Abstract
NRL proteins coordinate different aspects of phototropin signaling through signaling processes that are conserved in land plants and algae.
Plasma membrane receptors play fundamental roles in shaping plant growth and development. A large proportion of these are autophosphorylating Ser/Thr kinases (De Smet et al., 2009). Some function as dual-specificity kinases, autophosphorylating additionally on Tyr residues, one of which is the extensively studied steroid receptor BRASSINOSTEROID-INSENSITIVE1 (BRI1; Oh et al., 2009). Light regulation of plant growth also is mediated by plasma membrane-bound Ser/Thr kinases known as the phototropins (phots; Fankhauser and Christie, 2015). Seed plants contain two phots (phot1 and phot2) that have important roles in regulating leaf positioning and expansion, chloroplast photorelocation movement, stomatal opening, and phototropism, all of which serve to optimize photosynthetic efficiency (Christie et al., 2015). Phots are members of the AGCVIII kinase family (Rademacher and Offringa, 2012) but are distinct from transmembrane receptor kinases such as BRI1, as they are hydrophilic and bind to the intracellular side of the plasma membrane (Kong et al., 2013) to initiate signaling (Preuten et al., 2015). Although the ability to associate with the plasma membrane is conserved in algal phots (Sullivan et al., 2016a), the mechanism underlying this attachment is still not known, but it is thought to involve some form of lipid binding/modification.
Kinase-inactive versions of phot1 and phot2 are nonfunctional, highlighting the importance of receptor autophosphorylation in phot signaling (Inoue et al., 2008a, 2011). Autophosphorylation occurs predominantly on multiple Ser residues. At least 21 and 29 phosphorylation sites have been identified for Arabidopsis (Arabidopsis thaliana) phot1 and phot2, respectively (Christie et al., 2015). Most of these sites are found in the N terminus of the protein, which contains two light-sensing modules known as LIGHT, OXYGEN, OR VOLTAGE SENSING (LOV) domains known as LOV1 and LOV2 (Christie et al., 2012). Autophosphorylation of Ser-350, Ser-376, and Ser-410 within the LOV-linker region promotes the binding of 14-3-3 regulatory proteins to phot1 (Inoue et al., 2008a; Sullivan et al., 2009). However, the biological significance of this interaction is still not known. The occurrence of some these phosphorylation sites also is fluence rate dependent (Salomon et al., 2003) and is thought to play a role in receptor desensitization (Christie and Murphy, 2013). By contrast, the autophosphorylation of two conserved Ser residues within the activation loop of the C-terminal kinase domain (Ser-849 and Ser-851 in phot1 and Ser-761 or Ser-763 in phot2) is necessary for receptor signaling (Inoue et al., 2008a, 2011). Mutation of these sites to Ala impairs phot1 function in Arabidopsis, whereas phosphomimetic substitutions to Asp are without effect (Inoue et al., 2008a).
Despite considerable efforts over the last two decades, the signaling events that follow receptor autophosphorylation remain poorly understood for most phot-mediated responses. Yet, it is becoming increasingly apparent that members of the NPH3/RPT2-Like family (NRL) are required to elicit several phot responses. Moreover, accumulating evidence suggests that these plant-specific proteins act in concert with different AGCVIII kinases to regulate various aspects of auxin transport and signaling. In this review, we summarize our current understanding of the biochemical properties of the NRL family in Arabidopsis and how these proteins function to coordinate different aspects of phot signaling. We also discuss to what extent these signaling processes are conserved in land plants and algae.
STRUCTURE AND FUNCTION OF ARABIDOPSIS NRL PROTEINS
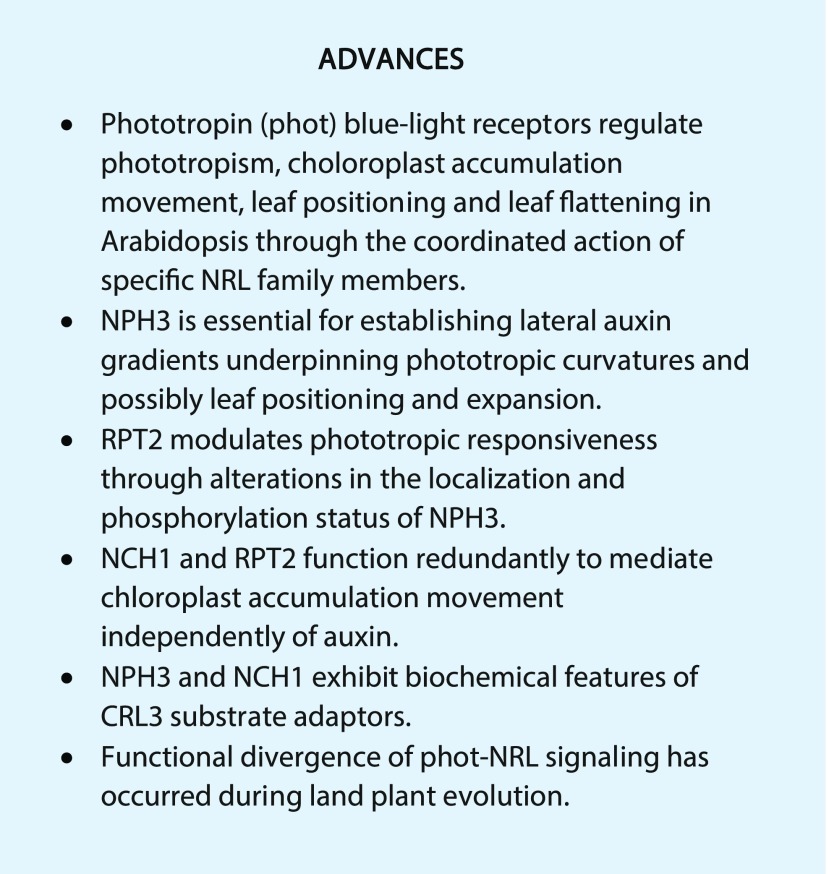
The NRL family is named after its two founding members, NONPHOTOTROPIC HYPOCOTYL3 (NPH3) and ROOT PHOTOTROPISM2 (RPT2; Liscum et al., 2014), which were first identified from genetic screens for Arabidopsis mutants impaired in hypocotyl and root phototropism (Okada and Shimura, 1992; Liscum and Briggs, 1995; Sakai et al., 2000). The primary amino acid structure of NRL proteins can be separated into three main parts based on sequence conservation: a BTB (bric-a-brac, tramtrack, and broad complex) domain at the N terminus, followed by an NPH3 domain and a C-terminal coiled-coil domain (Liscum et al., 2014). In addition to NPH3 and RPT2, Arabidopsis contains another 31 NPH3/RPT2-Like proteins (NRL1–NRL31), all of which are defined by the presence of the NPH3 domain (Pedmale et al., 2010). Ten of these members lack the C-terminal coiled-coli domain, whereas two are devoid of the BTB domain (Pedmale et al., 2010). The reasons for these differences in domain structure are not clear at present, because many of these NRL proteins have yet to be ascribed a biological function.
