COL12 is a substrate of the COP1/SPA ubiquitin ligase and regulates flowering time and plant architecture
Abstract
The ambient light environment controls many aspects of plant development throughout a plant’s life cycle. Such complex control is achieved because a key repressor of light signaling, the Arabidopsis (Arabidopsis thaliana) COP1/SPA E3 ubiquitin ligase causes the degradation of multiple regulators of endogenous developmental pathways. This includes the CONSTANS (CO) transcription factor that is responsible for photoperiodic control of flowering time. There are 16 CO-like proteins whose functions are only partly understood. Here, we show that 14 CO-like (COL) proteins bind CONSTITUTIVELY PHOTOMORPHOGENIC1 (COP1) and SUPPRESSOR OF PHYTOCHROME A-105 (SPA)1 in vitro. We subsequently focused on COL12 and show that COL12 binds COP1 and SPA proteins in vivo. The COL12 protein is degraded in darkness in a COP1-dependent fashion, indicating that COL12 is a substrate of the COP1/SPA ubiquitin ligase. Overexpression of COL12 causes late flowering specifically in long day conditions by decreasing the expression of FLOWERING LOCUS T. This phenotype is genetically dependent on CO. Consistent with this finding, COL12 physically interacts with CO in vivo, suggesting that COL12 represses flowering by inhibiting CO protein function. We show that COL12 overexpression did not alter CO protein stability. It is therefore likely that COL12 represses the activity of CO rather than CO levels. Overexpression of COL12 also affects plant architecture by increasing the number of rosette branches and reducing inflorescence height. These phenotypes are CO independent. Hence, we suggest that COL12 affects plant development through CO-dependent and CO-independent mechanisms.
Plants constantly monitor the ambient light environment to adjust growth and development to changing light conditions and seasons. Therefore, many developmental and metabolic processes are under the control of light. This includes seed germination, seedling de-etiolation, the shade avoidance response, and the control of flowering time by day length. To sense the light, plants have evolved several classes of photoreceptors: the red/far-red responsive phytochromes, the blue-light absorbing cryptochromes, phototropins and ZEITLUPE family, and the UV-B receptor UVR8 (Kami et al., 2010; Tilbrook et al., 2013).
Downstream of the phytochrome and cryptochrome photoreceptors, the CONSTITUTIVELY PHOTOMORPHOGENIC1/SUPPRESSOR OF PHYTOCHROME A-105 (COP1/SPA) E3 ubiquitin ligase acts to suppress light signaling in darkness. It polyubiquitinates positive regulators of light signaling, mainly transcription factors, and thereby targets those for degradation in the 26S proteasome. In the light, the phytochrome and cryptochrome photoreceptors bind to the COP1/SPA complex and inhibit its activity via multiple mechanisms. As a consequence, the target transcription factors accumulate and initiate photomorphogenesis (Menon et al., 2016; Hoecker, 2017). The COP1/SPA complex consists of two COP1 proteins and two SPA proteins of the four-member SPA protein family (SPA1–SPA4; Zhu et al., 2008). Genetic analysis indicates that both COP1 and SPA proteins are required for full activity of this E3 ubiquitin ligase (Deng et al., 1991; Laubinger et al., 2004; Ordoñez-Herrera et al., 2015). Both COP1 and SPA proteins carry a C-terminal WD-repeat domain responsible for interactions with most substrates and a central coiled-coil domain required for COP1/SPA complex formation. In their respective N termini, COP1 and SPA proteins differ, with COP1 carrying a RING finger domain and SPA proteins carrying a kinase-like domain (Menon et al., 2016; Hoecker, 2017).
A key substrate of the COP1/SPA ubiquitin ligase is the transcription factor CONSTANS (CO), which controls photoperiodic flowering (Laubinger et al., 2006; Jang et al., 2008; Liu et al., 2008). Arabidopsis (Arabidopsis thaliana) is a facultative long-day plant, that is it flowers considerably earlier under long-day than under short-day conditions. Long-day-promoted flowering involves the coincidence between endogenous expression of CO and light-dependent stabilization of the CO protein. Since daily CO expression commences not before the late afternoon, this coincidence occurs only under long-day conditions. The CO protein is degraded in darkness via the COP1/SPA complex and stabilized in the light through cry2- and phyA-mediated inhibition of the COP1/SPA E3 ligase (Andrés and Coupland, 2012; Song et al., 2015; Xu et al., 2016b).
CO is a B-box transcription factor that also contains a domain named CONSTANS, CONSTANS-LIKE, TOC1 (CCT). It initiates flowering in long day by activating the expression of FLOWERING LOCUS T (FT) in leaf phloem companion cells. The FT protein subsequently moves to the shoot apical meristem where it activates floral meristem identity genes to induce flowering (Andrés and Coupland, 2012). CO domain mapping demonstrated that the CCT domain of CO interacts with the WD-repeat domains of COP1 and SPA proteins (Laubinger et al., 2006; Jang et al., 2008; Liu et al., 2008). CO is a member of a gene family comprising 16 CO-like (COL) genes (COL1-COL16, also named BBX2-BBX17), which are subclassified into three groups. There are additional B-box transcription factors that do not contain a CCT domain (BBX18-BBX32; Khanna et al., 2009; Crocco and Botto, 2013; Gangappa and Botto, 2014). Probably due to functional redundancy among COL or BBX genes, there is limited understanding of the functions of these genes. Nevertheless, several genetic studies have shown functions of COLs in seedling de-etiolation, root elongation, shade avoidance, flowering time, branching, abiotic stress response, and circadian clock function (Ledger et al., 2001; Cheng and Wang, 2005; Datta et al., 2006; Hassidim et al., 2009; Wang et al., 2013; Zhang et al., 2014; Min et al., 2015; Tripathi et al., 2017). Among the COL family, only CO has been shown to be a substrate of the COP1/SPA E3 ubiquitin ligase (Laubinger et al., 2006; Jang et al., 2008; Liu et al., 2008). COL3/BBX4 interacts with COP1, but whether its protein stability is controlled by COP1 is not known (Datta et al., 2006). Among the BBX proteins that do not contain a CCT domain, BBX20-BBX22, BBX24, and BBX25 have been shown to be ubiquitination targets of COP1 (Gangappa and Botto, 2014; Xu et al., 2016a). BBX19 interacts with both COP1 and ELF3. It promotes hypocotyl elongation by mediating ELF3 degradation in a COP1-dependent fashion (Wang et al., 2015). BBX19 also represses photoperiodic flowering through an interaction with CO (Wang et al., 2014).
Here, we show that 14 COL proteins, like CO, interact with COP1 and SPA1 in vitro, suggesting that all COLs might be substrates of COP1/SPA. We further analyze interactions and function of COL12, a so-far unstudied COL. We demonstrate that COL12 is a substrate of the COP1/SPA E3 ubiquitin ligase and, thus, is degraded in darkness. Functionally, COL12 is a potent repressor of flowering by inhibiting CO activity. It also affects plant architecture in a CO-independent fashion.
RESULTS
COL Proteins Interact with COP1 and SPA1 in Vitro
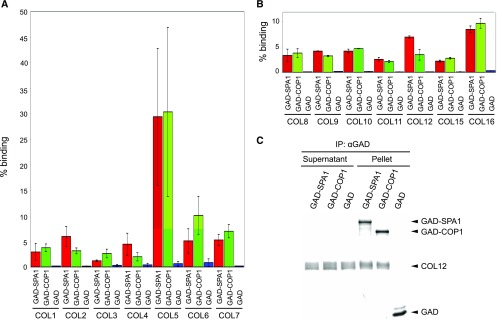
Previously, CO and COL3 were shown to interact with COP1 and/or SPA1 (Datta et al., 2006; Laubinger et al., 2006; Jang et al., 2008; Liu et al., 2008). We therefore tested whether other members of the COL family also interact with the COP1 and SPA1. To this end, we performed coimmunoprecipitations with recombinantly synthesized proteins. Figure 1 shows that all tested COLs (COL1-COL12, COL15, COL16) interacted with COP1 as well as with SPA1 in vitro. Figure 1C shows an example of the COL12 coimmunoprecipitation reactions that were separated on a protein gel. COL13 and COL14 were not investigated because no cDNA could be isolated. These results show that these, if not all, COLs are capable of interacting with COP1 and SPA proteins and thus might be substrates of the COP1/SPA complex.
Figure 1.
SPA1 and COP1 interact with COL proteins in vitro. A to C, All proteins were expressed by coupled in vitro transcription and translation in the presence of 35S-labeled Met. Coimmunoprecipitation was performed using α-GAD antibodies. Radioactively labeled proteins were detected after PAGE separation using a phosphoimager. The supernatant fraction indicates unbound proteins, the pellet fraction indicates coimmunoprecipitated proteins. Percent binding refers to the fraction of the COL prey that was coimmunoprecipitated by the respective bait. Error bars in A and B indicate the se of two to nine replicate experiments.
