Recent advances in understanding photosynthetic responses to dynamic light environments reveal opportunities to improve crop plant photosynthetic efficiency.
Abstract
Rapidly changing light conditions can reduce carbon gain and productivity in field crops because photosynthetic responses to light fluctuations are not instantaneous. Plant responses to fluctuating light occur across levels of organizational complexity from entire canopies to the biochemistry of a single reaction and across orders of magnitude of time. Although light availability and variation at the top of the canopy are largely dependent on the solar angle and degree of cloudiness, lower crop canopies rely more heavily on light in the form of sunflecks, the quantity of which depends mostly on canopy structure but also may be affected by wind. The ability of leaf photosynthesis to respond rapidly to these variations in light intensity is restricted by the relatively slow opening/closing of stomata, activation/deactivation of C3 cycle enzymes, and up-regulation/down-regulation of photoprotective processes. The metabolic complexity of C4 photosynthesis creates the apparently contradictory possibilities that C4 photosynthesis may be both more and less resilient than C3 to dynamic light regimes, depending on the frequency at which these light fluctuations occur. We review the current understanding of the underlying mechanisms of these limitations to photosynthesis in fluctuating light that have shown promise in improving the response times of photosynthesis-related processes to changes in light intensity.
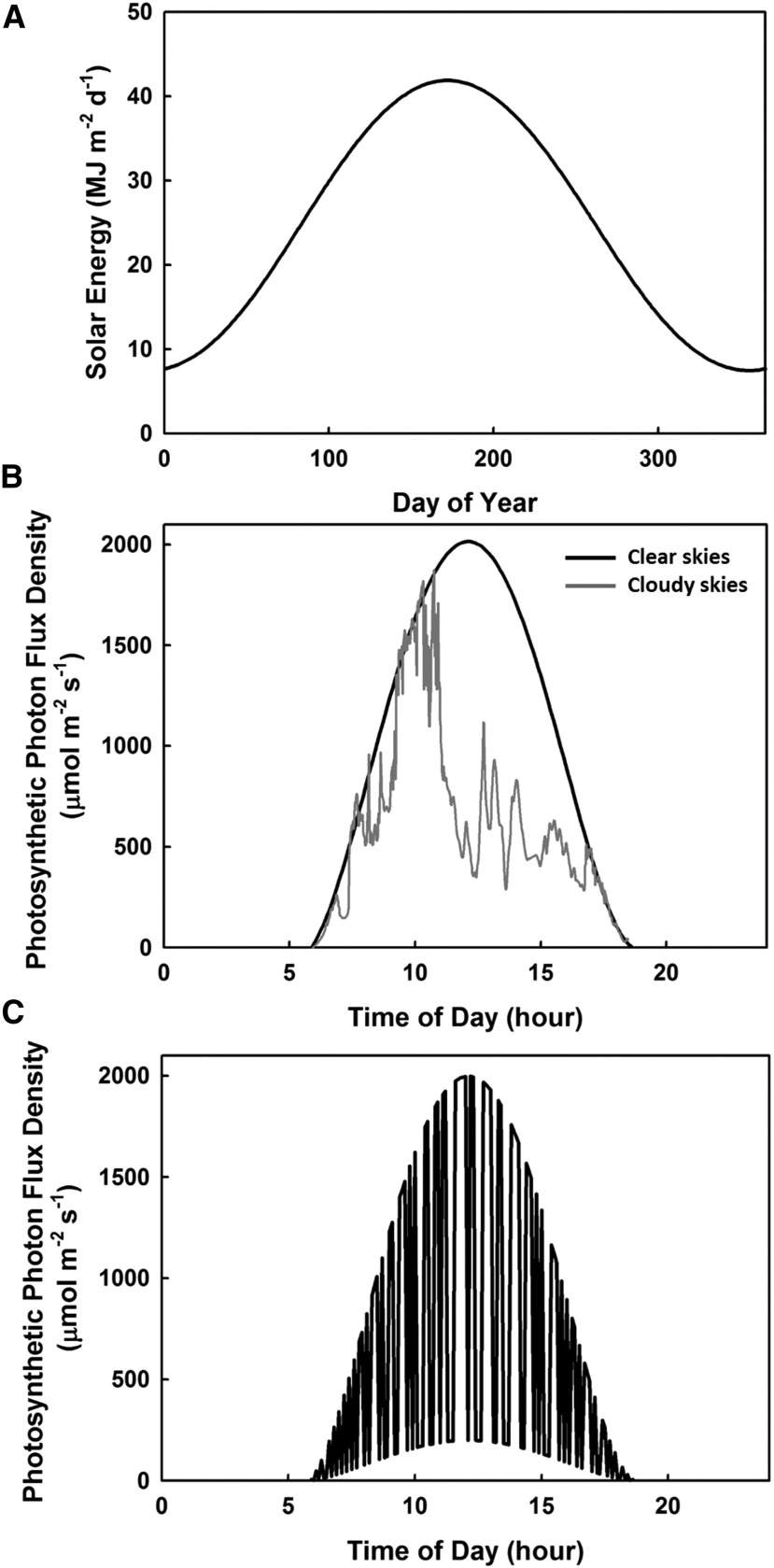
As sessile organisms, plants must respond to dynamically and rapidly changing environmental conditions. Light intensity is the most dynamic condition to which plants must respond and can change at time scales from a season to less than 1 s (Assmann and Wang, 2001). The maximum amount of light incident at any point in time for a given area of ground is determined seasonally (Fig. 1A), but within seasonal variation, there is also variation as a function of the time of day from complete darkness to full sunlight (Fig. 1B). Clouds can substantially reduce incident light, and their intermittency introduces dynamic intensity changes (Fig. 1B). Atmospheric aerosols, which are a complex and dynamic mixture of solid and liquid particles from natural and anthropogenic sources, also can interact with the Earth’s radiation budget and climate directly by reflecting light back into space and indirectly by changing how the clouds reflect and absorb sunlight. These sources of variation in incident light upon a canopy can have a large impact on canopy photosynthesis because the upper canopy drives ∼75% of crop canopy carbon gain (Long et al., 2006). While the amount of sunlight reaching lower leaves within a canopy depends on many factors, sunflecks provide the majority of light in the lower canopy (Fig. 1C). Despite the transient nature of sunflecks, they can provide up to 90% of the available light in lower layers of dense canopies, highlighting the importance of harnessing these rapid fluctuations of light for canopy productivity (Chazdon, 1988; Knapp and Smith, 1989; Pearcy, 1990; Chazdon and Pearcy, 1991; Way and Pearcy, 2012).
Figure 1.
Dynamics of light intensity above and within crop canopies. A, Maximum solar energy incident upon a canopy over the course of a year at 50°N. B, Light intensity at the top of a canopy on a clear sunny day (black line) and on a day with intermittent cloud cover (gray line). C, Light reaching a midcanopy leaf on a clear sunny day. Graphs are based on SURFRAD data from Bondville, Illinois (40°N latitude; B), and Zhu et al. (2004; C).
The importance of within-canopy photosynthetic performance on crop photosynthetic performance has been long recognized (Ort and Baker, 1988), and there is a growing realization of the contribution from within-canopy fluctuating light on crop productivity (Murchie et al., 2009; Lawson et al., 2012). For example, concurrent to many reviews focusing on the effects of sunflecks on forest understories (Chazdon, 1988; Chazdon and Pearcy, 1991; Pearcy and Pfitsch, 1995; Way and Pearcy, 2012), there have been advances in examining the same illumination dynamics in major crop species such as soybean (Glycine max; Pearcy et al., 1990, 1997), rice (Oryza sativa; Nishimura et al., 1998, 2000), and maize (Zea mays; Wang et al., 2008) using experimental and modeling approaches. Given the importance of dynamic light in crop canopies, various light-sensitive factors have been identified as targets to improve crop photosynthesis and productivity in fluctuating light conditions. For example, changes in canopy and leaf morphology can improve sunfleck abundance and distribution throughout canopy layers, while stomatal opening/closing kinetics determine carbon supply and water use during these transient periods of light. Delays in C3 cycle enzyme activation reduce the efficiency with which available CO2 is used during transitions to high light, and the slow relaxation of photoprotection lowers light use efficiency upon transitions to low light. The coordination of C3 and C4 cycles in C4 crops presents an additional layer of complexity during dynamic light conditions. Although large metabolite pools may aid C4 photosynthesis in acclimating rapidly to fluctuating light, some evidence suggests the contrary. Thus, the mechanisms of these processes are currently being studied in more detail under fluctuating light conditions and are the focus of this Update.
SEEING THE SUN THROUGH THE TREES
The degree of variation in light intensity incident on a given leaf is strongly dependent on the structure of the canopy around it, especially within the dense canopy structures typical of mature crop stands. Light variation within the canopy is affected by leaf shading by other leaves, which varies rapidly (Pearcy et al., 1990) and depends on a host of environmental, physiological, and morphological factors. As compared with forests, crop canopies have steeper angles of light penetration due to their short, dense canopies, resulting in fewer partially shaded regions. Thus, a large proportion of within-canopy fluctuating illumination comes from high-intensity, lower-canopy sunflecks, especially in crops, such as soybean, as compared with forest canopies (Pearcy et al., 1990). Although the total number of sunflecks may decrease as canopies become denser, sunflecks still contribute a large percentage of total light intercepted by the canopy, even with relatively dense leaf area index values greater than five. Leaf arrangement affects the abundance of sunflecks in lower canopies. The quantity of sunflecks decreases with greater leaf clumping in modeled, artificial, and experimentally manipulated tree and crop canopies under a fixed leaf area index (Chen and Black, 1992).
Leaf angle also can determine sunfleck frequency depending on what proportion of incoming light is diffuse. For example, leaf angle and orientation in rice influences the dynamic nature of incident light under sunny but not overcast diffuse light conditions (Nishimura et al., 1998). An increased proportion of diffuse light allows the penetration of light to deeper layers of a crop canopy, which improves light use efficiency and photosynthesis at the canopy level (Sinclair and Muchow, 1999; Roderick et al., 2001; Li et al., 2014). Diffuse light also reduces the temporal variation in light intensity, thus reducing the relative contribution of sunflecks to canopy photosynthesis and dampening the effects of fluctuating light on canopies (Pearcy and Pfitsch, 1995; Way and Pearcy, 2012; Li et al., 2016). The relative proportion of light incident as either sunflecks or diffuse light varies depending on canopy structure and depth. In layers of a soybean canopy where sunflecks occur, sunflecks can account for 20% to 93% of the total available light on cloudless days, with the remainder being diffuse (Pearcy et al., 1990). However, there is evidence that radiation incident on Earth’s surface is becoming more diffuse as a result of anthropogenic aerosol emission and changes in weather patterns, resulting in a global dimming of global surface radiation and possible changes to primary productivity (Mercado et al., 2009; Wild, 2009). Therefore, while diffuse light is important to overall canopy photosynthesis, it is not a focus of this review on fluctuating light in crop canopies other than to highlight its general importance and mention that it serves to dampen the impact of sunflecks.
