Abstract
Light signaling can affect root development and plasticity, either directly or through shoot-root communication via sugars, hormones, light, or other mobile factors.
Light is the energy source for plants as it drives photosynthesis to produce sugars. Given the obvious fact that light mostly occurs above ground and not in the soil, most interactions of plants with light have been studied in shoot parts of the plant. Research over more than a century has yielded tremendous insights into how light not only drives photosynthesis but also acts as an environmental cue that informs plants about their environment. Light quality and duration, for example, drive major developmental changes such as photomorphogenesis, photoperiodic induction of flowering, phototropism, and shade avoidance (see, for example, the following recent reviews: Wu, 2014; Fankhauser and Christie, 2015; Xu et al., 2015; Ballaré and Pierik, 2017). The picture that has emerged is that plants have very detailed light signaling mechanisms, with photoreceptors dedicated to different wavelengths in the light spectrum and interactions between these photoreceptors themselves and their downstream signal transduction pathways.
Studies have accumulated over the past 15 years, and intensified in recent years, showing pronounced effects of light on root physiology and development. Although some effects of light availability on root growth will be the simple consequence of differential sugar availability to the roots due to photosynthesis in the shoot, there is substantial evidence for more sophisticated signaling impacts of different aspects of the light environment.
In this Update, we will briefly review the core light signaling mechanisms, their impact on root development and plasticity, and the functional implications of these aboveground-belowground interactions.
LIGHT SIGNALING
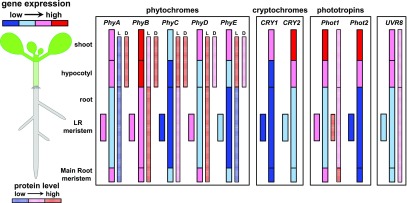
Different wavelengths of light are associated with various functions in plant development, and plants have a range of photoreceptors to detect these wavelengths. UV-B RESISTANCE LOCUS8 (UVR8) is sensitive to UV-B light, cryptochromes (CRYs) and phototropins (PHOTs) detect UV-A and blue light, and phytochromes (PHYs) sense red (R) and far-red (FR) light. Photoreceptors occur all over the plant body, and although they are most abundant in the shoot, they are also expressed in the roots (Fig. 1). Photoreceptors share downstream signaling hubs, notably the CONSTITUTIVE PHOTOMORPHOGENESIS1 (COP1)/SUPPRESSOR OF PHYTOCHROME A (SPA) complex and PHYTOCHROME INTERACTING FACTORs (PIFs), which will be discussed first, followed by the properties of each photoreceptor group.
Figure 1.
Relative amounts of photoreceptors in Arabidopsis tissues. The graph displays the relative expression of photoreceptors across seedling tissues. Both gene expression and protein (when data are available) abundance are shown. The box that groups classes of receptors indicates that intensities within can be compared. In the PHY box, “L” stands for protein levels in light and “D” for protein levels in the dark. Source data: for all, BAR eFP browser (http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi), PHYs (Somers and Quail, 1995; Goosey et al., 1997; Tóth et al., 2001; Sharrock and Clack, 2002; Salisbury et al., 2007), PHOTs (Sakamoto and Briggs, 2002; Moni et al., 2015), CRYs (Tóth et al., 2001), and UVR8 (Rizzini et al., 2011).
Shared Signaling Hubs
COP1-SPA-HY5
COP1 functions as a ubiquitin E3 ligase that targets proteins required for photomorphogenesis for degradation, such as the bZIP transcription factor ELONGATED HYPOCOTYL5 (HY5; Osterlund et al., 2000; Saijo et al., 2003) and HY5-HOMOLOG (HYH; Holm et al., 2002). Especially, HY5 is a key regulator in photomorphogenesis, and it serves as the center of a transcriptional network hub of hormone and light signaling (Gangappa and Botto, 2016). Besides HY5, COP1 also targets factors like the MYB transcription factor LONG AFTER FAR-RED LIGHT1 (Seo et al., 2003), the basic helix-loop-helix protein LONG HYPOCOTYL IN FAR-RED1 (Duek et al., 2004), and even phytochrome A (phyA) and phyB photoreceptors (Seo et al., 2004; Jang et al., 2010). COP1 activity relies on physical interaction with SPAs in the COP1/SPA complex. The CRY photoreceptors CRY1 and CRY2, upon blue light activation, can bind to SPAs, and this results in reduced COP1-SPA interaction, leading to stabilization of COP1 targets (Lian et al., 2011; Liu et al., 2011; Zuo et al., 2011). Comparable interaction mechanisms exist between PHYs and SPAs (Zheng et al., 2013; Sheerin et al., 2015), and UVR8 and SPAs (Favory et al., 2009; Huang et al., 2013). Another mechanism that controls COP1 activity is its light-based nuclear exclusion that affects HY5 degradation (von Arnim and Deng, 1994; Pacín et al., 2014). COP1 and HY5 are well-known regulators of the transition from skoto- to photomorphogenesis; for a detailed overview, we refer to Huang et al. (2014).
PIFs
PIFs are a group of basic helix-loop-helix transcription factors that mediate various physiological responses, such as seed germination, seedling photomorphogenesis, shade avoidance, and shoot architectural responses to elevated temperature (Koini et al., 2009; Chen and Chory, 2011; Hornitschek et al., 2012; Jeong and Choi, 2013; Xu et al., 2015). PIFs are regulated by light, temperature, and the circadian clock, and these cues can lead to PIF degradation by concerted action of the PHYs and the E3-ligase Light-Response Bric-a-Brack/Tramtrack/Broad (Leivar and Monte, 2014; Ni et al., 2014; Jung et al., 2016; Legris et al., 2016). The PHY and CRY photoreceptors can physically interact with specific sets of PIF proteins and thereby regulate PIF phosphorylation. The phosphorylation status of PIFs subsequently determines their activity and in most instances their stability (Paik et al., 2017). ChIP-seq studies on PIFs have identified a very broad range of target genes, including those associated with cell wall modifications, auxin biology and several other transcription factors. This broad range of targets combined with the multitude of signals that impact on PIF stability makes PIF proteins a signaling hub to integrate environmental conditions (Leivar and Monte, 2014; Paik et al., 2017).
Photoreceptors
UVR8
The effect of UV-B radiation on plant growth is dual. High fluence UV-B exposure causes photodamage, but low fluence UV-B contributes to photomorphogenesis and increases the resistance to herbivorous insects and pathogens (Ballaré et al., 2012; Galvão and Fankhauser, 2015). UV-B (280–315 nm) is perceived by UVR8, which uses a structure based on tryptophans and a complex salt bridge network. Upon sensing UV-B light, the salt bridges are disrupted, inducing dimer dissociation into UVR8 monomers and initiating signal transduction (Christie et al., 2012). The UV-B-induced responses include hypocotyl growth inhibition, altered leaf morphogenesis, stomatal closure, and compound synthesis associated with the prevention and repair of UV damage (Binkert et al., 2014; Galvão and Fankhauser, 2015). UVR8 regulates UV-B responses by negatively affecting COP1 function through the UVR8-SPA interaction (Osterlund et al., 2000; Favory et al., 2009; Huang et al., 2013; Hayes et al., 2014). Formation of UVR8-SPA leads to inhibition of COP1 function, and this results in stabilization of, among others, HY5 and HYH, thereby inducing genes downstream associated with UV-B signaling (Osterlund et al., 2000; Rizzini et al., 2011; Hayes et al., 2014).