Functions for 10 out of the 33 Arabidopsis NRL proteins have been identified so far. Phylogenetic analysis has shown that NRL proteins can be classified into seven distinct clades in land plants (Suetsugu et al., 2016). However, in angiosperms such as Arabidopsis, only six of these clades are present (Fig. 1). NRL proteins from three of these clade classifications (Suetsugu et al., 2016) are known to interact with the phots. These include NPH3 (Motchoulski and Liscum, 1999), RPT2 (Inada et al., 2004), NRL PROTEIN FOR CHLOROPLAST MOVEMENT1 (NCH1/NRL31; Suetsugu et al., 2016), and NRL2 (Sullivan et al., 2009). NRL2 was identified as a phot1-interacting protein through yeast-two hybrid screening (Sullivan et al., 2009). However, its functional role in phot signaling remains undetermined. NPH3 and RPT2 are required for several auxin-mediated growth processes, including phototropism, leaf positioning, and leaf expansion (Christie et al., 2015). A central role for coordinating auxin distribution also has been linked to other members of the NRL family, which function independently from the phots. For instance, DEFECTIVELY ORGANIZED TRIBUTARIES3/NRL23 is associated with vascular development and leaf vein patterning (Petricka et al., 2008), whereas NRL proteins (NRL6, NRL7, NRL20, NRL21, and NRL30) known as NAKED PINS IN YUCCA (NPY; Fig. 1) function redundantly to regulate auxin movements required for organogenesis and root gravitropism in concert with AGCVIII kinases other than the phots (Cheng et al., 2008; Li et al., 2011). NRL8 (otherwise known as SETH6) is involved in pollen tube growth (Lalanne et al., 2004), which also requires AGC kinases activity (Zhang et al., 2008). Consequently, NRL proteins are proposed to function as AGC kinase modules that regulate various aspects of auxin trafficking and signaling. However, as discussed below, new evidence for the function of NRL proteins in phot signaling has begun to break down the paradigm that these proteins are solely mediators of auxin-dependent processes.
Figure 1.
Bayesian phylogenetic tree of the NRL protein family in Arabidopsis. The tree was modified from that of Suetsugu et al. (2016) with Archaeopteryx software. Six of the seven major clades of land plant NRL proteins are color coded. RPT2/NCH1, NPH3, and NPY reside in different clades.
NRL-DEPENDENT AND -INDEPENDENT PHOT RESPONSES
Phots regulate a range of physiological responses in Arabidopsis. These are listed in Table I and can be separated into two categories depending on the involvement of NRL proteins (NRL dependent and NRL independent). Phototropism (Motchoulski and Liscum, 1999; Sakai et al., 2000), petiole positioning and leaf expansion (Inoue et al., 2008b), and chloroplast accumulation (Suetsugu et al., 2016) are examples of NRL-dependent responses, whereas chloroplast-avoidance movement (Kong and Wada, 2014) and stomatal opening (Inoue and Kinoshita, 2017) are NRL independent. While several of these responses can be described as NRL independent, it is worth remembering that functions for 23 members of the NRL family are still lacking. Whether these processes are truly devoid of NRL involvement awaits further genetic characterization of these remaining family members. In the following sections, we summarize progress made to date in uncovering the role of NRL proteins in phot signaling.
Table I. NRL-dependent and -independent phot-mediated responses in Arabidopsis.
| Phot Response | NRL Dependent | NRL Independent | References |
|---|---|---|---|
| Phototropism | Requires NPH3 and RPT2 | Motchoulski and Liscum (1999); Sakai et al. (2000) | |
| Petiole positioning | Requires NPH3 and RPT2 | Inoue et al. (2008); Harada et al. (2013) | |
| Leaf expansion | Requires NPH3 and RPT2 | Inoue et al. (2008); Harada et al. (2013) | |
| Chloroplast accumulation | Requires RPT2 and NCH1 (NRL31) | Suetsugu et al. (2016) | |
| Chloroplast avoidance | Does not require NPH3, RPT2, or NCH1 (NRL31) | Suetsugu et al. (2016) | |
| Nuclear avoidance | Does not require NPH3 | Higa et al. (2014) | |
| Stomatal opening | Does not require NPH3, RPT2, or NCH1 (NRL31) | Suetsugu et al. (2016) | |
| Destabilization of Lhcb mRNA | Requires NPH3 | Folta and Kaufman (2003) | |
| Inhibition of hypocotyl elongation | Does not require NPH3 | Folta and Spalding (2001) | |
| Circadian control of PSII photosynthetic efficiency | Does not require NPH3 | Litthauer et al. (2015) |
NPH3 AND ITS ROLE IN PHOTOTROPISM
A central role for NPH3 in phototropism has been firmly established for some time now. Arabidopsis mutants lacking NPH3 fail to exhibit phototropism under a variety of different light conditions (Liscum et al., 2014; Fankhauser and Christie, 2015). A detailed discussion of phototropism is beyond the scope of this review, and readers are directed to other articles that provide a comprehensive overview of the different types of photophysiological responses involved (Sakai and Haga, 2012; Christie and Murphy, 2013). While the biochemical function of NPH3 remains poorly understood, it appears to be instrumental for establishing lateral auxin gradients required for this differential growth response (Haga et al., 2015). NPH3 is localized to the plasma membrane and has been shown to interact directly with phot1 via its C-terminal region (Motchoulski and Liscum, 1999). Like the phots, NPH3 contains no obvious transmembrane-spanning domain, so how it associates with the plasma membrane is still not known. However, truncation and transient expression analysis of NPH3-GFP in stomatal guard cells of Vicia faba suggests that the C terminus, including the coiled-coil domain, is important for localization to the plasma membrane (Inoue et al., 2008b).
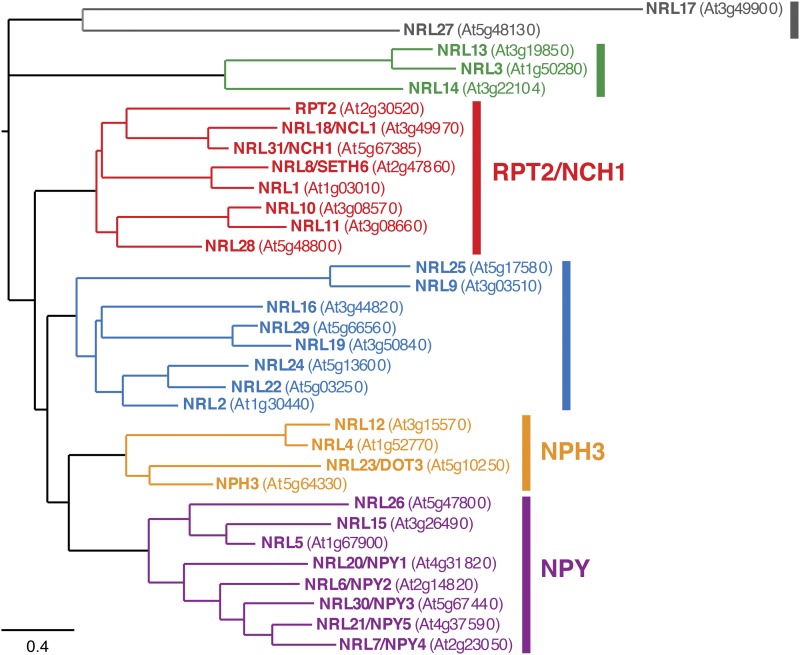
A recent in vivo coimmunoprecipitation analysis indicates that phot1-NPH3 interactions at the plasma membrane are transiently disrupted upon irradiation (Haga et al., 2015). Light not only impacts the ability of NPH3 to interact with phot1 but also leads to dynamic changes in its subcellular localization. The expression of YFP-NPH3 under the control of the constitutive cauliflower mosaic virus 35S promoter restores hypocotyl phototropism in the nph3 mutant (Haga et al., 2015). YFP-NPH3 is localized to the plasma membrane in darkness but is rapidly (within minutes) internalized into microdomain aggregates upon phot1 activation by blue light (Haga et al., 2015). The appearance of these microdomains is diminished in periods of prolonged irradiation or when seedlings are returned to darkness, suggesting that their formation is reversible (Haga et al., 2015). The biological significance and the role of these microdomains in phot signaling are currently unknown. However, biochemical fractionation experiments indicate that these NPH3 aggregates accumulate in the cytosol (Haga et al., 2015). These findings agree with observations that cytoskeleton inhibitors or inhibitors of vesicle trafficking fail to affect NPH3 microdomain formation (Haga et al., 2015).
