Abstract
The decision of a quiescent axillary bud to commit to regrowth is governed by both metabolic and signaling functions, driven by light, energy, and oxygen availability.
The hierarchy of events governing the resumption of growth of a quiescent axillary bud are poorly understood. During quiescence, a homeostasis exists in phytohormone and source/sink regulation, which represses the metabolic and mitotic progression of the bud. Environmental change and shoot development can alter the homeostasis, leading to a binary state change and the commitment to growth. Within this context, light and oxygen availability can serve both metabolic and signaling functions. However, the question of substrate versus signal has proven challenging to resolve; in the case of sugars, there are disparities in the data from apical and axillary buds in juvenile shoots, while in postdormant perennial buds, light has only a facultative role in the decision, but signaling may still be essential for bud fate. We briefly update the roles and hierarchies of light-, energy-, and oxygen-dependent functions in axillary bud outgrowth of annual shoots, before focusing discussion on the role of chloroplast-to-nucleus retrograde signaling genes such as GENOMES UNCOUPLED4 (GUN4) and ELONGATED HYPOCOTYL5 (HY5) in bud burst responses to light, examining available transcriptome data from postdormant grapevine (Vitis vinifera) buds. We discuss the evidence implicating cryptochromes (CRY) in the activation of HY5 expression in grapevine, leading to chloroplast biogenesis in the buds, and that this occurs via a biogenic rather than an adaptive developmental process. The cytokinin (CK) signaling pathways and the light-regulated expression of chloroplast processes, especially those involved in carbon and oxygen metabolism, may also play an important role in bud burst.
The mechanisms that control apical dominance in juvenile or annual shoots are well characterized. Removing the apex can result in axillary bud outgrowth, as can changes in light intensity and quality. Here, axillary bud outgrowth is regulated by signals arising from the apex, which contain several light quality and quantity sensing pigments. Of these, phytochromes (PHY) are perhaps the best characterized. PHY sense red and far-red light, while cryptochromes and phototropins are involved in the perception of blue light. These photoreceptors regulate the expression of different transcription factors to coordinate light-dependent photomorphogenesis. Some plant species require light for axillary bud outgrowth (annual shoots), but in others the requirement for light is facultative (Leduc et al., 2014). In addition, the buds of many perennial plants can resume growth following a period of dormancy. In this case, apical suppression may temporarily break down, and the axillary bud may be considered more independent, at least until a new homeostasis is established along the shoot. Moreover, there is no evidence postdormant perennial buds require light, although increased light intensity can accelerate bud burst in a range of species (Maynard et al., 1990; Rageau et al., 1998; Søgaard et al., 2008; Caffarra and Donnelly, 2011).
The influence of light on meristem activity involves at least two distinct but possibly cross-regulatory processes: direct regulation of gene expression via photoreceptors, and an indirect process involving the generation of energy through photosynthesis and respiration. A potential third pathway is the signaling of tissue oxygen status, which has been shown to be a primary cue for developmental transitions in plants, including photomorphogenesis (Considine et al., 2017).
In this Update, we consider the respective roles of light, energy, and oxygen as primary cues for axillary bud outgrowth, with a particular focus on the signaling pathways that trigger the resumption of growth following quiescence. We provide a concise overview of (1) the physiology of axillary meristems and buds, focusing on genotypic differences in bud requirements for light and energy to trigger outgrowth, and (2) the importance of transcriptional regulation of plastid functions in the resumption of growth in quiescent grapevine buds following dormancy.
LIGHT AND ENERGY DEPENDENCY OF AXILLARY BUD OUTGROWTH, AND A PUTATIVE ROLE FOR OXYGEN-DEPENDENT SIGNALING
Vascular plants display indeterminate growth and a branched root and shoot structure, which is enabled by the spatial distribution and activation of meristems (Sussex and Kerk, 2001). Most terrestrial species exhibit axillary branching rather than the more ancestral dichotomous branching. Axillary buds are classed as sylleptic or proleptic, and both types may be quiescent for sustained periods of time, being able to resume growth immediately upon perception of appropriate developmental, metabolic, or environmental cues. Additionally, proleptic buds of some species possess the ability to exhibit true dormancy, which is a developmental and internally repressed condition that requires environmental entrainment to enable a transition to quiescence (Considine and Considine, 2016). Dormant buds are metabolically isolated from the shoot by physiological barriers such as the deposition of callose. In this situation, apical dominance in its strictest sense may not apply, at least until dormancy is relieved. In the following discussion, we focus on quiescence and the role of light in the processes promoting axillary bud outgrowth, particularly in intact juvenile or annual shoots.
The dominance behavior of the apical meristem, which enforces and maintains axillary bud quiescence, is facilitated by mobile signals such as Suc and phytohormones, particularly auxin. The role of apically derived auxin in maintaining axillary bud quiescence was established nearly a century ago (Thimann and Skoog, 1934; for a detailed review, refer to Rameau et al., 2015). However, auxin signaling intersects with other phytohormones such as strigolactones and CKs to regulate the outgrowth of axillary buds. Each phytohormone functions downstream of light signaling pathways initiated by photoreceptors (Leduc et al., 2014). Phytohormone signaling pathways are thought to converge at the level of the BRANCHED1 transcription factor (and homologs), which is a central repressor of axillary bud outgrowth (Dun et al., 2012). However, auxin transport may be too slow to account for observed bud outgrowth kinetics, while Suc availability may provide a more rapid regulatory trigger (Renton et al., 2012; Mason et al., 2014). The application of Suc results in a dose-dependent activation of bud outgrowth, a process that apparently antagonizes auxin- and strigolactone-mediated signaling, although Suc effects were at least partly independent of these pathways (Barbier et al., 2015a).
Light and Suc can act both as signals and sources of energy for bud growth. Suc functions both as a metabolic substrate and signal controlling development, notably via the TARGET OF RAPAMYCIN (TOR) kinase and SUCROSE NONFERMENTING1-RELATED KINASE1 (SnRK1). Several species such as Rosa sp. and pea (Pisum sativum) require light for axillary bud outgrowth, while others have varying facultative requirements for light (Leduc et al., 2014). In axillary buds of Rosa sp., the expression of genes involved in Suc hydrolysis and mobilization is promoted by light; however, Suc cannot compensate for light in activating bud outgrowth (Girault et al., 2008). Application of Suc and nonmetabolizable analogs such as palatinose promotes the rate of bud outgrowth in Rosa, Arabidopsis (Arabidopsis thaliana), and pea when light is present (Rabot et al., 2012; Barbier et al., 2015b). These data suggest that photoreceptor-mediated signaling is a primary requirement for bud outgrowth, and that Suc synthesis and metabolism via photosynthesis are essential downstream components.
Several lines of evidence suggest that Suc may function as a signal rather than energy substrate in augmenting bud outgrowth (Barbier et al., 2015a). The altered shoot branching phenotype of Arabidopsis mutants deficient in trehalose-6-P (T6P) cannot be explained by metabolic or energy functions because T6P only accumulates to low concentrations even in wild-type plants (Chary et al., 2008). Overexpression of HEXOKINASE1 leads to increased bud outgrowth and expression of genes involved in abscisic acid-related processes, together with reduced expression of auxin-related genes (Kelly et al., 2012). Nevertheless, other studies have linked the effects of Suc to metabolic requirements (Leduc et al., 2014; Otori et al., 2017).
