Abstract
Introduction: The structured Benefit–risk Action Team (BRAT) approach aims to assist healthcare decision makers in treatment assessments. We applied BRAT to compare the benefit–risk profile of ranibizumab 0.5 mg versus laser photocoagulation for the treatment of diabetic macular oedema (DMO). Methods: One-year data for the ranibizumab 0.5 mg pro re nata (PRN) and laser arms of the phase III trials RESPOND (NCT01135914; n=220), RESTORE (NCT00687804; n=345), and REVEAL (NCT00989989; n=396) were included in the analysis. The benefit measures included ≥10 letters gain/avoidance of loss in best-corrected visual acuity (BCVA), achieving central retinal thickness (CRT) <275 μm, and 25-item Visual Function Questionnaire (VFQ-25) outcomes. The risks measures included endophthalmitis, intraocular pressure increase, hypertension, proteinuria, arterial/venous thromboembolic events and deaths. Results: Ranibizumab treatment provided significant benefits compared with laser for ≥10 letter BCVA gain at month 12 (387/1,000 versus 152/1,000 patients), CRT <275 μm at 12 months (474/1,000 versus 348/1,000 patients), and improvement of ≥6.06 on the VFQ-25 near activities subscale (325/1,000 versus 245/1,000 patients). Results for the risk measures were similar for both treatments. Conclusions: Superior clinically relevant outcomes were observed with ranibizumab 0.5 mg PRN compared with laser without compromising on safety. This analysis further supports the positive benefit–risk profile of ranibizumab 0.5 mg PRN.
Keywords: Ranibizumab, laser, diabetic macular oedema, benefit–risk profile
Diabetic retinopathy is a common microvascular complication of diabetes, characterised by one or more features including retinal microaneurysms, cotton wool spots, microvascular abnormalities, hard exudates and haemorrhage, and, in the most severe form of the condition, neovascularisation.1 Diabetic macular oedema (DMO) is a manifestation of diabetic retinopathy characterised by swelling of the macula due to a breakdown in the blood—retinal barrier, leading to hyperpermeability (Figure 1).2 The retinal swelling caused by DMO is considered clinically significant if it involves or threatens the fovea, necessitating treatment to avoid potentially permanent vision loss.3 Central retinal thickness (CRT) can be measured using optical coherence tomography (OCT). OCT is a non-invasive imaging technique, and this parameter is often used alongside functional measures of vision (such as the number of letters that can be read from a vision chart) in order to monitor disease progression and response to therapy in patients with DMO. Without treatment, between a quarter and a third of eyes with clinically significant DMO will have significant vision loss within 3 years.4
Figure 1: Clinical presentation of diabetic macular oedema.
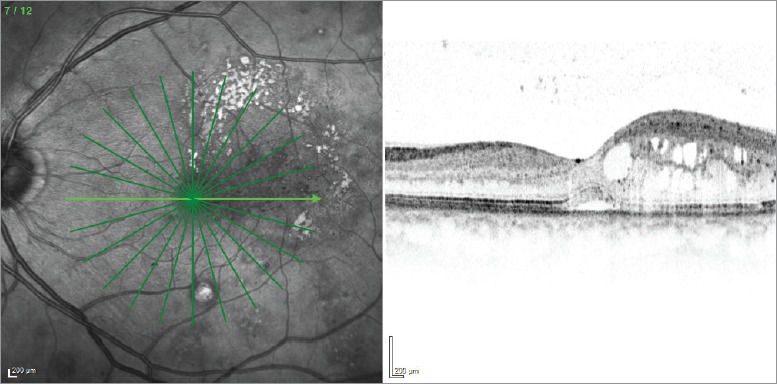
Optical coherence tomography image presenting characteristic features of diabetic macular oedema.
In 1985, the Early Treatment Diabetic Retinopathy Study (ETDRS) demonstrated a net benefit of laser photocoagulation for the treatment of DMO,3 establishing laser as the gold standard for treatment at the time due to the technique’s ability to stabilise vision in many patients, although improvements in visual function were uncommon.5 In a trial comparing laser with intravitreal triamcinolone acetate, treatment group differences at three years slightly favoured the laser group.6 However, laser treatment can cause atrophic changes in the retinal pigment epithelium (RPE), which may increase in size over time,7 thereby resulting in the formation of scotoma, a partial loss of vision or blind spot in an otherwise normal visual field.
Vascular endothelial growth factor (VEGF)-A plays a vital role within the vascular system as a regulator of developmental angiogenesis, vascular permeability, and inflammation.8 However, elevated levels ofVEGF-A within the eye have been implicated in the progression of hyperpermeability, and, therefore, in the evolution of DMO.9 Inhibition of VEGF-A, through the use of anti-VEGF treatments given as intravitreal injections, therefore provides an alternative strategy to laser photocoagulation. Ranibizumab, a humanised monoclonal antibody fragment, was the first such anti-VEGF treatment licensed for the treatment of DMO and is now approved for several retinal diseases.10 The binding of ranibizumab to active forms of VEGF-A prevents interaction of VEGF-A with its receptors on endothelial cells, thereby reducing endothelial cell proliferation, vascular leakage, and new blood vessel formation. Ranibizumab treatment has been shown to lead to clinically significant improvements in visual acuity and has shown superiority on markers of visual acuity over laser therapy in a number of clinical trials.11–16 Since its introduction, anti-VEGF therapy has come to be considered by the majority of clinicians as first-line therapy for the treatment of DMO, with one survey of US retinal specialists reporting an increase in the use of anti-VEGF as the initial treatment for DMO from 25% in 2007 to over 60% in 2015.17
Owing to the crucial part that VEGF-A plays within the vascular system, the systemic safety of anti-VEGF therapies is an important consideration. When used systemically in an oncology setting, the anti-VEGF agent bevacizumab is associated with an increased risk of various vascular events.18 While the doses delivered to the eye for intravitreal use are much lower than those used to treat cancer, some anti-VEGF agents have been detected in the systemic circulation up to one month after intravitreal injection.19 Concerns that this systemic exposure may lead to an increase in vascular events, above that already anticipated in this higher-risk population, have been raised in a number of studies.20,21 The systemic exposure of an anti-VEGF agent is affected by its molecular structure; due to lack of the fragment crystallisable (Fc) portion of the parent antibody, the molecular structure of ranibizumab is small and it has low systemic exposure.19
In clinical practice, a range of different factors must be taken into account when making treatment decisions relating to patients with DMO, including reimbursement and/or insurance considerations, patient profile and situation (for example their history of comorbidities and whether they are retired or of working age), and the benefits and risks of the different therapeutic options must be balanced against each other. Therefore, in this analysis we report a structured benefit—risk analysis conducted using three controlled, clinical studies of ranibizumab 0.5 mg given according to a pro re nata (PRN) regimen versus laser treatment in patients with DMO. This analysis uses pooled patient-level data combined together across studies, which allows more in-depth analyses than the analysis of published study-level data typical of most published metaanalyses. The results of this study are discussed within the context of other studies in DMO.
