An energy signaling pathway, photoperiod, and light intensity regulate sugar-induced hypocotyl elongation.
Abstract
Emerging seedlings respond to environmental conditions such as light and temperature to optimize their establishment. Seedlings grow initially through elongation of the hypocotyl, which is regulated by signaling pathways that integrate environmental information to regulate seedling development. The hypocotyls of Arabidopsis (Arabidopsis thaliana) also elongate in response to sucrose. Here, we investigated the role of cellular sugar-sensing mechanisms in the elongation of hypocotyls in response to Suc. We focused upon the role of SnRK1, which is a sugar-signaling hub that regulates metabolism and transcription in response to cellular energy status. We also investigated the role of TPS1, which synthesizes the signaling sugar trehalose-6-P that is proposed to regulate SnRK1 activity. Under light/dark cycles, we found that Suc-induced hypocotyl elongation did not occur in tps1 mutants and overexpressors of KIN10 (AKIN10/SnRK1.1), a catalytic subunit of SnRK1. We demonstrate that the magnitude of Suc-induced hypocotyl elongation depends on the day length and light intensity. We identified roles for auxin and gibberellin signaling in Suc-induced hypocotyl elongation under short photoperiods. We found that Suc-induced hypocotyl elongation under light/dark cycles does not involve another proposed sugar sensor, HEXOKINASE1, or the circadian oscillator. Our study identifies novel roles for KIN10 and TPS1 in mediating a signal that underlies Suc-induced hypocotyl elongation in light/dark cycles.
Emerging seedlings monitor the environment to optimize their establishment and out-compete neighboring plants (Salter et al., 2003; Weinig et al., 2007; Koini et al., 2009; Keuskamp et al., 2010; Crawford et al., 2012). Seedlings grow initially through cell expansion within the hypocotyl, which elongates rapidly to optimize light capture by the cotyledons. Hypocotyl elongation is controlled by several signaling pathways that converge upon phytohormones to regulate cell expansion (Lincoln et al., 1990; Collett et al., 2000). Examples of signals that adjust hypocotyl elongation include phytochrome-mediated signals concerning the ratio of red to far red light (Casal, 2013), blue light (Liscum and Hangarter, 1991), UV-B light (Kim et al., 1998; Hayes et al., 2014), temperature (Koini et al., 2009; Wigge, 2013; Mizuno et al., 2014), photoperiod and the circadian oscillator (Dowson-Day and Millar, 1999; Más et al., 2003; Nusinow et al., 2011). These signals are integrated by the PHYTOCHROME INTERACTING FACTOR (PIF) family of basic helix-loop-helix transcription factors. The PIFs are signaling hubs that control plant development through genomewide transcriptional alterations. One outcome of these PIF-mediated transcriptional changes is the alteration in phytohormone signaling that regulates hypocotyl elongation (Lorrain et al., 2008; Leivar and Quail, 2011).
Hypocotyl length is also increased by exogenous and endogenous sugars (Kurata and Yamamoto, 1998; Takahashi et al., 2003; Zhang et al., 2010, 2015, 2016; Liu et al., 2011; Stewart et al., 2011; Lilley et al., 2012). Under light/dark cycles, exogenous sugars are proposed to cause hypocotyl elongation by inducing auxin signals through the PIF-mediated gene regulation (Stewart et al., 2011; Lilley et al., 2012). Under extended darkness, brassinosteroid and gibberellin (GA) phytohormones are involved in sugar-induced hypocotyl elongation, which may also involve the target of rapamycin (TOR) kinase regulator of energy- and nutrient-responses (Zhang et al., 2010, 2015, 2016; Dobrenel et al., 2011). This elongation phenotype in darkness is thought to form a response to the starvation conditions that arise when plants are cultivated under periods of darkness exceeding the length of the daily light/dark cycle (Graf et al., 2010; Zhang et al., 2016). In comparison to these known roles for phytohormones and transcriptional regulators, the contribution of sugar sensing mechanisms to sucrose-induced hypocotyl elongation remains unknown.
Several sugar- or energy-signaling mechanisms underlie the metabolic and developmental responses of plants to sugars. One mechanism involves the sucrose nonfermenting1 (Snf1)-related protein kinase SnRK1 (Baena-González et al., 2007; Baena-González and Sheen, 2008), and another involves HEXOKINASE1 (Jang et al., 1997; Moore et al., 2003). SnRK1 controls metabolic enzymes directly by protein phosphorylation (Baena-González and Sheen, 2008). It also regulates greater than 1000 transcripts in response to carbohydrate availability, for example by adjusting bZIP transcription factor activity (Baena-González et al., 2007; Smeekens et al., 2010; Delatte et al., 2011; Matiolli et al., 2011; Mair et al., 2015). Both SnRK1- and hexokinase-mediated sugar signaling involve specific sugars functioning as signaling molecules that provide cellular information concerning sugar availability. For example, SnRK1 activity is thought to be regulated by trehalose-6-P (Tre6P), whose concentration tracks the cellular concentration of Suc (Lunn et al., 2006; Zhang et al., 2009; Nunes et al., 2013a, 2013b; Yadav et al., 2014). Tre6P is synthesized from UDP Glc and Glc-6-P, which are derived from mobilized and transported Suc, and also directly from photosynthesis. In Arabidopsis (Arabidopsis thaliana), Tre6P is synthesized by trehalose-6-P synthase (TPS). Of 11 TPS homologs encoded by the Arabidopsis genome, TREHALOSE-6-PHOSPHATE SYNTHASE1 (TPS1) synthesizes Tre6P in plants (Gómez et al., 2010; Vandesteene et al., 2010), and TPS2 and TPS4 are catalytically active in yeast complementation assays (Delorge et al., 2015). Tre6P is believed to regulate SnRK1-mediated signaling by suppressing the activity of SNF1-RELATED PROTEIN KINASE1.1 (KIN10/AKIN10/SnRK1.1), which is a catalytic subunit of SnRK1 that is fundamental to the signaling role of SnRK1 (Baena-González et al., 2007; Zhang et al., 2009; Nunes et al., 2013a, 2013b).
Manipulation of Tre6P metabolism in plants alters developmental phenotypes. For example, tps1 knockout mutants undergo seedling developmental arrest (Gómez et al., 2006), expression of bacterial Tre6P synthase (otsA) or phosphatase (otsB) affects leaf senescence (Wingler et al., 2012), and Tre6P and KIN10 act within a photoperiod-response pathway that controls the induction of flowering (Baena-González et al., 2007; Gómez et al., 2010; Wahl et al., 2013). Signaling by Tre6P and KIN10 is also important for the regulation of growth rates. Growth is increased by Suc in the presence of Tre6P (Schluepmann et al., 2003; Paul et al., 2010), but the lack of a quantitative (correlative) relationship between relative growth rates and [Tre6P] suggests that a threshold [Tre6P] is required for growth to occur (Nunes et al., 2013a, 2013b). Therefore, it has been suggested that control of KIN10/11 by [Tre6P] may prime the regulation of growth-related genes to capitalize upon increased energy availability, rather than by inducing growth directly (Nunes et al., 2013a, 2013b). Remarkably, the impact of this pathway is sufficiently global that its manipulation can increase maize (Zea mays) yields by almost 50% (Nuccio et al., 2015) and increase the yield and drought tolerance of wheat (Triticum aestivum; Griffiths et al., 2016).
Given the importance of Tre6P metabolism and SnRK1 for growth regulation under cycles of light and dark, we wished to determine whether this energy-signaling mechanism is important for the regulation of Suc-induced hypocotyl elongation. Moreover, because Tre6P signaling is reported to act upon GA and auxin signaling genes (Paul et al., 2010; Li et al., 2014) and these phytohormones are involved in Suc-induced hypocotyl elongation (Zhang et al., 2010; Lilley et al., 2012), we reasoned that SnRK1 might act upon these phytohormones to regulate Suc-induced hypocotyl elongation.
Here, we identified a novel role for Tre6P and KIN10 in the mechanisms that cause Suc-induced hypocotyl elongation. We focused upon light/dark cycles rather than conditions of extended darkness (Zhang et al., 2010, 2015, 2016), because we wished to identify mechanisms that regulate growth and development under regimes more representative of real-world growing conditions that do not elicit prolonged starvation. We found that the sensitivity of hypocotyl elongation to sugars depends on the photoperiod and light intensity. We identified that KIN10 is important for expression of transcripts encoding auxin-induced expansins. Our data reveal a new mechanistic link among carbohydrate supply, energy sensing, and phytohormone signaling during seedling emergence.
RESULTS
KIN10 and TPS1 Are Required for Suc-Induced Hypocotyl Elongation in Light/Dark Cycles
We investigated whether KIN10 and TPS1 contribute to Suc-induced hypocotyl elongation under light/dark cycles (Kurata and Yamamoto, 1998; Takahashi et al., 2003; Stewart et al., 2011; Lilley et al., 2012). We studied hypocotyl elongation in transgenic Arabidopsis where KIN10 activity was manipulated by overexpressing the catalytic subunit of KIN10 (KIN10-ox; Baena-González et al., 2007). Although KIN10 activity is regulated posttranslationally by Tre6P (Zhang et al., 2009), KIN10 overexpression alone alters the abundance of energy-response transcripts in protoplasts (Baena-González et al., 2007). We used KIN10 overexpression rather than knockouts, because KIN10/11 double knockouts disrupt pollen production and are lethal (Zhang et al., 2001; Baena-González et al., 2007). We also used hypomorphic Targeted Induced Local Lesions In Genomes (TILLING) mutants with reduced TPS1 activity (tps1-11, tps1-12; Gómez et al., 2006, 2010), which is preferable to tps1 loss-of-function mutants that cause seedling developmental arrest (Gómez et al., 2006).
