SUMMARY
Although autoimmune disorders are a significant source of morbidity and mortality in older individuals, the mechanisms governing age-associated increases in susceptibility remain incompletely understood. Central T cell tolerance is mediated through presentation of self-antigens by cells constituting the thymic microenvironment, including epithelial cells, dendritic cells, and B cells. Medullary thymic epithelial cells (mTECs) and B cells express distinct cohorts of self-antigens, including tissue-restricted self-antigens (TRAs), such that developing T cells are tolerized to antigens from peripheral tissues. We find that expression of the TRA transcriptional regulator Aire, as well as Aire-dependent genes, declines with age in thymic B cells in mice and humans and that cell-intrinsic and cell-extrinsic mechanisms contribute to the diminished capacity of peripheral B cells to express Aire within the thymus. Our findings indicate that aging may diminish the ability of thymic B cells to tolerize T cells, revealing a potential mechanistic link between aging and autoimmunity.
In Brief
Mechanisms governing age-associated increases in autoimmunity remain elusive. Expression of Aire and downstream self-antigens by thymic B cells helps tolerize developing T cells. Cepeda et al. report age-associated declines in expression of Aire and self-antigen genes in thymic B cells concomitant with increases in T-bet and IgG2a expression.
INTRODUCTION
Aging is associated with diminished immune responses to new infections and vaccines, as well as increased susceptibility to many autoimmune diseases (reviewed in Goronzy and Weyand, 2012, and Cooper and Stroehla, 2003). The mechanisms governing increased susceptibility to autoimmune disease are not fully understood, but age-associated thymic atrophy has been proposed to contribute to declines in central T cell tolerance induction (e.g., see Müller and Pawelec, 2015). In support of this notion, we have shown that in addition to loss of mass during aging, the thymus also loses primary functions, including the expression of tissue-restricted antigens (TRAs) (Griffith et al., 2012). TRA expression in the thymus allows the presentation of self-antigen that would normally be expressed in only one or a few tissues, such that T cells bearing potentially autoreactive T cell receptors may be negatively selected or diverted to the regulatory T cell (Treg) lineage (Derbinski et al., 2001; reviewed in Klein et al., 2014). The significance of Aire expression in the thymus is revealed in humans by autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED), in which mutation of AIRE (Autoimmune regulator), a transcriptional regulator required for expression of a large cohort of TRAs, results in spontaneous glandular autoimmunity (Anderson et al., 2002). Aire is estimated to regulate the expression of approximately 40% of all TRAs (St-Pierre et al., 2015), with the remaining 60% regulated by Aire-independent mechanisms.
In the course of our previous studies of thymic aging, we used an informatics-based approach to generate a non-presumptive list of TRA genes expressed in microdissected whole thymus medulla, which included both Aire-dependent and Aire-independent TRAs. Because some B cell-specific genes fit the criteria we used to define our TRA list, we also observed an increase in expression of B cell genes in the thymic medulla with age, though the biological significance of this increase was unclear at the time (Griffith et al., 2012). The presence of B cells in the young, steady-state thymus (Isaacson et al., 1987; Miyama-Inaba et al., 1988) and age-associated increases in thymic B cell frequency have been described in mice and humans for decades (Flores et al., 1999, 2001). Evidence supports contributions from both intrathymic development (Akashi et al., 2000; Perera et al., 2013) and recirculation (Yamano et al., 2015) to the thymic B cell population in the young thymus. Increased B cell frequency in the thymus is also a common feature of autoimmune disease in both mice and humans (Habu et al., 1971; Tamaoki et al., 1971).
Over the past several years, critical roles for thymic B cells in T cell tolerance induction have emerged. Thymic B cells have been shown to mediate negative selection of self-reactive T cells (Fujihara et al., 2014; Perera et al., 2013; Yamano et al., 2015), as well as diversion of developing T cells to the Treg lineage (Lu et al., 2015; Walters et al., 2014; Xing et al., 2015). B cells in the thymus tend to be self-reactive and can present cognate antigen, often self-antigen, to mediate negative selection of T cells bearing receptors that recognize those cognate antigens (Perera et al., 2013, 2016). A recent study demonstrated that B cells can also be “licensed” to express Aire and Aire-dependent genes in the young, steady-state thymus in mice (Yamano et al., 2015). Moreover, the cohort of Aire-dependent genes expressed in thymic B cells is distinct from the cohort of Aire-dependent genes expressed in mTECs (Yamano et al., 2015), such that B cell-specific Aire-dependent genes constitute a unique constellation of potential self-antigens to which T cells can be tolerized in the thymus. Given the recently established roles for B cells in thymus function, the present study was undertaken to characterize changes in thymic B cell phenotype and function during aging.
RESULTS
Age-Associated Changes in Thymic B Cell Frequency and Phenotype
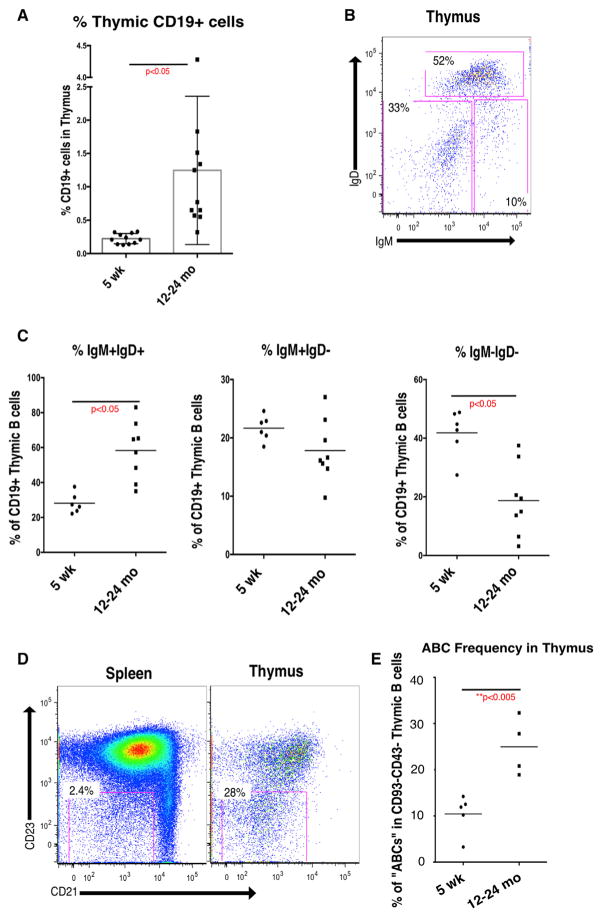
Our previous aging studies revealed increases in B cell-specific gene expression in microdissected whole medullary tissue from thymus and in the frequency of cells expressing B220 in aged mice by immunofluorescence (Griffith et al., 2012). Using flow cytometry, we found that CD19+ B cells represented about 0.2% of total thymic cellularity in young mice (Figure 1A), which is consistent with previous reports (Akashi et al., 2000; Perera et al., 2013; Yamano et al., 2015). B cell frequency in aged mice is variable but averages about 1.2% of total cellularity, an increase of more than 6-fold. Despite the substantial increase in frequency, the total number of B cells is unchanged, because of the precipitous decline in overall thymus size with age (Figure S1A). Increased frequency of B cells implies that the relative frequency of other key stromal cells, such as mTECs and dendritic cells, declines with age. Indeed, thymic B cells come to predominate individual medullary islets in some cases (Figures S1B–S1E).
Figure 1. Increased B Cell Frequency and Altered Phenotype in the Aged Mouse Thymus.
(A) Frequency (percentage of viable singlets) expressing surface CD19 cells in young (5 weeks) and aged (10–24 months) mouse thymus (n = 11).