It has long been recognized that wind plays an integral role in driving within-canopy light variation, but its effects are difficult to model, given the static and averaging nature of previous canopy models when representing plant structure and carbon assimilation. Recent advances in ray-tracing algorithms facilitated by improved imaging and computational abilities have enabled models with greater ability to account for the actual structure of a crop canopy and more precise localization of light regimes (Song et al., 2013; Burgess et al., 2015). These models have been leveraged to produce a representation of the impact of wind speed, variability, and direction on intercanopy radiation regimes and the net assimilation of a wheat canopy (Burgess et al., 2016). This work reveals that an increase in light penetration into the canopy due to wind-driven canopy movement can increase total canopy carbon gain by up to 17% and raises interesting possibilities for the improvement of canopy photosynthesis through canopy structural modifications.
Individual leaf morphology, diurnal response, and ultrastructure also can impact the response of crop plants to variable light regimes. For example, long-term exposure to either high or low light affects aspects of leaf development and morphology, such as size, thickness, and shape, that impact light absorption (Boardman, 1977). Diurnal changes in light can affect leaf movement and orientation, allowing leaves to track the movements of the sun across the sky or avoid direct sunlight in times of stress (Raven, 1989; Ehleringer and Forseth, 1990). In a matter of minutes following an alteration in light intensity, chloroplasts rearrange within cells, from the upper surfaces of the cell in low light to the sides of the cell in high light (Wada, 2013). These effects and responses are important considerations for how crop plants respond to seasonal, diurnal, and transient changes in light intensity at the leaf and canopy levels, with biochemical considerations constituting the focus of the remaining discussion.
THE PHYSIOLOGICAL POLITICS OF STOMATA
Stomata present the initial limitation in both C3 and C4 plants by controlling the entry of CO2 into the leaf and, therefore, the substrate supply for photosynthesis. Stomata also are responsible for balancing CO2 uptake for photosynthesis with water loss from the leaf through transpiration. Stomatal closure conserves water when there is no need for CO2 entry into the leaf for photosynthesis, but stomata must open to provide the CO2 necessary for the carbon reduction cycle (Lawson and Blatt, 2014). The opening and closing of stomata are regulated by guard cells, the cells that surround the stomatal pore and regulate the pore aperture. Blue and red light-mediated processes result in an influx of solute and, therefore, water into the guard cells, causing an increase in turgor that opens the stomata. When solute concentrations decline, guard cells lose water, resulting in stomata closure. Other external signals also are related to stomata aperture control, including CO2 concentration, humidity, temperature, and abscisic acid concentrations (Lawson, 2009).
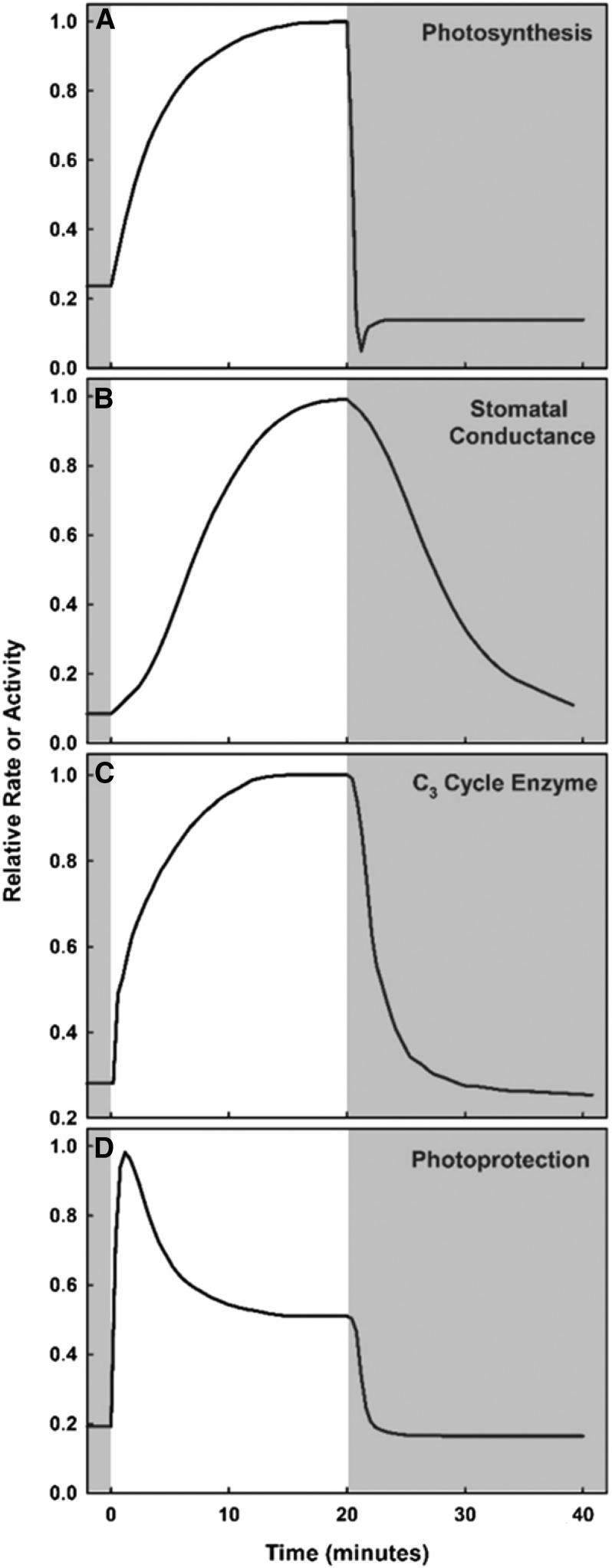
While stomatal conductance and photosynthesis are generally well coordinated in steady-state conditions (Wong et al., 1979; Farquhar and Sharkey, 1982), the lag in photosynthetic response during transitions from low to high light (Fig. 2A) often is due to an insufficient supply of CO2 needed for the carbon cycle. This is because the opening of stomata occurs at a much slower rate than the initial up-regulation of photosynthetic electron transport (Fig. 2B). In times of rapidly changing light conditions, this limitation can represent a substantial inefficiency in photosynthesis and, therefore, crop productivity. A recent study by McAusland et al. (2016) measured a 10% to 15% limitation to photosynthesis across several C3 and C4 crop species during the time it took leaves to reach steady-state conditions upon transfer from low light to high light. However, the limitation to carbon assimilation by stomatal conductance during transitions from low to high light can vary depending on the initial stomatal conductance in low light. If stomatal conductance is greater in low light, which might occur if water is not limiting (Lawson et al., 2012; Way and Pearcy, 2012) and was the case in the McAusland et al. (2016) study, stomatal limitation is likely to be less during the transition, whereas lower stomatal conductance in low light, which might be evident in water-limited conditions (Knapp and Smith, 1989), likely leads to much higher stomatal limitation to photosynthetic recovery. It should be noted that, in fluctuating light, greater stomatal conductance at low light also could alleviate temperature stress via evaporative cooling upon sudden exposure to high light intensity (Schymanski et al., 2013) but at the expense of water use efficiency.
Figure 2.
Schematic showing the relative induction and relaxation rates of photosynthesis-related processes during changes in light intensity. Relative rates of photosynthesis (A), stomatal conductance (B), C3 cycle enzymes (C), and NPQ (D) are shown as functions of time during transitions from low light (gray background) to high light (white background) and from high light to low light. Curves are based on data from McAusland et al. (2016), Sassenrath-Cole and Pearcy (1994), and Kromdijk et al. (2016).
A sluggish stomatal response when leaves are rapidly shaded by a cloud or overhead foliage also can cause excessive water losses due to a lack of coordination between assimilatory demand for CO2 and stomatal conductance. Thus, when stomata do not close rapidly enough, water is lost disproportionately relative to carbon gained, which reduces water use efficiency during the transition (Lawson and Blatt, 2014; McAusland et al., 2016). Upon low to high light transitions in some species, stomata conductance continues to climb, even after maximum rates of photosynthesis have been reached; thus, these species overshoot the rate of stomatal conductance that achieves maximum photosynthesis, which also reduces water use efficiency (McAusland et al., 2016). Some C3 crops bred for high photosynthesis rates display lower water use efficiency in fluctuating light (McAusland et al., 2016), likely because selection for yield in non-water-limiting conditions does not penalize accessions with inferior water use efficiencies (Fischer et al., 1998; Koester et al., 2016). Given that crop productivity and yield often are water limited and are likely to become more so (Ort and Long, 2014), improving stomatal closure kinetics and regulation in response to fluctuating light regimes could substantially improve canopy water use efficiency and yield.
Careful coordination of stomatal kinetics and photosynthesis are needed to both ensure CO2 availability for photosynthesis and limit water loss, especially for C3 crops. While some studies suggest that many small stomata could achieve this end (Drake et al., 2013; Raven, 2014), other research suggests that stomata type and biochemistry may be more influential. A smaller change in turgor can have larger effects on stomatal aperture in dumbbell- versus elliptical-shaped guard cells, leading to more rapid opening/closing kinetics (Hetherington and Woodward, 2003; McAusland et al., 2016). In addition, equal and opposite transfer of turgor from subsidiary epidermal cells to guard cells during opening and closing, rather than constant pressure in the subsidiary cells, not only enhances stomatal aperture by allowing displacement of the subsidiary cell but also increases the rate of stomatal movement (Franks and Farquhar, 2007; Raissig et al., 2017). The rate of solute transport into and out of guard cells also presents potential targets for modification to obtain more rapid opening upon transitions to high or low light (Lawson and Blatt, 2014; Wang et al., 2014b), but the intuitive solution of increasing the ratio of individual ion channels per surface area often has the opposite effect (Wang et al., 2014b, 2014c). However, modeling suggests that manipulating the gating of these ion channels may have more favorable consequences on stomatal kinetics (Wang et al., 2014b).
