Ethylene and light are major determinants of an altered root system architecture in flooded rice plants.
Abstract
Rice (Oryza sativa) is a semiaquatic plant that is well adapted to partial flooding. Rice stems develop adventitious root (AR) primordia at each node that slowly mature but emerge only when the plant gets flooded, leading to the formation of a whole new secondary root system upon flooding. AR growth is induced by ethylene that accumulates in submerged plant tissues due to its lowered diffusion rate in water. Here, we report that the architecture of the secondary root system in flooded rice plants is controlled not only by altered gas diffusion but also by gravity and light. While ethylene promotes the emergence and growth of ARs, gravity and light determine their gravitropic setpoint angle (i.e. the deviation of growth direction relative to vertical). ARs grow upward at about 120° in the dark and downward at 54° in the light. The upward growth direction is conserved in indica and japonica rice varieties, suggestive of a conserved trait in rice. Experiments with a klinostat and with inverted stem orientation revealed that gravity promotes upward growth by about 10°. Red, far-red, and blue light lead to negative phototropism in a dose-dependent manner, with blue light being most effective, indicating that phytochrome and blue light signaling control AR system architecture. The cpt1 (coleoptile phototropism1) mutant, which lacks one of the phototropin-interacting CPT proteins, shows reduced sensitivity to blue light. Hence, the gravitropic setpoint angle of rice ARs is controlled by genetic and environmental factors that likely balance the need for oxygen supply (upward growth) with avoidance of root desiccation (downward growth).
Root system architecture is determined by a number of parameters, including root growth rate, the rate at which lateral roots and adventitious roots (ARs) are formed, and the angles at which lateral roots and ARs grow with respect to the gravity vector. Root structure is highly adaptive. It is shaped by environmental signals such as gravity, light, nutrients, and water availability to ensure plant survival and propagation (Atkinson et al., 2014; Bellini et al., 2014). Gravity is an invariant physical signal and, hence, well suited to provide spatial orientation for plants. Gravity is sensed by starch statoliths in specialized root cap cells termed statocytes (Baldwin et al., 2013). Displacement of the statoliths as a result of an altered root orientation with regard to gravity triggers a signal in columella cells that causes an asymmetric distribution of auxin between the upper and lower sides of the lateral root cap and the epidermis of the root elongation zone. The auxin gradient causes differential growth on the upper and lower root sides and, eventually, root bending (Ottenschläger et al., 2003; Swarup et al., 2005; Sato et al., 2015). The mechanism of gravity sensing by displacement of statoliths is well suited to explain the positive gravitropic growth of primary roots that ensures penetration into soil. By contrast, many lateral roots and ARs grow at a nonvertical angle that has been termed the gravitropic setpoint angle (GSA; Digby and Firn, 1995). The branching of secondary roots ensures radial exploration of the soil. The nonvertical growth of lateral roots is species specific and genetically determined (i.e. independent of gravity; Guyomarc’h et al., 2012). However, the angle at which lateral roots grow also is environmentally controlled, as was shown for low-phosphate conditions in Arabidopsis (Arabidopsis thaliana) that cause a more vertical growth of lateral roots (Bai et al., 2013; Roychoudhry et al., 2017). However, relatively few studies have focused on the endogenous and environmental control of the GSA so far, with most of the information gained from Arabidopsis. In order to learn more about the control of the GSA and to identify general regulatory mechanisms that control secondary root architecture, it is useful to extend the studies to other plant species. Rice (Oryza sativa) is a monocot model plant that is well suited to extend our understanding of GSA regulation to an agriculturally important crop species and provide insights that may contribute to crop improvement.
Light is a key environmental cue for plants. It provides orientation for the shoot and root and triggers developmental reprogramming such as deetiolation in seedlings. Plants have different photoreceptors with characteristic action spectra. They perceive red light via the phytochrome receptor. The inactive phytochrome Pr is converted to its active form Pfr by red light absorbance. This conversion is reversible. Absorbance of far-red light by Pfr converts the active phytochrome to its inactive form Pr (Li et al., 2011). In rice, phytochromes are encoded by three genes, PhyA to PhyC, whereby PhyA is responsible mainly for mediating photomorphogenic responses (Takano et al., 2009; Roy et al., 2013). Some physiological responses, such as shade avoidance, depend on the ratio of Pr to Pfr. In shade avoidance, light gradients that are perceived by leaves induce the growth of petioles or leaf blades toward higher light intensities to optimize photosynthesis (Briggs, 2014). Shade avoidance is triggered by an increased ratio of far-red to red light that naturally occurs in the shade that is perceived by phytochrome (de Wit et al., 2016).
UV-A/blue light is perceived via the blue light receptors phototropin and cryptochrome, whereby phototropin is the main blue light receptor mediating phototropic responses (Fankhauser and Christie, 2015). Positive phototropism also serves to orient the shoot in the direction of light for efficient photosynthesis by means of directed growth (Fankhauser and Christie, 2015). While shoots display positive phototropism, roots most often respond to light by negative phototropic growth, driving them into the dark. In Arabidopsis, negative root phototropism was shown to have a beneficial effect under drought conditions with increased plant size at maturity (Galen et al., 2007), indicating that the control of root growth direction by light can help the plant to adapt to adverse environmental conditions. The blue light receptor phototropin is encoded by two genes in flowering plants, PHOT1 and PHOT2, whereby PHOT1 is responsible mainly for root phototropism (Fankhauser and Christie, 2015). In Arabidopsis roots, light was shown to redirect auxin flux by altering the polarized distribution of the auxin-transporting PIN proteins such that one side of the root grows faster while the other side grows slower, resulting in phototropic root bending (Ding et al., 2011; Sassi et al., 2012).
Root system architecture is determined not only by primary and lateral roots but also by shoot-borne roots. The formation of ARs can be developmentally programmed, as is the case for crown and brace roots of cereals, or be induced by environmental signals, such as flooding (Bellini et al., 2014). Submergence poses a number of challenges to plants, the most obvious of which is reduced oxygen availability. In flooding-adapted plants, postsubmergence root architecture differs largely from the primary root system. Flooding promotes the establishment of an AR system that takes on the task to supply the plant with water, nutrients, and anchorage (Ayi et al., 2016). It can support or even replace the soil root system that suffers most from oxygen shortage during flooding (Sauter, 2013). In semiaquatic rice, AR primordia are constitutively formed at the stem nodes without emerging from them as long as the node is not submerged (Lorbiecke and Sauter, 1999). Upon submergence, the gaseous hormone ethylene accumulates in the submerged plant parts due to its lowered diffusion rate in water as compared with air. Ethylene acts as a hormonal trigger that promotes the growth and emergence of ARs in concert with gibberellic acid and abscisic acid (Steffens et al., 2006).
While deep rooting is a beneficial trait for drought resistance, it is unfavorable during flooding, where shallow rooting helps avoid hypoxic or anoxic soil layers. In contrast to the primary root system, ARs grow in the oxygen-rich surface layers of the flood water, from which they can take up oxygen produced in underwater photosynthesis (Winkel et al., 2013; Ayi et al., 2016). ARs benefit from oxygen dissolved in flood water yet need to avoid desiccation in air. Light may be a helpful signal to guide root orientation under water. In this study, we report on the regulation of AR growth direction in rice by gravity and light signals.
RESULTS
Growth of Adventitious Rice Roots Is Induced in the Dark
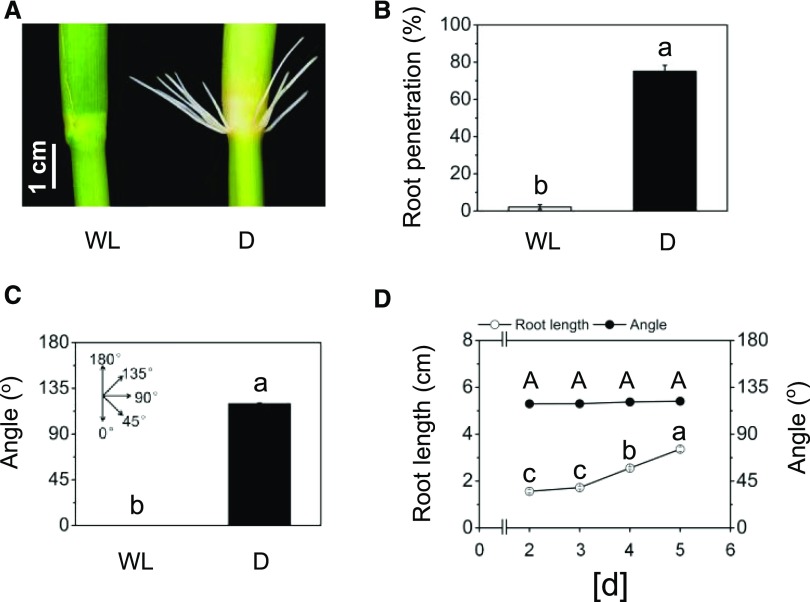
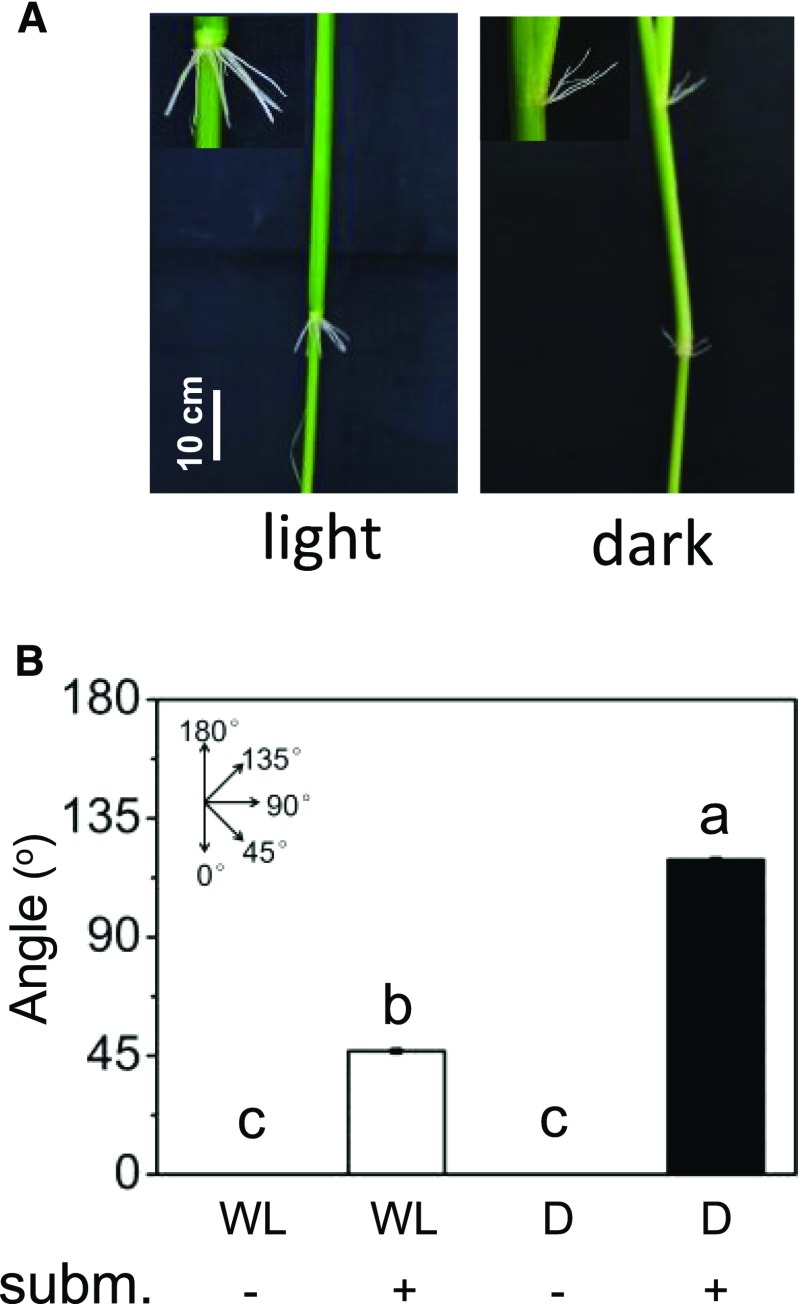
Rice plants form AR primordia at their stem nodes that emerge to develop into ARs upon plant submergence. AR growth is promoted by ethylene that accumulates in submerged tissue (Lorbiecke and Sauter, 1999). In this study, we show that exposure of excised deepwater rice stems of the cv PG56 to continuous darkness for 2 d likewise results in AR growth (Fig. 1, A and B). In the dark, 75% of root primordia grew out as compared with only 2.2% when stems were kept in the light. Interestingly, the angle at which ARs grew in the dark was, on average, 119.8° such that roots pointed upward (Fig. 1, A and C). A time-course analysis revealed that the upward angle was maintained while ARs continued to elongate for at least 5 d (Fig. 1D). To test if a similar growth behavior was observed in intact plants under flooding conditions, plants were partially submerged or kept in air in the light or in the dark for 3 d (Fig. 2). Partial submergence in either continuous light or continuous darkness induced AR growth. While the average growth angle in continuous light was 46.8°, it was 119.3° in the dark, confirming that the response observed in excised stem sections was a physiological response that likewise occurs in flooded plants. In the dark, no ARs emerged from intact plants. In rice plants, senescence-induced ethylene formation was measured no earlier than after 6 d of complete darkness (Fukao et al., 2012). When plants are submerged, ethylene that is continuously produced is trapped to physiologically active levels more rapidly.