The above phot1-driven changes in NPH3 localization correlate well with alterations in NPH3 phosphorylation status. NPH3 is phosphorylated in darkness and becomes rapidly (within minutes) dephosphorylated following phot1 activation (Pedmale and Liscum, 2007). This dephosphorylation process is tissue, and most likely cell, autonomous (Sullivan et al., 2016b) and does not appear to be initiated by phot2 (Inada et al., 2004; Pedmale and Liscum, 2007; Tsuchida-Mayama et al., 2008; Haga et al., 2015). As found for YFP-NPH3 microdomain formation, NPH3 dephosphorylation is recoverable either in darkness (Pedmale and Liscum, 2007) or over periods of prolonged irradiation (Haga et al., 2015). The kinetics for NPH3 dephosphorylation and rephosphorylation correlate closely with the time scale described for the changes in YFP-NPH3 localization (Haga et al., 2015). This has led to the proposal that the phosphorylation of NPH3 is necessary for it to form an active signaling complex with phot1 at the plasma membrane (Fig. 2). Phot1-NPH3 interactions are disrupted following NPH3 dephosphorylation and its release from the plasma membrane (Haga et al., 2015), leading to a cessation of receptor signaling. Thus, reassembly of this complex would be necessary to reestablish and sustain phototropic signaling, especially under continuous irradiation (Fig. 2).
Figure 2.
Roles of NPH3 and RPT2 in mediating phototropic signaling in Arabidopsis. The model was modified from Haga et al. (2015), where phot1 and NPH3 form a plasma membrane (PM) complex in darkness. Phot1-NPH3 interactions are diminished upon phot1 activation and dephosphorylation of NPH3. However, efficient signaling under continuous blue light conditions requires the reconstitution of a phot1-NPH3 complex that involves RPT2 at higher blue light intensities.
The identity of the kinase(s) responsible for phosphorylating NPH3 is still not known. Moreover, studies have yet to examine whether NPH3 is a substrate for phot1 kinase activity. However, pharmacological studies have implicated a role for a type 1 protein phosphatase in dephosphorylating NPH3 (Pedmale and Liscum, 2007). To date, 18 phosphorylation sites have been identified within NPH3 through global phosphoproteomic approaches (Heazlewood et al., 2008). The contribution of some of these sites to phototropic signaling has been investigated through structure-function analysis. Ser-212, Ser-222, and Ser-236 downstream of the BTB domain are phosphorylated in darkness and become dephosphorylated following phot1 activation (Tsuchida-Mayama et al., 2008). Collective mutation of these sites to Ala or deletion of this region (Δ212–238) does not adversely affect the ability of NPH3 to mediate hypocotyl phototropism in Arabidopsis (Tsuchida-Mayama et al., 2008). Therefore, phosphorylation sites other than Ser-212, Ser-222, and Ser-236 are clearly important (Haga et al., 2015). Identifying these sites and dissecting how changes in their phosphorylation status impact NPH3 function present a formidable challenge for future research. However, recent work has now demonstrated that the second founding member of the NRL family, RPT2, has a role in modulating the phosphorylation status and localization dynamics of NPH3 in Arabidopsis.
CONTRIBUTION OF RPT2 TO PHOT SIGNALING
RPT2 was identified originally from a genetic screen for Arabidopsis mutants impaired in root phototropism (Okada and Shimura, 1992; Sakai et al., 2000) and has been shown to interact directly with both NPH3 (Inada et al., 2004) and phot1 (Inada et al., 2004; Sullivan et al., 2009). The transposon insertion mutant rpt2-2 lacks the RPT2 protein (Inada et al., 2004) and is impaired in hypocotyl phototropism at blue light intensities of 0.17 μmol m−2 s−1 or greater (Haga et al., 2015). By contrast, the phototropic response of rpt2-2 mutants is indistinguishable from that of wild-type seedlings at blue light intensities of 0.017 μmol m−2 s−1 or less (Haga et al., 2015). A requirement for RPT2 in regulating phototropism to higher light correlates well with its expression profile. The expression of RPT2 is barely detectable in etiolated seedlings but increases in response to red and blue light (Inada et al., 2004; Tsuchida-Mayama et al., 2008; Haga et al., 2015) in a fluence rate-dependent manner (Sakai et al., 2000). As indicated above, reconstitution of a phot1-NPH3 complex is considered necessary to sustain effective phototropic signaling under continuous irradiation (Fig. 2). The presence of RPT2 is necessary to facilitate this process at high light intensities (Haga et al., 2015). Based on these findings, RPT2 is proposed to contribute to photosensory adaption and promote efficient phototropism under brighter light conditions (Fig. 2).
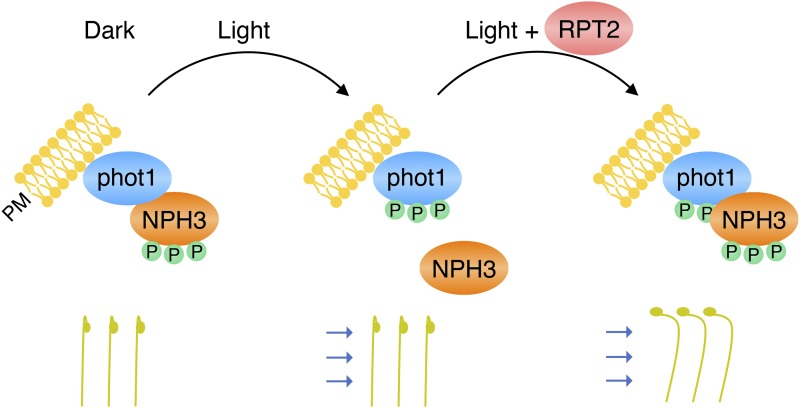
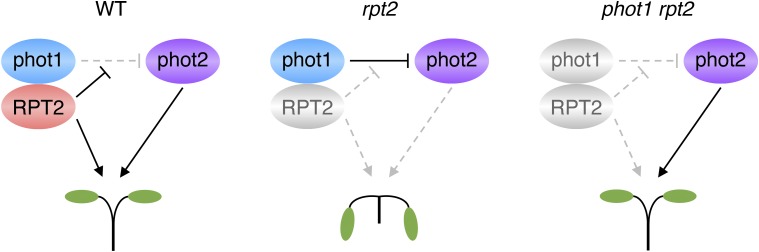
Mutants deficient in either NPH3 or RPT2 exhibit leaf positioning and leaf expansion phenotypes that resemble those of the phot1 phot2 double mutant (Inoue et al., 2008b; Harada et al., 2013). These findings demonstrate that NPH3 and RPT2 have roles in phot signaling besides phototropism. Leaf positioning and leaf expansion both require differential growth and most likely arise from alterations in auxin distribution. The severity of the rpt2 phenotype for these responses (Harada et al., 2013) is reduced dramatically under moderate light conditions when crossed with the phot1 single mutant. This phenotype of the phot1 rpt2 double mutant has uncovered a level of complexity regarding the contribution of RPT2 to phot signaling. RPT2 acts positively in conjunction with phot1 to mediate leaf positioning and leaf expansion. In contrast, RPT2 is not required for phot2 signaling. Instead, RPT2 appears to have some role in suppressing the ability of phot1 to inhibit phot2 signaling for these responses. In this model (Fig. 3), both phot1 and phot2 signaling pathways would be impaired in the rpt2 mutant. The phot1 pathway would be inactive, since its signaling is dependent on RPT2, whereas phot2 signaling is impaired, owing to the suppressive action of phot1. Likewise, both phot1 and phot2 signaling pathways would be inactive in a phot2 rpt2 double mutant. However, phot2 signaling for leaf positioning and leaf expansion still would be viable in this model for the phot1 rpt2 double mutant consistent with the phenotype observed, since the inhibitory action of phot1 is removed (Fig. 3).