COL12 Interacts with COP1 and SPA1 in Vivo
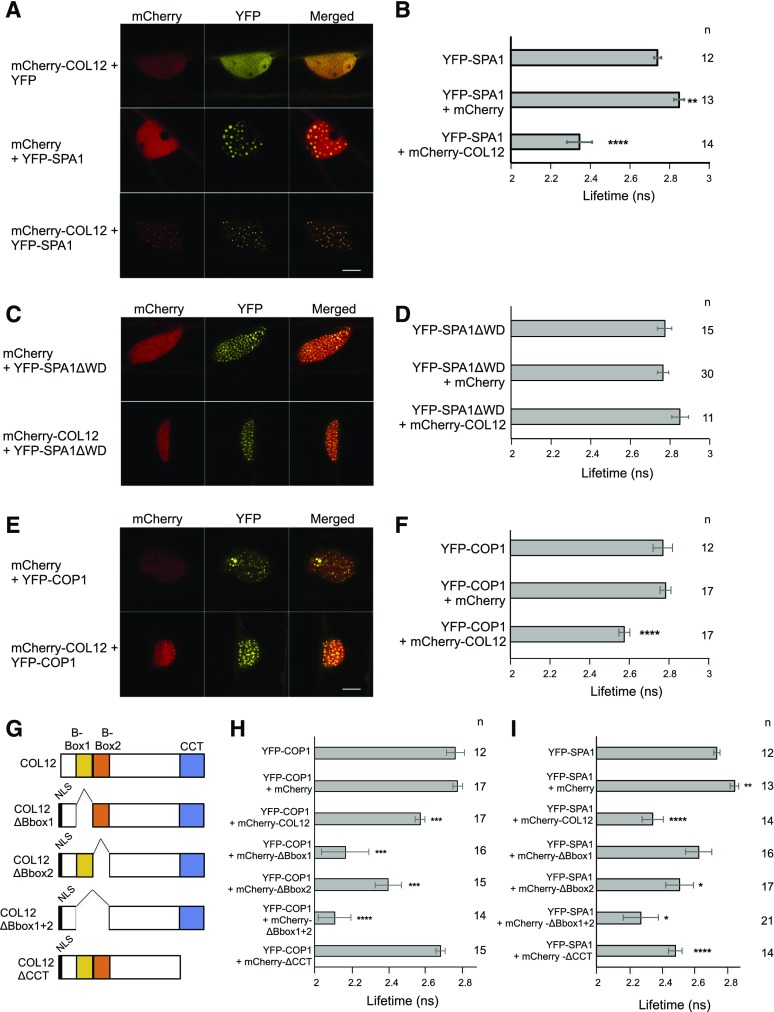
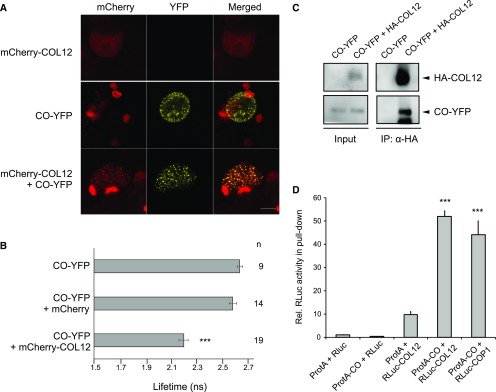
We subsequently focused further studies on COL12/BBX10, because its function has not been described so far. To investigate whether COL12 interacts with COP1 and SPA1 also in vivo, we performed FRET-FLIM experiments after expressing proteins in bombarded leek cells. The lifetime of the donor fluorescence (YFP) was reduced when YFP-SPA1 or YFP-COP1 were coexpressed with mCherry-COL12, while coexpression of mCherry did not change the lifetime of the respective donor fluorescence (Fig. 2, B and F). Confocal microscopy images further show that YFP-SPA1 recruited mCherry-COL12 into nuclear speckles (Fig. 2A): when expressed alone, mCherry-COL12 was diffusely distributed in the nucleus without forming distinct speckles. YFP-SPA1 and YFP-COP1, in contrast, form speckles when expressed alone. When YFP-SPA1 or YFP-COP1 was coexpressed with mCherry-COL12, the YFP and mCherry signals colocalized in nuclear speckles, indicating that mCherry-COL12 is now localized in the YFP-SPA1 and YFP-COP1 speckles. Taken together, these data indicate that COL12 interacts with SPA1 and COP1 in vivo.
Figure 2.
SPA1 and COP1 interact with COL12 in vivo. A, C, and E, Representative confocal images of leek cells coexpressing YFP- and mCherry-tagged proteins after particle bombardment. Plant cells were imaged using confocal microscopy at the indicated channels and images were subsequently merged. B, D, and F, Lifetime of the YFP donor measured by FRET-FLIM inside nuclear bodies. G, Schematic representation of COL12 and COL12 deletion proteins. Deletion constructs contained an artificial nuclear localization signal (NLS) to ensure nuclear import. H and I, The B-boxes and the CCT domains of COL12 are not required for the interaction with COP1 (H) or SPA1 (I). Lifetime of the YFP donor measured by FRET-FLIM inside nuclear bodies. Error bars indicate the sem. n indicates the number of measured cells. Differences between donor alone and donor + acceptor were estimated using Student’s t test and the P value is depicted: ****P ≤ 0.0001, ***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.1. Data in B and I as well as in F and H are in part identical because measurements of all experiments were combined for statistical analyses (see Methods).
The WD-Repeat Domain of SPA1 Is Necessary for the Interaction between SPA1 and COL12
The WD-repeat domains of SPA1 and COP1 have been identified as substrate-recognition domains for many COP1/SPA substrates, for example HY5, PAP1, PAP2, etc. (Holm et al., 2001; Saijo et al., 2003; Maier et al., 2013). We therefore investigated whether COL12 binds to the WD-repeat domain of SPA1. Indeed, a SPA1 deletion-derivative lacking the WD-repeat domain failed to interact with COL12 in FRET-FLIM analyses, since the lifetime of YFP-SPA1ΔWD was not reduced by coexpression of mCherry-COL12 (Fig. 2D). YFP-SPA1ΔWD also did not colocalize with COL12 in nuclear speckles (Fig. 2C). Hence, the WD-repeat domain of SPA1 is necessary for the interaction of SPA1 with COL12.
Analysis of the COP1/SPA1-Interaction Domain in COL12
COL12 carries two B-boxes and a CCT domain. It was shown previously that COP1 and SPA1 interact with the CCT domain of CO (Laubinger et al., 2006; Jang et al., 2008; Liu et al., 2008). Since BBX family members lacking a CCT domain are capable of interacting with COP1 (Gangappa and Botto, 2014; Wang et al., 2015; Xu et al., 2016a), alternative interaction domains might exist for COL12. We therefore tested several deletion-derivatives of COL12 for their interaction with COP1 and SPA1 using FRET-FLIM (Fig. 2, G–I) and colocalization (Supplemental Fig. S1).
Deleting one or both B-boxes of COL12 did not impair the interaction with COP1 (Fig. 2H). On the contrary, COL12 lacking B-box1 or both B-boxes interacted even more strongly with COP1 than the full-length COL12 protein. All B-box deletion proteins also colocalized with COP1 in nuclear speckles (Supplemental Fig. S1, A–C). This indicates that the B-boxes are not necessary for the interaction of COL12 with COP1. The CCT-domain of COL12, in contrast, was required for the interaction with COP1, because COL12-∆CCT did not interact with COP1 in FRET-FLIM nor did it colocalize with COP1 (Fig. 2H; Supplemental Fig. S1D).
Mapping the SPA1-interacting domain of COL12 derived somewhat different results. None of the tested domains of COL12 was essential for the interaction of SPA1 with COL12. COL12 lacking both B-boxes interacted with SPA1 at a similar strength as full-length COL12 (Fig. 2I). Also, colocalization of these proteins was observed (Supplemental Fig. S1C). Surprisingly, deleting only one B-box impaired the interaction with SPA1, as measured by FRET-FLIM (Fig. 2I). However, since the mCherry-tagged COL12∆Bbox1 and COL12∆Bbox2 proteins still showed complete colocalization with YFP-SPA1 (Supplemental Fig. S1, A and B), we conclude that the B-boxes of COL12 are not essential for binding SPA1, though they may affect the structure of the protein complex since deletion of both B-boxes caused the production of larger speckles, possibly protein aggregates. Deletion of the CCT domain of COL12 also did not abolish the interaction with SPA1 (Fig. 2I). The respective fluorescently tagged proteins also exhibited complete colocalization (Supplemental Fig. S1D). Together, these results suggest that either both the B-boxes and the CCT domain of COL12 are redundantly involved in SPA1 binding or the SPA1-binding domain of COL12 is located outside these two domains, for example in the middle portion of COL12.
