Boron is preferentially delivered in rice to developing tissues by OsNIP3;1 located in the nodes, which is regulated at both the transcriptional and protein levels in response to external boron concentration.
Abstract
Boron is especially required for the growth of meristem and reproductive organs, but the molecular mechanisms underlying the preferential distribution of B to these developing tissues are poorly understood. Here, we show evidence that a member of nodulin 26-like intrinsic protein (NIP), OsNIP3;1, is involved in this preferential distribution in rice (Oryza sativa). OsNIP3;1 was highly expressed in the nodes and its expression was up-regulated by B deficiency, but down-regulated by high B. OsNIP3;1 was polarly localized at the xylem parenchyma cells of enlarged vascular bundles of nodes facing toward the xylem vessels. Furthermore, this protein was rapidly degraded within a few hours in response to high B. Knockout of this gene hardly affected the uptake and root-to-shoot translocation of B, but altered B distribution in different organs in the above-ground parts, decreased distribution of B to the new leaves, and increased distribution to the old leaves. These results indicate that OsNIP3;1 located in the nodes is involved in the preferential distribution of B to the developing tissues by unloading B from the xylem in rice and that it is regulated at both transcriptional and protein level in response to external B level.
Boron (B) is an essential micronutrient for plant growth and development. Its major physiological function is to maintain the structure of the cell wall by cross linking pectic polysaccharides through borate-diol bonding of two rhamnogalacturonan II molecules (Matoh 1997; O’Neill et al., 2001). The requirement of B ranges from 5 mg/kg to 100 mg/kg, depending on plant species (Marschner 2012). There is a good correlation between B requirement and pectin content of the cell wall (Matoh et al., 1993). Except some plant species producing sugar alcohols such as sorbitol and mannitol, B is immobile in most plant species (Brown and Shelp, 1997). Therefore, a continuous supply of B is required to maintain growth of newly developing tissues, and deficiency of B will result in the cessation of root elongation, reduced leaf expansion, and loss of fertility (Dell and Huang 1997; Loomis and Durst 1992; Marschner 1995). On the other hand, B also shows toxicity to plants when present in excess, which symptoms are characterized by chlorosis and necrosis at the tips of older leaves (Schnurbusch et al., 2010). Both B deficiency and toxicity cause crop losses in many areas of the world (Nable et al., 1997; Shorrocks, 1997).
Plant roots take up B in the form of boric acid. Two different types of transporters for B uptake have been identified: NIP5;1 and BOR1 in Arabidopsis (Arabidopsis thaliana; Takano et al., 2008; Reid 2014). NIP5;1 is a member of the major intrinsic protein family, belonging to subgroup II of nodulin 26-like intrinsic protein (NIP; Takano et al., 2006; Mitani et al., 2008). It functions as a boric acid channel (Takano et al., 2006). On the other hand, BOR1 functions as a boric acid/borate exporter (Takano et al., 2002), which is similar to bicarbonate transporters in animal cells. In Arabidopsis, NIP5;1 and BOR1 are polarly localized at the distal and proximal side of the plasma membrane of the root cells, respectively (Takano et al., 2010), and their cooperative transport is required for efficient and directional uptake of B in the roots especially under a B-limited condition (Takano et al., 2008; Reid 2014). Homologs of Arabidopsis NIP5;1 and BOR1 have been functionally characterized in other plant species such as rice (Oryza sativa), barley (Hordeum vulgare), rapeseed (Brassica napus), and maize (Zea mays; Nakagawa et al., 2007; Sutton et al., 2007; Chatterjee et al., 2014; Durbak et al., 2014; Hanaoka et al., 2014; Leonard et al., 2014; Zhang et al., 2017). Basically, they have a similar role in B uptake, although the expression patterns differ somewhat within plant species. Recently, two maize mutants (tls1 and rte) defective in vegetative and inflorescence development were also found to be caused by loss of function of genes homologous to NIP5;1 and BOR1, respectively (Chatterjee et al., 2014; Durbak et al., 2014), highlighting the importance of B in plant growth and development. In addition, some PIPs (plasma membrane intrinsic proteins) such as OsPIP2;4 and OsPIP2;7 in rice (Kumar et al., 2014), ZmPIP1 in maize (Dordas and Brown, 2001), and HvPIP1;3 and HvPIP1;4 in barley (Fitzpatrick and Reid, 2009), have been implicated in B uptake. However, their exact role and contribution to B-uptake are still unclear.
On the other hand, differential expression level of BOR1 homolog (Bot1/BOR2) was involved in tolerance to B toxicity in barley (Reid 2007; Sutton et al., 2007). B-toxicity tolerant cultivar shows higher expression of Bot1/BOR2 than B-toxicity sensitive cultivar of barley due to increased gene copy number. Different from BOR1, Bot1/BOR2 in barley plays an important role in excluding B from the cytosol to the rhizospheres probably due to different cellular localization (Takano et al., 2002; Sutton et al., 2007). This is similar to AtBOR4 in Arabidopsis, which overexpression resulted in increased tolerance to B toxicity (Miwa et al., 2007). In addition to NIP5;1, HvLsi1, a silicon transporter in barley (Chiba et al., 2009), was found to be involved in the B uptake and its differential expression is responsible for genotypic difference in B-toxicity tolerance (Schnurbusch et al., 2010).
After B uptake mediated by NIP5;1 and BOR1, it will be loaded to the xylem, followed by translocation from the roots to the shoots by transpirational mass flow (Marschner 2012). For a long time, it has been believed that the distribution of B in the shoots is mainly determined by relative transpiration rather than demand (Shelp et al., 1995). However, on the other hand, it was observed that B was preferentially delivered to developing tissues in many plant species such as Arabidopsis (Takano et al., 2001), sunflower (Helianthus; Matoh and Ochiai, 2005), wheat (Triticum aestivum; Huang et al., 2001), and broccoli (Brassica oleracea, var. italica; Marentes et al., 1997), which have very low or no transpiration. Therefore, there must be a system for such preferential distribution of B especially under B-limited condition, but for a long time it has been a mystery on how B taken up by the roots is preferentially distributed to the developing tissues. Recently, nodes in gramineous plants were found to play an important role in distribution of mineral elements (Yamaji and Ma, 2014; 2017). Several transporters involved in the intervascular transfer from enlarged vascular bundle to diffuse vascular bundle for preferential distribution have been identified, but most transporters localized in the nodes have not been functionally characterized. Our genomewide transcriptional analysis with rice node found that a member of nodulin 26-like intrinsic protein (NIP), OsNIP3;1, was highly expressed in the node (Yamaji et al., 2013). OsNIP3;1 is the closest homolog of AtNIP5;1 (Mitani et al., 2008). It is localized to the plasma membrane and also permeable to boric acid in yeast (Saccharomyces cerevisiae; Hanaoka et al., 2014) as well as arsenite when expressed in Xenopus oocytes (Ma et al., 2008). Knockout of this gene resulted in retarded growth (Liu et al., 2015). It has been speculated that OsNIP3;1 is involved in B uptake, mobility, and distribution among shoot tissues (Hanaoka et al., 2014; Liu et al., 2015), but the exact role of this gene is unknown. By detailed functional analysis, we found that OsNIP3;1 was highly expressed in the rice nodes and did not contribute to B uptake and translocation. Its main role is to preferentially deliver B to the developing tissues. Furthermore, we found that B distribution is regulated at both the transcriptional and protein levels.