Methods
The Benefit—risk Action Team (BRAT) structured approach was developed by the Pharmaceutical Research and Manufacturers of America (PhRMA) BRAT, in order to present a structured framework to facilitate regulatory decision making.22 The BRAT framework enables the selection, organisation, summarisation and interpretation of data to provide a rationale for decision-making based on benefit—risk assessments.22 The structured benefit—risk analysis reported here was performed based upon the steps of this BRAT framework.
Selection of comparisons to be made
Ranibizumab 0.5 mg PRN was compared to laser photocoagulation as the previous gold standard therapy, with benefit and risk outcomes collated from trials which included a controlled period of 1 year (Table 1 and 2).
Table 1: Key clinical trials of ranibizumab in patients with diabetic macular oedema.
| Study (reference) | ClinicalTrials.gov registration | Study design | Treatment (number of patients enrolled) |
|---|---|---|---|
| Studies comparing ranibizumab versus laser | |||
| RESPOND11,12 | NCT01135914 | 12-month, prospective, multicentre, randomised, open-label study using three parallel treatment arms | Ranibizumab 0.5 mg monotherapy (n=71); ranibizumab in combination with laser (n=70); laser monotherapy (n=62) |
| RESTORE13 | NCT00687804 | 12-month, randomised, double-masked, multicentre study | Ranibizumab 0.5 mg (n=116); ranibizumab in combination with laser (n=118); laser monotherapy (n=111) |
| REVEAL14 | NCT00989989 | 12-month, randomised, double-masked, multicentre, active-controlled study | Ranibizumab 0.5 mg monotherapy (n=133); ranibizumab in combination with laser (n=132); laser monotherapy (n=131) |
| Studies comparing ranibizumab versus sham | |||
| RESOLVE15 | NCT00284050 | Randomised, double-masked, multicentre, sham-controlled, phase II study | Ranibizumab 0.3 mg (n=51); ranibizumab 0.5 mg (n=51); sham (n=49) |
| RISE16,25 | NCT00473382 | Randomised, multicentre, double-masked, 3-year trial, sham-controlled phase III study | Ranibizumab 0.3 mg (n=125); ranibizumab 0.5 mg (n=127); sham (n=130) |
| RIDE16,25 | NCT00473330 | Randomised, multicentre, double-masked, 3-year trial, sham-controlled phase III study | Ranibizumab 0.3 mg (n=125); ranibizumab 0.5 mg (n=125); sham (n=127) |
| Other comparisons | |||
| DRCR.net Protocol I33 | NCT00445003 | 12-month, multicentre, randomised phase III study | Sham + prompt laser (n=293); ranibizumab + prompt laser (n=187); ranibizumab + deferred laser (n=188); triamcinolone + prompt laser (n=186) |
| DRCR.net Protocol S35 | NCT01489189 | 2-year, multicentre, randomised, phase III study in patients with proliferative diabetic retinopathy | Pan retinal photocoagulation (n=203 eyes); ranibizumab 0.5 mg + deferred pan retinal photocoagulation (n=191 eyes) |
| DRCR.net Protocol T21 | NCT01627249 | 2-year, multicentre, randomised, phase III study | Aflibercept 2.0 mg (n=224); bevacizumab 1.25 mg (n=218); ranibizumab 0.3 mg (n=218) |
| RETAIN37 | NCT01171976 | 2-year, single-masked, multicentre phase IIIb study | T&E regimen of ranibizumab with laser (n=121); treat-and-extend regimen of ranibizumab monotherapy (n=128); PRN regimen of ranibizumab monotherapy (n=123) |
PRN = pro re nata; T&E = Treat and Extend.
Table 2: Efficacy outcomes from key clinical trials.
