Abstract
Purpose of the review
To update major new findings and concepts introduced during the past year on the role of angiotensin II (Ang II) subtype 2 receptors (AT2Rs) in the control of blood pressure (BP) and renal function.
Recent findings
AT2R activation prevents sodium (Na+) retention and lowers BP in the Ang II infusion model of experimental hypertension and prevents salt-sensitive hypertension in the obese Zucker rat model of obesity and the metabolic syndrome. Ang II metabolite, des-aspartyl1-Ang II (Ang III) is the predominant AT2R agonist in the kidney and possibly also in the vasculature; a novel synthetic Ang III peptide, β-Pro-Ang III, is vasodepressor and lowers BP in conscious spontaneously hypertensive rats (SHR) in the presence of low-level Ang II type 1 receptor (AT1R) blockade. Because nitric oxide (NO) is a product of AT2R activation, a potential feed forward loop, wherein NO increases AT2R transcription, may reinforce AT2R beneficial actions long term. AT2R activation also reduces proteinuria and oxidative stress in glomerulosclerotic kidneys of high salt obese Zucker rats.
Summary
Studies during the past year have helped clarify the physiological and pathophysiological roles of AT2Rs and have enhanced the promise of AT2R agonists in cardiovascular and renal disease.
Keywords: Angiotensin, angiotensin receptors, blood pressure, hypertension, kidney function, sodium excretion, natriuresis, kidney protection
INTRODUCTION
The renin-angiotensin system (RAS) is a complex hormonal cascade governing cardiovascular and renal function (1,2). The RAS is composed of a major effector peptide, angiotensin II (Ang II), formed from renin-induced catalytic cleavage of angiotensinogen (renin substrate) to produce the decapeptide Ang I and subsequent cleavage by angiotensin converting enzyme (ACE) to synthesize the octapeptide Ang II. Ang II acts mainly via AT1 receptors (AT1Rs) to initiate and maintain actions that can be detrimental to health, such as vasoconstriction, antinatriuresis, aldosterone secretion, sympathetic nervous system activation, cellular dedifferentiation and growth, inflammation and target organ damage. The RAS is also composed of several other peptide metabolites and receptors, many of which oppose these actions. Most important and best studied are the ACE-2-Ang (1–7)-Mas receptor pathway and the Ang II/Ang III-AT2 receptor (AT2R) pathway, both of which work to offset the actions of Ang II via AT1Rs. This review will focus on major new findings and concepts introduced during the past year on the role of AT2Rs in the control of BP and renal function.
AT2R AGONIST - INDUCED NATRIURESIS AND BP REDUCTION
AT2Rs are expressed throughout the adult kidney at vascular and tubule sites, albeit in smaller quantities than AT1Rs (3,4). In particular, AT2Rs are highly expressed in the renal proximal tubule (4). AT2R-induced natriuresis was first suggested 17 years ago when AT2R-null mice were shown to have pressor and antinatriuretic hypersensitivity to exogenous Ang II, indicating that pressure-natriuresis is reduced in the absence of AT2Rs (5). The antinatriuresis in these animals was attributed to the marked reduction in renal bradykinin (BK), nitric oxide (NO) and guanosine cyclic 3′,5′-monophosphate (cGMP) in AT2R-null compared to wild type animals at baseline and in response to Ang II (5). Subsequently, studies found that the formal pressure-natriuresis relationship is indeed shifted to the right (less sensitive) in AT2R-null mice with and without L-NAME-induced hypertension (6,7).
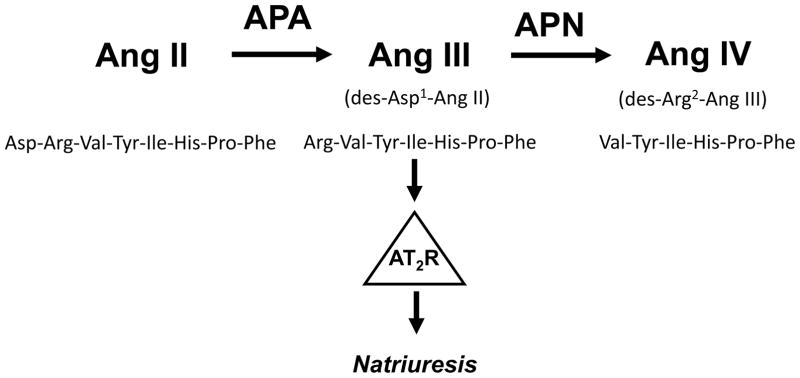
Unequivocal evidence now exists that renal AT2Rs mediate natriuresis (8–16). AT2Rs thereby oppose the actions of Ang II via AT1Rs, which increase renal tubule sodium (Na+) reabsorption and induce antinatriuresis (17–19). Studies have demonstrated that the predominant, and possibly exclusive, endogenous AT2R agonist for the natriuretic response is not Ang II, but the Ang II metabolite [des-aspartyl1]-Ang II (Ang III) (Figure 1) (9,10,16). In normal animals, the demonstration of Ang III mediated natriuresis via AT2Rs requires the presence of concurrent low level systemic AT1R blockade or inhibition of aminopeptidase N, which catabolizes Ang III to inactive metabolites (8–10,16). However, in obese Zucker rats or streptozotocin-induced diabetic rats, concomitant AT1R blockade is not required because AT2Rs exhibit increased renal proximal tubule AT2R expression in this model (11–15). Interestingly, selective intrarenal AT1R blockade induces natriuresis by an AT2R-dependent mechanism that is blocked with AT2R antagonist PD-123319 (PD) (8). Although not formally examined, the mechanism of this acute response is likely related to increased endogenous intrarenal Ang III levels with consequent activation of unblocked AT2Rs (8–10,16).