RESULTS
OsNIP3;1 Showed Higher Expression in the Nodes
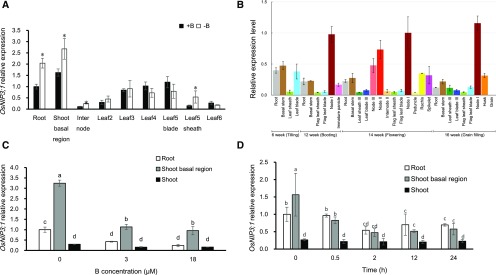
Although the expression pattern of OsNIP3;1 was investigated in previous studies (Hanaoka et al., 2014; Liu et al., 2015), they only focused on the roots and shoots. However, we found that the expression was higher in the nodes than in the roots and shoots at both the vegetative and reproductive stage (Fig. 1, A and B). Especially at reproductive stage, the expression in the uppermost node I of rice grown in rice field was much higher than that in other organs (Fig. 1B). At the vegetative growth stage, the expression in the roots and shoot basal regions including basal nodes were up-regulated by B deficiency, but that in the leaves was unaffected (Fig. 1A). The expression of OsNIP3;1 in the roots and shoot basal regions was down-regulated by 44% and 47%, respectively, at high external B concentrations compared with -B, but the expression level was similar at 3 µM and 18 µM B (Fig. 1C). A time-course experiment showed that the expression of OsNIP3;1 in the roots and shoot basal regions responded quickly to external B; at 30 min after exposure to 3 μM B, the expression level of OsNIP3;1 in the shoot basal region was reduced to one-half (Fig. 1D). The expression level was not further decreased after exposure to B for 2 h and longer.
Figure 1.
Expression pattern of OsNIP3;1 in rice. A, Expression of OsNIP3;1 in different organs of plants with or without B at vegetative growth stage. Seedlings (22-d-old, cv Hwayoung) were cultured in a solution containing 0 μM (−B) or 3 μM B (+B) for 7 d. Different organs were sampled for expression analysis. B, Expression of OsNIP3;1 in different organs of rice grown in a field at different growth stage. Different organs of rice (cv Nipponbare) grown in a paddy field were sampled at different growth stage shown. C, Effect of different B concentration on OsNIP3;1 expression. Seedlings (32-d-old, cv Hwayoung) precultured in B-free solution for 3 d, were exposed to a solution containing 0 μM, 3 μM, or 18 μM of B for 24 h. Different organs including roots, shoot basal region, and shoots were sampled. D, Time-dependent expression of OsNIP3;1 in response to external B. Seedlings (16-d-old, cv Hwayoung) precultured in B-free solution for 7 d, were exposed to 3 μM B for different times indicated, and the roots, shoot basal region, and shoots were sampled. The expression level was determined by real time RT-PCR. HistoneH3 and Ubiquitin were used as internal standards. Expression relative to the roots is shown. Data are means ± sd (n = 3). Asterisk shows significant difference at P < 0.05 between +B and −B. Different small letter indicates significant difference at P < 0.05 by Duncan’s test.
OsNIP3;1 Protein was Mainly Localized at the Xylem Region of the Node
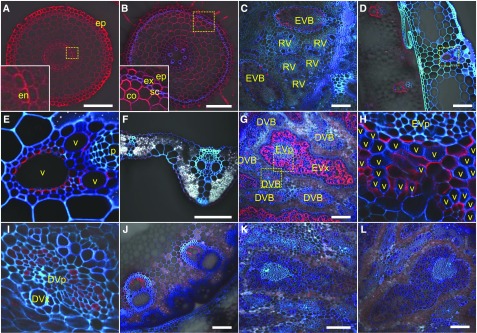
To investigate tissue and cell specificity localization of OsNIP3;1 protein, we performed immunostaining using an antibody against OsNIP3;1. At the vegetative stage, the signal was detected in all cells of the root tips (2 mm from root tip) and root mature region (20 mm from tip). Furthermore, it showed polar localization at the distal side (Fig. 2, A and B). A stronger signal was observed in the basal node and leaf sheath (Fig. 2, C to E). At the basal node, OsNIP3;1 was localized at the xylem parenchyma cells including transfer cells of enlarged vascular bundle (Fig. 2C). In the leaf sheath, OsNIP3;1 was localized at the xylem region (Fig. 2, D and E), but signal was very weak in the leaf blade (Fig. 2F). Furthermore, OsNIP3;1 also showed polar localization at the side facing toward the vessels in all xylem cells (Fig. 2, C to E).
Figure 2.
Tissue and cell specificity of localization of OsNIP3;1. Immunostaining using an antibody against OsNIP3;1 was performed in root tips (A), root mature region (B), basal node (C), leaf sheath (D and E), and leaf blade (F) of young seedlings (wild type), and node I (G to I) and peduncle (J) of plants (wild type) and node I (K and L) of two knockout lines at heading stage. Red color shows signal from OsNIP3;1 and blue/cyan color from cell wall autofluorescence. Insets of (A) and (B), (E), (H), and (I) are a magnified image of the yellow box area in (A), (B), (D), and (G), respectively. The xylem and phloem region of the enlarged vascular bundle and the diffuse vascular bundle (EVx, EVp, DVx, and DVp) are indicated. Scale bars = 100 μm. DVB, diffuse vascular bundle; EVB, enlarged vascular bundle; RV, regular vascular bundle in basal node. co, cortex cell; en, endodermis; ep, epidermis; ex, exodermis; p, phloem region; sc, sclerenchyma; v, xylem vessel.
At the reproductive growth stage, a strong signal was observed in the xylem parenchyma cells of enlarged vascular bundles of node I (Fig. 2, G and H). A weak signal was also observed in the phloem region of diffuse vascular bundle (Fig. 2I). Signal was also observed in the xylem region of the peduncle (Fig. 2J). In these tissues, OsNIP3;1 also showed polar localization at the sides facing toward the xylem vessels. No signals were detected at the nodes of two independent knockout lines (Fig. 2, K and L), indicating the specificity of this antibody against OsNIP3;1.
Rapid Degradation of OsNIP3;1 Protein in Response to High External B
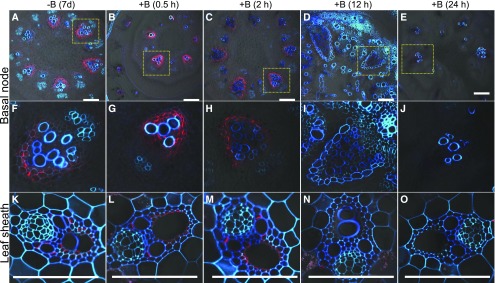
To investigate the response of OsNIP3;1 protein to external B, we traced the protein change after the B-deficient plants were transferred to B solution using the same timescale for the transcript experiment (Fig. 3). At the basal node and leaf sheath of young seedlings, OsNIP3;1 signal became weak after exposure to B solution for 2 h (Fig. 3, C, H, and M). At 12 h after the exposure, the signal completely disappeared. This result is consistent with western blot and coimmunostaining results (Supplemental Figs. S1 and S2). Western-blot analysis detected two bands at approximately 50 kD and 75 kD, which were supposed to be a dimer and trimer of OsNIP3;1 (Supplemental Fig. S1). The signal of OsNIP3;1 protein disappeared after exposure to B solution for 12 h (Supplemental Fig. S1). Coimmunostaining showed that the signal of Lsi6, a Si transporter (Yamaji et al., 2008), did not respond to high B (Supplemental Fig. S2), but that of OsNIP3;1 was degraded upon B exposure (Supplemental Fig. S2).