Figure 1.
ARs of rice emerge and grow upward in the dark. A, cv PG56 rice stem sections were exposed to white light (WL) or darkness (D) for 2 d. ARs emerged in the dark but not in the light. B, Percentage of penetrated roots. C, Average angle of ARs at the third youngest node. Bars in B and C indicate means ± se from four independent experiments, with 30 stem sections analyzed in total per treatment. Different letters indicate significantly different values between treatments (P < 0.05, Student’s t test). D, Time-course analysis of AR elongation and root growth angle in the dark. Values are means ± se of three independent experiments, with 18 stems analyzed per time point. Uppercase letters indicate significantly different values of root angle between different time points, and different lowercase letters indicate significantly different values of root length between different time points (P < 0.05, ANOVA with Tukey’s test).
Figure 2.
The direction of AR growth in partially flooded plants is determined by light. A, cv PG56 rice plants were partially submerged for 3 d in the light or in the dark. ARs grow downward in the light and upward in the dark when flooded. B, Mean ± se angles of ARs that emerged at the third youngest node were determined in three independent experiments from 18 to 24 plant stems exposed to white light (WL) or darkness (D). Different letters indicate statistically significant differences (P < 0.05, ANOVA with Tukey’s test).
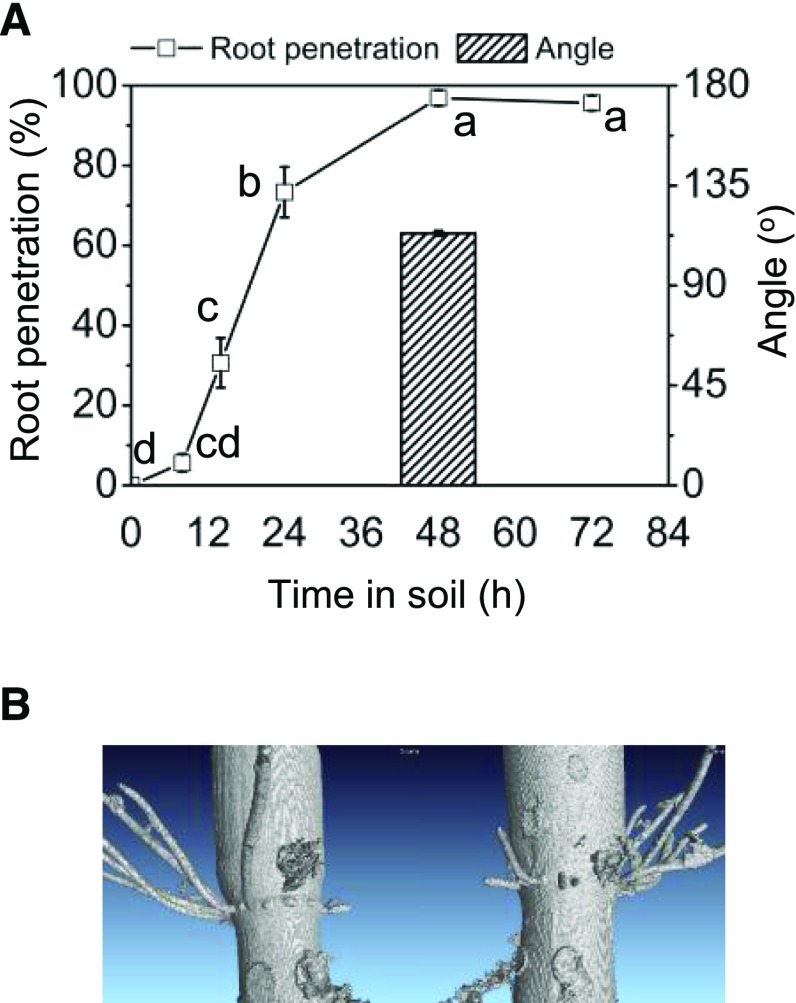
To test if the upward growth of ARs occurred only in a gaseous or liquid medium or also in solid medium such as soil, we tested AR growth behavior in cv PG56 stems kept in soil for up to 3 d (Fig. 3). Stem nodes were placed 8 cm below the soil surface. The number of emerged roots at this node increased from 0% at 0 h to 30.6% after 14 h, to 73.3% after 24 h, and to 96.9% after 2 d (Fig. 3A). To analyze the root growth angle, we carefully dug out stems from the soil after 2 d. The average length of ARs after 2 d was 1.6 cm and the average growth angle was 113.5°, indicating that upward growth of AR in rice was independent of the physical nature of the medium. To visualize AR growth direction in solid medium, we placed the lower 10-cm part of rice stem sections in Styrofoam beads for 3 d and analyzed the AR system in situ with x-ray computer tomography (Fig. 3B). ARs grew at an upward angle in situ as observed in air and in water, supporting the conclusion that the angle of AR growth is not determined by the medium in which roots grow.
Figure 3.
ARs emerge and grow upward in soil. A, AR penetration at the third node of cv PG56 stem sections that were buried in soil was monitored for up to 72 h. The AR growth angle was determined after 48 h. Values are means ± se analyzed from 15 stems in three independent experiments. Different letters indicate significantly different values between each time point (P < 0.05, ANOVA with Tukey’s test). B, CT image of third nodes with ARs visualized after 3 d in styrofoam beads.
Upward Growth of ARs in the Dark Is a Conserved Developmental Trait in Rice
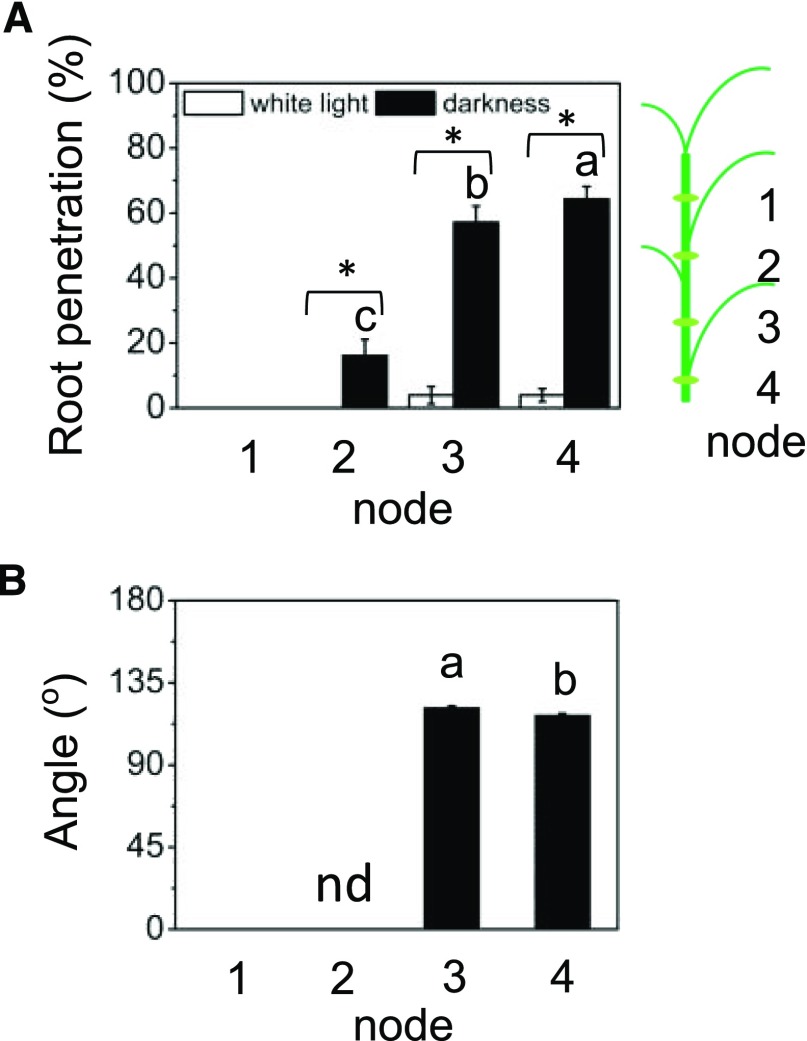
We next investigated AR development by studying different age nodes. ARs form at the youngest node and slowly grow over time without penetrating the epidermis as long as the node is kept in the light and not exposed to ethylene (Lorbiecke and Sauter, 1999). We analyzed ARs at the first (i.e. youngest) to the fourth (i.e. oldest) nodes of cv PG56 after exposure to continuous darkness or to light as a control for 2 d (Fig. 4). The percentage of roots that had penetrated after 2 d in the dark increased with developmental age of the node. None of the root primordia had emerged at the youngest node, while 64.3% of all primordia had emerged at the oldest node (Fig. 4A). Only ARs at the third and fourth nodes were long enough to determine the growth angles (Fig. 4B). ARs grew upward at both nodes, with similar angles of 121.4° at the third node and 117.4° at the fourth node. Taken together, the rate of emergence of ARs is dependent on their developmental stage, whereas the growth angle is independent of the development age of the node.