Figure 1.
Schematic representation of the metabolism of Ang II to Ang III by aminopeptidase A (APA) and Ang III to Ang IV by aminopeptidase N (APN) with peptide sequences. Ang III is the major endogenous AT2R agonist inducing natriuresis in rodents. Ang II: angiotensin II; Ang III: angiotensin III; AT2R: angiotensin subtype 2 receptor.
Recent studies of AT2R-induced natriuresis have employed the highly selective nonpeptide AT2R agonist Compound 21 (C-21) (20,21). C-21 administered acutely to normal rats (systemically or intrarenally) induced natriuresis by an action at the renal proximal tubule (RPT) (22). The C-21-induced natriuresis was mediated by AT2R activation, which was dependent upon a BK-NO-cGMP signaling pathway, as previously described (23,24). Acute C-21 administration recruited AT2Rs from intracellular sites to the apical plasma membranes of renal proximal tubule (RPT) cells, a possible mechanism for reinforcing and sustaining the AT2R natriuretic response (22). C-21 also internalized and inactivated major RPT Na+ transporters Na+-hydrogen exchanger-3 (NHE-3) and Na+/K+ATPase (NKA), indicating that reduced RPT Na+ transport is the mechanism for the natriuresis (22). Since no effective diuretic/natriuretic agents are available that act at the RPT, these observations suggested that AT2R activation might be additive to diuretics acting in the distal portions of the nephron (distal and connecting tubules and/or cortical collecting duct).
Recent studies have confirmed the natriuretic response of acute AT2R activation with intrarenal administration of highly selective AT2R synthetic peptide agonist CGP42112A (CGP) (*25). In this study, AT2R-induced natriuresis was associated with AT2R receptor recruitment to the apical plasma membranes and inactivation of NKA in RPT cells (*25). Interestingly, co-stimulation of the renal dopamine D3 receptor (D3R), a member of the D2-like receptor family, enhanced the CGP-induced natriuretic response, indicating the possibility that receptor heterodimerization on the plasma membrane is at least partially responsible for the enhancement. This finding reflects on earlier observations that stimulation of the RPT dopamine D1-like receptor (D1 and D5 receptors) family, appears to be absolutely dependent on renal AT2R activation (26,27). The interactions among angiotensin and dopamine receptors on natriuresis may be key considerations in the regulation of the natriuretic response and require further study.
Building on the findings that acute AT2R activation induces natriuresis, Kemp et al. (**28) demonstrated in the Ang II infusion model of experimental hypertension that chronic AT2R activation with C-21 prevented the initial (24h) Ang II-induced reduction in Na+ excretion, induced sustained negative Na+ balance and lowered BP to near-control levels during a 7-day infusion period. As with acute C-21 administration, concurrent AT1R blockade was not required to unmask the natriuretic and hypotensive actions of chronic C-21. Again, similar to the acute studies, chronic administration of C-21 was equally effective administered intrarenally or systemically, translocated AT2Rs to the apical plasma membranes and internalized/inactivated NHE-3 and NKA in RPT cells. C-21-induced natriuresis derived from RPT inhibition of Na+ reabsorption because it was additive to that of chlorothiazide and amiloride (**28). In addition to preventing Na+ retention and hypertension, the results demonstrated that C-21 was equally effective in lowering BP once the Ang II-dependent hypertension had been established. This study strongly suggests that AT2R agonists can be effective natriuretic and diuretic agents that improve the pressure-natriuresis relationship and may be effective antihypertensive agents in settings in which the RAS is activated.