CRYs
Blue light (320–500 nm) is used for photosynthesis and also serves as a signal for shade, photoperiodism, and directional light. Blue light sensing is performed by three groups of light receptors: CRYs, PHOTs, and other LOV domain-containing receptors such as ZEITLUPE (ZTL). CRYs are blue light receptors found in a broad range of organisms, including bacteria, fungi, animals, and plants. Three CRYs are encoded in the Arabidopsis (Arabidopsis thaliana) genome: CRY1, CRY2, and CRY3. CRY1 and CRY2 act redundantly in promoting flower induction, sensing blue light as input to circadian clock, and stomatal opening in Arabidopsis (Li and Yang, 2007; Galvão and Fankhauser, 2015; Mo et al., 2015). Both CRY1 and CRY2 regulate primary root elongation, but CRY1 promotes primary root elongation in blue light, whereas CRY2 has the opposite effect (Canamero et al., 2006). Mainly, CRY2 is expressed in Arabidopsis roots (Fig. 1), and CRY1 and CRY2 inhibit root growth via modulation of free auxin levels and polar auxin transport (Mo et al., 2015). The biological function of CRY3 is yet unclear (Song et al., 2006; Klar et al., 2007; Mo et al., 2015). CRYs are nuclear flavoproteins, composed of two domains, an N-terminal photolyase-related region and a C-terminal domain of varying size. The photolyase-related region binds the chromophore FAD and serves as the light-sensing part. Upon absorbing blue/UV-A light, CRY is phosphorylated and can interact with other protein partners, such as COP1, SPAs, and PIFs (Lian et al., 2011; Liu et al., 2011; Zuo et al., 2011; de Wit et al., 2016; Pedmale et al., 2016).
PHOTs
PHOTs are blue light receptors that mediate phototropism, chloroplast movement, and stomatal opening in Arabidopsis (Briggs and Christie, 2002). There are two PHOTs identified in Arabidopsis, phot1 and phot2, with similar function and structure. Besides their function in the shoot, phot1 is also expressed in the roots, regulating root bending (Wan et al., 2012; Zhang et al., 2013). In contrast to other photoreceptors, which are present in the nucleus or cytoplasm, PHOTs are located in the plasma membrane (Sakamoto and Briggs, 2002). When phot1 is activated by blue light, it phosphorylates PHYTOCHROME KINASE SUBSTRATE4 (PKS4), and interacts with NON-PHOTOTROPIC HYPOCOTYL3 (NPH3) to form a phot1-PKS-NPH3 protein complex (Pedmale and Liscum, 2007; Demarsy et al., 2012). This modulates auxin transport and thus underlies plant phototropism (Fankhauser and Christie, 2015). PHOTs have N-terminal FMN chromophore-binding light oxygen voltage (LOV1 and LOV2) domains for light absorption and a C-terminal AGC-type Ser/Thr protein kinase signaling domain (Briggs and Christie, 2002).
The ZTL, FLAVIN-BINDING, KELCH REPEAT, F-BOX, and LOV KELCH PROTEIN2 are blue light photoreceptors involved in circadian clock and photoperiodic flowering regulation, with a structure similar to PHOTs but with only one LOV domain followed by an F-box and six Kelch repeats (Galvão and Fankhauser, 2015).
PHYs
PHYs are photoreceptors sensing R and FR light that occur in plants as well as in fungi and prokaryotes (Burgie and Vierstra, 2014). Plants use R/FR light signaling through phytochromes to regulate germination, de-etiolation, stomatal development, flowering transition, senescence, and shade avoidance (Franklin and Quail, 2010). Five PHYs are identified in Arabidopsis, phyA through phyE. PHYs use the N-terminal covalently linked phytochromobilin to sense light and the C terminus to transmit the light signal. PHYs undergo reversible conformation changes: The inactive Pr form absorbs R light (max = 660 nm) that leads to its photoconversion into the active Pfr form that can then absorb FR light (max = 730 nm) to be inactivated. Upon activation, Pfr translocates from the cytosol to the nucleus, where it interacts with PIFs, modulating their activity (Chen and Chory, 2011; Leivar and Quail, 2011; Xu et al., 2015). PhyA, PhyB, PhyD, and PhyE are expressed in the root, while PhyC expression in the root is hardly detectable (Fig. 1). Phyb mutants have been shown to produce fewer lateral roots (Salisbury et al., 2007), and Phyb and Phya single and double mutants have reduced root elongation compared with the wild type (Correll and Kiss, 2005; Silva-Navas et al., 2015). Recently, PhyB has been identified to act as a temperature sensor (Jung et al., 2016; Legris et al., 2016), which is interesting since heat stress is known to lead to increased main root growth (Hanzawa et al., 2013). If this occurs through PhyB signaling has yet to be resolved, but since high temperatures destabilize PhyB and PhyB mutants have reduced root lengths, this may not be likely.
IMPACT OF DIRECT LIGHT SIGNALING ON ROOT DEVELOPMENT AND PLASTICITY
Root Development in Dark Versus Light
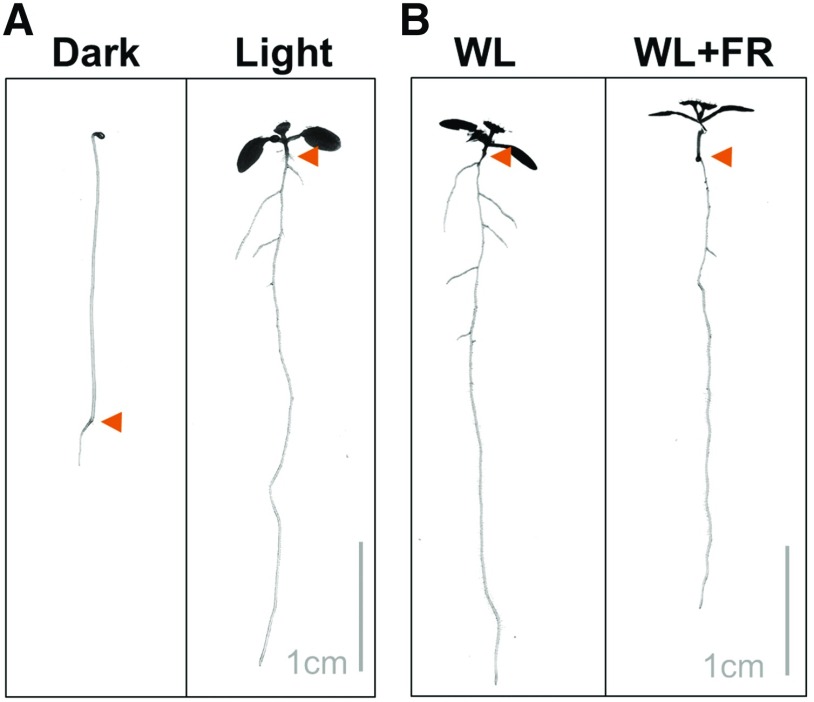
Root development (Box 1) starts at embryogenesis (Scheres et al., 1994), and all root layers are continuously formed from stem cells at the tip (Petricka et al., 2012) that are maintained by a constant auxin flow (Blilou et al., 2005; Galinha et al., 2007; Stepanova et al., 2008; Mähönen et al., 2014) while cytokinin promotes differentiation of these root layers (Dello Ioio et al., 2007, 2008). Lateral roots form after embryogenesis and are primed in the differentiation zone of the meristem (De Smet et al., 2007; De Rybel et al., 2010). Subsequently, they emerge through the outer layers of the main root (Lavenus et al., 2013) upon modification of these layers (Péret et al., 2013; Vermeer et al., 2014). In total darkness, the Arabidopsis seedling elongates its hypocotyl in an attempt to penetrate the soil, but its root stays very small (Fig. 2). Roots of dark-grown seedlings are much shorter and have a much thinner diameter than those of light-grown seedlings (Laxmi et al., 2008; Dyachok et al., 2011). When etiolated seedlings are exposed to light, they inhibit hypocotyl elongation, develop their cotyledons, and start to photosynthesize (Wu, 2014). Cotyledon-derived sugars are essential for the start of root growth, and when young seedlings are decapitated, root growth is slowed dramatically, also when grown in the light (Kircher and Schopfer, 2012). Besides Suc, the basipetal flow of auxin is necessary to facilitate root growth in seedlings (Bhalerao et al., 2002), and in the dark this basipetal auxin transport is very low due to the depletion of PIN-FORMED (PIN) auxin efflux carriers from the plasma membrane (Laxmi et al., 2008; Sassi et al., 2012). Light induces root growth by providing sugars and auxin to the young root, and specifically R and blue light exhibit a positive effect on root elongation when compared with darkness (Sweere, 2001; Canamero et al., 2006; Costigan et al., 2011; Fig. 2). However, the addition of Suc to the agar medium can sometimes reverse this effect (Correll and Kiss, 2005). Supplementation of white light with FR light reduces root growth compared with normal white light (Salisbury et al., 2007; Fig. 2), and UVB has a strong inhibiting effect on root growth, either when supplied to the whole seedling or only the root (Tong et al., 2008; Leasure et al., 2009; Silva-Navas et al., 2015).