Figure 4.
Developmental gradient of ARs in the dark. A, cv PG56 stem sections were excised 2 cm below the first, second, third, or fourth node and kept in white light or darkness for 2 d. Subsequently, root penetration was determined. B, Mean ± se root angles at each node were determined from 16 stems analyzed in three independent experiments. Different letters indicate statistically significant differences between nodes in the dark (n = 16; P < 0.05, ANOVA with Tukey’s test). Asterisks indicate significant differences between dark and light treatments at each node (P < 0.05, Student’s t test; nd, no data).
The studies described so far were carried out with the deepwater rice variety cv PG56, which raised the question of whether the observed trait was a characteristic of rice that is regularly exposed to long-term (several months) severe flooding (several meters) or whether the upward growth of ARs was generally conserved in rice. To answer this question, we studied five lowland rice cultivars that are adapted to short-term (fewer than 2 weeks), less severe (less than 1 m) flooding events. The deepwater and lowland rice varieties that we studied are not only adapted to different flooding regimes but also belong to different rice subspecies (Table I). The rice cv PG56 belongs to the indica subspecies, whereas the lowland rice cultivars belong to the japonica subspecies.
Table I. Upward growth of ARs in the dark is a conserved trait in rice.
ARs of the six rice genotypes, cv Pin Gaew 56 (PG56), Zhonghua 11 (ZH11), Taichung 65 (T65), Nipponbare, Bomba, and Kinmaze, were analyzed. Rice stem sections including the third youngest node were cut and incubated in white light with 150 μm ethephon (WL+E) or in the dark for 4 d before growth angles were determined. Angle values indicate means ± se from 15 to 30 stems analyzed in three independent experiments. Letters a and b indicate significant differences between treatments in a given variety (P < 0.05, Student’s t test).
| Cultivar | Subspecies | Ecotype | Angle |
|
|---|---|---|---|---|
| Dark | WL+E | |||
| ° | ||||
| PG56 | indica | Deepwater | 120.7 ± 0.6 a | 57.8 ± 1.2 b |
| ZH11 | japonica | Lowland | 104.6 ± 0.5 a | 60.2 ± 1.9 b |
| T65 | japonica | Lowland | 110.8 ± 0.9 a | 59.7 ± 0.9 b |
| Nipponbare | japonica | Lowland | 113.9 ± 0.9 a | 62.7 ± 1.7 b |
| Bomba | japonica | Lowland | 115.5 ± 1.2 a | 48.8 ± 1.3 b |
| Kinmaze | japonica | Lowland | 112.3 ± 1.0 a | 64.6 ± 1.2 b |
Rice stems from lowland rice cv ZH11, T65, Nipponbare, Bomba, and Kinmaze and from the deepwater rice cv PG56 were exposed to continuous darkness for 3 d. Darkness induced AR emergence in all cultivars (Table I). The angles of the lowland rice cultivars varied between 104.6° and 115.5° and, hence, were slightly lower than the 120.7° measured for cv PG56. Nonetheless, roots grew upward in all genotypes even prior to internode elongation (Supplemental Fig. S1, A and B). In conclusion, dark-induced AR growth in an upward direction is conserved in rice, suggesting that it is of benefit to its semiaquatic lifestyle.
Dark-Induced AR Growth Is Mediated by Ethylene Signaling
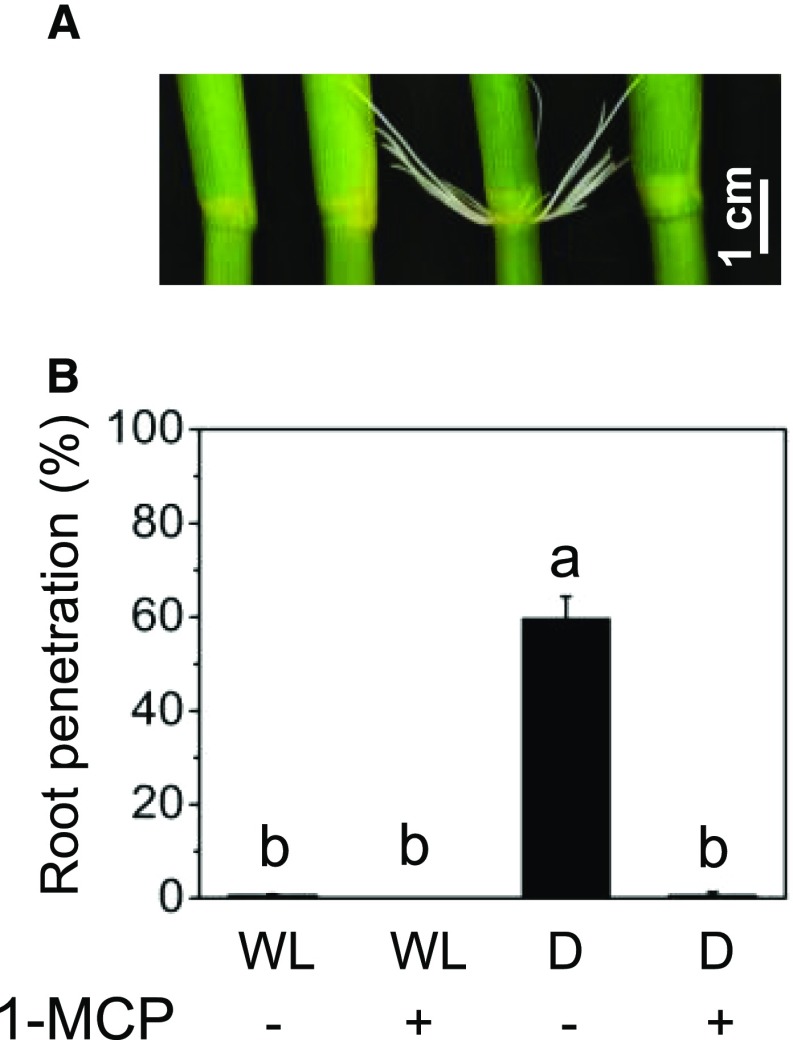
It has been reported previously that, in rice, an extended dark period results in ethylene formation, which, in turn, promotes dark-induced senescence (Fukao et al., 2012). To answer the question of whether ethylene was a trigger for AR growth in the dark, we exposed cv PG56 stems kept in air to 5 µL L−1 1-methylcyclopropene (1-MCP), an inhibitor of ethylene perception, and kept them either in the light as a control or in the dark (Fig. 5A). In the light, no roots penetrated regardless of the 1-MCP treatment (Fig. 5B). In the dark, AR growth was induced in untreated stems whereas no ARs penetrated in the dark when stems had been pretreated with 1-MCP, indicating that AR growth in the dark is mediated by ethylene signaling.
Figure 5.
AR growth in darkness is suppressed by 1-MCP. A, Stem sections of rice cv PG56 were kept in white light (WL) or darkness (D) with or without 5 µL L−1 1-MCP for 2 d to inhibit ethylene perception. B, Percentage of penetrated roots. Results are means ± se from three independent experiments, with 20 to 22 stems analyzed. Different letters indicate statistically significant differences (P < 0.05, ANOVA with Tukey’s test).
AR Emergence and the AR Growth Angle Are Controlled by Red and Blue Light Signaling
To better understand which light signaling pathways control AR emergence and the growth angle of ARs, we studied the effects of red, far-red, and blue light. As controls, cv PG56 stems were exposed to continuous darkness or treated with 150 µm of the ethylene-releasing compound ethephon in white light for 3 d to induce AR emergence. Ethephon treatment in white light caused about 80% of all AR primordia that were present at the third node to emerge, while no roots emerged in white light without ethephon (Fig. 6A; Steffens et al., 2006).
Figure 6.