Whether AT2R agonists are antihypertensive by directly reducing peripheral vascular resistance, independently at least in part of their effects on renal Na+ excretion, has been controversial (29,30). Clear results have been hindered by lack of highly selective AT2R agonists for study. In order to circumvent this problem, Jones et al. (31) applied a novel β-amino acid substitution to Ang II that resulted in >1,000-fold selectivity of AT2R over AT1R binding. This compound, β-Ile5-Ang II, exhibited AT2R-dependent vasodepressor actions in vitro and was antihypertensive in spontaneously hypertensive rats (SHR) in vivo in the presence of background low-level AT1R blockade (31). Interestingly, previous studies showed that the heptapeptide Ang II metabolite, Ang III, elicited a biphasic BP response with an initial pressor response followed by a depressor response that was blocked by AT2R antagonist PD (32). Building on these findings, Del Borgo and colleagues (**33) recently synthesized a new AT2R ligand, β-Pro-Ang III, with >20,000-fold AT2R to AT1R selectivity. This AT2R agonist evoked vasorelaxation in vitro and lowered BP acutely by ~ 35 mmHg during low-level AT1R blockade in conscious SHR. These vascular actions of β-Pro-Ang III were abolished by AT2R blockade with PD and also by BK B2R antagonist icatibant or NOS inhibitor L-NAME, indicating that they were caused by AT2R activation via the well-recognized BK-NO AT2R signaling pathway in the vasculature (**33). Of primary importance, the vasodepressor actions of Ang III and β-Pro-Ang III were much more impressive than those of Ang II under the same experimental conditions (in the presence of low-level AT1R blockade) (**33). Similar to reports on natriuresis, these results demonstrate the differential effects of Ang III over Ang II on AT2R activation in the vasculature (8–10,15, **33).
POSITIVE NO FEEDBACK SIGNALING LOOP MAY REGULATE AT2R EXPRESSION
Among the cell signaling mechanisms that have been implicated in AT2R-induced biological responses in multiple organs and tissues, the BK-NO-cGMP pathway is of paramount importance (34,35). AT2Rs can either activate NOS directly or indirectly via increased BK production and subsequent activation of its B2Rs (36). Indeed, mice lacking the B2R have normal BP and renal function, so direct NOS activation may serve as the default signaling pathway (37).
Surprisingly, as reported recently, NO has been shown to upregulate the expression of AT2Rs in endothelial cells primarily by increasing AT2R gene expression (transcription) (*38). Pharmacological inhibition of NOS with L-NAME reversed the upregulation of aortic AT2R expression in eNOS transgenic animals (*38). As reported earlier, AT2R upregulation was associated with reduced activity of angiotensin converting enzyme (ACE) (39). Mice with increased AT2R expression had reduced ACE activity whereas AT2R-null mice manifested increased ACE activity (*38). Thus, AT2Rs appear to be ACE inhibitors. When NO increased the AT2R message, the signaling pathway involved was increased soluble guanylyl cyclase activity increasing cGMP production, activation of protein kinase G (PKG) and p38 MAP kinase (*38). Because AT2R activation increases synthesis and release of NO and cGMP, this finding raises the interesting possibility that a selective AT2R agonist, via a feed-forward mechanism involving NO generation, may in turn increase AT2R transcription, thus reinforcing vascular responses to the agonist chronically. This putative positive feedback mechanism might engender a favorable AT2R:AT1R ratio that could contribute to a sustained reduction of BP in response to chronic agonist administration. This exciting concept deserves further validation with experiments testing BP responses to chronic AT2R agonist administration. Whether this feed-forward concept also applies to cells and tissues other than endothelium awaits further study.
BENEFICIAL EFFECTS OF AT2R ACTIVATION IN OBESITY/METABOLIC SYNDROME
The obese Zucker rat is an established model of obesity with insulin resistance and mild hyperglycemia that approximates the metabolic syndrome in humans. This model has elevated BP and renal dysfunction (40). Previous studies have shown that AT2R activation in the obese Zucker rat induces natriuresis by inactivating RPT NKA and exerts a protective role in lowering BP (11,13–15,41). As indicated above, in the obese Zucker rat, activation of AT2Rs alone appears sufficient to lower BP without the addition of concurrent AT1R blockade (42). This may be due to upregulation of AT2R expression by hyperglycemia. Furthermore, chronic AT2R blockade with PD increased BP in obese rats, suggesting a tonic protective role for AT2Rs on BP in this experimental model (41).