Further insights into the question of whether Suc acts as a signal rather than a substrate come from studies of the shoot apical meristem (SAM). Auxin- and Suc-mediated pathways independently promote the cell cycle by activating TOR kinase, which in turn directly activates key cell cycle regulators, as well as the stem cell identity protein WUSCHEL (WUS; Pfeiffer et al., 2016; Li et al., 2017). The fact that both auxin and Suc are required is particularly interesting for two reasons. First, the auxin response in the SAM is dependent on a small GTPase Rho-like protein (ROP2). This protein was shown to be activated by both the direct application of auxins and the light-induced auxins in shoot apices (Li et al., 2017). In addition, the application of auxin effectively substituted light to activate the TOR-dependent formation of true leaves, when Suc was present (Li et al., 2017). The ROP2 was shown to directly interact with TOR kinase, promoting its kinase activity (Cai et al., 2017). ROP2 also functions in oxygen- and redox-dependent survival (Baxter-Burrell et al., 2002). The expression of ROP2 is promoted by HYPOXIA RESPONSIVE UNIVERSAL STRESS PROTEIN1 that transduces the hypoxic cue via Group VII ETHYLENE RESPONSE FACTORs (ERF-VII), which are stabilized in hypoxic conditions (Gonzali et al., 2015). Hence, these data strongly suggest auxin and Suc pathways converge with oxygen signaling upstream of TOR kinase (Considine, 2017). We will return to oxygen signaling below. Second, the Suc effect on TOR and WUS is consistent with a metabolic function because Glc, not palatinose, is able to substitute for Suc (Pfeiffer et al., 2016), which conflicts with reports on axillary buds (Rabot et al., 2012; Barbier et al., 2015b).
The above points demonstrate the incomplete nature of current understanding of how auxin and Suc function together in axillary bud outgrowth. Interestingly, the addition of Suc is sufficient to trigger the growth of the root apical meristem but not the SAM. This finding may be explained by the relatively higher concentrations of auxin in the root apical meristem compared with the SAM, and also the light dependency of auxin synthesis in the SAM (Li et al., 2017). Increased auxin synthesis and transport from the axillary buds occur during the transition to bud outgrowth, suggesting that photoreceptor-dependent auxin synthesis in the axillary bud meristems may be a primary trigger for bud outgrowth. However, strigolactone has also been suggested to be a signal output from photosynthesis. Increased axillary branching is evident in an Arabidopsis mutant lacking the PsbP Domain Protein5 (PPD5), which is a key component of photosystem II (Roose et al., 2011). While PPD5 is essential for autotrophic metabolism and optimal oxygen-evolving activity, the ppd5 mutants are able to sustain electron transport, and the phenotype can be rescued by the application of strigolactone, indicating that the phenotype is more likely to be due to hormone defects than energy deficits. Perhaps also relevant, axis initiation in tomato (Solanum lycopersicum) requires light signaling via phytochromes but not photosynthesis (Yoshida et al., 2011). Meristems cultured with Suc in darkness, or in the presence of the carotenoid inhibitor norflurazon in the light, fail to initiate new leaf primordia. Nevertheless, axis initiation is a different process than organ development, i.e. the resumption of growth following quiescence.
LIGHT, OXYGEN, AND CHLOROPLAST FUNCTIONS IN PERENNIAL BUD BURST—AN ILLUSTRATION WITH GRAPEVINE BUDS
In many perennial species, proleptic buds resume growth following a prolonged period of dormancy (Considine and Considine, 2016). The dormant bud becomes desiccated and metabolically isolated by callose deposition in the plasmodesmata (Rinne et al., 2011). In this state, the meristem tissues are enclosed, typically by lignified bracts and scales (Fig. 1). Following dormancy, the bud resumes a quiescent but receptive state with a connected symplast. Studies of several woody species have shown that the internal tissues and leaf primordia of quiescent buds are largely etiolated and lack chlorophyll (Solymosi et al., 2012). The plastids in such buds, however, exist in different developmental stages that are partly related to the nature of the tissues in which they reside (Solymosi et al., 2012). For example, proplastid-like and etio-chloroplasts, respectively, were identified in the inner and outer leaf primordia of compactly closed common ash (Fraxinus excelsior) buds (Solymosi et al., 2012). After bud burst, the emerging leaves contain regular chloroplasts, although they are not fully developed (Solymosi et al., 2012). However, in horse chestnut (Aesculus hippocastanum), closed buds contain proplastids, and the leaf primordia of the opening buds contain etioplasts or etio-chloroplasts, but not chloroplasts (Solymosi et al., 2006). In tree-of-heaven (Ailanthus altissima) buds, both inner and outer leaf primordia contain chloroplasts and etio-chloroplasts (Solymosi et al., 2012). Hence, outer leaf primordia do not always contain more developed plastids than the inner leaf primordia.
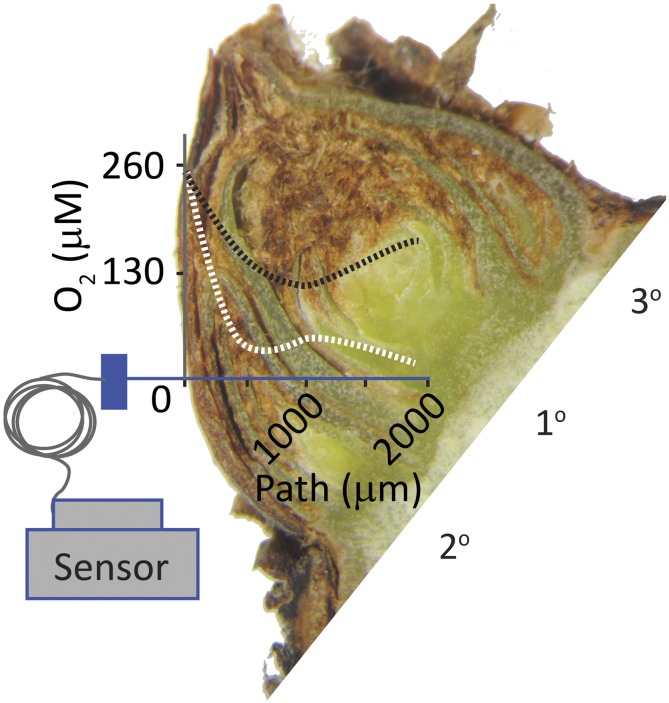
Figure 1.
Morphology, tissue oxygen status, and light-affected growth of single-node cuttings of postdormant grapevine buds. Shown is a longitudinal section of a quiescent grapevine bud with three preformed shoots (1°, 2°, and 3°), enclosed by layers of bracts, hairs, and lignified scales. A stylized plot of the tissue oxygen concentration of a bud during quiescence (dotted white line) and bud burst (dotted black line), as determined by an oxygen microsensor, is overlaid. The path of the probe, from external scales to the core of the primary meristem, is the x axis (blue line), and 260 μm [O2] approximates the air-saturated concentration in water at standard temperature and pressure (refer to Meitha et al., 2015).