Figure 2.
Root growth is affected by light quantity and quality. A, Eight-day-old seedlings grown on half-strength Murashige and Skoog medium in darkness and white light (WL; 140 μm m−2 s−1 photosynthetically active radiation). B, Eight-day-old seedling grown in white light (140 μm m−2 s−1) or white light plus FR (WL+FR; R:FR 0.1). The arrowheads point to the root-shoot junctions.
Many experiments addressing root development have been performed in the presence of light on the whole seedling since root development is typically studied in vertical agar plate setups with transparent medium. In field conditions, the top part of the root system will grow in minimal light and the lower part will even develop in darkness, while the shoot can be exposed to various different light conditions (Smith, 1982). Several solutions to these undesirable lab conditions have been postulated, including dark agar plugs (Sassi et al., 2012), black-colored vertical plates (Xu et al., 2013), and the D-Root system consisting of plate inserts plus cover slips (Silva-Navas et al., 2015). In the D-Root system, seedlings grow on medium in vertical square plates, but an insert at the root-shoot junction and an external cover slip prevent light exposure of the roots, whereas the shoot is exposed to the ambient light conditions. Compared with plants with roots grown in fully exposed light conditions, plants with roots in darkness have increased main root length and lateral root number, while root hair length decreased. When dark-grown roots were exposed to light for a duration longer than 8 h, their growth rate and root meristem size declined (Silva-Navas et al., 2016). Interestingly, shielding roots from light decreased the sensitivity of the main root to the plant hormones abscisic acid, brassinolide, and 6-benzyladenine, while the sensitivity to indole-3-acetic acid was increased (Silva-Navas et al., 2015). Interestingly, dark-exposed roots were less sensitive to salt and low nitrogen conditions compared with light-exposed roots (Silva-Navas et al., 2015). These data show that direct perception of light by the root is physiologically relevant in Arabidopsis seedlings.
Light Affects Root Developmental Plasticity
The root system can change the direction of growth in response to stimuli such as gravity (Morita, 2010) or light (Kutschera and Briggs, 2012). The movement of the root away from light sources, or root negative phototropism, is dependent upon blue light perception by PHOTs (Wan et al., 2012). Contrary to the negative response to blue light, a positive growth response of the root to R light has also been observed (Kiss et al., 2001), but this response is weak and can only be observed in the absence of gravity sensing (Ruppel et al., 2001; Kiss et al., 2003). Downstream of light perception, root phototropism impinges on elements involved in root gravitropism (Kiss et al., 2003; Kutschera and Briggs, 2012).
Root Negative Phototropism
Root negative phototropism is induced by blue and white light, and the photoreceptors involved are mainly phot1 and phot2, with minor roles for phyA and cry1/cry2 (Boccalandro et al., 2008; Wan et al., 2012; Silva-Navas et al., 2016). NPH3, a target of phot1 and phot2 involved in hypocotyl phototropism, also is an important player in root negative phototropism (Wan et al., 2012). Downstream of directional light perception, polar auxin transport directs auxin away from the illuminated side. This auxin transport gradient requires rootward plasma membrane localization of the auxin efflux carrier PIN1 in the stele, lateral relocalization of PIN3 in the columella, and rootward relocalization of PIN2 in the epidermis (Wan et al., 2012; Zhang et al., 2013, 2014). The relocalization of PIN2 and PIN3 is dependent upon the recycling and targeted degradation (vacuolar targeting) of these PIN carriers from one side of the membrane to the other (Wan et al., 2012; Zhang et al., 2013). The initial perception of gravitropism and phototropism is different, but the downstream signaling events of negative phototropism are very similar to those found in root gravitropism, involving comparable PIN protein and auxin transport dynamics (Friml et al., 2002; Abas et al., 2006; Baster et al., 2013). Most root phototropism experiments have been performed under conditions where the whole seedling was directionally illuminated. When roots that had been grown in the dark were stimulated by a one-sided white light stimulus while the shoot still remained in the dark, the negative phototropism persisted and an important role for flavonols was uncovered in the regulation of this response (Silva-Navas et al., 2016). White light, together with cytokinins, stimulates the accumulation of flavonols on the lighted side of the root, which induces cell elongation and stimulates PIN1 plasma membrane abundance (Buer and Muday, 2004; Silva-Navas et al., 2016).
Interaction of Light and Gravitropism
As mentioned above, both root negative phototropism and gravitropism rely on polar auxin transport. Light influences the direction of polar auxin transport by controlling the plasma membrane abundance of PIN proteins (Laxmi et al., 2008; Sassi et al., 2012; Wan et al., 2012; Zhang et al., 2013, 2014). An interesting example of how light interacts with the gravitropic output is the U-turn that an inverted maize (Zea mays) seedling root makes when growing in a glass tube exposed to light (Burbach et al., 2012; Suzuki et al., 2016). When these seedlings are inverted in the dark, they do not show this strong gravitropic response (U-turn), indicating that light can increase gravitropism. In accordance with this, Arabidopsis root slanting (on agar plates) and the root gravitropic response are reduced in the D-Root system where roots are kept in darkness (Silva-Navas et al., 2015). Light stimulates the gravitropic response through the increase of flavonol biosynthesis, which increases root auxin levels (Buer and Muday, 2004; Silva-Navas et al., 2016). Interestingly, FR light enrichment of Arabidopsis seedlings grown fully in the light leads to reduced activity of the auxin reporter pDR5::GUS (Salisbury et al., 2007), showing how light quality can markedly change root auxin homeostasis. Another way that light signaling influences auxin transport in the root is by controlling the removal of PIN proteins from the plasma membrane via the process of vacuolar degradation. Since PINs are transmembrane proteins, their degradation occurs through vacuolar targeting and subsequent degradation of multivesicular bodies containing PINs (Korbei and Luschnig, 2013). This vacuolar degradation of PINs is an important process in changing PIN polarity and is essential for the regulation of gravitropism (Baster et al., 2013). PIN2-GFP vacuolar targeting is controlled by the COP1/CSN complex in a light-dependent manner (Laxmi et al., 2008; Sassi et al., 2012). When a seedling is incubated in darkness for several hours, PIN2-GFP is targeted toward the vacuole (Kleine-Vehn et al., 2008), but this does not occur when only the seedling shoot is given a light treatment, or in the cop1-6 mutant (Sassi et al., 2012), showing how (COP1-mediated) light signaling in the shoot can affect root development.