COL12 Is Degraded in Darkness in a COP1-Dependent Fashion
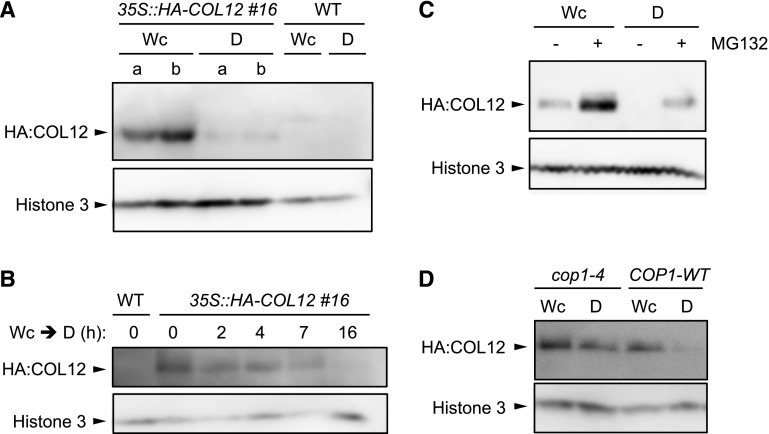
If COL12 were a substrate of the COP1/SPA complex, we would expect an effect of light on COL12 protein levels. To investigate this possibility, we generated transgenic lines expressing HA-tagged COL12 under the control the constitutive 35S promoter. To be able to detect the HA-COL12 protein by western blotting, nuclear-enriched protein extracts had to be used. HA-COL12 accumulated only in light-grown seedlings and not in seedlings exposed to darkness for 24 h (Fig. 3A; Supplemental Fig. S2 for an independent transgenic line). Upon light to dark transfer, HA-COL12 protein levels decreased within 2 h of exposure to darkness (Fig. 3B). When treated with a proteasome inhibitor, HA-COL12 also accumulated in dark-grown seedlings, suggesting that the lack of HA-COL12 accumulation in darkness is due to proteasomal degradation of the protein (Fig. 3C). Similar to the exposure to white light, irradiation of seedlings with red light or blue light also stabilized the HA-COL12 protein (Supplemental Fig. S3).
Figure 3.
COL12 is degraded in darkness in a COP1-dependent fashion. A and B, COL12 protein levels are strongly reduced in dark-grown seedlings. Homozygous 35S::HA-COL12 seedlings (line #16) were grown in continuous white light (Wc, 80 μmol m−2 s−1) for 10 d and subsequently transferred to darkness (D) for 24 h (A) or the indicated time (B). A and b in A indicate two biological replicates. C, COL12 is degraded by the 26S-proteasome. Homozygous 35S::HA-COL12 seedlings (line # 20) were grown in Wc for 16 d then treated with MG132 and subsequently kept in Wc or transferred to darkness (D) for 14 h. D, Degradation of COL12 in darkness requires COP1. The 35S::HA-COL12 transgene (line # 8) was crossed into cop1-4. Homozygous 35S::HA-COL12 seedlings in the COP1 wild-type or cop1-4 mutant background were grown in Wc for 10 d and subsequently transferred to darkness for 14 h. Nuclear proteins were isolated. COL12-HA protein was detected using α-HA antibodies. Histone H3 served as a loading control.
To investigate whether the COP1/SPA E3 ubiquitin ligase is responsible for degradation of COL12 in darkness, we crossed the HA-COL12 transgene into a cop1-4 mutant background. In this background, the HA-COL12 protein accumulated in dark-grown seedlings (Fig. 3D), indicating that COL12 is degraded in darkness in a COP1-dependent fashion. Since COL12 interacts with COP1 and SPA1, this suggests that COL12 is a direct substrate of the COP1/SPA E3 ubiquitin ligase.
Overexpression of COL12 Causes Late Flowering in Long Day, Dwarfism, and Increased Branching
To determine the function of COL12, we analyzed the phenotype of COL12-overexpression lines (35S::HA-COL12; Supplemental Fig. S4C). We also identified a col12 mutant that we named col12-1. It carries a T-DNA insertion in the third exon of COL12 (Supplemental Fig. S4, A and B). However, truncated COL12 transcript still accumulated in col12-1 (Supplemental Fig. S4C). Hence, truncated COL12 protein lacking the CCT domain might still accumulate in col12-1, suggesting that this mutant might not carry a null allele.
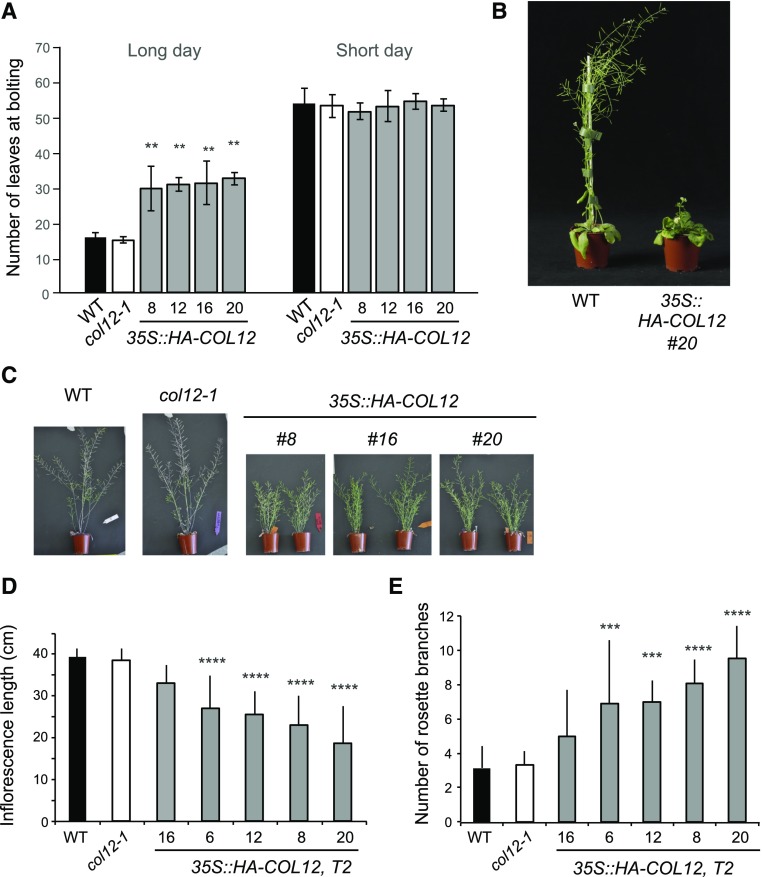
COL12 overexpression caused late flowering in long-day, but not in short-day conditions (Fig. 4, A and B). In long day, COL12-overexpressing plants needed approximately twice as long for the initiation of flowering compared to wild-type plants. Inflorescences of mature 35S::HA-COL12 plants were much shorter than those of wild-type plants (Fig. 4, C and D). Moreover, the COL12-overexpressing plants exhibited increased shoot branching compared to the wild type (Fig. 4, C and E). Seedlings of 35S::HA-COL12 lines were indistinguishable from wild type in darkness, red, far-red, and blue light (Supplemental Fig. S5), suggesting that COL12 overexpression only affects adult traits.
Figure 4.
Overexpression of COL12 causes late flowering in long day, dwarfism, and increased branching. A, Flowering time of the indicated genotypes grown in long day or short day. Line numbers indicate independent T2 transgenic lines. B, Visual phenotype of a wild-type and a COL12 overexpressing plant grown in long day for 45 d. C, Visual phenotype of 10-week-old plants of the indicated genotypes grown in long day. All plants had ceased to grow and had therefore reached their final size. D and E, Final inflorescence length (D) and number of rosette branches (E) of plants grown as in C. Error bars indicate the sd of the mean. Differences between wild type and other genotypes were estimated using ANOVA followed by Tukey’s post hoc test: ****P ≤ 0.0001, ***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.1.
While COL12 overexpression had a strong effect on plant growth, col12-1 mutants were indistinguishable from the wild type. col12-1 mutants exhibited normal flowering time, inflorescence length, branching, and seedling growth (Fig. 4, A–E; Supplemental Fig. S5).
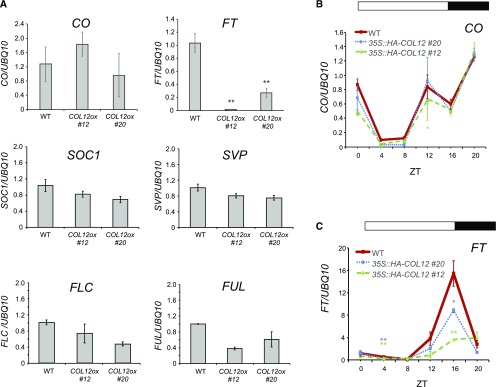
To uncover the molecular mechanism of late flowering in 35S::HA-COL12 plants, we determined the transcript levels of key flowering time regulators. COL12 overexpression strongly decreased the mRNA levels of FT, while those of CO, SOC1, SVP, FLC, and FUL were not or only weakly altered (Fig. 5A). A diurnal analysis of FT and CO transcript levels confirms that CO transcript levels were unchanged in 35S::HA-COL12 compared to the wild type (Fig. 5B). FT transcript levels, in contrast, were strongly reduced in 35S::HA-COL12 lines, primarily at ZT16, but also at ZT4 (Fig. 5C). Hence, late flowering of 35S::HA-COL12 plants correlated with a reduced expression of FT.
Figure 5.
COL12 down-regulates FT transcript levels without changing CO transcript levels. A, Transcript levels of the indicated flowering time genes in plants harvested at ZT 16. B, CO transcript levels in plants harvested at the indicated ZTs. C, FT transcript levels in plants harvested at the indicated ZTs. Transcript levels were analyzed by qRT-PCR in 12-d-old plants of the wild type and two independent transgenic 35S::HA-COL12 lines (COL12ox, #12 and #20). Plants were grown in long day with a white light fluence rate of 200 μmol m−2 s−1. Transcript levels were normalized to UBQ10 transcript levels. Error bars show the sem of 2 to 4 biological replicates (**P < 0.005, *P < 0.05 for difference to the wild type).