There is also evidence of regulated oxygenation during bud burst in grapevine. The postdormant bud is hypoxic (<10% saturation; Fig. 1), and oxygen concentration gradually increases in a spatially regulated manner during the first week of bud burst, prior to leaf emergence (Meitha et al., 2015, 2018). Independent studies show the seeds and fruits of several species show spatially and developmentally regulated tissue oxygen status (Verboven et al., 2008; Borisjuk and Rolletschek, 2009; Cukrov et al., 2016). In grapevine buds as in seeds, the outer scales were shown to be a barrier to oxygen diffusion; however, this did not explain the elevated levels of oxygen in the primary bud after bud burst commenced, particularly where the oxygen minimum was not at the core of the bud (Fig. 1; Meitha et al., 2015). Although not yet demonstrated in buds, the low oxygen status (hypoxia) of seeds is reflected in the spatial patterns of metabolic control, particularly in relation to anaerobic glycolysis and energy status (Borisjuk and Rolletschek, 2009). It has since emerged that oxygen status (and nitric oxide) has a regulatory role in seed dormancy and germination, where the oxygen-dependent degradation of ERF-VII regulates the effective transition from anaerobic to aerobic metabolism and quiescence to growth (Holman et al., 2009; Gibbs et al., 2014). No such research has been applied directly to bud outgrowth; however, it is notable that Arabidopsis mutants impaired in the regulated degradation of the ERF-VII transcription factors show reduced apical dominance (Graciet et al., 2009).
Gene expression data of grapevine buds may provide some insight into the roles of light and oxygen in regulating bud burst. Postdormant grapevine buds do not require light to burst; however, dark-grown buds are impaired in chlorophyll synthesis and develop an etiolated phenotype (Meitha et al., 2018). We have contrasted the gene expression of buds, grown in single-node cuttings, during bud burst in the presence (DL) and absence of light (D) at 72 and 144 h (Supplemental Table S1; fold change [FC] ≥ |2|, false discovery rate [FDR] P ≤ 0.05), which preceded leaf emergence (data available at NCBI BioProject PRJNA327467, http://www.ncbi.nlm.nih.gov/bioproject/327467). A complementary study investigated the developmental control of gene expression and primary metabolism (Meitha et al., 2018). Interestingly, there were few changes in physiological status or global transcript profiles of light- and dark-grown buds over the term; a total of 436 genes were differentially expressed at one or both time points, 47 genes consistently regulated at both (Supplemental Table S1). A small subset of genes showed quite starkly differential expression in response to light, and these will now be discussed in detail.
A key component of photomorphogenesis is HY5, a bZIP transcription factor known to bind the promoters of light-inducible genes to activate their expression (Chattopadhyay et al., 1998). This transcription factor is activated by different types of light, through the action of the photoreceptors PHYA, PHYB, CRY1, and CRY2 (Eberhard et al., 2008), at least in part due to their negative regulation of CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1, also known as FUSCA1), which targets HY5 to the proteasome (Ang et al., 1998). Although the function of HY5 in seedling photomorphogenesis in Arabidopsis has been reported, its expression and response to light in perennial buds had not been described. From the transcriptome analysis of the grapevine buds (Supplemental Table S1), we observed that the expression of genes coding for the HY5, or the upstream photoreceptors PHYA, PHYB, CRY1, and CRY2, was not differentially regulated by the presence of light at 72 h of growth. However, the expression of two CRY genes and HY5 was increased at 144 h in the buds exposed to light (Supplemental Table S1; Fig. 2). In rose species and cultivars, blue light is sufficient to promote bud outgrowth until flowering (Girault et al., 2008; Abidi et al., 2013). Together, this evidence suggests that in perennials buds, CRY photoreceptors are capable of stimulating bud burst by promoting HY5 expression. The data also suggest that in grapevine buds, light penetration and perception is not appreciable until after 72 h growth.
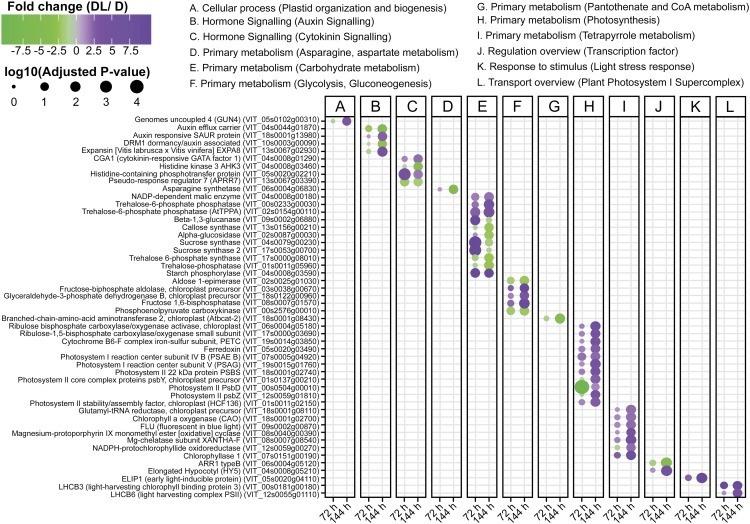
Figure 2.
Scatterplot showing the differential expression and functional category of grapevine genes specifically discussed here. Full data presented in Supplemental Table S1. Differential expression analysis was carried out from grapevine buds grown at 22°C in the presence (DL) or absence (D) of light at 72 and 144 h following removal from 4°C storage (FC ≥ |2|, FDR P ≤ 0.05). Letters from A to L summarize the functional categories. Size of dots represents the log10(adjusted P value). Color scale proportional to FC values: green (down-regulated genes), gray (not differentially expressed), and purple (up-regulated genes).
Known HY5 target genes encode proteins involved in chlorophyll biosynthesis, light harvesting, and the Calvin cycle (Eberhard et al., 2008). The expression of many genes involved in these processes was up-regulated at 144 h in illuminated buds compared with those kept in continuous darkness (Figs. 2 and 3). Homologs of many of the light-regulated genes in grapevine buds are also induced during photomorphogenesis in Arabidopsis (Ghassemian et al., 2006) and in rice (Oryza sativa; Kleffmann et al., 2007; Su et al., 2007). The subset of light-regulated genes in these species includes those coding for photosystem components (PsaD, PsaG, PsaH, PsaK, PsaL, and PsaN; PsbS; LHCA1, LHCA2, LHCA4, and LHCA6; and LHCII B2 and B3), as well as ATP synthase epsilon, Ferredoxin, Ferredoxin NADP-reductase, Rubisco subunits, and chlorophyll biosynthesis. Moreover, the expression of genes encoding two ankyrin domain-containing proteins, which are involved in successful insertion of light harvesting complex (LHC) components in the thylakoid membrane, was up-regulated in grapevine buds at 144 h under illumination (Supplemental Table S1). Furthermore, the levels of transcripts encoding several enzymes of the Calvin cycle were also higher in illuminated buds at 144 h, as described in further detail below.
