The renin-angiotensin system (RAS) is the most thoroughly studied and important hormonal system regulating blood pressure (BP) and renal function. The RAS is composed of a single substrate, angiotensinogen, and multiple enzymes [prorenin, renin, angiotensin converting enzyme (ACE), ACE-2, cathepsin B, chymase, and aminopeptidases A and N], peptides [angiotensins (Ang) I, II, III, (1–7), (1–12) and alamandine] and receptors [AT1, AT2, Mas and MrgD] (1,2). Despite the complexity of the system, however, the vast majority of the actions of the RAS, including vasoconstriction, are propagated by Ang II via AT1 receptors (AT1Rs), and AT1R blockers comprise an effective first line pharmacologic class in the treatment of hypertension.
Worldwide, hypertension is the most important risk factor for cardiovascular disease, stroke and death and affects approximately one-third of the adult population. Surprisingly, therefore, the fundamental mechanisms by which hypertension develops and is sustained are not understood. A major proposed mechanism for the initiation of hypertension is a fundamental defect in the capacity of the kidneys to excrete sodium (Na+). Over time, a compensatory increase in renal perfusion pressure permits appropriate Na+ excretion (pressure-natriuresis), but also induces hypertension.
Ang II, the primary peptide mediating the effects of the RAS, acts at two major receptors, AT1R and AT2R (1). The functional role of AT2Rs was clarified by studies in the mid-1990s and early 2000s demonstrating that receptor activation induces a “vasodilator cascade” by activating kininogen, increasing, bradykinin (BK) and facilitating BK action at its B2 receptors, resulting in nitric oxide (NO) production and, consequently, cyclic GMP (cGMP) formation (3). The vasodilator cascade could function either in the presence or absence of BK. NO and cGMP were established as common mediators of the majority of AT2R actions. By demonstrating that genetic deletion of AT2Rs resulted in pressor and antinatriuretic hypersensitivity to Ang II, we generated the hypothesis that AT2Rs might be endogenous natriuretic receptors which could be important in the pathophysiology of hypertension (4).
In addition to vasoconstriction, Ang II acts via AT1Rs to induce Na+ retention. Renal AT1Rs are both necessary and sufficient for inducing and sustaining hypertension during Ang II infusion, and increased Na+ reabsorption in the renal proximal tubule (RPT) is a major determinant of this response (5,6). In contrast, the role of AT2Rs in the control of Na+ excretion and BP has been less clearly defined. AT2Rs are expressed in the adult kidney primarily in the RPT (7).
Our recent studies have provided clear evidence for a major role of RPT AT2Rs in inhibition of Na+ reabsorption (8–11). These studies indicate that in normal Sprague-Dawley rats, Ang II must be metabolized to des-aspartyl1-Ang II (Ang III) in order to induce an AT2R-mediated natriuretic response (11). We established that intrarenal Ang III (1) inhibits Na+ reabsorption predominantly at the RPT, (2) induces natriuresis by a cGMP-dependent mechanism, (3) induces natriuresis in the absence of systemic AT1R blockade when Ang III metabolism by aminopeptidase N is blocked and (4) is the preferred endogenous AT2R agonist inducing natriuresis (8–11). For decades, Ang II has been considered the major agonist for both AT1Rs and AT2Rs. Our studies demonstrated for the first time that Ang III is the preferred endogenous agonist for AT2R-induced natriuresis, likely due to preferential agonist binding to AT2Rs. Receptor crystallization in the presence of different endogenous peptide agonists will be required to determine the structural characteristics of receptorbinding pocket-peptide interaction that facilitates Ang III over Ang II.
Importantly, we have also shown that the natriuretic response to Ang III is accompanied by translocation of AT2Rs from intracellular sites along the microtubules to the apical plasma membranes of RPT cells and that the natriuresis is accompanied by internalization and inactivation of major RPT Na+ transporter molecules Na+-H+ exchanger-3 (NHE-3) and Na+/K+ATPase (NKA) (11). Consistent with these observations, we also demonstrated that acute activation of RPT AT2Rs with exogenous AT2R non-peptide agonist Compound-21 (C-21) induces natriuresis in normotensive rats via the BK-NO-cGMP-dependent signaling pathway accompanied by recruitment of AT2Rs to RPT apical plasma membranes and internalization and inactivation of NHE-3 and NKA (12). C-21 is able to reduce BP in this model in the absence of concurrent AT1R blockade. Most recently, we have confirmed and extended these studies in a model of Ang II-dependent hypertension (the Ang II infusion model) (13). Here we have shown that chronic AT2R activation with C-21 prevented the initial (24h) Ang II-induced Na+ retention, induced sustained negative Na+ balance and lowered BP to near-control levels during a 7-day infusion period (13). As with the aforementioned acute studies (12), chronic administration of C-21 was equally effective administered intrarenally or systemically, translocated AT2Rs to the apical plasma membranes and internalized/inactivated NHE-3 and NKA in RPT cells (13). C-21-induced natriuresis derived selectively from RPT inhibition of Na+ reabsorption because it was additive to that of diurectics acting in the distal tubule (chlorothiazide) or cortical collecting duct (amiloride). In addition to preventing Na+ retention and hypertension, C-21 was equally effective in normalizing BP once the Ang II-induced hypertension had been established (13). These results strongly suggest that AT2R agonists are effective natriuretic and diuretic agents that improve the pressure-natriuresis relationship and, therefore, are potential therapeutic agents in Na+ retaining states and hypertension.