COL12 Interacts with CO
Since CO is a key regulator of photoperiodic flowering, we asked whether COL12 interacts with CO. Indeed, COL12 interacted with CO in FRET-FLIM studies conducted in plant cells (Fig. 6, A and B). We confirmed this result by coexpressing COL12 and CO in transfected tobacco leaves: COL12 successfully coimmunoprecipitated CO (Fig. 6C). Split luciferase assays in transfected tobacco (Nicotiana benthamiana) leaves also showed an interaction between CO and COL12 (Supplemental Fig. S6). These results demonstrate that CO and COL12 interact in vivo. We also used an ex planta system to analyze the COL12-CO protein interaction by expressing recombinant proteins in mammalian HEK293 cells. In this system, protein A-tagged CO coimmunoprecipitated luciferase-tagged COL12. The apparent strength of the CO-COL12 interaction was similar to that of the CO-COP1 interaction that we used as a positive control (Fig. 6D).
Figure 6.
COL12 interacts with CO. A, Representative confocal images of leek cells coexpressing YFP- and mCherry-tagged proteins after particle bombardment. Plant cells were imaged using confocal microscopy at the indicated channels and images were subsequently merged. B, Lifetime of the YFP donor measured by FRET-FLIM inside nuclear bodies. Error bars indicate the sem. n indicates the number of measured cells. Differences between donor alone and donor + acceptor were estimated using Student’s t test (***P < 0.005). C, YFP-CO is pulled down by HA-COL12 in vivo. Proteins were coexpressed in N. benthamiana by Agrobacterium-mediated transfection. HA-COL12 was immunoprecipitated using α-HA-coupled beads. Proteins were detected using α-HA and α-GFP antibodies. D, COL12 interacts with CO in a LUMIER assay performed in a mammalian cell culture. COL12 fused to Renilla luciferase (RLuc-COL12), protein A (ProtA)-tagged CO (ProtA-CO), and the respective negative controls were coexpressed in mammalian HEK293TN cells. ProtA-tagged proteins were immuno-precipitated and RLuc activity was determined in the input and pull-down fractions. RLuc activity in the pull-down fraction was normalized to the input fraction and subsequently normalized to the negative control ProtA + RLuc (= 1). ***indicates significant difference to negative control (P < 0.001).
To map the CO-interacting domain in COL12, we tested COL12-deletion proteins lacking conserved domains (the CCT domain, one or both of the B-boxes) for an interaction with CO using FRET-FLIM analysis (Supplemental Fig. S7, A and B). Deletion of the CCT domain of COL12 had only a mild effect on the lifetime of the donor fluorescence in FRET-FLIM experiments, indicating that the CCT domain is not essential for the interaction of COL12 with CO. Deletion of B-boxes, in contrast, more strongly affected the lifetime when compared to full-length COL12. Deleting B-box 1 or 2 fully abolished or strongly reduced FRET efficiency, respectively, suggesting that B-box 1 is involved in the COL12-CO interaction. On the other hand, COL12 lacking both B-boxes retained some FRET and partial colocalization with CO (Supplemental Fig. S7, A and B). However, COL12 lacking B-box 2 or both B-boxes formed very large speckles reminiscent of protein aggregates compared to those formed by full-length COL12 or COL12∆Bbox1. It is therefore questionable whether these deletion proteins form native structures or coaggregate with CO. We therefore conclude that B-boxes may be involved in CO-binding but are likely complemented by an additional, so far unknown, domain in COL12.
Genetic Interaction between COL12 and co
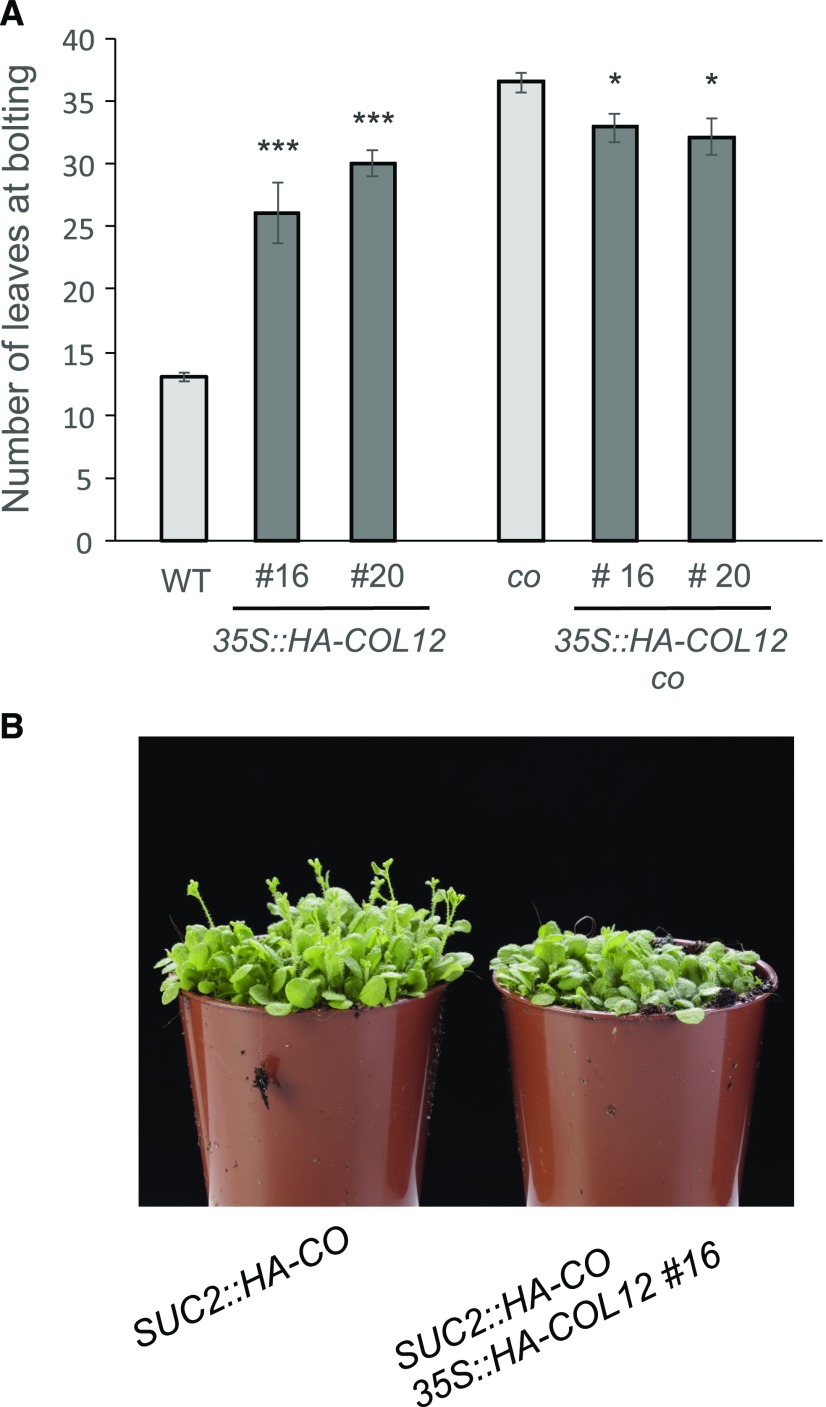
Since COL12 physically interacts with CO, we postulated that COL12 affects CO function. If so, we would expect that the late-flowering phenotype of 35S::HA-COL12 plants depends on CO. Alternatively, if COL12 acted independently of CO, we would expect additive effects of the co mutation and COL12 overexpression, that is later flowering of the doubly line compared to the co mutant as for example observed in fca mutants and short-day-grown ga1 mutants (Reeves and Coupland, 2001). To test this hypothesis, we crossed the 35S::HA-COL12 transgene of two independent 35S::HA-COL12 lines (#16 and #20) into a co mutant background. Indeed, co 35S::HA-COL12 plants did not flower later than the respective co mutant plants (Fig. 7A). These results suggest that the late-flowering phenotype of COL12-overexpressing plants depends on CO.
Figure 7.
Late flowering in 35S::HA-COL12 depends on CO. A, Flowering time of the indicated genotypes grown in long day. Error bars show the sem (*P < 0.05, ***P < 0.0005 calculated with Student’s t test between 35S::HA-COL12 lines and the respective controls). B, Visual phenotype of 35S::HA-COL12 plants (line #16) in a SUC2::HA-CO background.
CO controls flowering in the companion cells of phloem tissue (An et al., 2004). To test whether COL12 affects CO function in the phloem, we analyzed the influence of COL12 expression in a SUC2::HA-CO line that expresses CO under the control of the phloem-specific SUC2-promoter. Also in this background, COL12 overexpression delayed flowering (Fig. 7B; Supplemental Table S1). Taken together, these results suggest that COL12 can act in the phloem to reduce FT expression by attenuating CO function.