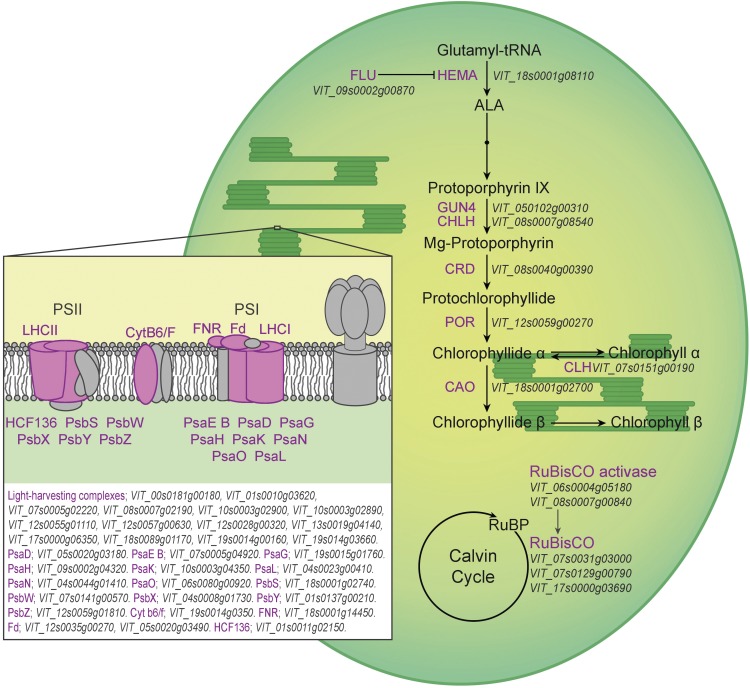
Figure 3.
Differential expression of genes during grapevine bud burst coding for photosynthetic and chlorophyll metabolic functions at 144 h in the presence (DL) or absence (D) of light. Purple color indicates up-regulation at 144 h of DL with respect to D. Abbreviations not defined in the text: ALA, aminolevulinic acid; CAO, CHLOROPHYLL A OXYGENASE; CHL, Mg-CHLOROPHYLLASE1; CHLH, Mg-CHELATASE subunit; CRD, Mg-PROTOPORPHYRIN IX MONOMETHYLESTER CYCLASE; CytB6/F, CYTOCHROME b6-F COMPLEX IRON-SULFUR subunit (PETC); Fd, FERREDOXIN; FLU, FLUORESCENT IN BLUE LIGHT; FNR, Fd NADP+ OXIDOREDUCTASE; HCF136, PSII STABILITY/ASSEMBLY FACTOR; HEMA, GLUTAMYL-TRNA REDUCTASE; POR, NADPH-PROTOCHLOROPHYLLIDE OXIDOREDUCTASE; PSI, PHOTOSYSTEM I; PSII, PHOTOSYSTEM II; PsaD, PSI REACTION CENTRE (RC) subunit II, chloroplast precursor; PsaE B, PSI RC subunit IV B; PsaG, PSI RC subunit V; PsaH, PSI RC subunit VI; PsaK, PSI subunit X; PsaL, PSI subunit XI; PsaN, PSI RC subunit N; PsaO, PSI subunit O; PsbS, PSII 22-kD protein; PsbW, PSII RC W; PsbX, PSII subunit X; PsbY, PSII CORE COMPLEX PROTEIN (chloroplast precursor); PsbZ, PSII core complex proteins; Rubisco, RIBULOSE BISPHOSPHATE CARBOXYLASE; RuBP, Ribulose 1,5-bisphosphate.
From the up-regulated genes in DL condition at 144 h, a total of 48 genes contained the target G-Box sequence (CACGTG) of HY5 (Supplemental Table S2), including homologs of genes known to be regulated by HY5 as well as likely candidates in light- and energy-dependent functions. This includes genes coding for two T6P phosphatases, the malic enzyme, the CK-responsive GATA factor 1, cryptochrome, and GUN4, among others (Fig. 2; Supplemental Table S2). Some evidence has been provided that links CK signaling pathways with HY5 (Vandenbussche et al., 2007; Das et al., 2012). It may be that the CK-responsive GATA factor 1 is responsible for this cross talk. In further studies, it would be interesting to evaluate whether HY5 can modulate the expression of these genes.
Early markers of light perception or prolonged darkness were differentially expressed according to the presence of light. For example, the expression of a homolog of EARLY LIGHT-INDUCIBLE PROTEIN was up-regulated in the light (Fig. 2; Supplemental Table S2). Conversely, the expression of a homolog of DARK-INDUCED6 (DIN6, also known as ASPARAGINE SYNTHETASE1) was progressively down-regulated in the presence of light, relative to continuous darkness. The up-regulation of DIN6 is a hallmark of stresses such as extended darkness and hypoxia, which limit photosynthesis and/or respiration (Baena-González et al., 2007). The expression of DIN6 is repressed by Suc and Glc, and is specifically induced by the Arabidopsis homologs of the catalytic subunits of SnRK1 (KIN10, KIN11), a conserved hub for starvation signaling (Baena-González et al., 2007).
These facets of the transcript profiles of developing grapevine buds further demonstrate that a light-dependent photomorphogenesis becomes apparent at 144 h of exposure of the buds to environmental favorable conditions, but not earlier (i.e. 72 h). This finding suggests that at the beginning of bud burst other environmental cues, such as temperature, are required to promote skotomorphogenic development. Thereafter, growth in the light provides signals that induce photomorphogenic development.
Chloroplast-to-nucleus and mitochondria-to-nucleus retrograde signals are very important for organelle development (Chan et al., 2016). Components that act as retrograde signals participate in biogenic and operational processes. Some genes that are involved in retrograde signaling, such as GUN4 and HY5, are differentially expressed in grapevine buds in response to light. The gun mutants of Arabidopsis are defective in tetrapyrrole metabolism, suggesting that this pathway is important in biogenic signaling. The expression of six genes involved in tetrapyrrole metabolism was changed in grapevine buds in response to light at 144 h. In particular, GUN4 participates in the biosynthesis of Mg-Protoporphyrin-IX, which in turn binds to a Heat Shock 90-type protein and interacts with HY5 to regulate the expression of photosynthesis-associated nuclear genes (Chan et al., 2016). The expression of Protoporphyrin-IX biosynthetic genes and HY5 was up-regulated by light in grapevine buds at 144 h, suggesting that the retrograde activation of photosynthesis-associated nuclear genes occurs in illuminated buds. Hence, the plastids in the buds of perennials species may be undergoing a biogenic process rather than an operational adaptation to the environmental conditions at the early stages of bud burst.
Light adaptation also occurs through the induction of CK signaling pathways in plants. The expression of a gene coding for a His-containing phosphotransfer protein was up-regulated by light at 72 h in grapevine buds (Fig. 2; Supplemental Table S1). This protein plays a key role in propagating CK signal transduction (Hwang et al., 2002). The expression of the CK-responsive GATA factor 1 is known to respond to light and CKs (Naito et al., 2007). It also plays a role in chloroplast development (Hudson et al., 2013). The expression of the CK-responsive GATA factor 1 was increased at 144 h DL in grapevine buds (Fig. 2). This transcription factor represses gibberellic acid signaling downstream of PIF and DELLA regulators (Richter et al., 2010). The expression of genes coding for repressors of CK signaling, such as ARR1 type B and APRR7, was down-regulated by light in grapevine buds. These findings suggest that the influence of light on grapevine buds involves CK signaling pathways. The expression of two other components (ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER1 [AHP1] and HISTIDINE KINASE3 [AHK3]) involved in CK signaling was down-regulated by light. Since there is considerable redundancy in the functions of the different AHP proteins (AHP1, AHP2, AHP3, and AHP5), which act as positive regulators of CK signaling to promote development, the significance of this observation is uncertain (Hutchison et al., 2006).