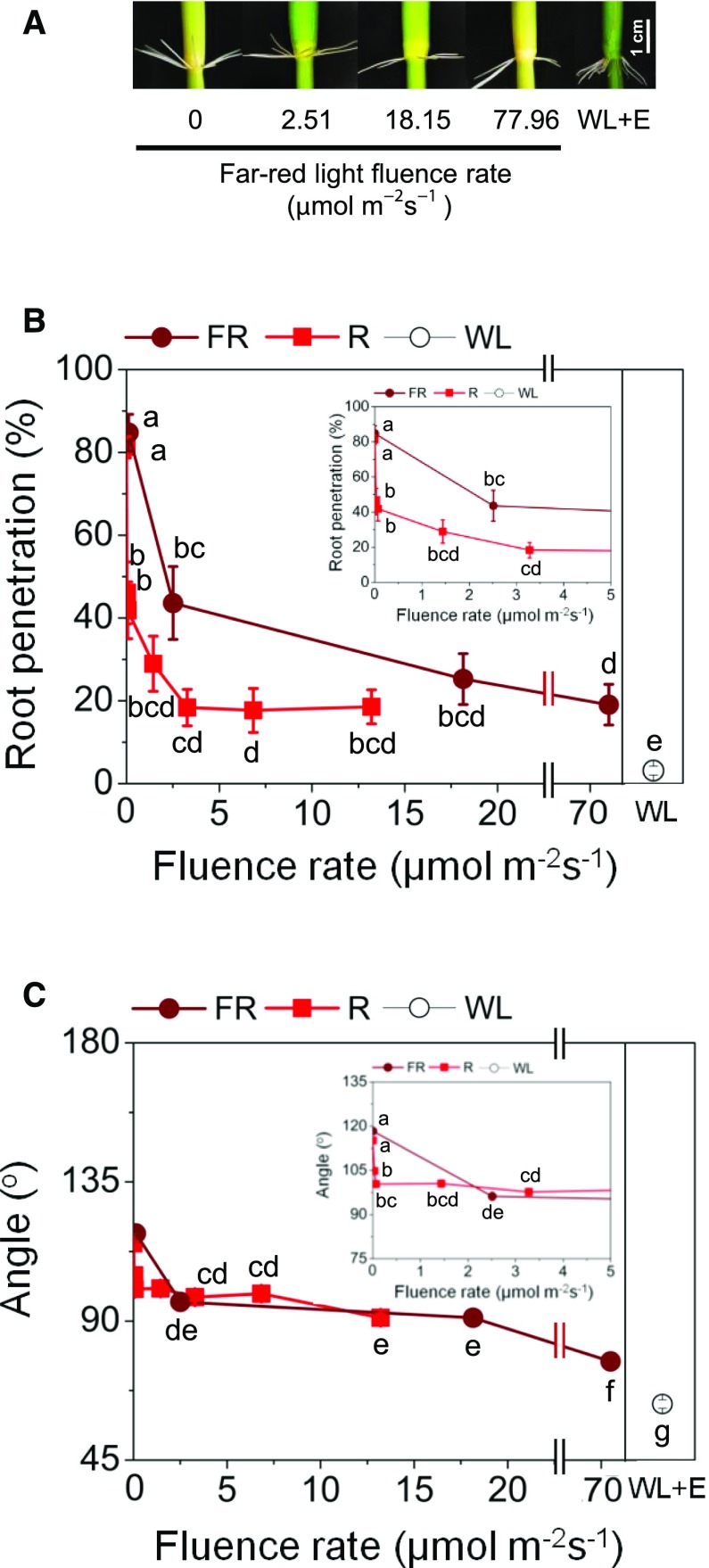
AR emergence and the growth angle of ARs are controlled by red and far-red light signaling. A, Stem sections including the third node show a fluence rate-dependent change in growth angle when exposed to far-red light. For comparison, a stem is shown that was exposed for 3 d to white light in the presence of 150 μm ethephon (WL+E). Ethephon releases ethylene that promotes AR growth even in the light. B, Percentage of ARs that penetrated at the third node in darkness (0), red light (R), far-red light (FR), or white light (WL) after 3 d. The inset shows a higher resolution of the low fluence range. C, Angle of ARs at the third node of stems exposed as described in A. The inset shows a higher resolution of the low fluence range. In B and C, results are means ± se from four independent experiments, with 18 to 30 stems analyzed per treatment. Different letters indicate statistically significant differences (P < 0.05, ANOVA with Tukey’s test).
Exposure to far-red light reduced root penetration with increasing fluence rate, from 84.8% in the dark to 19.1% at 78 µmol m−2 s−1, and was 2.2% in 43 µmol m−2 s−1 white light (Fig. 6B). In red light, we also observed decreasing AR penetration rates with increasing light intensity, a response that leveled out at 18.4% root penetration in stems exposed to 3.3 µmol m−2 s−1 and higher red light fluences (Fig. 6B). Red light had a stronger effect at low fluence rates than far-red light, while red and far-red light had similar inhibitory effects at higher fluence rates. The growth angle of emerged roots declined from 118.3° in the dark to 76.9° at 78 µmol m−2 s−1 far-red light (Fig. 6C). The angle of emerged ARs was likewise decreased by red light in a dose-dependent manner, from 104.8° at 0.03 µmol m−2 s−1 to 91° at 13.2 µmol m−2 s−1 (Fig. 6C), but again, red light appeared to be more efficient than far-red light at low light intensities. Roots that emerged in the presence of ethylene in white light grew at an angle of 54.2°. The results revealed that both Pr (favored by far-red light exposure) and Pfr (favored by red light exposure) regulate AR emergence and growth angle. Taken together, our analysis revealed an inhibitory effect of phytochrome signaling on AR emergence and upward growth. The results further show that inhibition of AR penetration is more sensitive to red light than to far-red light at low fluence rates. However, neither far-red nor red light signaling in the low (1–1,000 µmol m−2) and very low (0.1–1 µmol m−2) fluence ranges that were analyzed here (Li et al., 2011) can fully account for light regulation of AR penetration and growth angle, suggesting that another light signaling pathway may be involved.
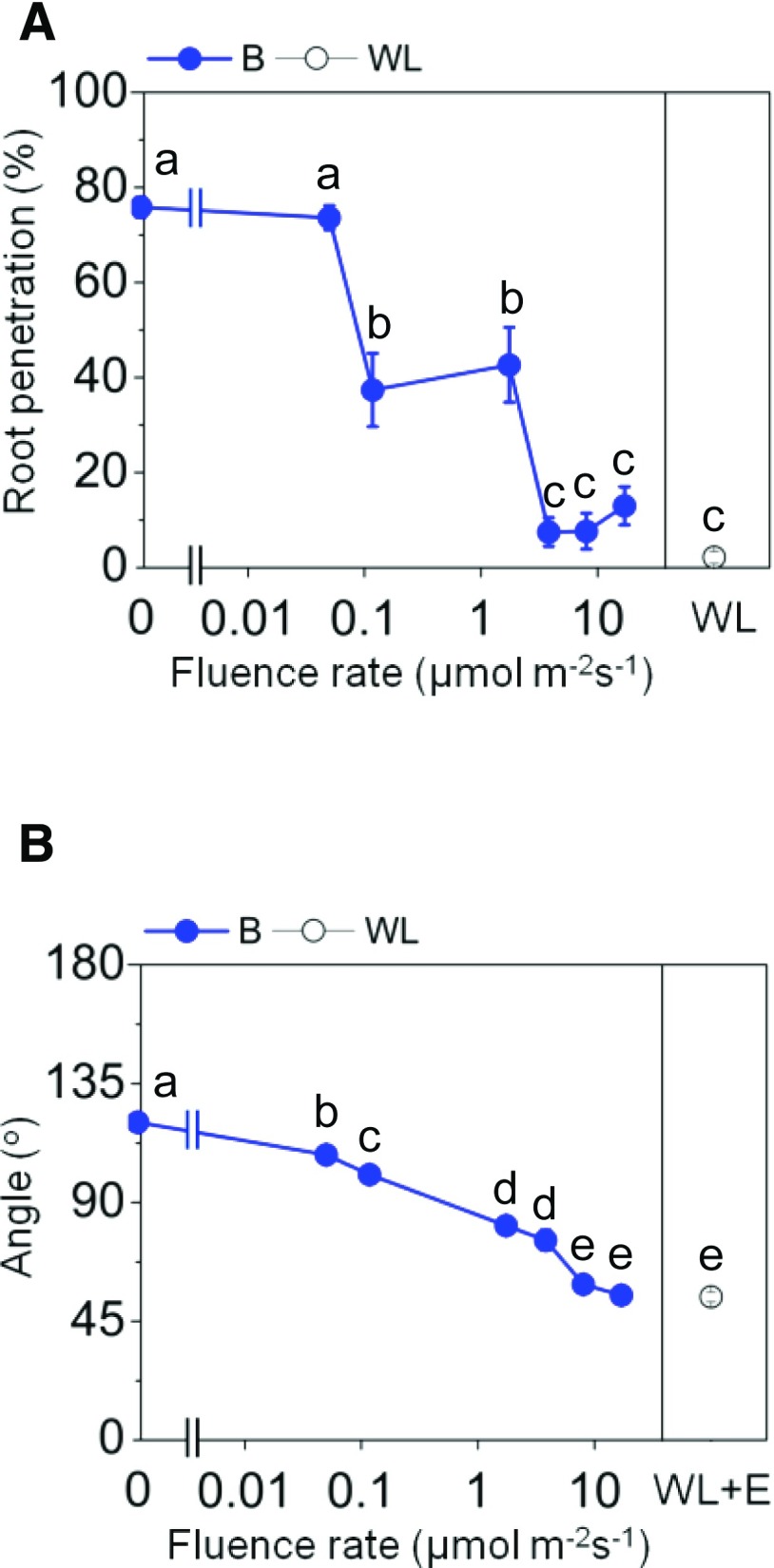
Since phototropic responses are commonly regulated by the blue light receptor phototropin, we tested the effect of blue light on AR penetration and growth angle (Fig. 7). The cv PG56 stems were exposed to white light, to darkness, or to different intensities of blue light for 3 d. Following these treatments, root penetration rates (Fig. 7A) and the angle of penetrated roots (Fig. 7B) were determined. Root penetration was inhibited in a dose-dependent manner. At 3.8 µmol m−2 s−1 and higher blue light intensities, the percentage of emerged roots was as low as in white light. Similarly, the angle of ARs decreased in a dose-dependent manner and reached 58.9° at 8 µmol m−2 s−1 blue light, which was comparable to the 54.2° observed in 43 µmol m−2 s−1 white light, suggesting that blue light signaling is a major signal regulating AR emergence and growth direction.
Figure 7.
Blue light fully inhibits the emergence and upward growth of ARs. A, Percentage of penetrated roots at the third node of cv PG56 stems exposed to darkness (0), blue light (B), or white light (WL) for 3 d. B, Average root growth angles in increasing blue light fluence rates and in white light in the presence of 150 μm ethephon (WL+E). Values are means ± se from three independent experiments, with 16 to 20 stems analyzed per treatment. Different letters indicate statistically significant differences (P < 0.05, ANOVA with Tukey’s test).