Figure 3.
Response of OsNIP3;1 protein to B supply. Seedlings (16-d-old, cv Hwayoung) precultured in B-free solution for 7 d were exposed to a solution containing 3 μM B. At different times indicated, both basal nodes (A to J) and leaf sheath (K to O) were sampled for immunostaining of OsNIP3;1. F to J, Magnified image of xylem area of EVB from above panel (A to E). Red color shows signal from OsNIP3;1 and blue/cyan color from cell wall autofluorescence. Scale bars = 100 μm.
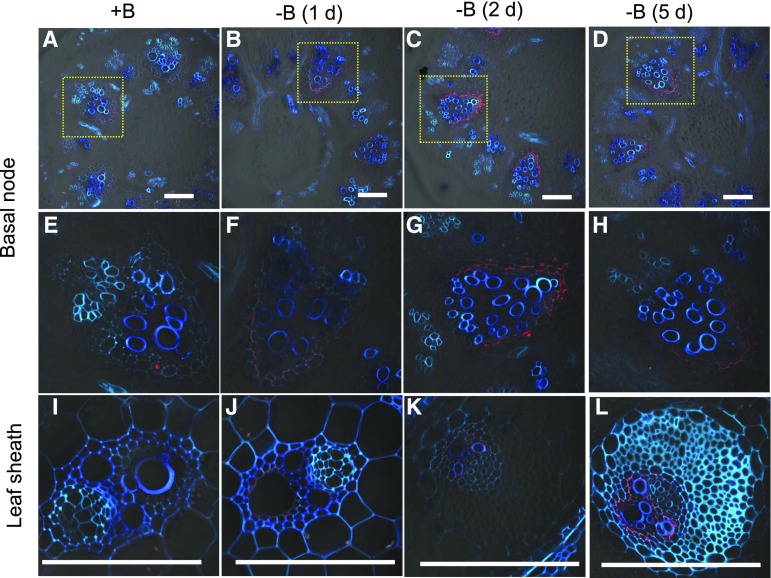
Conversely, when B-sufficient plants were exposed to a solution free of B, OsNIP3;1 was detected in the basal node and leaf sheath at d 1 (Fig. 4). The signal became stronger at d 2 and thereafter. These results indicate that OsNIP3;1 protein was degraded or synthesized in response to external B concentration. OsNIP3;1 in the roots and node I was also degraded in response to high B (Supplemental Figs. S3 and S4).
Figure 4.
Response of OsNIP3;1 protein localized in the basal node and leaf sheath to B deficiency. Seedlings (16-d-old, cv Hwayoung) precultured in 3 μM B solution were exposed to B-free solution. At d0, d1, d2, and d5, the basal node (A to H) and leaf sheath (I to L) were sampled for immunostaining of OsNIP3;1. Red color shows signal from OsNIP3;1 and blue/cyan color shows cell wall autofluorescence. E to H, Magnified image of xylem area of EVB in basal nodes shown above panel. Scale bars = 100 μm.
Phenotypic Analysis of OsNIP3;1 Knockout Lines
To examine the role of OsNIP3;1 in B-transport in rice, we obtained two independent knockout lines with T-DNA insertion (Supplemental Fig. S5, A and B). When grown at 0 μM and 0.3 μM B for 30 d, the growth of two mutants were severely inhibited compared with their wild types (Supplemental Fig. S5C). However, at 3 µM B, the growth became similar between mutants and wild types. This phenotype is similar to previous studies using RNAi lines and a different mutant, dte1 (Hanaoka et al., 2014; Liu et al., 2015). We further investigated the effect of OsNIP3;1 knockout on the development of individual leaf. For this purpose, plants precultured with 3 μM B were transferred to a solution containing 0 μM or 3 μM B and individual leaves were compared between wild types and mutants after 11 d. At 0 μM B, the young leaves (leaf 6 and leaf 7) that appeared after transfer to –B solution were much smaller in the mutant than the wild type (Fig. 5, A and B; Supplemental Fig. S6A), showing chlorosis and necrosis at the tips, which are typical B-deficiency symptoms. However, at 3 μM B, the growth of fully expanded young leaf (leaf 6) did not differ between wild type and mutant (Fig. 5, C and D; Supplemental Fig. S6D).
Figure 5.
Phenotypic analysis of OsNIP3;1 knockout lines. A and D, Phenotype of OsNIP3;1 mutant at different B. Seedlings (17-d-old) of wild type (A and C) and mutant (B and D) precultured with 3 μM B were exposed to a solution containing 0 μM (A and B) or 3 μM B (C and D). After 11 d, shoot basal region and individual leaf (leaf 2 to leaf 7, from old to young) were separated and photographed. E and F, Distribution of B in different organs of two mutants (nip3;1-1 and nip3;1-2) and their corresponding wild types (WT1 and WT2) at 0 (E) and 3 µM B (F). Distribution was calculated based on B accumulation in different organs. Data are means ± sd (n = 3). Asterisk shows significant difference at P < 0.05 between wild type and mutant. Scale bars = 10 cm.
We also compared B concentration and accumulation in different leaves. At 0 μM B, the concentration of B in each leaf did not differ between wild types and mutants (Supplemental Figure S6B), but the B-accumulation in leaf 6 and leaf 7 was significantly reduced in the mutants compared with the wild types (Supplemental Fig. S6C). As a result, the distribution ratio of B in young leaves (leaf 6 and leaf 7) was decreased in the mutants, but that in the old leaf (leaf 4) was increased (Fig. 5E). At 3 µM B, the difference in both B concentration and accumulation in each leaf was not evident between WTs and mutants (Supplemental Fig. S6, E and F). There was also no difference in the distribution, uptake, and root-to-shoot translocation of B between wild types and mutants (Fig. 5F; Supplemental Fig. S7). These results indicate that OsNIP3;1 is involved in the distribution of B to the developing tissues under B-limited conditions, rather than in B uptake by the roots and root-to-shoot translocation.
Stable Isotope-Labeling Experiment
To further support the above conclusion, we performed a short-term labeling experiment by using stable isotopes of B, 10B, and 11B. Plants precultured with 11B were exposed to a solution containing 0 μM or 3 μM 11B for 3 d and then subjected to a solution containing 0.3 μM 10B together with rubidium (Rb) and strontium (Sr) for 10 h. Rb and Sr are known as markers of phloem and xylem transport (Kuppelwieser and Feller, 1991), respectively. The uptake and the root-to-shoot translocation of 10B during 10 h was similar between WTs and mutants (Supplemental Fig. S8, A and B). There was also no difference in B uptake between B-sufficient and -deficient plants of each line. However, there was a clear difference in the concentration and distribution of 10B in some leaves of plants pretreated with −B between WTs and mutants. Knockout of OsNIP3;1 resulted in a lower 10B-concentration in the shoot basal regions and the youngest leaf (leaf 6; Fig. 6A). The distribution of 10B to the older leaves (leaf 2 to leaf 4) increased, but that to the young leaf significantly decreased in the mutants (Fig. 6B). However, neither the concentration nor the distribution of either Rb or Sr was altered in the mutants (Supplemental Fig. S9). In plants pretreated with sufficient B, decreased 10B concentration in the shoot basal region was also found in the mutants, although the difference was not as large as in plants pretreated with –B (Fig. 6C). No difference was found in the distribution of 10B to different leaves between WTs and mutants pretreated with B (Fig. 6D). There was also no difference in the concentration and distribution of Rb and Sr between WTs and mutants pretreated with B (Supplemental Fig. S9, C, D, G, and H). These results further indicate that OsNIP3;1 is required for preferential distribution of B to the developing tissues under the B-limited condition.
