Abstract
The roles played by cholesterol in cancer development and the potential of therapeutically targeting cholesterol homeostasis is a controversial area in the cancer community. Several epidemiological studies report an association between cancer and serum cholesterol levels or statin use, while others suggest that there is not one. Furthermore, the Cancer Genome Atlas (TCGA) project using next-generation sequencing has profiled the mutational status and expression levels of all the genes in diverse cancers, including those involved in cholesterol metabolism, providing correlative support for a role of the cholesterol pathway in cancer development. Finally, preclinical studies tend to more consistently support a role of cholesterol in cancer with several demonstrating that cholesterol homeostasis genes can modulate development. Due to space limitations, this review provides selected examples of the epidemiological, TCGA and preclinical data, focusing on alterations in cholesterol homeostasis and its consequent effect on patient survival. In melanoma, this focused analysis, demonstrated that enhanced expression of cholesterol synthesis genes was associated with decreased patient survival. Collectively, the studies in melanoma and other cancer types, suggested a potential role of disrupted cholesterol homeostasis in cancer development but that additional studies are needed to link population based epidemiological data, the TCGA database results and preclinical mechanistic evidence to concretely resolve this controversy.
Keywords: Cholesterol, cancer, statin, cholesterol transport inhibitor
Introduction
Cholesterol level tends to be high in cancer cells but it is currently controversial as to what this means (1,2). Some epidemiological studies, suggest a positive association between elevated serum cholesterol level and risk for certain cancer types (3–5). For example, a 10 mg/dl increase in cholesterol was associated with a 9% increase in prostate cancer recurrence (5). Furthermore, another study suggests that statin use was associated with lowered risk of melanoma, non-Hodgkin lymphoma, endometrial and breast cancers (6–8), while another report documents a dose-dependent reduction in colorectal cancer mortality with statin use (9). Recently, a case-control study with 295,925 cancer patients, suggested a link between statin use and a slight reduction in cancer-related mortality for 13 different cancer types (9). While these epidemiological studies suggest a possible role for cholesterol involvement in cancer, they have been criticized for having intrinsic limitations and a solely retrospective focus (9). Surprisingly, an equal number of epidemiological studies suggest no association between cholesterol and cancer (9–12). In fact, in some cases, cancer was linked to low cholesterol levels and statins were speculated to have carcinogenic properties (13–15). This conflicting epidemiological evidence is the major reason for the uncertainty regarding a role for cholesterol in cancer development and its currently unclear as to how this could be resolved.
The role of dietary cholesterol in cancer development is also controversial. Many case-control studies suggested a positive correlation between risks of several malignancies and dietary cholesterol uptake (16–18). However, the conclusiveness of these studies is arguable, being depended on dietary surveys that are notoriously unreliable. Preclinical studies tend to be more supportive of a role of dietary cholesterol in cancer development. For example, controlled experiments in mice suggest an association between dietary cholesterol and cancer, but extrapolation to humans is difficult since dietary cholesterol has limited effect on blood cholesterol levels in humans (19). Thus, while dietary cholesterol might be indicative of a lifestyle prone to health related problems, including cancer, dietary cholesterol alone seems unlikely to promote cancer development.
While the contradictory epidemiological studies fuel the controversy regarding a role for cholesterol in cancer, preclinical studies more consistently suggest involvement. Multiple mechanisms promoting deregulation of cholesterol homeostasis have been identified that could lead to cancer development (1,20–24). Recent studies also suggest that intracellular cholesterol levels in the evolving cancer cell might be more important than serum cholesterol (25,26). Furthermore, intracellular cholesterol homeostasis varies among different cancer types, therefore cholesterol could play differing roles dependent on cancer type (27). Thus, intracellular cholesterol levels appear more important than dietary cholesterol in cancer development.
Cholesterol metabolism and its role in cancer
Normal cholesterol homeostasis
Cholesterol is an essential lipid for maintaining cellular homeostasis (28). Besides being a precursor for steroid hormones, and being an essential component of plasma membranes, it is also enriched in lipid rafts and plays a key role in intracellular signal transduction (28). Cholesterol is primarily synthesized in the liver and transported to cells around the body through the bloodstream as a low density lipoprotein (LDL) bound form (29). LDL is taken into cells by clathrin-mediated endocytosis, and transported to the lysosomes through the endocytic pathway, where is then hydrolyzed to free cholesterol molecules, which are shuttled to the cell membrane and other cell membrane bound organelles (28,29).
Modulation of cholesterol homeostasis
Cholesterol homeostasis is tightly regulated by a complex protein network, which involves its import, synthesis, export, metabolism and esterification (28). Sterol regulatory element-binding protein transcription factor 2 (SREBF2) and liver X receptors (LXRs) act as key regulators of cholesterol homeostasis (28). Endoplasmic reticulum (ER) cholesterol levels serve as a sensor for intracellular cholesterol homeostasis. A decrease in ER cholesterol triggers translocation of SREBF2 from ER to golgi and then to the nucleus to activate transcription of genes involved in cholesterol synthesis (e.g., HMGCR) and import into cells (e.g., LDL receptors) (28). On the other hand, increased intracellular cholesterol levels shut down cholesterol synthesis and facilitates its export via activation of LXR receptors by oxysterols, oxidized derivatives of cholesterol (30).