A Role for CPT1 in Blue Light Regulation of the AR Growth Angle
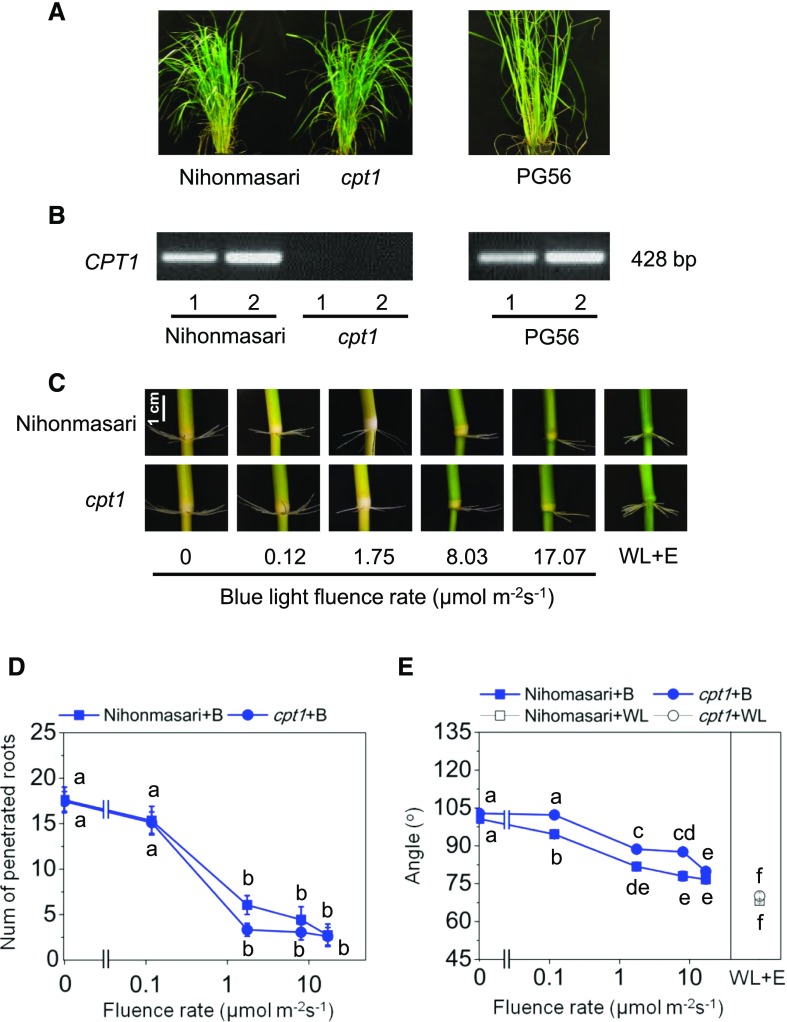
NONPHOTOTROPIC HYPOCOTYL3 (NPH3) has been identified as a signaling component involved in hypocotyl and root phototropism in Arabidopsis (Motchoulski and Liscum, 1999). The rice COLEOPTILE PHOTOTROPISM1 (CPT1) protein is an NPH3 ortholog (Haga et al., 2005). Seedlings of the cpt1 deletion mutant of the rice cv Nihonmasari are unresponsive to blue light with regard to the induction of positive phototropism of the coleoptile and show decreased blue light sensitivity in negative phototropism of the primary root (Haga et al., 2005). We employed the cpt1 mutant to answer the question of whether AR phototropism involves signaling via CPT1. To verify the CPT1 deletion, DNA was isolated from wild-type cv Nihonmasari, from cpt1 plants, and from cv PG56 plants. A DNA fragment within the deleted region was amplified with gene-specific primers from cv Nihonmasari and PG56 but not from cpt1 plants (Fig. 8, A and B), confirming the CPT1 gene deletion. Next, stem sections from wild-type cv Nihonmasari and from the cpt1 mutant were kept in the dark, exposed to blue light, or exposed to white light with 150 µm ethephon for 7 d (Fig. 8C). Root penetration was high in the dark and decreased in both cv Nihonmasari and cpt1 in a dose-dependent manner, with no statistically significant difference between genotypes (Fig. 8D). ARs grew upward in the dark with no difference in growth angle between cv Nihonmasari and cpt1. The growth angle decreased with increasing blue light intensity in both genotypes (Fig. 8E). However, cpt1 displayed reduced sensitivity to blue light such that angles were larger in cpt1 as compared with cv Nihonmasari at any given intermediary blue light intensity. ARs of cv Nihonmasari reached the lowest angle of 81.8° at 1.8 µmol m−2 s−1, whereas ARs of cpt1 reached 79.9° at 17.1 µmol m−2 s−1. These results suggest that CPT1 participates in blue light regulation of AR growth angle but not of AR emergence and, hence, suggest a specific role for CPT1 in negative AR phototropism in rice.
Figure 8.
Blue light signaling of AR growth direction is partially impaired in the cpt1 mutant. A, A cpt1 plant, its wild-type background cv Nihonmasari, and a cv PG56 rice plant. B, A 428-bp fragment within the CPT1 gene was amplified from cv PG56 and Nihonmasari but not from cpt1. DNA was isolated from two individual plants for each genotype. C, Emerged ARs at the third node of cv Nihonmasari and cpt1. cpt1 is less sensitive to low blue light fluence rates. D, Number of ARs penetrated at the third node of stems that were exposed to increasing blue light (B) intensities for 6 d. E, Average root angles at the third node of stems that were exposed to increasing blue light intensities for 6 d or kept in white light in the presence of 150 μm ethephon (WL+E). Results in D and E are means ± se obtained from three independent experiments, with 18 stems analyzed per treatment. Different letters indicate significantly different values (P < 0.05, ANOVA with Tukey’s test).
The Growth Direction of ARs Is Reversibly Controlled by Light
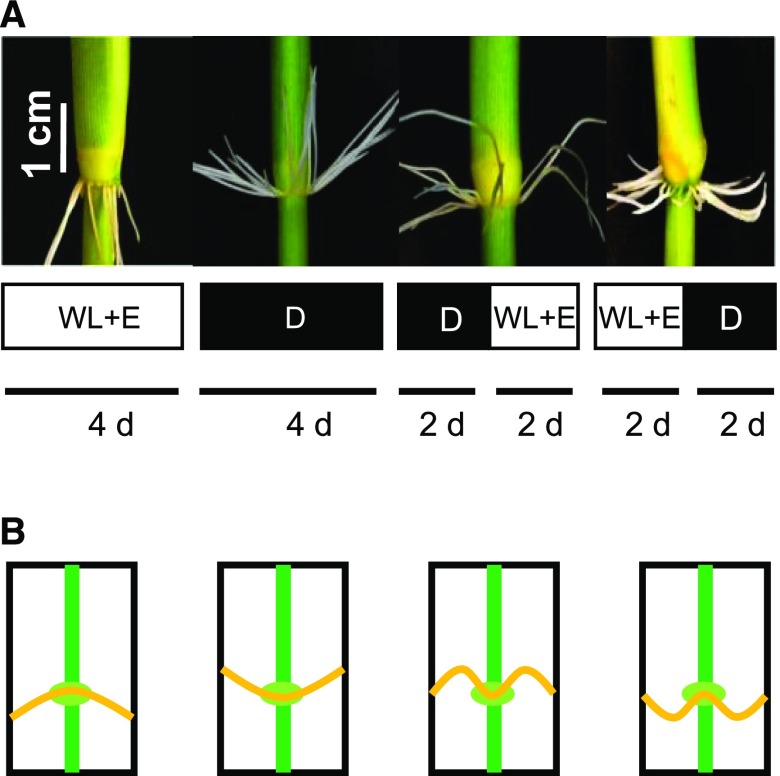
We next tested whether regulation of the AR growth angle by light was a reversible process. We exposed stem sections to either continuous white light combined with 150 µm ethephon to promote downward growth of ARs or to continuous darkness to promote upward growth. In addition, we exposed stems to 2 d of darkness followed by 2 d of light plus ethephon treatment, or to 2 d of light plus ethephon followed by 2 d of darkness (Fig. 9). ARs that grew upward in the dark changed their growth direction when transferred to the light, and ARs that grew downward in the light changed their growth direction to an upward angle when moved to the dark (Fig. 9A). The changes in growth angle are depicted as cartoon drawings (Fig. 9B).
Figure 9.
The growth direction of ARs is reversibly regulated by light. A, Rice stem sections were exposed to white light + ethephon (WL+E) and dark (D) regimes as indicated: WL+E or D for 4 d, D for 2 d followed by WL+E for 2 d, or WL+E for 2 d followed by 2 d of D. Ethephon was applied at a final concentration of 150 μm. The white light intensity was 43 µmol m−2 s−1. B, Schematic diagrams indicating the changes in root growth directions as shown in A.
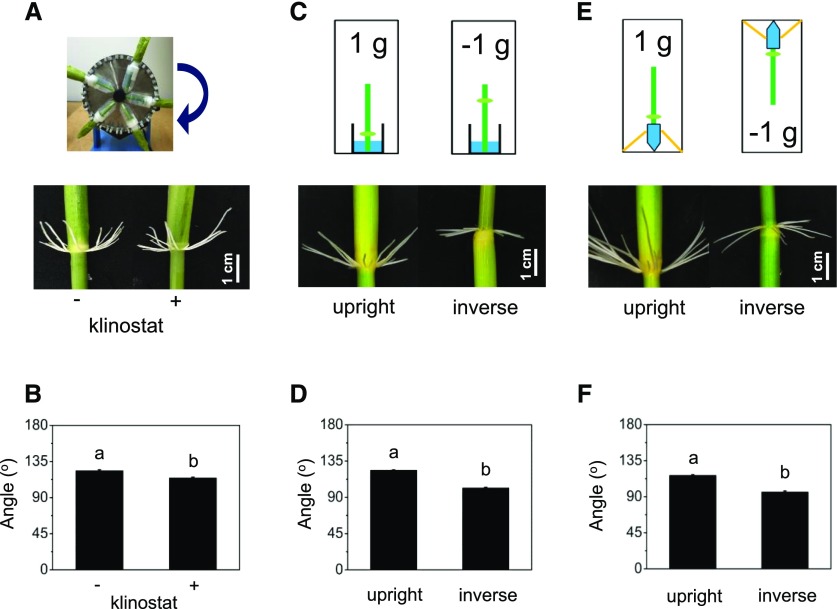
Gravity Plays a Minor Role in Determining AR Growth Direction
Plants are constantly exposed to gravity. Primary roots and shoots orient their growth direction along the gravity vector either in a positive or negative manner, while the angle of lateral roots or ARs deviates from the gravity vector. To investigate the impact of gravity on the GSA of ARs, we performed a klinostat experiment in which stem sections were vertically rotated at 5 rpm in the dark for 3 d (Fig. 10A). The angle of rotated roots was 106° as compared with 120.4° in nonrotated roots, indicating that gravity induced a negative gravitropic response of 14.4° (Fig. 10B). To further test the effect of gravity, we performed two experiments in which stem sections were oriented in an upright or inverse orientation (Fig. 10, C and E). In one setup, the stems were placed in a beaker in either orientation (Fig. 10C). In a second experiment, water was supplied in a test tube to the lower cut surface of the stem irrespective of its orientation. In stems kept in a beaker (Fig. 10, C and D), the angle changed from 123.4° in the upright orientation with roots exposed to 1 g to an angle of 101.3° in inverse oriented stems with roots exposed to −1 g. Hence, applying gravity from the opposite direction caused a change in growth angle of 22.1°. A similar result was obtained with stems supplied with water from test tubes at the lower cut surface (Fig. 10E). An angle of 116.4° was measured in the upright orientation and one of 96.6° in the inverse orientation, resulting in a difference of 19.8° (Fig. 10, E and F).
Figure 10.