Evidence for a role of AT2Rs in BP control in the obese Zucker model has now been reported (**43). Chronic administration of C-21 over a 2-week period prevented a high salt diet (HSD)-induced increase in BP in these animals (**43). Ang II levels in the renal cortex were approximately 4-fold higher in the HSD-fed rats than in their normal salt diet (NSD) controls. C-21 partially blocked the HSD-induced increase in renal Ang II levels and reduced renal AT1R protein expression by Western blot analysis (**43). However, antibodies employed for AT1R detection have been criticized as being non-specific (44). Interestingly, C-21 significantly increased 24h urine Na+ excretion in both control and HSD-fed animals chronically (on days 11–14 of the study) (**43). This observation is similar to earlier findings of an enhanced natriuresis in response to C-21 at 2 weeks in this model (42) and is reminiscent of the chronic negative Na+ balance induced by C-21 in the Ang II infusion model of experimental hypertension (**28), with obese Zucker rats and Ang II-infused rats having increased Ang II levels in common. Thus, pathophysiologic states characterized as having an activated RAS may respond best to the chronic natriuretic and BP lowering power of AT2R activation. Since AT2Rs are translocated to the apical plasma membranes of RPT cells (22,**28) and do not internalize in renal epithelial cells (45), these receptors likely remain active in promoting natriuresis without desensitization and, thereby, reducing BP over a prolonged period of time (46).
In addition to a progressive Na+-dependent increase in BP, obese Zucker rats also manifest salt-sensitive renal morphological changes of focal glomerulosclerosis (47). AT2Rs have anti-inflammatory, anti-proliferative and anti-fibrotic effects and may protect against oxidative stress (48–50). A recent report sheds light on the pathophysiology of the renal dysfunction in obese Zucker rats fed HSD and demonstrates the attenuation of the renal target organ damage with AT2R activation via C-21 (**51). HSD rats exhibited an increase in cortical nicotinamide adenine dinucleotide phosphate osidase activity, urinary hydrogen peroxide, and 8-isoprostanes and severe glomerulosclerosis, interstitial fibrosis reduction in glomerular filtration rate, urinary protein leak, and activity of N-acetyl-β-D-glucosaminidase, a lysosomal marker of tubular damage (**51). C-21 significantly attenuated these changes. Although further work needs to be done, particularly as to whether these C-21 effects are independent of reductions in BP, the results clearly show that AT2R activation protects against HSD-induced renal target organ damage in obesity.
CONCLUSION
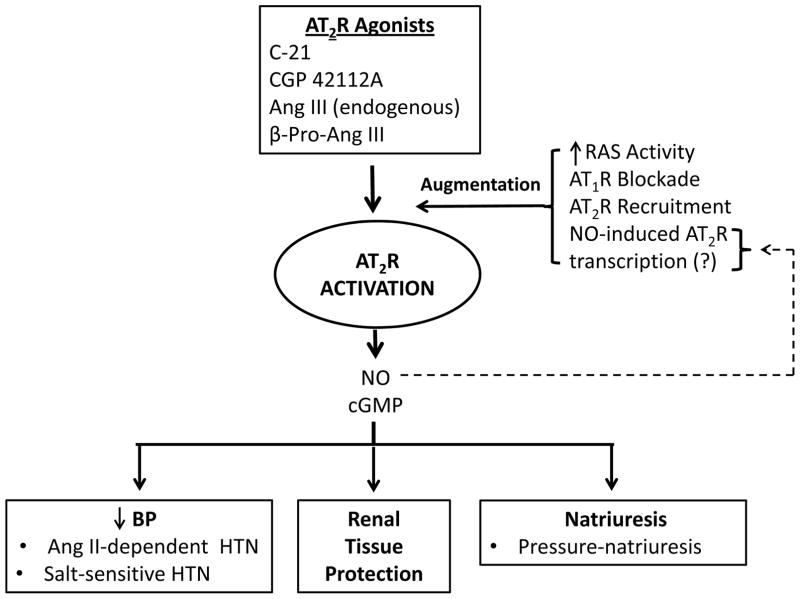
AT2Rs constitute an important component of the “protective arm” of the RAS, counter-balancing the untoward actions the main RAS effector peptide, Ang II, via AT1Rs. The functions of AT2Rs have been more difficult to elicit than those of AT1Rs, at least in part due to the relatively low expression level of AT2Rs compared to AT1Rs in adult cardiovascular and renal cells. With the recent development of highly selective AT2R agonists, however, the physiological and pathophysiological roles of these receptors are beginning to be clarified. Several concepts governing the role of AT2Rs in biological actions have emerged (Figure 2). First, AT2R activation seems to exert a beneficial role, such as natriuresis and/or hypotension, when the RAS is activated, as in Ang II-dependent hypertension or obesity with the metabolic syndrome. Second, AT2R beneficial effects are also observed when AT1Rs are blocked, so that AT2R responses are not swamped by AT1Rs and concurrently by facilitating stimulation of the unblocked AT2Rs. Third, the endogenous AT2R agonist (predominant or exclusive) appears to be the Ang II metabolite, Ang III. Fourth, because AT2Rs are recruited to plasma membranes and do not internalize, at least in renal epithelial cells, AT2R activation can sustain long-term beneficial effects, such as natriuresis, without desensitization. Fifth, AT2R activation has the capacity to improve or abolish target organ damage in certain cardiovascular and renal disease states. Many of these principles have been derived from work during the past year, which when coupled with past studies, have improved our understanding of the role of AT2Rs in pathophysiology and highlighted the promise of AT2R agonist therapy in the near future.