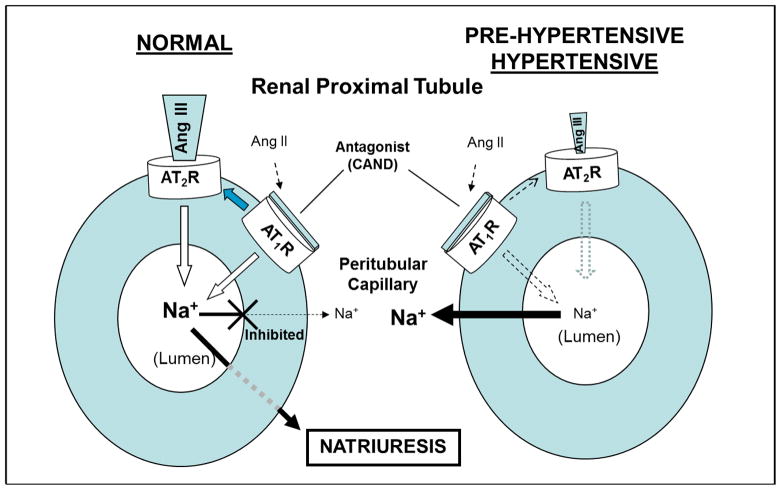
Returning to the role of endogenous Ang III and AT2Rs in the pathogenesis of hypertension, we have been unable to show a natriuretic responseeither to exogenous or endogenous (intrarenal AT1R blockade) Ang III in the hypertensive or pre-hypertensive spontaneously hypertensive rat (SHR) whereas their Wistar-Kyoto (WKY) and SD controls have robust natriuretic responses (14,15). These results strongly suggest a defect in AT2R-mediated natriuresis in SHR that predates the hypertension (Figure 1). Studies are underway to establish the mechanisms that lead to defective AT2R-mediated natriuresis in hypertension. These mechanisms most likely involve a pre-receptor defect, in which there may be accelerated intrarenal Ang III metabolism by aminopeptidase N or, alternatively, an AT2R/post-receptor signaling defect in hypertension.
Figure 1.
Schematic representation of ligand-receptor interactions mediating natriuresis in the RPT in normal rodents (left) and absence of natriuresis in pre-hypertensive and hypertensive rodents (right). CAND: candesartan, AT1R antagonist; Ang III: angiotensin III, preferred AT2R agonist; Ang II: angiotensin II; Na+: sodium; ⇨: AT2R-dependent inhibition of Na+ reabsorption;
 :reduced inhibition of Na+ reabsorption;
:reduced inhibition of Na+ reabsorption;
 response reduced.
response reduced.
References
- 1.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24:261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 2.Carey RM. Newly discovered components and actions of the renin-angiotensin system. Hypertension. 2013;62:818–822. doi: 10.1161/HYPERTENSIONAHA.113.01111. [DOI] [PubMed] [Google Scholar]
- 3.Carey RM, Padia SH. AT2 receptors: beneficial counter-regulatory role in cardiovascular and renal function. Pflugers Arch. 2013;465:99–110. doi: 10.1007/s00424-012-1146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc Natl Acad Sci USA. 96:6506–6510. doi: 10.1073/pnas.96.11.6506. 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowley SD, Gurley SB, Hererra MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Hasse VH, Snouwart JN, Le TH, McDonough AA, Koller BH, Coffman TM. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13:469–475. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozono R, Wang Z-Q, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype-2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30:1238–1246. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- 8.Padia SH, Howell NL, Siragy HM, Carey RM. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension. 2006;47:537–544. doi: 10.1161/01.HYP.0000196950.48596.21. [DOI] [PubMed] [Google Scholar]
- 9.Padia SH, Kemp BA, Howell NL, Siragy HM, Fournie-Zaluski M-C, Roques BP, Carey RM. Intrarenal aminopeptidase N inhibition augments natriuretic responses to angiotensin III in angiotensin type 1 receptor-blocked rats. Hypertension. 2007;49:625–630. doi: 10.1161/01.HYP.0000254833.85106.4d. [DOI] [PubMed] [Google Scholar]
- 10.Padia SH, Kemp BA, Howell NL, Fournie-Zaluski M-C, Roques BP, Carey RM. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-induced natriuresis in rats. Hypertension. 2008;51:460–465. doi: 10.1161/HYPERTENSIONAHA.107.103242. [DOI] [PubMed] [Google Scholar]
- 11.Kemp BA, Bell JF, Rottkamp DM, Howell NL, Shao W, Navar LG, Padia SH, Carey RM. Intrarenal angiotensin III is the predominant agonist for proximal tubule angiotensin type 2 receptors. Hypertension. 2012;60:387–395. doi: 10.1161/HYPERTENSIONAHA.112.191403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kemp BA, Howell NL, Gildea JJ, Keller SR, Padia SH, Carey RM. AT2 receptor activation induces natriuresis and lowers blood pressure. Circ Res. 2014;115:388–399. doi: 10.1161/CIRCRESAHA.115.304110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp BA, Howell NL, Keller SR, Gildea JJ, Padia SH, Carey RM. AT2 receptor activation prevents sodium retention and reduces blood pressure in angiotensin II-dependent hypertension. Circ Res. 2016;119:532–543. doi: 10.1161/CIRCRESAHA.116.308384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padia SH, Kemp BA, Howell NL, Gildea JJ, Keller SR, Carey RM. Intrarenal angiotensin III infusion induces natriuresis and AT2 receptor translocation in WKY but not in SHR. Hypertension. 2009;53(part 2):338–343. doi: 10.1161/HYPERTENSIONAHA.108.124198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padia SH, Howell NL, Kemp BA, Fournie-Zaluski M-C, Roques BP, Carey RM. Intrarenal aminopeptidase N inhibition restores defective angiotensin AT2 receptor-mediated natriuresis in SHR. Hypertension. 2010;55:474–480. doi: 10.1161/HYPERTENSIONAHA.109.144956. [DOI] [PMC free article] [PubMed] [Google Scholar]