| Studies comparing ranibizumab versus laser | ||||
|---|---|---|---|---|
| Study | Characteristics | Ranibizumab 0.5 mg + laser | Ranibizumab 0.5 mg | Laser |
| RESPOND11,12† | Mean BCVA gain, ETDRS letters | +8.2 | +8.9 | +0.3 |
| BCVA gain of ≥10 ETDRS letters | 34.4% | 52.1% | 16.1% | |
| Avoided BCVA loss of ≥10 ETDRS letters | - | - | - | |
| Mean CRT change, μm | -152.2 | -143.5 | -107.1 | |
| RESTORE13† | Mean BCVA gain, ETDRS letters | +5.9 | +6.1 | +0.8 |
| BCVA gain of ≥10 ETDRS letters | 43.2% | 37.4% | 15.5% | |
| Avoided BCVA loss of ≥10 ETDRS letters | 95.8% | 96.5% | 87.3% | |
| Mean CRT change, μm | -118.7 | -128.3 | -61.3 | |
| REVEAL14† | Mean BCVA gain, ETDRS letters | +6.4 | +6.8 | +1.8 |
| BCVA gain of ≥10 ETDRS letters | 37.2% | 33.8% | 13.3% | |
| Avoided BCVA loss of ≥10 ETDRS letters | 94.6% | 97.0% | 93.8% | |
| Mean CRT change, μm | -163.8 | -148.0 | -57.1 | |
| Studies comparing ranibizumab versus sham | ||||
| Study | Characteristics | Ranibizumab 0.5 mg | Sham | |
| RESOLVE15† | Mean BCVA gain, ETDRS letters | +10.3 | -1.4 | |
| BCVA gain of ≥10 ETDRS letters | 60.8% | 18.4% | ||
| Avoided BCVA loss of ≥10 ETDRS letters | 95.1% | 75.5% | ||
| Mean CRT change, μm | -194.2 | -48.4 | ||
| RISE16* | Mean BCVA gain, ETDRS letters | +12.5 | +2.6 | |
| BCVA gain of ≥10 ETDRS letters | 62.4% | 29.9% | ||
| Avoided BCVA loss of ≥10 ETDRS letters | - | - | ||
| Mean CFT change, μm | -253.1 | -133.4 | ||
| RIDE16* | Mean BCVA gain, ETDRS letters | +12.0 | +2.3 | |
| BCVA gain of ≥10 ETDRS letters | 64.4% | 25.4% | ||
| Avoided BCVA loss of ≥10 ETDRS letters | - | - | ||
| Mean CFT change, μm | -270.7 | -125.8 | ||
†Data for Month 12; *Data for Month 24; BCVA = best-corrected visual acuity; CFT = central foveal thickness; Cl = confidence interval; CRT = central retinal thickness; ETDRS = Early Treatment Diabetic Retinopathy Study.
Data to be included
Studies included in this analysis were limited to those pharmaceutical company-sponsored trials comparing a control laser arm with ranibizumab therapy from which the authors had full access to patient-level data, in order provide the format laid out by BRAT, which is not available from published studies. Studies fulfilling the selection criteria were RESTORE (a phase III, multicentre, 12-month, double-masked, randomised study, predominantly [>94%] Caucasian patients; n=345),13 REVEAL (a 12-month, phase III, multicentre, double-masked, randomised, laser-controlled study in Asian patients; n=396)14 and RESPOND (a 12-month, multicentre, open-label, parallel-group, randomised, active-controlled study, also predominately [87.7%] Caucasian patients; n=220).11,12 Additional published controlled studies of anti-VEGF therapy in DMO are included in the discussion section of this paper where relevant, but are not included in the pooled analysis for the following reasons:
Studies where the authors did not have access to patient-level data and used anti-VEGF other than ranibizumab, for example DA VINCI, VIVID and VISTA.23,24
Studies in DMO that included a sham injection control arm rather than laser, for example RISE and RIDE.25
Studies in DMO with only active (anti-VEGF) control arms, for example Protocol T.21
Single-arm studies in DMO with no control arm, for example RELIGHT.26
Outcome measures
The outcome measures chosen by the authors for presentation in this structured benefit—risk analysis were selected from the predefined primary endpoints within the contributing studies, predefined patient-related outcomes, and the important safety topics included within the ranibizumab Risk Management Plan.27 The selected endpoints were considered by the authors to be of high clinical relevance. In order to permit presentation of all selected endpoints on the same scale, a number of efficacy endpoints were transformed to a binary scale. For scores on the 25-item version of the National Eye Institute’s Visual Function Questionnaire (VFQ-25), responsiveness thresholds were used.28 The VFQ-25 can be divided into several subscales, each assessing a different aspect of visual function. In addition to the overall (composite) VFQ-25 score, three of the VFQ-25 subscales were included separately in the analysis as key aspects of vision-related quality of life for patients with DMO: near activities (e.g., reading), distance activities (e.g., ability to read street signs), and driving ability. The responsiveness threshold for the near activities subscale of the VFQ-25 was a change of 6.06. This was chosen on the basis of it being a clinically relevant difference equivalent to a 15-letter gain in visual acuity in the study eye at 12 months in the MARINA pivotal phase III study of ranibizumab in neovascular age-related macular degeneration.29
The selected benefits and risks presented in this benefit—risk analysis are (Figure 2):
Figure 2: Decision tree for the use of ranibizumab in patients with diabetic macular oedema.
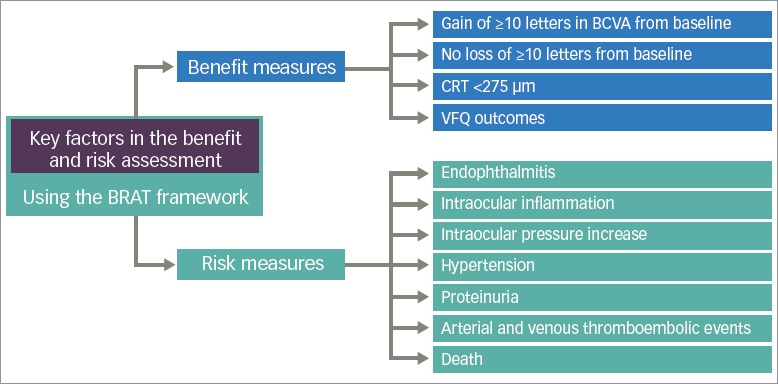
BCVA = best-corrected visual acuity; BRAT = benefit-risk action team; CRT = central retinal thickness; VFQ = Visual Functional Questionnaire.
Benefits: gain of ≥10 letters in BCVA from baseline and avoidance of loss of ≥10 letters from baseline (well-recognised functional endpoints that provide a clinically meaningful measure); CRT <275 μm (an accepted anatomical limit of normal retinal size); patient-orientated VFQ-25 quality of life outcomes.
Risks associated with intravitreal injection into the eye: endophthalmitis and the more general term of intraocular inflammation; intraocular pressure (IOP) increase.
Risks known to be associated with systemic inhibition of VEGF: hypertension, proteinuria, myocardial infarction, non-myocardial arterial thromboembolic events (a composite endpoint that includes strokes, transient ischaemic attacks and arterial peripheral vascular events), venous thromboembolic events.