Despite the complexity and lack of established approaches to manipulate stomatal kinetics, recent studies have shown that variation exists within and among certain species that could guide future engineering efforts or enable optimization by selective breeding. McAusland et al. (2016) showed substantial variation in stomatal responses across 13 crop species, and measurements conducted by Qu et al. (2016) showed variation in stomatal kinetics among 204 rice accessions. Noncrop species also may provide clues for ways in which to improve stomatal kinetics. For example, unlike the delayed increase in stomatal conductance compared with photosynthesis seen in several crops (McAusland et al., 2016), the stomatal response to increased light of the C4 shade-tolerant Microstegium vimineum is faster than the photosynthetic response following low-light acclimation (Horton and Neufeld, 1998). Other traits may be important to study in crops, such as the degree of anisohydry, or the tendency of stomata to remain open for CO2 entry into the leaf despite a decline in leaf water content, across species, as stomatal kinetics are more rapid in tree species with more anisohydric tendencies (Meinzer et al., 2017). In addition, variation could be explained by differences in the speed of signaling components driving the mechanistic changes. Therefore, identifying the sources of variation in stomatal kinetics will be crucial for mechanistic understanding and further engineering efforts to increase the rate and coordination of stomatal opening and closing with light intensity.
Mesophyll conductance presents an additional limitation to carbon supply to the chloroplast during fluctuating light. Mesophyll conductance varies within minutes when leaves are exposed to changes in light, temperature, and CO2 concentration (Flexas et al., 2007, 2008; Tholen et al., 2008; Evans and von Caemmerer, 2013), and more recent research has shown reductions in mesophyll conductance when plants are grown in fluctuating light conditions (Huang et al., 2015; Vialet-Chabrand et al., 2017). Although the mechanisms for these reductions are not yet understood, aquaporin levels, carbonic anhydrase concentration, and leaf and cell anatomy may contribute to variation in mesophyll conductance (Flexas et al., 2012). In a recent study, growth in fluctuating light resulted in thinner palisade and spongy mesophyll layers and altered cell shape as compared with steady-light conditions (Vialet-Chabrand et al., 2017), but a direct relationship to overall mesophyll conductance was not investigated. Thus, elucidating the mechanisms of mesophyll conductance also is necessary for engineering to ensure sufficient carbon supply to photosynthesis during fluctuating light conditions.
THE CONTROL OF CARBON REDUCTION ENZYMES IN THE FACE OF STOP-AND-GO TRAFFIC
While stomatal and mesophyll conductance kinetics determine the supply of CO2 for photosynthesis during dynamic light conditions, the activation rate of C3 cycle enzymes determines how rapidly available CO2 is reduced to form sugars. Upon the transition from low light to high light, such as occurs when a shaded leaf is exposed to a sunfleck, electron transport responds almost instantaneously. However, the relatively slower induction of the carbon reduction cycle (Fig. 2C) limits photosynthesis, thereby limiting total carbon gain by the leaf and canopy. In tobacco (Nicotiana tabacum), the time needed to reach maximum stomatal conductance in response to light (∼40 min; McAusland et al., 2016) is greater than the time needed to reach maximum Rubisco activation (∼7 min; Hammond et al., 1998) and is likely more limiting in some situations. However, in conditions favoring higher initial stomatal conductance, such as the brief periods between high-frequency sunflecks when biochemical enzymes relax more quickly than stomatal conductance, or in high CO2 concentrations, such as those present in the lower canopy, C3 cycle enzyme regulation likely limits photosynthesis to a greater degree than stomatal conductance (Sassenrath-Cole and Pearcy, 1992; Tomimatsu and Tang, 2012; Graham et al., 2017).
While gene expression and protein synthesis of enzymes involved in carbon reduction determine enzyme concentrations and, therefore, activity at longer time scales, reversible posttranslational modifications are responsible for the activation/deactivation of these enzymes during the more rapid transitions between light and dark conditions or even due to more modest changes in light intensity. The key enzymes controlling the C3 cycle during light intensity transitions include Rubisco, Rubisco activase (Rca), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Fru-1,6-bisphosphatase (FBPase), sedoheptulose-1,7-bisphosphatase (SBPase), and phosphoribulokinase (PRK).
Before Rubisco can catalyze the carboxylation reaction of photosynthesis, the enzyme must undergo a series of reactions to reach its activated state. A Lys residue in Rubisco must be carbamylated by CO2 and then stabilized by Mg2+ before it combines ribulose-1,5-bisphosphate with CO2 in photosynthesis (or oxygen in photorespiration). However, the activation process can be inhibited by the binding of sugar phosphates, such as ribulose-1,5-bisphosphate, to Rubisco’s active site, which prevents carbamylation. Thus, a separate process is needed to free the Rubisco active site for activation and is achieved through the activity of Rca. Rca hydrolyzes ATP to remove sugar phosphates from Rubisco, thus allowing Rubisco to proceed through the reactions necessary for full carbamylation and activation (Wang and Portis, 1992; Portis, 2003). But what activates Rca? In many species, there are two forms of Rca: a longer α-isoform, which contains two Cys residues that, when reduced by thioredoxin, render the enzyme less sensitive to inhibition by ADP and, when oxidized, render the enzyme more strongly inhibited by ADP; and a shorter β-isoform, which lacks this redox regulatory component. Therefore, as light levels increase, the ATP/ADP ratio and the redox poise of thioredoxin increase levels of activating Rca in species containing the α-isoform. However, this may not be the only mechanism for enabling Rca to function, as some species contain only the shorter β-isoform version of Rca, which is not directly redox regulated but responds to ATP/ADP ratios in a species-dependent manner and also is able to activate Rubisco in light (Portis, 2003; Portis et al., 2008).
Rubisco and its activation by Rca are important potential targets for crop improvement, especially with regard to photosynthesis during changes in the light environment (Parry et al., 2013; Carmo-Silva et al., 2015). While Rca quantity is not usually considered limiting to Rubisco activation unless reduced by more than 60%, these results only refer to steady-state conditions (Carmo-Silva et al., 2015). Under dynamic conditions, tobacco expressing antisense Rca constructs shows a linear response between Rca content and the initial slope of carbon fixation during the transition from low to high light under a broad range of Rca contents, indicating that Rca indeed limits the induction of carbon fixation (Hammond et al., 1998). However, the reduction in carbon assimilation upon the transition from high to low light is not sensitive to Rca content (Hammond et al., 1998). A modeling study by Mott and Woodrow (2000) shows that a greater allocation of resources to Rca at the expense of Rubisco benefits the induction time of photosynthesis in fluctuating light conditions. This greater investment in Rca is a typical characteristic of shade leaves (von Caemmerer and Quick, 2000), which ensures more efficient use of available light in the form of sunflecks (Pearcy, 1990). Experimental overexpression of Rca in rice increases Rubisco activation and the rate of photosynthesis induction upon the transition from low to high light (Fukayama et al., 2012; Yamori et al., 2012). However, overexpression of Rca in nonfluctuating light conditions leads to a decrease in total Rubisco content and a small reduction in net carbon assimilation (Fukayama et al., 2012), confirming that Rca is only limiting in fluctuating light conditions (Yamori et al., 2012). Modifying the regulation of Rca may present a more attractive option to increasing the rate of photosynthetic induction upon light transitions. In an Arabidopsis transformant containing only the non-redox-regulated Rca β-isoform, which is insensitive to physiologically relevant ATP/ADP ratios, Rca remains activated in low light, resulting in an instantaneous activation of Rubisco upon the transition to high light. This leads to faster rates of photosynthetic induction, which is correlated with large increases in biomass in fluctuating light compared with nonfluctuating light conditions that are not seen in the wild type (Carmo-Silva and Salvucci, 2013). The presence of two Rca isoforms with different regulatory properties raises the question of why Rca is regulated at all if constant activation is beneficial for carbon assimilation. The answer may lie, at least in part, in the observation that some inhibitors of Rubisco, like carboxy-d-arabinitol 1-phosphate, bind Rubisco during dark periods and may help protect against proteolysis (Andralojc et al., 1994; Khan et al., 1999). Therefore, given the cost of Rubisco biosynthesis, efforts to improve net assimilation through constant Rubisco activation should examine the impact on Rubisco turnover as well. In addition, inactivation of Rca in the dark would limit unnecessary ATP hydrolysis to maintain Rca activation under non-carbon-fixing conditions.
As with the α-isoform of Rca, numerous other enzymes of the C3 cycle are at least partially regulated by the ferredoxin-thioredoxin system, including the key enzymes involved in the carbon reduction cycle: GAPDH, FBPase, SBPase, and PRK. Enzyme activation by the chloroplast ferredoxin-thioredoxin system can take minutes for full activation when leaves are transferred from low to high light, thereby creating a lag in reaching full photosynthetic capacity (Sassenrath-Cole et al., 1994; Sassenrath-Cole and Pearcy, 1994). While there are several isoforms and subtypes of thioredoxins present in plant cells, recent studies have identified numerous thioredoxin proteins responsible for the activation of C3 cycle enzymes in the light. Specific f-type thioredoxin proteins have been identified in the direct activation of C3 cycle enzymes (Thormählen et al., 2013; Yoshida et al., 2015; Naranjo et al., 2016). In addition, m-type thioredoxins have been shown to have no effect on photosynthesis in steady-state conditions but alter photosynthetic efficiency in fluctuating light and, therefore, are essential in rapidly changing light conditions (Thormählen et al., 2017).