Identification of cholesterol synthesis pathway deregulation in cancer using the Cancer Genome Atlas (TCGA)
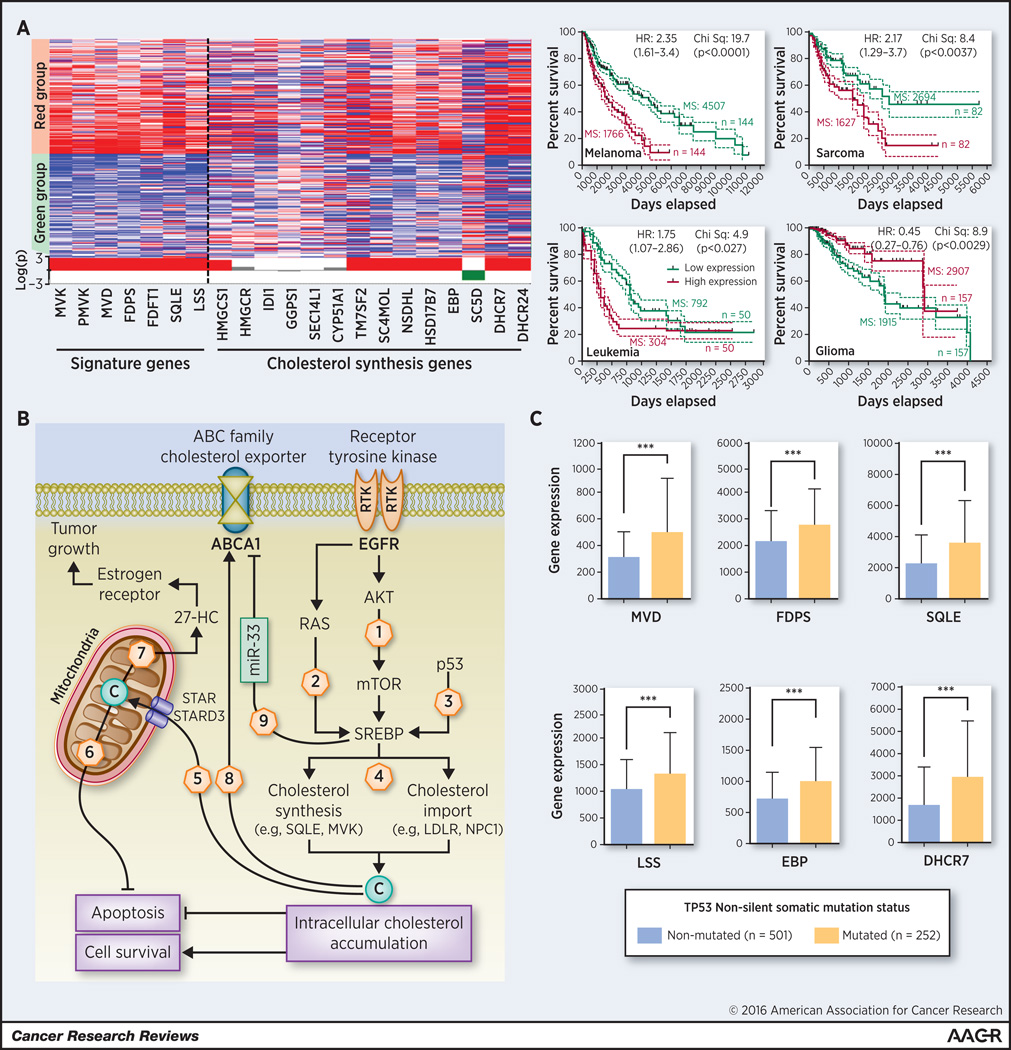
The TCGA database has profiled RNA expression levels and DNA mutational status for thousands of genes in tumors, enabling correlative analysis of particular cellular pathway involvement in cancer development (31). We have used the TCGA database to determine whether a prognostic signature of cholesterol synthesis genes could be correlated with patient survival and identified 7 cholesterol synthesis genes correlated with patient survival (Figure 1A). In sarcoma, acute myeloid leukemia and melanoma, increased activity of the cholesterol synthesis pathway was correlated with decreased patient survival while in lower grade glioma it was associated with enhanced survival (Figure 1A). Thus, based on the TCGA database, there appears to be a correlative link between cholesterol synthesis pathway and prognostic outcome that could be cancer-type specific. Several oncogenic signals, such as PI3K/AKT/mTOR, RTK/RAS and p53, have been shown to modulate cholesterol synthesis in cancer cells but due to space limitations only selected examples are discussed below (Figure 1B). Other examples can be seen in the following references (32–37).
Figure 1. Cancer patient survival and cholesterol synthesis pathway activity.
A) Expression of a gene signature representing the activity of the cholesterol synthesis pathway was analyzed using the UCSC Cancer Genomic Browser. Statistically significant differences in survival of patients were observed between high (red) and low expressing (green) groups in melanoma, sarcoma, leukemia and glioma (right panel). In left panel, the expression heatmap of various cholesterol synthesis genes are shown for melanoma. The statistical track displayed under the heatmap shows the logarithmic plot of p-values for each genomic position and represents the statistical difference between the two subgroups (Student t-test with Bonferroni correction). A bar above the centerline indicates that the expression of a particular gene is higher in the red group compared to the green group; and a bar below the center line indicates that the expression of the particular gene is higher in the green group compared to the red group. The data is available through the UCSC Cancer Brower (goo.gl/56UxKy). HR: Hazard ratio (Mantel-Haenszel) of red vs green group (95% Confidence Interval); p=p value of Mantel-Cox log-rank test; MS: Median-survival; n= number of patients. B) Oncogenic signals initiated from RTK/AKT/mTOR (1), RTK/RAS (2) or mutated p53 (3) induce the activity of SREBP transcription factor, the major regulator of genes encoding cholesterol synthesis as well as import proteins (4). Intracellular cholesterol is transported to the mitochondria by START domain family of proteins (5). Accumulation of cholesterol in mitochondria can suppress apoptosis by inhibiting release of apoptotic proteins from mitochondria (6). However, in mitochondria cholesterol is also metabolized to 27-hydroxycholesterol 27-HC), which induces tumor growth in certain cancers (7). Under steady state conditions, excess intracellular cholesterol is exported out by ABC-transporter family proteins, mainly by ABCA1 (8). Oncogenic signals may inhibit ABCA1 expression by inducing miR-33 leading to intracellular cholesterol accumulation (9). C) Breast cancer patients with mutated p53 showed increased expression of various cholesterol synthesis genes; Error bars show standard deviation. *** Student t-test p < 0.001.