Gravity plays a minor role in determining the setpoint angle of ARs. A, Photograph showing the klinostat setup. B, Angle of ARs growing at the third node of PG56 stems that were rotated at 5 rpm on a klinostat for 3 d in the dark. Means ± se were analyzed in three independent experiments. Different letters indicate different values between treatments (n = 15; P < 0.05, Student’s t test). C, Schematic showing stems that were placed upright or in an inverse orientation in a beaker. D, Average angle of ARs grown for 3 d in the dark as indicated in C. Means ± se were analyzed in three independent experiments. Different letters indicate different values between treatments (n = 16; P < 0.05, Student’s t test). E, Schematic indicating stems that were placed upright or in an inverse orientation. Water was supplied through a 10-mL tube attached to the lower cut surface in either setup. F, Average root angle of stems kept for 3 d in the dark as indicated in E. Means ± se were analyzed in three independent experiments on a total of 14 stems per treatment. Different letters indicate significant differences between treatments (P < 0.05, Student’s t test).
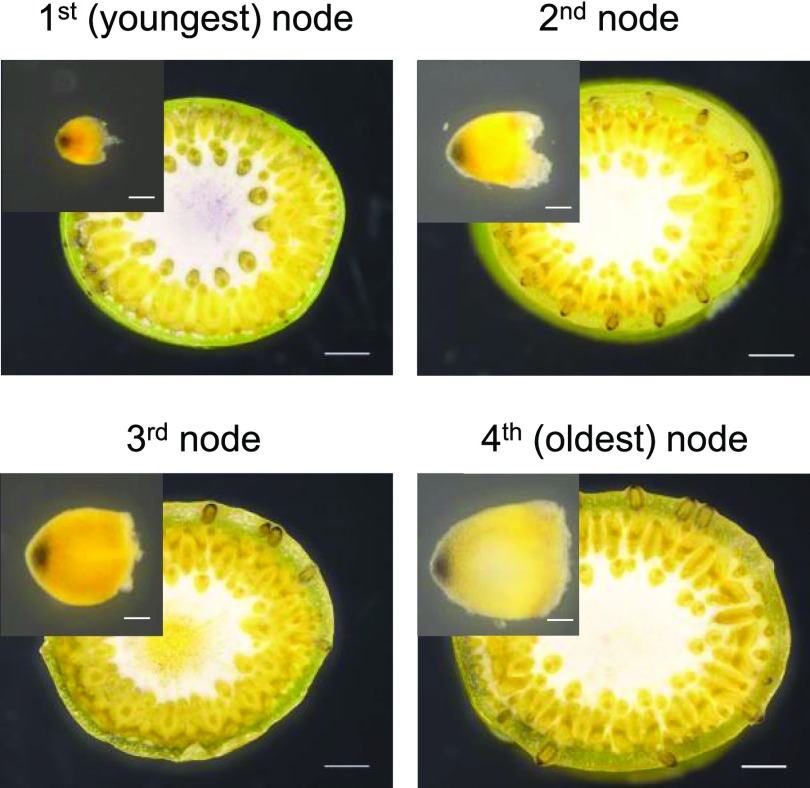
Gravity is sensed by starch-containing statoliths in specialized root cap cells called statocytes. We performed starch staining of ARs to analyze at which developmental stage the statocytes develop in AR primordia. We obtained cross sections from each node that were stained with Lugols’ solution to visualize starch as a brownish stain (Fig. 11). We also isolated AR primordia from each node for staining. The results revealed that statoliths were present in the root cap of nonemerged ARs at all nodes, including the youngest node (Fig. 11). These observations suggested that AR primordia can sense gravity at a very early stage. However, the GSA is determined by gravity to only a minor degree whereby ARs respond to gravity with a negative gravitropic response.
Figure 11.
AR primordia possess statoliths. Cross sections of the first, second, third, and fourth nodes of rice cv PG56 were stained with Lugols’ solution to visualize starch-containing statoliths in the root caps of AR primordia. Bars = 1 mm. Insets show enlarged images of isolated AR primordia. Bars = 0.1 mm.
DISCUSSION
Physiological Function of Upward AR Growth
Rice is a semiaquatic plant. With the exception of upland rice, many rice varieties are cultivated in paddy fields. Depending on the ecological niche to which a particular rice variety is adapted, it can, on top, encounter more or less severe flooding events. In waterlogged or flooded soil, gas diffusion is limited and roots compete with microorganisms for oxygen. For roots, a continuous supply of oxygen from the shoot via internal air spaces becomes crucial (Pedersen et al., 2009). When fully submerged, shoots do not have access to atmospheric oxygen; however, submerged shoots can photosynthesize and thereby increase endogenous oxygen levels and enrich the flood waters with oxygen. During flooding, the original root system in the soil is supported or replaced by aquatic ARs that emerge from the stem nodes. ARs are more easily supplied with oxygen, as they are closer to the photosynthesizing oxygen-producing shoot, and aquatic ARs can take up oxygen from the flood water (Ayi et al., 2016). The survival of Alternanthera philoxeroides plants was higher with aquatic ARs as compared with plants with aquatic ARs removed (Ayi et al., 2016). Root oxygen in fully submerged rice plants was shown to be determined by underwater photosynthesis (Winkel et al., 2013). For the wetland grass Meionectes brownii, Rich et al. (2013) showed that, irrespective of partial or full submergence, the deeper parts of submerged stems and aquatic roots received their oxygen from underwater photosynthesis during the day and from the oxygen-enriched flood water at night. Hence, light is a determinant of oxygen supply under water.
We observed that rice ARs show an unusual growth behavior in the dark: they grow upward. In the light, the growth direction changes, resulting in downward growth, a response that indicates negative phototropism. Phototropism is generally seen as a means to optimize plant growth and development. Shoot phototropism serves to optimize the photosynthetic ability of plants. Unlike shoots, roots in general display negative phototropism that directs roots into the soil to avoid desiccation and ensure that roots can take up nutrients and water and provide anchorage. In addition, root growth is directed in a positive gravitropic manner that further drives downward growth. Phototropic and gravitropic responses are mediated by auxin that can be transported polarly by auxin efflux carriers. Directed auxin flow allows for an asymmetric auxin distribution and, hence, for differential growth rates on the upper and lower sides of a root or on the shoot side facing the light versus the shaded side and, hence, to organ bending (Fankhauser and Christie, 2015; Su et al., 2017). In rice, AR growth direction is determined by gravity to only a minor degree. In fact, ARs display negative gravitropism. Loss of directional gravity in a klinostat resulted in a reduced upward growth angle in the deepwater rice cv PG56 by 14°, and reversion of the gravity vector with respect to stem orientation reduced upward growth by 20° to 22°. Hence, the gravitational signal enhances upward AR growth direction. Deeper rooting1 (DRO1) is a rice gene that makes roots grow deeper into soil and, thereby, provides drought avoidance ability in upland rice (Uga et al., 2013). IR64 is a paddy field rice variety with a shallow root system. Interestingly, roots of the DRO1-near isogenic IR64 line that was equipped with the DRO1 trait had a root system that reached twice as deep without having a higher root dry mass and with only marginally longer roots, suggesting a function of DRO1 in the determination of the root growth angle and, hence, genetic control of the root system architecture and the function of the root system.
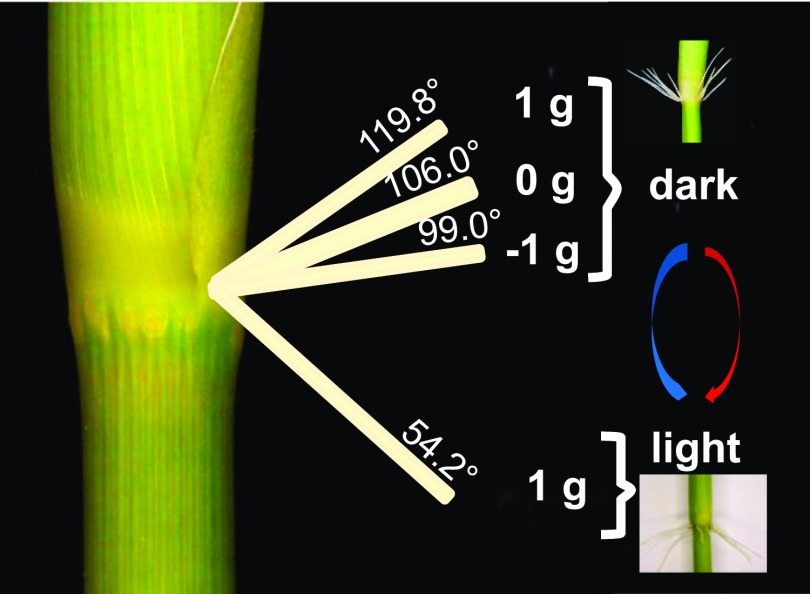
With regard to environmental control of the root system, we found that light had a strong impact. In nonflooded conditions, light fully inhibited AR emergence. In partially submerged plants or in the dark, ARs emerged due to the activity of ethylene that accumulates in these conditions (Steffens et al., 2006; Fukao et al., 2012). The growth angle of emerged ARs changed from about 120° in the dark to 54° in white light, thus reversing the growth direction from upward to downward (Fig. 12). Taking previous and our findings together, we conclude that the growth angle that deviates from the vertical, the GSA, is, in part, genetically programmed (e.g. via DRO1) and environmentally controlled by gravity and light, with light exerting the stronger environmental effect. We hypothesize that absence of light is an indicator for oxygen-deficient soil that ARs try to avoid through upward growth as an escape response. Light indicates an environment that could be in flood water but also could be in air, which bears the danger of desiccation for the root. Hence, in the light, ARs grow downward.
Figure 12.
Scheme summarizing the impact of light and gravity on AR growth direction. The growth angle of 119.8° at 1 g in the dark was taken from Figure 1C, that of 106° at 0 g in the dark was taken from Figure 10B, that of 99° at −1 g in the dark was taken from Figure 10, D and F, and that of 54.2° in white light + ethephon was taken from Figure 7B.
Regulation of AR Growth by Light
Plants sense light by a number of photoreceptors, including phytochromes, cryptochromes, and phototropins. Plant photoreceptors are specialized to perceive different light qualities (wavelengths) and to measure light intensity (fluence rate). The information gained from light signals helps plants to adapt to their environment. The red light receptor phytochrome exists in two isoforms that are interconvertible, the far-red light receptor Pfr and the red light receptor Pr (Li et al., 2011). Rice has three phytochrome genes, PHYA, PHYB, and PHYC. PHYA and PHYC interact to mediate red and far-red light responses, likely by stabilization of PHYC by PHYA (Takano et al., 2005; Xie et al., 2014). PHYB modulates red light signaling by regulating the Arabidopsis response regulator ARR4 (Sweere et al., 2001). Cryptochrome and phototropin are blue light receptors with distinct functions (Christie et al., 2015). While cryptochrome regulates photomorphogenesis, phototropic responses are generally controlled by phototropin that is encoded by two genes, PHOT1 and PHOT2, in flowering plants (Fankhauser and Christie, 2015). In Arabidopsis, root phototropism is mediated by PHOT1 that is active over a broad range of blue light intensities (Christie et al., 2015). In addition, the red light receptor phytochrome also has been implicated in the regulation of phototropism.