Mortality endpoints: vascular and non-vascular deaths.
Adverse events were identified using pre-defined searches based upon appropriate Medical Dictionary for Regulatory Activities (MedDRA) hierarchy. Where possible, standard MedDRA queries were used.
The variables included in the structured benefit—risk assessment are included in a Forest plot output (Figure 2). The relative risk was expressed as risk difference, which is always defined, including if the response or event rate is 0 in one or both treatment groups. Exact 95% confidence intervals (CI) were obtained by iterative computation.
Results
Benefit—risk analysis — efficacy outcomes
The selected endpoint of visual improvement showed increased benefits for ranibizumab versus laser (Table 3 and Figure 3). At month 12, the difference between ranibizumab and laser in the incidence rate of a gain from baseline in BCVA of ≥10 letters was significant, with an additional 235 subjects for every 1,000 treated achieving a gain from baseline in BCVA of ≥10 letters with ranibizumab (387 versus 152 per 1,000 patients for ranibizumab and laser, respectively; 95% CI 158.5, 310.2). There was a trend toward patients given ranibizumab being more likely to avoid a loss of 10 letters or more at 12 months (incidence rate of 969 versus 897 per 1,000 patients for ranibizumab and laser, respectively; 95% CI -5.8, 149.8). Furthermore, for every 1,000 patients, significantly more had a CRT of <275 μm at 12 months with ranibizumab than with laser (474 versus 348 per 1,000 patients, respectively; 95% CI 47.5, 202.2). Similar outcomes were seen for the comparisons between ranibizumab and laser on selected items of the quality of life VFQ-25 instrument, with a significant result on the rate of improvement on the near activities subscale by 6.06 or more at month 12 (325 versus 245 per 1,000 patients for ranibizumab and laser, respectively; 95% CI 1.7, 157.2).
Table 3: Benefit-risk analysis — efficacy outcomes.
| Benefit measures at month 12 | Ranibizumab 0.5 mg PRN (n=323) | Laser (n=310) | Difference in incidence rate per 1,000 patients, ranibizumab 0.5 mg PRN versus laser (95% CI) | ||
|---|---|---|---|---|---|
| Number of patients | Incidence rate per 1,000 patients | Number of patients | Incidence rate per 1,000 patients | ||
| Gain of ≥10 letters in BCVA from baseline at month 12 | 125 | 387 | 47 | 152 | 235 (158.5, 310.2) |
| Without loss of ≥10 letters in BCVA from baseline at month 12 | 313 | 969 | 278 | 897 | 72 (-5.8, 149.8) |
| CRT <275 μm at month 12 | 153 | 474 | 108 | 348 | 125 (47.5, 202.2) |
| VFQ-25: near-activities subscale improved by ≥6.06 at month 12 | 105 | 325 | 76 | 245 | 80 (1.7, 157.2) |
| VFQ-25: distance activities subscale improved by ≥5.38 at month 12 | 89 | 276 | 68 | 219 | 56 (-21.8, 133.9) |
| VFQ-25: patients who could drive at month 12 | 122 | 378 | 108 | 348 | 29 (-48.8, 107.1) |
Discrepancies in language translation in one country have been identified since the original RESTORE analysis; therefore, mental health dependency related VFQ questions 20–25 were excluded for RESTORE. BCVA = best-corrected visual acuity; CI = confidence interval; CRT = central retinal thickness; PRN = pro re nata; VFQ = Visual Functional Questionnaire.
Figure 3: Forest plot of efficacy and safety endpoints of ranibizumab versus laser treatment in patients with diabetic macular oedema.
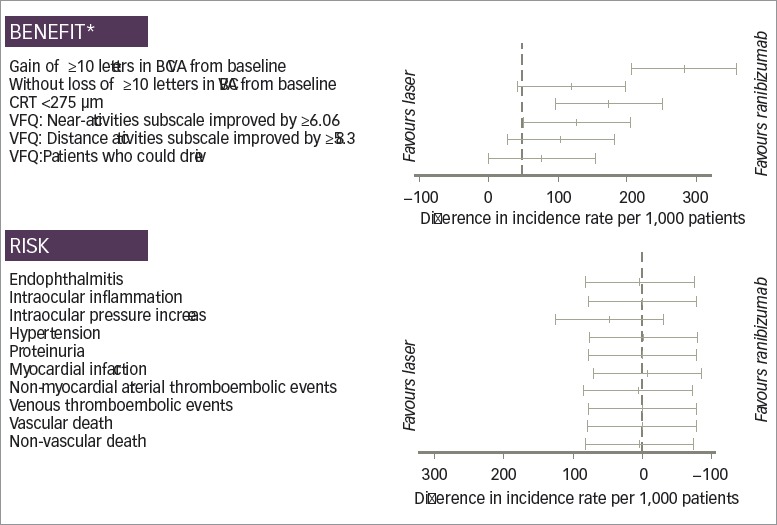
*At month 12; BCVA = best-corrected visual acuity; CRT = central retinal thickness; M = month; VFQ = Visual Functional Questionnaire.
Benefit—risk analysis — safety outcomes
At month 12, there were no statistically significant differences observed in the rates of risk of selected ocular and systemic safety outcomes between ranibizumab 0.5 mg and laser treatment (Figure 3 and Table 4 and 5). Raised IOP was reported as an adverse event with ranibizumab treatment but not with laser (46 versus 0 per 1,000 patients, respectively; 95% CI -31.5, 124.2).