Figure 6.
Concentration and distribution of 10B in different organs. A and C, 10B concentration in different organs of OsNIP3;1 knockout lines (osnip3;1-1 and osnip3;1-2) and their corresponding wild types (WT1 and WT2). B and D, Distribution of 10B in different organs. Seedlings (22-d-old) precultured with 3 μM 11B were exposed to a solution containing 0 μM (A and B) or 3 μM 11B (C and D) for 3 d. These seedlings were then exposed to a solution containing 0.3 μM 10B. After 10 h, each organ was separately harvested and subjected to 11B and 10B determination by ICP-MS. Data are means ± sd (n = 3). Asterisk shows significant difference at P < 0.05 between wild type and mutant.
Silicon Transporter Lsi1 Largely Contributes to B Uptake
OsNIP3;1 was previously proposed to be involved in the B uptake in rice (Hanaoka et al., 2014). However, our results showed that the contribution of OsNIP3;1 to total B uptake was negligible (Supplemental Figs. S7A and S8A). This raises a question: which transporter is responsible for B uptake in rice? Previous studies showed that Si transporter Lsi1 is also permeable to boric acid in Xenopus oocyte (Mitani et al., 2008) and yeast (Schnurbusch et al., 2010). By using our lsi1 mutant, it was also reported that the mutant contained less B compared with its wild type (Bienert et al., 2008; Schnurbusch et al., 2010). To further investigate the involvement of Lsi1 in B uptake, we grew both lsi1 mutant and its wild type at different B concentrations. At 0 μM B, the youngest leaf of the mutant showed a typical B-deficiency symptom, which is characterized by the retarded growth of the leaf meristem (Supplemental Fig. S10). However, such a symptom was not observed in the wild type. At 0.3 μM and 3 μM B, the growth became similar between the wild type and the lsi1 mutant (Supplemental Fig. S10). The B concentration in the shoot was significantly lower in the mutant than in the wild type at 0.3 μM and 3 μM B (Fig. 7A), but the difference in the root B concentration was not large (Fig. 7B). The total B uptake of lsi1 was only 49% and 44% of wild type, respectively, at 0.3 μM B and 3 μM B (Fig. 7C). More than 94% of total B was translocated to the shoots, but there was no difference in the root-to-shoot translocation of B between wild type and lsi1 (Fig. 7D).
Figure 7.
Concentration, uptake, and root-to-shoot translocation of B in the lsi1 knockout line and its wild type. A and B, B concentration in the shoots (A) and roots (B). C and D, Uptake (C) and root-to-shoot translocation (D). Seedlings (6-d-old) of the lsi1 mutant and its wild type (cv Oochikara) were grown in a nutrient solution containing 0 μM, 0.3 μM, or 3 μM B. After 14 d, the plants were harvested and subjected to B determination by ICP-MS. Data are means ± sd (n = 3). Asterisk shows significant difference at P < 0.05 between wild type and mutant.
Because the expression level of Lsi1 was much higher than that of OsNIP3;1 in the roots (Ma et al., 2008), there is a possibility that the contribution of OsNIP3;1 to B uptake may be overlapped by Lsi1. Because Lsi1 is mainly expressed in mature root but not in root tip (Yamaji and Ma, 2007), we therefore performed a short-term (6 h) uptake experiment using 10B and compared the 10B concentration of the root tips (0 to 0.5 cm) between osnip3;1 mutants and their wild types. The 10 B concentration in the root tips was significantly lower in the mutants than in the wild types (Supplemental Fig. S11). This result indicates that OsNIP3;1 locally contributes to B uptake in the root tips, although its contribution to total B uptake is small.
We also investigated the response of Lsi1 expression to different B concentrations. Unlike OsNIP3;1, the expression of Lsi1 was constant and not affected by the external B concentrations (Supplemental Fig. S12).
DISCUSSION
OsNIP3;1 Is Responsible for Preferential Distribution of B to Developing Tissues
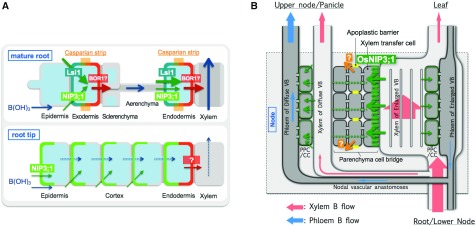
Previous physiological studies have shown that B taken up by the roots is preferentially delivered to the developing tissues such as new leaves and reproductive organs for their active growth (Marentes et al., 1997; Takano et al., 2001; Huang et al., 2001; Matoh and Ochiai, 2005), but the molecular mechanism underlying this preferential distribution is poorly understood. In this study, we found that OsNIP3;1 localized in the nodes is required for this preferential distribution of B in rice. Nodes are the hub of distribution of mineral elements (Yamaji and Ma, 2014), which have a complexed but well organized vascular system. We found that OsNIP3;1 is highly expressed in the nodes at both the vegetative and reproductive growth stage (Fig. 1, A and B). In the nodes, OsNIP3;1 is mainly localized in the xylem parenchyma cells (Fig. 2, C and G). Furthermore, it is polarly localized at the side, facing toward the xylem vessel (Fig. 2, C, G, and H). Knockout of OsNIP3;1 resulted in decreased distribution of B to the developing tissues under B-limited condition (Fig. 6B). These results together indicate that OsNIP3;1 is involved in unloading B in the xylem of enlarged vascular bundle (Fig. 8B), which is the first step for the intervascular transfer. In addition, OsNIP3;1 is also localized in the phloem region of diffuse vascular bundles of node I (Fig. 2I). This also facilitates preferential distribution of B to the developing tissues by loading B into the phloem companion cells (Fig. 8B). However, because OsNIP3;1 is a passive channel for boric acid, a cooperative efflux transporter for the intervascular transfer of B is required as a driving force (Yamaji and Ma, 2014; Yamaji et al., 2015; Yamaji and Ma, 2017), which remains to be identified. Localization of OsNIP3;1 is also observed in the xylem region of leaf sheath and peduncle (Fig. 2, D and J), indicating its similar role in unloading B from the xylem in these organs. A higher B ratio of leaf blade/ sheath in RNAi lines observed previously (Hanaoka et al., 2014) is probably the result of decreased unloading of B within the leaf sheath.
Figure 8.
Schematic presentation of role of OsNIP3;1 in uptake and distribution of B in rice. A, Role of OsNIP3;1 in B uptake. OsNIP3;1 polarly localized at the distal side of cells in the root tips locally contributes to B influx into the cells. In the root mature region, Lsi1 polarly localized at the distal side of exodermis and endodermis mediates B influx to the root cells. OsBOR1 is proposed to be involved in B efflux from the root cells. B, Role of OsNIP3;1 in preferential distribution of B to developing tissues. OsNIP3;1 polarly localized at the xylem transfer cells of enlarged vascular bundle mediates unloading of B from the xylem for intervascular transfer. Efflux B transporter for the intervascular transfer is unidentified. OsNIP3;1 located at the phloem cell of diffuse vascular bundle also facilitates B influx to the phloem for preferential distribution. OsNIP3;1 is regulated at both transcriptional and protein level in response to environmental B change.