Activation of cholesterol synthesis by PI3K/AKT/mTOR signaling
Constitutive activation of PI3K/AKT signaling promotes intracellular cholesterol levels by inducing cholesterol synthesis through activation of SREBP, by inducing LDL receptor mediated cholesterol import; and inhibiting ABCA1 mediated cholesterol export in an mTORC1 dependent manner (38,39) (Figure 1B). The expression of SREBP target genes and ABCA1 mediated cholesterol efflux was suppressed by rapamycin, the mTORC1 inhibitor (39). Studies in cultured cells and in animals suggested that induction of cholesterol synthesis by the AKT/mTORC1/SREBP pathway contributed to cell growth (38). In prostate cancer, AKT mediated upregulation of intracellular cholesterol levels promoted cancer aggressiveness and bone metastases (40,41). In glioblastoma, expression of LDL receptors were induced by AKT and pharmacological targeting of LDL receptors effectively promoted tumor cell death (42).
Activation of cholesterol synthesis through p53
Another example of a gene deregulating the cholesterol pathway in cancer cells is p53 (Figure 1B). p53 is the most frequently mutated gene in cancer and is a poor prognostic indicator (43). Loss of p53 function unregulated the cholesterol synthesis pathway in breast cancers, which was necessary and sufficient for disruption of breast tissue architecture (25,26). Genetic knockdown of mutant p53 or pharmacological inhibition of the cholesterol synthesis pathway reverted the disorganized morphology of breast cancer cells in a 3D culture model to a more normal phenotype (25). Prenylation of proteins (a process utilized by products of the cholesterol synthesis pathway) was essential for the phenotype. p53 mediated activation of cholesterol synthesis has also been found to induce proliferation and self-renewal of breast cancer cells via prenylation of Rho GTPases (26).
Data from the TCGA database supports the preclinical studies suggesting a role for p53 in upregulation of cholesterol synthesis genes, including FDPS (also a key protein for the prenylation), in p53 mutated breast cancer samples (Figure 1C). As a key tumor suppressor for a wide variety of cancers, p53-mediated modulation of cholesterol homeostasis could contribute to the progression of other malignancies, which requires further investigation (28).
Deregulation of mitochondrial cholesterol levels in cancer
In several cancer types, elevated mitochondrial cholesterol levels induced resistance to apoptotic signals (1,20). STAR and STARD3 are two essential proteins that regulate cholesterol import to the mitochondria (Figure 1B) (20,22). In hepatocellular carcinoma, increased mitochondrial cholesterol content was associated with increased expression of STAR and knockdown increased sensitivity to chemotherapeutic agents (20). In contrast, STARD3 was associated with a poor prognosis for breast cancer patients (44). Decreasing STARD3 levels reduced cell proliferation and increased cell death in HER2-positive breast cancer cell lines while it was ineffective in HER2-negative cells (44). Furthermore, STARD3 overexpression decreased the adhesiveness of breast cancer cells thereby modulating metastases (22).
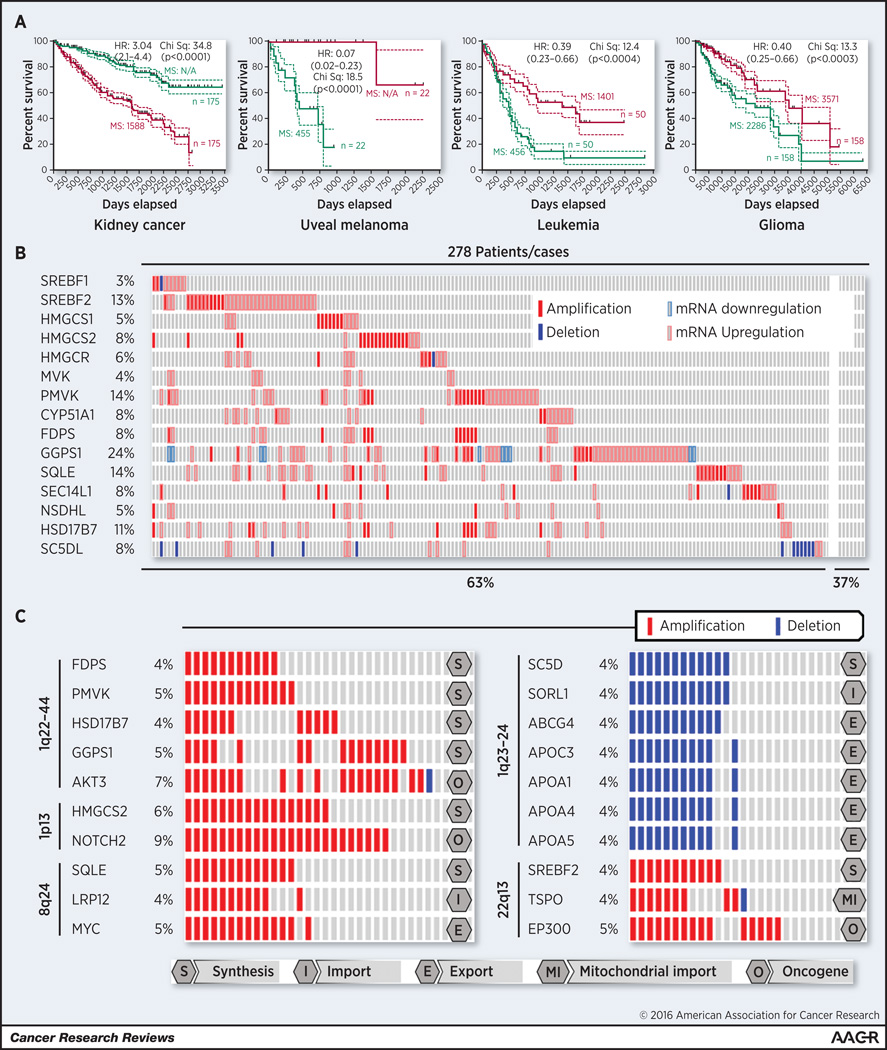
Analysis of STAR and STARD3 in the TCGA database further supports an important role in cancer development. These genes were upregulated or amplified in ~30 % of the TCGA breast cancer cohort, which is in agreement with the published preclinical studies (goo.gl/2IZM6S). However, no correlation was found between the expression of these two mitochondrial cholesterol importers and patient survival either in breast cancer or hepatocellular carcinoma. Moreover, in several malignancies elevated expression of STAR and STARD3 was correlated with increased patient survival (Figure 2A). This was not the case for kidney cancer where it was associated with a worse prognosis. The contradictory evidence involving STAR and STARD3 in cholesterol and cancer is an example where further study is needed. Another complication is that the STAR and STARD3 genes are located in the same amplicon with two well-known cancer genes, EIF4EBP1 and HER2, respectively. Therefore, it is possible that copy number increases of these genes might occur as a bystander effect but this would require validation.