Table 4: Benefit-risk analysis — ocular safety outcomes.
| Risk measures at month 12 | Ranibizumab 0.5 mg PRN (n=323) | Laser (n=312) | Difference in incidence rate per 1,000 patients, ranibizumab 0.5 mg PRN versus laser (95% CI) | ||
|---|---|---|---|---|---|
| Number of patients with at least one event | Incidence rate* per 1,000 patients | Number of patients with at least one event | Incidence rate* per 1,000 patients | ||
| Endophthalmitis | 1 | 3 | 0 | 0 | 3 (-74.9, 81.1) |
| Intraocular inflammation including uveitis | 3 | 9 | 3 | 10 | 0 (-78.2, 77.7) |
| Retinal detachment, retinal tear and RPE tears | 0 | 0 | 0 | 0 | 0 |
| Intraocular pressure increase | 15 | 46 | 0 | 0 | 46 (-31.5, 124.2) |
*Incidence rate of patients with one or more relevant events per 1,000 patients. CI = confidence interval; PRN = pro re nata; RPE = retinal pigment epithelium.
The number of events of endophthalmitis and intraocular inflammation in ranibizumab-treated patients was low (1 and 3 in a total of 323 patients, respectively; Table 4). Hypertension was the most common systemic adverse event reported in both ranibizumab and laser groups (68 versus 71 per 1,000 patients, respectively; 95% CI -80.4, 75.4). Rates of all other systemic adverse events assessed (proteinuria, myocardial infarction, non-myocardial arterial thromboembolic events [including strokes, transient ischemic attacks and arterial peripheral vascular events], venous thromboembolic events, vascular and non-vascular deaths) were extremely low and balanced between ranibizumab and laser-treated patients — between 3 and 19 events per 1,000 patients for ranibizumab and between 3 and 22 events per 1,000 patients for laser (Table 5).
Table 5: Benefit-risk analysis — systemic safety outcomes.
| Risk measures at month 12 | Ranibizumab 0.5 mg PRN (n=323) | Laser (n=312) | Difference in incidence rate per 1,000 patients, ranibizumab 0.5 mg PRN versus laser (95% CI) | ||
|---|---|---|---|---|---|
| Number of events | Incidence rate per 1,000 patients | Number of events | Incidence rate per 1,000 patients | ||
| Hypertension | 22 | 68 | 22 | 71 | -2 (-80.4, 75.4) |
| Proteinuria | 2 | 6 | 2 | 6 | 0 (-78.2, 77.8) |
| Myocardial infarction | 5 | 15 | 7 | 22 | -7 (-84.7, 71.2) |
| Non-myocardial arterial thromboembolie events | 6 | 19 | 4 | 13 | 6 (-72.1, 83.8) |
| Venous thromboembolie events | 2 | 6 | 2 | 6 | 0 (-78.2, 77.8) |
| Vascular death | 1 | 3 | 1 | 3 | 0 (-78.1,77.9) |
| Non-vascular death | 2 | 6 | 1 | 3 | 3 (-75.0, 81.0) |
CI = confidence interval; PRN = pro re nata.
Treatment exposure
The mean number of ranibizumab 0.5 mg injections administered in the patients from the ranibizumab 0.5 mg monotherapy group was 7.0 to 9.2. The mean number of laser treatments received by patients in the laser monotherapy group ranged from 1.9 to 2.6.
Discussion
Benefit-risk analysis of ranibizumab 0.5 mg versus laser
This benefit-risk analysis pooled data from three phase III clinical studies of ranibizumab in DMO11–14 in a patient-level data meta-analysis that utilised the BRAT framework to select for analysis those efficacy and safety endpoints which the authors considered important for clinical decision making (Figure 2). Based upon the identified endpoints, the analysis supports the superior efficacy of ranibizumab 0.5 mg versus laser and demonstrates a similar safety profile of the two treatments, giving ranibizumab 0.5 mg a superior benefit—risk profile relative to laser therapy in the treatment of patients with DMO.
For the benefits analysed, significant differences were observed in favour of ranibizumab 0.5 mg PRN treatment at month 12 in three key areas: overall visual function, measured as the ability to read an additional 2 lines (gain of ≥10 letters in BCVA from baseline) on an ETDRS vision chart; anatomical improvement in terms of reduction of the thickness of the macular oedema (measured by the number of patients who achieved a CRT <275 μm); and quality of life as measured using patient-reported outcomes scales (improvement on the VFQ-25 near activities subscale by ≥6.06).
There are two elements that should be considered when assessing these efficacy gains with anti-VEGF therapy compared with laser: improvements due to inhibition of neovascularisation and oedema associated with DMO, and the reduction in focal scarring that is associated with focal laser.