In Arabidopsis, AtNIP6;1 was implicated in the preferential distribution of B to growing shoot tissues (Tanaka et al., 2008). AtNIP6;1 is predominantly expressed in nodal regions of shoots, especially in the phloem region of vascular tissues. Knockout of AtNIP6;1 resulted in lower B concentration in the young rosette leaves (Tanaka et al., 2008). Based on this evidence, it was suggested that AtNIP6;1 is involved in xylem-phloem transfer of B at the nodal regions. These findings indicate that Arabidopsis has a different system for preferential distribution of B from rice. In fact, the node structure is totally different between Arabidopsis and rice (Yamaji and Ma, 2017). Unlike rice, Arabidopsis node does not have developed vascular system.
OsNIP3;1 Is Regulated at Both Transcriptional and Protein Level
The expression of OsNIP3;1 was down-regulated by B supply in the roots (Hanaoka et al., 2014; Liu et al., 2015). In this study, we found that expression of OsNIP3;1 in the nodes was also rapidly down-regulated in response to B supply (Fig. 1C). A time-course experiment showed that the expression level in the nodes decreased half at 30 min after exposure to B (Fig. 1D) and maintained at a constant level after 2 h. AtNIP5;1 in Arabidopsis was also down-regulated by B (Takano et al., 2006). The 5′ UTR was involved in the regulation of AtNIP5;1 expression (Tanaka et al., 2011). It seems that high B triggers AtNIP5;1 mRNA degradation. Furthermore, it was recently found that the minimum open reading frame, AUG-stop induced B-dependent ribosome stalling and mRNA degradation of AtNIP5;1 (Tanaka et al., 2016). The 5′ UTR is highly conserved between AtNIP5;1 and OsNIP3;1 (Tanaka et al., 2011). This suggests that similar mechanism is involved in the regulation of OsNIP3;1 in rice although further confirmation is required in future.
The OsNIP3;1 protein localized in the nodes also showed rapid response to external B change; the protein was rapidly degraded in response to high B (Fig. 3; Supplemental Figs. S1 and S2), whereas it was generated in response to B deficiency (Fig. 4). At 12 h after exposure to high B, OsNIP3;1 was completely degraded although its transcript was still detected (Figs. 1D and Fig. 3; Supplemental Figs. S1 and S2). This means that absence of OsNIP3;1 protein at high B is not solely due to its decreased transcript accumulation, but to another unknown mechanism for the degradation. BOR1 in Arabidopsis also undergoes degradation in response to high B (Takano et al., 2010). BOR1 is transferred via endosomes to the vacuole for degradation upon high B supply (Takano et al., 2005) and Tyr residues in the large loop region of BOR1 are required for the vacuolar pathway (Takano et al., 2010). Unlike BOR1, we did not observe dotlike structures of OsNIP3;1 in the cytoplasm during degradation process in the time-course experiment (Fig. 3), suggesting that a different mechanism for degradation of OsNIP3;1 is involved.
Different Regulation of B Accumulation in Rice and Arabidopsis
In natural systems, B concentrations vary from vanishingly small to very high (Tanaka et al., 2011; Reid 2014), but the range between deficiency and toxicity of B is very narrow. Therefore, plants must have a system to maintain B homeostasis by regulating the uptake, mobilization, distribution, and sequestration. In Arabidopsis, B homeostasis seems to be maintained through regulating B uptake mediated by NIP5;1 and BOR1. NIP5;1 undergoes transcriptional regulation (Takano et al., 2010; Tanaka et al., 2011), whereas BOR1 is regulated at posttranscriptional regulation in response to external B change (Takano et al., 2005). Rice seems to have a different mechanism for B homeostasis. OsNIP3;1, the closest homolog of AtNIP5;1, did not contribute to total B uptake (Supplemental Figs. S7A and S8A), but a Si transporter Lsi1 is largely involved in B uptake in rice roots (Fig. 7C). Lsi1 is localized at the distal side of exodermal and endodermal cells of the mature root region (Ma et al., 2006), which is responsible for transport of B from external solution to the root cells (Fig. 8A). However, unlike AtNIP5;1, the expression of Lsi1 was not affected by external B level (Supplemental Fig. S12). A homolog of BOR1 in rice, OsBOR1, was reported as an efflux B transporter (Nakagawa et al., 2007), although it is not clear whether OsBOR1 is localized at the proximal side of exodermal and endodermal cells for cooperative transport with Lsi1. The accumulation of OsBOR1 transcripts was also not affected by B deprivation, but the protein seems to be enhanced (Nakagawa et al., 2007). Therefore, there is a possibility that the B uptake is regulated by OsBOR1 at posttranscriptional level. However, we found that there was no large difference in B uptake between B-sufficient and -deficient rice plants (Supplemental Fig. S8A), suggesting that unlike Arabidopsis, B uptake is not regulated by external B supply. By contrast, it is likely that B homeostasis in rice is regulated at the distribution step mediated by OsNIP3;1 in the nodes. Under the B-limited condition, OsNIP3;1 mediates preferential distribution to developing tissues to meet their requirement (Figs. 6B and 8B). However, under the high B condition, OsNIP3;1 is rapidly degraded to avoid excess B to be delivered to the developing tissues. One possible explanation for such different regulation between rice and Arabidopsis is that Lsi1 responsible for Si and B uptake shows relatively wide transport substrates and rice accumulates much more Si than B (Ma and Yamaji, 2015), therefore it is difficult to control B influx at the uptake step. Moreover, Arabidopsis does not have Si channel homologous to rice Lsi1 in the genome (Ma and Yamaji, 2015). This regulation mechanism is similar to that for Mn in rice (Yamaji et al., 2013; Shao et al., 2017). OsNramp3 in the node required for Mn distribution, rather than OsNramp5 and OsMTP9 in the roots for Mn uptake, is regulated at the protein level in response to environmental Mn change (Shao et al., 2017).
In conclusion, OsNIP3;1 mainly localized in the nodes mediates intervascular transfer of B by unloading B from xylem of enlarged vascular bundles and is required for preferential distribution of B to the developing tissues. OsNIP3;1 is regulated at both transcriptional and protein level in response to external B-concentration change.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Two T-DNA insertion mutant lines of OsNIP3;1 (4A-01956/nip3;1-1 and 2A-00764/nip3;1-2) from RISD (http://cbi.khu.ac.kr/RISD_DB.html) and their wild-type rice (Oryza sativa; WT1; cv Dongjin, WT2; cv Hwayoung); lsi1 mutant and its wild type (cv Oochikara), were used in this study. Seeds were soaked in water for 2 d at 30°C in the dark and then the geminated seeds were transferred to nylon nets floating on a solution containing 0.5 mm CaCl2 in a 1.2-L pot and grown for 5 d. The seedlings were transferred to a 1/2 Kimura B-nutrient solution (pH 5.6) containing the following macronutrients: MgSO4 (0.28 mM), (NH4)2SO4 (0.18 mM), Ca(NO3)2 (0.18 mM), KNO3 (0.09 mM), and KH2PO4 (0.09 mM); and micronutrients: Fe-EDTA (20 μM), H3BO3 (3 μM), MnCl2 (0.5 μM), CuSO4 (0.2 μM), ZnSO4 (0.4 μM), and (NH4)6Mo7O24 (1 μM). The solution was renewed every 2 d. All experiments were conducted in a greenhouse at 25°C to 30°C under natural sunlight with three replicates.
