Seasonal influenza and RSV were associated with 23.0 and 13.2 allcause deaths/100 000 population annually. The peak mortality rate was in the elderly for influenza and in infants for RSV. And 63% of seasonal influenza and 48% of RSV-associated deaths occurred out-of-hospital.
Keywords: Influenza, respiratory syncytial virus, mortality, children, South Africa
Abstract
Background
Estimates of influenza- and respiratory syncytial virus (RSV)-associated mortality burden are important to guide policy for control. Data are limited on the contribution of out-of-hospital deaths to this mortality.
Methods
We modeled excess mortality attributable to influenza and RSV infection by applying regression models to weekly deaths from national vital statistics from 2009 through 2013, using influenza and RSV laboratory surveillance data as covariates. We fitted separate models for in- and out-of-hospital deaths.
Results
There were 509791 average annual deaths in South Africa, of which 44% (95% confidence interval [CI] 43%–45%) occurred out-of-hospital. Seasonal influenza and RSV all-cause mortality rates were 23.0 (95% CI 11.0–30.6) and 13.2 (95% CI 6.4–33.8) per 100000 population annually (2.3% [95%CI 2.3%–2.4%] and 1.3% [95% CI 1.2%–1.4%] of all deaths respectively). The peak mortality rate was in individuals aged ≥75 years (386.0; 95% CI 176.5–466.3) for influenza and in infants (143.4; 95% CI 0–194.8) for RSV. Overall, 63% (95% CI 62%–-65%) of seasonal influenza and 48% (95% CI 47%–49%) of RSV-associated deaths occurred out-of-hospital. Among children aged <5 years, RSV-associated deaths were more likely to occur in-hospital, whereas influenza-associated deaths were more likely to occur out-of-hospital. The mortality rate was 6.7 (95% CI 6.4–33.8) in the first influenza A(H1N1)pdm09 wave in 2009 and 20.9 (95% CI 6.4–33.8) in the second wave in 2011, with 30% (95% CI 29%–32%) of A(H1N1)pdm09-associated deaths in 2009 occurring out-of-hospital.
Discussion
More than 45% of seasonal influenza- and RSV-associated deaths occur out-of-hospital in South Africa. These data suggest that hospital-based studies may substantially underestimate mortality burden.
It is important to quantify the burden of influenza- and respiratory syncytial virus (RSV)-associated mortality in order to guide prioritization of interventions. Data on influenza- and RSV-associated mortality from low- and middle-income countries and especially in Africa are limited [1]. Human immunodeficiency virus (HIV) is an important risk factor for influenza- and RSV-associated mortality in South Africa, with a national HIV prevalence of 12.2% in 2012 [2–4]. South Africa has published estimates of influenza- and RSV-associated mortality, but these do not include the years following the global emergence of influenza A(H1N1)pdm09 in 2009 [5, 6]. Factors that could have impacted influenza- and RSV-associated mortality in South Africa since 2009 include recent improvements in the prevention and treatment of HIV and introduction of the pneumococcal conjugate vaccine into routine immunisation programme in 2009 [7–9]. In addition, influenza vaccine became available through publically funded programmes in 2010, with approximately one million doses distributed annually.
Approximately 44% of deaths in South Africa occur outside of the hospital [10]. An understanding of the proportion of influenza- and RSV-associated deaths occurring outside of the hospital is important for the interpretation of global and regional estimates of influenza and RSV-associated mortality [11]. Out-of-hospital deaths will be missed in traditional hospital-based influenza surveillance programs, potentially leading to underestimation of influenza-associated mortality burden [4]. Quantification of the proportion out-of-hospital deaths can provide multipliers to account for this.
Influenza A(H1N1)pdm09-associated mortality in 2009 followed the age shift described in previous pandemics predominantly affecting individuals aged <65 years [11]. In some settings the second wave of influenza A(H1N1)pdm09 has been suggested to have been associated with a greater mortality burden than the first wave [12]. It is unclear how large the burden of the second wave of influenza A(H1N1)pdm09 was in Africa and whether the classic “pandemic age shift” was observed in subsequent waves.
We aimed to estimate the rate of influenza- and RSV-associated excess deaths in- and out-of-hospital in South Africa from 2009 to 2013 by age group. In addition, we aimed to estimate age-specific excess mortality associated with influenza A(H1N1)pdm09 and describe the burden and age distribution of deaths between successive pandemic waves.
METHODS
Sources of Data
We obtained data on underlying causes of death and population denominators from Statistics South Africa from 2009 to 2013 [10, 13]. We used the International Classification of Diseases, Tenth Revision (ICD-10) codes to compile age-specific (<1, 1-4,5-19, 20–44, 45–64, 65–74, and ≥75 years) weekly mortality time series for all-cause (ICD-10: any), all-respiratory (ICD-10: J00-J99), all-circulatory (ICD-10: I00-I99) as well as pneumonia and influenza (P&I) (ICD-10: J10-J18; a subset of all-respiratory) deaths. Place of death is reported in the vital statistics data set [10]. We obtained influenza virus types and subtypes and RSV data from severe acute respiratory illness surveillance at 5 sites [3].
Estimation of Influenza- and Respiratory Syncytial Virus-associated Mortality
To estimate influenza virus type/subtype- and RSV-associated mortality, we fitted age-specific regression models to the rate of weekly deaths, using a semiparametric generalized additive model. We included independent variables for each influenza virus type/subtype and RSV for each year. A natural cubic smoothing spline of weeks as the non-parametric variable was included. The spline accounts and adjusts for time and trend in non-influenza associated mortality in the model. The number of nodes was selected using the Akaike Information Criteria. This model was found to provide an improved fit for influenza-associated mortality compared with more conventional modelling approaches [14].
The model is provided below:
| (1) |
where E(mortality rate) is the expected respiratory mortality rate, t was the sequential week number of the weekly time series, Influenza A(H1N1)pdm09 is the proxy for influenza A(H1N1)pdm09, Influenza A(H3N2) is the proxy for influenza A(H3N2), Influenza B is the proxy for influenza B, RSV is the proxy for RSV, spline is the spline curve for t and is specified with 31 degrees of freedom, which achieved a degree of control for autocorrelation (<0.2). One degree of freedom is allocated to the parametric linear time variable (β1t) and the remaining degrees of freedom are distributed at 6 per year for the spline [14]. The model correlates changes in influenza and RSV detection to changes in number of deaths over time and attributes influenza- and RSV-associated deaths over total death above an estimated mortality baseline.