Figure 3.
Functional complexity for RPT2 in controlling phot-mediated leaf positioning in Arabidopsis. The model was adapted from Harada et al. (2013). Sold black lines/arrows illustrate the signaling pathways operational in wild-type (WT) plants and mutant backgrounds. Nonoperational pathways in each of the genotypes are grayed out for clarity.
Additional evidence suggests that this complex interplay between phot1 and phot2 signaling may occur for other phot responses. The rpt2-1 mutant shows impaired hypocotyl phototropism under low and high fluence rates of unilateral blue light (Inada et al., 2004). Phototropic responsiveness was restored at higher fluence rates in the phot1-101 rpt2-1 double mutant, indicative of functional phot2 signaling. Similarities can be drawn between these results and those observed for the leaf positioning and leaf expansion phenotypes of the phot1 rpt2 mutant (Harada et al., 2013). However, some caution should be exercised when comparing these findings, given the difference in ecotypes used as well as the apparent leakiness of the rpt2-1 mutant (Haga et al., 2015).
PHYTOCHROME KINASE SUBSTRATE PROTEINS
NPH3 and RPT2 are not the only mediators of phototropism, leaf positioning, and leaf expansion in Arabidopsis. PHYTOCHROME KINASE SUBSRATE proteins (PKS1–PKS4) also play a role in establishing these processes. PKS1 was identified originally as a kinase substrate for phytochrome A (phyA; Fankhauser et al., 1999). Whether phyA is bona fide protein kinase is still debated, although recent work has offered renewed support for its role as a protein kinase in regulating photomorphogenesis (Shin et al., 2016). While PKS proteins are required for phytochrome signaling (Lariguet et al., 2003; Schepens et al., 2008), some of the phenotypes associated with pks mutants resemble those of nph3, rpt2, and phot-deficient mutants. For instance, the pks1 pks2 pks4 triple mutant exhibits reduced phototropic curvature (Lariguet et al., 2006; Kami et al., 2014). PKS1 and PKS2 also act in concert with NPH3 to establish leaf positioning and leaf expansion (de Carbonnel et al., 2010). Their role in phot signaling appears to be restricted to these processes, since pks mutants are not impaired in blue light-induced stomatal opening and chloroplast photorelocation movement (de Carbonnel et al., 2010). Consistent with their involvement in phot signaling, PKS proteins are known to localize to the plasma membrane, where they are known to associate with phot1, phot2, and NPH3 (Lariguet et al., 2006; de Carbonnel et al., 2010; Demarsy et al., 2012) Furthermore, studies have shown that PKS4 is a substrate target for phot1 kinase activity (Demarsy et al., 2012). Together, these data clearly demonstrate that PKS proteins are integral components of the phot signaling pathways associated with NPH3 and RPT2. Resolving the biochemical functions of these proteins and how they integrate with NPH3 and RPT2 will be important to fully understand how phots initiate signaling from the plasma membrane. Like NPH3, PKS proteins are suggested to modulate auxin transport or signaling (de Carbonnel et al., 2010; Kami et al., 2014). Yet, their mechanism of action in this regard remains to be elucidated.
NCH1 AND RPT2 ARE MEDIATORS OF CHLOROPLAST ACCUMULATION MOVEMENT
Phot-induced chloroplast accumulation movement was shown recently to involve the coaction of two NRL proteins. At low blue light intensities, phot1 and phot2 overlap in function to direct chloroplast relocation to the upper and lower cell surfaces to maximize light capture for photosynthesis (Sakai et al., 2001). Chloroplast-avoidance movement is mediated solely by phot2 (Kagawa et al., 2001) and serves to prevent photodamage of the photosynthetic apparatus in excess light (Kasahara et al., 2002). Chloroplast movement is achieved by the formation of chloroplast actin (cp-actin) filaments that polymerize on the leading edge of the chloroplast (Kadota et al., 2009). Polymerization of cp-actin requires the action of CHLOROPLAST UNUSUAL POSITIONING1 (CHUP1; Oikawa et al., 2003) and KINESIN-LIKE PROTEIN FOR ACTIN-BASED CHLOROPLAST MOVEMENT (KAC) proteins (Suetsugu et al., 2010). PLASTID MOVEMENT IMPAIRED1 (PMI1) also is required to stabilize cp-actin filaments (Suetsugu et al., 2015). Consequently, cp-actin formation, and thus chloroplast accumulation and avoidance movement, are impaired in chup1, pmi1, and kac1 kac2 mutants.
How light perception by phots is coupled to the activity of CHUP1, PMI1, and KAC proteins is not known. At least for chloroplast accumulation movement, the NRL proteins NCH1 (NRL31) and RPT2 have been shown to function as key mediators of this response (Suetsugu et al., 2016). NCH1 and RPT2 reside within the same clade of the NRL family tree in land plants (Fig. 1). Both nch1 and rpt2 mutants exhibit a weaker chloroplast accumulation response compared with wild-type plants (Suetsugu et al., 2016). However, this process is abolished completely in the rpt2 nch1 double mutant, indicating that NCH1 functions redundantly with RPT2 to mediate chloroplast accumulation movement (Suetsugu et al., 2016). Moreover, chloroplast-avoidance movement is unaltered in the rpt2 nch1 double mutant, demonstrating that NCH1 and RPT2 are only necessary for the accumulation response. While these findings have uncovered a new function for NRL proteins in phot signaling, they also demonstrate that RPT2 plays a central role in establishing several different phot responses. RPT2 could possibly mediate additional aspects of phot signaling in conjunction with other members of the NRL family. Further genetic analysis of the NRL family in combination with the rpt2 mutant will be required to shed light on this possibility.