Gene Expression Pattern of OsNIP3;1 and OsLsi1
To investigate the expression of OsNIP3;1 in different organs at vegetative growth stage, seedlings (22-d-old, cv Hwayoung) were cultured in a solution containing 0 (−B) or 3 μM B (+B) for 7 d. Different organs including roots, shoot basal region, and different leaves were sampled and immediately frozen in liquid nitrogen until extraction of RNA. The effect of different B concentrations on OsNIP3;1 expression was investigated by exposing seedlings (32-d-old, cv Hwayoung) precultured in B free solution for 3 d, to a solution containing 0 μM, 3 μM, and 18 μM B. After 24 h, roots, shoot basal region, and shoots were sampled. Furthermore, a time-course experiment of OsNIP3;1 expression was performed by exposing seedlings (16-d-old, cv Hwayoung) precultured in B-free solution for 7 d, to a solution containing 3 μM B. At times indicated, the roots, shoot basal region, and shoots were sampled. The expression of OsNIP3;1 was also investigated in different organs at different growth stages in rice grown in a paddy field using the same samples described previously (Yamaji et al., 2013).
Effect of different B concentrations on Lsi1 expression was investigated in plants (27-d-old, cv Hwayoung) exposed to a solution containing 0 μM, 3 μM, and 18 μM B. After exposure for 24 h, the roots were sampled for RNA extraction.
Total RNA of samples harvested was extracted by using an RNeasy Plant Mini Kit (Qiagen), followed by DNase I treatment. Conversion to cDNA was performed using the protocol supplied by the manufacturer of SuperScript II (Invitrogen). Specific cDNAs were amplified by Sso Fast EvaGreen Supermix (Bio-Rad). The expression was determined by quantitative RT-PCR using primers (F1) 5′-AATCAGTCGTGTCATCCTTGGG-3′ (forward) and (R1) 5′-CCCATCTACGTGAATGGACCG-3′ (reverse) for OsNIP3;1, 5′-GCCAGCAACAACTCGAGAACAA-3′ (forward) and 5′-CATGGTAGGCATGGTGCCGT-3′ (reverse) for Lsi1 on CFX384 (Bio-Rad). HistoneH3 and Ubiquitin were used as internal standards with primers 5′-AGTTTGGTCGCTCTCGATTTCG-3′ (forward) and 5′-TCAACAAGTTGACCACGTCACG-3′ (reverse) for HistoneH3, 5′-GCTCCGTGGCGGTATCAT-3′ (forward) and 5′-CGGCAGTTGACAGCCCTAG-3′ (reverse) for Ubiquitin. The relative expression was normalized based on these two genes by ∆∆Ct method using the CFX Manager software (Bio-Rad).
Immunohistological Staining of OsNIP3;1
The synthetic peptide C-LRDENGETPRPQRSFRR (positions 293 to 309 of OsNIP3;1) was used to immunize rabbits to obtain antibodies against OsNIP3;1. The obtained antiserum was purified through a peptide affinity column before use. Different organs including roots, basal node, leaf sheath, blade, node I, and peduncle of both wild types and mutants were used for immunostaining of OsNIP3;1 protein as described previously (Yamaji and Ma, 2007; Yamaji et al., 2008).
To investigate the response of OsNIP3;1 protein to B supply, seedlings (16-d-old, cv Hwayoung) precultured in B-free solution for 7 d were exposed to a solution containing 3 μM B. At different times indicated, basal nodes and leaf sheath were sampled for immunostaining of OsNIP3;1 as described above. To trace the response of OsNIP3;1 protein to B deficiency, seedlings (16-d-old, cv Hwayoung), precultured in 3 μM B-solution, were exposed to B-free solution. At d0, d1, d2, and d5, the basal nodes and leaf sheath were sampled for immunostaining of OsNIP3;1. Response of OsNIP3;1 protein localized in the roots in response to high B was examined in seedlings (5-d-old) of both wild type (cv Donging) and osnip3;1 mutant, which were exposed to a solution containing 0 μM or 10 μM B. After 12 h, different root segments (2 mm and 20 mm from the root tip) were sampled for immunostaining. To compare OsNIP3;1 in node I between B-sufficient and -deficient plants, plants were hydroponically cultivated until the heading stage. Before sampling node I for immunostaining, the plants were subjected to 0 μM or 3 μM B for 3 d. Observation was made with a confocal laser scanning microscope (TCS SP8x; Leica Microsystems).
For coimmunostaining of OsNIP3;1 and Lsi6 as a positive control, we used a Zenon Rabbit IgG Labeling Kit (Molecular Probes) in accordance with the manufacturer’s instructions. Seedlings (15-d-old) of both the WT2 (cv Hwayoung) and the mutant (osnip3;1-2) were precultured in B-free solution for 7 d, followed by exposing to a solution containing 3 µM B. Basal nodes were sampled for coimmunostaining at 0 h, 2 h, 12 h, and 24 h after exposure to B. Fluorescence signals were observed with a confocal laser-scanning microscopy (TCS SP8x; Leica Microsystems). Signal intensities of each channel were measured by LAS X Software (Leica Microsystems) and the ratio relative to control −B condition (0 h) were calculated in each xylem region of the enlarged vascular bundles. A total of 12 to approximately 25 regions from two to approximately four sections were used for the calculation.
Western-blot Analysis
For western-blot analysis, seedlings (15-d-old) of both the WT2 (cv Hwayoung) and the mutant (osnip3;1-2) were precultured in a B-free solution for 7 d, followed by exposure to a solution containing 3 μM B. At different times as described above, shoot basal regions (1 cm from the root to shoot junction) were sampled and subjected to protein extraction as described previously (Mitani et al., 2009). The microsome fraction was obtained by ultracentrifugation at 100,000g for 40 min. The same amounts (10 μg) of microsome protein were subjected to SDS-PAGE using 5% to 20% gradient polyacrylamide gels (e-PAGEL, ATTO; http://www.atto.co,jp) and subsequently to immune-blotting. The membrane was treated with OsNIP3;1 antibody (1:500 dilutions). Anti-rabbit IgG (H+L) HRP Conjugate (1:10,000 dilution; Promega; http://www.promega.com) was used as a secondary antibody. The membrane was finally subjected to Coomassie Brilliant Blue staining. The ECL Plus Western Blotting Detection System (GE Healthcare; http://www.gehealthcare.com) was used for detection via chemiluminescence.
Phenotypic Analysis of OsNIP3;1 Knockout Lines and lsi1 Mutant
For phenotypic analysis, seedlings (17-d-old) of both knockout lines and their wild types were exposed to various B-concentrations (0 μM, 0.3 μM, and 3 μM). After exposure for 11 d, the roots were washed with 5 mm CaCl2 solution for three times and separated from the shoots. In above-ground parts, shoot basal region (0 cm to 0.5 cm from the root-shoot junction) and different leaves (leaf 2 to leaf 7) of one plant each were separately harvested for B determination and photographed. To observe the prolonged effect of B deficiency on plant growth, some seedlings were exposed to –B solution for 30 d and then photographed.