Separate models were fitted for each age group and cause of death. We estimated the age-specific excess mortality associated with influenza virus types/subtypes and RSV each week by subtracting an expected baseline from the weekly mortality predictions of the model. The baseline was obtained by setting the relevant viral covariates to 0 (i.e., to obtain a baseline for influenza, we set the seasonal influenza proxy to 0 for each type/subtype). Annual excess mortality was estimated as the sum of the weekly excess mortality for each year. Negative estimates were not set to zero. For the estimation of seasonal influenza-associated mortality we included influenza A(H1N1)pdm09-associated deaths after 2009 as seasonal influenza-associated deaths. As a validation, we estimated the excess mortality associated with cancer deaths (ICD-10 C00.0-C97), which are not expected to vary with influenza and RSV circulation [15].
For the estimation of in- and out-of-hospital mortality we fitted separate age- and cause of death-specific models for in- and out-of-hospital deaths as recorded in the vital statistic data. We restricted the analysis of in- and out-of-hospital deaths to the broad groups of all-cause, all-circulatory and all-respiratory because we felt that a specific ICD code of pneumonia and influenza was unreliable for out-of-hospital deaths [10]. Data on the location of death were missing for 20% of individuals overall, 14% of respiratory deaths and 14% of circulatory deaths. We adjusted the final excess mortality estimates to account for missing data assuming that the percent of deaths in- and out-of-hospital was similar in those with missing data to those with available data.
We obtained the 95% confidence interval (CI) for the estimated excess mortality using bootstrap resampling on blocks of calendar years over 1000 replications [5, 6]. For each resampled dataset we refitted the regression model and the 95% CI were obtained from the 2.5th and 97.5th percentiles of the estimated influenza- and RSV-associated mortality from the 1000 resampled data sets. The statistical analysis was implemented using STATA version 14 (StataCorp, College Station, Texas).
Ethics
Because this analysis used only publicly available mortality data and de-identified and aggregated laboratory data, the study was considered to be exempt from human subjects’ review.
RESULTS
Deaths and Mortality Rates
The population of South Africa increased from 50020918 in 2009 to 53192216 in 2013, with approximately 10% of individuals aged <5 years and 5% aged ≥65 years. From 2009 through 2013, a mean of 509791 deaths occurred annually, of which 109032 (21%) were attributable to respiratory and 123453 (24%) to circulatory causes (Supplementary Table 1). The mean annual mortality rate per 100000 for all-cause death was highest in individuals aged ≥75 years, 65–74 years, and <1 year. All-cause deaths rates per 100000 population declined by 25% from 1141 in 2009 to 858 in 2013. The largest reductions were in individuals aged 20–44 years (34%) and <1 year (32%) and the smallest in individuals aged ≥65 years (4%). Deaths peaked seasonally each winter (Supplementary Figure 1).
Influenza and Respiratory Syncytial Virus Surveillance
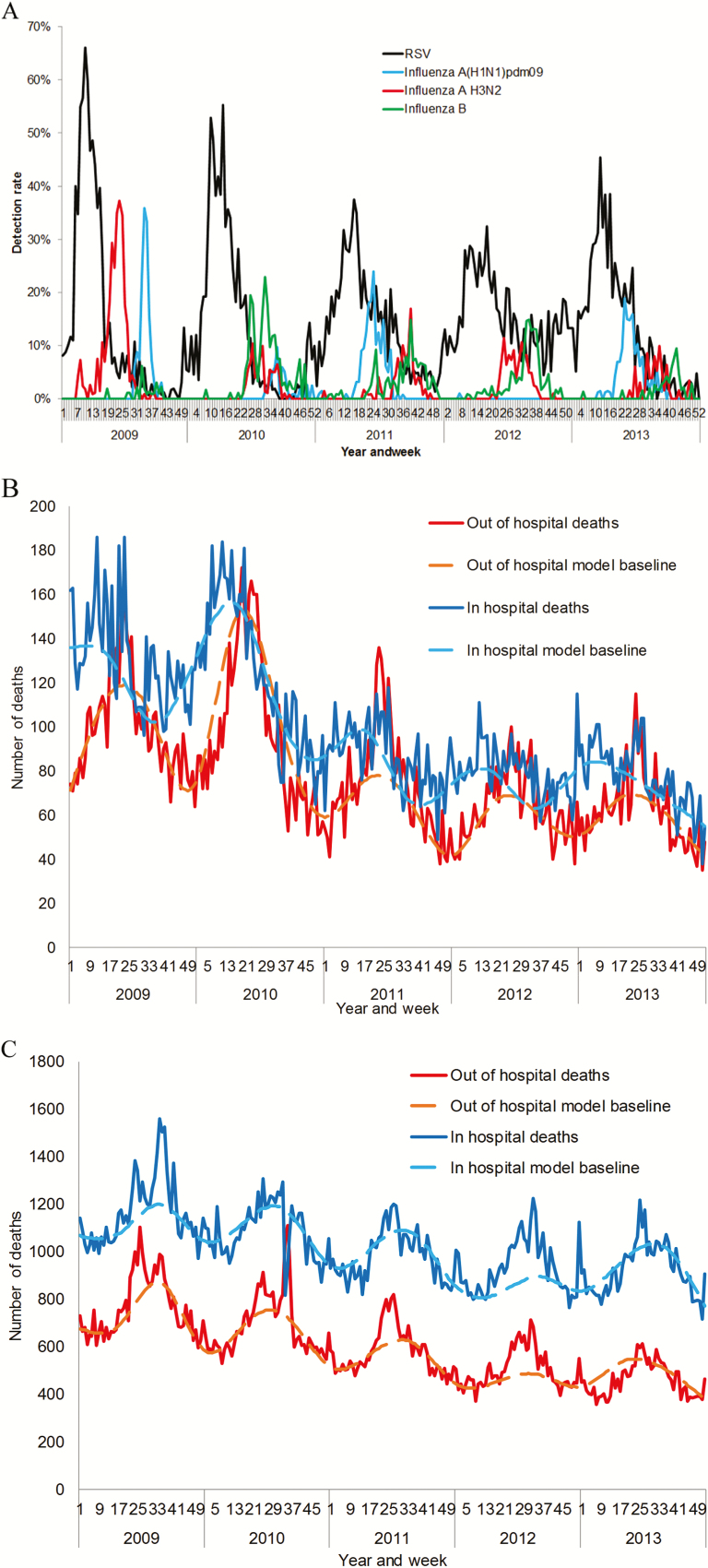
A mean of 4378 (range 3002–5204) specimens were tested for influenza and RSV annually with an annual average of 8% (95% confidence interval (CI) 7%-8%) and 15% (95% CI 14%–16%) testing positive for influenza and RSV respectively. The influenza season peaked between June and August (winter) (Figure 1A). In 2009, influenza A(H3N2) peaked in June followed by influenza A(H1N1)pdm09 which peaked in August. RSV peak activity was observed between March and May (autumn).