Additional systems have been shown to contribute to the regulation of carbon reduction enzymes in fluctuating light. The NADPH-thioredoxin reductase C (NTRC) pathway, which likely uses NADPH indirectly as an electron donor, plays a distinct but cooperative role with the ferredoxin-thioredoxin system in modulating chloroplast functions and in regulating FBPase and SBPase activity (Yoshida and Hisabori, 2016). The NTRC pathway also plays a key role in maintaining photosynthetic efficiency in fluctuating light by controlling the NADPH redox status of the stroma (Thormählen et al., 2017). Dissociation from large protein complexes also is involved in C3 cycle enzyme activation. Although PRK and GAPDH are reduced directly by thioredoxins (Marri et al., 2009), they are not fully activated until dissociated from the complex they form with a small protein, CP12. The level of dissociation correlates well with light levels, thus allowing a more rapid activation/deactivation response during dynamic light conditions than redox regulation alone (Howard et al., 2008; Marri et al., 2009). However, the presence of this complex is not universal across species (Howard et al., 2011), suggesting that the regulation of these enzymes may be even more intricate and diverse. Reversible phosphorylation also may contribute to enzyme regulation, as Rca phosphorylation seems to occur in dark but not light conditions (Kim et al., 2016). While the effects of phosphorylation on Rca activity are not known yet, this suggests a potential method of regulation for carbon reduction enzymes in addition to the thioredoxin-mediated regulation discussed above. Other posttranslational regulatory mechanisms directly affect Rubisco (Houtz et al., 2008), such as acetylation (Gao et al., 2016), but their effects in dynamic light have yet to be examined. As more of the key players are identified and the mechanisms by which they regulate carbon metabolism in rapidly changing light conditions are elucidated, they will likely be used to enhance the responsiveness of carbon reduction enzymes in fluctuating light.
IS PHOTOPROTECTION OVERPROTECTIVE?
The photosynthetic response to light is linear at low light intensities but becomes saturated as light increases. In many crops, light saturation occurs by 25% of full sunlight in the absence of other stresses (Long et al., 2006). When leaves are experiencing additional stress or light increases more rapidly than photochemical capacity (e.g. slow responses of stomata and C3 cycle enzymes, as detailed above), light saturation may occur at even lower light levels (Fig. 2D). As a result, leaves at the top of the canopy experience excessive amounts of light during the majority of daylight hours (Ort, 2001). When absorbed light is in excess of photosynthetic capacity, it has the potential to damage photosynthetic proteins and membranes. Thus, plants have evolved various photoprotective mechanisms, the most universal and important of which is called NPQ, which enables chloroplasts to safely dissipate excess light as heat (Niyogi, 1999).
By engaging NPQ in high-light conditions, leaves achieve protection from photodamage without any cost to carbon gain. Photoprotection is beneficial to leaves at high light because it prevents irreversible damage to PSII that must be repaired through protein synthesis. However, when NPQ remains engaged under nonsaturating light conditions, it limits carbon gain by lowering the maximum quantum yield of PSII and, thereby, the quantum efficiency of CO2 assimilation (Long et al., 1994). While NPQ inductions can occur within a few seconds upon the transition to high light, the relaxation process during the transition from high light to low light is much slower and can take minutes to hours to occur. As a result, when plants are in a photoprotected state and light levels decline rapidly to nonsaturating levels, some of the available light that could be safely harnessed for photochemical processes is dissipated as heat and, therefore, wasted (Fig. 2D). For crops grown in the field, fluctuations in light, such as when a leaf shades another leaf due the continuous diurnal change in solar azimuth or when a passing cloud occludes the sun, present opportunities for this inefficiency to significantly limit leaf and canopy carbon gain. In fact, it has been estimated that the slow relaxation of NPQ can limit the daily canopy carbon uptake of crops grown in the field by up to 32% (Zhu et al., 2004).
NPQ is composed of several processes involved with protecting the photosynthetic machinery from excess light. The most rapid of these processes is energy-dependent quenching, or qE, which engages on the scale of seconds to minutes (Müller et al., 2001). qE is initiated when protons accumulate in the lumen, thereby reducing the lumen pH. PsbS, a protein associated with PSII, senses the low pH and signals a conformation change in the LHCII protein (Li et al., 2000; Sacharz et al., 2017). This conformational change occurs in concert with the xanthophyll cycle, through which violaxanthin is converted to zeaxanthin by violaxanthin deepoxidase in high light (Demmig-Adams, 1990). Quenching via state transitions, or qT, also diverts excitation energy away from PSII, engages on a slightly longer time scale (5–10 min) than qE, but accounts for a very small portion of NPQ in higher plants (Horton et al., 1996). In addition to enhancing qE, the zeaxanthin pool size is associated with qZ, or zeaxanthin-dependent quenching (Nilkens et al., 2010), which arises on a slower time scale (10–15 min) than qE and qT. qI is the slowest onset process, requiring up to hours to appear, and often is associated with the accumulation of photodamage to PSII (Müller et al., 2001).
Due to its complexity, manipulations of single facets of NPQ have not been sufficient for improving the relaxation kinetics of NPQ while maintaining full capacity for CO2 assimilation at high light. Overexpression of PsbS increases the induction and relaxation rates and increases the maximum capacity of qE (Li et al., 2002a; Zia et al., 2011; Hubbart et al., 2012), representing a potential benefit to plant biomass under stressful light conditions (Li et al., 2002b) but at a cost to CO2 assimilation under less stressful conditions, as qE remains higher than needed (Hubbart et al., 2012). While an increase in zeaxanthin concentration can improve stress resistance (Johnson et al., 2007), it slows the kinetics of NPQ, demonstrating that zeaxanthin concentration is less important for induction/relaxation rates than the ratio of zeaxanthin to violaxanthin or the deepoxidation state of the xanthophyll cycle (Johnson et al., 2008). A lingering pool of zeaxanthin, which increases the deepoxidation state, may serve as a memory of previous high-light conditions (Murchie et al., 2009) that would delay the relaxation rate of NPQ in anticipation of another high-light event. Overexpression of an ion antiporter helps increase the lumen pH more quickly upon the transfer of leaves to low light, thus speeding up the relaxation of qE (Armbruster et al., 2014). However, overexpression also leads to photoinhibitory effects at high light, thus requiring more sophisticated regulation of the protein for increasing relaxation kinetics. To overcome the limits of altering single processes within NPQ, a recent study by Kromdijk et al. (2016) used a multitarget approach to increase NPQ kinetics by transforming tobacco to overexpress PsbS, violaxanthin deepoxidase, and zeaxanthin epoxidase. The overexpression of violaxanthin deepoxidase and zeaxanthin epoxidase increased the kinetics of the xanthophyll cycle, which led to a lower deepoxidation state, while overexpression of Psbs maintained the amplitude of qE in high light when the deepoxidation state was low. When grown in chambers under fluctuating light, the transformants show increased photosynthetic efficiency and reduced average NPQ compared with the wild type. Moreover, this more complex approach of simultaneously altering the expression of multiple enzymes related to NPQ leads to a 15% increase in plant biomass when grown in field conditions under natural light fluctuations (Kromdijk et al., 2016). Since the mechanisms of NPQ are conserved across most plant species, altering NPQ kinetics will likely lead to increased yields in many other crops.
C4 PHOTOSYNTHESIS: ADDING COMPLEXITY UNDER FLUCTUATING LIGHT
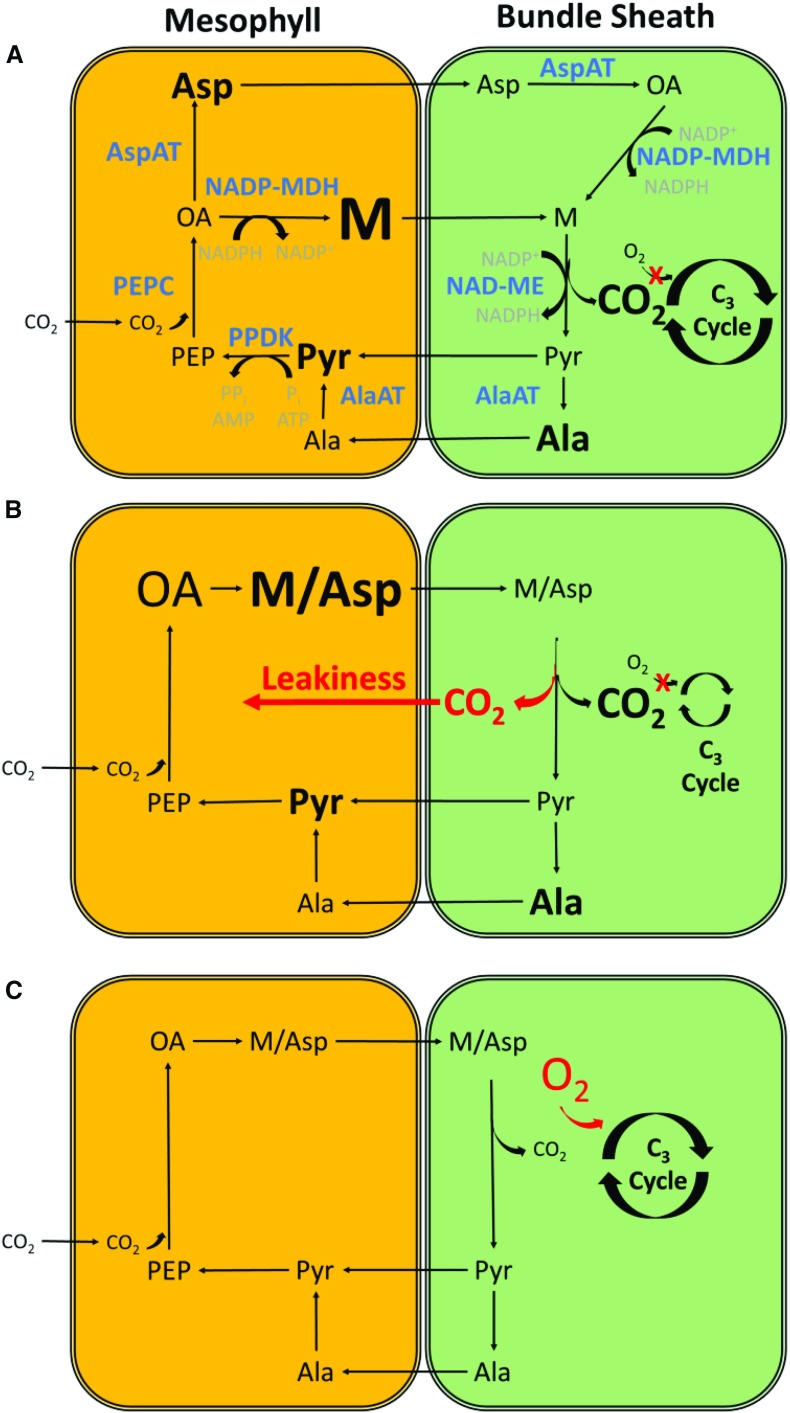
While the processes described above may apply to both C3 and C4 plants, the unique metabolism of C4 plants may further impact their response to the fluctuating light of field conditions. Plants performing C4 photosynthesis are among the most important crop species globally due to the high efficiency with which they can capture and reduce CO2 with decreased water use and nitrogen investment in Rubisco. C4 crops, such as maize, sugarcane (Saccharum officinarum), millet (Pennisetum glaucum), and sorghum (Sorghum bicolor), achieve higher carbon fixation rates by first carboxylating phosphoenolpyruvate (PEP) with atmospheric CO2 to form oxaloacetate via PEP carboxylase (Fig. 3A). The carbon in oxaloacetate is then either reduced with NADPH into malate or converted into Asp before moving into the bundle sheath cell, where either substrate is decarboxylated. Thus, the concentration of CO2 around Rubisco increases, which minimizes photorespiration in the coupled C3 cycle. To maintain the cycle, reduced carbon must be transported from the bundle sheath cells back into the mesophyll cells to replace the carbon transported by the original C4 carbonic acid. This return transport can occur directly via pyruvate produced following malate decarboxylation, via Ala produced from pyruvate through Ala aminotransferase, and/or via the shuttling of 3-phosphoglycerate from the C3 cycle in the bundle sheath cells. PEP is then regenerated from pyruvate at the cost of ATP, which imposes an additional energetic cost to C4 metabolism. C4 species have traditionally been classified by the different decarboxylating enzymes employed (i.e. NADP-ME, NAD-ME, and PEP-CK), but there is a growing consensus that C4 plants like maize use multiple decarboxylation pathways in parallel (Pick et al., 2011; Arrivault et al., 2017). However, there are few examples of a crop plant exclusively using PEP-CK as a decarboxylation enzyme, which suggests that PEP-CK functions predominantly as a supplemental pathway supporting NADP-ME and NAD-ME decarboxylation (Wang et al., 2014a). Thus, only the NADP-ME and NAD-ME pathways are presented here (Fig. 3A).