To compare growth and B uptake between the lsi1 mutant and its wild type (cv Oochikara) under different B concentrations, the seedlings (6-d-old) were exposed to a nutrient solution containing 0 µM, 0.3 µM, and 3 µM B for 14 d (one plant each for different B concentrations, three replicates). After the plants were photographed, the roots were washed with 5 mm CaCl2 three times, separately from the shoots.
Labeling Experiment with the Stable Isotope 10B
To investigate the distribution pattern of B, seedlings (22-d-old) of two independent mutants and their wild types precultivated in a nutrient solution containing 1 mm Si and 3 μM 11B (99% enriched boric acid; Cambridge Isotope Laboratories) were exposed to 0 μM (−B) or 3 μM 11B (+B) for 3 d. These seedlings were then exposed to 0.3 μM 10B (99% enriched boric acid; Cambridge Isotope Laboratories) with 1 μM Rb and Sr in a nutrient solution. After 10 h, the roots were washed with 5 mm CaCl2 solution three times. Roots, shoot basal region (0 cm to 0.5 cm from the root-shoot junction), and different leaves (leaf 2 to leaf 6) were separately sampled and subjected to determination of 10B as described below. Leaf 1 was too small to be sampled.
Determination of 10B Concentration in Root Tips
To investigate B uptake in root tips, seedlings (24-d-old) of two knockout lines and their wild types precultured in 1/2 Kimura B-solution containing 3 μM B, were exposed to B-free (−B) solution for 3 d. These seedlings were then exposed to 0.3 μM 10B. After 6 h, the roots were washed with 5 mm CaCl2 solution for three times and the root tips (0 cm to 0.5 cm, 20 to 30 root tips for one replicate) were excised by a razor. Their fresh weight was immediately recorded. After digestion, 10B concentration in the root tips was determined as described below.
Measurement of B Concentration
All samples harvested were dried at 70°C in an oven for 3 d. The samples were digested with HNO3 (60%) and H2O2 mixture (HNO3: H2O2=1:1) at a temperature up to 110°C in 15-mL plastic tubes. After dilution, the concentration of B as well as Rb and Sr was determined by inductively coupled plasma mass spectrometry (ICP-MS, 7700X; Agilent Technologies). An isotope mode was used for quantification of 10B and 11B in the digest solution.
Statistical Analysis of Data
Data were analyzed using Student’s t test and Duncan’s test. Significance was defined as P < 0.05.
Accession Number
Sequence data from this article can be found in the GenBank/EMBL databases under accession no. AB856420 for NIP3;1.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Western-blot analysis of OsNIP3;1 in nodes of wild-type rice and mutant.
Supplemental Figure S2. Time-dependent degradation of OsNIP3;1 in response to high B.
Supplemental Figure S3. Response of OsNIP3;1 protein localized in the roots in response to high B.
Supplemental Figure S4. Response of OsNIP3;1 protein localized in node I in response to high B.
Supplemental Figure S5. Position of T-DNA insertion (A) and isolation of OsNIP3;1 mutants (B). Phenotype of osnip3;1 mutants at different B concentrations (C).
Supplemental Figure S6. Dry weight, concentration, and content of B in different organs.
Supplemental Figure S7. Uptake and root-to-shoot translocation of B in osnip3;1 knockout lines and their wild types.
Supplemental Figure S8. Uptake and root-to-shoot translocation of 10B in osnip3;1 knockout lines and their wild types.
Supplemental Figure S9. Concentration and distribution of Rb and Sr in different organs of osnip3;1 knockout lines and their wild types.
Supplemental Figure S10. Phenotype of lsi1 knockout line and its wild type at different B concentrations.
Supplemental Figure S11. 10B concentration in the root tips (0 cm to 0.5 cm) of osnip3;1 mutants and their wild types.
Supplemental Figure S12. Response of Lsi1 expression to different B concentrations.
Acknowledgments
We thank Junpei Takano for providing 11B and 10B compounds and Sanae Rikiishi for ICP-MS determination.
Footnotes
This work was supported by Grant-in-Aid for Specially Promoted Research (JSPS KAKENHI grant no. 16H06296 to J.F.M. and 15H04469 to N.Y.).
Articles can be viewed without a subscription.
References
- Bienert GP, Schüssler MD, Jahn TP (2008) Metalloids: essential, beneficial or toxic? Major intrinsic proteins sort it out. Trends Biochem Sci 33: 20–26 [DOI] [PubMed] [Google Scholar]
- Brown PH, Shelp BJ (1997) Boron mobility in plants. Plant Soil 193: 85–101 [Google Scholar]
- Chatterjee M, Tabi Z, Galli M, Malcomber S, Buck A, Muszynski M, Gallavotti A (2014) The boron efflux transporter ROTTEN EAR is required for maize inflorescence development and fertility. Plant Cell 26: 2962–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y, Mitani N, Yamaji N, Ma JF (2009) HvLsi1 is a silicon influx transporter in barley. Plant J 57: 810–818 [DOI] [PubMed] [Google Scholar]
- Dell B, Huang LB (1997) Physiological response of plants to low boron. Plant Soil 193: 103–120 [Google Scholar]
- Dordas C, Brown PH (2001) Evidence for channel mediated transport of boric acid in squash (Cucurbita pepo). Plant Soil 235: 95–103 [Google Scholar]
- Durbak AR, Phillips KA, Pike S, O’Neill MA, Mares J, Gallavotti A, Malcomber ST, Gassmann W, McSteen P (2014) Transport of boron by the tassel-less1 aquaporin is critical for vegetative and reproductive development in maize. Plant Cell 26: 2978–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick KL, Reid RJ (2009) The involvement of aquaglyceroporins in transport of boron in barley roots. Plant Cell Environ 32: 1357–1365 [DOI] [PubMed] [Google Scholar]
- Hanaoka H, Uraguchi S, Takano J, Tanaka M, Fujiwara T (2014) OsNIP3;1, a rice boric acid channel, regulates boron distribution and is essential for growth under boron-deficient conditions. Plant J 78: 890–902 [DOI] [PubMed] [Google Scholar]
- Huang L, Bell RW, Dell B (2001) Boron supply into wheat (Triticum aestivum L. cv. Wilgoyne) ears whilst still enclosed within leaf sheaths. J Exp Bot 52: 1731–1738 [PubMed] [Google Scholar]
- Kumar K, Mosa KA, Chhikara S, Musante C, White JC, Dhankher OP (2014) Two rice plasma membrane intrinsic proteins, OsPIP2;4 and OsPIP2;7, are involved in transport and providing tolerance to boron toxicity. Planta 239: 187–198 [DOI] [PubMed] [Google Scholar]
- Kuppelwieser H, Feller U (1991) Transport of Rb and Sr to the ear in mature, excised shoots of wheat - effects of temperature and stem length on Rb removal from the xylem. Plant Soil 132: 281–288 [Google Scholar]
- Leonard A, Holloway B, Guo M, Rupe M, Yu G, Beatty M, Zastrow-Hayes G, Meeley R, Llaca V, Butler K, Stefani T, Jaqueth J, et al. (2014) tassel-less1 encodes a boron channel protein required for inflorescence development in maize. Plant Cell Physiol 55: 1044–1054 [DOI] [PubMed] [Google Scholar]
- Liu K, Liu LL, Ren YL, Wang ZQ, Zhou KN, Liu X, Wang D, Zheng M, Cheng ZJ, Lin QB, Wang JL, Wu FQ, et al. (2015) Dwarf and tiller-enhancing 1 regulates growth and development by influencing boron uptake in boron limited conditions in rice. Plant Sci 236: 18–28 [DOI] [PubMed] [Google Scholar]