ABOVEGROUND LIGHT REGULATES ROOT DEVELOPMENT: MECHANISMS AND CONSEQUENCES
As mentioned above, it is critical to research the effects of light signaling on root developmental plasticity under conditions where roots are not directly exposed to the light environment as they are typically shielded from by the soil under natural conditions. Indeed, root systems develop differently between dark and light conditions of the roots themselves. Since plants constantly coordinate growth and development of root and shoot in response to their highly dynamic environment, they do need to translate important information about their light environment to the root system. This is of particular importance under dense planting conditions, such as in most agricultural fields, where both above- and belowground competition for resources occurs (Pierik et al., 2013; Gundel et al., 2014). Below ground, plants compete for water and nutrients, whereas above ground they struggle for light. Belowground competition is size-symmetric, which means that resource acquisition is proportional to the size of the root system of a given individual in a dense stand. Competition for light is size-asymmetric: A plant that is only slightly taller than its neighbors can put its leaves above those neighbors, thereby severely limiting their total access to light while itself not being affected at all by its neighbors (Weiner, 1985; Weiner and Thomas, 1986). Above ground, plants maximize their competitive performance against neighbors by activating the so-called shade avoidance response (Box 2). Briefly, FR light reflected by neighbor plants inactivates the PHY photoreceptors, and this relieves their repression of PIF4, PIF5, and PIF7. PIFs then accumulate, induce auxin biosynthesis and transport, and auxin subsequently induces elongation of hypocotyls, stems, and petioles as well as upward leaf movement (Lorrain et al., 2008; Keuskamp et al., 2010; Casal, 2013; Gommers et al., 2013; Kohnen et al., 2016; de Wit et al., 2016; Ballaré and Pierik, 2017; Michaud et al., 2017; Pantazopoulou et al., 2017). In this section, we briefly review the currently established mechanisms of light signal information transfer from the shoot to the root, followed by a discussion on its functional importance under dense planting conditions and plant competition.
Mechanisms of Light Signal Information Transfer from Shoot to Root
Stem-Piped Light
Stem-piped light refers to the light transmitted through the internal tissues of the plants from the shoot to the root. This manner of light transmission through the interiors of the plant has been described for woody and herbaceous species. Light piping is wavelength specific, and long wavelengths such as FR and near infrared light are transmitted relatively well, while shorter wavelengths such as blue and green light are less effectively transmitted (Sun et al., 2003, 2005). Stem-piped light can activate root-expressed phyB, which on its turn regulates HY5 in the Arabidopsis root (Lee et al., 2016). HY5 is involved in root growth in response to light, modulating, for example, root gravitropism and nitrogen uptake (Cluis et al., 2004; Lee et al., 2007; Huang et al., 2015; Chen et al., 2016). Therefore, stem-piped light might communicate information about the aboveground light environment to the root (Fig. 3). However, the light transmission goes down to 1% when conduction distances increase to 3 cm in herbaceous plants (Sun et al., 2005), which suggests that this mechanism might play a relatively modest role in mature planting systems.
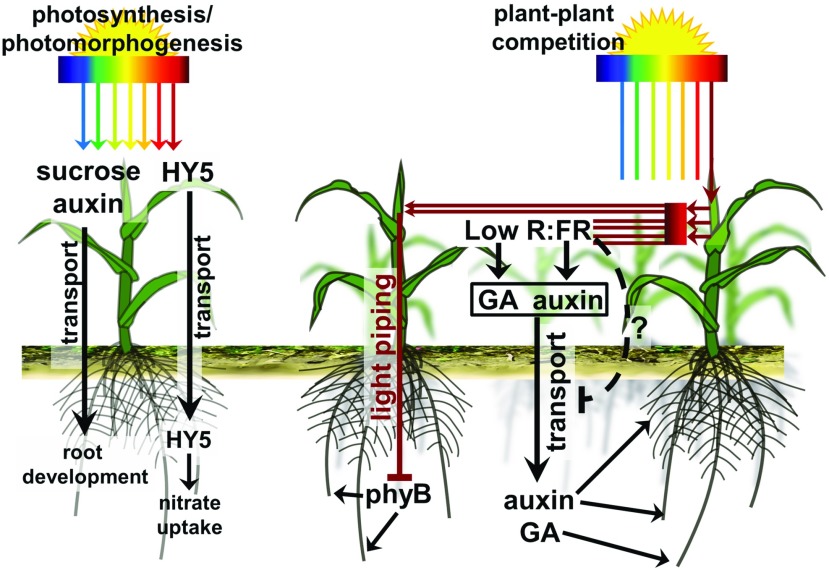
Figure 3.
The aboveground regulation of root development in dense vegetation involves photosynthesis, light piping, hormones, and mobile factors. The primary effect of light is to enable photosynthesis, which leads to the production of sugars (sucrose) that enable the root to grow. Photomorphogenic development is associated with the production of auxins in the shoot, which are transported rootward and enable root development. HY5 is stabilized in the shoot during photomorphogenesis and is transported rootward, where it regulates nitrate uptake and root development. Light is used as a cue to detect neighboring plant competition via sensing of the R:FR ratio. Plant tissues reflect FR, which lowers the nearby R:FR ratio, leading to shade avoidance responses mediated by, among others, auxin and GA. These hormones can be transported rootward, where they affect root development. There also are indications that shade avoidance responses, in a negative feedback mechanism, decrease the amount of rootward auxin transport. FR light itself also can be transmitted directly through woody, vascular tissues from the shoot to the root, where it can affect root-localized PHYs. Plant vector drawing: Lobet (2017).
Mobile (Signaling) Chemicals
Although various components are transported from the shoot to the root, only a few of them have been directly linked to light signaling in the shoot. These include sugars derived from photosynthesis and transported through the phloem, plant hormones, and HY5. These will be briefly discussed below.
Sugars
Once a seed is germinated, it first invests energy in hypocotyl growth. Upon penetrating the soil and perceiving light, photomorphogenesis is initiated and the seedling acquires the ability to conduct photosynthesis, followed by root growth (Kircher and Schopfer, 2012). It was shown in Arabidopsis seedlings that blocking photosynthesis inhibits root growth, just like in darkness, and that adding Suc to the growth medium can rescue root growth. Interestingly, Suc addition could not induce additional root growth in photosynthesis positive seedlings (Kircher and Schopfer, 2012). These data confirm that Suc is needed for root growth and development, and indicate that Suc can serve as a long-distance signal from the shoot to inform the root system about light availability above ground (Fig. 3).
Auxin
Young shoot tissues are the main source of the plant hormone auxin that is then transported to the root system, where it regulates root development, including lateral root formation (Reed et al., 1998; Bhalerao et al., 2002; Fig. 3). Interestingly, light signaling affects auxin biosynthesis and transport, implicating this hormone as a potential integrator of light signaling and root development. PHY inactivation in low R:FR light triggers auxin biosynthesis in the shoot (Hornitschek et al., 2012), which is then transported laterally through the hypocotyl during low R:FR conditions by PIN3 as well as PIN4 and PIN7 (Keuskamp et al., 2010; Kohnen et al., 2016) and rootward by PIN1 and PIN2 in response to light (Sassi et al., 2012). In darkness, the expression of PIN1 is largely reduced, thus reducing auxin delivery to the root system (Sassi et al., 2012).
Gibberellic Acid
The plant hormone gibberellic acid (GA) mediates various growth and developmental processes, such as seed germination, cell elongation, and reproductive development (Hedden and Sponsel, 2015). Low R:FR induces GA biosynthesis, at least partly through elevated expression of GA20OX genes (Hisamatsu et al., 2005). GAs are diterpenoid tetracyclic carboxylic acids, but only a few are biological active, such as GA1 and GA4 (Hedden and Thomas, 2012). Interestingly, biologically inactive GA12 has been identified as a long-distance growth signal that is transported from the shoot to the root. Here, GA12 is converted into active form by GA20ox and GA3ox enzymes and subsequently promotes root growth in Arabidopsis (Regnault et al., 2015; Fig. 3). It is yet unknown if low R:FR-induced production of bioactive GAs in the shoot affects downward GA12 transport.
HY5
The transcription factor HY5 is a key integrator of photomorphogenesis and is involved in light, hormone, and stress signaling (Cluis et al., 2004; Lee et al., 2007; Gangappa and Botto, 2016). HY5 was recently shown to be transported upon light activation from the shoot to the root (Chen et al., 2016). In the root system, HY5 activates its own expression and that of HYH, creating a positive feedback loop after shoot-to-root transport of HY5 (Zhang et al., 2017). HY5 and HYH modulate lateral root development and lateral root gravitropism (Oyama et al., 1997; Sibout et al., 2006). Interestingly, R:FR ratios influence the expression and stability of HY5, and this transcription factor is, therefore, together with auxin and gibberellin, a candidate regulator of root developmental adjustments to shoot-sensed R:FR light ratios, which indicate the presence of neighboring competitors.