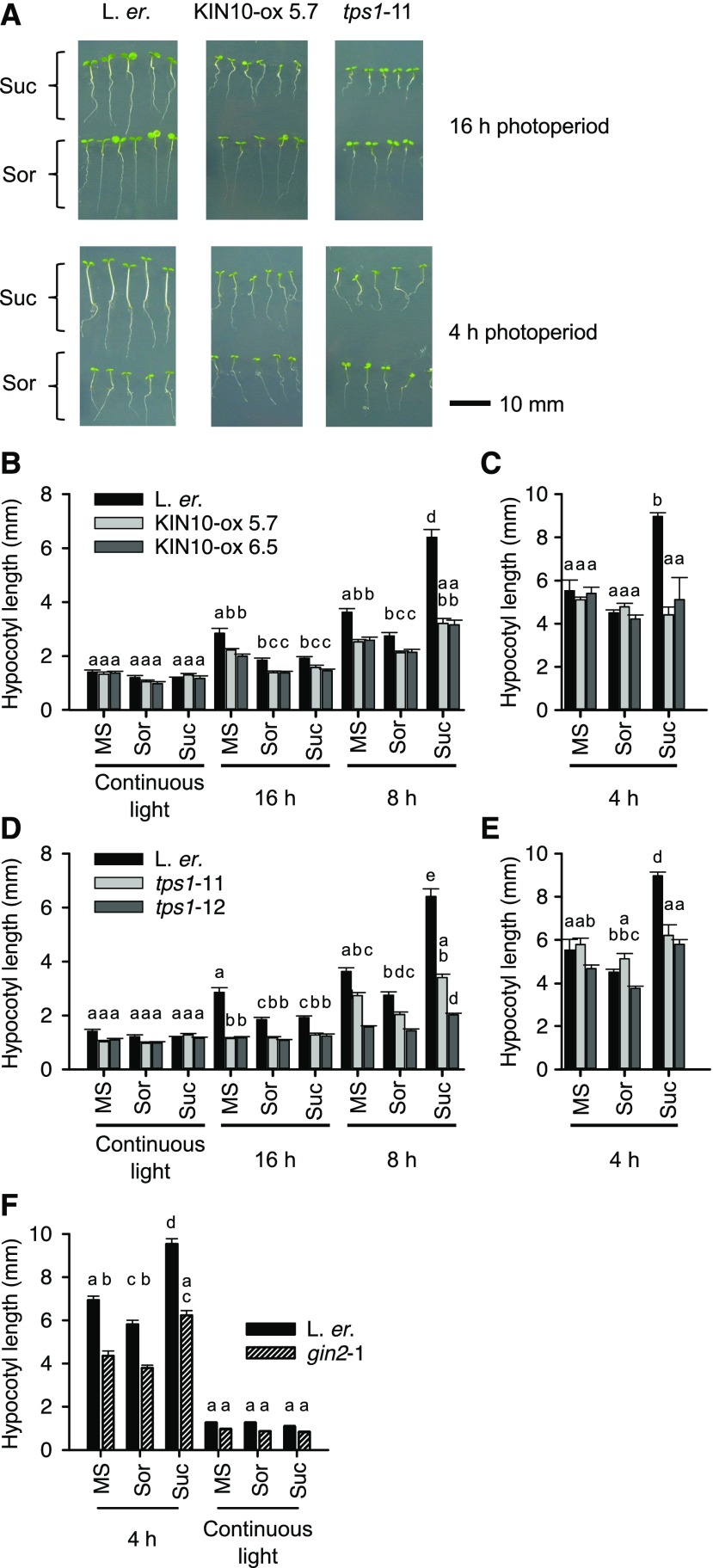
First, we investigated the effect of exogenous Suc upon hypocotyl elongation in a variety of photoperiods (Fig. 1). Under 4-h and 8-h photoperiods, Suc supplementation of wild-type seedlings caused a significant increase in hypocotyl length relative to the sorbitol control (2.1-fold and 2.3-fold relative to sorbitol controls, under 4-h and 8-h photoperiods, respectively; Fig. 1, A to E). In comparison, under 16-h photoperiods and constant light conditions, exogenous Suc did not promote hypocotyl elongation (Fig. 1, A to E).
Figure 1.
KIN10 and TPS1 participate in Suc-induced hypocotyl elongation. A, Representative images of L. er. wild-type, KIN10-ox, and tps1 seedlings cultivated under a variety of photoperiods, with and without supplementation with 3% (w/v) Suc. All panels scaled identically. Images are a subset of seedlings used to generate data in (B) to (E). B to E, Lengths of hypocotyls of seedlings grown under (B and D) constant light, 16-h and 8-h photoperiods and (C and E) 4-h photoperiods. Photoperiods are indicated underneath graphs. F, Effect of Suc supplementation upon gin2-1 hypocotyl length. SEM is small under continuous light (0.03 mm to 0.05 mm), so is not visible on graphs. Data were analyzed with ANOVA and Tukey’s posthoc tests (n = 10 (B to E) or n = 20 (F) seedlings in three independent experiments; mean ± se). Different letters indicate statistically significant differences between means, specifically within each light condition (P < 0.05). B to E, MS is half-strength MS media, and Suc and Sor are 0.5 MS supplemented with 3% (w/v) Suc or equimolar sorbitol (87.6 mm osmotic control), respectively.
Next, we investigated roles of KIN10 in Suc-induced hypocotyl elongation under light/dark cycles. Under 8-h photoperiods, the hypocotyls of two KIN10-ox lines (Baena-González et al., 2007) did not elongate significantly in response to exogenous Suc relative to the MS control (Fig. 1B). Both KIN10-ox lines elongated 1.5-fold in response to Suc relative to the sorbitol control (Fig. 1B). Exogenous Suc caused no significant increase in the hypocotyl length of KIN10-ox seedlings under 4-h photoperiods (Fig. 1C). Hypocotyls of the L. er. background and KIN10-ox appeared shorter when supplemented with exogenous Suc in constant light and 16-h photoperiods. However, this could be an osmotic effect rather than a Suc response because hypocotyl elongation responded identically to Suc and the sorbitol control (Fig. 1B).
Because KIN10 activity is thought to be regulated by Tre6P (Zhang et al., 2009), we investigated the role of the Tre6P biosynthetic enzyme TPS1 in Suc-induced hypocotyl elongation under light/dark cycles. In two tps1 TILLING mutants under 8-h photoperiods, Suc supplementation caused a significant 2.3-fold increase in hypocotyl length in the wild type relative to the sorbitol control, compared with 1.6-fold and 1.3-fold increases in hypocotyl length in tps1-11 and tps1-12, respectively (Fig. 1D). Under 4-h photoperiods, Suc caused a significant 2-fold increase in hypocotyl length of the wild type relative to the sorbitol control, compared with no significant increase in length in tps1-11 but a significant 1.5-fold increase in hypocotyl length in tps1-12 (Fig. 1E). Together, these experiments with KIN10 overexpressors and tps1 mutants indicate that TPS1 and KIN10 are involved in one or more mechanisms that increase hypocotyl length in response to exogenous Suc. This suggests that SnRK1-mediated energy signaling regulates hypocotyl elongation in response to Suc supplementation.
HEXOKINASE1 Is Not Required for Suc-Induced Hypocotyl Elongation under Light/Dark Cycles
Hexokinase is thought to function as a sugar sensor that regulates development in response to the concentration of Glc (Jang et al., 1997; Moore et al., 2003), so we investigated whether hexokinase-based signaling also contributes to Suc-induced hypocotyl elongation. For this, we measured the elongation of hypocotyls in response to exogenous Suc in the glucose insensitive2 (gin2-1) mutant of HEXOKINASE1. Overall, gin2-1 hypocotyls were slightly shorter than the wild type under all conditions tested (Fig. 1F). Exogenous Suc caused a significant increase in hypocotyl length of wild-type and gin2-1 seedlings, producing hypocotyls 63% and 67% longer than the osmotic control in the wild type and gin2-1, respectively (Fig. 1F). Therefore, Suc caused a similar magnitude of hypocotyl elongation in gin2-1 and the wild type. This suggests that interconversion of Suc to Glc, and therefore hexokinase-based Glc signaling, does not contribute to Suc-induced hypocotyl elongation in short photoperiods.