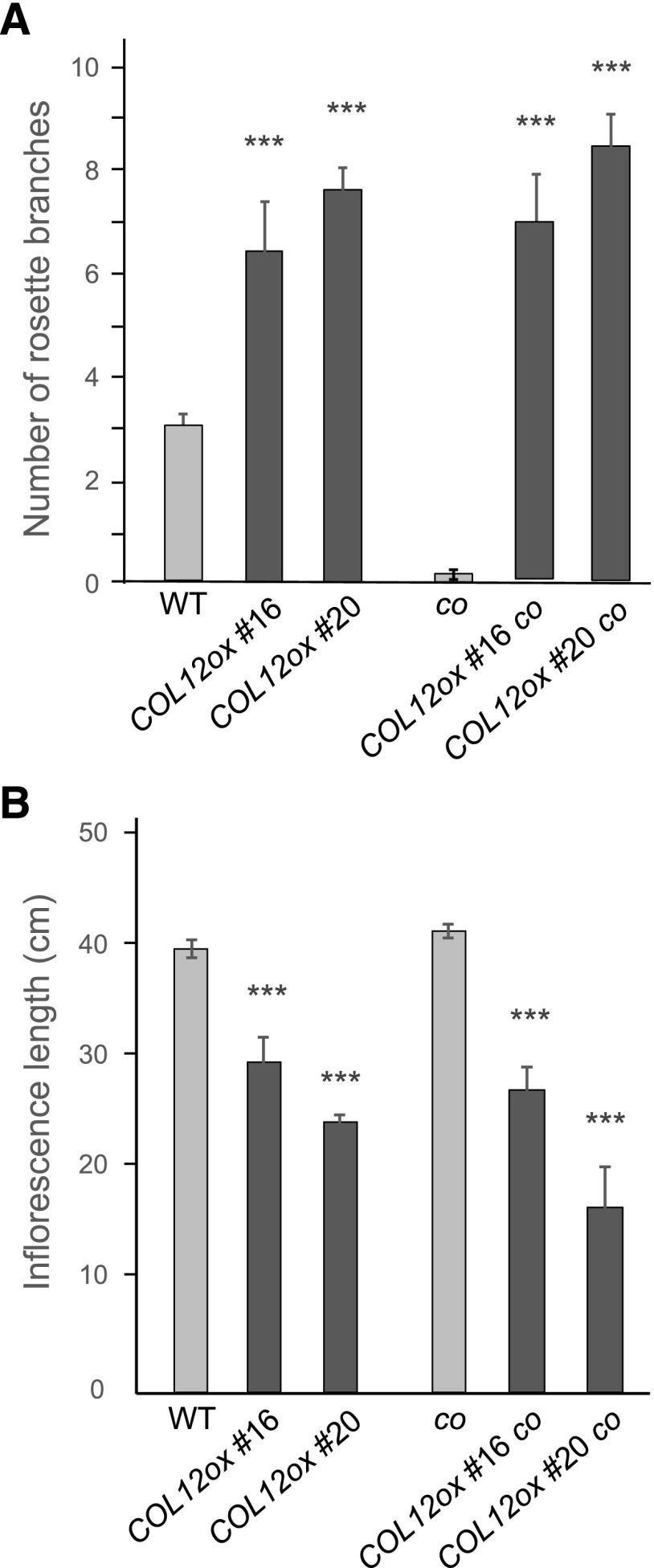
We also analyzed whether the reduced inflorescence height and increased branching observed in 35S::HA-COL12 lines depends on CO. co 35S::HA-COL12 plants exhibited a similar increase in branching and a similar reduction in inflorescence length as 35S::HA-COL12 plants (Fig. 8, A and B). This demonstrates that these phenotypes of COL12-overexpressing plants are independent of CO. Hence, COL12 affects plant development through CO-dependent and CO-independent pathways.
Figure 8.
Enhanced branching and reduced inflorescence height in 35S::HA-COL12 are independent of CO. A, Number of rosette branches of the indicated genotypes (COL12ox = 35S::HA-COL12). B, Inflorescence length of the indicated genotypes. Plants were grown in long day for 10 weeks, that is until they had reached maturity and their final size. Error bars show the sem (*P < 0.05, ***P < 0.0005 calculated with Student’s t test, compared to the respective background control, i.e. wild type or co).
COL12 Is Ubiquitously Expressed
To analyze the endogenous expression pattern of COL12, we performed qRT-PCR experiments on different tissues of wild-type plants. COL12 was expressed in seedlings, rosette leaves, the inflorescence stem, and flowers (Supplemental Fig. S8A). An analysis of the diurnal expression of COL12 in long-day-grown plants indicates that COL12 transcript levels exhibited weak diurnal regulation with a peak at ZT12 (Supplemental Fig. S8C). COL12 was not present on the AtGenExpress microarray, but RNAseq data on Genevestigator indicate low to medium level expression of COL12 throughout vegetative development and after the floral transition that is in agreement with our experimental data.
The closest homolog of COL12, COL11/BBX9, was only expressed in flowers. COL11 transcripts were undetectable in seedlings, rosette leaves, and inflorescence stems (Supplemental Fig. S8B). This finding agrees with data from AtGenExpress showing that COL11 transcripts were present mainly in stamens.
COL12 Does Not Alter CO Protein Levels
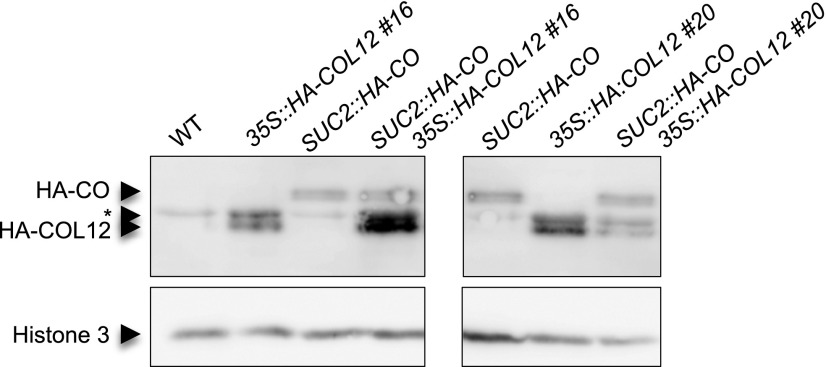
To analyze the effect of COL12 on CO protein levels we determined HA-CO protein levels in a 35S::HA-COL12 background (Fig. 9). HA-CO levels were unchanged by overexpression of COL12. This suggests that COL12 does not alter CO protein stability.
Figure 9.
COL12 does not affect CO protein levels. HA-CO and HA-COL12 levels were analyzed in two 35S::HA-COL12 lines (#16 and #20), a SUC2::HA-CO line, and the respective doubly transgenic SUC2::HA-CO 35S::HA-COL12 lines. Seedlings were grown in long day for 10 d and harvested at ZT16. Nuclear proteins were isolated and HA-CO and HA-COL12 proteins were detected using an α-HA antibody. Histone H3 levels served as a control. * indicates a nonspecific signal underlying one HA-COL12 signal.
DISCUSSION
CO is a key transcription factor controlling photoperiodic flowering in Arabidopsis. co mutants are, therefore, insensitive to day length and flower equally late in long-day and short-day conditions. Since CO is essential for photoperiodic control of flowering time, it can be assumed that the 16 CO-like genes that are present in the genome of Arabidopsis have functions that are distinct from CO and thus nonredundant with CO. On the other hand, the mechanism of CO function in photoperiodic flowering strongly depends on the COP1/SPA-mediated degradation of CO in darkness (Andrés and Coupland, 2012; Xu et al., 2016b). This posttranslational control of CO function might be shared by CO family members.
Here, we have shown that 14 COL proteins strongly interact with COP1 and SPA1 in vitro, indicating that the interaction with the COP1/SPA complex is conserved among CO family members. Indeed, COL3 was previously shown to interact with COP1 (Datta et al., 2006). Whether all COL proteins are also targeted for degradation by the COP1/SPA complex needs to be investigated but can be considered very likely. COL7 protein levels were previously shown to rapidly increase upon light exposure (Wang et al., 2013). Also consistent with this idea, we have shown that the COL12 protein is degraded in darkness in a COP1-dependent fashion. Since COL12 interacts with both COP1 and SPA1 in vivo, we conclude that it is directly destabilized in darkness by the COP1/SPA E3 ubiquitin ligase. Degradation of COL12 was repressed by white, red, and blue light. While CO is also stabilized by white and blue light, it is still degraded through a phyB-dependent effect when seedlings were transferred to red light (Valverde et al., 2004). Hence, the light-regulation of CO and COL12 stability is not identical.
Overexpression of COL12 caused late flowering in long day, a reduction in apical dominance, and a smaller inflorescence size. Seedling de-etiolation, in contrast, was not altered by COL12 overexpression. A col12 T-DNA mutant did not show a detectable mutant phenotype, suggesting that COL12 may act redundantly with other COL family members. The most closely related COL, COL11, unlikely masks a col12 mutant phenotype, because this gene is specifically expressed in flowers whereas COL12 is ubiquitously expressed. COL3 and COL9, on the other hand, have been shown to also repress flowering and thus may act redundantly with COL12, though likely via at least partially distinct mechanisms affecting FT expression: COL9 was shown to repress flowering by inhibiting the expression of CO, which we do not observe for COL12. COL3 represses flowering in long day and short day that is also distinct from the long-day-specific effect of COL12 overexpression (Cheng and Wang, 2005; Datta et al., 2006). Since COL3 and COL12 belong to different subgroups among the COL proteins (Crocco and Botto, 2013; Gangappa and Botto, 2014), it cannot be excluded that other COLs, and maybe also BBX genes, act redundantly with COL12. Nevertheless, COL proteins do not have identical activities in flowering time, since the expression of several COLs, such as COL1, COL2, and COL16 did not affect flowering time (Ledger et al., 2001; Kim et al., 2013; Simon et al., 2015; Graeff et al., 2016) or promoted flowering, such as COL5 (Hassidim et al., 2009). Alternatively, col12-1 may not show a mutant phenotype, because it still expresses a truncated transcript containing the B-box coding sequences. Hence, it may not be null in activity, as it was similarly reported for a truncated BBX19 transcript (Wang et al., 2014). Unfortunately, we were not successful in generating a null allele through RNAi nor CRISPR-Cas.