Functional Implications in Dense Vegetation
R:FR light conditions above ground are a reliable cue for the presence of competing neighbors and effectively trigger shade avoidance (Box 2). Interestingly, if the R:FR ratio signals neighbor proximity, it would also signal the likely presence of neighbors below ground competing for water and nutrients (Gundel et al., 2014). Competition experiments in Arabidopsis have shown that root biomass decreases when plants are competing in dense stands and that there is a separate transcriptomic regulation in the root versus the shoot (Masclaux et al., 2012). In controlled growth on medium, seedlings experiencing a low R:FR ratio have a lower lateral root number and main root length (Salisbury et al., 2007). However, as argued previously, in soil conditions the roots are not directly exposed to low R:FR light, and the shoot might thus relay information about nearby competitors to the root system either by direct piping or through secondary mobile messengers.
An important question that remains is what are the functional implications of light signal transmission from the shoot to the root? One clear functional example is that HY5 can stimulate nitrate uptake via NRT2;1 and will do so upon light-activation in the shoot (Chen et al., 2016; Fig. 3). This will help keep the carbon and nitrogen acquisition in tune. In accordance with these findings, direct shading in different phases of the growth period of field-grown maize was shown to suppress root mass, length, and absorptive area, suggesting reduced nutrient and/or water uptake rates (Gao et al., 2017). In addition to true shade, a reduced R:FR light ratio also affects root growth in different species (Kasperbauer and Hunt, 1992, 1994; Salisbury et al., 2007). The effects of reduced R:FR ratios on lateral root formation (Salisbury et al., 2007) may go at the expense of nutrient uptake rates, and might reflect a resource prioritization strategy at the whole plant level, ensuring that resources are invested to consolidate light capture in a growing vegetation with increasing competition for light. It is presently unknown if the root architecture responses to low R:FR affect nutrient acquisition at all, but the resulting reduced root length would suggest so. Nevertheless, much more detailed research into nutrient and water uptake rates of plants under high and low R:FR conditions is needed to assess the precise functional implications of shoot-to-root communication of the aboveground light climate.
Alternatively, plants may also sense neighbors below ground through, for example, nutrient depletion zones and chemical exudates. It is unknown if these cues are transferred to the shoot to prepare for upcoming competition or to adjust shoot responses to light cues from neighbors. Finally, abiotic stresses, for example, drought, occur primarily in the belowground environment and may interact with aboveground light responses. Although little is known about interactions between abiotic stresses and shade responses of the shoot, there is ample information about the interaction of biotic stress and neighbor detection through light cues. The emerging picture is that shade avoidance responses and PHY signaling dominate plant defenses against pathogenic microorganisms and insects (de Wit et al., 2013; Ballaré, 2014), thus prioritizing light interception. It will be interesting to study if, next to biotic stresses, responses against abiotic stresses are suppressed to accommodate shade avoidance responses at high plant density.
CONCLUDING REMARKS
Plants have a variety of photoreceptors that control many different aspects of plant life, including root development. Recently, several novel mechanisms have been discovered that allow plants to relay information from the shoot and its environment to the root system. These include direct light transmission, hormones, and mobile proteins. Although research into these different mechanisms will continue to identify novel mechanisms, it is also crucial to establish the functional implications of this information transfer. It is therefore pertinent that ecologists, agronomists, and plant scientists join forces to unravel both the mechanisms and functional implications of shoot-root communication induced by light cues from the environment.
Footnotes
This work was supported by the Netherlands Organisation for Scientific Research (open competition grant 823.01.013 to R.P., K.v.G.) and the Ministry of Education, Taiwan (Scholarship of Government Sponsorship for Overseas Study, admission no. 0991167-2-UK-004, to C.K.).
Articles can be viewed without a subscription.
References
- Abas L, Benjamins R, Malenica N, Paciorek T, Wiśniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C (2006) Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol 8: 249–256; erratum Abas L, Benjamins R, Malenica N, Paciorek T, Wiśniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C (2006) Nat Cell Biol 8: 424 [DOI] [PubMed] [Google Scholar]
- Ballaré CL. (2014) Light regulation of plant defense. Annu Rev Plant Biol 65: 335–363 [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Mazza CA, Austin AT, Pierik R (2012) Canopy light and plant health. Plant Physiol 160: 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré CL, Pierik R (2017) The shade-avoidance syndrome: multiple signals and ecological consequences. Plant Cell Environ 40: 2530–2543 [DOI] [PubMed] [Google Scholar]
- Baster P, Robert S, Kleine-Vehn J, Vanneste S, Kania U, Grunewald W, De Rybel B, Beeckman T, Friml J (2013) SCF(TIR1/AFB)-auxin signalling regulates PIN vacuolar trafficking and auxin fluxes during root gravitropism. EMBO J 32: 260–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalerao RP, Eklöf J, Ljung K, Marchant A, Bennett M, Sandberg G (2002) Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29: 325–332 [DOI] [PubMed] [Google Scholar]
- Binkert M, Kozma-Bognár L, Terecskei K, De Veylder L, Nagy F, Ulm R (2014) UV-B-responsive association of the Arabidopsis bZIP transcription factor ELONGATED HYPOCOTYL5 with target genes, including its own promoter. Plant Cell 26: 4200–4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 [DOI] [PubMed] [Google Scholar]
- Boccalandro HE, De Simone SN, Bergmann-Honsberger A, Schepens I, Fankhauser C, Casal JJ (2008) PHYTOCHROME KINASE SUBSTRATE1 regulates root phototropism and gravitropism. Plant Physiol 146: 108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR, Christie JM (2002) Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci 7: 204–210 [DOI] [PubMed] [Google Scholar]
- Buer CS, Muday GK (2004) The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 16: 1191–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach C, Markus K, Zhang Y, Schlicht M, Baluška F (2012) Photophobic behavior of maize roots. Plant Signal Behav 7: 874–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgie ES, Vierstra RD (2014) Phytochromes: an atomic perspective on photoactivation and signaling. Plant Cell 26: 4568–4583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canamero RC, Bakrim N, Bouly JP, Garay A, Dudkin EE, Habricot Y, Ahmad M (2006) Cryptochrome photoreceptors cry1 and cry2 antagonistically regulate primary root elongation in Arabidopsis thaliana. Planta 224: 995–1003 [DOI] [PubMed] [Google Scholar]
- Casal JJ. (2013) Photoreceptor signaling networks in plant responses to shade. Annu Rev Plant Biol 64: 403–427 [DOI] [PubMed] [Google Scholar]
- Chen M, Chory J (2011) Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol 21: 664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yao Q, Gao X, Jiang C, Harberd NP, Fu X (2016) Shoot-to-root mobile transcription factor HY5 coordinates plant carbon and nitrogen acquisition. Curr Biol 26: 640–646 [DOI] [PubMed] [Google Scholar]
- Christie JM, Arvai AS, Baxter KJ, Heilmann M, Pratt AJ, O’Hara A, Kelly SM, Hothorn M, Smith BO, Hitomi K, et al. (2012) Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science 335: 1492–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluis CP, Mouchel CF, Hardtke CS (2004) The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J 38: 332–347 [DOI] [PubMed] [Google Scholar]