Relationship among Day-Length, Light Intensity, and Suc-Induced Hypocotyl Elongation
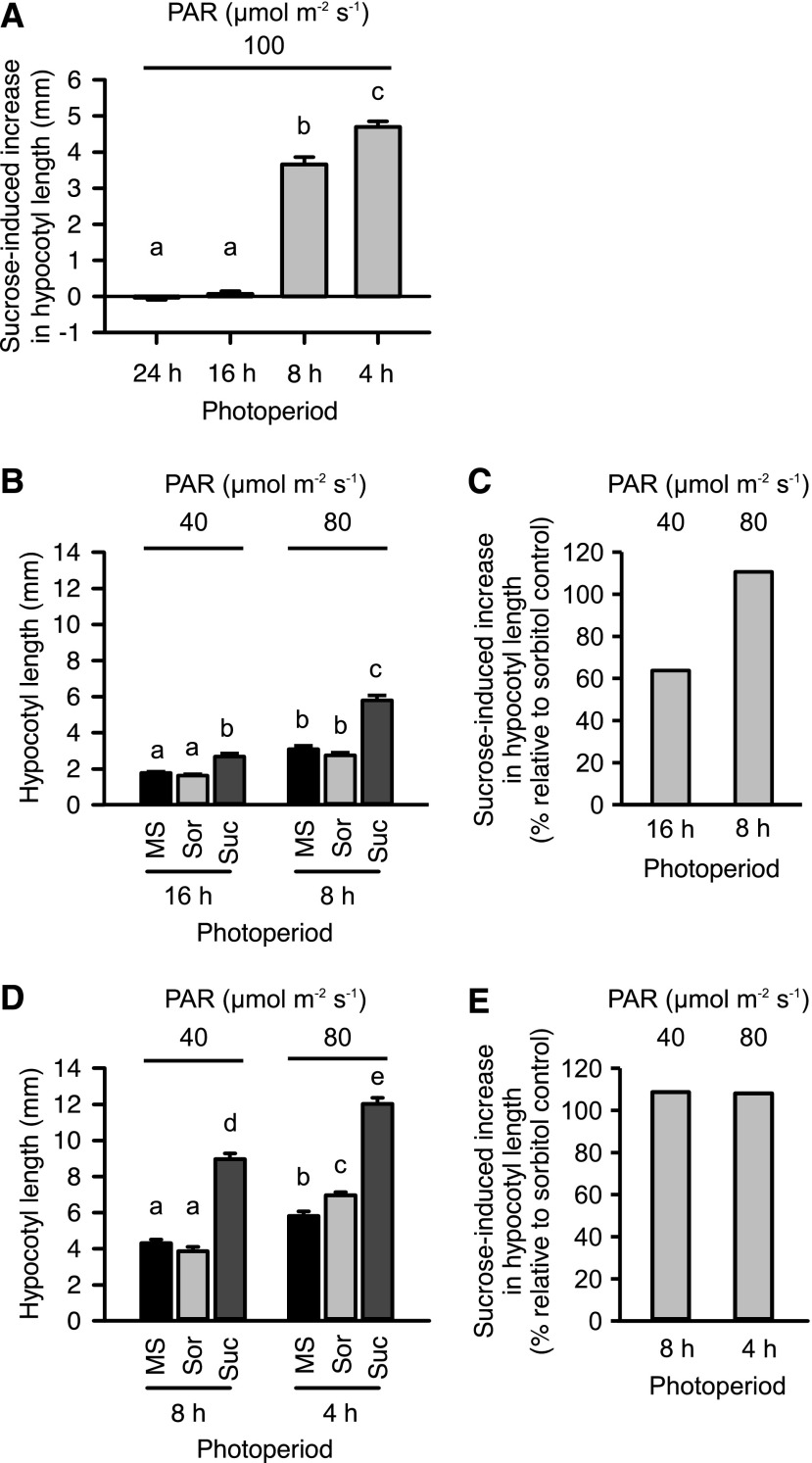
Our data suggest that the magnitude of the Suc-induced increase in hypocotyl length depends upon the photoperiod or the quantity of light received. In the wild type, Suc increased hypocotyl length under short (4 h or 8 h) but not long (16 h or constant light) photoperiods under photosynthetically active radiation (PAR) of 100 μmol m−2 s−1 (Figs. 1, B to E and 2A). In addition, Suc caused significantly greater hypocotyl elongation under 4-h photoperiods compared with 8-h photoperiods of 100 μmol m−2 s−1 (Fig. 2A). We reasoned that these varying responses to Suc might arise from differences in total daily PAR received under each of these conditions, or alternatively from the sensing of photoperiod length. To investigate this, we compared the magnitude of Suc-induced hypocotyl elongation under the same total daily integrated PAR, under longer photoperiods (16 h at 40 μmol m−2 s−1 and 8 h at 80 μmol m−2 s−1) and under shorter photoperiods (8 h at 40 μmol m−2 s−1 and 4 h at 80 μmol m−2 s−1). Under a 16-h photoperiod at 40 μmol m−2 s−1, Suc caused a significant increase in hypocotyl length (Fig. 2, B and C). This contrasts a 16-h photoperiod at 100 µmol m−2 s−1, where Suc did not promote hypocotyl elongation (Figs. 1 and 2A). This suggests that the quantity of light received influences the sensitivity of hypocotyl elongation to Suc. Under 8-h photoperiods, Suc caused greater hypocotyl elongation under 40 µmol m−2 s−1 (mean 4.1 mm increase) than under 80 μmol m−2 s−1 (mean 3.3 mm increase), which also suggests that hypocotyl elongation is more responsive to Suc under lower light conditions (Fig. 2, B and D). When daily integrated PAR was the same under 4-h and 8-h photoperiods, there was no difference in the increase in hypocotyl length caused by Suc (Fig. 2, D and E). These responses suggest that daily integrated PAR influences the magnitude of Suc-induced hypocotyl elongation. However, the magnitude of Suc-induced hypocotyl elongation was significantly less under 16-h photoperiods at 40 μmol m−2 s−1 than 8-h photoperiods at 80 μmol m−2 s−1 (Fig. 2, B and C), suggesting that under long photoperiods, the magnitude of Suc-induced hypocotyl elongation could be also determined by a photoperiod-response mechanism acting independently from daily integrated PAR. These data provide the insight that the photoperiod-sensitivity of Suc-induced hypocotyl elongation is determined by both the absolute photoperiod and the amount of light received.
Figure 2.
Day-length dependency of Suc-induced hypocotyl elongation in wild-type seedlings. A, Increase in hypocotyl length caused by Suc under range of photoperiods (data derived from Fig. 1, plotted relative to sorbitol control). B to E, Comparison of (B) and (D) absolute hypocotyl length and (C) and (E) proportional increase in hypocotyl length caused by Suc supplementation under specified PAR and photoperiod. Mean ± se (A, C to E), n = 10 seedlings in two independent experiments; (B) n = 20 seedlings. Data analyzed using ANOVA followed by posthoc Tukey test. Different letters indicate statistically significant differences between means (P < 0.05).
Interaction between Hypocotyl Elongation by Exogenous Suc and the Circadian Oscillator
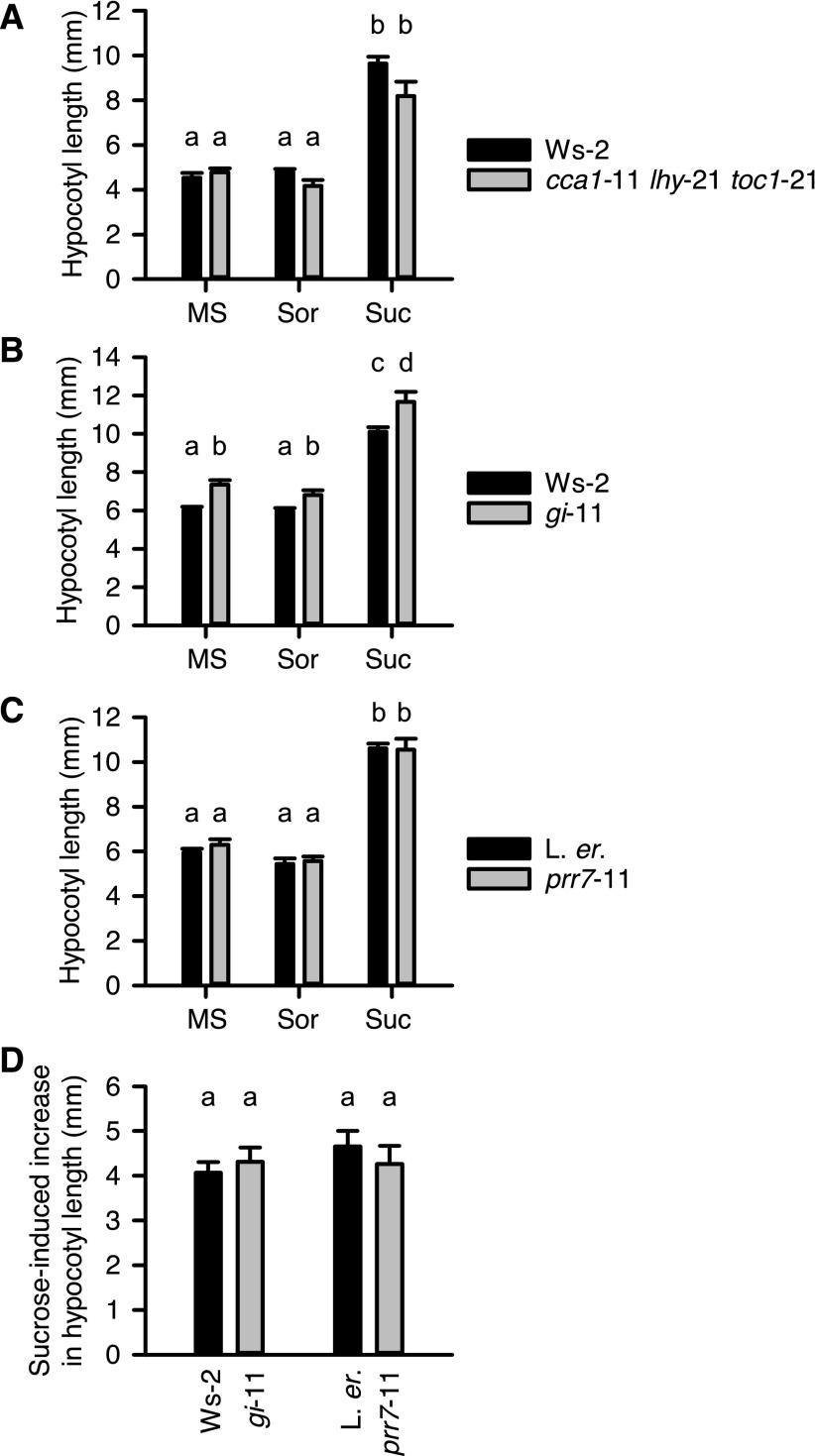
The circadian oscillator regulates hypocotyl elongation because the accumulation of PIF proteins is restricted to the end of the night (Nozue et al., 2007; Nusinow et al., 2011). Because the circadian oscillator responds to exogenous and endogenous sugars (Dalchau et al., 2011; Haydon et al., 2013) and KIN10 overexpression can lengthen circadian period (Shin et al., 2017), we investigated whether Suc-induced increases in hypocotyl length under short photoperiods involve the circadian oscillator. First, we tested whether the circadian oscillator components CIRCADIAN CLOCK ASSOCIATED1 (CCA1), LATE ELONGATED HYPOCOTYL (LHY), and TIMING OF CAB2 EXPRESSION1 (TOC1) are required for Suc-induced hypocotyl elongation using the cca1-11 lhy-21 toc1-21 triple mutant (Ding et al., 2007). cca1-11 lhy-21 toc1-21 causes circadian arrhythmia under constant light and temperature, and disrupts rhythms of oscillator transcripts, including evening complex components that regulate hypocotyl elongation (Ding et al., 2007). Under 4-h photoperiods, the magnitude of the Suc-induced increase in hypocotyl length was unaltered in cca1-11 lhy-21 toc1-21 (Fig. 3A; Supplemental Fig. S1). Under 40-h photoperiods, the hypocotyls of cca1-11 lhy-21 toc1-21 were of similar length to the wild type (Fig. 3A), whereas under 8-h photoperiods, cca1-11 lhy-21 toc1-21 has longer hypocotyls than the wild type (Ding et al., 2007).
Figure 3.
The circadian oscillator does not participate in Suc-induced hypocotyl elongation under short photoperiods. Suc-induced change in hypocotyl length of (A) a circadian oscillator triple mutant (cca1-11 lhy-21 toc1-21, background Ws-2) and (B and C) two oscillator components participating in Suc regulation of the circadian oscillator. D, Change in hypocotyl length caused by Suc supplementation in gi-11 and prr7-11, expressed relative to 0.5 MS control. MS is 0.5 MS media, and Suc and Sor are 0.5 MS supplemented with 3% (w/v) Suc and sorbitol (87.6 mM, osmotic control), respectively. Data are mean ± se (n = 10 − 16), analyzed with (A to C) ANOVA and posthoc Tukey tests and (D) two-sample t test comparing mutant with wild type for each treatment. Data show one of three independent repeats of the experiment, conducted under 4-h photoperiods. Different letters indicate statistically significant differences between means (P < 0.05).