Figure 2.
Schematic representation of the current understanding of the role of AT2R activation in the control of BP and renal function on the basis of studies published during the past year. The ability of an AT2R agonist to activate the receptor is dependent on at least four factors shown at the upper right of the diagram. The biological effects are shown at the bottom of the figure. Ang II: angiotensin II; Ang III: angiotensin III; AT1R: angiotensin subtype 1 receptor; AT2R: angiotensin subtype 2 receptor; BP: blood pressure; β-Pro-Ang III: beta-proline-angiotensin III; C-21: Compound 21; cGMP: guanosine cyclic 3′,5′-monophosphate; HTN: hypertension; NO: nitric oxide; RAS: renin-angiotensin system; (?): putative.
KEY POINTS.
Angiotensin AT2 receptor (AT2R) activation induces natriuresis, maintains negative Na+ balance and lowers blood pressure chronically in angiotensin-dependent hypertension due to reduced Na+ reabsorption at the renal proximal tubule.
AT2R activation prevents salt-sensitive hypertension in the obese Zucker rat model of obesity and the metabolic syndrome.
Nitric oxide is both a product and a stimulator of AT2Rs, potentially reinforcing the AT2R biological response.
β-Pro-angiotensin III, a novel synthetic AT2R peptide agonist, reduces blood pressure in spontaneous hypertension.
AT2R activation improves renal structural and functional damage high salt diet-fed obese Zucker rats.
Acknowledgments
The author acknowledges Brandon A. Kemp, Nancy L. Howell and Drs. Susanna R. Keller and John J. Gildea in the preparation of this manuscript.
Financial Support and Sponsorship: This review was supported by National Institutes of Health research grant 1-R01-HL-128189.
Footnotes
Conflicts of Interest: None
References
- 1.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24:261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 2.Carey RM. Newly discovered components and actions of the renin-angiotensin system. Hypertension. 2013;62:818–822. doi: 10.1161/HYPERTENSIONAHA.113.01111. [DOI] [PubMed] [Google Scholar]
- 3.Ozono R, Wang Z-Q, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30:1238–1246. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- 4.Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol Renal Physiol. 1999;277:F437–F446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 5.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc Nat Acad Sci USA. 1999;96:6506–6510. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross V, Schunck WH, Honeck H, Milia AF, Kargel E, Walther T, Bader M, Inagami T, Schneider W, Luft FC. Inhibition of pressure natriuresis in mice lacking the AT2 receptor. Kidney Int. 2000;57:191–202. doi: 10.1046/j.1523-1755.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- 7.Obst M, Gross V, Janke J, Wellner M, Schneider W, Luft FC. Pressure natriuresis in AT2 receptor-deficient mice with L-NAME hypertension. J Am Soc Nephrol. 2003;14:303–310. doi: 10.1097/01.asn.0000043904.26730.11. [DOI] [PubMed] [Google Scholar]
- 8.Padia SH, Howell NL, Siragy HM, Carey RM. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension. 2006;47:537–544. doi: 10.1161/01.HYP.0000196950.48596.21. [DOI] [PubMed] [Google Scholar]
- 9.Padia SH, Kemp BA, Howell NL, Siragy HM, Fournie-Zaluski M-C, Roques BP, Carey RM. Intrarenal aminopeptidase N inhibition augments natriuretic responses to angiotensin III in angiotensin type 1 receptor-blocked rats. Hypertension. 2007;49:625–630. doi: 10.1161/01.HYP.0000254833.85106.4d. [DOI] [PubMed] [Google Scholar]
- 10.Padia SH, Kemp BA, Howell NL, Fournie-Zaluski M-C, Roques BP, Carey RM. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-induced natriuresis in rats. Hypertension. 2008;51:460–465. doi: 10.1161/HYPERTENSIONAHA.107.103242. [DOI] [PubMed] [Google Scholar]
- 11.Hakam AC, Hussain T. Renal angiotensin II type-2 receptors are upregulated and mediate the candesartan-induced natriuresis in obese Zucker rats. Hypertension. 2005;45:270–275. doi: 10.1161/01.HYP.0000151622.47814.6f. [DOI] [PubMed] [Google Scholar]