Figure 1.
A, Detection rate (i.e., weekly number of positive specimens divided by annual number of specimens tested) of influenza (by type/subtype) and respiratory syncytial virus (all ages), Severe Acute Respiratory Illness Surveillance (SARI) programme, South Africa, 2009–2013. B, Number of deaths in- and out-of-hospital by year and week, children aged <5 years, South Africa, 2009–2013. C, Number of deaths in- and out-of-hospital by year and week, individuals aged ≥5 years, South Africa, 2009–2013.
Seasonal Influenza and Respiratory Syncytial Virus-associated Deaths
For seasonal influenza-associated all-cause deaths, the highest mortality rate per 100000 population was in individuals aged ≥75 years (386.0; 95% CI: 176.5–466.3), followed by infants (87.3; 95% CI: 5.2–118.5) (Table 1, Supplementary Table 2, Supplementary Figure 2). For RSV-associated deaths, the peak mortality rate was in infants (143.4; 95% CI: 0–194.8), followed by individuals aged ≥75 years (81.7; 95% CI: 68.8–337.9). Influenza-associated mortality was highest for influenza A(H3N2), followed by influenza A(H1N1)pdm09 and influenza B (Supplementary Table 3).
Table 1.
Estimated Mean Annual Rate of RSV- and Seasonal Influenza-associated Excess Deaths by Cause of Death and Age Group in South Africa, 2009–2013
| Cause of death | ||||
|---|---|---|---|---|
| Age group, years | All-causes, Ratea (95% CI)b | All-circulatory, Ratea (95% CI)b | All-respiratory, Ratea (95% CI)b | Pneumonia and influenza, Ratea (95% CI)b |
| RSV | ||||
| <1 | 143.4 (0.0–195.4) | 1.5 (0.0–6.8) | 42.0 (0.0–63.9) | 30.4 (0.0–42.1) |
| 1–4 | 7 (3.9–17.0) | 0.3 (0.0–1.3) | 5 (2.5–7.9) | 3.3 (2.2–5.8) |
| 5–19 | 3.7 (2.5–8.4) | 0.6 (0.2–0.9) | 0.6 (0.3–1.4) | 0.6 (0.1–1.0) |
| 20–44 | 9.9 (5.5–26.0) | 0.8 (0.6–2.7) | 1.2 (0.4–4.6) | 0.9 (0.2–4.1) |
| 45–64 | 15.7 (10.4–53.6) | 4.5 (3.7–16.4) | 4.6 (2.7–14.3) | 2.4 (1.1–6.8) |
| 65–74 | 22 (14.5–67.6) | 8.4 (5.5–30.7) | 4.2 (0.0–34.3) | 5.1 (1.1–17.7) |
| ≥75 | 81.7 (72.7–339.5) | 41.3 (36.0–189.1) | 16 (13.0–88.1) | 15.7 (6.4–67.4) |
| <5 | 35.2 (3.1–53.8) | 0.6 (0.0–2.5) | 12.7 (2.0–19.4) | 8.9 (1.8–13.3) |
| ≥5 | 10.7 (6.8–31.5) | 2.4 (1.8–8.5) | 2.0 (0.9–7.7) | 1.5 (0.4–5.1) |
| All ages | 13.2 (6.4–33.8) | 2.2 (1.6–7.9) | 3.1 (1.0–8.9) | 2.2 (0.6–5.9) |
| Influenza | ||||
| <1 | 87.3 (58.2–118.6) | 2.2 (1.2–4.5) | 33.6 (25.7–45.1) | 25.4 (18.3–34.6) |
| 1–4 | 6.8 (2.8–8.9) | 0.8 (0.3–1.5) | 3.4 (2.1–4.9) | 2.8 (2.1–4.1) |
| 5–19 | 2.7 (1.2–3.7) | 0.2 (0.1–0.4) | 0.8 (0.5–1.2) | 0.6 (0.3–1.0) |
| 20–44 | 15.4 (7.4–22.6) | 1.3 (0.4–2.3) | 4.4 (2.1–7.6) | 2.9 (1.4–5.2) |
| 45–64 | 34.3 (14.8–46.3) | 10.7 (3.3–14.8) | 13.9 (6.9–18.7) | 6.6 (3.9–9.7) |
| 65–74 | 73.5 (34.5–98.1) | 34.2 (16.7–47.1) | 27.8 (19.0–38.0) | 11.7 (5.7–16.0) |
| ≥75 | 386 (193.4–473.1) | 183.9 (81.2–224.7) | 115.7 (36.1–140.9) | 79.5 (44.5–94.2) |
| <5 | 23.4 (14.2–31.6) | 1.1 (0.5–2.1) | 9.7 (7.0–13.2) | 7.5 (5.5–10.4) |
| ≥5 | 22.9 (10.7–30.5) | 6.9 (2.8–9.1) | 7.6 (3.6–10.7) | 4.4 (2.3–6.4) |
| All ages | 23.0 (11.0–30.6) | 6.3 (2.5–8.4) | 7.8 (3.9–10.9) | 4.7 (2.7–6.8) |
Abbreviations: CI, confidence interval; RSV, respiratory syncytial virus.
aDeath rates per 100000 person-years.
b95% confidence intervals estimated using bootstrap resampling on blocks of calendar years over 1000 replications.
Rates of influenza-associated circulatory deaths were highest in individuals aged ≥75 (183.9; 95% CI: 81.2–224.7) (Table 1). Influenza-associated respiratory death rates were highest in the ≥75 years age group (115.7; 95% CI: 36.1–140.9), followed by <1 year (33.6; 95% CI: 25.7–45.1), with a similar pattern for P&I deaths.
Similar to influenza, rates of RSV-associated circulatory deaths were highest in individuals aged ≥75 (41.3; 95 CI: 36.0–189.0). RSV-associated respiratory deaths were highest in infants (42.0; 95% CI: 0.0–63.9) and ≥75 years (16.0; 95% CI: 13.0–88.1) age groups, with a similar pattern for P&I deaths.
An estimated 3.7% and 1.4% of all annual respiratory deaths were associated with influenza and RSV respectively (4.6% and 6.0% in children aged <5 years for influenza and RSV respectively)(Supplementary Table 2). We did not estimate any significant excess cancer deaths associated with influenza or RSV (data not shown).