- Correll MJ, Kiss JZ (2005) The roles of phytochromes in elongation and gravitropism of roots. Plant Cell Physiol 46: 317–323 [DOI] [PubMed] [Google Scholar]
- Costigan SE, Warnasooriya SN, Humphries BA, Montgomery BL (2011) Root-localized phytochrome chromophore synthesis is required for photoregulation of root elongation and impacts root sensitivity to jasmonic acid in Arabidopsis. Plant Physiol 157: 1138–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B, Vassileva V, Parizot B, Demeulenaere M, Grunewald W, Audenaert D, Van Campenhout J, Overvoorde P, Jansen L, Vanneste S, et al. (2010) A novel aux/IAA28 signaling cascade activates GATA23-dependent specification of lateral root founder cell identity. Curr Biol 20: 1697–1706 [DOI] [PubMed] [Google Scholar]
- De Smet I, Tetsumura T, De Rybel B, Frei dit Frey N, Laplaze L, Casimiro I, Swarup R, Naudts M, Vanneste S, Audenaert D, et al. (2007) Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134: 681–690 [DOI] [PubMed] [Google Scholar]
- de Wit M, Keuskamp DH, Bongers FJ, Hornitschek P, Gommers CMM, Reinen E, Martínez-Cerón C, Fankhauser C, Pierik R (2016) Integration of phytochrome and cryptochrome signals determines plant growth during competition for light. Curr Biol 26: 3320–3326 [DOI] [PubMed] [Google Scholar]
- de Wit M, Spoel SH, Sanchez-Perez GF, Gommers CMM, Pieterse CMJ, Voesenek LA, Pierik R (2013) Perception of low red:far-red ratio compromises both salicylic acid- and jasmonic acid-dependent pathogen defences in Arabidopsis. Plant J 75: 90–103 [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Linhares FS, Scacchi E, Casamitjana-Martinez E, Heidstra R, Costantino P, Sabatini S (2007) Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr Biol 17: 678–682 [DOI] [PubMed] [Google Scholar]
- Dello Ioio RD, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S (2008) A genetic framework for the control of cell division and differentiation in the root meristem. Science 322: 1380–1384 [DOI] [PubMed] [Google Scholar]
- Demarsy E, Schepens I, Okajima K, Hersch M, Bergmann S, Christie J, Shimazaki K, Tokutomi S, Fankhauser C (2012) Phytochrome Kinase Substrate 4 is phosphorylated by the phototropin 1 photoreceptor. EMBO J 31: 3457–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duek PD, Elmer MV, van Oosten VR, Fankhauser C (2004) The degradation of HFR1, a putative bHLH class transcription factor involved in light signaling, is regulated by phosphorylation and requires COP1. Curr Biol 14: 2296–2301 [DOI] [PubMed] [Google Scholar]
- Dyachok J, Zhu L, Liao F, He J, Huq E, Blancaflor EB (2011) SCAR mediates light-induced root elongation in Arabidopsis through photoreceptors and proteasomes. Plant Cell 23: 3610–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Christie JM (2015) Plant phototropic growth. Curr Biol 25: R384–R389 [DOI] [PubMed] [Google Scholar]
- Favory J-J, Stec A, Gruber H, Rizzini L, Oravecz A, Funk M, Albert A, Cloix C, Jenkins GI, Oakeley EJ, et al. (2009) Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J 28: 591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Quail PH (2010) Phytochrome functions in Arabidopsis development. J Exp Bot 61: 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, Scheres B (2007) PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 449: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Galvão VC, Fankhauser C (2015) Sensing the light environment in plants: photoreceptors and early signaling steps. Curr Opin Neurobiol 34: 46–53 [DOI] [PubMed] [Google Scholar]
- Gangappa SN, Botto JF (2016) The multifaceted roles of HY5 in plant growth and development. Mol Plant 9: 1353–1365 [DOI] [PubMed] [Google Scholar]
- Gao J, Shi J, Dong S, Liu P, Zhao B, Zhang J (2017) Grain yield and root characteristics of summer maize (Zea mays L.) under shade stress conditions. J Agron Crop Sci 203: 562–573 [Google Scholar]
- Gommers CM, Visser EJ, St Onge KR, Voesenek LA, Pierik R (2013) Shade tolerance: when growing tall is not an option. Trends Plant Sci 18: 65–71 [DOI] [PubMed] [Google Scholar]
- Goosey L, Palecanda L, Sharrock RA (1997) Differential patterns of expression of the Arabidopsis PHYB, PHYD, and PHYE phytochrome genes. Plant Physiol 115: 959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundel PE, Pierik R, Mommer L, Ballaré CL (2014) Competing neighbors: light perception and root function. Oecologia 176: 1–10 [DOI] [PubMed] [Google Scholar]
- Hanzawa T, Shibasaki K, Numata T, Kawamura Y, Gaude T, Rahman A (2013) Cellular auxin homeostasis under high temperature is regulated through a sorting NEXIN1-dependent endosomal trafficking pathway. Plant Cell 25: 3424–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S, Velanis CN, Jenkins GI, Franklin KA (2014) UV-B detected by the UVR8 photoreceptor antagonizes auxin signaling and plant shade avoidance. Proc Natl Acad Sci USA 111: 11894–11899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P, Sponsel V (2015) A century of gibberellin research. J Plant Growth Regul 34: 740–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P, Thomas SG (2012) Gibberellin biosynthesis and its regulation. Biochem J 444: 11–25 [DOI] [PubMed] [Google Scholar]
- Hisamatsu T, King RW, Helliwell CA, Koshioka M (2005) The involvement of gibberellin 20-oxidase genes in phytochrome-regulated petiole elongation of Arabidopsis. Plant Physiol 138: 1106–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm M, Ma L-G, Qu L-J, Deng X-W (2002) Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev 16: 1247–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornitschek P, Kohnen MV, Lorrain S, Rougemont J, Ljung K, López-Vidriero I, Franco-Zorrilla JM, Solano R, Trevisan M, Pradervand S, et al. (2012) Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J 71: 699–711 [DOI] [PubMed] [Google Scholar]
- Huang L, Zhang H, Zhang H, Deng XW, Wei N (2015) HY5 regulates nitrite reductase 1 (NIR1) and ammonium transporter1;2 (AMT1;2) in Arabidopsis seedlings. Plant Sci 238: 330–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Ouyang X, Deng XW (2014) Beyond repression of photomorphogenesis: role switching of COP/DET/FUS in light signaling. Curr Opin Plant Biol 21: 96–103 [DOI] [PubMed] [Google Scholar]
- Huang X, Ouyang X, Yang P, Lau OS, Chen L, Wei N, Deng XW (2013) Conversion from CUL4-based COP1-SPA E3 apparatus to UVR8-COP1-SPA complexes underlies a distinct biochemical function of COP1 under UV-B. Proc Natl Acad Sci USA 110: 16669–16674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang I-C, Henriques R, Seo HS, Nagatani A, Chua N-H (2010) Arabidopsis PHYTOCHROME INTERACTING FACTOR proteins promote phytochrome B polyubiquitination by COP1 E3 ligase in the nucleus. Plant Cell 22: 2370–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J, Choi G (2013) Phytochrome-interacting factors have both shared and distinct biological roles. Mol Cells 35: 371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J-H, Domijan M, Klose C, Biswas S, Ezer D, Gao M, Khattak AK, Box MS, Charoensawan V, Cortijo S, et al. (2016) Phytochromes function as thermosensors in Arabidopsis. Science 354: 886–889 [DOI] [PubMed] [Google Scholar]
- Kasperbauer MJ, Hunt PG (1992) Root size and shoot/root ratio as influenced by light environment of the shoot. J Plant Nutr 15: 685–697 [Google Scholar]
- Kasperbauer MJ, Hunt PG (1994) Shoot/root assimilate allocation and nodulation of Vigna unguiculata seedlings as influenced by shoot light environment. Plant Soil 161: 97–101 [Google Scholar]