(B) Representative surface IgD and IgM staining (gated on CD19+ viable singlets) on intrathymic B cells in aged (12 months) mouse thymus. Data are mean ± SEM.
(C) Frequency of IgM+IgD+, IgM+IgD−, and IgM−IgD− cells among CD19+ viable singlets in young (5 weeks, n = 6) and aged (10–24 months, n = 8) mouse thymus.
(D) Representative surface CD23 and CD21 staining (gated on CD19+CD93−CD43− viable singlets) in spleen and thymus from a 12-month-old mouse.
(E) Frequency of CD21−CD23− “ABCs” among CD19+CD93−CD43− viable singlets in thymus of 5-week-old (n = 5) and 12- to 24-month-old (n = 4) mice.
p values were calculated using unpaired two-tailed Student’s t test. See also Figure S1.
Because Aire expression has been characterized in young thymic B cells according to expression of IgM and IgD (Yamano et al., 2015), we evaluated the frequency of these B cell subsets in aged mice. Representative surface IgM and IgD staining (gated on CD19+ thymocytes) from a 12-month-old mouse are shown in Figure 1B. We found that the IgM+IgD+ subset, which was found to express the lowest levels of Aire in young mice, increased in frequency with age, while the frequency of the Aire-expressing IgM−IgD− subset declined with age (Figure 1C). As was reported for young mice (Yamano et al., 2015), we found that the IgM−IgD− subset was predominately IgG class switched in aged mice (data not shown).
To further characterize the phenotype of thymic B cells in young and aged mice, we evaluated the frequency of B cells with a phenotype described in a population of aging peripheral B cells, designated “age-associated B cells” (ABCs; Hao et al., 2011; Rubtsov et al., 2011; Rubtsova et al., 2015). Figure 1D shows representative surface expression of CD23 and CD21 (gated on CD19+CD43−CD93− cells) from spleen and thymus in aged mice. The frequency of ABCs increases in thymus with age (Figure 1E), consistent with changes found in peripheral B cells (Hao et al., 2011). The ABC subset has been characterized by expression of T-bet (Rubtsov et al., 2011), which also increases in thymic B cells during aging (see Figure 2C). Together, these data show that aging is associated with an increase in the frequency of thymic B cells, as well as significant changes in thymic B cell phenotype, including relative reduction of B cell subsets most likely to express Aire.
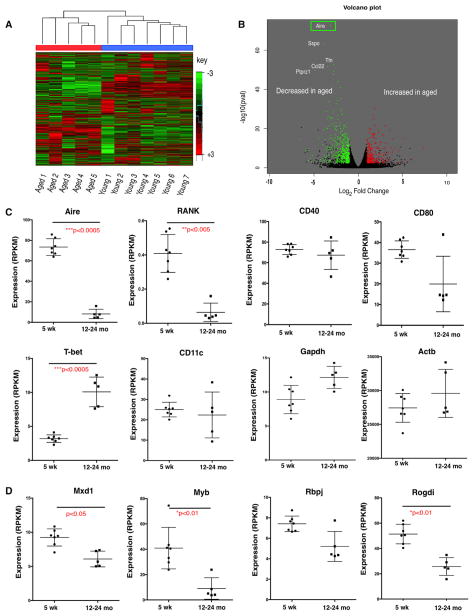
Figure 2. Distinct Gene Expression Signature and Loss of Aire Expression in Aged Intrathymic B Cells.
Gene expression in sorted (≥98.5% purity) CD19+ intrathymic B cells was analyzed using RNA-seq in young (5 weeks) and aged (12–24 months) thymus.
(A) Unsupervised hierarchical clustering of genes (log2 signal SD > 0.5) distinguishes B cells from young (5 weeks, n = 7) and aged (12–24 months, n = 5) thymus.
(B) Analysis of differentially expressed genes (false discovery rate [FDR] value < 0.05, fold change > 2). Colors indicate statistically significantly decreases (green) or increases (red) in gene expression with age. Top five most significantly changed genes are labeled. Aire was revealed as the gene with the most statistically significant change (decrease) in aged intrathymic B cells.
(C) RNA-seq RPKM expression values for Aire, RANK, CD40, CD80, T-bet, and CD11c in sorted (≥98.5% purity) intrathymic B cells from 5-week-old (n = 7) and 12- to 24-month-old (n = 5) mice. Bars indicate mean ± SEM.
(D) RNA-seq RPKM expression values for Mxd1, Myb, Rbpj, and Rogdi as described in (A). Bars indicate mean ± SEM.
p values were calculated using limma. See also Figure S2.
Broad Transcriptional Changes Associated with Age in Thymic B Cells Include Conspicuous Decrease in Expression of Aire
Given the decreased frequency of thymic B cell subsets shown to express the highest levels of Aire, we analyzed the transcriptome of purified thymic B cells from young and aged mice in order to assess expression of Aire and TRA genes. Thymic B cells were enriched using magnetic beads, followed by purification by sorting of CD19+ cells. Samples with at least 98.5% purity from young (4–6 weeks, n = 7) or aged (12–24 months, n = 5) mice were submitted for RNA-sequencing (RNA-seq) analysis. As shown in Figure 2A, aged and young samples are easily distinguished by unsupervised hierarchical clustering, indicating substantial age-associated changes.
As shown in the volcano plot in Figure 2B, differential gene expression analysis identified Aire as the gene changed with greatest statistical significance during aging. The DESeq package in R was used to identify changes in gene expression with a magnitude of at least 2-fold (decreases in green and increases in red) with a corrected p value of 0.05 or below (Anders and Huber, 2010) (Figure 2B). The top five most significant changes are labeled by gene symbol and include not only Aire as the gene with the most significant decrease but also decreased expression of Titin (Ttn), which is an important antigen in late-onset myasthenia gravis (Skeie et al., 1995) (previously identified as a TRA using an informatics-based approach; Griffith et al., 2012).
Age-Associated Changes in Gene Expression Suggest that B Cell-Intrinsic Mechanisms Contribute to Loss of Aire Expression
To learn more about mechanisms that may govern changes in TRA expression with age, we evaluated a manually curated list of genes related to Aire expression in mouse B cells (Figure 2C). Along with declining Aire expression, we found loss of Rank (Tnfrs11a) but not CD40 expression. Because CD40 but not RANK signaling is required for Aire expression in young thymic B cells (Yamano et al., 2015), it may be that thymic B cell licensing requirements change during aging, such that loss of RANK becomes critical with age. CD80 expression is increased when thymic B cells are signaled to express Aire in young mice (Yamano et al., 2015), and we found a downward trend in CD80 expression, but the change was not statistically significant. We also evaluated changes in expression of several genes recently shown to regulate Aire expression (Herzig et al., 2017) and found that expression of four of these positive Aire regulators tended to decline with age. Expression of Mxd1, Myb, and Rogdi declined significantly in aged thymic B cells (Figure 2D). We also evaluated changes in genes expressed by ABCs (Rubtsov et al., 2011) and found that expression of T-bet (Tbx21), but not CD11c (Itgax), was increased with age (Figure 2C), as expected on the basis of the increased frequency of B cells expressing the ABC phenotype in aged thymi (Figure 1). Expression of housekeeping genes Gapdh and Actb were unchanged in aged samples (Figure 2C). We confirmed RNA-seq data for five of these genes by qRT-PCR (see Figure S2). Together, these data indicate that B cell-intrinsic changes (such as diminished expression of RANK and other Aire regulators) may contribute to age-associated declines in function.