Deaths Associated With Influenza A(H1N1)pdm09
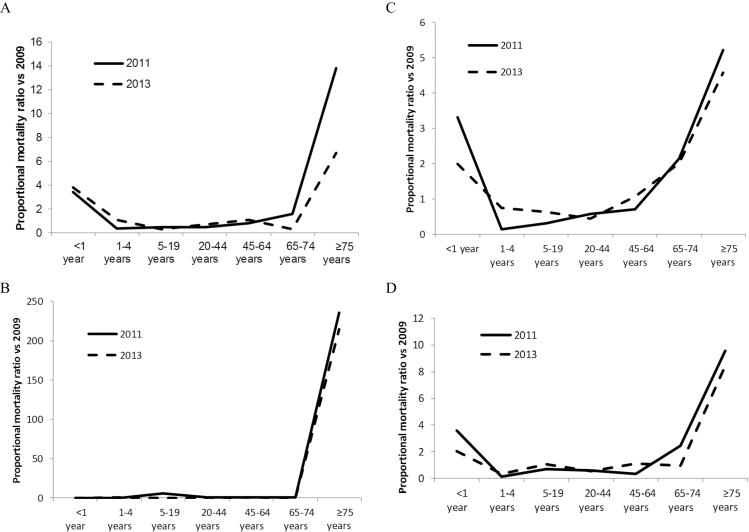
We estimated that there were 3460 (95% CI: 3010–6572) deaths associated with the first wave of influenza A(H1N1)pdm09 in 2009, 10753 (95% CI: 2922–14489) deaths in the second wave in 2011 and 5938 (95% CI: 2465–8573) in the 3rd wave in 2013 (Supplementary Table 4). The proportion of deaths in the extremes of age (<1 year and ≥75 years) increased from 5% in 2009 to 38% in 2011 and 25% in 2013, with concomitant reductions in the percent of deaths in older children and young adults (Table 2, Supplementary Table 4, Figure 2, Supplementary Figures 3–4). Circulatory deaths accounted for 15% (525/3460) of all deaths in 2009, 30% (3228/10753) in 2011 and 19% (1125/5938) in 2013.
Table 2.
Estimated Influenza A(H1N1)pdm09 Mortality Rates in South Africa in 2009 (1st wave), 2011 (2nd wave), 2013 (3rd wave) by Age Group
| 2009 | 2011 | 2013 | Proportional mortality ratio vs. 2009 (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Cause of death, age group, years | Ratea (95% CI)b | %b | Ratea (95% CI)c | %b | Ratea (95% CI) c | %b | 2011 | 2013 |
| All-causes | ||||||||
| <1 | 10.4 (0–13.0) | 3 | 109.2 (44.2–141.0) | 11 | 63.1 (52.6–95.0) | 12 | 3.4 (2.8–4.2) | 3.8 (3.1–4.7) |
| 1–4 | 2.0 (0.0–3.5) | 2 | 2.3 (0.0–5.5) | 1 | 3.6 (0.0–5.4) | 3 | 0.4 (0.3–0.5) | 1.1 (0.8–1.4) |
| 5–19 | 1.3 (1.2–1.9) | 6 | 1.8 (1.2–2.7) | 3 | 0.6 (0.-12) | 2 | 0.5 (0.4–0.6) | 0.3 (0.2–0.4) |
| 20–44 | 7.8 (7.6–13.3) | 46 | 11 (1.2–16.6) | 21 | 9.2 (3.3–13.2) | 33 | 0.5 (0.4–0.5) | 0.7 (0.7–0.8) |
| 45–64 | 14.8 (14.6–26.6) | 33 | 34.7 (1.4–46.5) | 25 | 25.2 (13.0–31.8) | 35 | 0.8 (0.7–0.8) | 1.1 (1.0–1.1) |
| 65–74 | 14.7 (12.2–38.3) | 8 | 72.4 (9.1-101-4) | 12 | 6.9 (0–19.0) | 2 | 1.6 (1.4–1.8) | 0.3 (0.2–0.4) |
| ≥75 | 9.0 (0–99.0) | 2 | 381.6 (228.0–453.8) | 27 | 94.6 (14.5–134.0) | 13 | 13.8 (10.8–17.8) | 6.7 (5.2–8.7) |
| <5 | 3.7 (3.1–53.8) | 6 | 24.5 (3.1–53.8) | 12 | 17.0 (3.1–53.8) | 15 | 2.1 (1.8–2.5) | 2.7 (2.3–3.1) |
| ≥5 | 7.1 (6.8–31.5) | 94 | 20.5 (6.8–31.5) | 88 | 11.0 (6.8–31.5) | 85 | 0.9 (0.8–0.97) | 0.9 (0.8–0.9) |
| All ages | 6.7 (6.4–33.8) | 100 | 20.9 (6.4–33.8) | 100 | 11.6 (6.4–33.8) | 100 | 1.0 | 1.0 |
| All-circulatory | ||||||||
| <1 | 1.5 (0.7–2.1) | 3 | 0.0 (0.0–0.8) | 0 | 0.0 (0.0–0.0) | 0 | 0.0 (0–0.04) | 0.0 (0.0–0.1) |
| 1–4 | 0.5 (0.3–0.5) | 4 | 0.0 (0.0–0.0) | 0 | 0.8 (0.0–1.0) | 3 | 0.0 (0.0–0.3) | 0.7 (0.4–1.4) |
| 5–19 | 0.0 (0.0–0.0) | 0 | 0.2 (0.1–0.7) | 1 | 0.0 (0.0–0.3) | 0 | 5.9 (1.0–237.6) | 0.0 (0.0–18.2) |
| 20–44 | 0.5 (0.2–1.0) | 19 | 1.2 (0.0–2.0) | 8 | 0.0 (0.0–0.2) | 0 | 0.4 (0.3–0.5) | 0.0 (0.0–0.1) |
| 45–64 | 3.4 (2.6–5.6) | 50 | 11.0 (0.9–15.7) | 26 | 7.9 (3.1–11.0) | 54 | 0.5 (0.5–0.6) | 1.1 (0.9–1.3) |
| 65–74 | 7.0 (5.0–19.4) | 23 | 36.3 (0.6–48.8) | 20 | 2.0 (0.0–8.9) | 3 | 0.8 (0.7–1.0) | 0.1 (0.1–0.2) |
| ≥75 | 0.1 (0.0–33.6) | 0 | 192.3 (114.0–230.0) | 45 | 59.4 (26.8–87.2) | 40 | 235.3 (42.1–9301.7) | 208.6 (37.2–8257.5) |
| <5 | 0.7 (0.4–0.9) | 7 | 0.0 (0.0–0.2) | 0 | 0.6 (0.0–0.8) | 3 | 0.0 (0.0–0.02) | 0.4 (0.2–0.7) |