COL12 and CO have opposite effects on flowering time in long day: CO promotes flowering, whereas COL12 represses flowering. Taken together, our results suggest that COL12 overexpression delays flowering by suppressing CO function: (1) COL12 overexpression only affected flowering time in long day and not in short day, indicating that only the photoperiodic pathway in which CO is a central player is affected by COL12. (2) COL12 overexpression inhibited the expression of FT, the direct target gene of CO, without affecting CO transcript levels. This finding is consistent with COL12 affecting CO protein activity rather than CO expression. (3) COL12 did not alter the transcript levels of other key regulators of flowering time (SOC1, SVP, FLC, FUL). (4) COL12 and CO proteins directly interact in vivo. (5) In the absence of CO, that is in a co mutant, COL12 overexpression did not delay flowering, suggesting that COL12 action in flowering time control requires CO. While we consider this scenario very likely, we do not fully exclude the possibility that COL12 overexpression alters flowering time in part independently of CO, for example by acting as a transcription factor on FT.
We considered two mechanisms through which COL12 might repress CO function. (1) COL12 might enhance the degradation of CO, possible by enhancing the recruitment of CO to the COP1/SPA complex. (2) COL12 might reduce the ability of CO to transcriptionally activate FT, either by inhibiting DNA-binding or transcription activation activity. By analyzing CO protein levels in a COL12 overexpression line, we showed that CO protein levels are unchanged by COL12 overexpression. These results indicate that COL12 does not affect CO protein stability. We therefore suggest that COL12, through binding to CO, directly reduces the ability of CO to transcriptionally activate FT. This hypothesis is also consistent with our observation that the effect of COL12 overexpression on flowering time is much weaker in a SUC2::CO background, which overexpresses CO, compared to a CO wild-type background. This suggests that overexpression of CO can at least partially outcompete for the effects of COL12. Hence, this finding is consistent with the model that COL12 protein inhibits the activity of CO by heterodimerization. A similar repressive effect on CO activity was recently reported for BBX19, a CO-interacting B-box transcription factor lacking a CCT domain (Wang et al., 2014). Similarly, the B-box-containing microProteins miP1a and miP1b repress flowering by interacting with CO (Graeff et al., 2016). In total, these results suggest that BBX19, miP1 proteins, and COL12 can sequester CO into nonfunctional complexes. The inhibitory mechanisms of these proteins may, however, be distinct. miP1 proteins repress flowering through a PFVFL motif that is not present in COLs and BBX proteins. This motif recruits the transcriptional corepressor TOPLESS and, therefore, presents a mechanism that is specific to the miP1 proteins among the B-box proteins (Graeff et al., 2016). Both BBX19 and the miP1 proteins lack the CCT domain, which, in contrast, is present in COL12. Since the CCT domain of CO is responsible for binding the FT promoter (Tiwari et al., 2010; Gnesutta et al., 2017), the lack of a CCT domain might be important for the dominant negative effect of BBX19, but not of COL12. The sequence specificity in DNA-binding is conferred by the CCT domain (Gnesutta et al., 2017); hence, the sequence of the CCT domain of COL12 may be important for its dominant-negative effect on CO.
Even though both CO and COL12 are degradation targets of the COP1/SPA ubiquitin ligase, they act antagonistically in the control of flowering time. Because CO expression is circadianly regulated with very low expression levels in mid- to late morning, the main function of COP1/SPA is to mediate CO degradation in darkness in short-day-grown plants (Andrés and Coupland, 2012). COL12, in contrast, is expressed throughout the 24-h cycle with only weak diurnal regulation. The observed stabilization of COL12 by light might therefore be a mechanism to repress the activity of CO protein during the day in long-day-grown plants. We therefore hypothesize that COL12 serves to balance CO activity in long day to prevent premature induction of flowering. In addition, since COL12 is stabilized by red light while CO is degraded in red light through a phyB- and HOS1-dependent mechanism (Valverde et al., 2004; Lazaro et al., 2015), COL12 might serve to repress the activity of residual CO protein present in the early morning hours. Hence, COL12 might reinforce CO inactivation during this critical period of the day in particular in short-day-grown plants.
COL12 overexpression also affected the architecture of adult plants, whereas seedling de-etiolation was unchanged compared to the wild type. Shoot branching was also shown to be positively regulated by COL7 overexpression. However, in contrast to COL12 function that was limited to the adult plant, COL7 also affected seedling growth by promoting de-etiolation under high R:FR conditions (Wang et al., 2013). COL7 overexpression reduces the expression of the auxin marker DR5::GFP. Moreover, the expression of SUR2, a suppressor of auxin biosynthesis, is enhanced by COL7 overexpression in high R:FR. This suggests that COL7 regulates plant architecture and seedling de-etiolation in high R:FR by suppressing auxin biosynthesis (Zhang et al., 2014). A similar mechanism may be involved in the activity of COL12 in shoot branching, but not in seedling de-etiolation.
Strikingly, the effect of COL12 on plant architecture was CO independent. Interestingly, COL7 was shown to transactivate a CCAAT box-containing minimal promoter after expression in Nicotiana benthamiana (Zhang et al., 2014). Hence, COL12 might also directly act as a transcription factor. Consistent with this idea, COL3 was found to repress flowering and FT expression by directly binding to the FT promoter in the presence of BBX32 (Tripathi et al., 2017). Alternatively, COL12 might affect plant architecture by interacting with other transcription factors than CO. BBX proteins have been shown to interact with HY5 (Gangappa and Botto, 2014, 2016). Additional, so far unknown COL-interacting proteins might exist that regulate COL-dependent plant architecture. Future studies are necessary to unravel the function of the COL signaling network(s) in the control of plant architecture.
MATERIALS AND METHODS
Plant Material and Growth Conditions
ft-10, co-10 (also called co-SAIL), and SUC2::HA-CO were described previously (Yoo et al., 2005; Laubinger et al., 2006; Jang et al., 2009). The col12-1 allele was identified from a SAIL T-DNA insertion population (N862394) and carries a T-DNA insertion at position 1,089 bp after the ATG start codon with respect to the genomic sequence. The 35S::HA-COL12 transgene was transformed into the Col-0 background.
For analysis of adult phenotypes, plants were grown on soil in a climate-controlled growth chamber under long day (16 h light, 8 h darkness) or short day (8 h light, 16 h darkness) at 21°C. White light was provided by Fluora L58W/77 fluorescent tubes. For protein experiments, seedlings were grown in a Percival growth chamber under continuous white light of the indicated fluence rates. Red and blue light were provided by LED light sources as described previously (Baumgardt et al., 2002; Laubinger et al., 2004). For MG132 treatment, seedlings were grown on solid Murashige and Skoog plates (without Suc), then transferred to water containing either 50 µm MG132 or the corresponding volume of dimethyl sulfoxide and kept under the otherwise same growth conditions for 4 h followed by harvesting of the tissue.
Phenotype Analyses
Flowering time was measured by counting the number of rosette leaves formed at the time of bolting, as defined when the first inflorescence was seen by eye. Final inflorescence length was measured when plants had started to senesce and had reached their final size (10-week-old plants). In the same plants, the number of branches that had emerged from the rosette were counted to determine branching. Hypocotyl length was determined as described previously (Chen et al., 2015).
Plasmid Constructions
Plasmid constructions are described in the Supplemental Materials and Methods.
Coimmunoprecipitations and Immunodetection of Proteins
For analysis of HA-COL12 protein levels, nuclear proteins were isolated from transgenic plants as described in (Fernández et al., 2016). For coimmunoprecipitation experiments, HA-COL12 and YFP-CO were coexpressed in Nicotiana benthamiana through Agrobacterium tumefaciens-mediated transfection. Proteins were isolated from transfected tissue as described in (Fernández et al., 2016). The lysate was mixed with 15 µL of anti-HA magnetic μMACS microbeads (Miltenyi Biotec) and incubated at 4°C for 30 min in a circulating rotor. Next, μ-columns were placed in the magnetic field of the μMACSTM separator (Miltenyi), and the μ-columns were moisturized by adding 200 μL of sonication buffer used during protein isolation, except that no protease inhibitors were added. The mixture of lysate and microbeads was loaded onto the μ-columns and let run through. The columns were rinsed with washing buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.5% [v/v] NP40) four times. The immunoprecipitated fraction was eluted into 60 μL of elution buffer provided by the μMACS Epitope Tag Protein Isolation Kit (Miltenyi Biotec). After western blotting, proteins were detected using α-HA antibodies (Roche Diagnostics) or α-histone H3 antibodies (Abcam).