Figure 3.
Schematic showing the relevant components of C4 biochemistry and the theoretical impacts of light fluctuations on C4 and C3 cycle coordination. A, A simplified diagram shows two types of C4 pathways, NADP-ME and NAD-ME. The PEP-CK pathway is absent. B, A high to low light transition results in a transient overpumping of CO2 into and subsequent CO2 leakage out of the bundle sheath. C, A low to high light transition results in higher rates of Rubisco oxygenation. The relative substrate size represents the concentration gradient between the mesophyll and bundle sheath cells based on measured data (see text). Metabolites are black, enzymes are blue, and cofactors are gray. Terms are abbreviated as follows: oxaloacetate (OA), malate (M), pyruvate (Pyr), PEP carboxylase (PEPC), NADP-malate dehydrogenase (NADP-MDH), Asp aminotransferase (AspAT), NAD malic enzyme (NAD-ME), Ala aminotransferase (AlaAT), and Pyr phosphate dikinase (PPDK).
The efficiency of C4 photosynthesis depends on the coordination of C4 carbonic acid production within the mesophyll cells with C3 carbon reduction within the bundle sheath cells. There is ample theoretical and experimental evidence indicating that this coordination is impacted to some degree by variations in light regimes. For example, during high to low light transitions, accumulated malate and/or Asp are still available for decarboxylation in the bundle sheath cell, but there is insufficient photophosphorylation to generate the ATP necessary for C3 carbon fixation. This could result in a transient overpumping of CO2 into the bundle sheath cells and subsequent leaking of the CO2 back into the mesophyll cells (Fig. 3B). This leakiness is energetically costly, since PEP must still be regenerated at the cost of ATP without the usual fixation of carbon (von Caemmerer and Furbank, 1999; Sage and McKown, 2006). Transient increases in leakiness are reported in many C4 plants in response to low light shifts (Cousins et al., 2006, 2008; Kubásek et al., 2007; Tazoe et al., 2008; Pengelly et al., 2010), especially under light values lower than 100 µmol m−2 s−1 (Kromdijk et al., 2010; Bellasio and Griffiths, 2014a), although it should be noted that the 13CO2 discrimination assumptions used to interpret earlier reports can overstate the impact of transient light to leakiness (Ubierna et al., 2011, 2013; Kromdijk et al., 2014). While leakiness in the C4 carbon pump results in inefficient carbon fixation and transient decreases in the quantum efficiency of C4 carbon fixation, these losses appear small. But what about low to high light transitions?
The coordination of C4 and C3 cycles is complicated during the transition from low to high light due to the reliance of C4 acid transport into the bundle sheath cell on concentration gradients (Fig. 3C). The transport of C4 acids into bundle sheath cells is thought to occur mainly via diffusion along concentration gradients between the mesophyll and bundle sheath cells, which need to be a minimum of 5 to 10 mm to facilitate rapid transport in C4 metabolism (Hatch and Osmond, 1976). Gradients between the two cell types have been determined experimentally for malate in maize and range between 6 and 88 mm (Leegood, 1985; Stitt and Heldt, 1985; Arrivault et al., 2017), indicating that large active C4 acid pools are required. Therefore, during a low to high light transition, these large C4 acid pools must accumulate to optimal values before the C4 cycle and the C3 cycle are synchronized. Before optimal pool sizes are established for a given light intensity, suboptimal CO2 concentrations near Rubisco would increase rates of oxygenation, leading to increased photorespiration and, thus, incurring the double costs of C4 carbon pumping and C3 photorespiration (Fig. 3C; de Veau and Burris, 1989; von Caemmerer and Furbank, 2003; Sage and McKown, 2006; Bellasio and Griffiths, 2014b). The transport mechanisms returning C3 acids into the mesophyll following decarboxylation in the bundle sheath cell are less clear. Ala and triose phosphate seem to require modest gradients between the mesophyll and bundle sheath cell to facilitate return, but pyruvate shows small to even reverse gradients between the two cell types (Arrivault et al., 2017).
C4 METABOLIC POOL SIZES IN FLUCTUATING LIGHT: A TALE OF TWO HYPOTHESES
The reliance of malate and Asp on gradient-driven transport and the pool sizes that enable this transport lead to two potentially contradictory hypotheses concerning the resilience of C4 photosynthesis during low to high light transitions. One hypothesis, as suggested above, is that C4 photosynthesis is more negatively impacted by fluctuating light than C3 photosynthesis due to the transient incoordination between the C3 and C4 cycles occurring while transport gradients either collapse or reestablish. An alternative hypothesis suggests that the large pool sizes are beneficial from a photoprotective role. Under this hypothesis, C4 metabolism can store excess harvested ATP and NADPH within the large pools of carbon-shuttling intermediates without having to resort to NPQ during transient increases in light energy capture, resulting in a higher quantum yield of photosynthesis and effectively using C4 intermediates transiently as alternative electron acceptors or donors for the coupled C3 cycle (Stitt and Zhu, 2014). The evidence for these contrasting hypotheses is discussed below.
There are several lines of experimental evidence indicating that C4 photosynthesis is less resilient than C3 photosynthesis to rapid fluctuations in light. For example, C4 species relax their photosynthetic capacity more rapidly under decreasing light and take longer to reach high rates of photosynthesis under return to high light, resulting in a decreased ability of C4 plants to rapidly reinduce photosynthesis in response to sunflecks (Chazdon and Pearcy, 1986; Horton and Neufeld, 1998; Sage and McKown, 2006). This is consistent with a requirement to first reestablish large malate or Asp pools upon illumination to achieve the optimal transport of carbon into the bundle sheath. While this may explain why examples of understory or shade-tolerant C4 plants are few, it also suggests that the shaded regions within C4 crop canopies would be less able to take advantage of rapidly fluctuating light regimes and frequent sunflecks that can occur in lower C4 canopies (Tang et al., 1988). This relative difference also is seen in the slower response of maize leaves to simulated sunflecks as compared with soybean (Pons and Pearcy, 1992; Krall and Pearcy, 1993). A direct comparison of C3 and C4 responses to fluctuating light also has shown that plant biomass is reduced to a greater degree in C4 plants (58% reduction) as compared with C3 plants (30%–51% reduction) when grown under dynamic light conditions (Kubásek et al., 2013). Additionally, C4 photosynthetic rates decrease during low-light periods in fluctuating light environments compared with steady-state light conditions, whereas C3 photosynthetic rates increase, and dynamic light regimes decrease the photochemical efficiency of C4 plants more than C3 plants due, in part, to C4 leakiness, which also increases under dynamic light conditions (Kubásek et al., 2013).
Given the above observations, can the large metabolite pools involved in C4 photosynthesis act to buffer energy supply and demand during fluctuating light in a way that confers any advantage to these crop species? While there are many potential advantages to the newly recognized flexibility of C4 photosynthesis to utilize various metabolites with different energy balances (Stitt and Zhu, 2014), we focus here on malate transport, since we have the most data concerning its presence, specifically to estimate the total electron-buffering capacity of malate and its effective time scale. The total active malate pool was recently reported as ∼5 μmol g−1 fresh weight (Arrivault et al., 2017), which translates to 1,500 μmol m−2, assuming a fresh weight specific leaf area of 300 g m−2. Since each malate carries the reductive power of two electrons and four electrons are required to reduce one CO2 molecule, this malate pool contains enough reductive power to reduce ∼750 μmol CO2 m−2, or enough reductant to support the electron demands of carbon fixation occurring at a rate of 50 μmol m−2 s−1 for 15 s. Thus, the active pool of malate could contain a sufficient supply of reductant to buffer against transitions from high to low light, perhaps explaining why greater leakiness is not observed during these transitions, as discussed above. Of course, the estimate of 15 s is an upper boundary for the ability of the active malate pool to supply energy to the C3 cycle during high to low light transitions, since malate transport would slow as pool sizes within the mesophyll and bundle sheath cell come into equilibrium and C3 carbon fixation becomes limited by ATP availability.
Using the same logic, we can estimate the potential of C4 metabolite pools to buffer against rapid shifts from low to high light by acting as electron sinks by determining the immediate capacity for carboxylation and reduction. The total active pool size of metabolites upstream of malate (only PEP, pyruvate, Ala, and 3-PGA; oxaloacetate was not determined) presented by Arrivault et al. (2017) is 3.6 μmol g−1 or ∼1,000 μmol m−2. This represents the capacity to provide alternative electron acceptors for ∼2,000 electrons while the C3 cycle activates and malate pools establish, or the capacity to provide 10 s of alternative electron acceptors, assuming an electron transport rate of 200 e− m−2 s−1 during a low to high light transition without having to initiate NPQ in the bundle sheath. Again, this represents an upper boundary to the possible buffering capacity, since all upstream metabolites would not be immediately transported into the bundle sheath and converted to oxaloacetate. Naturally, both calculations above depend upon the light conditions of Arrivault et al. (2017), which were moderate (∼500 μmol photons m−2 s−1). However, these calculations serve as initial estimates of the impact that gradient formation and pool sizes can have on C4 photosynthesis during light transitions.