The discovery of NCH1 and its role in chloroplast accumulation movement has now changed our view of how different NRL proteins contribute to phot and AGCVIII kinase signaling. Chloroplast photorelocation movement is cell autonomous and occurs independently of gene expression (Kong and Wada, 2016; Wada, 2016). Thus, auxin trafficking and auxin-mediated transcription are not required for this processes, in contrast to multicellular responses like phototropism, leaf positioning, and leaf expansion. To our knowledge, these findings are the first to demonstrate that NRL proteins can function with AGCVIII kinases to elicit responses independently of auxin.
BIOCHEMICAL FUNCTION OF NRL PROTEINS
Molecular genetic analysis in Arabidopsis has been successful in identifying physiological functions for almost one-third of the NRL family. Nevertheless, it is still not clear how these proteins operate at the biochemical level. The majority of NRL proteins contain a BTB domain that can function as a substrate adaptor to recruit specific proteins for ubiquitination and degradation (Hua and Vierstra, 2011). Indeed, NPH3 has been reported to interact with CULLIN3a (CUL3a) when coexpressed in insect cells and may form part of the CUL3 RING E3 UBIQUITIN LIGASE (CRL3) complex that directs the ubiquitination of signaling targets (Roberts et al., 2011). Biochemical and mass spectrometry analyses have shown that phot1 becomes ubiquitinated by a CRLNPH3 complex following blue light activation (Deng et al., 2014). CRL3NPH3-mediated polyubiquitination of phot1 is associated with its degradation in response to prolonged irradiation, whereas monoubiquitination is proposed to stimulate the rapid trafficking of phot1 from the plasma membrane in response to low light intensities (Roberts et al., 2011). Whether this light-activated change in subcellular localization plays a role in phot1 signaling is still open to question (Liscum, 2016). Recent approaches aimed at tethering phot1 to the plasma membrane by myristoylation or farnesylation suggest this not to be the case, as incorporation of these modifications severely diminishes the light-induced internalization of phot1 without impacting its functionality in Arabidopsis (Preuten et al., 2015). So far, no other targets besides phot1 have been identified for the CRL3NPH3 complex. Yet, it is worth noting that phot1-driven changes in subcellular trafficking of the auxin efflux carrier PIN2 are dependent on NPH3 (Wan et al., 2012). Since dynamic adjustments in PIN2 distribution and turnover have been linked to the ubiquitination of Lys-63 (Leitner et al., 2012), it is tempting to speculate that auxin transporters could be CRL3NPH3 targets. That said, there is currently no published evidence indicating that NPH3 can interact physically with auxin transport proteins.
NPH3 is not the only NRL protein to exhibit features of a CRL3 substrate adaptor. NCH1 (NRL31) was identified previously as AtSR1 INTERACTING PROTEIN1 (SR1IP1; Zhang et al., 2014). SR1IP1 and CUL3a interact when transiently coexpressed in Nicotiana benthamiana. SR1IP1 is responsible for ubiquitination and subsequent degradation of the Arabidopsis Ca2+/calmodulin-binding transcription factor SIGNAL RESPONSIVE1 (AtSR1), which functions to suppress plant defense responses to bacterial pathogens. Consequently, sr1ip1 mutants are more susceptible to Pseudomonas syringae infection, indicating that SR1IP1 serves as a positive regulator of plant immunity (Zhang et al., 2014). By contrast, virus-induced gene silencing of SR1IP1 orthologs in N. benthamiana reduces susceptibility to Phytophthora infestans infection, suggesting that SR1IP1 also can act as a negative regulator of plant defense (Yang et al., 2016). Clearly, more work is needed to elucidate the role of SR1IP1 (NCH1) in plant immunity and how this function integrates with its role in regulating chloroplast accumulation movement (Suetsugu et al., 2016). Chloroplasts can aid the transport of prodefense signals to the nucleus via highly dynamic connections known as stromules (Caplan et al., 2015). Therefore, it will be of interest to examine whether NRL proteins such as NCH1 are involved, especially since light is known to impact stromule formation (Gray et al., 2012; Brunkard et al., 2015).
EVOLUTIONARY CONSERVATION OF PHOT SIGNALING
Phots are restricted to the green plant lineage from photosynthetic algae to flowering plants (Fig. 3), but they are absent from red algae (Li et al., 2015). In contrast to land plants, green algae typically contain one phot (Huang et al., 2002; Prochnik et al., 2010; Sullivan et al., 2016), although two phots have been identified in Zygnematales including Mougeotia scalaris, both of which might function to mediate chloroplast photorelocation movement in this alga (Suetsugu et al., 2005; Li et al., 2015). Several functions have been ascribed to phot from Chlamydomonas reinhardtii. These include the light regulation of various aspects of the sexual life cycle (Huang et al., 2002; Huang and Beck, 2003; Ermilova et al., 2004), the control of eyespot development and phototactic behavior (Trippens et al., 2012), and the transcriptional regulation of enzymes involved in chlorophyll and carotenoid biosynthesis (Im et al., 2006). More recently, C. reinhardtii phot was shown to be pivotal for modulating the abundance of key molecular effectors required for photoprotection of the photosynthetic machinery. As a result, the phot-deficient mutants of C. reinhardtii displays reduced fitness under excessive light conditions (Petroutsos et al., 2016).
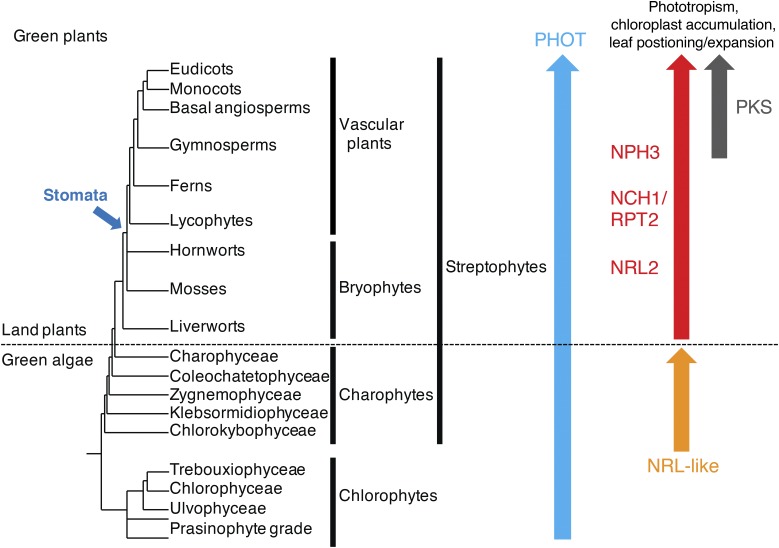
Despite its divergent functions, C. reinhardtii phot can successfully restore phototropism, chloroplast photorelocation movement, and stomatal opening when expressed in the phot1 phot2 double mutant of Arabidopsis (Onodera et al., 2005). These findings suggest that the mode of action between plant and algal phots is highly conserved. However, recent studies centered on phot from the marine picoalga Ostreococcus tauri are not consistent with this conclusion. While O. tauri phot is capable of mediating chloroplast accumulation movement, stomatal opening, as well as leaf positioning and leaf expansion when expressed in the phot1 phot2 double mutant of Arabidopsis, it fails to restore phototropism and chloroplast-avoidance movement (Sullivan et al., 2016a). At least for phototropism, this lack of functionality correlates with the inability of O. tauri phot to complex with NPH3 (Sullivan et al., 2016a). These findings fall more in line with what is known regarding the evolutionary conservation of NPH3 and other NRL proteins involved in phot signaling. NPH3, RPT2, and NCH1 are prevalent in land plants, including liverworts and mosses (Suetsugu et al., 2016), but they have not been identified in green algae (Fig. 4). The presence of these NRLs in land plants would coincide with their cooption to modulate multicellular (e.g. phototropism) as well as cell-autonomous (e.g. chloroplast movement and stomatal opening) responses.