In rice, we observed two distinct responses to light, AR emergence and a change in AR growth angle. Root penetration was inhibited from about 80% in the dark to 2.2% in white light and was reduced to 18.4% in red light and to 19.1% in far-red light, clearly supporting a role of both red and far-red light signaling in the control of AR emergence. Root emergence is furthermore controlled by blue light that reduced the number of penetrated roots in a dose-dependent manner, with a significant reduction observed at 0.12 µmol m−2 s−1 blue light and a full inhibition to 7.5% at 3.8 µmol m−2 s−1. Hence, light of different quality is able to prevent root penetration, with blue light being more effective than red/far-red light. In rice seedlings, continuous white light irradiation was shown to inhibit the growth of seminal roots (Shimizu et al., 2009). Partial inhibition of seminal root growth was observed with red, far-red, and blue light. Analysis of phytochrome mutants revealed that far-red light was perceived exclusively by PhyA, whereas red light was perceived by both PhyA and PhyB. Little or no role was assigned to PhyC in growth inhibition of the seminal roots. Light can penetrate soils to some degree, with red light penetrating deeper (several centimeters) than blue light that is perceived in shallow soil. In flood waters, numerous factors, including turbidity and chlorophyll content, determine the quality and intensity of penetrating light (Dev and Shanmugam, 2014). It may be advantageous for plants to perceive a whole range of light with different photoreceptors to control AR growth in an environment where not only light intensity but also light quality varies.
As long as the rice stem does not get flooded or buried in soil, ARs will not grow. In flooded plants, emergence of root primordia is promoted even in the light by ethylene that gets trapped naturally due to its low gas diffusion rate in water. In prolonged darkness, plants start to senesce, a process that is promoted by ethylene (Johnson and Ecker, 1998; Wang et al., 2002). Ethylene accumulation during an extended dark period has been shown previously for rice and Arabidopsis (Fukao et al., 2012; Rasmussen et al., 2017). In this study, we show that inhibition of ethylene signaling abolishes dark-induced AR emergence, supporting the conclusion that AR growth is mediated by ethylene both in the dark and during submergence. Interestingly, the SUB1A-1 locus, which confers submergence tolerance in rice, dampens ethylene formation and delays senescence-associated processes such as chlorophyll breakdown (Fukao et al., 2012). It is conceivable that rice genotypes that carry the SUB1A-1 locus have delayed AR growth in the dark compared with rice varieties that follow an escape strategy that is driven by ethylene. In rice seedlings, seminal root growth is inhibited by white light. Analysis of phytochrome mutants revealed that seminal roots were inhibited by far-red light perceived by PHYA and by red light through both PHYA and PHYB signaling, whereas PHYC played no role in this response (Shimizu et al., 2009). In addition, blue light partially inhibited the growth of seminal roots, indicating that, in rice, phytochrome and blue light signaling regulate both seminal and AR growth, but to different degrees.
The growth angle of ARs was reversed in light in a gradual, dose-dependent manner, indicating that the AR system can adjust to a wide range of light intensities. Blue light was as effective as white light in redirecting ARs downward, whereas red and far-red light caused a partial redirection of ARs at the light intensities analyzed. Taken together, AR emergence and growth angle are under the control of red and blue light receptors and are inhibited by red and blue light to different degrees.
Blue light signal transduction via phototropin involves NPH3 of the NRL (NPH3/RPT2-like) family (Holland et al., 2009) that interacts directly with PHOT1 (Motchoulski and Liscum, 1999). NPH3 acts as a substrate adapter in a CULLIN3-based E3 ubiquitin ligase (Roberts et al., 2011), with PHOT1 being a substrate in Arabidopsis. Both NPH3 and CUL3 were shown to be required for PHOT1 ubiquitination following blue light irradiation and may serve to internalize or degrade PHOT1 depending on the degree of ubiquitination (Roberts et al., 2011). CPT1 is a rice ortholog of Arabidopsis NPH3 (Haga et al., 2005). The cpt1 mutant displays reduced negative phototropism of the primary root of rice seedlings in response to unilateral blue light, while the gravitropic response was not changed. Analysis of AR growth angle in the wild-type cv Nihonmasari and in cpt1 mutant plants revealed no difference in blue light inhibition of AR emergence. However, cpt1 plants showed a decreased sensitivity toward blue light with respect to the negative phototropic response, indicating that the regulation of the AR growth angle is mediated in part by CPT1. The cpt1 mutant was still responsive to higher fluence rates of blue light, possibly due to the activity of redundant NRL family members. Haga et al. (2005) predicted 26 CPT1 homologs in rice.
In summary, we have shown that secondary root system architecture in rice is altered substantially during flooding. Ethylene promotes AR growth, and far-red, red, and blue light signaling control AR growth direction (Fig. 12). We propose that the environmental control of the AR system serves to keep ARs close to the waterlogged soil surface and, thus, to escape oxygen shortage, which, in turn, supports plant survival during flooding.
CONCLUSION
Rice is a semiaquatic plant that generates AR primordia at each stem node that emerges upon flooding. For rice plants, stem nodes experience extended darkness when they are buried in soil or submerged in very turbid flood waters. In either case, the nodes are likely to become oxygen deficient. In the dark, ARs emerge and grow upward, possibly to escape this low-oxygen environment. The AR growth angle is modulated moderately by gravity, whereas the decision between upward and downward growth is regulated by light signaling through red and blue light receptors (Fig. 12). Hence, light is the most important environmental signal that shapes AR architecture in rice during flooding. We propose that light is an indirect cue for oxygen availability. Lack of this signal in the dark triggers an escape response.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Seeds of rice (Oryza sativa indica) cv PG56 were originally obtained from the International Rice Research Institute. The japonica cv Nihonmasari and the cpt1 mutant were donated by Moritoshi Iino (Osaka City University). The japonica cv Kinmaze was provided by Ko Shimamoto (Plant Molecular Genetics, Nara Institute of Science and Technology). The japonica cv ZH11 was obtained from Ping Wu (Zhejiang University). The japonica cultivar T65 was obtained from Motoyuki Ashikari (Nagoya University). The japonica cv Nipponbare was obtained from the Genome Research Centre. And the japonica cv Bomba was obtained from José L. García-Martínez (Instituto de Biología Molecular y Celular de Plantas, Universidad Politécnica de Valencia). Plants were grown for 12 to 20 weeks according to Sauter (1997). Stem sections with a total length of 20 cm were cut 2 cm below the third node and incubated in a 150-mL beaker with 20 mL of tap water and 150 µm ethephon where indicated. A plastic cylinder covered the beaker to ensure high humidity (Steffens and Sauter, 2005). Stem sections were kept in a growth chamber at 27°C and 70% humidity. To inhibit ethylene perception, stems were treated with 5 µL L−1 1-MCP.
For partial submergence of rice plants, a 600-L tank was filled with tap water 2 d before plant treatment to adjust the water temperature to an average of 26.8°C. Experiments were carried out in the same growth chamber as experiments with stem sections at 27°C and 70% relative humidity. During partial submergence, ∼30% of the leaves remained above the water surface (Sasidharan et al., 2017).
Light Treatments
For soil experiments, 20-cm-long stem sections were placed in soil to a depth of 8 cm to monitor ARs at the third node that was 2 cm above the lower cut surface (6 cm below the soil surface). After the treatment, stems were removed from the soil, washed twice with tap water, and ARs were analyzed. Partially submerged plants were exposed to either darkness or light. The white light intensity perceived by leaves that remained in air was at least 362.3 µmol m−2 s−1 (EYE CERA ARC CMT220/W/BH; IWASAKI).
To incubate stem sections in the dark, the cylinder with which stems were covered was wrapped in aluminum foil. For treatment with white light, stems were exposed to 43 µmol m−2 s−1 white light (EYE CERA ARC CMT220/W/BH; IWASAKI). For far-red light treatment, a light-emitting diode (LED) lamp (Philips; LED E27 far-red light lamp) with a maximum wavelength of 730 nm was used. To provide blue or red light, an LED light (Thomann; LED Flood Panel 150) was used accordingly. The wavelengths of red light irradiance were 620 to 640 nm, and the wavelengths of blue light were 460 to 480 nm. The LED lamps were fixed 1 m above the cylinder, and light intensity was measured 2 cm below the third node of the stem sections. Five different light intensities were used for red and blue light treatments. Light intensities were measured from 250 to 799 nm with a double monochromator (DMc150; Bentham). Irradiance was measured every 1 nm as skip size and was normalized as mW m−2 nm−1.
Measurement of Root Penetration Rate, Root Angle, and Root Length
The total number of AR primordia and the number of emerged roots were counted at each node, and the percentage of emerged ARs was calculated from that. The numbers given in the graphs are average percentages obtained from all stem sections (i.e. nodes) analyzed. The number of stems analyzed is provided for each experiment in the figure legends. Images of ARs were taken with a digital camera (PowerShot; SX220HS). The root angle was measured using ImageJ software (version 146r; National Institutes of Health). To determine AR lengths, the roots were excised and their lengths were measured with a ruler.
X-Ray Computed Tomography Imaging
Twenty-centimeter-long stem sections were cut such that the third node was 4 cm above the lower cut surface. Stems were placed up to 9 cm in Styrofoam. After 3 d of incubation in the dark, the buried part of the stem was scanned in a 180-kV nanofocus computed tomography system (GE Sensing & Inspection Technologies) and visualized with Volume Graphics VGStudio MAX software (GE Sensing & Inspection Technologies) at a voxel resolution of 120 µm or less.
Gravity Experiments and Statolith Staining
For clinostat experiments, each stem section was fixed in a 10-mL tube (Falcon) filled with 10 mL of tap water such that the third node remained outside the tube. Tubes were sealed with Parafilm to prevent water leakage. In order to provide an atmosphere of high humidity, a 50-mL centrifuge tube (Falcon) was placed above this setup. Stem sections were rotated vertically at a speed of 5 rpm in the dark. For inverse experiments, in one setup, stem sections were placed in a beaker in either upright or inverse orientation. In another setup, a stem section was fixed with its lower cut surface in a 10-mL tube (Falcon) filled with 10 mL of tap water and placed upright or inverted in a cylinder to ensure high humidity. In either setup, stems were incubated in the dark for 3 d. Subsequently, AR growth angles were determined.
In order to visualize the starch-containing statocytes in the root cap, cross sections including the AR primordia were cut from the first, second, third, and fourth nodes of cv PG56. In addition, we isolated intact ARs from each node. The samples were stained with Lugols’ solution for 1 min and washed once with distilled water for 1 min.