| ≥5 | 1.1 (0.7–2.7) | 93 | 7.0 (2.1–9.4) | 100 | 2.4 (1.0–3.8) | 97 | 1.1 (0.9–1.2) | 1.0 (0.9–1.2) |
| All ages | 1.0 (0.7–2.5) | 100 | 6.3 (1.9–8.4) | 100 | 2.2 (0.9–3.5) | 100 | 1.0 | 1.0 |
| All-respiratory | ||||||||
| <1 | 8.9 (7.0–14.0) | 5 | 52.3 (43.5–66.2) | 17 | 24.8 (13.3–32.0) | 10 | 3.3 (2.7–4.2) | 2.0 (1.6–2.5) |
| 1–4 | 1.5 (1.2–2.3) | 3 | 0.4 (0.0–2.0) | 0 | 1.6 (0.0–1.7) | 2 | 0.1 (0.1–0.2) | 0.7 (0.5–1.1) |
| 5–19 | 0.6 (0.6–0.8) | 5 | 0.3 (0.0–0.6) | 2 | 0.6 (0.3–1.0) | 3 | 0.3 (0.2–0.4) | 0.6 (0.5–0.8) |
| 20–44 | 3.9 (3.5–4.6) | 43 | 4.0 (0.0–5.7) | 25 | 2.5 (0.0–3.5) | 19 | 0.6 (0.5–0.6) | 0.4 (0.4–0.5) |
| 45–64 | 7.8 (5.6–9.8) | 33 | 9.7 (0.0–12.7) | 23 | 11.7 (5.7–13.6) | 35 | 0.7 (0.6–0.8) | 1.1 (1.0–1.2) |
| 65–74 | 6.8 (6.0–13.6) | 6 | 25.8 (10.5–34.9) | 14 | 19.3 (9.3–22.6) | 13 | 2.1 (1.7–2.6) | 2.0 (1.6–2.5) |
| ≥75 | 9.0 (5.6–40.9) | 4 | 83.1 (0.0–107.5) | 19 | 57.9 (5.7–73.9) | 17 | 5.2 (4.1–6.8) | 4.6 (3.5–6.0) |
| <5 | 3.0 (2.4–4.7) | 9 | 11.1 (9.0–15.3) | 18 | 6.4 (2.7–7.9) | 13 | 2.1 (1.7–2.5) | 1.5 (1.2–1.8) |
| ≥5 | 3.7 (3.0–5.1) | 91 | 5.9 (0.4–8.0) | 82 | 4.9 (1.5–6.2) | 87 | 0.9 (0.8–0.96) | 1.0 (0.9–1.0) |
| All ages | 3.6 (3.0–5.1) | 100 | 6.4 (1.3–8.7) | 100 | 5.1 (1.7–6.4) | 100 | 1.0 | 1.0 |
| Pneumonia and influenza | ||||||||
| <1 | 6.5 (5.2–9.9) | 6 | 40.2 (28.2–51.5) | 22 | 18.4 (9.8–23.4) | 12 | 3.6 (2.8–4.7) | 2.0 (1.5–2.7) |
| 1–4 | 1.5 (1.2–2.1) | 6 | 0.4 (0.0–2.0) | 1 | 0.8 (0.0–1.0) | 2 | 0.2 (0.1–0.3) | 0.4 (0.2–0.6) |
| 5–19 | 0.4 (0.4–0.5) | 6 | 0.5 (0.1–0.7) | 4 | 0.6 (0.5–1.3) | 6 | 0.7 (0.5–1.0) | 1.0 (0.7–1.4) |
| 20–44 | 2.7 (2.5–3.2) | 49 | 2.7 (0.0–3.9) | 29 | 2.1 (0.0–2.8) | 27 | 0.6 (0.5–0.7) | 0.5 (0.5–0.6) |
| 45–64 | 3.8 (2.7–4.9) | 26 | 2.2 (0.0–4.2) | 9 | 5.8 (5.1–6.8) | 28 | 0.3 (0.3–0.4) | 1.1 (0.9–1.3) |
| 65–74 | 3.5 (1.5–6.1) | 5 | 15.0 (7.5–17.6) | 13 | 4.8 (0.0–7.4) | 5 | 2.5 (1.9–3.3) | 1.0 (0.7–1.4) |
| ≥75 | 3.5 (2.3–22.7) | 2 | 57.1 (8.5–71.6) | 22 | 41.1 (17.3–48.5) | 19 | 9.6 (6.4–14.8) | 8.4 (5.6–13.1) |
| <5 | 2.5 (2.0–3.7) | 12 | 8.6 (5.8–12.2) | 23 | 4.4 (2.0–5.6) | 14 | 2.0 (1.6–2.4) | 1.2 (1.0–1.5) |
| ≥5 | 2.2 (1.8–3.0) | 88 | 3.3 (0.5–4.5) | 77 | 3.0 (1.3–3.9) | 86 | 0.9 (0.8–0.9) | 1.0 (0.9–1.1) |
| All ages | 2.2 (1.8–3.1) | 100 | 3.8 (1.0–5.3) | 100 | 3.1 (1.4–4.1) | 100 | 1.0 | 1135 (921–1597) |
Abbreviation: CI, confidence interval.
aDeath rates per 100000 person-years.
bPercent of deaths in each age group.
c95% confidence intervals estimated using bootstrap resampling on blocks of calendar years over 1000 replications.
Figure 2.
Ratio of the proportion of influenza A(H1N1)pdm09-associated deaths in each age group in 2011 (2nd wave) and 2013 (3rd wave) vs 2009 (1st wave)*. A, All-cause; B, All-circulatory; C, All-respiratory; D, Pneumonia and influenza. Proportionate mortality ratio = (number of influenza A(H1N1)pdm09-associated deaths in age group i in 2011 or 2013/total influenza A(H1N1)pdm09-associated deaths in 2011 or 2013)/(number of influenza A(H1N1)pdm09-associated deaths in age group i in 2009/total influenza A(H1N1)pdm09-associated deaths in 2009).