As described above, evidence now suggests that Suc and light-dependent auxin signaling converge upon meristem activators in Arabidopsis, promoting meristem growth. We found few primarily auxin-related functions in the grapevine data shown here (Fig. 2; Supplemental Table S1). Auxin has previously been shown to function in the removal of dormancy callose in grapevine buds and to accumulate during bud swell; however, direct application has apparently little effect (Aloni et al., 1991; Lavee and May 1997, and references therein). A more recent, limited transcript analysis in developing grapevine buds (predormant, paradormant) showed no relationship between genes selected as auxin and Suc function markers, or with auxin-function markers and the outgrowth potential (He et al., 2012). Nevertheless, none of these studies were designed to elaborate auxin or Suc functions, and hence any relationships may be obscured.
SUGAR METABOLISM IS REGULATED BY LIGHT AT EARLY STAGES OF GRAPEVINE BUD BURST
Several transcripts encoding enzymes involved in starch, Suc, and hexose metabolism were strongly regulated by light in grapevine buds at 72 h in the light. These include homologs of STARCH PHOSPHORYLASE, BETA-1,3-GLUCANASE, and two SUCROSE SYNTHASE (Fig. 2; Supplemental Table S1). The light-induced activation of expression of starch and Suc hydrolytic genes was largely attenuated at 144 h, although STARCH PHOSPHORYLASE transcripts remained at higher levels at 144 h. Transcripts encoding a homolog of CALLOSE SYNTHASE were decreased in the buds in the light at 144 h. In Rosa sp., the light-dependent up-regulation of VACUOLAR INVERTASE is considered to be important in promoting sugar degradation and bud burst (Girault et al., 2008; Henry et al., 2011). The finding that the expression of a VACUOLAR INVERTASE, GIN2, was not differentially regulated by light in grapevine may partially explain the differences in the light requirements of bud burst in Rosa sp. and grapevine.
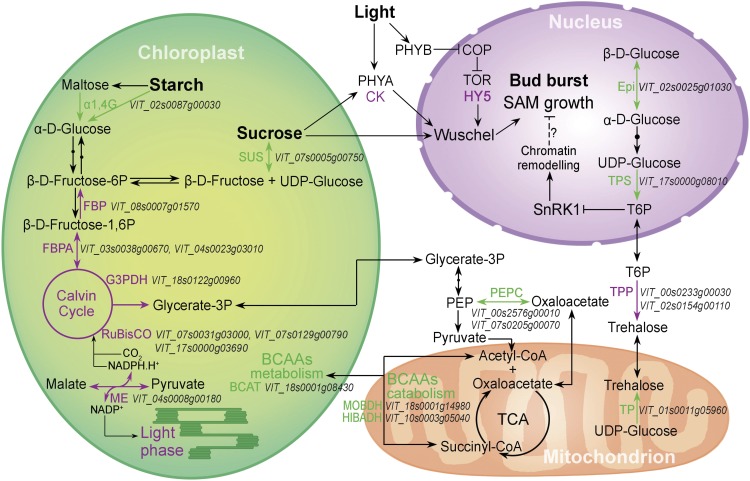
As illustrated in Figure 4, the expression of genes encoding plastid carbon metabolism enzymes in grapevine buds was clearly up-regulated by light at 144 h. Moreover, transcripts encoding a homolog of the plastid-localized NADP+-dependent MALIC ENZYME were increased in the light, suggesting a need for regulation of NADP+/NADPH homeostasis and provision of reducing power for Calvin cycle activity (Wheeler et al., 2005). In contrast, the up-regulated expression of genes encoding proteins involved in the catabolism of branched-chain amino acids in the plastid was increased in the dark, as were the levels of transcripts encoding a cytosolic PHOSPHOENOLPYRUVATE CARBOXYKINASE (Fig. 4). These findings suggest a requirement for alternative substrates to fuel the mitochondrial tricarboxylic acid pathway in the dark-treated condition (Araújo et al., 2012; Avin-Wittenberg et al., 2015).
Figure 4.
Differential expression of genes during grapevine bud burst coding for carbon- and energy-related functions at 144 h in the presence (DL) or absence (D) of light. Processes and reactions in purple and green reflect up- and down-regulation, respectively, at 144 h in the DL/D comparison. Abbreviations not defined in the text: α1,4G, α-1,4-GLUCOSIDASE; BCAAs, branched-chain amino acids; BCAT, BRANCHED-CHAIN AMINO ACID AMINOTRANSFERASE; Epi, ALDOSE 1-EPIMERASE; FBP, FRUCTOSE 1,6-BISPHOSPHATASE; FBPA, FRUCTOSE-BISPHOSPHATE ALDOLASE; G3PDH, GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE B; ME, NADP-DEPENDENT MALIC ENZYME; PEPC, PHOSPHOENOLPYRUVATE CARBOXYKINASE; Rubisco, RIBULOSE BISPHOSPHATE CARBOXYLASE; SUS, SUCROSE SYNTHASE; TP, TREHALOSE-PHOSPHATASE.
T6P is a primary sensor of cellular energy status. Transcripts encoding two T6P PHOSPHATASE (TPP) homologs were increased by light at 144 h, while TPP and T6P SYNTHASE (TPS) mRNAs were decreased in abundance (and increased in the dark; Figs. 2 and 4). These transcriptional differences suggest that reduced T6P levels or alternatively, increased T6P turnover, occurs in the buds in the light compared with the dark condition.
We then compared the grapevine bud differential gene expression at 144 h (Supplemental Table S1) against the public data of the Arabidopsis transcriptional perturbation database in Genevestigator (Hruz et al., 2008). We used the accession identifiers of the Arabidopsis homologs of the grapevine differentially expressed genes and selected unique genes, leaving 317 differentially expressed genes (Supplemental Table S3A). The corresponding Arabidopsis accessions were entered using the Signature tool and compared with all available Arabidopsis data using the Perturbations profile, with the Manhattan Distance algorithm (Affymetrix Arabidopsis ATH1 Genome Array, all genetic backgrounds, 9,552 samples). Some of the Arabidopsis accessions submitted did not match a probe from the ATH1 microarray, leaving 306 probes (Supplemental Table S3B). Nearly all of the top 50 most similar of 3,020 Perturbation studies attended to postgermination photomorphogenesis. Each of the top five most similar were wild-type studies that investigated light signaling and contrasted light conditions against continuous darkness (Supplemental Table S3B), for example, the role of plastid biogenesis in mediating light-dependent signaling (GEO accession GSE24517; Ruckle et al., 2012) and the role of light-dependent translational regulation in photomorphogenesis (GEO accession GSE29657; Liu et al., 2012). Several of the studies involving mutant lines that had similar profiles to grapevine buds (BioProject PRJNA327467) also related to light and carbon signaling, for example, a study of the role of the COP1 in coordinating light-dependent signaling (GEO accession GSE22983; Chang et al., 2011) and a study identifying CARBON AND LIGHT INSENSITIVE (CLI186) mutants (ArrayExpress accession E-MEXP-1112; Thum et al., 2008).
We then constrained our query of the Genevestigator data to developmental studies of germination or postgermination seedlings, which retrieved 136 perturbations (Supplemental Table S3C). The similarity of our data with comparisons from Narsai et al. (2011; GEO accession GSE30223) of germinating seed against dark-stratified seed suggested the DL condition in our study was more developmentally advanced than the D condition. Also of interest were comparisons of Glc-treated against control seedlings of wild-type or conditional mutants of the TOR protein kinase, indicating the DL condition was consistent with active metabolism of sugars (GEO accession GSE40245; Xiong et al., 2013).