When the retina is treated with laser, a decrease in retinal sensitivity related to laser coagulation spots is seen, and there is the potential for lesions to enlarge over time to involve the fovea.30,31 The non-destructive nature of treatment with ranibizumab appears in accordance with the lower rate of visual loss (≥10 letters) seen with ranibizumab in the studies pooled for this analysis and the reduced reading speed seen in some patients treated with laser photocoagulation, reported elsewhere.32
Potential additional benefits of ranibizumab treatment
Intravitreal ranibizumab 0.5 mg was compared with laser/grid laser alone for the treatment of DMO in the Protocol I trial carried out by the Diabetic Retinopathy Clinical Research Network (DRCR.net).33 Diabetic retinopathy progression from baseline to the 1-year primary outcome visit was less likely to occur in eyes treated with ranibizumab compared with those given sham plus prompt laser. Similarly, in the RISE and RIDE trials, significantly more patients treated with ranibizumab 0.5 mg versus sham achieved a two- or three-step improvement on the ETDRS diabetic retinopathy severity scale (a scoring system which classifies the stages of the disease) at month 24 (35.9% versus 5.4% for a two-step improvement, and 14.5% versus 1.3% for a three-step improvement for ranibizumab and laser, respectively, both p<0.001).34 Since the additional benefits of ranibizumab over laser are seen not only on DMO but also on diabetic retinopathy disease progression, these findings suggest that ranibizumab may have a disease modifying effect on the underlying diabetic retinopathy pathology. Indeed, the Protocol S trial, also carried out by the DRCR.net, has recently reported statistically significantly greater gains in visual acuity over 2 years with ranibizumab versus pan-retinal photocoagulation (PRP; the application of many small laser spots across the retina but avoiding the central area) in patients with proliferative diabetic retinopathy (PDR; 2.8 versus 0.2 letters, p<0.001), with ranibizumab remaining more effective than PRP in the treatment of PDR, even in patients without DMO.35
The current EU label for ranibizumab allows for a flexible, individualised treatment approach with monitoring and treatment intervals based on disease activity, which enables physicians to choose a treatment schedule with reduced visit burden.36,37 This flexibility of dosing is particularly valuable in the management of patients with DMO, as longterm clinical trial evidence shows that the number of treatments required to maintain visual gains from anti-VEGF therapy reduces markedly over time, from 8–9 injections in the first year of treatment, to 0–1 injections in years 4 and 5.38
Ophthalmic risks of ranibizumab
Endophthalmitis is a severe inflammation of the anterior and/or posterior chambers of the eye and is a potential risk following any intravitreal injection into the eye. It may be sterile or associated with bacterial infection (e.g., staphylococci or streptococci), with the risk minimised through proper injection technique and aseptic procedure. Patients must be made aware of the potential for endophthalmitis, to allow timely assessment, diagnosis and treatment. Rates of endophthalmitis following intravitreal injection are extremely low, with only one instance of endophthalmitis (in a patient given ranibizumab) reported across the three studies included in this analysis (Table 4). Outside the studies included in this analysis, the rate of endophthalmitis over 3 years in the RISE and RIDE studies was 1.2%, or around 0.06% per injection,25 the rate over two years in Protocol T was 0.45%, evenly split across all treatment arms,21 while no cases of endophthalmitis were observed over 100 weeks in the VIVID and VISTA studies.24 In the LUMINOUS 30,000-patient, real-world evidence observational study, the overall rate of endophthalmitis was three events in 4,710 patients with DMO (0.06%) or three cases in 19,258 injections (0.016%).39
Transient increases in IOP have been seen within 60 minutes of injection of ranibizumab.40 In this analysis, IOP was reported as an adverse event in 4.6% of subjects receiving ranibizumab and in no patients receiving laser. Following the administration of intravitreal injections, IOP and the perfusion of the optic nerve head must be checked.
Systemic risks of ranibizumab
VEGF plays a crucial role within the cardiovascular system as a regulator of normal and pathological angiogenesis, vascular permeability and inflammation. Systemic VEGF inhibition using intravenous bevacizumab for the management of colon cancer has been associated with hypertension, thrombo-embolism (arterial and venous), haemorrhage, congestive heart failure, cardiac arrhythmia, proteinuria, gastrointestinal perforation, surgery and wound healing complications.18 For the treatment of DMO, anti-VEGF therapy is administered as an intravitreal injection in order to deliver effective drug concentrations to the retina while minimising any systemic exposure. Only a fraction of the administered dose of ranibizumab reaches the systemic circulation, and even then it has a very short half-life (around 2 hours), as a result of the molecule’s small size and lack of the Fc portion of the antibody,40 giving it a low systemic exposure.41 Even so, it is important to be mindful of any potential risk associated with even low levels of exposure when considering the large DMO patient population receiving intravitreal anti-VEGF in clinical practice, and the high risk of systemic comorbidities including cardiovascular and cerebrovascular events in patients with DMO.42
The comparison of systemic risks between ranibizumab and laser across the three trials included in this analysis does not show significant differences in systemic safety between subjects receiving ranibizumab and those randomised to laser. Both myocardial arterial thrombotic events (ATEs) and non-myocardial ATEs (a collection of adverse events that include strokes, transient ischemic attacks and peripheral vascular events) were reported at similarly low rates in both ranibizumab and laser-treated subjects, with no overall excess compared with laser treatment over the 1 year of observation in these trials.
Additional meta-analyses have been conducted to pool data from controlled studies in which ranibizumab was compared with sham or laser treatment. In a meta-analysis of six controlled phase II and III clinical trials conducted by Novartis and Genentech (1,767 patients in total, 936 treated with ranibizumab 0.5 mg [1095 patient-years]), no meaningful differences in cardiovascular or cerebrovascular safety were observed for ranibizumab 0.5 mg compared with sham and laser control participants (Table 6).43 In another recent pooled analysis, Avery and colleagues have published data from four pivotal registration trials for Lucentis (RISE/RIDE) and Eylea (VISTA/VIVID).20 These trials were sham injection controlled and required monthly dosing. Avery et al. have reported an increase in stroke, but not myocardial infarction, with both agents, and concluded that caution may be required when using these agents in the subgroup of what was described as ‘high risk patients’ requiring monthly injections.23 Clinical studies that compare the safety of the available anti-VEGF agents within the same study are limited.
Table 6: Pairwise cardiovascular and cerebrovascular safety comparisons for ranibizumab 0.5 mg versus control and ranibizumab 0.3 mg versus control from a pooled analysis of six phase II and III clinical trials (n=1,767)43.
| Adverse event | Ranibizumab 0.5 mg versus control | Ranibizumab 0.3 mg versus control | ||
|---|---|---|---|---|
| Ranibizumab 0.5 mg, rate per 100 patient-years (n/N) | Control, rate per 100 patient-years (n/N) | Ranibizumab 0.3 mg, rate per 100 patient-years (n/N) | Control, rate per 100 patient-years (n/N) | |
| Stroke plus transient ischemic attack | 1.38 (15/936) | 1.76 (13/581) | 1.29 (6/250) | 2.43 (11/251) |
| Stroke excluding transient ischemic attack | 1.29 (14/936) | 0.94 (7/581) | 0.64 (3/250) | 1.09 (5/251) |
| Myocardial infarction | 1.47 (16/936) | 2.02 (15/581) | 2.62 (12/250) | 2.87 (13/251) |
| APTC events | 2.96 (32/936) | 3.12 (23/581) | 3.72 (17/250) | 3.78 (17/251) |
| Vascular deaths | 0.73 (8/936) | 0.40 (3/581) | 1.07 (5/250) | 0.43 (2/251) |
APTC = Antiplatelet Trialists Collaboration.