Figure 2. Genetic alterations in cholesterol homeostasis genes in the melanoma patient cohort of the TCGA database.
A) Survival of cancer patients based on the STAR+STARD3 gene signature (goo.gl/6zT8kM); B) Around sixty percent of the tumors from 278 melanoma patients in the TCGA cohort, displayed increased gene copy number or expression of cholesterol synthesis genes (goo.gl/tqbV4h); C) Copy number increases of cholesterol homeostasis genes can be linked to amplification sites of known oncogenes, such as AKT3, NOTCH2, MYC or EP300 or deleted with along with genes linked to cancer. SC5D is an example of a gene co-deleted together with several cholesterol export related genes. HR: Hazard ratio (Mantel-Haenszel) of red vs green group (95% Confidence Interval); p=p value of Mantel-Cox log-rank test; MS: Median-survival; n= number of patients.
Another example of a cholesterol homeostasis gene deregulated in cancer cells is ABCA1, a cell membrane bound cholesterol exporter (Figure 1B). Decreased activity of ABCA1 promoted cancer cell survival by increasing mitochondrial cholesterol levels (1). ABCA1 activity is reduced colorectal cancer cells either through loss of function mutations or gene down regulation (1). Transformation of colon epithelial cells by expression of mutant p53 and RAS decreased ABCA1 levels and ectopic expression of ABCA1 in p53/RAS transformed cells, decreased xenografted tumor growth (1). Interestingly, growing tumors had 3-fold lower levels of ABCA1 expression compared to the original cells, indicating a selection process for tumor growth. Furthermore, ectopic expression of the loss of function mutants of ABCA1 did not reduce tumor growth and tumors that did develop had ABCA1 levels similar to those observed in the original parental cells.
ABCA1 is an example where the preclinical and TCGA data are contradictory. The TCGA database suggests that only 6.6% of colorectal cancer patients harbor ABCA1 mutations, which was similar to the background somatic mutation rate of 6.7%. Discrepancy between preclinical data and the TCGA database demonstrates the need for the field to validate the clinical relevance of preclinical observations.
Role of cholesterol metabolites in cancer development
Cholesterol metabolites have also been associated with the development of various cancers (45,46). Mitochondrial cytochrome P450 family enzymes metabolize cholesterol to synthesize steroids and oxysterols. The involvement of certain steroids, such as estrogen, is well known in cancer development (see (47)), and will not be discussed here due to space limitations. Another example is oxysterols that play an essential role in cholesterol homeostasis. These metabolites inhibit cholesterol synthesis and enhance its export by activating LXRs (48,49). Many of the oxysterols (eg, 7α-, 7β-, 25-, -hydroxycholesterol) have anti-proliferative effects in various cancer types (46). However, 27-hydroxycholesterol (27HC) has recently been shown to act as an estrogen receptor agonist in breast cancer, inducing tumor growth and metastasis (45) (Figure 1B). In breast cancer, decreased expression of CYP7B1 triggers accumulation of 27HC (50).
Involvement of cholesterol metabolites in cancer development is an example where the TCGA data is in agreement with the preclinical studies. Lower levels of CYP7B1 are observed in breast cancer compared to normal breast tissue (goo.gl/m2j9k8). However, the role of cholesterol metabolites in cancer development needs expansion as well as the involvement of different metabolites in various cancer types. Targeting the synthesis, transport or metabolites of the cholesterol homeostasis pathways are options for controlling cancer development. Due to space limitations, selected examples of targeting these processes are provided below. Other examples can be seen if the following references (37,42,51–53).