In addition, the comparison to the core 600 putative targets of the Arabidopsis KIN10 (Baena-González et al., 2007) corroborated the identification of components involved in the catabolism of branched-chain amino acids and the regulation of TPS expression under continuous darkness (repressed in DL/D). Furthermore, this analysis supported conclusions regarding light-mediated regulation of DORMANCY/AUXIN ASSOCIATED1, two genes coding for thioredoxins, and two members of the NBS-LRR Leu-rich repeat superfamily, each implicated in sugar starvation responses (Baena-González et al., 2007).
Together, these data suggest that transcriptional changes induced by light in grapevine buds are similar to those observed in Arabidopsis, providing evidence of a prominent role for chloroplast processes in carbon and oxygen (energy) metabolism during bud burst and the requirement for light to orchestrate chloroplast biogenesis. It also provides considerable evidence of the effect of light on sugar signaling. Alternative pathways for catabolism became evident under continuous darkness, suggesting catabolism of branched-chain amino acids to fuel the mitochondrial tricarboxylic acid cycle.
CONCLUDING REMARKS
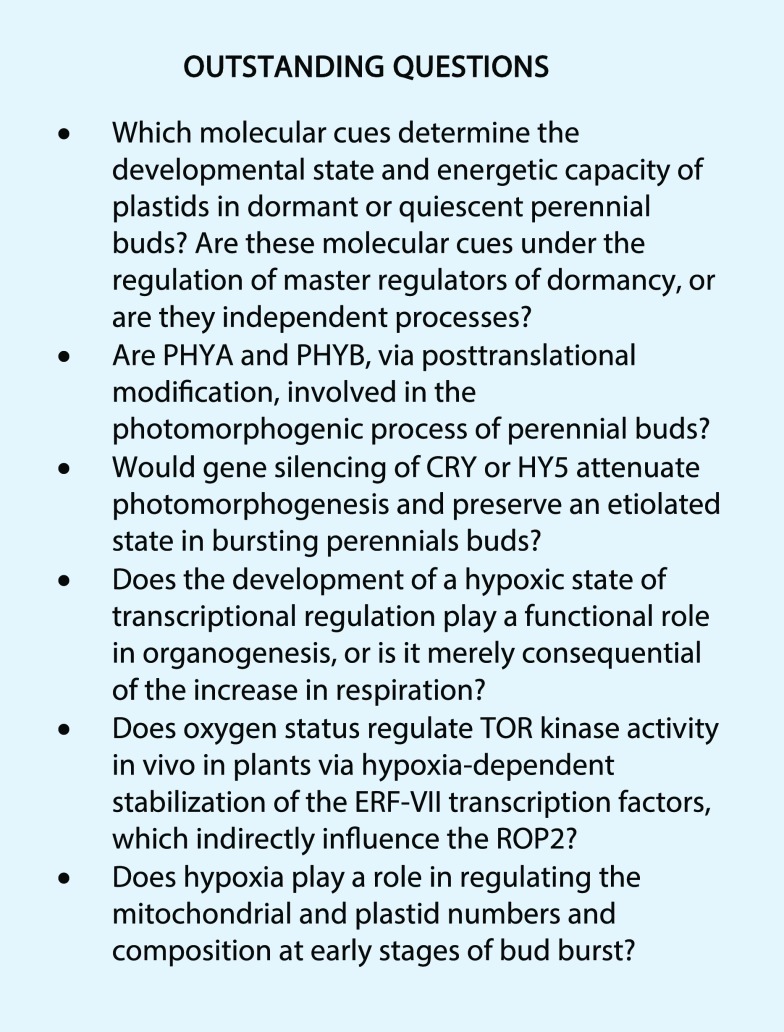
The commitment to resume growth of postdormant perennial buds is driven by developmental activators such as CKs and auxins. While light can function as an upstream regulator of these phytohormones, light is only a facultative requirement for the decision in many species. The body of evidence discussed here demonstrates that light promotes/enhances rather than drives photomorphogenic development, while other cues such as temperature promote the initial skotomorphogenic outgrowth. Suc, resulting from emerging photosynthesis, may also participate in the light-independent activation process, acting as both a metabolite and signaling molecule. While the present discussion has focused on the importance of white light, blue light may also play a key role in bud burst. Accumulating evidence supports the function of CRY photoreceptors in blue light perception resulting in HY5 expression, which in turn activates photomorphogenic gene expression, stimulating bud outgrowth. PHYA and PHYB may also fulfill roles in light perception as they do in Arabidopsis seeds. The developmental stages of plastids of buds can vary between different perennial plants but also within different tissues of the same bud. The developmental regulation of the hypoxic state also plays important but largely undefined roles in bud burst. The role of hypoxia in regulating mitochondrial and plastid numbers and composition at the early stages of bud burst is largely unexplored. Finally, our analysis of the literature evidence highlights the conservation of light-induced signaling cascades and associated transcriptional changes that drive the resumption of growth after a period of quiescence in perennial buds and Arabidopsis seeds. Several exciting questions remain, particularly in regard to the role of light and oxygen in bud burst (see Outstanding Questions). Increasingly, the tools required to investigate them, even in perennials, are becoming available.
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. Differentially expressed genes (DEG) which met fold change and false discovery rate criteria (FC≥|2|, FDR P≤0.05) in the presence (DL) or absence (D) of light at 72 and 144 h and after functional enrichment.
Supplemental Table S2. List of genes containing the G-box sequence in upregulated genes at DL 144h from Supplemental Table S1.
Supplemental Table S3. Comparison of differentially expressed gene expression, after functional enrichment with public arabidopsis microarray data available at Genevestigator.