Statistical Analysis
Statistical analyses were performed using Minitab. Cell death rates were calculated with arcsine√(x/100) formation to obtain distributed data. Comparison of means was performed for statistical significance with an ANOVA Tukey’s test or two-sample Student’s t test. Constant variance and normal distribution of data were verified before statistical analysis. The P value was set to P < 0.05.
Accession Number
The accession number for CPT1 is AB186127.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. ARs growing at the first and second nodes of rice stems excised from deepwater rice plants.
Acknowledgments
We thank Moritoshi Iino (Osaka City University) for providing seeds of the rice wild-type cv Nihonmasari and the cpt1 mutant, Wolfgang Bilger (University of Kiel) for expert advice and help with light experiments, and Rainer Horn (University of Kiel) for computed tomography analysis.
Footnotes
This work was supported by the China Scholarship Council through a grant to C.L.
Articles can be viewed without a subscription.
References
- Atkinson JA, Rasmussen A, Traini R, Voß U, Sturrock C, Mooney SJ, Wells DM, Bennett MJ (2014) Branching out in roots: uncovering form, function, and regulation. Plant Physiol 166: 538–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayi Q, Zeng B, Liu J, Li S, van Bodegom PM, Cornelissen JH (2016) Oxygen absorption by adventitious roots promotes the survival of completely submerged terrestrial plants. Ann Bot 118: 675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Murali B, Barber K, Wolverton C (2013) Low phosphate alters lateral root setpoint angle and gravitropism. Am J Bot 100: 175–182 [DOI] [PubMed] [Google Scholar]
- Baldwin KL, Strohm AK, Masson PH (2013) Gravity sensing and signal transduction in vascular plant primary roots. Am J Bot 100: 126–142 [DOI] [PubMed] [Google Scholar]
- Bellini C, Pacurar DI, Perrone I (2014) Adventitious roots and lateral roots: similarities and differences. Annu Rev Plant Biol 65: 639–666 [DOI] [PubMed] [Google Scholar]
- Briggs WR. (2014) Phototropism: some history, some puzzles, and a look ahead. Plant Physiol 164: 13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Blackwood L, Petersen J, Sullivan S (2015) Plant flavoprotein photoreceptors. Plant Cell Physiol 56: 401–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev PJ, Shanmugam P (2014) New model for subsurface irradiance reflectance in clear and turbid waters. Opt Express 22: 9548–9566 [DOI] [PubMed] [Google Scholar]
- de Wit M, Galvão VC, Fankhauser C (2016) Light-mediated hormonal regulation of plant growth and development. Annu Rev Plant Biol 67: 513–537 [DOI] [PubMed] [Google Scholar]
- Digby J, Firn RD (1995) The gravitropic set-point angle (GSA): the identification of an important developmentally controlled variable governing plant architecture. Plant Cell Environ 18: 1434–1440 [DOI] [PubMed] [Google Scholar]
- Ding Z, Galván-Ampudia CS, Demarsy E, Łangowski Ł, Kleine-Vehn J, Fan Y, Morita MT, Tasaka M, Fankhauser C, Offringa R, et al. (2011) Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat Cell Biol 13: 447–452 [DOI] [PubMed] [Google Scholar]
- Fankhauser C, Christie JM (2015) Plant phototropic growth. Curr Biol 25: R384–R389 [DOI] [PubMed] [Google Scholar]
- Fukao T, Yeung E, Bailey-Serres J (2012) The submergence tolerance gene SUB1A delays leaf senescence under prolonged darkness through hormonal regulation in rice. Plant Physiol 160: 1795–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen C, Rabenold JJ, Liscum E (2007) Functional ecology of a blue light photoreceptor: effects of phototropin-1 on root growth enhance drought tolerance in Arabidopsis thaliana. New Phytol 173: 91–99 [DOI] [PubMed] [Google Scholar]
- Guyomarc’h S, Léran S, Auzon-Cape M, Perrine-Walker F, Lucas M, Laplaze L (2012) Early development and gravitropic response of lateral roots in Arabidopsis thaliana. Philos Trans R Soc Lond B Biol Sci 367: 1509–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K, Takano M, Neumann R, Iino M (2005) The rice COLEOPTILE PHOTOTROPISM1 gene encoding an ortholog of Arabidopsis NPH3 is required for phototropism of coleoptiles and lateral translocation of auxin. Plant Cell 17: 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland JJ, Roberts D, Liscum E (2009) Understanding phototropism: from Darwin to today. J Exp Bot 60: 1969–1978 [DOI] [PubMed] [Google Scholar]
- Johnson PR, Ecker JR (1998) The ethylene gas signal transduction pathway: a molecular perspective. Annu Rev Genet 32: 227–254 [DOI] [PubMed] [Google Scholar]
- Li J, Li G, Wang H, Deng XW (2011) Phytochrome signaling mechanisms. The Arabidopsis Book 9: e0148, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorbiecke R, Sauter M (1999) Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol 119: 21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motchoulski A, Liscum E (1999) Arabidopsis NPH3: a NPH1 photoreceptor-interacting protein essential for phototropism. Science 286: 961–964 [DOI] [PubMed] [Google Scholar]
- Ottenschläger I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K (2003) Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA 100: 2987–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen O, Rich SM, Colmer TD (2009) Surviving floods: leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice. Plant J 58: 147–156 [DOI] [PubMed] [Google Scholar]
- Rasmussen A, Hu Y, Depaepe T, Vandenbussche F, Boyer FD, Van Der Straeten D, Geelen D (2017) Ethylene controls adventitious root initiation sites in Arabidopsis hypocotyls independently of strigolactones. J Plant Growth Regul 36: 897–911 [Google Scholar]
- Rich SM, Pedersen O, Ludwig M, Colmer TD (2013) Shoot atmospheric contact is of little importance to aeration of deeper portions of the wetland plant Meionectes brownii: submerged organs mainly acquire O2 from the water column or produce it endogenously in underwater photosynthesis. Plant Cell Environ 36: 213–223 [DOI] [PubMed] [Google Scholar]
- Roberts D, Pedmale UV, Morrow J, Sachdev S, Lechner E, Tang X, Zheng N, Hannink M, Genschik P, Liscum E (2011) Modulation of phototropic responsiveness in Arabidopsis through ubiquitination of phototropin 1 by the CUL3-Ring E3 ubiquitin ligase CRL3(NPH3). Plant Cell 23: 3627–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Sahoo D, Tripathy BC (2013) Involvement of phytochrome A in suppression of photomorphogenesis in rice seedling grown in red light. Plant Cell Environ 36: 2120–2134 [DOI] [PubMed] [Google Scholar]
- Roychoudhry S, Kieffer M, Del Bianco M, Liao CY, Weijers D, Kepinski S (2017) The developmental and environmental regulation of gravitropic setpoint angle in Arabidopsis and bean. Sci Rep 7: 42664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasidharan R, Bailey-Serres J, Ashikari M, Atwell BJ, Colmer TD, Fagerstedt K, Fukao T, Geigenberger P, Hebelstrup KH, Hill RD, et al. (2017) Community recommendations on terminology and procedures used in flooding and low oxygen stress research. New Phytol 214: 1403–1407 [DOI] [PubMed] [Google Scholar]
- Sassi M, Lu Y, Zhang Y, Wang J, Dhonukshe P, Blilou I, Dai M, Li J, Gong X, Jaillais Y, et al. (2012) COP1 mediates the coordination of root and shoot growth by light through modulation of PIN1- and PIN2-dependent auxin transport in Arabidopsis. Development 139: 3402–3412 [DOI] [PubMed] [Google Scholar]
- Sato EM, Hijazi H, Bennett MJ, Vissenberg K, Swarup R (2015) New insights into root gravitropic signalling. J Exp Bot 66: 2155–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter M. (1997) Differential expression of a CAK (cdc2-activating kinase)-like protein kinase, cyclins and cdc2 genes from rice during the cell cycle and in response to gibberellin. Plant J 11: 181–190 [DOI] [PubMed] [Google Scholar]
- Sauter M. (2013) Root responses to flooding. Curr Opin Plant Biol 16: 282–286 [DOI] [PubMed] [Google Scholar]
- Shimizu H, Tanabata T, Xie X, Inagaki N, Takano M, Shinomura T, Yamamoto KT (2009) Phytochrome-mediated growth inhibition of seminal roots in rice seedlings. Physiol Plant 137: 289–297 [DOI] [PubMed] [Google Scholar]
- Steffens B, Sauter M (2005) Epidermal cell death in rice is regulated by ethylene, gibberellin, and abscisic acid. Plant Physiol 139: 713–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Wang J, Sauter M (2006) Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 223: 604–612 [DOI] [PubMed] [Google Scholar]
- Su SH, Gibbs NM, Jancewicz AL, Masson PH (2017) Molecular Mechanisms of Root Gravitropism. Current Biology. Curr Biol 27: R964–R972 [DOI] [PubMed] [Google Scholar]
- Swarup R, Kramer EM, Perry P, Knox K, Leyser HM, Haseloff J, Beemster GT, Bhalerao R, Bennett MJ (2005) Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat Cell Biol 7: 1057–1065 [DOI] [PubMed] [Google Scholar]
- Sweere U, Eichenberg K, Lohrmann J, Mira-Rodado V, Bäurle I, Kudla J, Nagy F, Schäfer E, Harter K (2001) Interaction of the response regulator ARR4 with phytochrome B in modulating red light signaling. Science 294: 1108–1111 [DOI] [PubMed] [Google Scholar]
- Takano M, Inagaki N, Xie X, Kiyota S, Baba-Kasai A, Tanabata T, Shinomura T (2009) Phytochromes are the sole photoreceptors for perceiving red/far-red light in rice. Proc Natl Acad Sci USA 106: 14705–14710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M, Inagaki N, Xie X, Yuzurihara N, Hihara F, Ishizuka T, Yano M, Nishimura M, Miyao A, Hirochika H, et al. (2005) Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice. Plant Cell 17: 3311–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, et al. (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45: 1097–1102 [DOI] [PubMed] [Google Scholar]
- Wang KL, Li H, Ecker JR (2002) Ethylene biosynthesis and signaling networks. Plant Cell (Suppl) 14: S131–S151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel A, Colmer TD, Ismail AM, Pedersen O (2013) Internal aeration of paddy field rice (Oryza sativa) during complete submergence: importance of light and floodwater O2. New Phytol 197: 1193–1203 [DOI] [PubMed] [Google Scholar]
- Xie X, Kagawa T, Takano M (2014) The phytochrome B/phytochrome C heterodimer is necessary for phytochrome C-mediated responses in rice seedlings. PLoS ONE 9: e97264. [DOI] [PMC free article] [PubMed] [Google Scholar]