We also investigated whether two proteins that confer sugar sensitivity to the circadian oscillator, GIGANTEA (GI) and PSEUDO-RESPONSE REGULATOR7 (PRR7; Dalchau et al., 2011; Haydon et al., 2013), contribute to Suc-induced hypocotyl elongation under short photoperiods. We tested this because the prr7-11 mutation renders the oscillator insensitive to sugar signals that entrain the oscillator (Haydon et al., 2013), and the gi-11 mutation alters oscillator responses to long-term exposure to exogenous Suc (Dalchau et al., 2011). In all cases, gi-11 had longer hypocotyls than the wild type (Fig. 3B), but the magnitude of the Suc-induced increase in hypocotyl length was unaltered in gi-11 relative to the wild type (Fig. 3D). Likewise, the prr7-11 mutant also did not alter the magnitude of Suc-induced increases in hypocotyl length (Fig. 3, C and D).
These experiments indicate that two mechanisms providing sugar inputs to the circadian oscillator (Dalchau et al., 2011; Haydon et al., 2013) and three core oscillator components do not contribute to Suc-induced increases in hypocotyl length under short photoperiods.
Phytohormone Signaling and Suc-Induced Hypocotyl Elongation under Light/Dark Cycles: Auxin
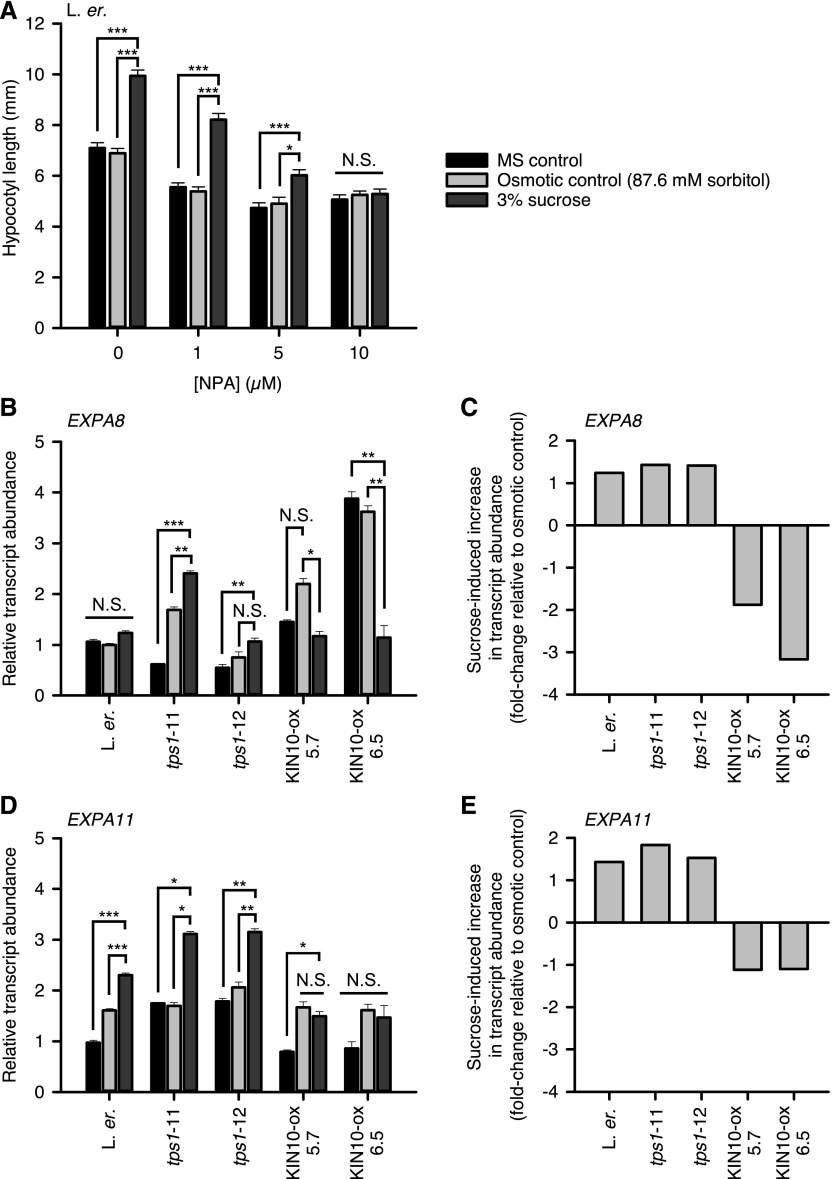
Suc-induced hypocotyl elongation in the light involves auxin and GA signaling (Zhang et al., 2010; Lilley et al., 2012). We investigated the involvement of phytohormones in Suc-induced hypocotyl elongation under light/dark cycles, and their relationship with SnRK1-mediated signaling. First, we examined the effect of the inhibitor of polar auxin transport 1-N-naphthylpthalamic acid (NPA) upon Suc-induced hypocotyl elongation. NPA inhibited Suc-induced hypocotyl elongation in a concentration-dependent manner, such that 10 μm NPA completely abolished Suc-induced elongation (Fig. 4A). Consistent with previous work (Lilley et al., 2012), this indicates that under light/dark cycles Suc-induced hypocotyl elongation is auxin-dependent. Next, we examined the responses of auxin- and PIF-dependent expansin transcripts to Suc. Expansins are a large family of cell wall modifying enzymes that allow turgor-driven cell expansion, and some expansin transcripts are up-regulated by auxins in a PIF-dependent manner during hypocotyl elongation (Li et al., 2002; Miyazaki et al., 2016; Gangappa and Kumar, 2017). We examined EXPANSIN A4 (EXPA4), EXPA8, and EXPA11 transcripts, which are auxin-induced in seedlings (Goda et al., 2004; Esmon et al., 2006; Winter et al., 2007; Lee et al., 2009). EXPA8 and EXPA11 transcripts were up-regulated by conditions of constant darkness, which also increases hypocotyl elongation (Supplemental Fig. S2A; Boylan and Quail, 1991), and down-regulated by 10 μM NPA, which suppresses hypocotyl elongation (Supplemental Fig. S2B; Lilley et al., 2012). EXPA4 was unaltered by these conditions (Supplemental Fig. S2). Therefore, EXPA8 and EXPA11 transcript abundance was increased by conditions that promote hypocotyl elongation, and reduced by conditions that suppress hypocotyl elongation. Next, we monitored the change in abundance of these two expansin transcripts in response to Suc under 4-h photoperiods. In the wild type, EXPA11 transcripts were up-regulated by 3% (w/v) Suc, whereas EXPA8 transcripts were not up-regulated by Suc relative to the controls (Fig. 4, B to E). In KIN10-ox, where Suc does not promote hypocotyl elongation under light/dark cycles, EXPA8 and EXPA11 transcripts were not increased by Suc (Fig. 4, B to E). EXPA8 was Suc-induced relative to the controls in tps1-11, but not in tps1-12 (Fig. 4, B and C). EXPA11 transcripts were Suc-induced in both tps1-11 and tps1-12 (Fig. 4, D and E). The induction of these two expansin transcripts by Suc in tps1 mutants was unexpected, because both KIN10-ox and tps mutants suppress Suc-induced hypocotyl elongation under short photoperiods (Fig. 1). We also examined several other transcripts associated with auxin biosynthesis or responses, but the osmotic controls caused substantial alterations in transcript abundance that prevented interpretation of their regulation by Suc (Supplemental Fig. S3).
Figure 4.
Auxin signaling underlies Suc-induced hypocotyl elongation and KIN10 regulates expansin gene expression. A, Hypocotyl length of seedlings cultivated with a range of concentrations of the inhibitor of polar auxin transport NPA, under 4-h photoperiods (mean ± se; n = 20). B to E, Suc-induced changes in expansin transcript abundance in elongating wild-type, tps1, and KIN10-ox seedlings under 4-h photoperiods. B and D, EXPA8 and EXPA11 transcript abundance relative to PP2AA3 (mean ± se; n = 3). C and E, The magnitude of Suc-induced change in transcript abundance in each genotype relative to the osmotic control. Data analyzed with ANOVA and posthoc Tukey tests, and with statistical significance indicated using starring (P > 0.05; * = P < = 0.05; ** = P < 0.01; *** = P < 0.001).
Phytohormone Signaling and Suc-Induced Hypocotyl Elongation under Light/Dark Cycles: Gibberellins
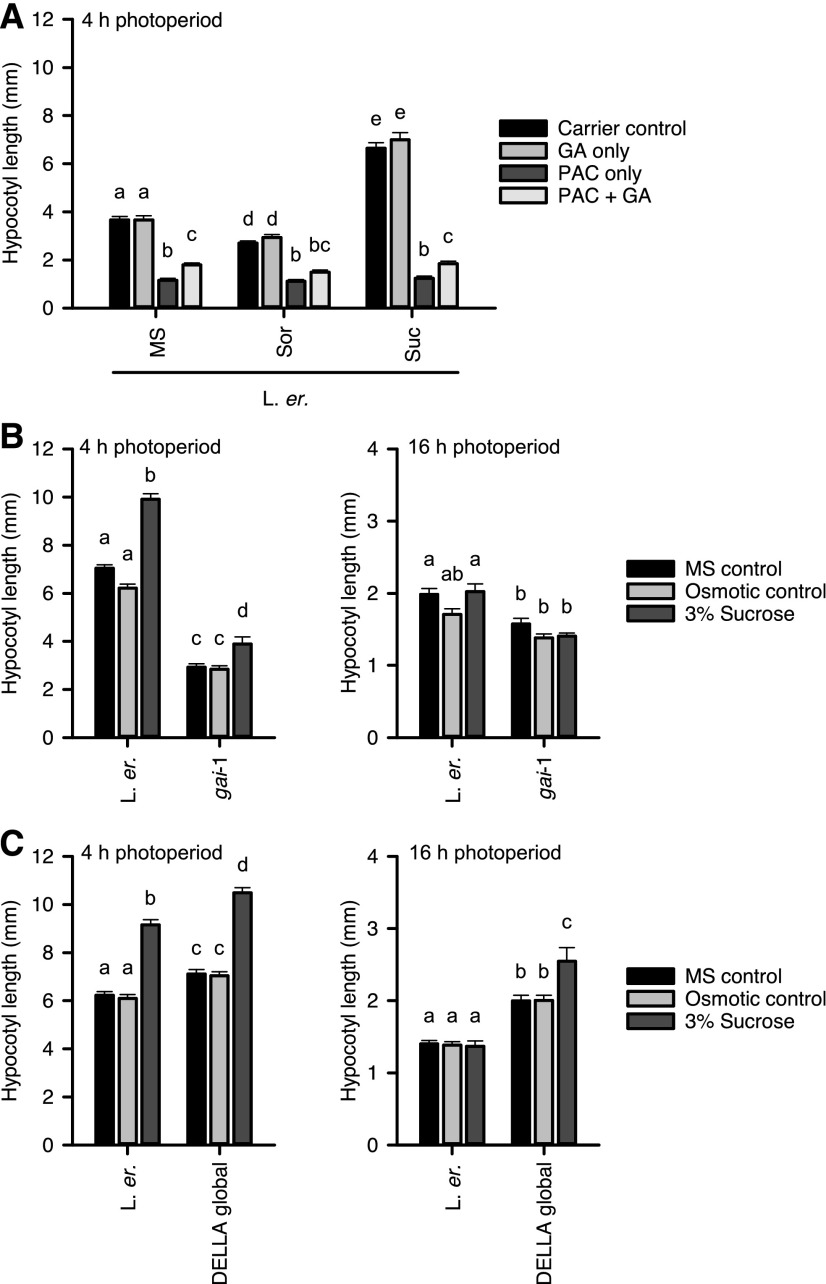
We tested whether GA signaling also contributes to Suc-induced hypocotyl elongation under short photoperiods. After germination, wild-type seedlings were transferred to media containing 3% (w/v) Suc or an osmotic control, supplemented with combinations of the GA biosynthesis inhibitor paclobutrazol (PAC), GA, or a carrier control. Consistent with previous studies, wild-type seedlings grown on media supplemented with PAC or PAC and GA had significantly shorter hypocotyls than controls (Fig. 5A; Cowling and Harberd, 1999; Liu et al., 2011). PAC abolished Suc-induced hypocotyl elongation, with a small hypocotyl length rescue occurring when GA was supplied in combination with PAC (Fig. 5A). We confirmed that the GA was active by demonstrating that, consistent with previous reports (Cowling and Harberd, 1999), hypocotyl length is increased by GA supplementation (Supplemental Fig. S4).