- 12.Hakam AC, Siddiqui AH, Hussain T. Renal angiotensin II AT2 receptors promote natriuresis in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol. 2006;290:F503–F508. doi: 10.1152/ajprenal.00092.2005. [DOI] [PubMed] [Google Scholar]
- 13.Hakam AC, Hussain T. Angiotensin II AT2 receptors inhibit proximal tubular Na+K+ATPase activity via a NO/cGMP-dependent pathway. Am J Physiol Renal Physiol. 2006;290:F1430–F1436. doi: 10.1152/ajprenal.00218.2005. [DOI] [PubMed] [Google Scholar]
- 14.Hakam AC, Hussain T. Angiotensin type 2 receptor agonist directly inhibits proximal tubule sodium pump activity in obese but not lean Zucker rats. Hypertension. 2006;47:1117–1124. doi: 10.1161/01.HYP.0000220112.91724.fc. [DOI] [PubMed] [Google Scholar]
- 15.Ali Q, Hussain T. AT2 receptor non-peptide agonist C21 promotes natriuresis in obese Zucker rats. Hypertens Res. 2012;35:654–660. doi: 10.1038/hr.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kemp BA, Bell JF, Rottkamp DM, Howell NL, Shao W, Navar LG, Padia SH, Carey RM. Intrarenal angiotensin III is the predominant agonist for proximal tubule angiotensin type 2 receptors. Hypertension. 2012;60:387–395. doi: 10.1161/HYPERTENSIONAHA.112.191403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson MD, Malvin RL. Stimulation of renal sodium reabsorption by angiotensin II. Am J Physiol. 1977;232:F298–F306. doi: 10.1152/ajprenal.1977.232.4.F298. [DOI] [PubMed] [Google Scholar]
- 18.Van der Mark J, Kline RL. Altered pressure natriuresis in chronic angiotensin II hypertension in rats. Am J Physiol. 1994;266:R739–R748. doi: 10.1152/ajpregu.1994.266.3.R739. [DOI] [PubMed] [Google Scholar]
- 19.Mattson DL, Raff H, Roman RJ. Influence of angiotensin II on pressure natriuresis and renal hemodynamics in volume-expanded rats. Am J Physiol. 1991;260:R1200–1209. doi: 10.1152/ajpregu.1991.260.6.R1200. [DOI] [PubMed] [Google Scholar]
- 20.Wan Y, Wallinder C, Plouffe B. Design, synthesis and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem. 2004;47:5995–6008. doi: 10.1021/jm049715t. [DOI] [PubMed] [Google Scholar]
- 21.Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond) 2011;121:297–303. doi: 10.1042/CS20110036. [DOI] [PubMed] [Google Scholar]
- 22.Kemp BA, Howell NL, Gildea JJ, Keller SR, Padia SH, Carey RM. AT2 receptor activation induces natriuresis and lowers blood pressure. Circ Res. 2014;115:388–399. doi: 10.1161/CIRCRESAHA.115.304110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siragy HM, Jaffa AA, Margolius HS, Carey RM. Renin-angiotensin system modulates renal bradykinin production. Am J Physiol. 1996;271:R1090–R1095. doi: 10.1152/ajpregu.1996.271.4.R1090. [DOI] [PubMed] [Google Scholar]
- 24.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest. 1997;100:264–269. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Yang S, Han Y, Zheng S, Kou X, Asico LD, Huang H, Gao Z, Jose PA, Zeng C. Enhanced natriuresis and diuresis in Wistar rats caused by the costimulation of renal dopamine D3 and angiotensin II type 2 receptors. Am J Hypertens. 2015;28:1267–1276. doi: 10.1093/ajh/hpv018. This study confirms that AT2R activation induces natriuresis and indicates that co-stimulation of dopamine D3 receptors enhances the response, possibly by heterodimerizing with AT2Rs on the apical plasma membranes of RPT cells. More work is needed to clarify the importance of interaction of angiotensin and dopamine receptors, especially in the D1-like receptor family, on renal Na+ excretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salomone LJ, Howell NL, McGrath HE, Kemp BA, Keller SR, Gildea JJ, Felder RA, Carey RM. Intrarenal dopamine D1-like receptor stimulation induces natriuresis via an angiotensin type-2 receptor mechanism. Hypertension. 2007;49:155–161. doi: 10.1161/01.HYP.0000251881.89610.ee. [DOI] [PubMed] [Google Scholar]