Figure 4.
Schematic representation depicting the evolution of phot signaling components in the green plant lineage. The topology of the lineages is derived from Bowman et al. (2007). Arrows indicate the lineages in which orthologs of PHOT, NRL, and PKS proteins were identified from genomic and/or transcriptomic data. Stomata evolved after hornwort diversification.
Chloroplast photorelocation movement is prevalent in both land plants and green algae. The liverwort Marchantia polymorpha contains seven NRL proteins, one protein for each of the seven NRL clades (Suetsugu et al., 2016). The ortholog of NCH1 is essential for chloroplast accumulation movement in M. polymorpha but not for the avoidance response, indicating that the signaling events underlying chloroplast photorelocation movements are conserved in land plants (Suetsugu et al., 2016). In contrast to Arabidopsis, M. polymorpha contains one representative ortholog for the NPH3 clade and one for the NCH1/RPT2 clade, suggesting that the divergence of these NRL proteins occurred early in land plant evolution (Suetsugu et al., 2016). PKS proteins, by comparison, appear to have evolved later than NPH3 and NCH1/RPT2 in the land plant lineage (Fig. 4). CHUP1, KAC, PMI1, and NRL proteins are present in Streptophytes (including land plants and charophytes) and are essential factors for driving chloroplast photorelocation movement (Suetsugu and Wada, 2016). However, no orthologs of these genes have been found in chlorophytes (including C. reinhardtii and O. tauri). Thus, NRL-mediated chloroplast movement may have been acquired during evolution of the streptophyte lineage. Yet, the charophyte alga Klebsormidium flaccidum (charophyte) does contain two NRL-like proteins (Suetsugu et al., 2016). It is possible that these proteins mediate chloroplast photorelocation in this organism. Clearly, more work is needed to determine how light-induced chloroplast movement is achieved in photosynthetic algae.
CONCLUSION AND FUTURE PERSPECTIVES
Molecular genetic analysis in Arabidopsis has been instrumental in shedding light on the biological functions of NRL family members. Work so far has coordinated their mode of action with AGCVIII kinases and auxin signaling. Studies have now shown that NRL proteins can mediate signaling independently of auxin, at least in the case of chloroplast accumulation movement. A major challenge for future research will be to elucidate how NRL proteins function at the biochemical level and how changes in their localization and phosphorylation status can impact their activity. At least 24 out of the 33 NRL members are known to be phosphorylated (Heazlewood et al., 2008). Unlike NPH3 and NCH1, RPT2 does not exhibit E3 ubiquitin ligase activity in vitro (Roberts et al., 2011). Nor does its plasma membrane localization change in response to blue light treatment (Haga et al., 2015). Hence, the biochemical and localization properties for specific NRL family members are clearly different. Resolving the biochemical functions of NPH3 and RPT2 and their role in phot signaling will be essential if we are to understand how auxin gradients are established to promote responses such as phototropism, leaf positioning, and leaf expansion. NRL2 is known to interact with phot1 (Sullivan et al., 2009), but its biological role in phot signaling awaits additional molecular and genetic characterization. Further functional dissection of the Arabidopsis NRL family will be a demanding task, given the large size of this protein family and the redundancy between members. The reduced size and complexity of the NRL family in M. polymorpha now provides a tractable alternative to assigning functions to each of seven NRL clade classifications found in land plants.
Footnotes
This work was supported by funding from the U.K. Biotechnology and Biological Sciences Research Council (BB/M002128/1 to J.M.C.) and by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (15KK0254 to N.S.).
References
- Bowman JL, Floyd SK, Sakakibara K (2007) Green genes: comparative genomics of the green branch of life. Cell 129: 229–234 [DOI] [PubMed] [Google Scholar]
- Brunkard JO, Runkel AM, Zambryski PC (2015) Chloroplasts extend stromules independently and in response to internal redox signals. Proc Natl Acad Sci USA 112: 10044–10049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JL, Kumar AS, Park E, Padmanabhan MS, Hoban K, Modla S, Czymmek K, Dinesh-Kumar SP (2015) Chloroplast stromules function during innate immunity. Dev Cell 34: 45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Qin G, Dai X, Zhao Y (2008) NPY genes and AGC kinases define two key steps in auxin-mediated organogenesis in Arabidopsis. Proc Natl Acad Sci USA 105: 21017–21022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Blackwood L, Petersen J, Sullivan S (2015) Plant flavoprotein photoreceptors. Plant Cell Physiol 56: 401–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Gawthorne J, Young G, Fraser NJ, Roe AJ (2012) LOV to BLUF: flavoprotein contributions to the optogenetic toolkit. Mol Plant 5: 533–544 [DOI] [PubMed] [Google Scholar]
- Christie JM, Murphy AS (2013) Shoot phototropism in higher plants: new light through old concepts. Am J Bot 100: 35–46 [DOI] [PubMed] [Google Scholar]
- de Carbonnel M, Davis P, Roelfsema MR, Inoue S, Schepens I, Lariguet P, Geisler M, Shimazaki K, Hangarter R, Fankhauser C (2010) The Arabidopsis PHYTOCHROME KINASE SUBSTRATE2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiol 152: 1391–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarsy E, Schepens I, Okajima K, Hersch M, Bergmann S, Christie J, Shimazaki K, Tokutomi S, Fankhauser C (2012) Phytochrome Kinase Substrate 4 is phosphorylated by the phototropin 1 photoreceptor. EMBO J 31: 3457–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Oses-Prieto JA, Kutschera U, Tseng TS, Hao L, Burlingame AL, Wang ZY, Briggs WR (2014) Blue light-induced proteomic changes in etiolated Arabidopsis seedlings. J Proteome Res 13: 2524–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I, Voss U, Jürgens G, Beeckman T (2009) Receptor-like kinases shape the plant. Nat Cell Biol 11: 1166–1173 [DOI] [PubMed] [Google Scholar]
- Ermilova EV, Zalutskaya ZM, Huang K, Beck CF (2004) Phototropin plays a crucial role in controlling changes in chemotaxis during the initial phase of the sexual life cycle in Chlamydomonas. Planta 219: 420–427 [DOI] [PubMed] [Google Scholar]
- Fankhauser C, Christie JM (2015) Plant phototropic growth. Curr Biol 25: R384–R389 [DOI] [PubMed] [Google Scholar]
- Fankhauser C, Yeh KC, Lagarias JC, Zhang H, Elich TD, Chory J (1999) PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science 284: 1539–1541 [DOI] [PubMed] [Google Scholar]
- Folta KM, Kaufman LS (2003) Phototropin 1 is required for high-fluence blue-light-mediated mRNA destabilization. Plant Mol Biol 51: 609–618 [DOI] [PubMed] [Google Scholar]
- Folta KM, Spalding EP (2001) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26: 471–478 [DOI] [PubMed] [Google Scholar]