In vitro pull-down assays were performed as described previously (Hoecker and Quail, 2001). Briefly, all proteins were synthesized in the reticulocyte TnT in vitro transcription/translation system (Promega) in the presence of 35S-labeled Met according to the manufacturer's instructions. TnT-produced bait and prey proteins were added to 0.1 mL of binding buffer (20 mm Tris-HCl pH 7.5, 150 mm NaCl, 1 mm dithiothreitol, 0.1% Tween 20) with protease inhibitors (Complete, EDTA-free) and incubated on a rotating platform at 4°C for 2 to 3 h Following addition of 1 μg of monoclonal antibody against GAD (Santa Cruz Biotechnology) incubation continued for 1 more hour. Subsequently, 20 μL of protein A-coated magnetic beads (Dynal) was added. After another hour of incubation, beads were washed three times with 1 mL of binding buffer. Pellet and supernatant fractions were resolved on SDS-PA gels with 6% to 15% acrylamide and visualized using a phosphorimager (Fudji).
LUMIER protein-protein interaction assays in mammalian HEK293 cell culture was performed as described in (Holtkotte et al., 2016).
Colocalization and FRET-FLIM
For colocalization and FRET-FLIM analyses, YFP- and mCherry-tagged proteins were coexpressed in leek cells by particle bombardment. The fluorescent proteins were imaged using a confocal laser scanning microscope (SP8, Leica, Microsystems). For colocalization, the cells were imaged sequentially at both channels: YFP was excited with the pulsed picosecond laser emitting at 470 nm at a frequency of 40 MHz and detected in the 490- to 540-nm range; mCherry was excited with a laser emitting at 561 nm and detected in the range of 670 to 740 nm. The occurrence of FRET was inferred by comparing the fluorescence lifetime (FLIM) of the YFP donor alone or in presence of the coexpressed mCherry acceptor. For the FLIM measurements, excitation of the donor was conducted with a pulsed picosecond laser emitting at 470 nm at a frequency of 40 MHz (Leica, Microsystems). The fluorescence emission was acquired by the FLIM-PMT detector at a range of 490 to 540 nm. FLIM was determined by time-correlated single photon counting using a time-correlated single photon counting unit (PicoHarp 300, PicoQuant). The nuclear speckles were delimited as regions of interest to generate the time-correlated single photon counting histogram that were deconvoluted and fitted to monoexponential decay using the SymPhoTime software (PicoQuant). Thus, the lifetime and χ2 were obtained and only cells in which the χ2 was <1 were considered for the analysis. The experiments were conducted multiple times after independent particle bombardments. Since FRET-FLIM is independent of protein expression levels, data from several experiments were combined to increase statistical power.
Split Luciferase Assay
The GATEWAY cloning technology was used for binary expression vector cloning for the FLuCI system. The used destination vectors were published by (Gehl et al., 2011).
Transient transformation of wild-type N. benthamiana via A. tumefaciens strain C58C1/pMP90 was performed as described by (Kaufholdt et al., 2016). The interaction approach of the proteins of interest and the corresponding negative control were infiltrated with identical bacteria concentrations into the same leaf, each into one leaf half, to guarantee an optimal comparison of expression levels. Twenty-six analyzed leaves of six different plants in two independent experiments were used for interaction factor calculation.
The split-luciferase assay was performed as described in detail previously (Gehl et al., 2011; Kaufholdt et al., 2013). Between 3 and 6 d after transformation of N. benthamiana, a luciferin solution (10 mm MES [pH = 5.6], 10 mm MgCl2, 0.5% dimethyl sulfoxide, 0.1 mm luciferin) was infiltrated into the leaves just before luminescence measurement. Analyzed were within every measurement six leaf discs (12-mm diameter each) per leaf half (12-mm diameter each) with a luminometer (TriStar multimode reader, Berthold Technologies). The expression and transformation level of the leaf halves was adjusted by coexpressed β-glucuronidase. The Calcineurin B-like protein CBL10 (Ren et al., 2013) was used as noninteracting negative control protein. The N-terminal fragment of the fluorescence protein Venus was used as abundance control protein (Kaufholdt et al., 2016). The split-luciferase factor was calculated by dividing the luminescence of the interaction approach by the background value of the randomly reconstituted negative control constructs. The split-luciferase factor of the abundance control was calculated in the same manner, which defines the factor for noninteracting proteins in the interaction approach. An interaction takes place if the split-luciferase factor of the interaction approach is higher than the abundance factor. To allow an easy interpretation the split-luciferase factor of the interaction approach and the corresponding abundance control split-luciferase factor were calculated to show a ratio that is named interaction factor. The interaction factor shows how strong the interaction is, a value of 1.0 would define no interaction, and every value higher is an interaction of the proteins of interest.
RNA Isolation and qRT-PCR Analysis
Total RNA was isolated from the indicated tissues using the RNeasy Plant Mini Kit (Qiagen). One μg of RNA was treated with DNase I to remove contaminating genomic DNA. Successful removal of genomic DNA was confirmed by PCR analysis. DNase-treated RNA was subsequently used for reverse transcription with oligo(dT) primers. qPCR was performed using the SYBR FAST qPCR Mastermix (Kapa Biosystems via Sigma-Aldrich). Primer sequences are provided in Supplemental Table S2. Relative transcript levels were calculated using the 2ΔΔCT method (Schmittgen and Livak, 2008). For each experiment, two to four biological replicates were used with two technical replicates each. Each experiment was repeated at least twice.
Accession Numbers
COL12/BBX10 (At3g21880), COL11/BBX9 (At4g15250), SPA1 (At2g46340), COP1 (At2g32950), CO (AT5G15840).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The B-boxes and the CCT domain of COL12 are not required for the interaction with SPA1 or COP1.
Supplemental Figure S2. COL12 is degraded in darkness.
Supplemental Figure S3. COL12 is stabilized by red and blue light.
Supplemental Figure S4. Molecular characterization of the col12-1 mutant and 35S::HA-COL12 overexpression lines.
Supplemental Figure S5. De-eiolation of wild-type, col12-1 mutant, and 35S::HA-COL12 overexpressing seedlings.
Supplemental Figure S6. In vivo interaction studies of COL12 with CO via floated-leaf luciferase complementation imaging (FLuCI).
Supplemental Figure S7. COL12 domain mapping of the interaction with CO.
Supplemental Figure S8. Transcript levels of COL12 and its closest homolog, COL11.
Supplemental Table S1. Flowering time of a SUC2::CO 35S::HA-COL12 line grown in long day.
Supplemental Table S2. List of primers used for qRT-PCR.
Acknowledgments
We thank George Coupland and Franziska Turck for providing co-10, SUC2::HA-CO, and ft-10 seeds. We are grateful to George Coupland for providing Gateway Entry clones for COLs, to Virginia Fernandez for advice with nuclear protein preparations, and to Xu Holtkotte and Ryosuke Hayama for advice on FRET-FLIM and co-immunoprecipitation experiments. We thank Klaus Menrath, his green house staff, and many undergraduate students for expert care of our plants.