The 2-year analysis of Protocol T reported an increased rate of AntiPlatelet Trialists’ Collaboration (APTC) events in ranibizumab 0.3 mg-treated patients compared with those receiving bevacizumab or aflibercept; albeit, not statistically different from that of patients treated with the other anti-VEGF agents after adjusting for baseline risk factors.21 The authors noted the variability of their safety results compared with those consistently reported in previous clinical trials of ranibizumab 0.5 mg in DMO, and stated that the ‘inconsistencies in the totality of the evidence create uncertainty as to whether there is a true increased risk of APTC events with ranibizumab at this time’.21
Strengths and weaknesses of this analysis
The strengths of this analysis include the fact that it pools together patient-level data from three randomised, controlled, phase III trials, and presents in a single graphical format the key benefits and risks of the two treatment options considered.
In terms of weaknesses, this analysis does not include any of the other available treatment options for DMO (including other anti-VEGF agents or steroids), as the authors did not have access to patient-level data for any trials fitting the inclusion criteria, other than those sponsored by Novartis. This analysis includes only the dose of ranibizumab approved within Europe (0.5 mg) — the studies meeting the inclusion criteria for this analysis did not include the 0.3 mg dose approved for use in DMO and diabetic retinopathy for monthly use in the US. It was also not possible to include in this analysis a number of endpoints that may have proved important, including measures of retinal scarring (as scarring was not specifically defined or scored within the selected trials) and focal loss of vision (again, as this was not specifically tested within the trials). The analysis did not take into consideration any effect of injection frequency on level of systemic risk. The patient-level data included in our analysis may not be an accurate reflection of the general DMO population as a result of clinical trial exclusion criteria selecting against those at highest risk of systemic cardio- and cerebrovascular events. Finally, this analysis, like many other clinical trials and metaanalyses, lacks sufficient power to detect differences in absolute risk rates for rare safety events such as arterial thromboembolic events and vascular death. It might be that the risk—benefit profile is different for subgroups of individual patients.
In this benefit—risk analysis comparing ranibizumab 0.5 mg PRN with laser treatment, significant benefits were seen with ranibizumab 0.5 mg PRN over laser therapy, on benefit measures including gain of ≥10 letters in BCVA from baseline, achievement of CRT <275 µm, and patient-reported quality of life outcomes. There were no clinically relevant differences in key ocular and systemic safety endpoints. The authors consider that the BRAT framework, facilitating the summary of key efficacy and safety endpoints into a single chart, may help when counselling patients with regard to the specific benefits and risks of the laser and ranibizumab therapy approaches. Consultation and decision should be made on the basis of the individual scenario including adherence and reimbursement.
Acknowledgments
Medical writing assistance for this manuscript was provided by Catherine Amey at Touch Medical Media and was funded by Sanofi US. The contents of this paper and opinions expressed within are those of the authors, and it was the decision of the authors to submit the manuscript for publication. The authors were responsible for the conception of the article and contributed to the writing, including critical review and editing of each draft, and approval of the submitted version.
Funding Statement
Support: The publication of this article was supported by Novartis.
References
- 1.Kohner EM, Aldington SJ, Stratton IM. et al. United Kingdom Prospective Diabetes Study, 30: diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch Ophthalmol. 1998;116:297–303. doi: 10.1001/archopht.116.3.297. [DOI] [PubMed] [Google Scholar]
- 2.Ferris FL. 3rd. Patz A, Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol. 1984;28(Suppl):452–61. doi: 10.1016/0039-6257(84)90227-3. [DOI] [PubMed] [Google Scholar]
- 3.Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 4.Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54:1–32. doi: 10.1016/j.survophthal.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Dowler JG. Laser management of diabetic retinopathy. J R Soc Med. 2003;96:277–9. doi: 10.1258/jrsm.96.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diabetic Retinopathy Clinical Research Network. Beck RW, Edwards AR. et al. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245–51. doi: 10.1001/archophthalmol.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero-Aroca P, Reyes-Torres J, Baget-Bernaldiz M, Blasco-Sune C. Laser treatment for diabetic macular edema in the 21st century. Curr Diabetes Rev. 2014;10:100–12. doi: 10.2174/1573399810666140402123026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffy AM, Bouchier-Haye DJ, Harmey JH. Bioscience. Austin, TX, USA: 2000. Vascular endothelial growth factor (VEGF) and its role in non-endothelial cells: autocrine signalling by VEGF Madame Curie bioscience database [Internet] Available from: www.ncbi.nlm.nih.gov/books/NBK6482/ (accessed 20 July 2017) [Google Scholar]
- 9.Boyer DS, Hopkins JJ, Sorof J, Ehrlich JS. Antic-vascular endothelial growth factor therapy for diabetic macular edema. Ther Adv Endocrinol Metab. 2013;4:151–69. doi: 10.1177/2042018813512360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrara N, Damico L, Shams N. et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina (Philadelphia, Pa) 2006;26:859–70. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- 11.Sheidow T, Li R, Réhel B, de Takacsy F, Courseau AS. Seattle, WA, US: 2013. Canadian 12-month, open-label, multicenter study assessing the efficacy, safety and cost-efficacy of ranibizumab as combination and monotherapy in patients with visual impairment due to DME: Preliminary Analysis. Presented at: Association for Research in Vision and Ophthalmology Meeting. [Google Scholar]
- 12.Berger A, Sheidow T, Li R, Rehel B, deTakacsy F, Courseau AS. ACanadian 12-month, phase IIIb study of ranibizumab combination or monotherapy in visual impairment due to diabetic macular edema: preliminary analysis (“Respond”) Canadian Diabetes Association/Canadian Society of Endocrinology and Metabolism Meeting. Montreal, QC, October 17–19 2013. [Google Scholar]