Targeting cholesterol synthesis
The cholesterol synthesis pathway has more than 15 proteins that are potential targets to disrupt this pathway in cancer cells (29). The chemotherapeutic potential of targeting these cholesterol synthesis genes has been studies preclinically (54–56). Statins can have anti-tumor effects and can synergize with certain chemotherapeutic agents to decrease development of multidrug resistance (54,57). They are especially effective against mesenchymal-like cancer cells, and might potently kill cells having undergone the epithelial-to-mesenchymal transition to promote metastasis development (57). Several clinical trials have examined the potential chemopreventive and therapeutic efficacy of statins (Clinical trial identifier: NCT02534376, NCT02360618, NCT00584012, NCT01110785). A recent example of a trial that modulated cholesterol levels to control cancer, involved a short-term biomarker study involving Simvastatin, which reduced breast cancer recurrence by reducing serum estrone sulfate levels (58). However, long-term studies are needed to confirm this observation.
Bisphosphonates and tocotrienols are examples of downstream inhibitors of the cholesterol synthesis pathway, which in preclinical studies suppressed cultured cancer cell and tumor growth similar to that observed with statins (59,60). Geranylgeranylation of proteins, a branch of the cholesterol synthesis pathway, was found to be essential for maintaining stemness of basal breast cancer cells (56). GGTI-288, an inhibitor of the geranylgeranyl transferase I (GGTI) reduced the cancer stem cell subpopulation in primary breast cancer xenografts (56). Thus, preclinical studies suggest that targeting the cholesterol synthesis pathways could be useful for modulating cancer.
Targeting cholesterol transport and intestinal absorption
Recently, our group demonstrated the preclinical chemotherapeutic potential of disrupting intracellular cholesterol transport using a small lysosomotropic compound called leelamine, (61). Leelamine inhibits cholesterol egress from lysosomes reducing cholesterol levels in all membrane bound organelles in cancer cells (61,62). Inhibition of intracellular cholesterol transport consequently led to ER stress and autophagy (61,63–65). Melanoma cells were more sensitive to inhibition of intracellular cholesterol transport than normal skin cells suggesting this agent could be a useful therapeutic agent (61). Intracellular cholesterol transport inhibitors could also inhibit tumor cell metastasis by interfering with cholesterol levels in the trans-golgi network and reducing cell surface expression of integrins that are fundamental for cancer cell migration during metastasis (62,66,67). However, the potential of agents like leelamine to inhibit metastasis of melanoma cells remains to be demonstrated. Thus, while targeting cholesterol transport in cancer cells seems to a potentially important therapeutic approach, utility of this approach remains to be demonstrated clinically.
Targeting intestinal cholesterol absorption is another approach to reduce levels in cancer cells. For example, Ezetimibe, an FDA-approved drug, reduced preclinical prostate tumor growth by inhibiting intestinal cholesterol absorption (55,68). While this approach targets dietary uptake, it does not modulate liver produced levels in the serum. It might require inhibiting intestinal uptake and liver production to show clinical efficacy.
Concluding remarks and future directions
The TCGA database provides correlative evidence suggesting the involvement of the cholesterol homeostasis pathways in cancer development (31). Altered expression levels and mutations of genes involved in the cholesterol homeostasis pathways have been identified in cancer cells (68). These include increases in gene copy numbers, upregulation of cholesterol synthesis gene expression, enhanced cholesterol import by LDL receptors, and decreased transport of cholesterol, which promote increased cellular cholesterol levels to aid cancer cell proliferation (1,2,21,68). However, the field is still young and further research is needed to fully dissect the consequences of these changes and how they modulate cancer development. Furthermore, correlative TCGA database evidence suggesting deregulation of cholesterol homeostasis in cancer development needs validation in preclinical model systems and finally translation into useful practices in the clinic that could decrease cancer development.
The following are some questions needing evaluation in the cholesterol and cancer field. First, the role of the genetic alterations affecting the cholesterol pathways genes and function in cancer development needs investigation. For example, many cholesterol synthesis genes or mitochondrial cholesterol importers are upregulated through copy number increases but the effects on cancer development remain unknown. For example, approximately 60% of melanomas had increased expression or chromosomal copy number increases in at least one of the cholesterol synthesis genes (Figure 2B). Several of these alterations were associated with known chromosomal amplification sites that harbor well-characterized oncogenes (Figure 2C). Specifically, HMGCS2 & NOTCH2 and SQLE & MYC were co-localized to the same amplicons. Possibly, oncogenes and cholesterol synthesis genes cooperate to promote disease progression, but this needs demonstration. Similarly, SC5D, one of the key genes in the last steps of cholesterol synthesis pathway is localized to 11q23.3 and co-deleted with several cholesterol export genes (Figure 2C). The deletion of SC5D may contribute to cancer progression through a mechanism similar to that occurring with lathosterolosis, a disease resulting from the loss of SC5D function (69,70). Decreased SC5D activity in cancer might increase prenylation of many cancer genes such as RAS, RAC or RHOC thereby promoting cancer progression (69,70). This possibility is supported by data from the TCGA database where melanoma patients having reduced expression of SC5D had decreased survival (Figure 1A). Linking these data to preclinical models and clinical support is needed to validating the roles played by cholesterol in cancer.
A second question needing addressing is whether tumors could be classified into subclasses based on genetic abnormalities occurring in cholesterol homeostasis genes. This might facilitate development of precision medicine based approaches for preventing or treating particular subgroups of cancer. For examples, the efficacy of statins, squalene synthesis inhibitors, farnesyl or geranylgeranyl transferase inhibitors might be particularly effective for certain patients with characteristic genetic profiles.
In summary, while not conclusive, it appears that deregulation of cholesterol homeostasis is an important contributing factor to cancer development. Studies are needed to link population based epidemiological data, results from the TCGA database, and preclinical mechanistic evidence to more thoroughly dissect the involvement of cholesterol in cancer development.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by The Foreman Foundation for Melanoma Research, Geltrude Foundation, The Chocolate Tour Funds and NIH grants CA-127892, CA-136667, and CA-138634.