Influenza- and Respiratory Syncytial Virus-associated in- and out-of-hospital Deaths
Overall, 44% (95% CI: 43%–45%) of all-cause deaths, 45% (95% CI: 44%–46%) of circulatory deaths and 38% (95% CI: 37–39) of respiratory deaths occurred out of the hospital (Table 3, Supplementary Table 5). Among children aged <5 years, in-hospital deaths peaked between weeks 1 and 21 (January to May), coinciding with the period of RSV circulation, whereas out-of-hospital deaths peaked from weeks 17 to 29 (May to July), coinciding with the peak in influenza virus circulation (Figure 1B). In individuals aged ≥5 years the timing of the peak of in- and out-of-hospital deaths was similar and coincided with the period of influenza virus circulation (Figure 1C).
Table 3.
Estimates of Influenza- and RSV-associated Mortality Occurring In- and Out-of-hospital in South Africa, 2009–2013
| Cause of death | |||||||
|---|---|---|---|---|---|---|---|
| Age group, years | % of deaths out-of-hospital | Ratio of % of deaths due to pathogen out-of-hospital vs. all deaths (95% CI)c | |||||
| All | RSV | Influenzaa | H1N1 pdm09b |
RSV | Influenza a | H1N1 pdm09 b |
|
| All-causes | |||||||
| All ages | 44 (43–45) | 48 (47–49) | 63 (62–64) | 30 (29–32) | 1.1 (1.1–1.2) | 1.4 (1.4–1.5) | 0.7 (0.7-0.7) |
| <5 | 41 (40–42) | 26 (24–28) | 57 (54–60) | 21 (16–28) | 0.6 (0.6–0.7) | 1.4 (1.3–1.5) | 0.5 (0.4–0.7) |
| ≥5 | 44 (43–45) | 56 (55–58) | 64 (63–65) | 31 (30–33) | 1.3 (1.2–1.3) | 1.4 (1.4–1.5) | 0.7 (0.7–0.8) |
| All-circulatory | |||||||
| All ages | 45 (44–46) | 57 (54–60) | 57 (55–59) | Not estimated | 1.3 (1.2–1.4) | 1.3 (1.2–1.3) | Not estimated |
| <5 | 31 (30–32) | 22 (8–40) | 33 (22–47) | Not estimated | 0.7 (0.2–1.5) | 1.1 (0.7–1.7) | Not estimated |
| ≥5 | 45 (44–46) | 59 (56–62) | 57 (56–59) | Not estimated | 1.3 (1.2–1.4) | 1.3 (1.2–1.3) | Not estimated |
| All-respiratory | |||||||
| All ages | 38 (37–39) | 37 (35–40) | 51 (50–53) | 24 (22–26) | 1.0 (0.9–1.1) | 1.3 (1.3–1.4) | 0.6 (0.6–0.7) |
| <5 | 44 (43–45) | 33 (29–36) | 50 (46–55) | 26 (19–33) | 0.7 (0.6–0.8) | 1.1 (0.9–1.3) | 0.6 (0.4–0.8) |
| ≥5 | 37 (36–39) | 40 (37–43) | 51 (50–53) | 24 (22–26) | 1.1 (0.9–1.2) | 1.4 (1.3–1.4) | 0.6 (0.6–0.7) |
Abbreviations: CI, confidence interval; RSV, respiratory syncytial virus.
aSeasonal influenza.
bin the 2009 pandemic.
cRatio of percent of out-of-hospital deaths associated with influenza to all deaths (example of all-cause deaths) = (number of influenza-associated all-cause deaths out-of-hospital/total influenza-associated all-cause deaths)/(number of all-cause deaths out-of-hospital/total number of all-cause deaths).
Overall, 48% (95% CI: 47%–49%) of RSV-associated and 63% (95% CI: 62%–64%) of influenza-associated all-cause deaths occurred outside of the hospital (Table 3). For RSV-associated deaths, proportionately fewer deaths occurred out of the hospital compared to the overall percent of out-of-hospital all-cause deaths among the <5 years age group; for example, 26% (95% CI: 24–28) of RSV-associated all-cause deaths occurred out-of-hospital vs. 41% (95% CI: 40%–42%) of all-cause deaths overall (risk ratio (RR) 0.6; 95% CI: 0.6–0.7). In contrast, in the ≥5 years age group more or a similar proportion of RSV-associated deaths occurred outside of the hospital compared to all deaths (for all-cause deaths 56% (95% CI: 55%–58%) of RSV-associated deaths out-of-hospital vs. 44% (95% CI: 43%–45%) of all deaths RR 1.3; 95% CI:1.2–1.3).
For seasonal influenza-associated deaths, the percent of out-of-hospital deaths was greater than or similar to the overall percent of out-of-hospital deaths for all-cause of death and age groups (Table 3). In contrast, for influenza A(H1N1)pdm09 in 2009, for all-cause of death groups, proportionately fewer deaths occurred out-of-hospital.
DISCUSSION
We have estimated that from 2009 through 2013 there were approximately 11800 annual seasonal influenza- and 6800 RSV-associated deaths in South Africa, with influenza accounting for approximately 4% of respiratory deaths in all ages, and RSV for 6% in children aged <5 years. The peak rates of influenza-associated mortality were in older adults, whereas peak rates of RSV-associated mortality were in infants. The mortality impact of the 2009 influenza A(H1N1)pdm09 pandemic was greater in the second than the first wave, associated with a shift in the age distribution of mortality towards the extremes of age. More than 45% of influenza- and RSV-associated deaths occurred outside of the hospital.
We provide updated estimates of influenza- and RSV-associated mortality for South Africa in the period during and following the 2009 influenza pandemic. Compared to previous estimates, these estimates were obtained using a different modeling approach incorporating weekly (as opposed to monthly) data and including separate estimation of influenza-associated mortality by year and influenza type/subtype; however, our estimates are very similar to those previously published. For example, in children aged <5 years, we estimated a mean annual seasonal influenza- and RSV-associated all-respiratory death rate (per 100000 population) of 10 (95% CI: 7–13) and 13 (95% CI: 2–19), respectively, compared to 8 (95% CI: 4–13) and 10 (95%: CI 5–15) estimated for the period 1998–2009. Similarly, in individuals aged ≥5 years we estimated an annual average seasonal influenza- and RSV-associated all-respiratory death rate (per 100000 population) of 8 (95% CI: 4–11) and 2 (95% CI: 1–8) compared to 8 (95% CI: 6–12) and 1 (95% CI: 0–1) estimated for the period 1998–2009. Differences in the modeling approach used have been found to be less important than seasonal variation in disease burden in other settings [16]. There has been an overall decreasing mortality in children and adults in South Africa since 2006 largely as a result of interventions for prevention and treatment of HIV, and this has likely been associated with decreases in influenza- and RSV-associated mortality [7, 17]. Our previous study included a long period from the early years of the HIV epidemic where mortality was lower than in recent years through the peak in HIV-associated mortality [5, 6]. The present analysis included 5 years in the period of decreasing mortality following widespread HIV interventions, but mortality has still not reduced to the levels seen in the early HIV epidemic. This likely contributed to the overall similar estimated excess mortality levels. An analysis of a longer time series including data from the early, peak, and post-peak periods of the HIV epidemic, and incorporating a consistent modeling approach to account for the estimated contribution of HIV to mortality would be useful to directly address the impact of the HIV epidemic on absolute and proportionate influenza- and RSV-associated mortality burden [6, 18].