References
- An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, Piñeiro M, Hepworth S, Mouradov A, Justin S, Turnbull C, et al. (2004) CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131: 3615–3626 [DOI] [PubMed] [Google Scholar]
- Andrés F, Coupland G (2012) The genetic basis of flowering responses to seasonal cues. Nat Rev Genet 13: 627–639 [DOI] [PubMed] [Google Scholar]
- Baumgardt RL, Oliverio KA, Casal JJ, Hoecker U (2002) SPA1, a component of phytochrome A signal transduction, regulates the light signaling current. Planta 215: 745–753 [DOI] [PubMed] [Google Scholar]
- Chen S, Lory N, Stauber J, Hoecker U (2015) Photoreceptor specificity in the light-induced and COP1-mediated rapid degradation of the repressor of photomorphogenesis SPA2 in Arabidopsis. PLoS Genet 11: e1005516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng XF, Wang ZY (2005) Overexpression of COL9, a CONSTANS-LIKE gene, delays flowering by reducing expression of CO and FT in Arabidopsis thaliana. Plant J 43: 758–768 [DOI] [PubMed] [Google Scholar]
- Crocco CD, Botto JF (2013) BBX proteins in green plants: insights into their evolution, structure, feature and functional diversification. Gene 531: 44–52 [DOI] [PubMed] [Google Scholar]
- Datta S, Hettiarachchi GH, Deng XW, Holm M (2006) Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell 18: 70–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X-W, Caspar T, Quail PH (1991) cop1: a regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev 5: 1172–1182 [DOI] [PubMed] [Google Scholar]
- Fernández V, Takahashi Y, Le Gourrierec J, Coupland G (2016) Photoperiodic and thermosensory pathways interact through CONSTANS to promote flowering at high temperature under short days. Plant J 86: 426–440 [DOI] [PubMed] [Google Scholar]
- Gangappa SN, Botto JF (2014) The BBX family of plant transcription factors. Trends Plant Sci 19: 460–470 [DOI] [PubMed] [Google Scholar]
- Gangappa SN, Botto JF (2016) The multifaceted roles of HY5 in plant growth and development. Mol Plant 9: 1353–1365 [DOI] [PubMed] [Google Scholar]
- Gehl C, Kaufholdt D, Hamisch D, Bikker R, Kudla J, Mendel RR, Hänsch R (2011) Quantitative analysis of dynamic protein-protein interactions in planta by a floated-leaf luciferase complementation imaging (FLuCI) assay using binary Gateway vectors. Plant J 67: 542–553 [DOI] [PubMed] [Google Scholar]
- Gnesutta N, Kumimoto RW, Swain S, Chiara M, Siriwardana C, Horner DS, Holt BF III, Mantovani R (2017) CONSTANS imparts DNA sequence specificity to the histone fold NF-YB/NF-YC dimer. Plant Cell 29: 1516–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff M, Straub D, Eguen T, Dolde U, Rodrigues V, Brandt R, Wenkel S (2016) MicroProtein-mediated recruitment of CONSTANS into a TOPLESS trimeric complex represses flowering in Arabidopsis. PLoS Genet 12: e1005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassidim M, Harir Y, Yakir E, Kron I, Green RM (2009) Over-expression of CONSTANS-LIKE 5 can induce flowering in short-day grown Arabidopsis. Planta 230: 481–491 [DOI] [PubMed] [Google Scholar]
- Hoecker U. (2017) The activities of the E3 ubiquitin ligase COP1/SPA, a key repressor in light signaling. Curr Opin Plant Biol 37: 63–69 [DOI] [PubMed] [Google Scholar]
- Hoecker U, Quail PH (2001) The phytochrome A-specific signaling intermediate SPA1 interacts directly with COP1, a constitutive repressor of light signaling in Arabidopsis. J Biol Chem 276: 38173–38178 [DOI] [PubMed] [Google Scholar]
- Holm M, Hardtke CS, Gaudet R, Deng XW (2001) Identification of a structural motif that confers specific interaction with the WD40 repeat domain of Arabidopsis COP1. EMBO J 20: 118–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtkotte X, Dieterle S, Kokkelink L, Artz O, Leson L, Fittinghoff K, Hayama R, Ahmad M, Hoecker U (2016) Mutations in the N-terminal kinase-like domain of the repressor of photomorphogenesis SPA1 severely impair SPA1 function but not light responsiveness in Arabidopsis. Plant J 88: 205–218 [DOI] [PubMed] [Google Scholar]
- Jang S, Marchal V, Panigrahi KC, Wenkel S, Soppe W, Deng XW, Valverde F, Coupland G (2008) Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO J 27: 1277–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Torti S, Coupland G (2009) Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. Plant J 60: 614–625 [DOI] [PubMed] [Google Scholar]
- Kami C, Lorrain S, Hornitschek P, Fankhauser C (2010) Light-regulated plant growth and development. Curr Top Dev Biol 91: 29–66 [DOI] [PubMed] [Google Scholar]
- Kaufholdt D, Baillie CK, Meyer MH, Schwich OD, Timmerer UL, Tobias L, van Thiel D, Hänsch R, Mendel RR (2016) Identification of a protein-protein interaction network downstream of molybdenum cofactor biosynthesis in Arabidopsis thaliana. J Plant Physiol 207: 42–50 [DOI] [PubMed] [Google Scholar]
- Kaufholdt D, Gehl C, Geisler M, Jeske O, Voedisch S, Ratke C, Bollhöner B, Mendel RR, Hänsch R (2013) Visualization and quantification of protein interactions in the biosynthetic pathway of molybdenum cofactor in Arabidopsis thaliana. J Exp Bot 64: 2005–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Kronmiller B, Maszle DR, Coupland G, Holm M, Mizuno T, Wu SH (2009) The Arabidopsis B-box zinc finger family. Plant Cell 21: 3416–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Park HY, Jang YH, Lee JH, Kim JK (2013) The sequence variation responsible for the functional difference between the CONSTANS protein, and the CONSTANS-like (COL) 1 and COL2 proteins, resides mostly in the region encoded by their first exons. Plant Sci 199-200: 71–78 [DOI] [PubMed] [Google Scholar]
- Laubinger S, Fittinghoff K, Hoecker U (2004) The SPA quartet: a family of WD-repeat proteins with a central role in suppression of photomorphogenesis in arabidopsis. Plant Cell 16: 2293–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubinger S, Marchal V, Le Gourrierec J, Wenkel S, Adrian J, Jang S, Kulajta C, Braun H, Coupland G, Hoecker U (2006) Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability. Development 133: 3213–3222 [DOI] [PubMed] [Google Scholar]
- Lazaro A, Mouriz A, Piñeiro M, Jarillo JA (2015) red light-mediated degradation of CONSTANS by the E3 ubiquitin ligase HOS1 regulates photoperiodic flowering in Arabidopsis. Plant Cell 27: 2437–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledger S, Strayer C, Ashton F, Kay SA, Putterill J (2001) Analysis of the function of two circadian-regulated CONSTANS-LIKE genes. Plant J 26: 15–22 [DOI] [PubMed] [Google Scholar]
- Liu LJ, Zhang YC, Li QH, Sang Y, Mao J, Lian HL, Wang L, Yang HQ (2008) COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis. Plant Cell 20: 292–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A, Schrader A, Kokkelink L, Falke C, Welter B, Iniesto E, Rubio V, Uhrig JF, Hülskamp M, Hoecker U (2013) Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. Plant J 74: 638–651 [DOI] [PubMed] [Google Scholar]
- Menon C, Sheerin DJ, Hiltbrunner A (2016) SPA proteins: SPAnning the gap between visible light and gene expression. Planta 244: 297–312 [DOI] [PubMed] [Google Scholar]
- Min JH, Chung JS, Lee KH, Kim CS (2015) The CONSTANS-like 4 transcription factor, AtCOL4, positively regulates abiotic stress tolerance through an abscisic acid-dependent manner in Arabidopsis. J Integr Plant Biol 57: 313–324 [DOI] [PubMed] [Google Scholar]
- Ordoñez-Herrera N, Fackendahl P, Yu X, Schaefer S, Koncz C, Hoecker U (2015) A cop1 spa mutant deficient in COP1 and SPA proteins reveals partial co-action of COP1 and SPA during Arabidopsis post-embryonic development and photomorphogenesis. Mol Plant 8: 479–481 [DOI] [PubMed] [Google Scholar]
- Reeves PH, Coupland G (2001) Analysis of flowering time control in Arabidopsis by comparison of double and triple mutants. Plant Physiol 126: 1085–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XL, Qi GN, Feng HQ, Zhao S, Zhao SS, Wang Y, Wu WH (2013) Calcineurin B-like protein CBL10 directly interacts with AKT1 and modulates K+ homeostasis in Arabidopsis. Plant J 74: 258–266 [DOI] [PubMed] [Google Scholar]
- Saijo Y, Sullivan JA, Wang H, Yang J, Shen Y, Rubio V, Ma L, Hoecker U, Deng XW (2003) The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev 17: 2642–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108 [DOI] [PubMed] [Google Scholar]
- Simon S, Rühl M, de Montaigu A, Wötzel S, Coupland G (2015) Evolution of CONSTANS regulation and function after gene duplication produced a photoperiodic flowering switch in the Brassicaceae. Mol Biol Evol 32: 2284–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YH, Shim JS, Kinmonth-Schultz HA, Imaizumi T (2015) Photoperiodic flowering: time measurement mechanisms in leaves. Annu Rev Plant Biol 66: 441–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilbrook K, Arongaus AB, Binkert M, Heijde M, Yin R, Ulm R (2013) The UVR8 UV-B photoreceptor: perception, signaling and response. Arabidopsis Book 11: e0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SB, Shen Y, Chang HC, Hou Y, Harris A, Ma SF, McPartland M, Hymus GJ, Adam L, Marion C, et al. (2010) The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element. New Phytol 187: 57–66 [DOI] [PubMed] [Google Scholar]
- Tripathi P, Carvallo M, Hamilton EE, Preuss S, Kay SA (2017) Arabidopsis B-BOX32 interacts with CONSTANS-LIKE3 to regulate flowering. Proc Natl Acad Sci USA 114: 172–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G (2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303: 1003–1006 [DOI] [PubMed] [Google Scholar]
- Wang CQ, Guthrie C, Sarmast MK, Dehesh K (2014) BBX19 interacts with CONSTANS to repress FLOWERING LOCUS T transcription, defining a flowering time checkpoint in Arabidopsis. Plant Cell 26: 3589–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CQ, Sarmast MK, Jiang J, Dehesh K (2015) The transcriptional regulator BBX19 promotes hypocotyl growth by facilitating COP1-mediated EARLY FLOWERING3 degradation in Arabidopsis. Plant Cell 27: 1128–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang Z, Li H, Zhao X, Liu X, Ortiz M, Lin C, Liu B (2013) CONSTANS-LIKE 7 regulates branching and shade avoidance response in Arabidopsis. J Exp Bot 64: 1017–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Jiang Y, Li J, Lin F, Holm M, Deng XW (2016a) BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc Natl Acad Sci USA 113: 7655–7660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Zhu D, Deng XW (2016b) The role of COP1 in repression of photoperiodic flowering. F1000 Res 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SK, Chung KS, Kim J, Lee JH, Hong SM, Yoo SJ, Yoo SY, Lee JS, Ahn JH (2005) CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiol 139: 770–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Ji R, Li H, Zhao T, Liu J, Lin C, Liu B (2014) CONSTANS-LIKE 7 (COL7) is involved in phytochrome B (phyB)-mediated light-quality regulation of auxin homeostasis. Mol Plant 7: 1429–1440 [DOI] [PubMed] [Google Scholar]
- Zhu D, Maier A, Lee JH, Laubinger S, Saijo Y, Wang H, Qu LJ, Hoecker U, Deng XW (2008) Biochemical characterization of Arabidopsis complexes containing CONSTITUTIVELY PHOTOMORPHOGENIC1 and SUPPRESSOR OF PHYA proteins in light control of plant development. Plant Cell 20: 2307–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]