- Gray JC, Hansen MR, Shaw DJ, Graham K, Dale R, Smallman P, Natesan SK, Newell CA (2012) Plastid stromules are induced by stress treatments acting through abscisic acid. Plant J 69: 387–398 [DOI] [PubMed] [Google Scholar]
- Haga K, Tsuchida-Mayama T, Yamada M, Sakai T (2015) Arabidopsis ROOT PHOTOTROPISM2 contributes to the adaptation to high-intensity light in phototropic responses. Plant Cell 27: 1098–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Takemiya A, Inoue S, Sakai T, Shimazaki K (2013) Role of RPT2 in leaf positioning and flattening and a possible inhibition of phot2 signaling by phot1. Plant Cell Physiol 54: 36–47 [DOI] [PubMed] [Google Scholar]
- Heazlewood JL, Durek P, Hummel J, Selbig J, Weckwerth W, Walther D, Schulze WX (2008) PhosPhAt: a database of phosphorylation sites in Arabidopsis thaliana and a plant-specific phosphorylation site predictor. Nucleic Acids Res 36: D1015–D1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa T, Suetsugu N, Wada M (2014) Plant nuclear photorelocation movement. J Exp Bot 65: 2873–2881 [DOI] [PubMed] [Google Scholar]
- Hua Z, Vierstra RD (2011) The cullin-RING ubiquitin-protein ligases. Annu Rev Plant Biol 62: 299–334 [DOI] [PubMed] [Google Scholar]
- Huang K, Beck CF (2003) Phototropin is the blue-light receptor that controls multiple steps in the sexual life cycle of the green alga Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 100: 6269–6274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Merkle T, Beck CF (2002) Isolation and characterization of a Chlamydomonas gene that encodes a putative blue-light photoreceptor of the phototropin family. Physiol Plant 115: 613–622 [DOI] [PubMed] [Google Scholar]
- Im CS, Eberhard S, Huang K, Beck CF, Grossman AR (2006) Phototropin involvement in the expression of genes encoding chlorophyll and carotenoid biosynthesis enzymes and LHC apoproteins in Chlamydomonas reinhardtii. Plant J 48: 1–16 [DOI] [PubMed] [Google Scholar]
- Inada S, Ohgishi M, Mayama T, Okada K, Sakai T (2004) RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. Plant Cell 16: 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Kinoshita T, Matsumoto M, Nakayama KI, Doi M, Shimazaki K (2008a) Blue light-induced autophosphorylation of phototropin is a primary step for signaling. Proc Natl Acad Sci USA 105: 5626–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Kinoshita T, Takemiya A, Doi M, Shimazaki K (2008b) Leaf positioning of Arabidopsis in response to blue light. Mol Plant 1: 15–26 [DOI] [PubMed] [Google Scholar]
- Inoue S, Matsushita T, Tomokiyo Y, Matsumoto M, Nakayama KI, Kinoshita T, Shimazaki K (2011) Functional analyses of the activation loop of phototropin2 in Arabidopsis. Plant Physiol 156: 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue SI, Kinoshita T (2017) Blue light regulation of stomatal opening and the plasma membrane H+-ATPase. Plant Physiol 174: 531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota A, Yamada N, Suetsugu N, Hirose M, Saito C, Shoda K, Ichikawa S, Kagawa T, Nakano A, Wada M (2009) Short actin-based mechanism for light-directed chloroplast movement in Arabidopsis. Proc Natl Acad Sci USA 106: 13106–13111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Kami C, Allenbach L, Zourelidou M, Ljung K, Schütz F, Isono E, Watahiki MK, Yamamoto KT, Schwechheimer C, Fankhauser C (2014) Reduced phototropism in pks mutants may be due to altered auxin-regulated gene expression or reduced lateral auxin transport. Plant J 77: 393–403 [DOI] [PubMed] [Google Scholar]
- Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M (2002) Chloroplast avoidance movement reduces photodamage in plants. Nature 420: 829–832 [DOI] [PubMed] [Google Scholar]
- Kong SG, Suetsugu N, Kikuchi S, Nakai M, Nagatani A, Wada M (2013) Both phototropin 1 and 2 localize on the chloroplast outer membrane with distinct localization activity. Plant Cell Physiol 54: 80–92 [DOI] [PubMed] [Google Scholar]
- Kong SG, Wada M (2014) Recent advances in understanding the molecular mechanism of chloroplast photorelocation movement. Biochim Biophys Acta 1837: 522–530 [DOI] [PubMed] [Google Scholar]
- Kong SG, Wada M (2016) Molecular basis of chloroplast photorelocation movement. J Plant Res 129: 159–166 [DOI] [PubMed] [Google Scholar]
- Lalanne E, Honys D, Johnson A, Borner GH, Lilley KS, Dupree P, Grossniklaus U, Twell D (2004) SETH1 and SETH2, two components of the glycosylphosphatidylinositol anchor biosynthetic pathway, are required for pollen germination and tube growth in Arabidopsis. Plant Cell 16: 229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariguet P, Boccalandro HE, Alonso JM, Ecker JR, Chory J, Casal JJ, Fankhauser C (2003) A growth regulatory loop that provides homeostasis to phytochrome A signaling. Plant Cell 15: 2966–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariguet P, Schepens I, Hodgson D, Pedmale UV, Trevisan M, Kami C, de Carbonnel M, Alonso JM, Ecker JR, Liscum E, et al. (2006) PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc Natl Acad Sci USA 103: 10134–10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner J, Petrášek J, Tomanov K, Retzer K, Pařezová M, Korbei B, Bachmair A, Zažímalová E, Luschnig C (2012) Lysine63-linked ubiquitylation of PIN2 auxin carrier protein governs hormonally controlled adaptation of Arabidopsis root growth. Proc Natl Acad Sci USA 109: 8322–8327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FW, Rothfels CJ, Melkonian M, Villarreal JC, Stevenson DW, Graham SW, Wong GK, Mathews S, Pryer KM (2015) The origin and evolution of phototropins. Front Plant Sci 6: 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Dai X, Cheng Y, Zhao Y (2011) NPY genes play an essential role in root gravitropic responses in Arabidopsis. Mol Plant 4: 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E. (2016) Blue light-induced intracellular movement of phototropins: functional relevance or red herring? Front Plant Sci 7: 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Askinosie SK, Leuchtman DL, Morrow J, Willenburg KT, Coats DR (2014) Phototropism: growing towards an understanding of plant movement. Plant Cell 26: 38–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Briggs WR (1995) Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7: 473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litthauer S, Battle MW, Lawson T, Jones MA (2015) Phototropins maintain robust circadian oscillation of PSII operating efficiency under blue light. Plant J 83: 1034–1045 [DOI] [PubMed] [Google Scholar]
- Motchoulski A, Liscum E (1999) Arabidopsis NPH3: a NPH1 photoreceptor-interacting protein essential for phototropism. Science 286: 961–964 [DOI] [PubMed] [Google Scholar]