Figure 5.
Gibberellin signals contribute to Suc-induced hypocotyl elongation under short photoperiods. A, The GA biosynthesis inhibitor PAC at 20 μm inhibits Suc-induced hypocotyl elongation. Seedlings were germinated on MS agar and transferred to treatment media after germination; carrier control was 0.12% (v/v) methanol. B, Suc-induced hypocotyl elongation was attenuated in gai-1 mutant seedlings. C, Suc-induced hypocotyl elongation was unaltered in a DELLA global knockout mutant. Experiments performed under 4-h photoperiods. Data are mean ± se (n = 20) from one of two independent repeats, analyzed with ANOVA and posthoc Tukey tests. Different letters indicate statistically significant differences between means (P < 0.05). Osmotic control was 87.6 mm sorbitol.
GA increases growth by causing degradation of DELLA growth repressor proteins, and also through DELLA-independent mechanisms (Peng et al., 1997; Fu et al., 2002; Cheng et al., 2004; Cao et al., 2006). Therefore, we investigated the involvement of DELLA proteins in Suc-induced hypocotyl elongation under light/dark cycles. The gai-1 mutant harbors a deletion within the DELLA domain of GIBBERELLIC ACID INSENSITIVE (GAI), which prevents GA-induced proteasomal degradation of GAI (Peng et al., 1997; Fu et al., 2002). Under 4-h photoperiods, Suc supplementation increased hypocotyl length in gai-1, but the magnitude of Suc-induced elongation in gai-1 was reduced compared with the wild type (hypocotyls became 36.5% longer in gai-1 in response to Suc, compared with 59.2% longer in the wild type; Fig. 5B). Under 16-h photoperiods, Suc did not induce hypocotyl elongation in the wild type or gai-1 (Fig. 5B), which is consistent with Figure 1, B and C. We also examined the effect of a mutant lacking all five DELLA proteins upon Suc-induced hypocotyl elongation under light/dark cycles (Koini et al., 2009). Under short photoperiods, Suc-induced hypocotyl elongation was unaltered in this mutant (Fig. 5C). Interestingly, under long photoperiods Suc promoted hypocotyl elongation in the DELLA global mutant, whereas Suc was without effect upon wild-type hypocotyls (Fig. 5C). The partial attenuation of Suc-induced hypocotyl elongation in gai-1 (Fig. 5B) combined with the derepression of Suc-induced hypocotyl elongation under long photoperiods in the DELLA global mutant (Fig. 5C) suggests that DELLA-mediated GA signaling contributes to, but does not exclusively control, Suc-induced hypocotyl elongation.
Phytohormone Signaling and Suc-Induced Hypocotyl Elongation under Light/Dark Cycles: Abscisic Acid
ABA suppresses seedling development (Belin et al., 2009) and several studies have linked Tre6P and abscisic acid (ABA) signaling (Avonce et al., 2004; Ramon et al., 2007; Gómez et al., 2010; Debast et al., 2011). Therefore, we investigated whether ABA signaling contributes to Suc-induced hypocotyl elongation under light/dark cycles. Suc-induced hypocotyl elongation was unaffected by the ABA receptor quadruple mutant pyr1-1 pyl1-1 pyl2-1 pyl4-1, which is highly ABA-insensitive (Park et al., 2009; Supplemental Fig. S5). This suggests that PYR/PYL-mediated ABA signaling does not participate in the mechanisms underlying Suc-induced hypocotyl elongation under light/dark cycles.
DISCUSSION
KIN10 and TPS1 Contribute to Sugar-Induced Hypocotyl Elongation under Light/Dark Cycles
Here, we make the new finding that a mechanism involving KIN10 activity and Tre6P metabolism regulates Suc-induced hypocotyl elongation under light/dark cycles. Although hypocotyl elongation arises from cell expansion rather than growth through increases in cell number (Gendreau et al., 1997), our data are consistent with studies demonstrating that Tre6P metabolism is a crucial regulator of growth responses to Suc. For example, Arabidopsis seedlings overexpressing the bacterial Tre6P phosphatase otsB, which reduces [Tre6P], accumulate less biomass compared with the wild type when supplemented with Suc (Schluepmann et al., 2003). The converse is also true; otsA (TPS) overexpressors, in which [Tre6P] is increased, accumulate more biomass than the wild type when supplemented with Suc (Schluepmann et al., 2003). Therefore, our data using tps1 mutants as a proxy for altered Tre6P metabolism provide new evidence to support the notion that Tre6P promotes growth under conditions of increased Suc availability (Schluepmann et al., 2003; Zhang et al., 2009).
Overexpression in Arabidopsis of the bacterial Tre6P synthase otsA has been reported to produce seedlings having shorter hypocotyls than the wild type (Paul et al., 2010). The Suc-insensitivity of hypocotyl elongation in tps1 mutants (Fig. 1) and the shorter hypocotyls in seedlings with increased [Tre6P] (otsA-ox) may appear to conflict with each other (Paul et al., 2010). However, the experiments are not directly comparable. We found that exogenous Suc only caused hypocotyl elongation under short photoperiods or lower light conditions (Fig. 2). In comparison, the otsA-ox experiments involved 16-h photoperiods at higher PAR (150 μmol m−2 s−1) and shaking liquid culture (Zhang et al., 2009), both of which could mask the hypocotyl elongation response that we investigated.
Our experiments suggest that increased KIN10 activity might attenuate the elongation response of hypocotyls to exogenous Suc under light/dark cycles. The KIN10-ox lines that we used overexpress the catalytic subunit of SnRK1 (Baena-González et al., 2007). KIN10 overexpression down-regulates transcripts associated with anabolic processes and up-regulates transcripts associated with energy starvation (Baena-González et al., 2007). Therefore, in our experiments KIN10 overexpression may have stopped seedlings from taking advantage of the greater energy availability caused by Suc supplementation, thereby preventing Suc-induced hypocotyl elongation in KIN10-ox (Fig. 1).
Photoperiod-Dependency of Sugar-Induced Hypocotyl Elongation
We made the new finding that under relatively high light, exogenous Suc increases hypocotyl length in photoperiods of 8 h and shorter, but not under long photoperiods or constant light (Figs. 1 and 2). These data reconcile differences between previous studies of Suc-induced hypocotyl elongation. Previous studies reporting Suc-insensitivity of hypocotyl elongation in the light were conducted in continuous light (Zhang et al., 2010), in which we also found Suc to be without effect upon hypocotyls (Figs. 1B and 2A). In comparison, studies reporting that Suc does promotes hypocotyl elongation in the light were conducted under 8-h photoperiods (Stewart et al., 2011; Lilley et al., 2012), where we likewise found that Suc causes hypocotyl elongation (Figs. 1B and 2). Therefore, the sensitivity of hypocotyls to Suc-induced elongation depends upon the photoperiod or the amount of light received each day.
One explanation for this response could be that the daily quantity of light determines the magnitude of Suc-induced hypocotyl elongation through the accumulation of photosynthetic metabolites. Our experiments indicate that under shorter photoperiods, the sensitivity of hypocotyl elongation to Suc depends upon the total amount of daily light (Fig. 2, A, D, and E). Furthermore, Suc-induced hypocotyl elongation under long photoperiods only occurred when the seedlings were under lower light conditions (Fig. 2, A to C). One interpretation is that under long photoperiods and higher light, cells are replete with sugars (Sulpice et al., 2014), therefore supplementation with exogenous Suc has a relatively small effect upon the hypocotyl length of already sugar-rich seedlings. In contrast, under short photoperiods or lower light, the background level of endogenous sugar is lower (Sulpice et al., 2014), so supplementation with exogenous Suc has a greater effect upon hypocotyl length.
An alternative interpretation is that PIFs integrate light signals derived from photoreceptors with SnRK1-mediated sugar signals to modulate the sensitivity of elongating hypocotyls to Suc, because PIFs are required for Suc-induced hypocotyl elongation (Stewart et al., 2011; Lilley et al., 2012). This might explain the PAR-independent reduction in Suc-induced hypocotyl elongation that occurred under long photoperiods (Fig. 2C). In the future, it will be informative to resolve the relative contributions of these mechanisms to Suc-induced hypocotyl elongation, given that Tre6P can regulate expression of both PIFs and auxin signaling genes (Paul et al., 2010). This could provide insights into the nature of the coupling of SnRK1-mediated sugar signaling and growth regulation by PIFs (Paul et al., 2010; Stewart et al., 2011; Lilley et al., 2012).