References
- Abidi F, Girault T, Douillet O, Guillemain G, Sintes G, Laffaire M, Ben Ahmed H, Smiti S, Huché-Thélier L, Leduc N (2013) Blue light effects on rose photosynthesis and photomorphogenesis. Plant Biol (Stuttg) 15: 67–74 [DOI] [PubMed] [Google Scholar]
- Aloni R, Raviv A, Peterson CA (1991) The role of auxin in the removal of dormancy callose and resumption of phloem activity in Vitis vinifera. Can J Bot 69: 1825–1832 [Google Scholar]
- Ang L-H, Chattopadhyay S, Wei N, Oyama T, Okada K, Batschauer A, Deng X-W (1998) Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol Cell 1: 213–222 [DOI] [PubMed] [Google Scholar]
- Araújo WL, Nunes-Nesi A, Nikoloski Z, Sweetlove LJ, Fernie AR (2012) Metabolic control and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant tissues. Plant Cell Environ 35: 1–21 [DOI] [PubMed] [Google Scholar]
- Avin-Wittenberg T, Bajdzienko K, Wittenberg G, Alseekh S, Tohge T, Bock R, Giavalisco P, Fernie AR (2015) Global analysis of the role of autophagy in cellular metabolism and energy homeostasis in Arabidopsis seedlings under carbon starvation. Plant Cell 27: 306–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-González E, Rolland F, Thevelein JM, Sheen J (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942 [DOI] [PubMed] [Google Scholar]
- Barbier FF, Lunn JE, Beveridge CA (2015a) Ready, steady, go! A sugar hit starts the race to shoot branching. Curr Opin Plant Biol 25: 39–45 [DOI] [PubMed] [Google Scholar]
- Barbier F, Péron T, Lecerf M, Perez-Garcia M-D, Barrière Q, Rolčík J, Boutet-Mercey S, Citerne S, Lemoine R, Porcheron B, et al. (2015b) Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J Exp Bot 66: 2569–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J (2002) RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296: 2026–2028 [DOI] [PubMed] [Google Scholar]
- Borisjuk L, Rolletschek H (2009) The oxygen status of the developing seed. New Phytol 182: 17–30 [DOI] [PubMed] [Google Scholar]
- Cai W, Li X, Liu Y, Wang Y, Zhou Y, Xu T, Xiong Y (2017) COP1 integrates light signals to ROP2 for cell cycle activation. Plant Signal Behav 12: e1363946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffarra A, Donnelly A (2011) The ecological significance of phenology in four different tree species: effects of light and temperature on bud burst. Int J Biometeorol 55: 711–721 [DOI] [PubMed] [Google Scholar]
- Chan KX, Phua SY, Crisp P, McQuinn R, Pogson BJ (2016) Learning the languages of the chloroplast: retrograde signaling and beyond. Annu Rev Plant Biol 67: 25–53 [DOI] [PubMed] [Google Scholar]
- Chang C-SJ, Maloof JN, Wu S-H (2011) COP1-mediated degradation of BBX22/LZF1 optimizes seedling development in Arabidopsis. Plant Physiol 156: 228–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chary SN, Hicks GR, Choi YG, Carter D, Raikhel NV (2008) Trehalose-6-phosphate synthase/phosphatase regulates cell shape and plant architecture in Arabidopsis. Plant Physiol 146: 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Ang LH, Puente P, Deng XW, Wei N (1998) Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression. Plant Cell 10: 673–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine MJ. (2017) Oxygen, energy, and light signalling direct meristem fate. Trends Plant Sci 23: 1–3 [DOI] [PubMed] [Google Scholar]
- Considine MJ, Considine JA (2016) On the language and physiology of dormancy and quiescence in plants. J Exp Bot 67: 3189–3203 [DOI] [PubMed] [Google Scholar]
- Considine MJ, Diaz-Vivancos P, Kerchev P, Signorelli S, Agudelo-Romero P, Gibbs DJ, Foyer CH (2017) Learning to breathe: developmental phase transitions in oxygen status. Trends Plant Sci 22: 140–153 [DOI] [PubMed] [Google Scholar]
- Cukrov D, Zermiani M, Brizzolara S, Cestaro A, Licausi F, Luchinat C, Santucci C, Tenori L, Van Veen H, Zuccolo A, et al. (2016) Extreme hypoxic conditions induce selective molecular responses and metabolic reset in detached apple fruit. Front Plant Sci 7: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das PK, Shin DH, Choi SB, Yoo SD, Choi G, Park YI (2012) Cytokinins enhance sugar-induced anthocyanin biosynthesis in Arabidopsis. Mol Cells 34: 93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun EA, de Saint Germain A, Rameau C, Beveridge CA (2012) Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol 158: 487–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard S, Finazzi G, Wollman FA (2008) The dynamics of photosynthesis. Annu Rev Genet 42: 463–515 [DOI] [PubMed] [Google Scholar]
- Ghassemian M, Lutes J, Tepperman JM, Chang HS, Zhu T, Wang X, Quail PH, Lange BM (2006) Integrative analysis of transcript and metabolite profiling data sets to evaluate the regulation of biochemical pathways during photomorphogenesis. Arch Biochem Biophys 448: 45–59 [DOI] [PubMed] [Google Scholar]
- Gibbs DJ, Md Isa N, Movahedi M, Lozano-Juste J, Mendiondo GM, Berckhan S, Marín-de la Rosa N, Vicente Conde J, Sousa Correia C, Pearce SP, et al. (2014) Nitric oxide sensing in plants is mediated by proteolytic control of group VII ERF transcription factors. Mol Cell 53: 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault T, Bergougnoux V, Combes D, Viemont JD, Leduc N (2008) Light controls shoot meristem organogenic activity and leaf primordia growth during bud burst in Rosa sp. Plant Cell Environ 31: 1534–1544 [DOI] [PubMed] [Google Scholar]
- Gonzali S, Loreti E, Cardarelli F, Novi G, Parlanti S, Pucciariello C, Bassolino L, Banti V, Licausi F, Perata P (2015) Universal stress protein HRU1 mediates ROS homeostasis under anoxia. Nat Plants 1: 15151. [DOI] [PubMed] [Google Scholar]
- Graciet E, Walter F, Ó’Maoiléidigh DS, Pollmann S, Meyerowitz EM, Varshavsky A, Wellmer F (2009) The N-end rule pathway controls multiple functions during Arabidopsis shoot and leaf development. Proc Natl Acad Sci USA 106: 13618–13623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D, Mathiason K, Fennell A (2012) Auxin and cytokinin related gene expression during active shoot growth and latent bud paradormancy in Vitis riparia grapevine. J Plant Physiol 169: 643–648 [DOI] [PubMed] [Google Scholar]
- Henry C, Rabot A, Laloi M, Mortreau E, Sigogne M, Leduc N, Lemoine R, Sakr S, Vian A, Pelleschi-Travier S (2011) Regulation of RhSUC2, a sucrose transporter, is correlated with the light control of bud burst in Rosa sp. Plant Cell Environ 34: 1776–1789 [DOI] [PubMed] [Google Scholar]
- Holman TJ, Jones PD, Russell L, Medhurst A, Ubeda Tomás S, Talloji P, Marquez J, Schmuths H, Tung SA, Taylor I, et al. (2009) The N-end rule pathway promotes seed germination and establishment through removal of ABA sensitivity in Arabidopsis. Proc Natl Acad Sci USA 106: 4549–4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P (2008) Genevestigator V3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics 2008: e420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson D, Guevara DR, Hand AJ, Xu Z, Hao L, Chen X, Zhu T, Bi Y-M, Rothstein SJ (2013) Rice cytokinin GATA transcription Factor1 regulates chloroplast development and plant architecture. Plant Physiol 162: 132–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison CE, Li J, Argueso C, Gonzalez M, Lee E, Lewis MW, Maxwell BB, Perdue TD, Schaller GE, Alonso JM, et al. (2006) The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell 18: 3073–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Chen HC, Sheen J (2002) Two-component signal transduction pathways in Arabidopsis. Plant Physiol 129: 500–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G, David-Schwartz R, Sade N, Moshelion M, Levi A, Alchanatis V, Granot D (2012) The pitfalls of transgenic selection and new roles of AtHXK1: a high level of AtHXK1 expression uncouples hexokinase1-dependent sugar signaling from exogenous sugar. Plant Physiol 159: 47–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleffmann T, von Zychlinski A, Russenberger D, Hirsch-Hoffmann M, Gehrig P, Gruissem W, Baginsky S (2007) Proteome dynamics during plastid differentiation in rice. Plant Physiol 143: 912–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavee S, May P (1997) Dormancy of grapevine buds - facts and speculation. Aust J Grape Wine Res 3: 31–46 [Google Scholar]