Expression of Aire-Dependent Genes Declines with Age
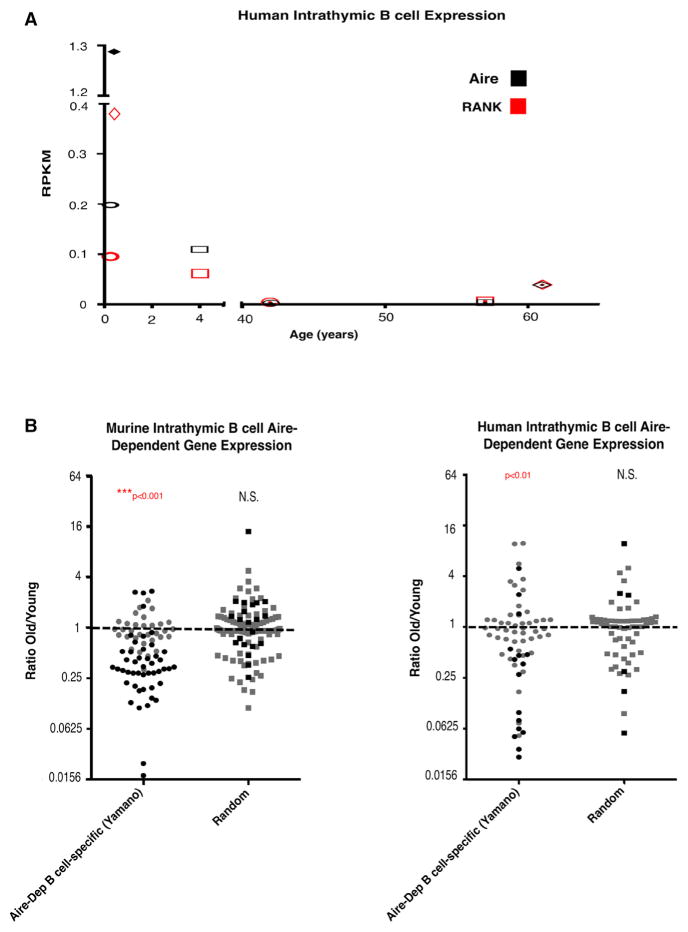
Given the reduction in Aire expression in aged thymic B cells, we sought to assess expression of Aire-dependent genes in mouse and human thymic B cells. Gies et al. (2017) recently reported expression of AIRE in human thymic B cells at both the transcript and protein levels, as well as expression of genes identified as B cell-specific Aire-dependent genes in mouse (Yamano et al., 2015). In order to assess gene expression in human thymic B cells from young (“young”: 3–5 months and 4 years) and older (“old”: 42, 57, and 61 years) patients, we purified thymic B cells by magnetic bead enrichment, followed by sorting of CD19+ cells. Samples of at least 98.5% purity from young and aged patients were submitted for RNA-seq analysis. RNA-seq analysis revealed very low AIRE expression, even in young patients, relative to the expression previously reported in human thymic B cells. This discrepancy may be because we sequenced purified total (all CD19+) B cells, whereas Gies et al. (2017) sequenced a CD19+CD27− subset of thymic B cells. Although expression levels are low, we find that AIRE expression declines significantly with age (p value for “young” versus “old” < 0.05) and correlated closely with RANK expression (Figure 3A). We evaluated changes in expression of a published Aire-dependent B cell-specific list of genes (“Aire-Dep B cell-specific [Yamano]”) (Yamano et al., 2015) in mice, and mapped the mouse list to human homologs to adapt the list for analysis of the human RNA-seq data. The Aire-dependent gene lists, mean RPKM signal values, and p values for comparison of young and old groups (see Experimental Procedures) are included in Table S1.
Figure 3. Aire and Aire-Dependent Gene Expression in Mouse and Human Intrathymic B Cells Diminishes with Age.
(A) RNA-seq RPKM values for Aire (black) and RANK (red) in sorted human intrathymic B cells (≥98.5% purity) from young (3 months, 5 months, and 4 years; n = 3) and aged (42–61 years, n = 3) patients. Individuals are represented by unique symbols.
(B) Relative expression of genes in the Aire-dependent B cell-specific gene list (Yamano) and a random list of similar size, in intrathymic B cells from young (5 weeks, n = 5) and aged (12–24 months, n = 7) mice (left) and young (3 months, 5 months, and 4 years; n = 3) and aged (42–61 years, n = 3) humans. Statistically significant changes (calculated using limma; see Experimental Procedures) are indicated in black, and changes not statistically significant are shown in gray.
p values for over-representation of up- or down-regulated genes were calculated by chi-square testing. See also Table S1.
Given the low expression levels expected for any particular TRA (Gotter et al., 2004), we set an arbitrary threshold at the top 75th percentile of RPKM signal value to define “present” genes (~0.14 and 0.016 RPKM in mouse and human datasets, respectively). This threshold captured expression of ~95% of the published B cell-specific Aire-dependent gene list (Yamano et al., 2015) in thymic B cells from young mice (see Table S1). We evaluated changes in gene expression with age in mice and humans, as shown in Figure 3B. Expression of Aire-dependent B cell-specific genes (first columns) declined significantly with age in both mouse and human thymic B cells. A random list of genes derived from the same dataset did not show significant increases or decreases in expression with age (“Random,” second columns). These results indicate that aging is associated with declining expression of Aire and Aire-dependent genes in mice and humans.
Aire-GFP Reporter Confirms that Aire Expression Declines in All IgMIgD Subsets during Aging
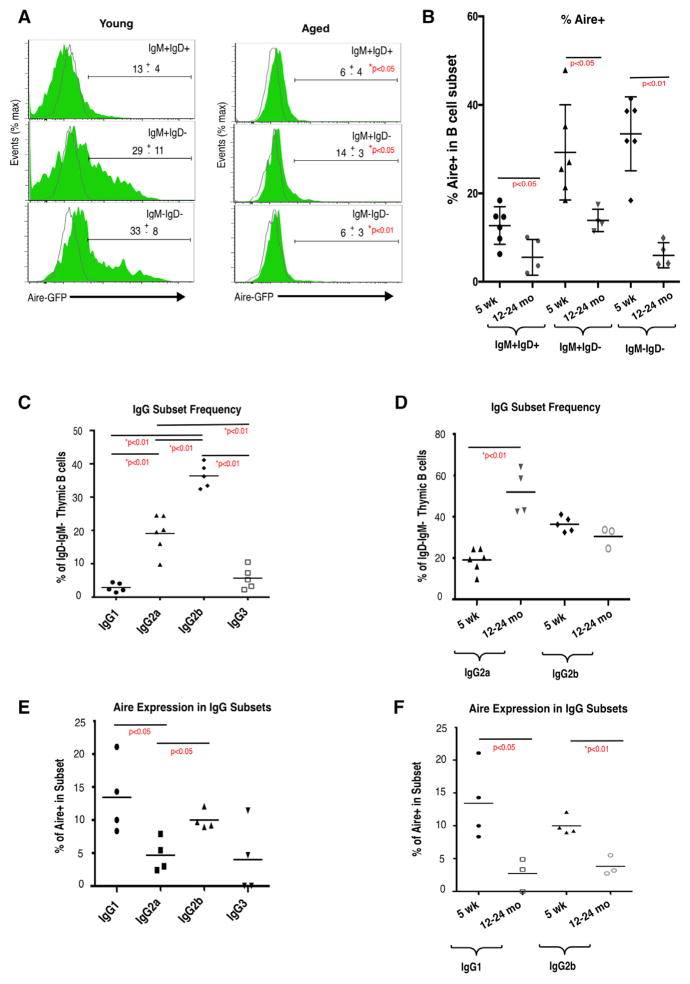
The decline in Aire expression identified in our RNA-seq analysis could be predicted on the basis of the decreased frequency of thymic B cell subsets known to express Aire (Figure 1C; Yamano et al., 2015). To evaluate changes in Aire expression within B cell subsets, and to confirm our RNA-seq results, we next measured Aire expression using Adig Aire-GFP reporter mice (Gardner et al., 2008). Thymic B cells from young and aged Adig mice were stained for surface CD19, IgM, and IgD expression, and Aire expression was evaluated by GFP expression. Figure 4A shows GFP expression within the three IgMIgD subsets described in Figure 1. We find significant declines in Aire-GFP expression in all three IgMIgD subsets in aged mice (12–24 months old) relative to young (5 weeks old) (Figures 4A and 4B). These results indicate that in addition to loss of the thymic B cell populations most likely to express Aire, aging is also associated with significant declines in Aire expression on a per cell basis. With increasing total B cell frequency, and decreasing frequency of Aire+ cells among B cells, it is possible that these changes would in effect compensate for each other, leaving the frequency of Aire+ B cells among total thymic cells essentially unchanged. To address this, we calculated the frequency of Aire+ B cells within total thymus viable singlet cells in young (5 weeks) and old (12 months) Adig mice and found that Aire+CD19+ cells decrease significantly in frequency from approximately 0.05% of total cells to approximately 0.02% (Figure S3). Thus the frequency of Aire-expressing B cells among developing T cells, which is likely important for cell-cell interactions required for negative selection, declines with age.