Given the above calculations, the buffering capacity of C4 photosynthesis during light fluctuations is expected to be limited to, at maximum, the first 10 to 15 s of a transition between light intensities. This is a much faster time scale than the minute time scales examined in 13CO2 discrimination-based measurements of leakiness (Cousins et al., 2006, 2008; Kubásek et al., 2007; Tazoe et al., 2008; Pengelly et al., 2010) or growth analysis (Kubásek et al., 2013) discussed above but on the order of sunfleck light availability (Pons and Pearcy, 1992; Krall and Pearcy, 1993). Therefore, we argue that C4 photosynthesis may be made both more and less resilient to dynamic light regimes, depending on the frequency at which these light fluctuations occur. We propose that a more systematic examination of the response of C4 photosynthesis to a range of dynamic light frequencies would help resolve the interaction between C4 photosynthesis and dynamic light.
CONCLUSION
To date, the majority of studies on crop photosynthesis have been performed in steady-state conditions, but more recent research has been trending toward exploring the responses of photosynthesis, especially in field-grown crops, to dynamic conditions. With this shift in emphasis has come the realization of the importance of the efficiency of photosynthesis in fluctuating light to the overall efficiency of canopy photosynthesis. The studies incorporating fluctuating light conditions have shown a lagging response of photosynthesis to both increasing and decreasing light intensity, presenting a significant limitation to crop productivity that, at the same time, reveals opportunities for improving performance. Going forward, it is clearly important to incorporate measurements of non-steady-state photosynthesis in the experimental design of field experiments and into system models of crop growth and performance. This may be even more important when assessing photosynthetic performance under both dynamic light and future climate conditions. The effects of elevated CO2 concentration on carbon gain in fluctuating light have been studied in limited species and show conflicting results (Tomimatsu and Tang, 2016; Kaiser et al., 2017), and the interaction of elevated CO2 with other factors, such as increased temperature and altered precipitation patterns, remains to be studied. Therefore, research in artificial light environments must either attempt to simulate dynamic light conditions similar to those experienced in the field or acknowledge the potential biases that may be present in experimental results from steady-state light conditions (Vialet-Chabrand et al., 2017).
Footnotes
This work was supported by the USDA/ARS (R.A.S. and D.R.O.), the Bill and Melinda Gates Foundation grant (OPP1060461) titled RIPE-Realizing Increased Photosynthetic Efficiency for Sustainable Increases in Crop Yield (D.R.O.), a postdoctoral research fellowship awarded by the Alexander von Humboldt Foundation (B.J.W.), the Cluster of Excellence on Plant Science (CEPLAS, EXC 1028; A.P.M.W.), and the Bundesministerium für Bildung und Forschung (BMBF) grant 031B0205A (A.P.M.W.).
Articles can be viewed without a subscription.
References
- Andralojc PJ, Dawson GW, Parry MA, Keys AJ (1994) Incorporation of carbon from photosynthetic products into 2-carboxyarabinitol-1-phosphate and 2-carboxyarabinitol. Biochem J 304: 781–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster U, Carrillo LR, Venema K, Pavlovic L, Schmidtmann E, Kornfeld A, Jahns P, Berry JA, Kramer DM, Jonikas MC (2014) Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nat Commun 5: 5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrivault S, Obata T, Szecówka M, Mengin V, Guenther M, Hoehne M, Fernie AR, Stitt M (2017) Metabolite pools and carbon flow during C4 photosynthesis in maize: 13CO2 labeling kinetics and cell type fractionation. J Exp Bot 68: 283–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM, Wang XQ (2001) From milliseconds to millions of years: guard cells and environmental responses. Curr Opin Plant Biol 4: 421–428 [DOI] [PubMed] [Google Scholar]
- Bellasio C, Griffiths H (2014a) Acclimation to low light by C4 maize: implications for bundle sheath leakiness. Plant Cell Environ 37: 1046–1058 [DOI] [PubMed] [Google Scholar]
- Bellasio C, Griffiths H (2014b) The operation of two decarboxylases, transamination, and partitioning of C4 metabolic processes between mesophyll and bundle sheath cells allows light capture to be balanced for the maize C4 pathway. Plant Physiol 164: 466–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman NK. (1977) Comparative photosynthesis of sun and shade plants. Annu Rev Plant Physiol 28: 355–377 [Google Scholar]
- Burgess AJ, Retkute R, Pound MP, Foulkes J, Preston SP, Jensen OE, Pridmore TP, Murchie EH (2015) High-resolution three-dimensional structural data quantify the impact of photoinhibition on long-term carbon gain in wheat canopies in the field. Plant Physiol 169: 1192–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess AJ, Retkute R, Preston SP, Jensen OE, Pound MP, Pridmore TP, Murchie EH (2016) The 4-dimensional plant: effects of wind-induced canopy movement on light fluctuations and photosynthesis. Front Plant Sci 7: 1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Silva AE, Salvucci ME (2013) The regulatory properties of Rubisco activase differ among species and affect photosynthetic induction during light transitions. Plant Physiol 161: 1645–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Silva E, Scales JC, Madgwick PJ, Parry MAJ (2015) Optimizing Rubisco and its regulation for greater resource use efficiency. Plant Cell Environ 38: 1817–1832 [DOI] [PubMed] [Google Scholar]
- Chazdon RL. (1988) Sunflecks and their importance to forest understory plants. Adv Ecol Res 18: 1–63 [Google Scholar]
- Chazdon RL, Pearcy RW (1986) Photosynthetic responses to light variation in rainforest species. II. Carbon gain and photosynthetic efficiency during lightflecks. Oecologia 69: 524–531 [DOI] [PubMed] [Google Scholar]
- Chazdon RL, Pearcy RW (1991) The importance of sunflecks for forest understory plants. Bioscience 41: 760–766 [Google Scholar]
- Chen JM, Black TA (1992) Foliage area and architecture of plant canopies from sunfleck size distributions. Agric Meteorol 60: 249–266 [Google Scholar]
- Cousins AB, Badger MR, von Caemmerer S (2006) Carbonic anhydrase and its influence on carbon isotope discrimination during C4 photosynthesis: insights from antisense RNA in Flaveria bidentis. Plant Physiol 141: 232–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins AB, Badger MR, von Caemmerer S (2008) C4 photosynthetic isotope exchange in NAD-ME- and NADP-ME-type grasses. J Exp Bot 59: 1695–1703 [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B. (1990) Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochim Biophys Acta 1020: 1–24 [Google Scholar]
- de Veau EJ, Burris JE (1989) Photorespiratory rates in wheat and maize as determined by o-labeling. Plant Physiol 90: 500–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake PL, Froend RH, Franks PJ (2013) Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. J Exp Bot 64: 495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer JR, Forseth IN (1990) Diurnal leaf movements and productivity in canopies. In Russell G, ed, Plant Canopies. Cambridge University Press, Cambridge, UK, pp 129–142 [Google Scholar]
- Evans JR, von Caemmerer S (2013) Temperature response of carbon isotope discrimination and mesophyll conductance in tobacco. Plant Cell Environ 36: 745–756 [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33: 317–345 [Google Scholar]
- Fischer RA, Rees D, Sayre KD, Lu ZM, Condon AG, Larque Saavedra A (1998) Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci 38: 1467–1475 [Google Scholar]
- Flexas J, Barbour MM, Brendel O, Cabrera HM, Carriquí M, Díaz-Espejo A, Douthe C, Dreyer E, Ferrio JP, Gago J, et al. (2012) Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci 193-194: 70–84 [DOI] [PubMed] [Google Scholar]
- Flexas J, Diaz-Espejo A, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbo M (2007) Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ 30: 1284–1298 [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H (2008) Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ 31: 602–621 [DOI] [PubMed] [Google Scholar]
- Franks PJ, Farquhar GD (2007) The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol 143: 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukayama H, Ueguchi C, Nishikawa K, Katoh N, Ishikawa C, Masumoto C, Hatanaka T, Misoo S (2012) Overexpression of Rubisco activase decreases the photosynthetic CO2 assimilation rate by reducing Rubisco content in rice leaves. Plant Cell Physiol 53: 976–986 [DOI] [PubMed] [Google Scholar]
- Gao X, Hong H, Li WC, Yang L, Huang J, Xiao YL, Chen XY, Chen GY (2016) Downregulation of Rubisco activity by non-enzymatic acetylation of RbcL. Mol Plant 9: 1018–1027 [DOI] [PubMed] [Google Scholar]
- Graham PJ, Nguyen B, Burdyny T, Sinton D (2017) A penalty on photosynthetic growth in fluctuating light. Sci Rep 7: 12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond ET, Andrews TJ, Mott KA, Woodrow IE (1998) Regulation of Rubisco activation in antisense plants of tobacco containing reduced levels of Rubisco activase. Plant J 14: 101–110 [DOI] [PubMed] [Google Scholar]
- Hatch M, Osmond C (1976) Compartmentation and transport in C4 photosynthesis. In Stocking C, Heber U, eds, Transport in Plants. III. Intracellular Interactions and Transport Processes. Springer, Berlin, pp 144–184 [Google Scholar]
- Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424: 901–908 [DOI] [PubMed] [Google Scholar]
- Horton JL, Neufeld HS (1998) Photosynthetic responses of Microstegium vimineum (Trin.) A. Camus, a shade-tolerant, C4 grass, to variable light environments. Oecologia 114: 11–19 [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47: 655–684 [DOI] [PubMed] [Google Scholar]