- Leduc N, Roman H, Barbier F, Péron T, Huché-Thélier L, Lothier J, Demotes-Mainard S, Sakr S (2014) Light signaling in bud outgrowth and branching in plants. Plants (Basel) 3: 223–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Cai W, Liu Y, Li H, Fu L, Liu Z, Xu L, Liu H, Xu T, Xiong Y (2017) Differential TOR activation and cell proliferation in Arabidopsis root and shoot apexes. Proc Natl Acad Sci USA 114: 2765–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MJ, Wu SH, Chen HM, Wu SH (2012) Widespread translational control contributes to the regulation of Arabidopsis photomorphogenesis. Mol Syst Biol 8: 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA (2014) Sugar demand, not auxin, is the initial regulator of apical dominance. Proc Natl Acad Sci USA 111: 6092–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard BK, Sun WQ, Bassuk NL (1990) Encouraging bud break in newly-rooted softwood cuttings. Proc Int Plant Propagators Soc 40: 597–602 [Google Scholar]
- Meitha K, Konnerup D, Colmer TD, Considine JA, Foyer CH, Considine MJ (2015) Spatio-temporal relief from hypoxia and production of reactive oxygen species during bud burst in grapevine (Vitis vinifera). Ann Bot 116: 703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitha K, Agudelo-Romero P, Signorelli S, Gibbs DG, Considine JA, Foyer CH, Considine MJ, (2018) Developmental control of hypoxia during bud burst in grapevine. Plant Cell Environ http://dx.doi.org/10.1111/pce.13141 [DOI] [PubMed]
- Naito T, Kiba T, Koizumi N, Yamashino T, Mizuno T (2007) Characterization of a unique GATA family gene that responds to both light and cytokinin in Arabidopsis thaliana. Biosci Biotechnol Biochem 71: 1557–1560 [DOI] [PubMed] [Google Scholar]
- Narsai R, Law SR, Carrie C, Xu L, Whelan J (2011) In-depth temporal transcriptome profiling reveals a crucial developmental switch with roles for RNA processing and organelle metabolism that are essential for germination in Arabidopsis. Plant Physiol 157: 1342–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otori K, Tamoi M, Tanabe N, Shigeoka S (2017) Enhancements in sucrose biosynthesis capacity affect shoot branching in Arabidopsis. Biosci Biotechnol Biochem 81: 1470–1477 [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Janocha D, Dong Y, Medzihradszky A, Schöne S, Daum G, Suzaki T, Forner J, Langenecker T, Rempel E, et al. (2016) Integration of light and metabolic signals for stem cell activation at the shoot apical meristem. eLife 5: e17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabot A, Henry C, Ben Baaziz K, Mortreau E, Azri W, Lothier J, Hamama L, Boummaza R, Leduc N, Pelleschi-Travier S, et al. (2012) Insight into the role of sugars in bud burst under light in the rose. Plant Cell Physiol 53: 1068–1082 [DOI] [PubMed] [Google Scholar]
- Rageau R, Bonhomme M, Richard JP, Erez A (1998) The climatic determinism of vegetative bud break on peach trees with no exposure to chilling: some experimental results. Acta Hortic 465: 511–520 [Google Scholar]
- Rameau C, Bertheloot J, Leduc N, Andrieu B, Foucher F, Sakr S (2015) Multiple pathways regulate shoot branching. Front Plant Sci 5: 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton M, Hanan J, Ferguson BJ, Beveridge CA (2012) Models of long-distance transport: How is carrier-dependent auxin transport regulated in the stem? New Phytol 194: 704–715 [DOI] [PubMed] [Google Scholar]
- Richter R, Behringer C, Müller IK, Schwechheimer C (2010) The GATA-type transcription factors GNC and GNL/CGA1 repress gibberellin signaling downstream from DELLA proteins and PHYTOCHROME-INTERACTING FACTORS. Genes Dev 24: 2093–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne PLH, Welling A, Vahala J, Ripel L, Ruonala R, Kangasjärvi J, van der Schoot C (2011) Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-β-glucanases to reopen signal conduits and release dormancy in Populus. Plant Cell 23: 130–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose JL, Frankel LK, Bricker TM (2011) Developmental defects in mutants of the PsbP domain protein 5 in Arabidopsis thaliana. PLoS One 6: e28624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckle ME, Burgoon LD, Lawrence LA, Sinkler CA, Larkin RM (2012) Plastids are major regulators of light signaling in Arabidopsis. Plant Physiol 159: 366–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard G, Johnsen O, Nilsen J, Junttila O (2008) Climatic control of bud burst in young seedlings of nine provenances of Norway spruce. Tree Physiol 28: 311–320 [DOI] [PubMed] [Google Scholar]
- Solymosi K, Bóka K, Böddi B (2006) Transient etiolation: protochlorophyll(ide) and chlorophyll forms in differentiating plastids of closed and breaking leaf buds of horse chestnut (Aesculus hippocastanum). Tree Physiol 26: 1087–1096 [DOI] [PubMed] [Google Scholar]
- Solymosi K, Morandi D, Bóka K, Böddi B, Schoefs B (2012) High biological variability of plastids, photosynthetic pigments and pigment forms of leaf primordia in buds. Planta 235: 1035–1049 [DOI] [PubMed] [Google Scholar]
- Su N, He K, Jiao Y, Chen C, Zhou J, Li L, Bai S, Li X, Deng XW (2007) Distinct reorganization of the genome transcription associates with organogenesis of somatic embryo, shoots, and roots in rice. Plant Mol Biol 63: 337–349 [DOI] [PubMed] [Google Scholar]
- Sussex IM, Kerk NM (2001) The evolution of plant architecture. Curr Opin Plant Biol 4: 33–37 [DOI] [PubMed] [Google Scholar]
- Thimann KV, Skoog F (1934) On the inhibition of bud development and other functions of growth substance in Vicia faba. Proc R Soc Lond B Biol Sci 114: 317–339 [Google Scholar]
- Thum KE, Shin MJ, Gutiérrez RA, Mukherjee I, Katari MS, Nero D, Shasha D, Coruzzi GM (2008) An integrated genetic, genomic and systems approach defines gene networks regulated by the interaction of light and carbon signaling pathways in Arabidopsis. BMC Syst Biol 2: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Habricot Y, Condiff AS, Maldiney R, Van der Straeten D, Ahmad M (2007) HY5 is a point of convergence between cryptochrome and cytokinin signalling pathways in Arabidopsis thaliana. Plant J 49: 428–441 [DOI] [PubMed] [Google Scholar]
- Verboven P, Kerckhofs G, Mebatsion HK, Ho QT, Temst K, Wevers M, Cloetens P, Nicolaï BM (2008) Three-dimensional gas exchange pathways in pome fruit characterized by synchrotron x-ray computed tomography. Plant Physiol 147: 518–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MCG, Tronconi MA, Drincovich MF, Andreo CS, Flügge U-I, Maurino VG (2005) A comprehensive analysis of the NADP-malic enzyme gene family of Arabidopsis. Plant Physiol 139: 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, McCormack M, Li L, Hall Q, Xiang C, Sheen J (2013) Glucose-TOR signalling reprograms the transcriptome and activates meristems. Nature 496: 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Mandel T, Kuhlemeier C (2011) Stem cell activation by light guides plant organogenesis. Genes Dev 25: 1439–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]