics of vancomycin from calcium phosphate cement and polymethylmethacrylate. J Orthop Sci. 2009;14(6):784–93. doi: 10.1007/s00776-009-1397-9. [DOI] [PubMed] [Google Scholar]
- 9.Anagnostakos K, Kelm J. Enhancement of antibiotic elution from acrylic bone cement. J Biomed Mater Res B Appl Biomater. 2009;90(1):467–75. doi: 10.1002/jbm.b.31281. [DOI] [PubMed] [Google Scholar]
- 10.Cai XZ, Chen XZ, Yan SG, et al. Intermittent watt-level ultrasonication facilitates vancomycin release from therapeutic acrylic bone cement. J Biomed Mater Res B Appl Biomater. 2009;90(1):11–17. doi: 10.1002/jbm.b.31288. [DOI] [PubMed] [Google Scholar]
- 11.Cai XZ, Yan SG, Wu HB, et al. Effect of delayed pulsed-wave ultrasound on local pharmacokinetics and pharmacodynamics of vancomycin-loaded acrylic bone cement in vivo. Antimicrob Agents Chemother. 2007;51(9):3199–204. doi: 10.1128/AAC.01465-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sariibrahimoglu K, An J, van Oirschot BA, et al. Tuning the degradation rate of calcium phosphate cements by incorporating mixtures of polylactic-co-glycolic acid microspheres and glucono-delta-lactone microparticles. Tissue Eng Part A. 2014;20(21–22):2870–82. doi: 10.1089/ten.TEA.2013.0670. [DOI] [PubMed] [Google Scholar]
- 13.An J, Leeuwenburgh SC, Wolke JG, Jansen JA. Effects of stirring and fluid perfusion on the in vitro degradation of calcium phosphate cement/PLGA composites. Tissue Eng Part C Methods. 2015;21(11):1171–77. doi: 10.1089/ten.TEC.2015.0016. [DOI] [PubMed] [Google Scholar]
- 14.Sedghizadeh PP, Sun S, Junka AF, et al. Design, synthesis, and antimicrobial evaluation of a novel bone-targeting bisphosphonate-ciprofloxacin conjugate for the treatment of osteomyelitis biofilms. J Med Chem. 2017;60(6):2326–43. doi: 10.1021/acs.jmedchem.6b01615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berebichez-Fridman R, Montero-Olvera P, Gómez-García R, Berebichez-Fastlicht E. An intramedullary nail coated with antibiotic and growth factor nanoparticles: An individualized state-of-the-art treatment for chronic osteomyelitis with bone defects. Med Hypotheses. 2017;105:63–68. doi: 10.1016/j.mehy.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Gundapaneni D, Goswami T. Thermal isotherms in PMMA and cell necrosis during total hip arthroplasty. J Appl Biomater Funct Mater. 2014;12(3):193–202. doi: 10.5301/jabfm.5000196. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki T, Ishibashi Y, Katano H, et al. In vitro elution of vancomycin from calcium phosphate cement. J Arthroplasty. 2005;20(8):1055–59. doi: 10.1016/j.arth.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Ensing GT, Hendriks JG, Jongsma JE, et al. The influence of ultrasound on the release of gentamicin from antibiotic-loaded acrylic beads and bone cements. J Biomed Mater Res B Appl Biomater. 2005;75(1):1–5. doi: 10.1002/jbm.b.30140. [DOI] [PubMed] [Google Scholar]
- 19.Hendriks JG, Ensing GT, van Horn JR, et al. Increased release of gentamicin from acrylic bone cements under influence of low-frequency ultrasound. J Control Release. 2003;92(3):369–74. doi: 10.1016/s0168-3659(03)00361-4. [DOI] [PubMed] [Google Scholar]
- 20.Sinisterra JV. Application of ultrasound to biotechnology: An overview. Ultrasonics. 1992;30(3):180–85. doi: 10.1016/0041-624x(92)90070-3. [DOI] [PubMed] [Google Scholar]
- 21.Runyan CM, Carmen JC, Beckstead BL, et al. Low-frequency ultrasound increases outer membrane permeability of Pseudomonas aeruginosa. J Gen Appl Microbiol. 2006;52(5):295–301. doi: 10.2323/jgam.52.295. [DOI] [PubMed] [Google Scholar]
- 22.Carmen JC, Nelson JL, Beckstead BL, et al. Ultrasonic-enhanced gentamicin transport through colony biofilms of Pseudomonas aeruginosa and Escherichia coli. J Infect Chemother. 2004;10(4):193–99. doi: 10.1007/s10156-004-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuang GM, Yau WP, Wu J, et al. Strontium exerts dual effects on calcium phosphate cement: Accelerating the degradation and enhancing the osteoconductivity both in vitro and in vivo. J Biomed Mater Res A. 2015;103(5):1613–21. doi: 10.1002/jbm.a.35298. [DOI] [PubMed] [Google Scholar]
- 24.Zhou ZQ, Ye DP, Liang WG, et al. Preparation and characterization of a novel injectable strontium-containing calcium phosphate cement with collagen. Chin J Traumatol. 2015;18(1):33–38. doi: 10.1016/j.cjtee.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Tarride JE, Hopkins RB, Blackhouse G, et al. Low-intensity pulsed ultrasound for treatment of tibial fractures: An economic evaluation of the TRUST study. Bone Joint J. 2017;99-B(11):1526–32. doi: 10.1302/0301-620X.99B11.BJJ-2017-0737. [DOI] [PubMed] [Google Scholar]
- 26.Guzmán HR, Nguyen DX, Khan S, Prausnitz MR. Ultrasound-mediated disruption of cell membranes. I. Quantification of molecular uptake and cell viability. J Acoust Soc Am. 2001;110(1):588–96. doi: 10.1121/1.1376131. [DOI] [PubMed] [Google Scholar]
- 27.Yu FT, Chen X, Wang J, et al. Low intensity ultrasound mediated liposomal doxorubicin delivery using polymer microbubbles. Mol Pharm. 2016;13(1):55–64. doi: 10.1021/acs.molpharmaceut.5b00421. [DOI] [PMC free article] [PubMed] [Google Scholar]