Figure 4. Aire Expression in Aged Intrathymic B Cells Is Diminished on a per Cell Basis and in All IgG Subclasses.
(A) Expression of Aire-GFP in IgM+IgD+, IgM+IgD−, and IgM−IgD− thymic B cells in Adig reporter mice (green) or reporter negative (gray) control mice. Representative gating strategy is shown on the left and Aire-driven GFP expression on the right.
(B) Frequency of Aire-GFP+ cells within each IgM/IgD subset of thymic B cells in 5-week-old (n = 6) and 12- to 24-month-old (n = 4) Adig mice. Bars indicate mean ± SEM. p values were calculated using unpaired two-tailed Student’s t test.
(C) Frequency of IgG1, IgG2a, IgG2b, and IgG3 class-switched cells within the CD19+IgM−IgD− subset of thymic B cells in 5-week-old (n = 5 or 6) mice.
(D) Frequency of IgG2a and IgG2b cells within the CD19+IgM−IgD− subset of thymic B cells in 5-week-old (n = 4–6) and 12- to 24-month-old (n = 3 or 4) mice.
(E) Frequency of Aire-GFP expression in each IgG subset in 5-week-old Adig mice (n = 4). Gated on viable singlet CD19+IgM−IgD− and respective IgG.
(F) Frequency of Aire-GFP+ cells in the IgG1 and IgG2b subsets in 5-week-old (n = 4) and 12- to 24-month-old (n = 3) Adig mice. p values were calculated using unpaired two-tailed Student’s t test. Gated on viable singlet CD19+IgM−IgD− and respective IgG.
See also Figure S3.
Aging Is Associated with an Increase in IgG2a+ Thymic B Cells and Diminished Aire Expression in All IgG Subsets
In addition to expression of Aire, B cells have also been shown to mediate negative selection of T cells bearing receptors that bind their cognate antigen (Perera et al., 2013). Perera et al. (2016) showed that B cells in the thymus tend to be autoreactive, and therefore presentation of cognate antigen likely often represents presentation of self-antigen. Given that individual IgG subclasses are associated with autoimmunity (e.g., IgG2a in Lupus; Ehlers et al., 2006) and with the ABC phenotype, we examined the frequency of IgG subsets in thymic B cells in young mice. As shown in Figure 4C, IgG2a and IgG2b are present at the highest frequency among young thymic B cells, which is consistent with results previously reported (Perera et al., 2016; Yamano et al., 2015). With age, we found an increase in IgG2a frequency and a decline in IgG2b frequency (Figure 4D). Increased IgG2a frequency is consistent with increased ABC frequency and T-bet expression with age, as T-bet expressing ABCs tend to express IgG2a (Gerth et al., 2003; Peng et al., 2002; Rubtsova et al., 2013). Given that thymic B cells “licensed” to express Aire undergo IgG class switching, we also measured Aire expression among IgG subsets using Adig mice. We found the highest levels of Aire expression in IgG1 and IgG2b subsets in young mice (Figure 4E) and that Aire expression declined with age in both subsets (Figure 4F).
B Cell-Intrinsic and B Cell-Extrinsic Changes Contribute to Diminished Capacity to Induce Aire in Aged B Cells In Vivo
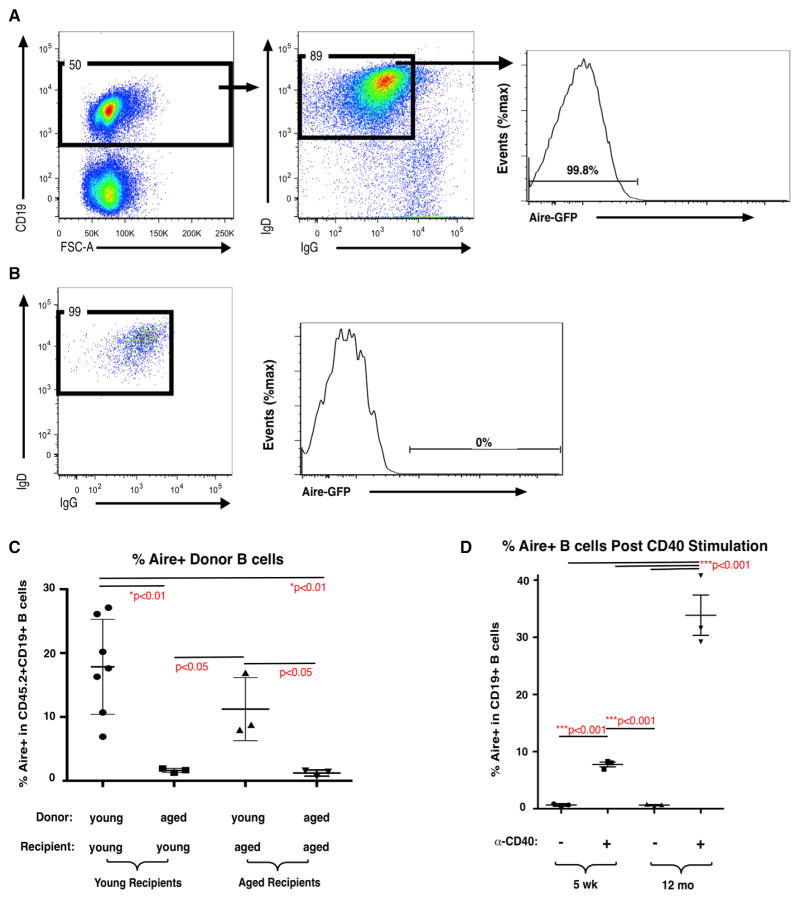
Given the evidence for B cell-intrinsic age-associated defects (Figures 2C and 2D), we sought to test the capacity of aged B cells to be licensed to express Aire. Yamano et al. (2015) showed that lymph node (LN) B cells could be induced to express Aire after intrathymic injection in young mice. To assess the relative contributions of B cell-intrinsic changes and changes in the thymic microenvironment on the capacity of peripheral B cells to induce Aire expression in the thymus, we sorted IgD+IgG−Aire-GFP− LN B cells from young (5–6 weeks) and aged (12 months) Adig mice for intrathymic injection into young and aged congenic recipients. Figure 5A shows representative sorting gates, and Figure 5B shows representative post-sort analysis. IgD+IgG−Aire-GFP− cells were transferred into young and aged CD45.1 congenic recipients by intrathymic injection. Seven days later, Aire-GFP expression was evaluated in donor-derived (CD45.2+) CD19+ B cells. Aire expression was induced in about 18% of B cells transferred from young donors to young recipients. In contrast, Aire expression was induced in approximately 3% of B cells from aged donors after intrathymic injection in young recipients (Figure 5C). These results indicate that B cell-intrinsic defects contribute to loss of Aire expression in aged mice. Although the sorted donor populations were restricted to IgD+IgG−Aire−CD19+ cells, we cannot rule out the possibility that other phenotypic differences in the young and aged donor populations were not detected and could have contributed to differences in Aire induction after intrathymic injection.