- Oh MH, Wang X, Kota U, Goshe MB, Clouse SD, Huber SC (2009) Tyrosine phosphorylation of the BRI1 receptor kinase emerges as a component of brassinosteroid signaling in Arabidopsis. Proc Natl Acad Sci USA 106: 658–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa K, Kasahara M, Kiyosue T, Kagawa T, Suetsugu N, Takahashi F, Kanegae T, Niwa Y, Kadota A, Wada M (2003) Chloroplast unusual positioning1 is essential for proper chloroplast positioning. Plant Cell 15: 2805–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Shimura Y (1992) Mutational analysis of root gravitropism and phototropism of Arabidopsis thaliana seedlings. Aust J Plant Physiol 19: 439–448 [Google Scholar]
- Onodera A, Kong SG, Doi M, Shimazaki K, Christie J, Mochizuki N, Nagatani A (2005) Phototropin from Chlamydomonas reinhardtii is functional in Arabidopsis thaliana. Plant Cell Physiol 46: 367–374 [DOI] [PubMed] [Google Scholar]
- Pedmale UV, Celaya RB, Liscum E (2010) Phototropism: mechanism and outcomes. The Arabidopsis Book 8: e0125, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedmale UV, Liscum E (2007) Regulation of phototropic signaling in Arabidopsis via phosphorylation state changes in the phototropin 1-interacting protein NPH3. J Biol Chem 282: 19992–20001 [DOI] [PubMed] [Google Scholar]
- Petricka JJ, Clay NK, Nelson TM (2008) Vein patterning screens and the defectively organized tributaries mutants in Arabidopsis thaliana. Plant J 56: 251–263 [DOI] [PubMed] [Google Scholar]
- Petroutsos D, Tokutsu R, Maruyama S, Flori S, Greiner A, Magneschi L, Cusant L, Kottke T, Mittag M, Hegemann P, et al. (2016) A blue-light photoreceptor mediates the feedback regulation of photosynthesis. Nature 537: 563–566 [DOI] [PubMed] [Google Scholar]
- Preuten T, Blackwood L, Christie JM, Fankhauser C (2015) Lipid anchoring of Arabidopsis phototropin 1 to assess the functional significance of receptor internalization: should I stay or should I go? New Phytol 206: 1038–1050 [DOI] [PubMed] [Google Scholar]
- Prochnik SE, Umen J, Nedelcu AM, Hallmann A, Miller SM, Nishii I, Ferris P, Kuo A, Mitros T, Fritz-Laylin LK, et al. (2010) Genomic analysis of organismal complexity in the multicellular green alga Volvox carteri. Science 329: 223–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher EH, Offringa R (2012) Evolutionary adaptations of plant AGC kinases: from light signaling to cell polarity regulation. Front Plant Sci 3: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D, Pedmale UV, Morrow J, Sachdev S, Lechner E, Tang X, Zheng N, Hannink M, Genschik P, Liscum E (2011) Modulation of phototropic responsiveness in Arabidopsis through ubiquitination of phototropin 1 by the CUL3-Ring E3 ubiquitin ligase CRL3(NPH3). Plant Cell 23: 3627–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Haga K (2012) Molecular genetic analysis of phototropism in Arabidopsis. Plant Cell Physiol 53: 1517–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98: 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Wada T, Ishiguro S, Okada K (2000) RPT2: a signal transducer of the phototropic response in Arabidopsis. Plant Cell 12: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon M, Knieb E, von Zeppelin T, Rüdiger W (2003) Mapping of low- and high-fluence autophosphorylation sites in phototropin 1. Biochemistry 42: 4217–4225 [DOI] [PubMed] [Google Scholar]
- Schepens I, Boccalandro HE, Kami C, Casal JJ, Fankhauser C (2008) PHYTOCHROME KINASE SUBSTRATE4 modulates phytochrome-mediated control of hypocotyl growth orientation. Plant Physiol 147: 661–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin AY, Han YJ, Baek A, Ahn T, Kim SY, Nguyen TS, Son M, Lee KW, Shen Y, Song PS, et al. (2016) Evidence that phytochrome functions as a protein kinase in plant light signalling. Nat Commun 7: 11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N, Higa T, Kong SG, Wada M (2015) PLASTID MOVEMENT IMPAIRED1 and PLASTID MOVEMENT IMPAIRED1-RELATED1 mediate photorelocation movements of both chloroplasts and nuclei. Plant Physiol 169: 1155–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N, Mittmann F, Wagner G, Hughes J, Wada M (2005) A chimeric photoreceptor gene, NEOCHROME, has arisen twice during plant evolution. Proc Natl Acad Sci USA 102: 13705–13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N, Takemiya A, Kong SG, Higa T, Komatsu A, Shimazaki K, Kohchi T, Wada M (2016) RPT2/NCH1 subfamily of NPH3-like proteins is essential for the chloroplast accumulation response in land plants. Proc Natl Acad Sci USA 113: 10424–10429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N, Wada M (2016) Evolution of the Cp-actin-based motility system of chloroplasts in green plants. Front Plant Sci 7: 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N, Yamada N, Kagawa T, Yonekura H, Uyeda TQ, Kadota A, Wada M (2010) Two kinesin-like proteins mediate actin-based chloroplast movement in Arabidopsis thaliana. Proc Natl Acad Sci USA 107: 8860–8865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S, Petersen J, Blackwood L, Papanatsiou M, Christie JM (2016a) Functional characterization of Ostreococcus tauri phototropin. New Phytol 209: 612–623 [DOI] [PubMed] [Google Scholar]
- Sullivan S, Takemiya A, Kharshiing E, Cloix C, Shimazaki KI, Christie JM (2016b) Functional characterization of Arabidopsis phototropin 1 in the hypocotyl apex. Plant J 88: 907–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S, Thomson CE, Kaiserli E, Christie JM (2009) Interaction specificity of Arabidopsis 14-3-3 proteins with phototropin receptor kinases. FEBS Lett 583: 2187–2193 [DOI] [PubMed] [Google Scholar]
- Trippens J, Greiner A, Schellwat J, Neukam M, Rottmann T, Lu Y, Kateriya S, Hegemann P, Kreimer G (2012) Phototropin influence on eyespot development and regulation of phototactic behavior in Chlamydomonas reinhardtii. Plant Cell 24: 4687–4702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida-Mayama T, Nakano M, Uehara Y, Sano M, Fujisawa N, Okada K, Sakai T (2008) Mapping of the phosphorylation sites on the phototropic signal transducer, NPH3. Plant Sci 174: 626–633 [Google Scholar]
- Wada M. (2016) Chloroplast and nuclear photorelocation movements. Proc Jpn Acad Ser B Phys Biol Sci 92: 387–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Jasik J, Wang L, Hao H, Volkmann D, Menzel D, Mancuso S, Baluška F, Lin J (2012) The signal transducer NPH3 integrates the phototropin1 photosensor with PIN2-based polar auxin transport in Arabidopsis root phototropism. Plant Cell 24: 551–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, McLellan H, Naqvi S, He Q, Boevink PC, Armstrong M, Giuliani LM, Zhang W, Tian Z, Zhan J, et al. (2016) Potato NPH3/RPT2-like protein StNRL1, targeted by a Phytophthora infestans RXLR effector, is a susceptibility factor. Plant Physiol 171: 645–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Wengier D, Shuai B, Gui CP, Muschietti J, McCormick S, Tang WH (2008) The pollen receptor kinase LePRK2 mediates growth-promoting signals and positively regulates pollen germination and tube growth. Plant Physiol 148: 1368–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Du L, Shen C, Yang Y, Poovaiah BW (2014) Regulation of plant immunity through ubiquitin-mediated modulation of Ca2+-calmodulin-AtSR1/CAMTA3 signaling. Plant J 78: 269–281 [DOI] [PubMed] [Google Scholar]