- Houtz RL, Magnani R, Nayak NR, Dirk LMA (2008) Co- and post-translational modifications in Rubisco: unanswered questions. J Exp Bot 59: 1635–1645 [DOI] [PubMed] [Google Scholar]
- Howard TP, Lloyd JC, Raines CA (2011) Inter-species variation in the oligomeric states of the higher plant Calvin cycle enzymes glyceraldehyde-3-phosphate dehydrogenase and phosphoribulokinase. J Exp Bot 62: 3799–3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard TP, Metodiev M, Lloyd JC, Raines CA (2008) Thioredoxin-mediated reversible dissociation of a stromal multiprotein complex in response to changes in light availability. Proc Natl Acad Sci USA 105: 4056–4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Hu H, Zhang SB (2015) Photorespiration plays an important role in the regulation of photosynthetic electron flow under fluctuating light in tobacco plants grown under full sunlight. Front Plant Sci 6: 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbart S, Ajigboye OO, Horton P, Murchie EH (2012) The photoprotective protein PsbS exerts control over CO2 assimilation rate in fluctuating light in rice. Plant J 71: 402–412 [DOI] [PubMed] [Google Scholar]
- Johnson MP, Davison PA, Ruban AV, Horton P (2008) The xanthophyll cycle pool size controls the kinetics of non-photochemical quenching in Arabidopsis thaliana. FEBS Lett 582: 262–266 [DOI] [PubMed] [Google Scholar]
- Johnson MP, Havaux M, Triantaphylidès C, Ksas B, Pascal AA, Robert B, Davison PA, Ruban AV, Horton P (2007) Elevated zeaxanthin bound to oligomeric LHCII enhances the resistance of Arabidopsis to photooxidative stress by a lipid-protective, antioxidant mechanism. J Biol Chem 282: 22605–22618 [DOI] [PubMed] [Google Scholar]
- Kaiser E, Zhou D, Heuvelink E, Harbinson J, Morales A, Marcelis LFM (2017) Elevated CO2 increases photosynthesis in fluctuating irradiance regardless of photosynthetic induction state. J Exp Bot 68: 5629–5640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Andralojc PJ, Lea PJ, Parry MA (1999) 2′-Carboxy-D-arabitinol 1-phosphate protects ribulose 1,5-bisphosphate carboxylase/oxygenase against proteolytic breakdown. Eur J Biochem 266: 840–847 [DOI] [PubMed] [Google Scholar]
- Kim SY, Bender KW, Walker BJ, Zielinski RE, Spalding MH, Ort DR, Huber SC (2016) The plastid casein kinase 2 phosphorylates Rubisco activase at the Thr-78 site but is not essential for regulation of Rubisco activation state. Front Plant Sci 7: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp AK, Smith WK (1989) Influence of growth form on ecophysiological responses to variable sunlight in subalpine plants. Ecology 70: 1069–1082 [Google Scholar]
- Koester RP, Nohl BM, Diers BW, Ainsworth EA (2016) Has photosynthetic capacity increased with 80 years of soybean breeding? An examination of historical soybean cultivars. Plant Cell Environ 39: 1058–1067 [DOI] [PubMed] [Google Scholar]
- Krall JP, Pearcy RW (1993) Concurrent measurements of oxygen and carbon dioxide exchange during lightflecks in maize (Zea mays L.). Plant Physiol 103: 823–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP (2016) Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354: 857–861 [DOI] [PubMed] [Google Scholar]
- Kromdijk J, Griffiths H, Schepers HE (2010) Can the progressive increase of C4 bundle sheath leakiness at low PFD be explained by incomplete suppression of photorespiration? Plant Cell Environ 33: 1935–1948 [DOI] [PubMed] [Google Scholar]
- Kromdijk J, Ubierna N, Cousins AB, Griffiths H (2014) Bundle-sheath leakiness in C4 photosynthesis: a careful balancing act between CO2 concentration and assimilation. J Exp Bot 65: 3443–3457 [DOI] [PubMed] [Google Scholar]
- Kubásek J, Setlík J, Dwyer S, Santrůcek J (2007) Light and growth temperature alter carbon isotope discrimination and estimated bundle sheath leakiness in C4 grasses and dicots. Photosynth Res 91: 47–58 [DOI] [PubMed] [Google Scholar]
- Kubásek J, Urban O, Šantrůček J (2013) C4 plants use fluctuating light less efficiently than do C3 plants: a study of growth, photosynthesis and carbon isotope discrimination. Physiol Plant 149: 528–539 [DOI] [PubMed] [Google Scholar]
- Lawson T. (2009) Guard cell photosynthesis and stomatal function. New Phytol 181: 13–34 [DOI] [PubMed] [Google Scholar]
- Lawson T, Blatt MR (2014) Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol 164: 1556–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Kramer DM, Raines CA (2012) Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Curr Opin Biotechnol 23: 215–220 [DOI] [PubMed] [Google Scholar]
- Leegood RC. (1985) The intercellular compartmentation of metabolites in leaves of Zea mays L. Planta 164: 163–171 [DOI] [PubMed] [Google Scholar]
- Li T, Heuvelink E, Dueck TA, Janse J, Gort G, Marcelis LFM (2014) Enhancement of crop photosynthesis by diffuse light: quantifying the contributing factors. Ann Bot 114: 145–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Kromdijk J, Heuvelink E, van Noort FR, Kaiser E, Marcelis LFM (2016) Effects of diffuse light on radiation use efficiency of two Anthurium cultivars depend on the response of stomatal conductance to dynamic light intensity. Front Plant Sci 7: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XP, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403: 391–395 [DOI] [PubMed] [Google Scholar]
- Li XP, Gilmore AM, Niyogi KK (2002a) Molecular and global time-resolved analysis of a psbS gene dosage effect on pH- and xanthophyll cycle-dependent nonphotochemical quenching in photosystem II. J Biol Chem 277: 33590–33597 [DOI] [PubMed] [Google Scholar]
- Li XP, Muller-Moule P, Gilmore AM, Niyogi KK (2002b) PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc Natl Acad Sci USA 99: 15222–15227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Humphries S, Falkowski PG (1994) Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Mol Biol 45: 633–662 [Google Scholar]
- Long SP, Zhu XG, Naidu SL, Ort DR (2006) Can improvement in photosynthesis increase crop yields? Plant Cell Environ 29: 315–330 [DOI] [PubMed] [Google Scholar]
- Marri L, Zaffagnini M, Collin V, Issakidis-Bourguet E, Lemaire SD, Pupillo P, Sparla F, Miginiac-Maslow M, Trost P (2009) Prompt and easy activation by specific thioredoxins of Calvin cycle enzymes of Arabidopsis thaliana associated in the GAPDH/CP12/PRK supramolecular complex. Mol Plant 2: 259–269 [DOI] [PubMed] [Google Scholar]
- McAusland L, Vialet-Chabrand S, Davey P, Baker NR, Brendel O, Lawson T (2016) Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytol 211: 1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer FC, Smith DD, Woodruff DR, Marias DE, McCulloh KA, Howard AR, Magedman AL (2017) Stomatal kinetics and photosynthetic gas exchange along a continuum of isohydric to anisohydric regulation of plant water status. Plant Cell Environ 40: 1618–1628 [DOI] [PubMed] [Google Scholar]
- Mercado LM, Bellouin N, Sitch S, Boucher O, Huntingford C, Wild M, Cox PM (2009) Impact of changes in diffuse radiation on the global land carbon sink. Nature 458: 1014–1017 [DOI] [PubMed] [Google Scholar]
- Mott KA, Woodrow IE (2000) Modelling the role of Rubisco activase in limiting non-steady-state photosynthesis. J Exp Bot 51: 399–406 [DOI] [PubMed] [Google Scholar]
- Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching: a response to excess light energy. Plant Physiol 125: 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie EH, Pinto M, Horton P (2009) Agriculture and the new challenges for photosynthesis research. New Phytol 181: 532–552 [DOI] [PubMed] [Google Scholar]
- Naranjo B, Diaz-Espejo A, Lindahl M, Cejudo FJ (2016) Type-f thioredoxins have a role in the short-term activation of carbon metabolism and their loss affects growth under short-day conditions in Arabidopsis thaliana. J Exp Bot 67: 1951–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilkens M, Kress E, Lambrev P, Miloslavina Y, Müller M, Holzwarth AR, Jahns P (2010) Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim Biophys Acta 1797: 466–475 [DOI] [PubMed] [Google Scholar]
- Nishimura S, Koizumi H, Tang Y (1998) Spatial and temporal variation in photon flux density on rice (Oryza sativa L.) leaf surface. Plant Prod Sci 1: 30–36 [Google Scholar]
- Nishimura S, Tang Y, Itoh K, Koizumi H (2000) Photosynthetic light-use efficiency in rice (Oryza sativa L.) leaf under light with fluctuating intensities at two different ambient humidities. Plant Prod Sci 3: 79–83 [Google Scholar]
- Niyogi KK. (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50: 333–359 [DOI] [PubMed] [Google Scholar]
- Ort DR. (2001) When there is too much light. Plant Physiol 125: 29–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort DR, Baker NR (1988) Consideration of photosynthetic efficiency at low light as a major determinant of crop photosynthetic performance. Plant Physiol Biochem 26: 555–565 [Google Scholar]
- Ort DR, Long SP (2014) Limits on yields in the Corn Belt. Science 344: 484–485 [DOI] [PubMed] [Google Scholar]
- Parry MAJ, Andralojc PJ, Scales JC, Salvucci ME, Carmo-Silva AE, Alonso H, Whitney SM (2013) Rubisco activity and regulation as targets for crop improvement. J Exp Bot 64: 717–730 [DOI] [PubMed] [Google Scholar]
- Pearcy R, Gross L, He D (1997) An improved dynamic model of photosynthesis for estimation of carbon gain in sunfleck light regimes. Plant Cell Environ 20: 411–424 [Google Scholar]
- Pearcy R, Roden J, Gamon J (1990) Sunfleck dynamics in relation to canopy structure in a soybean (Glycine max (L.) Merr.) canopy. Agric Meteorol 52: 359–372 [Google Scholar]
- Pearcy RW. (1990) Photosynthesis in plant life. Annu Rev Plant Physiol Plant Mol Biol 41: 421–453 [Google Scholar]
- Pearcy RW, Pfitsch WA (1995) The consequences of sunflecks for photosynthesis and growth of forest understory plants. In Schulze ED, Caldwell MM, eds, Ecophysiology of Photosynthesis. Springer-Verlag, Berlin, pp 343–359 [Google Scholar]