Figure 5. Age-Associated Decline in the Capacity of Peripheral B Cells to Be Licensed to Express Aire in Thymus Despite Increased CD40-Mediated Aire Induction.
(A) Representative sorting gates for donor LN B cells from Adig mice stained for surface CD19, IgD, and IgG.
(B) Representative post-sort re-analysis of purified CD19+IgD+IgG−Aire-GFP− LN B cells from Adig mice (CD45.2) before intrathymic injection into CD45.1 recipient mice.
(C) Frequency of Aire-GFP+ donor cells in donor B cells (gated on CD45.2+CD19+ viable singlets) 7 days after intrathymic injection of 5 × 105 CD19+IgD+IgG−Aire-GFP− LN B cells from young (5-week-old) or aged (12-month-old) Adig mice into young (5-week-old) or aged (12- to 17-month-old) CD45.1 recipient mice. Bars indicate mean ± SD. p values were calculated using unpaired two-tailed Student’s t test.
(D) Frequency of Aire-GFP+ cells in 5-week-old or 12-month-old Adig spleen B cells cultured for 3 days with or without agonistic anti-CD40 antibody (10 μg/mL) (n = 3). Bars indicate mean ± SEM. p values were calculated using unpaired two-tailed Student’s t test.
To determine whether age-associated microenvironmental changes in thymus also contribute to loss of Aire expression, we performed the reciprocal experiment and evaluated Aire-GFP induction in aged recipient mice receiving young or aged peripheral B cells by intrathymic injection. Aire-GFP was induced in approximately 10% of young donor B cells intrathymically injected into aged recipients. The diminished Aire induction in young donor B cells injected into aged thymi compared with induction in young donor B cells injected into young thymi, though not statistically significant, indicates that age-associated changes in the thymic microenvironment may also inhibit Aire induction. Together, these data support the notion that B cell-intrinsic changes, as well as changes in the thymic microenvironment, both contribute to declining Aire induction in thymic B cells.
CD40-Stimulated Aire Induction Is Increased in Aged B Cells In Vitro
Because in vitro CD40 stimulation is sufficient for Aire induction in peripheral B cells from young mice (Yamano et al., 2015), we hypothesized that diminished CD40 responsiveness could represent a B cell-intrinsic mechanism resulting in loss of Aire induction with age. To test this, Aire-GFP− peripheral B cells from young and aged Adig mice were sorted and stimulated with agonistic anti-CD40 antibody in vitro. Aire expression was induced in approximately 10% of B cells from young Adig mice, and at a significantly higher frequency, about 35%, in B cells from aged Adig mice (Figure 5D). Thus, we find that both CD40 expression and CD40-stimulated Aire expression are maintained in aged B cells. Further studies will be required to clarify whether the increased Aire induction in aged peripheral B cells after in vitro CD40 stimulation reflects increased Aire expression in identical B cell subpopulations or altered relative frequencies of Aire-expressing populations.
DISCUSSION
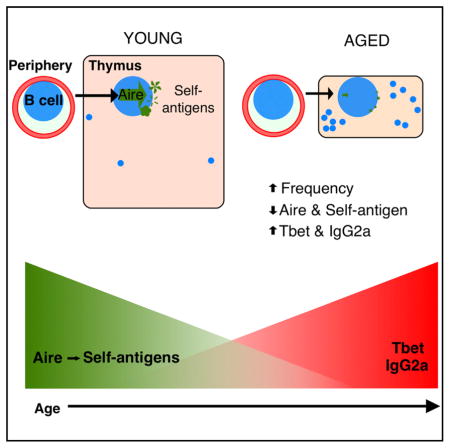
Autoimmune disease affects at least 3% of the population of the United States, and susceptibility generally increases with age (Cooper and Stroehla, 2003). However, the precise mechanisms by which aging is linked to autoimmune disease are not completely resolved. One hallmark of the aging immune system is the marked atrophy of the thymus, which begins relatively early in life, with concomitant reduction in production of new, naive T cells (Aspinall et al., 2010; Hartwig and Steinmann, 1994). In both humans and in mice, this results in diminished T cell receptor diversity with age, as diminished thymus output is compensated by homeostatic proliferation of memory T cells (Ernst et al., 1990; Hale et al., 2006; Haynes et al., 2000; Utsuyama et al., 1992). Resulting immunodeficiencies include diminished vaccine responsiveness and tumor surveillance and decreased response to new infections, especially viral infection. Paradoxically, aging is associated with both immunodeficiencies and increased autoimmunity. We have previously identified age-associated functional declines in the thymus that may impair central tolerance induction and promote autoimmune disease. In particular, decreased expression of TRAs in the medulla may contribute to a decline in the efficiency of negative selection of T cells bearing self-reactive TCRs and release of those potentially auto-reactive cells to the periphery (Griffith et al., 2012).
When we characterized the decrease in TRA expression that occurs in the thymic medulla during aging, we attributed the decline to changes in expression in medullary thymic epithelial cells (mTECs), because mTECs were the only cell type known to express TRA genes in the medulla at that time. However, given that thymic B cells are now known to express Aire and TRA genes (Gies et al., 2017; Yamano et al., 2015), we reasoned that changes in thymic B cells might also contribute to changes in TRA expression. Because mTECs and thymic B cells have been shown to express different cohorts of Aire-dependent genes (Yamano et al., 2015), even if thymic B cell function were unchanged with age, a relative expansion of thymic B cells into niche space previously occupied by mTECs and dendritic cells could alter the panel of TRAs presented to T cells. In fact, our data demonstrate that thymic B cells are altered with age, both phenotypically and functionally. Our findings reveal age-associated declines in expression of Aire and Aire-dependent genes (Table S1; Figure 3). Gies et al. (2017) previously reported AIRE expression in human B cells at the mRNA and protein level. Perhaps because our data were derived from bulk thymic B cells, we measured low levels of AIRE even in young patients. Nonetheless, confidence in the biological relevance of these measurements is increased by the correlation between expression of AIRE and RANK (Figures 2C and 3A) and the decrease in expression of Aire-dependent genes (Figure 3) in aged human samples.
We found evidence that the failure of aged B cells to induce Aire expression may be governed in part by B cell-intrinsic defects in mice, because aged LN B cells are diminished in their capacity to express Aire upon injection into young thymi (Figure 5C). A role for B cell-intrinsic changes is also supported by decreased expression of RANK, a positive regulator of Aire expression (Akiyama et al., 2008; Hikosaka et al., 2008; Rossi et al., 2007), in thymic B cells in aged mice. Yamano et al. (2015) demonstrated that, unlike mTECs, thymic B cells require CD40, rather than RANK, for Aire induction. Because expression of RANK, but not CD40, declines with age, it may be that aging alters signaling requirements for Aire induction. ABCs, which increase in frequency in the aged thymus, have impaired CD40 responsiveness (Hao et al., 2011). Thus, even without a decline in CD40 expression, we hypothesized that thymic B cell responsiveness to CD40 signaling, including Aire induction, may be impaired with age. This led us to investigate whether CD40 stimulation-induced Aire expression was diminished in aged B cells. In fact, aged peripheral B cells showed increased Aire induction upon CD40 stimulation (Figure 5), indicating that B cell-intrinsic capacity to induce Aire after CD40 stimulation remains intact with age. Thus, alterations independent of CD40 signaling contribute to B cell-intrinsic age-associated decreases in Aire induction. Expression of at least three positive regulators of Aire expression (Mxd1, Myb, and Rogdi; Herzig et al., 2017), in addition to RANK, declined in aged thymic B cells, which may indicate alternative mechanisms governing age-associated declines in Aire expression. Nonetheless, the extensive changes in the stromal microenvironment that occur during age-associated thymic atrophy are also likely to have an impact in addition to B cell-intrinsic defects, because young LN B cells injected into the thymi of aged mice are also somewhat diminished in their capacity to induce Aire (Figure 5C).