- Keuskamp DH, Pollmann S, Voesenek LACJ, Peeters AJM, Pierik R (2010) Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc Natl Acad Sci USA 107: 22740–22744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher S, Schopfer P (2012) Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proc Natl Acad Sci USA 109: 11217–11221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JZ, Mullen JL, Correll MJ, Hangarter RP (2003) Phytochromes A and B mediate red-light-induced positive phototropism in roots. Plant Physiol 131: 1411–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JZ, Ruppel NJ, Hangarter RP (2001) Phototropism in Arabidopsis roots is mediated by two sensory systems. Adv Space Res 27: 877–885 [DOI] [PubMed] [Google Scholar]
- Klar T, Pokorny R, Moldt J, Batschauer A, Essen LO (2007) Cryptochrome 3 from Arabidopsis thaliana: structural and functional analysis of its complex with a folate light antenna. J Mol Biol 366: 954–964 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Leitner J, Zwiewka M, Sauer M, Abas L, Luschnig C, Friml J (2008) Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc Natl Acad Sci USA 105: 17812–17817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnen MV, Schmid-Siegert E, Trevisan M, Petrolati LA, Sénéchal F, Müller-Moulé P, Maloof J, Xenarios I, Fankhauser C (2016) Neighbor detection induces organ-specific transcriptomes, revealing patterns underlying hypocotyl-specific growth. Plant Cell 28: 2889–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koini MA, Alvey L, Allen T, Tilley CA, Harberd NP, Whitelam GC, Franklin KA (2009) High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr Biol 19: 408–413 [DOI] [PubMed] [Google Scholar]
- Korbei B, Luschnig C (2013) Plasma membrane protein ubiquitylation and degradation as determinants of positional growth in plants. J Integr Plant Biol 55: 809–823 [DOI] [PubMed] [Google Scholar]
- Kutschera U, Briggs WR (2012) Root phototropism: from dogma to the mechanism of blue light perception. Planta 235: 443–452 [DOI] [PubMed] [Google Scholar]
- Lavenus J, Goh T, Roberts I, Guyomarc’h S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L (2013) Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci 18: 450–458 [DOI] [PubMed] [Google Scholar]
- Laxmi A, Pan J, Morsy M, Chen R (2008) Light plays an essential role in intracellular distribution of auxin efflux carrier PIN2 in Arabidopsis thaliana. PLoS One 3: e1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure CD, Tong H, Yuen G, Hou X, Sun X, He Z-H (2009) ROOT UV-B SENSITIVE2 acts with ROOT UV-B SENSITIVE1 in a root ultraviolet B-sensing pathway. Plant Physiol 150: 1902–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-J, Ha J-H, Kim S-G, Choi H-K, Kim ZH, Han Y-J, Kim J-I, Oh Y, Fragoso V, Shin K, et al. (2016) Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots. Sci Signal 9: ra106. [DOI] [PubMed] [Google Scholar]
- Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW (2007) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19: 731–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legris M, Klose C, Burgie ES, Rojas CCR, Neme M, Hiltbrunner A, Wigge PA, Schäfer E, Vierstra RD, Casal JJ (2016) Phytochrome B integrates light and temperature signals in Arabidopsis. Science 354: 897–900 [DOI] [PubMed] [Google Scholar]
- Leivar P, Monte E (2014) PIFs: systems integrators in plant development. Plant Cell 26: 56–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Quail PH (2011) PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci 16: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q-H, Yang H-Q (2007) Cryptochrome signaling in plants. Photochem Photobiol 83: 94–101 [DOI] [PubMed] [Google Scholar]
- Lian HL, He SB, Zhang YC, Zhu DM, Zhang JY, Jia KP, Sun SX, Li L, Yang HQ (2011) Blue-light-dependent interaction of cryptochrome 1 with SPA1 defines a dynamic signaling mechanism. Genes Dev 25: 1023–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Zuo Z, Liu H, Liu X, Lin C (2011) Arabidopsis cryptochrome 1 interacts with SPA1 to suppress COP1 activity in response to blue light. Genes Dev 25: 1029–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobet G. (2017): Schematic of a maize plant. figshare https://doi.org/10.6084/m9.figshare.4684996.v1 [Google Scholar]
- Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C (2008) Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Mähönen AP, Ten Tusscher K, Siligato R, Smetana O, Díaz-Triviño S, Salojärvi J, Wachsman G, Prasad K, Heidstra R, Scheres B (2014) PLETHORA gradient formation mechanism separates auxin responses. Nature 515: 125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masclaux FG, Bruessow F, Schweizer F, Gouhier-Darimont C, Keller L, Reymond P (2012) Transcriptome analysis of intraspecific competition in Arabidopsis thaliana reveals organ-specific signatures related to nutrient acquisition and general stress response pathways. BMC Plant Biol 12: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud O, Fiorucci A-S, Xenarios I, Fankhauser C (2017) Local auxin production underlies a spatially restricted neighbor-detection response in Arabidopsis. Proc Natl Acad Sci USA 114: 7444–7449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo M, Yokawa K, Wan Y, Baluška F (2015) How and why do root apices sense light under the soil surface? Front Plant Sci 6: 775; erratum Mo M, Yokawa K, Wan Y, Baluška F (2015) Front Plant Sci 6: 930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moni A, Lee AY, Briggs WR, Han IS (2015) The blue light receptor Phototropin 1 suppresses lateral root growth by controlling cell elongation. Plant Biol (Stuttg) 17: 34–40 [DOI] [PubMed] [Google Scholar]
- Morita MT. (2010) Directional gravity sensing in gravitropism. Annu Rev Plant Biol 61: 705–720 [DOI] [PubMed] [Google Scholar]
- Ni W, Xu S-L, Tepperman JM, Stanley DJ, Maltby DA, Gross JD, Burlingame AL, Wang Z-Y, Quail PH (2014) A mutually assured destruction mechanism attenuates light signaling in Arabidopsis. Science 344: 1160–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MT, Hardtke CS, Wei N, Deng XW (2000) Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405: 462–466 [DOI] [PubMed] [Google Scholar]
- Oyama T, Shimura Y, Okada K (1997) The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev 11: 2983–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacín M, Legris M, Casal JJ (2014) Rapid decline in nuclear costitutive photomorphogenesis1 abundance anticipates the stabilization of its target elongated hypocotyl5 in the light. Plant Physiol 164: 1134–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik I, Kathare PK, Kim J-I, Huq E (2017) Expanding roles of PIFs in signal integration from multiple processes. Mol Plant 10: 1035–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulou CK, Bongers FJ, Küpers JJ, Reinen E, Das D, Evers JB, Anten NPR, Pierik R (2017) Neighbor detection at the leaf tip adaptively regulates upward leaf movement through spatial auxin dynamics. Proc Natl Acad Sci USA 114: 7450–7455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedmale UV, Liscum E (2007) Regulation of phototropic signaling in Arabidopsis via phosphorylation state changes in the phototropin 1-interacting protein NPH3. J Biol Chem 282: 19992–20001 [DOI] [PubMed] [Google Scholar]
- Pedmale UV, Huang SC, Zander M, Cole BJ, Hetzel J, Ljung K, Reis PAB, Sridevi P, Nito K, Nery JR, et al. (2016) Cryptochromes interact directly with PIFs to control plant growth in limiting blue light. Cell 164: 233–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, Middleton AM, French AP, Larrieu A, Bishopp A, Njo M, Wells DM, Porco S, Mellor N, Band LR, et al. (2013) Sequential induction of auxin efflux and influx carriers regulates lateral root emergence. Mol Syst Biol 9: 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petricka JJ, Winter CM, Benfey PN (2012) Control of Arabidopsis root development. Annu Rev Plant Biol 63: 563–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Mommer L, Voesenek LA (2013) Molecular mechanisms of plant competition: neighbour detection and response strategies. Funct Ecol 27: 841–853 [Google Scholar]
- Reed RC, Brady SR, Muday GK (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118: 1369–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault T, Davière J-M, Wild M, Sakvarelidze-Achard L, Heintz D, Carrera Bergua E, Lopez Diaz I, Gong F, Hedden P, Achard P (2015) The gibberellin precursor GA12 acts as a long-distance growth signal in Arabidopsis. Nat Plants 1: 15073. [DOI] [PubMed] [Google Scholar]