- 27.Padia SH, Kemp BA, Howell NL, Keller SR, Gildea JJ, Carey RM. Mechanisms of dopamine D(1) and angiotensin type 2 receptor interaction in natriuresis. Hypertension. 2012;59:437–445. doi: 10.1161/HYPERTENSIONAHA.111.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28**.Kemp BA, Howell NL, Keller SR, Gildea JJ, Padia SH, Carey RM. AT2 receptor activation prevents sodium retention and reduces blood pressure in angiotensin II-dependent hypertension. Circ Res. 2016;119:532–543. doi: 10.1161/CIRCRESAHA.116.308384. This study demonstrates that AT2R agonist C-21 may be effective in the treatment of hypertension and/or edema-forming states by its RPT diuretic and natriuretic action. Inhibition of Na+ reabsorption at this nephron site was additive to the natriuretic actions of thiazide (chlorothiazide) and K+-sparing (amiloride) diuretics. AT2R activation, both systemically and intrarenally, was effective chronically in increasing Na+ excretion and lowering of BP in the Ang II infusion model of experimental hypertension and did not require concomitant AT1R blockade. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fouiquier S, Steckelings UM, Unger T. Impact of AT2 receptor agonist C21 on blood pressure and beyond. Curr Hypertens Rep. 2012;14:403–409. doi: 10.1007/s11906-012-0291-6. [DOI] [PubMed] [Google Scholar]
- 30.Sumners C, deKloet AD, Krause EG, Unger T, Steckelings UM. Angiotensin type 2 receptors: blood pressure regulation and end organ damage. Curr Opin Pharmacol. 2015;21:115–121. doi: 10.1016/j.coph.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones ES, Del Borgo MP, Kirsch JF, Clayton D, Bosnyak S, Welungoda I, Hausler N, Unabia S, Perlmutter P, Thomas WG, Aguilar MI, Widdop RE. A single beta-amino acid substitution to angiotensin II confers AT(2) receptor selectivity and vascular function. Hypertension. 2011;57:570–576. doi: 10.1161/HYPERTENSIONAHA.110.164301. [DOI] [PubMed] [Google Scholar]
- 32.Scheuer DA, Perrone MH. Angiotensin type 2 receptors mediate depressor phase of biphasic pressure responses to angiotensin. Am J Physiol. 1993;264:R917–R923. doi: 10.1152/ajpregu.1993.264.5.R917. [DOI] [PubMed] [Google Scholar]
- 33**.Del Borgo M, Wang Y, Bosnyak S, Khan M, Walters P, Spizzo I, Perlmutter P, Hilliard L, Denton K, Aguilar M-I, Widdop RE, Jones ES. B-Pro7 Ang II is a novel highly selective angiotensin II type 2 receptor (AT2R) agonist, which acts as a vasodepressor agent via the AT2R in conscious spontaneously hypertensive rats. Clin Sci. 2015;129:505–513. doi: 10.1042/CS20150077. This excellent study introduces a novel angiotensin peptide agonist, β-Pro-Ang III, with >20,000-fold selectivity for AT2Rs over AT1Rs. This agonist was vasodepressor in vitro and lowered BP in conscious SHR in the presence of low-level AT1R blockade. This study confirms the concept that Ang III is the preferred agonist for AT2Rs because β-Pro-Ang III was significantly more efficacious in causing vasodepressor responses and lowering BP than β-Pro-Ang II in the presence of low-level AT1R blockade. [DOI] [PubMed] [Google Scholar]
- 34.Jones ES, Vinh A, McCarthy CA, Gaspari TA, Widdop RE. AT2 receptors: functional relevance in cardiovascular disease. Pharmacol Ther. 2008;120:292–316. doi: 10.1016/j.pharmthera.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chow BSM, Allen TJ. Angiotensin II type 2 receptor (AT2R) in renal and cardiovascular disease. Clinical Science. 2016;130:1307–1326. doi: 10.1042/CS20160243. [DOI] [PubMed] [Google Scholar]
- 36.Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension. 2003;42:600–604. doi: 10.1161/01.HYP.0000090323.58122.5C. [DOI] [PubMed] [Google Scholar]
- 37.Milia AF, Gross V, Piehm R, DeSilva JA, Jr, Bader M, Luft FC. Normal blood pressure and renal function in mice lacking the bradykinin b(2) receptor. Hypertension. 2001;37:1473–1479. doi: 10.1161/01.hyp.37.6.1473. [DOI] [PubMed] [Google Scholar]
- 38*.Dao VT-V, Medini S, Bishna M, Balz V, Suvorava T, Bas M, Kojda G. Nitric oxide up-regulates endothelial expression of angiotensin type 2 receptors. Biochem Pharmacol. 2016;112:24–36. doi: 10.1016/j.bcp.2016.05.011. This novel study introduces for the first time that NO upregulates AT2R biosynthesis in vascular endothelial cells. This finding opens the door to consideration that NO, a well-established signaling molecule mediating the effects of AT2R activation, can participate in a positive feedback mechanism to strengthen and sustain AT2R vascular responses. Whether this finding is duplicated in other cell types, such as renal epithelial cells, required further investigation. [DOI] [PubMed] [Google Scholar]