- Pengelly JJL, Sirault XRR, Tazoe Y, Evans JR, Furbank RT, von Caemmerer S (2010) Growth of the C4 dicot Flaveria bidentis: photosynthetic acclimation to low light through shifts in leaf anatomy and biochemistry. J Exp Bot 61: 4109–4122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick TR, Bräutigam A, Schlüter U, Denton AK, Colmsee C, Scholz U, Fahnenstich H, Pieruschka R, Rascher U, Sonnewald U, et al. (2011) Systems analysis of a maize leaf developmental gradient redefines the current C4 model and provides candidates for regulation. Plant Cell 23: 4208–4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons TL, Pearcy RW (1992) Photosynthesis in flashing light in soybean leaves grown in different conditions. II. Lightfleck utilization efficiency. Plant Cell Environ 15: 577–584 [Google Scholar]
- Portis AR., Jr (2003) Rubisco activase: Rubisco’s catalytic chaperone. Photosynth Res 75: 11–27 [DOI] [PubMed] [Google Scholar]
- Portis AR Jr, Li C, Wang D, Salvucci ME (2008) Regulation of Rubisco activase and its interaction with Rubisco. J Exp Bot 59: 1597–1604 [DOI] [PubMed] [Google Scholar]
- Qu M, Hamdani S, Li W, Wang S, Tang J, Chen Z, Song Q, Li M, Zhao H, Chang T, et al. (2016) Rapid stomatal response to fluctuating light: an under-explored mechanism to improve drought tolerance in rice. Funct Plant Biol 43: 727–738 [DOI] [PubMed] [Google Scholar]
- Raissig MT, Matos JL, Anleu Gil MX, Kornfeld A, Bettadapur A, Abrash E, Allison HR, Badgley G, Vogel JP, Berry JA, et al. (2017) Mobile MUTE specifies subsidiary cells to build physiologically improved grass stomata. Science 355: 1215–1218 [DOI] [PubMed] [Google Scholar]
- Raven JA. (1989) Fight or flight: the economics of repair and avoidance of photoinhibition of photosynthesis. Funct Ecol 3: 5–19 [Google Scholar]
- Raven JA. (2014) Speedy small stomata? J Exp Bot 65: 1415–1424 [DOI] [PubMed] [Google Scholar]
- Roderick ML, Farquhar GD, Berry SL, Noble IR (2001) On the direct effect of clouds and atmospheric particles on the productivity and structure of vegetation. Oecologia 129: 21–30 [DOI] [PubMed] [Google Scholar]
- Sacharz J, Giovagnetti V, Ungerer P, Mastroianni G, Ruban AV (2017) The xanthophyll cycle affects reversible interactions between PsbS and light-harvesting complex II to control non-photochemical quenching. Nat Plants 3: 16225. [DOI] [PubMed] [Google Scholar]
- Sage RF, McKown AD (2006) Is C4 photosynthesis less phenotypically plastic than C3 photosynthesis? J Exp Bot 57: 303–317 [DOI] [PubMed] [Google Scholar]
- Sassenrath-Cole GF, Pearcy RW (1992) The role of ribulose-1,5-bisphosphate regeneration in the induction requirement of photosynthetic CO2 exchange under transient light conditions. Plant Physiol 99: 227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassenrath-Cole GF, Pearcy RW (1994) Regulation of photosynthetic induction state by the magnitude and duration of low light exposure. Plant Physiol 105: 1115–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassenrath-Cole GF, Pearcy RW, Steinmaus S (1994) The role of enzyme activation state in limiting carbon assimilation under variable light conditions. Photosynth Res 41: 295–302 [DOI] [PubMed] [Google Scholar]
- Schymanski SJ, Or D, Zwieniecki M (2013) Stomatal control and leaf thermal and hydraulic capacitances under rapid environmental fluctuations. PLoS ONE 8: e54231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair TR, Muchow RC (1999) Radiation use efficiency. Adv Agron 65: 215–265 [Google Scholar]
- Song Q, Zhang G, Zhu XG (2013) Optimal crop canopy architecture to maximise canopy photosynthetic CO2 uptake under elevated CO2: a theoretical study using a mechanistic model of canopy photosynthesis. Funct Plant Biol 40: 108–124 [DOI] [PubMed] [Google Scholar]
- Stitt M, Heldt HW (1985) Generation and maintenance of concentration gradients between the mesophyll and bundle sheath in maize leaves. Biochim Biophys Acta 808: 400–414 [Google Scholar]
- Stitt M, Zhu XG (2014) The large pools of metabolites involved in intercellular metabolite shuttles in C4 photosynthesis provide enormous flexibility and robustness in a fluctuating light environment. Plant Cell Environ 37: 1985–1988 [DOI] [PubMed] [Google Scholar]
- Tang YH, Washitani I, Tsuchiya T, Iwaki H (1988) Fluctuation of photosynthetic photon flux density within a Miscanthus sinensis canopy. Ecol Res 3: 253–266 [Google Scholar]
- Tazoe Y, Hanba YT, Furumoto T, Noguchi K, Terashima I (2008) Relationships between quantum yield for CO2 assimilation, activity of key enzymes and CO2 leakiness in Amaranthus cruentus, a C4 dicot, grown in high or low light. Plant Cell Physiol 49: 19–29 [DOI] [PubMed] [Google Scholar]
- Tholen D, Boom C, Noguchi K, Ueda S, Katase T, Terashima I (2008) The chloroplast avoidance response decreases internal conductance to CO2 diffusion in Arabidopsis thaliana leaves. Plant Cell Environ 31: 1688–1700 [DOI] [PubMed] [Google Scholar]
- Thormählen I, Ruber J, von Roepenack-Lahaye E, Ehrlich SM, Massot V, Hümmer C, Tezycka J, Issakidis-Bourguet E, Geigenberger P (2013) Inactivation of thioredoxin f1 leads to decreased light activation of ADP-glucose pyrophosphorylase and altered diurnal starch turnover in leaves of Arabidopsis plants. Plant Cell Environ 36: 16–29 [DOI] [PubMed] [Google Scholar]
- Thormählen I, Zupok A, Rescher J, Leger J, Weissenberger S, Groysman J, Orwat A, Chatel-Innocenti G, Issakidis-Bourguet E, Armbruster U, et al. (2017) Thioredoxins play a crucial role in dynamic acclimation of photosynthesis in fluctuating light. Mol Plant 10: 168–182 [DOI] [PubMed] [Google Scholar]
- Tomimatsu H, Tang Y (2012) Elevated CO2 differentially affects photosynthetic induction response in two Populus species with different stomatal behavior. Oecologia 169: 869–878 [DOI] [PubMed] [Google Scholar]
- Tomimatsu H, Tang Y (2016) Effects of high CO2 levels on dynamic photosynthesis: carbon gain, mechanisms, and environmental interactions. J Plant Res 129: 365–377 [DOI] [PubMed] [Google Scholar]
- Ubierna N, Sun W, Cousins AB (2011) The efficiency of C4 photosynthesis under low light conditions: assumptions and calculations with CO2 isotope discrimination. J Exp Bot 62: 3119–3134 [DOI] [PubMed] [Google Scholar]
- Ubierna N, Sun W, Kramer DM, Cousins AB (2013) The efficiency of C4 photosynthesis under low light conditions in Zea mays, Miscanthus × giganteus and Flaveria bidentis. Plant Cell Environ 36: 365–381 [DOI] [PubMed] [Google Scholar]
- Vialet-Chabrand S, Matthews JS, Simkin AJ, Raines CA, Lawson T (2017) Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiol 173: 2163–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Furbank RT (1999) Modeling C4 photosynthesis. In Sage RF, Monson RK, eds, C4 Plant Biology. Academic Press, San Diego, pp 173–211 [Google Scholar]
- von Caemmerer S, Furbank RT (2003) The C4 pathway: an efficient CO2 pump. Photosynth Res 77: 191–207 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Quick WP (2000) Rubisco: physiology in vivo. In Leegood RC, Sharkey TD, von Caemmerer S, eds, Photosynthesis: Physiology and Metabolism. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 85–113 [Google Scholar]
- Wada M. (2013) Chloroplast movement. Plant Sci 210: 177–182 [DOI] [PubMed] [Google Scholar]
- Wang X, Guo Y, Wang X, Ma Y, Li B (2008) Estimating photosynthetically active radiation distribution in maize canopies by a three-dimensional incident radiation model. Funct Plant Biol 35: 867–875 [DOI] [PubMed] [Google Scholar]
- Wang Y, Bräutigam A, Weber APM, Zhu XG (2014a) Three distinct biochemical subtypes of C4 photosynthesis? A modelling analysis. J Exp Bot 65: 3567–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hills A, Blatt MR (2014b) Systems analysis of guard cell membrane transport for enhanced stomatal dynamics and water use efficiency. Plant Physiol 164: 1593–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Noguchi K, Ono N, Inoue S, Terashima I, Kinoshita T (2014c) Overexpression of plasma membrane H+-ATPase in guard cells promotes light-induced stomatal opening and enhances plant growth. Proc Natl Acad Sci USA 111: 533–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Portis AR (1992) Dissociation of ribulose-1,5-bisphosphate bound to ribulose-1,5-bisphosphate carboxylase/oxygenase and its enhancement by ribulose-1,5-bisphosphate carboxylase/oxygenase activase-mediated hydrolysis of ATP. Plant Physiol 99: 1348–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way DA, Pearcy RW (2012) Sunflecks in trees and forests: from photosynthetic physiology to global change biology. Tree Physiol 32: 1066–1081 [DOI] [PubMed] [Google Scholar]
- Wild M. (2009) Global dimming and brightening: a review. J Geophys Res 114: D00D16 [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD (1979) Stomatal conductance correlates with photosynthetic capacity. Nature 282: 424–426 [Google Scholar]
- Yamori W, Masumoto C, Fukayama H, Makino A (2012) Rubisco activase is a key regulator of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature. Plant J 71: 871–880 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Hara S, Hisabori T (2015) Thioredoxin selectivity for thiol-based redox regulation of target proteins in chloroplasts. J Biol Chem 290: 14278–14288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Hisabori T (2016) Two distinct redox cascades cooperatively regulate chloroplast functions and sustain plant viability. Proc Natl Acad Sci USA 113: E3967–E3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XG, Ort DR, Whitmarsh J, Long SP (2004) The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis. J Exp Bot 55: 1167–1175 [DOI] [PubMed] [Google Scholar]
- Zia A, Johnson MP, Ruban AV (2011) Acclimation- and mutation-induced enhancement of PsbS levels affects the kinetics of non-photochemical quenching in Arabidopsis thaliana. Planta 233: 1253–1264 [DOI] [PubMed] [Google Scholar]