Although the changes we describe are indeed “age associated,” our studies do not attempt to distinguish between chronological age and repeated antigenic exposure. As a result, it is possible that differences we attribute to aging are caused by antigen exposure over time. Antigen exposure in our specific pathogen-free mouse colony will be low relative to that of pet store or feral mice as well as normal human populations (Beura et al., 2016). We also note that the human samples available to us were from neonatal (3–5 months) and pediatric (4 years) patients, whereas the “young” cohort of mice comprised young adults. We expect that the “aged” mouse samples (12–24 months) more closely reflect the age of our “aged” human samples (41–62 years). These caveats notwithstanding, our data reveal declines in critical functions within this population during aging under physiological conditions.
Several key questions arise from this study: What are the mechanisms governing the increased frequency of thymic B cells with age? Do increases in intrathymic differentiation and/or recirculation contribute? What are the effects of these changes on negative selection, Treg induction, and T cell tolerance? The perivascular space in the thymus was recently shown to be a reservoir for plasma cells in humans (Nuñez et al., 2016), raising the possibility that thymic B cells from different anatomical locations (i.e., perivascular versus central medulla versus cortico-medullary junction) may also serve unique functions, which may also vary depending on age and antigen exposure. Although decreases in Aire and TRA expression with age would predict diminished T cell tolerance induction, further work will be required to address whether these changes affect the repertoire of antigens to which developing T cells are exposed and whether downstream changes in negative selection and/or diversion to the Treg lineage occur with age.
EXPERIMENTAL PROCEDURES
Oversight
This study was approved by the Institutional Review Board at UT Health San Antonio and University Hospital. Written informed consent was obtained from the patients undergoing corrective cardiac surgery or from the patients’ parents. Animal studies and all procedures were approved by the Institutional Animal Care and Use Committee.
Mice
Young (5 weeks) C57BL/6J and B6.SJL-PtprcaPepcb/BoyJ (Jax strain 002014) male and female mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA), aged C57BL/6J (12–24 months) male and female mice were obtained from our colony and/or from the National Institute on Aging (NIA), and male and female Adig (Aire-Driven-Igrp-GFP) (5 weeks to 13 months) (Gardner et al., 2008) mice were provided by Dr. Mark Anderson (University of California, San Francisco, USA). Mice were bred and maintained at The University of Texas Health Science Center at San Antonio animal facility.
Cell Counting, Flow Cytometry, and Cell Sorting from Mouse Tissues
Upon euthanasia, thymi were removed and placed immediately in iced buffer (Hank’s balanced salt solution [HBSS] [GIBCO, Waltham, MA, USA], 5% fetal bovine serum [FBS], 5 μg/mL DNase). All subsequent steps were performed on ice. Thymi and spleens were gently but thoroughly pressed between two ice-cold frosted glass slides to generate a single-cell suspension. Cells were counted using a hemacytometer; viable cells were determined by exclusion of trypan blue (Sigma-Aldrich, St Louis, MO, USA). Red blood cells were lysed in spleen samples using ACK lysis buffer (Thermo Fisher Scientific, Waltham, MA, USA).
After counting, cells were stained with the following antibodies from BioLegend: CD19-A594 (6D5), IgD-APCCy7 (11-26c.2a), and IgM-A647 (RMM-1). For surface IgG phenotyping, the following antibodies were used: IgG1-Biotin (RMG1-1), IgG2a-Biotin (RMG2a-62), IgG2b-Biotin (RMG2b-1), and IgG3-Biotin (RMG3-1), followed by incubation with Streptavidin-PerCp-Cy5.5. For ABC phenotyping, cells were stained with the following antibodies from BioLegend (unless otherwise noted): CD11c-BV510 (N418), CD43-A647 (1B11), CD93-PE (AA4.1; Molecular Probes), CD21-PeCy7 (8D9; eBioscience), and CD23-PerCp-eFluor710 (B3B4; eBioscience). For all phenotyping experiments, dead cells were excluded using DAPI (Molecular Probes) in fluorescence-activated cell sorting (FACS) buffer (HBSS, 5% FBS, 0.5% DNase [1 mg/mL, pH 7.2]). Flow cytometry analysis was performed using a BD LSRII flow cytometer, and data were analyzed using BD FACSDiva and FlowJo flow cytometry analysis software (Tree Star).
Thymic single-cell suspensions from C57BL/6J mice (aged 5 weeks to 24 months) were stained with CD19-APC (MB19-1; eBioscience), followed by incubation with anti-APC magnetic MicroBeads (Miltenyi Biotec). CD19+ cells were subsequently magnetically enriched using a QuadroMACS separator with LS columns (Miltenyi Biotec). Enriched B cells were suspended in DAPI and sorted on CD19+ viable singlets using a BD FACSAria cell sorter (BD Biosciences) using the single-cell sorting mode. Post-sort analysis for sorted cell purity was accomplished using FACSDiva software. Sorted samples for RNA-seq analysis were selected to be at least 98.5% pure. RNA was isolated using the RNAqueous – Micro Kit (Invitrogen) as per manufacturer’s instructions.
Cell Sorting from Human Tissues
Human thymic tissue was obtained from pediatric (3 months, 5 months, and 4 years) and adult (42, 57, and 61 years) volunteers undergoing corrective cardiac surgery at University Hospital. Sections of human thymi were mechanically broken down into single-cell suspensions as described above. Cells (400 × 106) were stained with α-human CD19-APC (HIB19; BioLegend), followed by incubation with anti-APC magnetic MicroBeads. CD19+ cells were subsequently magnetically enriched using a QuadroMACS separator with LS columns. Human CD19+ cells were subsequently sorted as described for mouse cells above. Sorted samples for RNA-seq analysis were selected to be at least 98.5% pure. RNA was isolated using the RNAqueous – Micro Kit as per manufacturer’s instructions.
RNA Sequencing and Analysis
These steps were carried out by the Genome Sequencing Core Facility. RNA quality was analyzed using an Agilent Bioanalyzer (Santa Clara, CA, USA) and/or Qubit fluorometer (Life Technologies). Approximately 20–50 ng total RNA was used for RNA-seq library preparation following the KAPA Stranded RNA-Seq Kit with RiboErase (HMR) according to the manufacturer’s instructions (catalog no. KR1151). cDNA libraries were sequenced using 50 bp single-read sequencing runs on an Illumina HiSeq 3000 sequencer. Reads were aligned to mouse genome (University of California, Santa Cruz [UCSC], mm9 build) or human genome (UCSC hg19 build) using TopHat (Kim et al., 2013; Trapnell et al., 2009). The expression levels were extracted using HTSeq (Anders et al., 2015) with GENCODE annotation (GENCODE V24lift37; Harrow et al., 2012) and converted to reads per kilobase per million mapped reads (RPKM) units according to Mortazavi et al. (2008). Data were then normalized and tested for differential expression by DESeq in R (Anders and Huber, 2010). Data reported in this article were submitted to the NCBI’s GEO (GEO: GSE107112). Heatmap, hierarchical clustering (using Pearson correlation as distance function and average linkage as agglomeration method), and volcano plot were generated using R/Bioconductor (https://www.bioconductor.org). The mouse Aire-dependent B cell-specific list was mapped to human homologs using Homologene identifiers downloaded from MGI (http://www.informatics.jax.org). Genes from any list that did not map to gene symbols in our dataset were omitted from further analysis.