Footnotes
Conflict of Interest: None
REFRENCES
- 1.Smith B, Land H. Anticancer activity of the cholesterol exporter ABCA1 gene. Cell reports. 2012;2(3):580–590. doi: 10.1016/j.celrep.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krycer JR, Brown AJ. Cholesterol accumulation in prostate cancer: a classic observation from a modern perspective. Biochimica et biophysica acta. 2013;1835(2):219–229. doi: 10.1016/j.bbcan.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Shafique K, McLoone P, Qureshi K, Leung H, Hart C, Morrison DS. Cholesterol and the risk of grade-specific prostate cancer incidence: evidence from two large prospective cohort studies with up to 37 years' follow up. BMC Cancer. 2012;12:25. doi: 10.1186/1471-2407-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelton K, Freeman MR, Solomon KR. Cholesterol and prostate cancer. Current opinion in pharmacology. 2012;12(6):751–759. doi: 10.1016/j.coph.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, et al. Serum lipid profile and risk of prostate cancer recurrence: Results from the SEARCH database. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23(11):2349–2356. doi: 10.1158/1055-9965.EPI-14-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs EJ, Newton CC, Thun MJ, Gapstur SM. Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res. 2011;71(5):1763–1771. doi: 10.1158/0008-5472.CAN-10-2953. [DOI] [PubMed] [Google Scholar]
- 7.Murtola TJ, Visvanathan K, Artama M, Vainio H, Pukkala E. Statin use and breast cancer survival: a nationwide cohort study from Finland. PloS one. 2014;9(10):e110231. doi: 10.1371/journal.pone.0110231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardwell CR, Hicks BM, Hughes C, Murray LJ. Statin use after colorectal cancer diagnosis and survival: a population-based cohort study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(28):3177–3183. doi: 10.1200/JCO.2013.54.4569. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. The New England journal of medicine. 2012;367(19):1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- 10.Ravnskov U, Rosch PJ, McCully KS. Statins do not protect against cancer: quite the opposite. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(7):810–811. doi: 10.1200/JCO.2014.58.9564. [DOI] [PubMed] [Google Scholar]
- 11.Bjerre LM, LeLorier J. Do statins cause cancer? A meta-analysis of large randomized clinical trials. The American journal of medicine. 2001;110(9):716–723. doi: 10.1016/s0002-9343(01)00705-7. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen TR, Wilhelmsen L, Faergeman O, Strandberg TE, Thorgeirsson G, Troedsson L, et al. Follow-up study of patients randomized in the Scandinavian simvastatin survival study (4S) of cholesterol lowering. The American journal of cardiology. 2000;86(3):257–262. doi: 10.1016/s0002-9149(00)00910-3. [DOI] [PubMed] [Google Scholar]
- 13.Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs. Jama. 1996;275(1):55–60. [PubMed] [Google Scholar]
- 14.Ravnskov U, McCully KS, Rosch PJ. The statin-low cholesterol-cancer conundrum. QJM : monthly journal of the Association of Physicians. 2012;105(4):383–388. doi: 10.1093/qjmed/hcr243. [DOI] [PubMed] [Google Scholar]
- 15.Matsuzaki M, Kita T, Mabuchi H, Matsuzawa Y, Nakaya N, Oikawa S, et al. Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low-dose simvastatin therapy in Japanese patients with hypercholesterolemia. Circulation journal : official journal of the Japanese Circulation Society. 2002;66(12):1087–1095. doi: 10.1253/circj.66.1087. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, La Vecchia C, de Groh M, Negri E, Morrison H, Mery L, et al. Dietary cholesterol intake and cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23(2):491–500. doi: 10.1093/annonc/mdr155. [DOI] [PubMed] [Google Scholar]
- 17.Jarvinen R, Knekt P, Hakulinen T, Rissanen H, Heliovaara M. Dietary fat, cholesterol and colorectal cancer in a prospective study. British journal of cancer. 2001;85(3):357–361. doi: 10.1054/bjoc.2001.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Qin S, Wang M, Zhang T, Zhang S. Association between cholesterol intake and pancreatic cancer risk: evidence from a meta-analysis. Scientific reports. 2015;5:8243. doi: 10.1038/srep08243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keys A. Serum cholesterol response to dietary cholesterol. The American journal of clinical nutrition. 1984;40(2):351–359. doi: 10.1093/ajcn/40.2.351. [DOI] [PubMed] [Google Scholar]
- 20.Montero J, Morales A, Llacuna L, Lluis JM, Terrones O, Basanez G, et al. Mitochondrial cholesterol contributes to chemotherapy resistance in hepatocellular carcinoma. Cancer Res. 2008;68(13):5246–5256. doi: 10.1158/0008-5472.CAN-07-6161. [DOI] [PubMed] [Google Scholar]
- 21.Llaverias G, Danilo C, Mercier I, Daumer K, Capozza F, Williams TM, et al. Role of cholesterol in the development and progression of breast cancer. The American journal of pathology. 2011;178(1):402–412. doi: 10.1016/j.ajpath.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vassilev B, Sihto H, Li S, Holtta-Vuori M, Ilola J, Lundin J, et al. Elevated levels of StAR-related lipid transfer protein 3 alter cholesterol balance and adhesiveness of breast cancer cells: potential mechanisms contributing to progression of HER2-positive breast cancers. The American journal of pathology. 2015;185(4):987–1000. doi: 10.1016/j.ajpath.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Robinson DR, Kalyana-Sundaram S, Wu YM, Shankar S, Cao X, Ateeq B, et al. Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nature medicine. 2011;17(12):1646–1651. doi: 10.1038/nm.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yun SM, Yoon K, Lee S, Kim E, Kong SH, Choe J, et al. PPP1R1B-STARD3 chimeric fusion transcript in human gastric cancer promotes tumorigenesis through activation of PI3K/AKT signaling. Oncogene. 2014;33(46):5341–5347. doi: 10.1038/onc.2013.472. [DOI] [PubMed] [Google Scholar]