The highest burden of influenza-associated mortality is in adults aged ≥65 years, similar to other countries [11, 15, 19, 20]. In addition, we found a substantial mortality burden in children aged <5 years, particularly those aged <1 year and in adults aged 20–64 years for all-cause and all-respiratory cause of death groups. HIV infection is an important driver of this mortality, particularly in young adults [3–6]. In young children, high baseline mortality rates likely contribute to the high mortality burden, fueled by the presence of underlying conditions such as malnutrition and challenges in accessing care [3, 4, 21]. The majority of influenza-associated cardiovascular deaths were in individuals aged ≥65 years, likely reflecting the prevalence of underlying cardiac illness in this older population [22].
The highest RSV-associated mortality burden was in children aged <5 years, particularly those aged <1 year. This is in keeping with other studies from South Africa and elsewhere [18, 19, 23]. An increase in RSV-associated mortality was also observed in the elderly but to a lower extent than for influenza [6]. Importantly, in South Africa, the influenza and RSV seasons occur at different times of the year, and so it is possible to separately estimate the relative contributions of influenza and RSV to mortality more robustly than in settings where influenza and RSV co-circulate. We were not powered to evaluate the impact of the separation in timing of influenza and RSV outbreaks on excess mortality estimates as we only had 5 years of available data.
We estimated 3460 (95% CI: 3010–6572) influenza-associated all-cause deaths in the first wave of the influenza A(H1N1)pdm09 pandemic and 10753 (2922–14489) and 5938 (2465–8573) in the 2nd and 3rd waves, respectively. Although mortality point estimates were higher for the 2nd and 3rd waves compared to the 1st wave, they were within the range of mortality seen for seasonal influenza. We observed a marked shift in the age distribution of influenza A(H1N1)pdm09-associated mortality from the first wave which predominantly affected young and middle-aged adults to the second and third waves where mortality was concentrated in the extremes of age. This shift in age distribution has been described in other settings and likely contributed to the higher mortality in the second and third waves [24–26]. Postulated reasons for the shift in age distribution include increasing immunity in younger individuals and gradual drift of the virus. The timing of the first wave at the end of the influenza season in 2009 may also have contributed to a shorter epidemic duration in this year. In order to fully estimate the impact of the first wave of the pandemic, years of life lost might be a more robust measure [11].
We estimate that in South Africa more than half of all influenza- and adult RSV-associated deaths occur outside of the hospital. Strikingly, amongst children aged <5 years there was a difference in the location of influenza- and RSV-associated deaths with RSV-associated deaths occurring predominantly in-hospital and influenza-associated deaths occurring predominantly outside of the hospital. A contributing factor could be the fact that RSV predominantly affects infants aged <6 months, whereas influenza is more common in slightly older children [27]. Very young children may be more likely to be brought to hospital and to be admitted to hospital. Among hospitalized children aged <5 years with respiratory illness in South Africa, approximately 26% test positive for RSV and 7% test positive for influenza [27]. In contrast, our estimates of respiratory mortality in children aged <5 years were relatively similar for RSV (665; 95% CI: 105–1021) and influenza (508; 95% CI: 368–693). Although a high in-hospital case-fatality ratio for influenza compared to RSV may partly explain this difference, the substantial burden of influenza-associated mortality outside of the hospital may be an important additional contributing factor [3, 28].
In a study from Canada, with 70% influenza vaccine coverage in the community dwelling elderly, 57% percent of influenza-associated deaths (mainly in the elderly) occurred out-of-hospital, with 73% of deaths in those aged >90 years occurring out of hospital [23]. A high proportion of individuals dying of influenza had underlying lung or heart disease and sudden death due to a cardiac event may be more likely to occur out of hospital. It has been suggested that the South African elderly may be less likely to seek healthcare than younger individuals, although there are no published studies of health-seeking behavior in this age group [4]. Patients may be less likely to be admitted during peak influenza season as facilities may be full.
In the first wave of the 2009 pandemic we observed that influenza A(H1N1)pdm09-associated deaths were more likely to occur in-hospital, in contrast to the observation that seasonal influenza-associated deaths were more likely out of the hospital. Heightened awareness during the pandemic could have led to patients seeking care more expeditiously, although there were no available data from South Africa on health seeking or hospital procedures during the pandemic [29]. The age-shift in mortality away from the extremes of age may also have contributed as young adults may be more likely to seek care than the elderly [3].
Our study has several limitations. Although we used established modeling approaches to estimate influenza- and RSV-associated mortality, the data are ecologic and individuals were not tested for influenza. Approximately 13% of deaths were nonspecifically coded, and this percentage remained constant over the study period. There are no available data on reliability of classification of cause of death for in- and out-of-hospital deaths, but misclassification may be more likely for out-of-hospital deaths because when deaths occur in remote areas a tribal leader may complete the death certificate [30]. For this reason, we restricted the analysis of in-and out-of-hospital deaths to large cause of death groups such as respiratory and circulatory deaths. All-cause deaths should not be subject to this bias, and trends in in- and out-of-hospital mortality were similar for all-cause deaths. Weekly data on cause of death were only available from 2009 onward limiting the number of years of data available for inclusion. Since 2000, completeness of death registration in South Africa is estimated to exceed 95%, but estimates of completeness are not available for in- and out-of-hospital deaths separately [31]. Confidence intervals were estimated using bootstrap analysis, and we obtained wide confidence intervals for some estimates particularly for age groups and endpoints with small numbers.