- Loomis WD, Durst RW (1992) Chemistry and biology of boron. Biofactors 3: 229–239 [PubMed] [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M, (2006) A silicon transporter in rice. Nature 440: 688–691 [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, (2015) A cooperative system of silicon transport in plants. Trends Plant Sci 20: 435–442 [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ, (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA 105: 9931–9935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H. (1995). Mineral Nutrition of Higher Plants, 2nd Ed, Academic Press, San Diego, CA [Google Scholar]
- Marschner P. (2012). Mineral Nutrition of Higher Plants, 3rd Ed, Academic Press, London, United Kingdom [Google Scholar]
- Matoh T. (1997) Boron in plant cell walls. Plant Soil 193: 59–70 [Google Scholar]
- Matoh T, Ishigaki K, Ohno K, Azuma J (1993). Isolation and characterization of a boron-polysaccharide complex from radish roots. Plant Cell Physiol 34: 639–642 [Google Scholar]
- Matoh T, Ochiai K (2005) Distribution and partitioning of newly taken-up boron in sunflower. Plant Soil 278: 351–360 [Google Scholar]
- Marentes E, Shelp BJ, Vanderpool RA, Spiers GA (1997) Retranslocation of boron in broccoli and lupin during early reproductive growth. Physiol Plant 100: 389–399 [Google Scholar]
- Mitani N, Chiba Y, Yamaji N, Ma JF (2009) Identification and characterization of maize and barley Lsi2-like silicon efflux transporters reveals a distinct silicon uptake system from that in rice. Plant Cell 21: 2133–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani N, Yamaji N, Ma JF (2008) Characterization of substrate specificity of a rice silicon transporter, Lsi1. Pflugers Arch 456: 679–686 [DOI] [PubMed] [Google Scholar]
- Miwa K, Takano J, Omori H, Seki M, Shinozaki K, Fujiwara T (2007) Plants tolerant of high boron levels. Science 318: 1417. [DOI] [PubMed] [Google Scholar]
- Nable RO, Banuelos GS, Paull JG (1997) Boron toxicity. Plant Soil 193: 181–198 [Google Scholar]
- Nakagawa Y, Hanaoka H, Kobayashi M, Miyoshi K, Miwa K, Fujiwara T (2007) Cell-type specificity of the expression of Os BOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. Plant Cell 19: 2624–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MA, Eberhard S, Albersheim P, Darvill AG (2001) Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 294: 846–849 [DOI] [PubMed] [Google Scholar]
- Reid R. (2007) Identification of boron transporter genes likely to be responsible for tolerance to boron toxicity in wheat and barley. Plant Cell Physiol 48: 1673–1678 [DOI] [PubMed] [Google Scholar]
- Reid R. (2014) Understanding the boron transport network in plants. Plant Soil 385: 1–13 [Google Scholar]
- Schnurbusch T, Hayes J, Hrmova M, Baumann U, Ramesh SA, Tyerman SD, Langridge P, Sutton T (2010) Boron toxicity tolerance in barley through reduced expression of the multifunctional aquaporin HvNIP2;1. Plant Physiol 153: 1706–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao JF, Yamaji N, Shen RF, Ma JF (2017) The key to Mn homeostasis in plants: regulation of Mn transporters. Trends Plant Sci 22: 215–224 [DOI] [PubMed] [Google Scholar]
- Shelp BJ, Marentes E, Kitheka AM, Vivekanandan P (1995) Boron mobility in plants. Physiol Plant 94: 356–361 [Google Scholar]
- Shorrocks VM. (1997) The occurrence and correction of boron deficiency. Plant Soil 193: 121–148 [Google Scholar]
- Sutton T, Baumann U, Hayes J, Collins NC, Shi BJ, Schnurbusch T, Hay A, Mayo G, Pallotta M, Tester M, Langridge P (2007) Boron-toxicity tolerance in barley arising from efflux transporter amplification. Science 318: 1446–1449 [DOI] [PubMed] [Google Scholar]
- Takano J, Miwa K, Fujiwara T (2008) Boron transport mechanisms: collaboration of channels and transporters. Trends Plant Sci 13: 451–457 [DOI] [PubMed] [Google Scholar]
- Takano J, Miwa K, Yuan L, von Wirén N, Fujiwara T (2005) Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc Natl Acad Sci USA 102: 12276–12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Noguchi K, Yasumori M, Kobayashi M, Gajdos Z, Miwa K, Hayashi H, Yoneyama T, Fujiwara T (2002) Arabidopsis boron transporter for xylem loading. Nature 420: 337–340 [DOI] [PubMed] [Google Scholar]
- Takano J, Tanaka M, Toyoda A, Miwa K, Kasai K, Fuji K, Onouchi H, Naito S, Fujiwara T (2010) Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc Natl Acad Sci USA 107: 5220–5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Wada M, Ludewig U, Schaaf G, von Wirén N, Fujiwara T (2006) The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18: 1498–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Yamagami M, Noguchi K, Hayashi H, Fujiwara T (2001) Preferential translocation of boron to young leaves in Arabidopsis thaliana regulated by the BOR1 gene. Soil Sci Plant Nutr 47: 345–357 [Google Scholar]
- Tanaka M, Sotta N, Yamazumi Y, Yamashita Y, Miwa K, Murota K, Chiba Y, Hirai MY, Akiyama T, Onouchi H, Naito S, Fujiwara T (2016) The minimum open reading frame, AUG-stop, induces boron-dependent ribosome stalling and mRNA degradation. Plant Cell 28: 2830–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Takano J, Chiba Y, Lombardo F, Ogasawara Y, Onouchi H, Naito S, Fujiwara T (2011) Boron-dependent degradation of NIP5;1 mRNA for acclimation to excess boron conditions in Arabidopsis. Plant Cell 23: 3547–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Wallace IS, Takano J, Roberts DM, Fujiwara T (2008) NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. Plant Cell 20: 2860–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji N, Ma JF (2017) Node-controlled allocation of mineral elements in Poaceae. Curr Opin Plant Biol 39: 18–24 [DOI] [PubMed] [Google Scholar]
- Yamaji N, Ma JF (2007) Spatial distribution and temporal variation of the rice silicon transporter Lsi1. Plant Physiol 143: 1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji N, Ma JF (2014) The node, a hub for mineral nutrient distribution in graminaceous plants. Trends Plant Sci 19: 556–563 [DOI] [PubMed] [Google Scholar]
- Yamaji N, Mitani N, Ma JF (2008) A transporter regulating silicon distribution in rice shoots. Plant Cell 20: 1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji N, Sakurai G, Mitani-Ueno N, Ma JF (2015) Orchestration of three transporters and distinct vascular structures in node for intervascular transfer of silicon in rice. Proc Natl Acad Sci USA 112: 11401–11406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji N, Sasaki A, Xia JX, Yokosho K, Ma JF (2013) A node-based switch for preferential distribution of manganese in rice. Nat Commun 4: 2442. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Chen H, He M, Zhao Z, Cai H, Ding G, Shi L, Xu F (2017) The boron transporter BnaC4.BOR1;1c is critical for inflorescence development and fertility under boron limitation in Brassica napus. Plant Cell Environ 40: 1819–1833 [DOI] [PubMed] [Google Scholar]