- Rizzini L, Favory JJ, Cloix C, Faggionato D, O’Hara A, Kaiserli E, Baumeister R, Schafer E, Nagy F, Jenkins GI, et al. (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science 332: 103–106 [DOI] [PubMed] [Google Scholar]
- Ruppel NJ, Hangarter RP, Kiss JZ (2001) Red-light-induced positive phototropism in Arabidopsis roots. Planta 212: 424–430 [DOI] [PubMed] [Google Scholar]
- Saijo Y, Sullivan JA, Wang H, Yang J, Shen Y, Rubio V, Ma L, Hoecker U, Deng XW (2003) The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev 17: 2642–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Briggs WR (2002) Cellular and subcellular localization of phototropin 1. Plant Cell 14: 1723–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury FJ, Hall A, Grierson CS, Halliday KJ (2007) Phytochrome coordinates Arabidopsis shoot and root development. Plant J 50: 429–438 [DOI] [PubMed] [Google Scholar]
- Sassi M, Lu Y, Zhang Y, Wang J, Dhonukshe P, Blilou I, Dai M, Li J, Gong X, Jaillais Y, et al. (2012) COP1 mediates the coordination of root and shoot growth by light through modulation of PIN1- and PIN2-dependent auxin transport in Arabidopsis. Development 139: 3402–3412 [DOI] [PubMed] [Google Scholar]
- Scheres B, Wolkenfelt H, Willemsen V, Terlouw M, Lawson E, Dean C, Weisbeek P (1994) Embryonic origin of the Arabidopsis primary root and root meristem initials. Development 120: 2475–2487 [Google Scholar]
- Seo HS, Watanabe E, Tokutomi S, Nagatani A, Chua NH (2004) Photoreceptor ubiquitination by COP1 E3 ligase desensitizes phytochrome A signaling. Genes Dev 18: 617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Yang J-Y, Ishikawa M, Bolle C, Ballesteros ML, Chua N-H (2003) LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature 423: 995–999 [DOI] [PubMed] [Google Scholar]
- Sharrock RA, Clack T (2002) Patterns of expression and normalized levels of the five Arabidopsis phytochromes. Plant Physiol 130: 442–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheerin DJ, Menon C, zur Oven-Krockhaus S, Enderle B, Zhu L, Johnen P, Schleifenbaum F, Stierhof Y-D, Huq E, Hiltbrunner A (2015) Light-activated phytochrome A and B interact with members of the SPA family to promote photomorphogenesis in Arabidopsis by reorganizing the COP1/SPA complex. Plant Cell 27: 189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibout R, Sukumar P, Hettiarachchi C, Holm M, Muday GK, Hardtke CS (2006) Opposite root growth phenotypes of hy5 versus hy5 hyh mutants correlate with increased constitutive auxin signaling. PLoS Genet 2: e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Navas J, Moreno-Risueno MA, Manzano C, Pallero-Baena M, Navarro-Neila S, Téllez-Robledo B, Garcia-Mina JM, Baigorri R, Gallego FJ, del Pozo JC (2015) D-Root: a system for cultivating plants with the roots in darkness or under different light conditions. Plant J 84: 244–255 [DOI] [PubMed] [Google Scholar]
- Silva-Navas J, Moreno-Risueno MA, Manzano C, Téllez-Robledo B, Navarro-Neila S, Carrasco V, Pollmann S, Gallego FJ, Del Pozo JC (2016) Flavonols mediate root phototropism and growth through regulation of proliferation-to-differentiation transition. Plant Cell 28: 1372–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. (1982) Light quality, photoperception, and plant strategy. Annu Rev Plant Physiol 33: 481–518 [Google Scholar]
- Somers DE, Quail PH (1995) Temporal and spatial expression patterns of PHYA and PHYB genes in Arabidopsis. Plant J 7: 413–427 [DOI] [PubMed] [Google Scholar]
- Song S-H, Dick B, Penzkofer A, Pokorny R, Batschauer A, Essen L-O (2006) Absorption and fluorescence spectroscopic characterization of cryptochrome 3 from Arabidopsis thaliana. J Photochem Photobiol B 85: 1–16 [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie D-Y, Dolezal K, Schlereth A, Jürgens G, Alonso JM (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133: 177–191 [DOI] [PubMed] [Google Scholar]
- Sun Q, Yoda K, Suzuki H (2005) Internal axial light conduction in the stems and roots of herbaceous plants. J Exp Bot 56: 191–203 [DOI] [PubMed] [Google Scholar]
- Sun Q, Yoda K, Suzuki M, Suzuki H (2003) Vascular tissue in the stem and roots of woody plants can conduct light. J Exp Bot 54: 1627–1635 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Yokawa K, Nakano S, Yoshida Y, Fabrissin I, Okamoto T, Baluška F, Koshiba T (2016) Root cap-dependent gravitropic U-turn of maize root requires light-induced auxin biosynthesis via the YUC pathway in the root apex. J Exp Bot 67: 4581–4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweere U. (2001) Interaction of the response regulator ARR4 with phytochrome B in modulating red light signaling. Science 294: 1108–1111 [DOI] [PubMed] [Google Scholar]
- Tong H, Leasure CD, Hou X, Yuen G, Briggs W, He Z-H (2008) Role of root UV-B sensing in Arabidopsis early seedling development. Proc Natl Acad Sci USA 105: 21039–21044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth R, Kevei E, Hall A, Millar AJ, Nagy F, Kozma-Bognár L (2001) Circadian clock-regulated expression of phytochrome and cryptochrome genes in Arabidopsis. Plant Physiol 127: 1607–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer JEM, von Wangenheim D, Barberon M, Lee Y, Stelzer EHK, Maizel A, Geldner N (2014) A spatial accommodation by neighboring cells is required for organ initiation in Arabidopsis. Science 343: 178–183 [DOI] [PubMed] [Google Scholar]
- von Arnim AG, Deng XW (1994) Light inactivation of Arabidopsis photomorphogenic repressor COP1 involves a cell-specific regulation of its nucleocytoplasmic partitioning. Cell 79: 1035–1045 [DOI] [PubMed] [Google Scholar]
- Wan Y, Jasik J, Wang L, Hao H, Volkmann D, Menzel D, Mancuso S, Baluška F, Lin J (2012) The signal transducer NPH3 integrates the phototropin1 photosensor with PIN2-based polar auxin transport in Arabidopsis root phototropism. Plant Cell 24: 551–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. (1985) Size hierarchies in experimental populations of annual plants. Ecology 66: 743–752 [Google Scholar]
- Weiner J, Thomas SC (1986) Size variability and competition in plant monocultures. Oikos 47: 211–222 [Google Scholar]
- Wu S-H. (2014) Gene expression regulation in photomorphogenesis from the perspective of the central dogma. Annu Rev Plant Biol 65: 311–333 [DOI] [PubMed] [Google Scholar]
- Xu W, Ding G, Yokawa K, Baluška F, Li Q-F, Liu Y, Shi W, Liang J, Zhang J (2013) An improved agar-plate method for studying root growth and response of Arabidopsis thaliana. Sci Rep 3: 1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Paik I, Zhu L, Huq E (2015) Illuminating progress in phytochrome-mediated light signaling pathways. Trends Plant Sci 20: 641–650 [DOI] [PubMed] [Google Scholar]
- Zhang K-X, Xu H-H, Gong W, Jin Y, Shi Y-Y, Yuan T-T, Li J, Lu Y-T (2014) Proper PIN1 distribution is needed for root negative phototropism in Arabidopsis. PLoS One 9: e85720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K-X, Xu H-H, Yuan T-T, Zhang L, Lu Y-T (2013) Blue-light-induced PIN3 polarization for root negative phototropic response in Arabidopsis. Plant J 76: 308–321 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li C, Zhang J, Wang J, Yang J, Lv Y, Yang N, Liu J, Wang X, Palfalvi G, et al. (2017) Dissection of HY5/HYH expression in Arabidopsis reveals a root-autonomous HY5-mediated photomorphogenic pathway. PLoS One 12: e0180449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Wu S, Zhai H, Zhou P, Song M, Su L, Xi Y, Li Z, Cai Y, Meng F, et al. (2013) Arabidopsis phytochrome B promotes SPA1 nuclear accumulation to repress photomorphogenesis under far-red light. Plant Cell 25: 115–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z, Liu H, Liu B, Liu X, Lin C (2011) Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis. Curr Biol 21: 841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]