- 39.Hunley TE, Tamura M, Stoneking BJ, Nishimura T, Ichiki T, Inagami T, Kon V. The angiotensin type II receptor tonically inhibits angiotensin-converting enzyme in AT2 null mutant mice. Kidney Int. 2000;57:570–577. doi: 10.1046/j.1523-1755.2000.00877.x. [DOI] [PubMed] [Google Scholar]
- 40.Alonso-Galicia M, Brands MW, Zappe DH, Hall JE. Hypertension in obese Zucker rats. Role of angiotensin II and adrenergic activity. Hypertension. 1996;28:1047–1054. doi: 10.1161/01.hyp.28.6.1047. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqui AH, Ali Q, Hussain T. Protective role of angiotensin II subtype 2 receptor in blood pressure increase in obese Zucker rats. Hypertension. 2009;53:256–261. doi: 10.1161/HYPERTENSIONAHA.108.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali Q, Wu Y, Hussain T. Chronic AT2 receptor activation attenuates renal AT1 receptor function and blood pressure in obese Zucker rats. Kidney Int. 2013;84:931–939. doi: 10.1038/ki.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43**.Ali Q, Patel S, Hussain T. Angiotensin AT2 receptor agonist prevents salt-sensitive hypertension in obese Zucker rats. Am J Physiol Renal Physiol. 2015;308:F1379–F1385. doi: 10.1152/ajprenal.00002.2015. This study demonstrates for the first time that the BP increase following salt-loading in obese Zucker rats, can be prevented by exogenous AT2R activation with C-21. Compared with normal salt diet controls, salt-loaded rats had greater natriuresis and increased urinary levels of nitrates and these parameters were further increased by C-21 treatment. This study provides further confirmation that elevated intrarenal Ang II levels in salt-loaded obese Zucker rats may enable beneficial C-21 responses in the absence of concurrent AT1R blockade. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benicky J, Hafko R, Sanchez-Lemus E, Aguilera G, Saavedra JM. Six commercially available angiotensin II AT2 receptor antibodies are non-specific. Cell Mol Neurobiol. 2012;32:1353–1365. doi: 10.1007/s10571-012-9862-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hein L, Meinel L, Pratt RE, Dzau VJ, Koblika BK. Intracellular trafficking of angiotensin and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol Endocrinol. 1997;11:1266–1277. doi: 10.1210/mend.11.9.9975. [DOI] [PubMed] [Google Scholar]
- 46.Widdop RE, Matrougui K, Levy BI, Henrion D. AT2 receptor-mediated relaxation is preserved after long-term AT1 receptor blockade. Hypertension. 2002;40:516–520. doi: 10.1161/01.hyp.0000033224.99806.8a. [DOI] [PubMed] [Google Scholar]
- 47.Teschner M, Paczek L, Schaefer RM, Heidland A. Obese Zucker rat: potential role of intraglomerular proteolytic enzymes in the development of glomerulosclerosis. Res Exp Med (Berl) 1991;191:129–135. doi: 10.1007/BF02576668. [DOI] [PubMed] [Google Scholar]
- 48.Rehman A, Leibowitz A, Yamamoto N, Rautureau Y, Paradis P, Schiffrin EL. Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension. 2012;59:291–299. doi: 10.1161/HYPERTENSIONAHA.111.180158. [DOI] [PubMed] [Google Scholar]
- 49.Matavelli LC, Siragy HM. AT2 receptor activities and pathophysiological implications. J Cardiovasc Pharmacol. 2015;65:226–232. doi: 10.1097/FJC.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matavelli LC, Zatz R, Siragy HM. A nonpeptide angiotensin II type 2 receptor agonist prevents renal inflammation in early diabetes. J Cardiovasc Pharm. 2015;65:371–376. doi: 10.1097/FJC.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Patel S, Ali Q, Hussain T. Angiotensin II type 2-receptor agonist C21 reduces proteinuria and oxidative stress in kidney of high-salt-fed obese Zucker rats. Hypertension. 2016;67:906–915. doi: 10.1161/HYPERTENSIONAHA.115.06881. This study confirms the beneficial actions of AT2R activation on renal protection in salt-loaded obese Zucker rats. These animals develop rapid severe glomerulosclerosis with proteinuria and oxidative stress, all of which were improved by C-21 treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]