In Vitro Stimulation of B Cells
Splenic B cells from young (5 weeks) or aged (12 months) Adig mice were magnetically enriched using a mouse B cell Isolation Kit with a QuadroMACS separator and LS columns according to the manufacturer’s instructions. Enriched B cells were suspended in DAPI in FACS buffer and sorted on Aire-GFP− viable singlets. Subsequently, 2 × 105 sorted B cells were cultured for 3 days with or without agonistic anti-CD40 monoclonal antibody (FGK45; Bio X Cell) (10 μg/mL) and recombinant mouse IL-4 (PeproTech) (5 ng/mL) in flat-bottom 96-well plates. For qRT-PCR, RNA extraction, cDNA synthesis, and qPCR were performed on 2 × 105 cells using Taqman Gene Expression Probes (Thermo Fisher Scientific) for Aire and Hprt and gene expression values normalized as previously described.
For flow cytometry analysis, 6 × 105 to 1 × 106 stimulated B cells were stained with CD19-A594 (6D5; BioLegend) and suspended in DAPI in FACS buffer. Aire-GFP expression was analyzed on CD19+ viable singlets using a BD LSRII flow cytometer with BD FACSDiva and FlowJo® flow cytometry analysis software.
Validation of RNA-Seq by qRT-PCR
RNA from sorted murine thymic B cells with a purity of at least 98.5% was extracted using the method described above, and cDNA synthesis was performed using a Superscript VILO cDNA synthesis kit (Invitrogen) per manufacturer’s instructions. qPCR (cycle 1, 95°C for 10 min; cycle 2 [×40], 95°C for 15 s and 60°C for 1 min) was performed using a Bio-Rad CFX96 Real-Time System and C1000 Touch Thermal Cycler (Bio-Rad) using presynthesized Taqman Gene Expression Assay Probes to amplify the following gene sequences: Tbx21 (assay ID: Mm00450960_m1), CD80 (Mm00711660_m1), CD40 (Mm00441891_m1), Tnfrsf11a (Mm00437132_m1), Aire (Mm00477457_m1), and Hprt (Mm00446968_m1). Gene expression values were analyzed using Bio-Rad CFX Manager software and normalized to Hprt.
Intrathymic Injections
Single-cell suspensions were generated from LNs of young (6 weeks) and aged (12–13 months) Adig mice as described above. Cells were stained with the following antibodies from BioLegend: CD19-A594 (6D5), IgD-APCCy7 (11-26c.2a), IgG1-Biotin (RMG1-1), IgG2a-Biotin (RMG2a-62), IgG2b-Biotin (RMG2b-1), and IgG3-Biotin (RMG3-1), followed by incubation with Streptavidin-APC (BioLegend). CD19+IgD+IgG−Aire-GFP− cells were isolated at a purity of 98.5% or better for intrathymic injection. Young (4–6 weeks) or aged (13–17 months) B6.SJL-PtprcaPepcb/BoyJ CD45.1+ mice were placed under anesthesia using ketamine and xylazine, and a small incision was made in the skin of the upper thoracic region. A Hamilton Microlitersyringe needle was inserted in the second intercostal space at a 30°–40° angle, and 5 × 105 CD19+IgD+IgG−Aire− viable singlet lymphocytes (suspended in ~5 μL sterile PBS) were injected into one thymic lobe. After 7 days, thymocytes of recipient mice were stained with the following antibodies from BioLegend (unless otherwise specified): CD19-A594 (6D5), IgG1-Biotin (RMG1-1), IgG2a-Biotin (RMG2a-62), IgG2b-Biotin (RMG2b-1), IgG3-Biotin (RMG3-1), Streptavidin-A700 (Life Technologies), CD45.1-BV510 (A20), and CD45.2-APC (104; eBioscience), and Aire-GFP expression was analyzed in CD45.2+CD19+ donor cells.
Statistics
p values for differences in gene expression between young and aged samples in RNA-seq data were calculated using base 2 log scale RPKM values for the two groups with the R package limma, using the empirical Bayes method to calculate p values (Ritchie et al., 2015). p values for changes in the Aire-dependent gene lists during aging were calculated using the chi-square test. Scatterplots were generated in GraphPad Prism. p values for differences in surface phenotype (as in Figure 1) were calculated in GraphPad Prism using Student’s t test.
DATA AND SOFTWARE AVAILABILITY
The accession number for the RNA-seq data reported in this paper is GEO: GSE107112.
Supplementary Material
Highlights.
Expression of Aire and self-antigen genes decreases with age in thymic B cells
B cell-intrinsic and B cell-extrinsic mechanisms contribute to this decline in expression
The phenotype and transcriptome of thymic B cells change with age
T-bet expression and frequency of IgG2a expression increase in aged thymic B cells
Acknowledgments
We thank Drs. John Kearney, Howard Petrie, Lauren Ehrlich, Ellen Kraig, Michael Berton, Paolo Casali, and Zhenming Xu for technical advice and helpful discussions and Karla Gorena for cell sorting support. Data generated in the Flow Cytometry Shared Resource Facility were supported by the University of Texas Health Science Center at San Antonio (UTHSCSA), NIH National Cancer Institute (NCI) grant P30 CA054174-20 (Clinical and Translational Research Center [CTRC] at UTHSCSA), and UL1 TR001120 (Clinical and Translational Science Award). Special thanks to Drs. S. Adil Husain and Edward Sako for human thymus tissue. A.V.G. is supported by NIH grant R01AI121367, and S.C. is supported by NIH grant R01AI121367-S. RNA-seq analysis was performed by the Genome Sequencing Core Facility at UT Health San Antonio, which is supported by UT Health San Antonio, NIH/NCI Cancer Center Support Grant P30 CA054174, NIH Shared Instrument grant 1S10OD021805-01, and Cancer Prevention and Research Institute of Texas (CPRIT) Core Facility grant RP160732. We thank Yidong Chen, Zhao Lai, and Harry Chen for RNA-seq and informatics assistance.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, three figures, and one table and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.01.015.