- 25.Freed-Pastor WA, Mizuno H, Zhao X, Langerod A, Moon SH, Rodriguez-Barrueco R, et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell. 2012;148(1–2):244–258. doi: 10.1016/j.cell.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nature cell biology. 2014;16(4):357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- 27.Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Current opinion in clinical nutrition and metabolic care. 2006;9(4):358–365. doi: 10.1097/01.mco.0000232894.28674.30. [DOI] [PubMed] [Google Scholar]
- 28.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nature reviews Molecular cell biology. 2008;9(2):125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 29.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Rogers PM, Su C, Varga G, Stayrook KR, Burris TP. Regulation of cholesterologenesis by the oxysterol receptor, LXRalpha. The Journal of biological chemistry. 2008;283(39):26332–26339. doi: 10.1074/jbc.M804808200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cancer Genome Atlas Research N. Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nature genetics. 2013;45(10):1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown DN, Caffa I, Cirmena G, Piras D, Garuti A, Gallo M, et al. Squalene epoxidase is a bona fide oncogene by amplification with clinical relevance in breast cancer. Scientific reports. 2016;6:19435. doi: 10.1038/srep19435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sui Z, Zhou J, Cheng Z, Lu P. Squalene epoxidase (SQLE) promotes the growth and migration of the hepatocellular carcinoma cells. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36(8):6173–6179. doi: 10.1007/s13277-015-3301-x. [DOI] [PubMed] [Google Scholar]
- 34.Riganti C, Massaia M. Inhibition of the mevalonate pathway to override chemoresistance and promote the immunogenic demise of cancer cells: Killing two birds with one stone. Oncoimmunology. 2013;2(9):e25770. doi: 10.4161/onci.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maione F, Oliaro-Bosso S, Meda C, Di Nicolantonio F, Bussolino F, Balliano G, et al. The cholesterol biosynthesis enzyme oxidosqualene cyclase is a new target to impair tumour angiogenesis and metastasis dissemination. Scientific reports. 2015;5:9054. doi: 10.1038/srep09054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haskins JW, Zhang S, Means RE, Kelleher JK, Cline GW, Canfran-Duque A, et al. Neuregulin-activated ERBB4 induces the SREBP-2 cholesterol biosynthetic pathway and increases low-density lipoprotein uptake. Sci Signal. 2015;8(401):ra111. doi: 10.1126/scisignal.aac5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabitova L, Restifo D, Gorin A, Manocha K, Handorf E, Yang DH, et al. Endogenous Sterol Metabolites Regulate Growth of EGFR/KRAS-Dependent Tumors via LXR. Cell reports. 2015;12(11):1927–1938. doi: 10.1016/j.celrep.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell metabolism. 2008;8(3):224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong F, Mo Z, Eid W, Courtney KC, Zha X. Akt inhibition promotes ABCA1-mediated cholesterol efflux to ApoA-I through suppressing mTORC1. PloS one. 2014;9(11):e113789. doi: 10.1371/journal.pone.0113789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell metabolism. 2014;19(3):393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thysell E, Surowiec I, Hornberg E, Crnalic S, Widmark A, Johansson AI, et al. Metabolomic characterization of human prostate cancer bone metastases reveals increased levels of cholesterol. PloS one. 2010;5(12):e14175. doi: 10.1371/journal.pone.0014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo D, Reinitz F, Youssef M, Hong C, Nathanson D, Akhavan D, et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer discovery. 2011;1(5):442–456. doi: 10.1158/2159-8290.CD-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alpy F, Tomasetto CL. Cholesterol Transporters of the START Domain Protein Family in Health and Disease. Springer; 2014. STARD3: A Lipid Transfer Protein in Breast Cancer and Cholesterol Trafficking; pp. 119–138. [Google Scholar]
- 45.McDonnell DP, Park S, Goulet MT, Jasper J, Wardell SE, Chang CY, et al. Obesity, cholesterol metabolism, and breast cancer pathogenesis. Cancer Res. 2014;74(18):4976–4982. doi: 10.1158/0008-5472.CAN-14-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin CY, Huo C, Kuo LK, Hiipakka RA, Jones RB, Lin HP, et al. Cholestane-3beta, 5alpha, 6beta-triol suppresses proliferation, migration, and invasion of human prostate cancer cells. PloS one. 2013;8(6):e65734. doi: 10.1371/journal.pone.0065734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fox EM, Andrade J, Shupnik MA. Novel actions of estrogen to promote proliferation: integration of cytoplasmic and nuclear pathways. Steroids. 2009;74(7):622–627. doi: 10.1016/j.steroids.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.York AG, Bensinger SJ. Subverting sterols: rerouting an oxysterol-signaling pathway to promote tumor growth. The Journal of experimental medicine. 2013;210(9):1653–1656. doi: 10.1084/jem.20131335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dufour J, Viennois E, De Boussac H, Baron S, Lobaccaro JM. Oxysterol receptors, AKT and prostate cancer. Current opinion in pharmacology. 2012;12(6):724–728. doi: 10.1016/j.coph.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 50.Wu Q, Ishikawa T, Sirianni R, Tang H, McDonald JG, Yuhanna IS, et al. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell reports. 2013;5(3):637–645. doi: 10.1016/j.celrep.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J, Lee S-Y, Gu D, Song B, Chen S, Konieczny S, et al. Abstract B50: Abrogating cholesterol esterification suppresses pancreatic cancer growth and metastasis mediated by caveolin-1. Cancer Research. 2015;75(13 Supplement):B50–B50. [Google Scholar]