- 13.Mitchell P, Bandello F, Schmidt-Erfurth U. et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Ishibashi T, Li X, Koh A. et al. The REVEAL Study: ranibizumab monotherapy or combined with laser versus laser monotherapy in asian patients with diabetic macular edema. Ophthalmology. 2015;122:1402–15. doi: 10.1016/j.ophtha.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Massin P, Bandello F, Garweg JG. et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen QD, Brown DM, Marcus DM. et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 17.Rezaei KA, Stone TW. Chicago, IL: American Society of Retina Specialists; 2015. 2015. Global Trends in Retina Survey. Available from: www.asrs.org/content/documents/2015_ global_trends_in_retina_survey_-_for_website.pdf (accessed 10 June 2016) [Google Scholar]
- 18.Avastin: Summary of Product Characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000582/WC500029271.pdf (accessed 5 August 2015) [Google Scholar]
- 19.Avery RL, Castellarin AA, Steinle NC. et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol. 2014;98:1636–41. doi: 10.1136/bjophthalmol-2014-305252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avery RL, Gordon GM. systemic safety of prolonged monthly anti-vascular endothelial growth factor therapy for diabetic macular edema: a systematic review and meta-analysis. JAMA Ophthalmol. 2016;134:21–9. doi: 10.1001/jamaophthalmol.2015.4070. [DOI] [PubMed] [Google Scholar]
- 21.Wells JA, Glassman AR, Ayala AR. et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:1351–9. doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coplan PM, Noel RA, Levitan BS. et al. Development of a framework for enhancing the transparency, reproducibility and communication of the benefit-risk balance of medicines. Clin Pharmacol Ther. 2011;89:312–5. doi: 10.1038/clpt.2010.291. [DOI] [PubMed] [Google Scholar]
- 23.Do DV, Schmidt-Erfurth U, Gonzalez VH. et al. The DA VINCI Study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology. 2011;118:1819–26. doi: 10.1016/j.ophtha.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Brown DM, Schmidt-Erfurth U, Do DV. et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122:2044–52. doi: 10.1016/j.ophtha.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Brown DM, Nguyen QD, Marcus DM. et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013–22. doi: 10.1016/j.ophtha.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 26.Pearce I, Banerjee S, Burton BJ. et al. Ranibizumab 0.5 mg for diabetic macular edema with bimonthly monitoring after a phase of initial treatment: 18-month, multicenter, phase IIIB RELIGHT study. Ophthalmology. 2015;122:1811–9. doi: 10.1016/j.ophtha.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 27.European Medicines Agency Lucentis. European public assessment report (EPAR) 2014. Availalable from: www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000715/human_med_000890.jsp&mid=WC0b01ac058001d125 (accessed 24 August 2015) [Google Scholar]
- 28.Suner IJ, Kokame GT, Yu E. et al. Responsiveness of NEI VFQ-25 to changes in visual acuity in neovascular AMD: validation studies from two phase 3 clinical trials. Invest Ophthalmol Vis Sci. 2009;50:3629–35. doi: 10.1167/iovs.08-3225. [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeld PJ, Brown DM, Heier JS. et al. Ranibizumab for neovascular age-related macular degeneratio. N Eng J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 30.Kumar V, Ghosh B, Mehta DK, Goel N. Functional outcome of subthreshold versus threshold diode laser photocoagulation in diabetic macular oedema. Eye (Lond) 2010;24:1459–65. doi: 10.1038/eye.2010.53. [DOI] [PubMed] [Google Scholar]
- 31.Ishiko S, Ogasawara H, Yoshida A, Hanada K. The use of scanning laser ophthalmoscope microperimetry to detect visual impairment caused by macular photocoagulation. Ophthalmic Surg Lasers. 1998;29:95–8. [PubMed] [Google Scholar]
- 32.Pearce E, Sivaprasad S, Chong NV. Factors affecting reading speed in patients with diabetic macular edema treated with laser photocoagulation. PLoS One. 2014;9:e105696. doi: 10.1371/journal.pone.0105696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elman MJ, Aiello LP, Beck RW. et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–77. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ip MS, Domalpally A, Hopkins JJ. et al. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130:1145–52. doi: 10.1001/archophthalmol.2012.1043. [DOI] [PubMed] [Google Scholar]
- 35.Gross JG, Glassman AR. et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314:2137–46. doi: 10.1001/jama.2015.15217. Writing Committee for the Diabetic Retinopathy Clinical Research N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. LUCENTIS® (ranibizumab injection) Prescribing Information, 2014 www.gene.com/download/pdf/lucentis_prescribing.pdf (accessed 28 January 2015) [Google Scholar]
- 37.Prünte C, Fajnkuchen F, Mahmood S. et al. Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol. 2016;100:787–95. doi: 10.1136/bjophthalmol-2015-307249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bressler SB, Glassman AR, Almukhtar T. et al. Five year outcomes of ranibizumab with prompt or deferred laser versus laser or triamcinolone plus deferred ranibizumab for diabetic macular edema. Am J Ophthalmol. 2016;164:57–68. doi: 10.1016/j.ajo.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Novartis data on file. [Google Scholar]
- 40. LUCENTIS® Summary of Product Characteristics Novartis Pharma AG 2014 http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000715/WC500043546.pdf (Accessed on 15 April 2015) [Google Scholar]
- 41.Avery RL, Castellarin A, Steinle N, Annual Meeting of the Association for Research in Vision and Opthalmology (ARVO); Orlando, FL, US: 2014. May 4 8, 2014. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab, or aflibercept in patients with DME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen-Khoa BA, Goehring EL, Werther W. et al. Hospitalized cardiovascular events in patients with diabetic macular edema. BMC Ophthalmol. 2012;12:11. doi: 10.1186/1471-2415-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zarbin MA, Dunger-Baldauf C, Haskova Z. et al. Vascular safety of ranibizumab in patients with diabetic macular edema a pooled analysis of patient-level data from randomized clinical trials. JAMA Ophthalmol. 2017;135:424–31. doi: 10.1001/jamaophthalmol.2017.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]