AUTHOR CONTRIBUTIONS
S.C., M.S.A., and A.V.G. designed the study. S.C., C.C., S.O., and Y.X. designed and performed experiments and analyzed data. Z.B. and M.K.S. performed experiments. T.V. and A.V.G. analyzed bioinformatic data. S.C. and A.V.G. wrote the manuscript.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- Akashi K, Richie LI, Miyamoto T, Carr WH, Weissman IL. B lymphopoiesis in the thymus. J Immunol. 2000;164:5221–5226. doi: 10.4049/jimmunol.164.10.5221. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–437. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Aspinall R, Pitts D, Lapenna A, Mitchell W. Immunity in the elderly: the role of the thymus. J Comp Pathol. 2010;142(Suppl 1):S111–S115. doi: 10.1016/j.jcpa.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512–516. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2:119–125. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006;203:553–561. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst DN, Hobbs MV, Torbett BE, Glasebrook AL, Rehse MA, Bottomly K, Hayakawa K, Hardy RR, Weigle WO. Differences in the expression profiles of CD45RB, Pgp-1, and 3G11 membrane antigens and in the patterns of lymphokine secretion by splenic CD4+ T cells from young and aged mice. J Immunol. 1990;145:1295–1302. [PubMed] [Google Scholar]
- Flores KG, Li J, Sempowski GD, Haynes BF, Hale LP. Analysis of the human thymic perivascular space during aging. J Clin Invest. 1999;104:1031–1039. doi: 10.1172/JCI7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores KG, Li J, Hale LP. B cells in epithelial and perivascular compartments of human adult thymus. Hum Pathol. 2001;32:926–934. doi: 10.1053/hupa.2001.27106. [DOI] [PubMed] [Google Scholar]
- Fujihara C, Williams JA, Watanabe M, Jeon H, Sharrow SO, Hodes RJ. T cell-B cell thymic cross-talk: maintenance and function of thymic B cells requires cognate CD40-CD40 ligand interaction. J Immunol. 2014;193:5534–5544. doi: 10.4049/jimmunol.1401655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, Anderson MS. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerth AJ, Lin L, Peng SL. T-bet regulates T-independent IgG2a class switching. Int Immunol. 2003;15:937–944. doi: 10.1093/intimm/dxg093. [DOI] [PubMed] [Google Scholar]
- Gies V, Guffroy A, Danion F, Billaud P, Keime C, Fauny JD, Susini S, Soley A, Martin T, Pasquali JL, et al. B cells differentiate in human thymus and express AIRE. J Allergy Clin Immunol. 2017;139:1049–1052. e12. doi: 10.1016/j.jaci.2016.09.044. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol Life Sci. 2012;69:1615–1623. doi: 10.1007/s00018-012-0970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotter J, Brors B, Hergenhahn M, Kyewski B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J Exp Med. 2004;199:155–166. doi: 10.1084/jem.20031677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith AV, Fallahi M, Venables T, Petrie HT. Persistent degenerative changes in thymic organ function revealed by an inducible model of organ regrowth. Aging Cell. 2012;11:169–177. doi: 10.1111/j.1474-9726.2011.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habu S, Kameya T, Tamaoki N. Thymic lymphoid follicles in autoimmune diseases. I Quantitative studies with special reference to myasthenia gravis. Keio J Med. 1971;20:45–56. doi: 10.2302/kjm.20.45. [DOI] [PubMed] [Google Scholar]
- Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci USA. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, Aken BL, Barrell D, Zadissa A, Searle S, et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22:1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig M, Steinmann G. On a causal mechanism of chronic thymic involution in man. Mech Ageing Dev. 1994;75:151–156. doi: 10.1016/0047-6374(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Sempowski GD, Wells AF, Hale LP. The human thymus during aging. Immunol Res. 2000;22:253–261. doi: 10.1385/IR:22:2-3:253. [DOI] [PubMed] [Google Scholar]
- Herzig Y, Nevo S, Bornstein C, Brezis MR, Ben-Hur S, Shkedy A, Eisenberg-Bord M, Levi B, Delacher M, Goldfarb Y, et al. Transcriptional programs that control expression of the autoimmune regulator gene Aire. Nat Immunol. 2017;18:161–172. doi: 10.1038/ni.3638. [DOI] [PubMed] [Google Scholar]
- Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29:438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Isaacson PG, Norton AJ, Addis BJ. The human thymus contains a novel population of B lymphocytes. Lancet. 1987;2:1488–1491. doi: 10.1016/s0140-6736(87)92622-5. [DOI] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nat Rev Immunol. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu FT, Yang W, Wang YH, Ma HD, Tang W, Yang JB, Li L, Ansari AA, Lian ZX. Thymic B cells promote thymus-derived regulatory T cell development and proliferation. J Autoimmun. 2015;61:62–72. doi: 10.1016/j.jaut.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Miyama-Inaba M, Kuma S, Inaba K, Ogata H, Iwai H, Yasumizu R, Muramatsu S, Steinman RM, Ikehara S. Unusual phenotype of B cells in the thymus of normal mice. J Exp Med. 1988;168:811–816. doi: 10.1084/jem.168.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- Müller L, Pawelec G. As we age: Does slippage of quality control in the immune system lead to collateral damage? Ageing Res Rev. 2015;23(Pt A):116–123. doi: 10.1016/j.arr.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Nuñez S, Moore C, Gao B, Rogers K, Hidalgo Y, Del Nido PJ, Restaino S, Naka Y, Bhagat G, Madsen JC, et al. The human thymus perivascular space is a functional niche for viral-specific plasma cells. Sci Immunol. 2016;1:1. doi: 10.1126/sciimmunol.aah4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci U S A. 2002;99:5545–5550. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera J, Meng L, Meng F, Huang H. Autoreactive thymic B cells are efficient antigen-presenting cells of cognate self-antigens for T cell negative selection. Proc Natl Acad Sci U S A. 2013;110:17011–17016. doi: 10.1073/pnas.1313001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera J, Zheng Z, Li S, Gudjonson H, Kalinina O, Benichou JIC, Block KE, Louzoun Y, Yin D, Chong AS, et al. Self-antigen-driven thymic B cell class switching promotes T cell central tolerance. Cell Rep. 2016;17:387–398. doi: 10.1016/j.celrep.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi SW, Kim MY, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, McConnell FM, Scott HS, Penninger JM, Jenkinson EJ, et al. RANK signals from CD4(+)3(−) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med. 2007;204:1267–1272. doi: 10.1084/jem.20062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of auto-immunity. Blood. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, Marrack P. T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proc Natl Acad Sci U S A. 2013;110:E3216–E3224. doi: 10.1073/pnas.1312348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsova K, Rubtsov AV, Cancro MP, Marrack P. Age-associated B cells: a T-bet-dependent effector with roles in protective and pathogenic immunity. J Immunol. 2015;195:1933–1937. doi: 10.4049/jimmunol.1501209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeie GO, Mygland A, Aarli JA, Gilhus NE. Titin antibodies in patients with late onset myasthenia gravis: clinical correlations. Autoimmunity. 1995;20:99–104. doi: 10.3109/08916939509001933. [DOI] [PubMed] [Google Scholar]
- St-Pierre C, Trofimov A, Brochu S, Lemieux S, Perreault C. Differential features of AIRE-induced and AIRE-independent promiscuous gene expression in thymic epithelial cells. J Immunol. 2015;195:498–506. doi: 10.4049/jimmunol.1500558. [DOI] [PubMed] [Google Scholar]
- Tamaoki N, Habu S, Kameya T. Thymic lymphoid follicles in autoimmune diseases. II Histological, histochemical and electron microscopic studies. Keio J Med. 1971;20:57–68. doi: 10.2302/kjm.20.57. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsuyama M, Hirokawa K, Kurashima C, Fukayama M, Inamatsu T, Suzuki K, Hashimoto W, Sato K. Differential age-change in the numbers of CD4+CD45RA+ and CD4+CD29+ T cell subsets in human peripheral blood. Mech Ageing Dev. 1992;63:57–68. doi: 10.1016/0047-6374(92)90016-7. [DOI] [PubMed] [Google Scholar]
- Walters SN, Webster KE, Daley S, Grey ST. A role for intrathymic B cells in the generation of natural regulatory T cells. J Immunol. 2014;193:170–176. doi: 10.4049/jimmunol.1302519. [DOI] [PubMed] [Google Scholar]
- Xing C, Ma N, Xiao H, Wang X, Zheng M, Han G, Chen G, Hou C, Shen B, Li Y, Wang R. Critical role for thymic CD19+CD5+CD1dhiIL-10+ regulatory B cells in immune homeostasis. J Leukoc Biol. 2015;97:547–556. doi: 10.1189/jlb.3A0414-213RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano T, Nedjic J, Hinterberger M, Steinert M, Koser S, Pinto S, Gerdes N, Lutgens E, Ishimaru N, Busslinger M, et al. Thymic B cells are licensed to present self antigens for central T cell tolerance induction. Immunity. 2015;42:1048–1061. doi: 10.1016/j.immuni.2015.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.