Influenza and RSV cause substantial mortality in South Africa, a large proportion of which occurs out of the hospital. The mortality burden of influenza A(H1N1)pdm09 was greater in the second and third waves, accompanied by a marked shift in age distribution of deaths. These findings will be helpful to inform our understanding of global and regional mortality burden and for pandemic planning.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Financial disclosure. Since publicly available data were used, no funding was received for this work.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Gessner BD, Shindo N, Briand S. Seasonal influenza epidemiology in sub-Saharan Africa: a systematic review. Lancet Infect Dis 2011; 11:223–35. [DOI] [PubMed] [Google Scholar]
- 2. Shisana O, Rehle T, Simbayi L et al. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town: HSRC Press; 2014. [Google Scholar]
- 3. Cohen C, Moyes J, Tempia S et al. Severe influenza-associated respiratory infection in high HIV prevalence setting, South Africa, 2009–2011. Emerg Infect Dis 2013; 19:1766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen C, Moyes J, Tempia S et al. Mortality amongst patients with influenza-associated severe acute respiratory illness, South Africa, 2009–2013. PLoS One 2015; 10:e0118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tempia S, Walaza S, Viboud C et al. Mortality associated with seasonal and pandemic influenza and respiratory syncytial virus among children <5 years of age in a high HIV prevalence setting–South Africa, 1998–2009. Clin Infect Dis 2014; 58:1241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tempia S, Walaza S, Viboud C et al. Deaths associated with respiratory syncytial and influenza viruses among persons ≥5 years of age in HIV-prevalent area, South Africa, 1998–2009(1). Emerg Infect Dis 2015; 21:600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madhi SA, Cohen C, von Gottberg A. Introduction of pneumococcal conjugate vaccine into the public immunization program in South Africa: translating research into policy. Vaccine 2012; 30 Suppl 3:C21–7. [DOI] [PubMed] [Google Scholar]
- 8. National Department of Health South Africa. The South African Antiretroviral Treatment Guidelines 2013. 2013. [Google Scholar]
- 9. Barron P, Pillay Y, Doherty T et al. Eliminating mother-to-child HIV transmission in South Africa. Bull World Health Organ 2013; 91:70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Statistics South Africa. Mortality and causes of death in South Africa, 2013: Findings from death notification P0309.3. Pretoria: Statistics South Africa, 2014 2014. [Google Scholar]
- 11. Simonsen L, Spreeuwenberg P, Lustig R et al. ; GLaMOR Collaborating Teams. Global mortality estimates for the 2009 Influenza Pandemic from the GLaMOR project: a modeling study. PLoS Med 2013; 10:e1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mytton OT, Rutter PD, Mak M, Stanton EA, Sachedina N, Donaldson LJ. Mortality due to pandemic (H1N1) 2009 influenza in England: a comparison of the first and second waves. Epidemiol Infect 2012; 140:1533–41. [DOI] [PubMed] [Google Scholar]
- 13. Statistics South Africa. Census 2011 Statistical release- P0301.4. Statistics South Africa; 2012. [Google Scholar]
- 14. Muscatello DJ, Newall AT, Dwyer DE, Macintyre CR. Mortality attributable to seasonal and pandemic influenza, Australia, 2003 to 2009, using a novel time series smoothing approach. PLoS One 2014; 8:e64734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen C, Simonsen L, Kang JW et al. Elevated influenza-related excess mortality in South African elderly individuals, 1998–2005. Clin Infect Dis 2010; 51:1362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schanzer DL, Sevenhuysen C, Winchester B, Mersereau T. Estimating influenza deaths in Canada, 1992–2009. PLoS One 2013; 8:e80481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pillay-van Wyk V, Msemburi W, Laubscher R et al. Mortality trends and differentials in South Africa from 1997 to 2012: second National Burden of Disease Study. Lancet Glob Health 2016; 4:e642–53. [DOI] [PubMed] [Google Scholar]
- 18. Tempia S, Walaza S, Viboud C et al. Mortality associated with seasonal and pandemic influenza and respiratory syncytial virus among children <5 years of age in a high HIV prevalence setting–South Africa, 1998–2009. Clin Infect Dis 2014; 58:1241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thompson WW, Shay DK, Weintraub E et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–86. [DOI] [PubMed] [Google Scholar]
- 20. Vestergaard LS, Nielsen J, Krause TG et al. Excess all-cause and influenza-attributable mortality in Europe, December 2016 to February 2017. Euro Surveill 2017; 22:30506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tempia S, Walaza S, Moyes J et al. Risk factors for influenza-associated severe acute respiratory illness hospitalization in a high HIV prevalence setting—South Africa, 2012–2015. Open Forum Infect Dis 2017. doi: 10.1093/ofid/ofw262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abadom TR, Smith AD, Tempia S, Madhi SA, Cohen C, Cohen AL. Risk factors associated with hospitalisation for influenza-associated severe acute respiratory illness in South Africa: A case-population study. Vaccine 2016; 34:5649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schanzer DL, Langley JM, Tam TW. Co-morbidities associated with influenza-attributed mortality, 1994–2000, Canada. Vaccine 2008; 26:4697–703. [DOI] [PubMed] [Google Scholar]
- 24. Kwok KO, Riley S, Perera RAPM et al. Relative incidence and individual-level severity of seasonal influenza A H3N2 compared with 2009 pandemic H1N1. BMC Infect Dis 2017; 17:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borja-Aburto VH, Chowell G, Viboud C et al. Epidemiological characterization of a fourth wave of pandemic A/H1N1 influenza in Mexico, winter 2011–2012: age shift and severity. Arch Med Res 2012; 43:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Green HK, Andrews N, Fleming D, Zambon M, Pebody R. Mortality attributable to influenza in England and Wales prior to, during and after the 2009 pandemic. PLoS One 2013; 8:e79360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cohen C, Walaza S, Moyes J et al. Epidemiology of viral-associated acute lower respiratory tract infection among children <5 years of age in a high HIV prevalence setting, South Africa, 2009–2012. Pediatr Infect Dis J 2015; 34:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moyes J, Cohen C, Pretorius M et al. Epidemiology of respiratory syncytial virus-associated acute lower respiratory tract infection hospitalizations among HIV-infected and HIV-uninfected South African children, 2010–2011. J Infect Dis 2013; 208Suppl 3:S217–26. [DOI] [PubMed] [Google Scholar]
- 29. Archer BN, Timothy GA, Cohen C et al. Introduction of 2009 pandemic influenza A virus subtype H1N1 into South Africa: clinical presentation, epidemiology, and transmissibility of the first 100 cases. J Infect Dis 2012; 206Suppl 1:S148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Statistics South Africa. Mortality and causes of death in South Africa, 2014: Findings from death notification. Statistics South Africa. 2015. [Google Scholar]
- 31. Wang H, Dwyer-Lindgren L, Lofgren KT et al. Age-specific and sex-specific mortality in 187 countries, 1970–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2071–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.