Involvement of Phytohormone Signals in Suc-Induced Hypocotyl Elongation under Light/Dark Cycles
Auxin, GA, and brassinosteroids are reported to mediate Suc-induced hypocotyl elongation, with a role for auxin identified under light/dark cycles and roles for GA and brassinosteroids identified under extended darkness (de Lucas et al., 2008; Zhang et al., 2010, 2015, 2016; Liu et al., 2011; Stewart et al., 2011; Lilley et al., 2012;). Consistent with this, our data indicate that auxin signaling has a major role in Suc-induced hypocotyl elongation under light/dark cycles (Fig. 4A), with GA signaling also contributing to this process (Fig. 5, B and C). We suggest two possible reasons why paclobutrazol completely abolished Suc-induced hypocotyl elongation (Fig. 5A), whereas the gai-1 mutant only led to partial inhibition of this phenotype (Fig. 5B). One possibility is that DELLA-independent GA signaling contributes to Suc-induced hypocotyl elongation, because DELLA proteins control approximately 40% to 60% of GA-regulated transcripts (Cao et al., 2006). An alternative possibility is that these were off-target or ectopic effects of paclobutrazol, because the paclobutrazol-induced attenuation of hypocotyl elongation was not rescued fully by GA supplementation (Fig. 5A).
Auxin-induced expansins that are up-regulated during hypocotyl elongation were also induced by Suc supplementation (Fig. 4, B to E; Supplemental Fig. S2). Although EXPA11 was induced strongly by Suc, the small response of EXPA8 to Suc in the wild type makes it difficult to interpret the responses of EXPA8 to Suc in KIN10-ox and the tps1 mutants (Fig. 4, B and C). Interestingly, Suc induction of EXPA11 was abolished in KIN10-ox, suggesting a role for KIN10 in expansin gene expression within elongating hypocotyls. In comparison, these expansins were Suc-inducible in tps1-11 and tps1-12 (Fig. 4, B to E). One possible explanation is that KIN10-ox causes a much greater level of SnRK1 activity compared with the tps mutants, which are hypomorphic alleles that harbor reduced Tre6P concentrations (Gómez et al., 2010) and are not completely deficient in Suc-induced hypocotyl elongation (Fig. 1, D and E).
An alternative and speculative explanation for the different behavior of expansin transcripts in KIN10-ox and tps mutants could relate to Tre6P-KIN10 regulating growth through two broad processes—first, though direct signaling effects upon growth (e.g. by regulating auxin signals), and second, through metabolic effects, such as growth constraints due to altered nocturnal catabolism. This could point to TPS1 and SnRK1 making independent contributions to Suc-induced hypocotyl elongation under light/dark cycles, potentially through separate signaling and metabolic effects, rather than acting in series. Our data suggest that Suc-induced hypocotyl elongation under light/dark cycles includes a signaling effect, previously proposed to occur through PIF-regulated auxin signals (Stewart et al., 2011; Lilley et al., 2012). On the other hand, the unexpected behavior of expansin transcripts in tps1 mutants (Fig. 1, D and E) suggests that mechanisms additional to auxin/GA signaling might contribute to Suc-induced hypocotyl elongation under light/dark cycles. These additional mechanisms could involve brassinosteroid and/or TOR signaling, which are required for Suc-induced increases in hypocotyl length under extended darkness (Zhang et al., 2015, 2016). It would be informative in future to investigate the cross talk between SnRK1 and TOR energy signaling during hypocotyl elongation, to gain insights into the relative importance of these energy management pathways to the below-ground (darkness) and above-ground (light/dark cycles) stages of seedling establishment.
CONCLUSIONS
We identified a novel role for the SnRK1 energy signaling hub in the regulation of Suc-induced hypocotyl elongation under light/dark cycles. We propose that KIN10 could be positioned upstream from the auxin and GA signals that lead to Suc-induced hypocotyl elongation in the light (Liu et al., 2011; Stewart et al., 2011; Lilley et al., 2012). A question for future investigation concerns the functional organization of this pathway. In one scenario, KIN10-mediated energy signaling regulates hypocotyl elongation by acting upon phytohormone signaling, potentially through PIFs (Lilley et al., 2012). In a different and nonexclusive scenario, SnRK1-mediated alterations in metabolic enzyme activity and growth-related transcripts prime the hypocotyls to capitalize upon increased Suc availability (Nunes et al., 2013a). This is an interesting question in the case of hypocotyl elongation, which arises from cell expansion rather than growth through cell division and biomass accumulation per se (Gendreau et al., 1997). These two possibilities are nonexclusive, because the phenotypic differences that we report between KIN10-ox lines and tps1 mutants (e.g. expansin transcript accumulation; Fig. 4) could implicate more than one mechanism in Suc-induced hypocotyl elongation.
A further question for future investigation is of the nature of the interplay among KIN10/Tre6P, TOR, and brassinosteroids in the regulation of hypocotyl elongation in response to sugars. One speculative hypothesis is that under conditions of starvation, such as when a developing below-ground seedling is exhausting its seed-based energy store, brassinosteroid signaling produces a strong elongation cue to drive seedling emergence into the light (Zhang et al., 2015, 2016). Then, once the seedling has emerged into the daily cycles of light and dark, KIN10/Tre6P adjusts the elongation of hypocotyls to allow optimal seedling establishment under local light conditions (Figs. 1 and 2). It is possible that increased SnRK1 activity under conditions of transiently low light, for example due to unpredictable changes in the weather, operates alongside phototransduction pathways to prevent inappropriate etiolation after seedling emergence. Therefore, one potential function of the mechanism that we identified might be to adapt the rate of seedling development to optimize the use of seed and photosynthetic resources under fluctuating light environments.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana (L.) Heynh.) seeds were surface-sterilized and sown on half-strength Murashige & Skoog (MS) basal salt mixture (0.5 MS; Duchefa) with 0.8% (w/v) agar (Noordally et al., 2013). Seeds were then stratified (3 d at 4°C) and germinated and grown for 7 d under 100 µmol m−2 s−1 of white light at 19°C, except Figure 2, B to E, where PAR was reduced. Media was supplemented with either 3% (w/v) Suc (87.6 mM) or 87.6 mm sorbitol as an osmotic control, according to the experiment. For experiments investigating gibberellin signaling, media was supplemented with 20 μm paclobutrazol (PAC) and 100 μm gibberellic acid (GA3 form, both Sigma-Aldrich) with a methanol carrier. Paclobutrazol is effective for studies of GA signaling during development at the concentration of 20 μm (Penfield et al., 2004; MacGregor et al., 2015). For experiments investigating auxin signaling, media was supplemented with N-1-naphthylphthalamic acid (NPA) (Sigma-Aldrich) at up to 10 μm with a DMSO carrier. Controls were supplemented with the appropriate carrier at the same concentration as treatment media (0.1% (v/v) DMSO for NPA; 0.12% (v/v) methanol for PAC and GA).
To transfer growing seedlings to media containing GA or PAC, surface-sterilized and stratified seeds were pipetted onto 1-μm-pore-diameter nylon mesh (Normesh), on top of 0.5 MS 0.8% (w/v) agar, and allowed to germinate for 3 d. Seedlings were then transferred to 0.5 MS supplemented with either 3% (w/v) Suc (87.6 mM) or 87.6 mm sorbitol, plus 20 μm PAC, 100 μm GA, or both PAC and GA. Hypocotyls were measured after 5-d growth on treatment plates. For experiments with circadian oscillator mutants, we did not use arrhythmic CCA1-ox plants because overexpression of CCA1 causes very long hypocotyls (Wang and Tobin, 1998), which would confound investigation of the role of sugars in hypocotyl elongation.
Genotypes used were tps1 TILLING mutants (Gómez et al., 2010), KIN10-ox (Baena-González et al., 2007), gin2-1 (Moore et al., 2003), gai-1 (Koorneef et al., 1985), DELLA global mutant (Koini et al., 2009), pyr1 pyl1 pyl2 pyl4 (Park et al., 2009), cca1-11 lhy-21 toc1-21 (Ding et al., 2007), gi-11 (Richardson et al., 1998), and prr7-11 (Yamamoto et al., 2003; Nakamichi et al., 2005). In the KIN10-ox lines, KIN10 transcript abundance was 17-fold greater than the wild type in elongating hypocotyls (Supplemental Fig. S6A). In the tps1-11 and tps1-12 alleles, TPS1 transcript abundance was unchanged (tps1-11) or slightly increased (tps1-12) compared with the wild type (Supplemental Fig. S6B). This result for the tps1 alleles was unsurprising, because these are mis-sense mutants rather than insertion mutants (Gómez et al., 2010).
Hypocotyl Measurement
Seedlings were grown on square petri dishes within temperature-controlled growth chambers (MLR-352; Panasonic). Plates were angled at approximately 45 degrees to allow hypocotyls to elongate without touching lids. Hypocotyls were measured by positioning 7-d-old seedlings on the surface of 1% (w/v) agar for photography (D50; Nikon) and subsequent measurement using the ImageJ software (https://imagej.nih.gov/ij/).
RNA Extraction and qRT-PCR
RNA was extracted according to Noordally et al. (2013), using the Machery-Nagel Nucleospin II plant RNA extraction kit incorporating DNase I treatment (Thermo Fisher Scientific), except approximately 60 seedlings were used per RNA sample. cDNA was synthesized using the High Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Applied Biosystems), according to manufacturer’s instructions. cDNA was analyzed using an MXPro 3005 real time PCR system (Agilent) with Brilliant III Ultra-Fast SYBR qPCR mastermix (Agilent; primers are given in Supplemental Table S1). At least two technical repeats were performed for each qRT-PCR reaction. Data were analyzed using the ΔΔCt method, with PROTEIN PHOSPHATASE 2A SUBUNIT A3 (PP2AA3) as a reference transcript.
Accession Numbers
Arabidopsis Genome Initiative identifiers for the genes mentioned in this study are: KIN10 (At3g01090), TPS1 (At1g78580), HEXOKINASE1 (At4g29130), CCA1 (At2g46830), LHY (At1g01060), TOC1 (At5g61380), GI (At1g22770), PRR7 (At5g02810), EXPA4 (At2g39700), EXPA8 (At2g40610), EXPA11 (At1g20190), YUCCA8 (At4g28720), YUCCA9 (At1g04180), CYP79B3 (At2g22330), IAA29 (At4g32280), and SAUR15 (At4g38850).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The cca1-11 lhy-21 toc1-21 triple mutant does not alter Suc-induced hypocotyl elongation (direct repeat of Fig. 3A).