- 52.Yamaguchi R, Perkins G, Hirota K. Targeting cholesterol with beta-cyclodextrin sensitizes cancer cells for apoptosis. FEBS letters. 2015;589(24 Pt B):4097–4105. doi: 10.1016/j.febslet.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 53.Sukhanova A, Gorin A, Serebriiskii IG, Gabitova L, Zheng H, Restifo D, et al. Targeting C4-demethylating genes in the cholesterol pathway sensitizes cancer cells to EGF receptor inhibitors via increased EGF receptor degradation. Cancer discovery. 2013;3(1):96–111. doi: 10.1158/2159-8290.CD-12-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cruz PM, Mo H, McConathy WJ, Sabnis N, Lacko AG. The role of cholesterol metabolism and cholesterol transport in carcinogenesis: a review of scientific findings, relevant to future cancer therapeutics. Frontiers in pharmacology. 2013;4:119. doi: 10.3389/fphar.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solomon KR, Pelton K, Boucher K, Joo J, Tully C, Zurakowski D, et al. Ezetimibe is an inhibitor of tumor angiogenesis. The American journal of pathology. 2009;174(3):1017–1026. doi: 10.2353/ajpath.2009.080551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ginestier C, Monville F, Wicinski J, Cabaud O, Cervera N, Josselin E, et al. Mevalonate metabolism regulates Basal breast cancer stem cells and is a potential therapeutic target. Stem cells. 2012;30(7):1327–1337. doi: 10.1002/stem.1122. [DOI] [PubMed] [Google Scholar]
- 57.Warita K, Warita T, Beckwitt CH, Schurdak ME, Vazquez A, Wells A, et al. Statin-induced mevalonate pathway inhibition attenuates the growth of mesenchymal-like cancer cells that lack functional E-cadherin mediated cell cohesion. Scientific reports. 2014;4:7593. doi: 10.1038/srep07593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Higgins MJ, Prowell TM, Blackford AL, Byrne C, Khouri NF, Slater SA, et al. A short-term biomarker modulation study of simvastatin in women at increased risk of a new breast cancer. Breast cancer research and treatment. 2012;131(3):915–924. doi: 10.1007/s10549-011-1858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pavlakis N, Stockler M. Bisphosphonates for breast cancer. The Cochrane database of systematic reviews. 2002;(1) doi: 10.1002/14651858.CD003474. CD003474. [DOI] [PubMed] [Google Scholar]
- 60.Mo H, Elson CE. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Experimental biology and medicine. 2004;229(7):567–585. doi: 10.1177/153537020422900701. [DOI] [PubMed] [Google Scholar]
- 61.Kuzu OF, Gowda R, Sharma A, Robertson GP. Leelamine mediates cancer cell death through inhibition of intracellular cholesterol transport. Molecular cancer therapeutics. 2014;13(7):1690–1703. doi: 10.1158/1535-7163.MCT-13-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reverter M, Rentero C, Garcia-Melero A, Hoque M, Vila de Muga S, Alvarez-Guaita A, et al. Cholesterol regulates Syntaxin 6 trafficking at trans-Golgi network endosomal boundaries. Cell reports. 2014;7(3):883–897. doi: 10.1016/j.celrep.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 63.Ridsdale A, Denis M, Gougeon PY, Ngsee JK, Presley JF, Zha X. Cholesterol is required for efficient endoplasmic reticulum-to-Golgi transport of secretory membrane proteins. Molecular biology of the cell. 2006;17(4):1593–1605. doi: 10.1091/mbc.E05-02-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hammadi M, Oulidi A, Gackiere F, Katsogiannou M, Slomianny C, Roudbaraki M, et al. Modulation of ER stress and apoptosis by endoplasmic reticulum calcium leak via translocon during unfolded protein response: involvement of GRP78. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27(4):1600–1609. doi: 10.1096/fj.12-218875. [DOI] [PubMed] [Google Scholar]
- 65.Cenedella RJ. Cholesterol synthesis inhibitor U18666A and the role of sterol metabolism and trafficking in numerous pathophysiological processes. Lipids. 2009;44(6):477–487. doi: 10.1007/s11745-009-3305-7. [DOI] [PubMed] [Google Scholar]
- 66.Enrich C, Rentero C, Hierro A, Grewal T. Role of cholesterol in SNARE-mediated trafficking on intracellular membranes. Journal of cell science. 2015;128(6):1071–1081. doi: 10.1242/jcs.164459. [DOI] [PubMed] [Google Scholar]
- 67.Cubells L, Vila de Muga S, Tebar F, Wood P, Evans R, Ingelmo-Torres M, et al. Annexin A6-induced alterations in cholesterol transport and caveolin export from the Golgi complex. Traffic. 2007;8(11):1568–1589. doi: 10.1111/j.1600-0854.2007.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murai T. Cholesterol lowering: role in cancer prevention and treatment. Biological chemistry. 2015;396(1):1–11. doi: 10.1515/hsz-2014-0194. [DOI] [PubMed] [Google Scholar]
- 69.Jiang XS, Backlund PS, Wassif CA, Yergey AL, Porter FD. Quantitative proteomics analysis of inborn errors of cholesterol synthesis: identification of altered metabolic pathways in DHCR7 and SC5D deficiency. Molecular & cellular proteomics : MCP. 2010;9(7):1461–1475. doi: 10.1074/mcp.M900548-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berndt N, Hamilton AD, Sebti SM. Targeting protein prenylation for cancer therapy. Nature reviews Cancer. 2011;11(11):775–791. doi: 10.1038/nrc3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.