Supplemental Figure S2. Selection of expansin transcripts for experimentation.
Supplemental Figure S3. Suc supplementation of growth media did not alter abundance of auxin biosynthesis transcripts or auxin-responsive transcripts relative to osmotic controls.
Supplemental Figure S4. Efficacy of GA3 used for study.
Supplemental Figure S5. ABA signaling is not required for Suc-induced hypocotyl elongation under short photoperiods.
Supplemental Figure S6. KIN10 and TPS1 transcript abundance in KIN10-ox and tps1 TILLING mutants.
Supplemental Table S1. qRT-PCR primer sequences.
Acknowledgments
We thank Ian Graham (York), Filip Rolland (KU Leuven), Kerry Franklin (Bristol), Jean-Charles Isner (Bristol), Nicholas Harberd (Oxford), and Alex Webb (Cambridge) for seed donation, and Ian Graham for the discussion and encouragement that led to this work. We thank Kerry Franklin and Brendan Davies (Leeds) for critical feedback.
Footnotes
This research was funded by the Biotechnology and Biological Sciences Research Council (BBSRC grant BB/I005811/2; South-West Doctoral Training Partnership BB/J014400/1), the Lady Emily Smyth Agricultural Research Station (Bristol), the Wolfson Foundation and The Royal Society. A.N.D. is grateful to Kyoto University for awarding a Guest Professorship (Joint Usage/Research Program of the Center for Ecological Research, Kyoto University) and to The Royal Society for awarding a University Research Fellowship.
[CC-BY]: Article free via Creative Commons CC-BY 4.0 license.
These authors contributed equally to this article.
References
- Avonce N, Leyman B, Mascorro-Gallardo JO, van Dijck P, Thevelein JM, Iturriaga G (2004) The Arabidopsis trehalose-6-P synthase AtTPS1 gene is a regulator of glucose, abscisic acid, and stress signaling. Plant Physiol 136: 3649–3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-González E, Rolland F, Thevelein JM, Sheen J (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942 [DOI] [PubMed] [Google Scholar]
- Baena-González E, Sheen J (2008) Convergent energy and stress signaling. Trends Plant Sci 13: 474–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin C, Megies C, Hauserová E, Lopez-Molina L (2009) Abscisic acid represses growth of the Arabidopsis embryonic axis after germination by enhancing auxin signaling. Plant Cell 21: 2253–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan MT, Quail PH (1991) Phytochrome a overexpression inhibits hypocotyl elongation in transgenic Arabidopsis. Proc Natl Acad Sci USA 88: 10806–10810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Cheng H, Wu W, Soo HM, Peng J (2006) Gibberellin mobilizes distinct DELLA-dependent transcriptomes to regulate seed germination and floral development in Arabidopsis. Plant Physiol 142: 509–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ. (2013) Photoreceptor signaling networks in plant responses to shade. Annu Rev Plant Biol 64: 403–427 [DOI] [PubMed] [Google Scholar]
- Cheng H, Qin L, Lee S, Fu X, Richards DE, Cao D, Luo D, Harberd NP, Peng J (2004) Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 131: 1055–1064 [DOI] [PubMed] [Google Scholar]
- Collett CE, Harberd NP, Leyser O (2000) Hormonal interactions in the control of Arabidopsis hypocotyl elongation. Plant Physiol 124: 553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling RJ, Harberd NP (1999) Gibberellins control Arabidopsis hypocotyl growth via regulation of cellular elongation. J Exp Bot 50: 1351–1357 [Google Scholar]
- Crawford AJ, McLachlan DH, Hetherington AM, Franklin KA (2012) High temperature exposure increases plant cooling capacity. Curr Biol 22: R396–R397 [DOI] [PubMed] [Google Scholar]
- Dalchau N, Baek SJ, Briggs HM, Robertson FC, Dodd AN, Gardner MJ, Stancombe MA, Haydon MJ, Stan G-B, Gonçalves JM, Webb AAR (2011) The circadian oscillator gene GIGANTEA mediates a long-term response of the Arabidopsis thaliana circadian clock to sucrose. Proc Natl Acad Sci USA 108: 5104–5109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debast S, Nunes-Nesi A, Hajirezaei MR, Hofmann J, Sonnewald U, Fernie AR, Börnke F (2011) Altering trehalose-6-phosphate content in transgenic potato tubers affects tuber growth and alters responsiveness to hormones during sprouting. Plant Physiol 156: 1754–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte TL, Sedijani P, Kondou Y, Matsui M, de Jong GJ, Somsen GW, Wiese-Klinkenberg A, Primavesi LF, Paul MJ, Schluepmann H (2011) Growth arrest by trehalose-6-phosphate: an astonishing case of primary metabolite control over growth by way of the SnRK1 signaling pathway. Plant Physiol 157: 160–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorge I, Figueroa CM, Feil R, Lunn JE, van Dijck P (2015) Trehalose-6-phosphate synthase 1 is not the only active TPS in Arabidopsis thaliana. Biochem J 466: 283–290 [DOI] [PubMed] [Google Scholar]
- de Lucas M, Davière JM, Rodríguez-Falcón M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blázquez MA, Titarenko E, Prat S (2008) A molecular framework for light and gibberellin control of cell elongation. Nature 451: 480–484 [DOI] [PubMed] [Google Scholar]
- Ding Z, Doyle MR, Amasino RM, Davis SJ (2007) A complex genetic interaction between Arabidopsis thaliana TOC1 and CCA1/LHY in driving the circadian clock and in output regulation. Genetics 176: 1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrenel T, Marchive C, Sormani R, Moreau M, Mozzo M, Montané M-H, Menand B, Robaglia C, Meyer C (2011) Regulation of plant growth and metabolism by the TOR kinase. Biochem Soc Trans 39: 477–481 [DOI] [PubMed] [Google Scholar]
- Dowson-Day MJ, Millar AJ (1999) Circadian dysfunction causes aberrant hypocotyl elongation patterns in Arabidopsis. Plant J 17: 63–71 [DOI] [PubMed] [Google Scholar]
- Esmon CA, Tinsley AG, Ljung K, Sandberg G, Hearne LB, Liscum E (2006) A gradient of auxin and auxin-dependent transcription precedes tropic growth responses. Proc Natl Acad Sci USA 103: 236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Richards DE, Ait-Ali T, Hynes LW, Ougham H, Peng J, Harberd NP (2002) Gibberellin-mediated proteasome-dependent degradation of the barley DELLA protein SLN1 repressor. Plant Cell 14: 3191–3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangappa SN, Kumar SV (2017) DET1 and HY5 control PIF4-mediated thermosensory elongation growth through distinct mechanisms. Cell Reports 18: 344–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Höfte H (1997) Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol 114: 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda H, Sawa S, Asami T, Fujioka S, Shimada Y, Yoshida S (2004) Comprehensive comparison of auxin-regulated and brassinosteroid-regulated genes in Arabidopsis. Plant Physiol 134: 1555–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez LD, Baud S, Gilday A, Li Y, Graham IA (2006) Delayed embryo development in the ARABIDOPSIS TREHALOSE-6-PHOSPHATE SYNTHASE 1 mutant is associated with altered cell wall structure, decreased cell division and starch accumulation. Plant J 46: 69–84 [DOI] [PubMed] [Google Scholar]
- Gómez LD, Gilday A, Feil R, Lunn JE, Graham IA (2010) AtTPS1-mediated trehalose 6-phosphate synthesis is essential for embryogenic and vegetative growth and responsiveness to ABA in germinating seeds and stomatal guard cells. Plant J 64: 1–13 [DOI] [PubMed] [Google Scholar]
- Graf A, Schlereth A, Stitt M, Smith AM (2010) Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc Natl Acad Sci USA 107: 9458–9463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths CA, Sagar R, Geng Y, Primavesi LF, Patel MK, Passarelli MK, Gilmore IS, Steven RT, Bunch J, Paul MJ, Davis BG (2016) Chemical intervention in plant sugar signalling increases yield and resilience. Nature 540: 574–578 [DOI] [PubMed] [Google Scholar]
- Haydon MJ, Mielczarek O, Robertson FC, Hubbard KE, Webb AAR (2013) Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature 502: 689–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S, Velanis CN, Jenkins GI, Franklin KA (2014) UV-B detected by the UVR8 photoreceptor antagonizes auxin signaling and plant shade avoidance. Proc Natl Acad Sci USA 111: 11894–11899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JC, León P, Zhou L, Sheen J (1997) Hexokinase as a sugar sensor in higher plants. Plant Cell 9: 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuskamp DH, Pollmann S, Voesenek LACJ, Peeters AJM, Pierik R (2010) Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc Natl Acad Sci USA 107: 22740–22744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BC, Tennessen DJ, Last RL (1998) UV-B-induced photomorphogenesis in Arabidopsis thaliana. Plant J 15: 667–674 [DOI] [PubMed] [Google Scholar]
- Koini MA, Alvey L, Allen T, Tilley CA, Harberd NP, Whitelam GC, Franklin KA (2009) High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr Biol 19: 408–413 [DOI] [PubMed] [Google Scholar]
- Koorneef M, Elgersma A, Hanhart CJ, van Loenen-Martinet EP, van Rijn L, Zeevaart JAD (1985) A gibberellin insensitive mutant of Arabidopsis thaliana. Physiol Plant 65: 33–39 [Google Scholar]
- Kurata T, Yamamoto KT (1998) petit1, a conditional growth mutant of Arabidopsis defective in sucrose-dependent elongation growth. Plant Physiol 118: 793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Park JW, Lee HW, Kim J (2009) Genome-wide analysis of the auxin-responsive transcriptome downstream of iaa1 and its expression analysis reveal the diversity and complexity of auxin-regulated gene expression. J Exp Bot 60: 3935–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Quail PH (2011) PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci 16: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Darley CP, Ongaro V, Fleming A, Schipper O, Baldauf SL, McQueen-Mason SJ (2002) Plant expansins are a complex multigene family with an ancient evolutionary origin. Plant Physiol 128: 854–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, van den Ende W, Rolland F (2014) Sucrose induction of anthocyanin biosynthesis is mediated by DELLA. Mol Plant 7: 570–572 [DOI] [PubMed] [Google Scholar]
- Lilley JL, Gee CW, Sairanen I, Ljung K, Nemhauser JL (2012) An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiol 160: 2261–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M (1990) Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2: 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Hangarter RP (1991) Arabidopsis mutants lacking blue light-dependent inhibition of hypocotyl elongation. Plant Cell 3: 685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhang Y, Liu R, Hao H, Wang Z, Bi Y (2011) Phytochrome interacting factors (PIFs) are essential regulators for sucrose-induced hypocotyl elongation in Arabidopsis. J Plant Physiol 168: 1771–1779 [DOI] [PubMed] [Google Scholar]
- Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C (2008) Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JHM, Gibon Y, Morcuende R, Osuna D, Scheible WR, Carillo P, Hajirezaei MR, Stitt M (2006) Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor DR, Kendall SL, Florance H, Fedi F, Moore K, Paszkiewicz K, Smirnoff N, Penfield S (2015) Seed production temperature regulation of primary dormancy occurs through control of seed coat phenylpropanoid metabolism. New Phytol 205: 642–652 [DOI] [PubMed] [Google Scholar]
- Mair A, Pedrotti L, Wurzinger B, Anrather D, Simeunovic A, Weiste C, Valerio C, Dietrich K, Kirchler T, Nägele T, Vicente Carbajosa J, Hanson J, et al. (2015) SnRK1-triggered switch of bZIP63 dimerization mediates the low-energy response in plants. eLife 4: 4 10.7554/eLife.05828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Más P, Alabadí D, Yanovsky MJ, Oyama T, Kay SA (2003) Dual role of TOC1 in the control of circadian and photomorphogenic responses in Arabidopsis. Plant Cell 15: 223–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matiolli CC, Tomaz JP, Duarte GT, Prado FM, Del Bem LEV, Silveira AB, Gauer L, Corrêa LGG, Drumond RD, Viana AJC, Di Mascio P, Meyer C, et al. (2011) The Arabidopsis bZIP gene AtbZIP63 is a sensitive integrator of transient abscisic acid and glucose signals. Plant Physiol 157: 692–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Jikumaru Y, Takase T, Saitoh A, Sugitani A, Kamiya Y, Kiyosue T (2016) Enhancement of hypocotyl elongation by LOV KELCH PROTEIN2 production is mediated by auxin and phytochrome-interacting factors in Arabidopsis thaliana. Plant Cell Rep 35: 455–467 [DOI] [PubMed] [Google Scholar]
- Mizuno T, Nomoto Y, Oka H, Kitayama M, Takeuchi A, Tsubouchi M, Yamashino T (2014) Ambient temperature signal feeds into the circadian clock transcriptional circuitry through the EC night-time repressor in Arabidopsis thaliana. Plant Cell Physiol 55: 958–976 [DOI] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng W-H, Liu Y-X, Hwang I, Jones T, Sheen J (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300: 332–336 [DOI] [PubMed] [Google Scholar]
- Nakamichi N, Kita M, Ito S, Yamashino T, Mizuno T (2005) PSEUDO-RESPONSE REGULATORS, PRR9, PRR7 and PRR5, together play essential roles close to the circadian clock of Arabidopsis thaliana. Plant Cell Physiol 46: 686–698 [DOI] [PubMed] [Google Scholar]
- Noordally ZB, Ishii K, Atkins KA, Wetherill SJ, Kusakina J, Walton EJ, Kato M, Azuma M, Tanaka K, Hanaoka M, Dodd AN (2013) Circadian control of chloroplast transcription by a nuclear-encoded timing signal. Science 339: 1316–1319 [DOI] [PubMed] [Google Scholar]
- Nozue K, Covington MF, Duek PD, Lorrain S, Fankhauser C, Harmer SL, Maloof JN (2007) Rhythmic growth explained by coincidence between internal and external cues. Nature 448: 358–361 [DOI] [PubMed] [Google Scholar]
- Nuccio ML, Wu J, Mowers R, Zhou H-P, Meghji M, Primavesi LF, Paul MJ, Chen X, Gao Y, Haque E, Basu SS, Lagrimini LM (2015) Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions. Nat Biotechnol 33: 862–869 [DOI] [PubMed] [Google Scholar]
- Nunes C, O’Hara LE, Primavesi LF, Delatte TL, Schluepmann H, Somsen GW, Silva AB, Fevereiro PS, Wingler A, Paul MJ (2013a) The trehalose 6-phosphate/SnRK1 signaling pathway primes growth recovery following relief of sink limitation. Plant Physiol 162: 1720–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes C, Primavesi LF, Patel MK, Martinez-Barajas E, Powers SJ, Sagar R, Fevereiro PS, Davis BG, Paul MJ (2013b) Inhibition of SnRK1 by metabolites: tissue-dependent effects and cooperative inhibition by glucose 1-phosphate in combination with trehalose 6-phosphate. Plant Physiol Biochem 63: 89–98 [DOI] [PubMed] [Google Scholar]
- Nusinow DA, Helfer A, Hamilton EE, King JJ, Imaizumi T, Schultz TF, Farré EM, Kay SA (2011) The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature 475: 398–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, Alfred SE, Bonetta D, et al. (2009) Abscisic acid inhibits PP2Cs via the PYR/PYL family of ABA-binding START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Jhurreea D, Zhang Y, Primavesi LF, Delatte T, Schluepmann H, Wingler A (2010) Upregulation of biosynthetic processes associated with growth by trehalose 6-phosphate. Plant Signal Behav 5: 386–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S, Rylott EL, Gilday AD, Graham S, Larson TR, Graham IA (2004) Reserve mobilization in the Arabidopsis endosperm fuels hypocotyl elongation in the dark, is independent of abscisic acid, and requires PHOSPHOENOLPYRUVATE CARBOXYKINASE1. Plant Cell 16: 2705–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP (1997) The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev 11: 3194–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon M, Rolland F, Thevelein JM, van Dijck P, Leyman B (2007) ABI4 mediates the effects of exogenous trehalose on Arabidopsis growth and starch breakdown. Plant Mol Biol 63: 195–206 [DOI] [PubMed] [Google Scholar]
- Richardson K, Fowler S, Pullen C, Skelton C, Morris B, Putterill J (1998) T-DNA tagging of a flowering-time gene and improved gene transfer by in planta transformation of Arabidopsis. Funct Plant Biol 25: 125–130 [Google Scholar]
- Salter MG, Franklin KA, Whitelam GC (2003) Gating of the rapid shade-avoidance response by the circadian clock in plants. Nature 426: 680–683 [DOI] [PubMed] [Google Scholar]
- Schluepmann H, Pellny T, van Dijken A, Smeekens S, Paul M (2003) Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 6849–6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Sánchez-Villarreal A, Davis AM, Du SX, Berendzen KW, Koncz C, Ding Z, Li C, Davis SJ (2017) The metabolic sensor AKIN10 modulates the Arabidopsis circadian clock in a light-dependent manner. Plant Cell Environ 40: 997–1008 [DOI] [PubMed] [Google Scholar]
- Smeekens S, Ma J, Hanson J, Rolland F (2010) Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13: 274–279 [DOI] [PubMed] [Google Scholar]
- Stewart JL, Maloof JN, Nemhauser JL (2011) PIF genes mediate the effect of sucrose on seedling growth dynamics. PLoS One 6: e19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpice R, Flis A, Ivakov AA, Apelt F, Krohn N, Encke B, Abel C, Feil R, Lunn JE, Stitt M (2014) Arabidopsis coordinates the diurnal regulation of carbon allocation and growth across a wide range of photoperiods. Mol Plant 7: 137–155 [DOI] [PubMed] [Google Scholar]
- Takahashi F, Sato-Nara K, Kobayashi K, Suzuki M, Suzuki H (2003) Sugar-induced adventitious roots in Arabidopsis seedlings. J Plant Res 116: 83–91 [DOI] [PubMed] [Google Scholar]
- Vandesteene L, Ramon M, Le Roy K, van Dijck P, Rolland F (2010) A single active trehalose-6-P synthase (TPS) and a family of putative regulatory TPS-like proteins in Arabidopsis. Mol Plant 3: 406–419 [DOI] [PubMed] [Google Scholar]
- Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, Schmid M (2013) Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 339: 704–707 [DOI] [PubMed] [Google Scholar]
- Wang Z-Y, Tobin EM (1998) Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93: 1207–1217 [DOI] [PubMed] [Google Scholar]
- Weinig C, Johnston JA, Willis CG, Maloof JN (2007) Antagonistic multilevel selection on size and architecture in variable density settings. Evolution 61: 58–67 [DOI] [PubMed] [Google Scholar]
- Wigge PA. (2013) Ambient temperature signalling in plants. Curr Opin Plant Biol 16: 661–666 [DOI] [PubMed] [Google Scholar]
- Wingler A, Delatte TL, O’Hara LE, Primavesi LF, Jhurreea D, Paul MJ, Schluepmann H (2012) Trehalose 6-phosphate is required for the onset of leaf senescence associated with high carbon availability. Plant Physiol 158: 1241–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS One 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav UP, Ivakov A, Feil R, Duan GY, Walther D, Giavalisco P, Piques M, Carillo P, Hubberten H-M, Stitt M, Lunn JE (2014) The sucrose-trehalose 6-phosphate (Tre6P) nexus: specificity and mechanisms of sucrose signalling by Tre6P. J Exp Bot 65: 1051–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Sato E, Shimizu T, Nakamich N, Sato S, Kato T, Tabata S, Nagatani A, Yamashino T, Mizuno T (2003) Comparative genetic studies on the APRR5 and APRR7 genes belonging to the APRR1/TOC1 quintet implicated in circadian rhythm, control of flowering time, and early photomorphogenesis. Plant Cell Physiol 44: 1119–1130 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu Z, Wang J, Chen Y, Bi Y, He J (2015) Brassinosteroid is required for sugar promotion of hypocotyl elongation in Arabidopsis in darkness. Planta 242: 881–893 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu Z, Wang L, Zheng S, Xie J, Bi Y (2010) Sucrose-induced hypocotyl elongation of Arabidopsis seedlings in darkness depends on the presence of gibberellins. J Plant Physiol 167: 1130–1136 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RAC, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ (2009) Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol 149: 1860–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Shewry PR, Jones H, Barcelo P, Lazzeri PA, Halford NG (2001) Expression of antisense SnRK1 protein kinase sequence causes abnormal pollen development and male sterility in transgenic barley. Plant J 28: 431–441 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhu J-Y, Roh J, Marchive C, Kim S-K, Meyer C, Sun Y, Wang W, Wang Z-Y (2016) TOR signaling promotes accumulation of BZR1 to balance growth with carbon availability in Arabidopsis. Curr Biol 26: 1854–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]