The development of leaves requires photoreceptors to initiate auxin export, cytokinin action, and sugar-dependent signaling at dividing cells, energy signaling further adjusting growth to available light.
Abstract
The development of leaf primordia is subject to light control of meristematic activity. Light regulates the expression of thousands of genes with roles in cell proliferation, organ development, and differentiation of photosynthetic cells. Previous work has highlighted roles for hormone homeostasis and the energy-dependent Target of Rapamycin (TOR) kinase in meristematic activity, yet a picture of how these two regulatory mechanisms depend on light perception and interact with each other has yet to emerge. Their relevance beyond leaf initiation also is unclear. Here, we report the discovery that the dark-arrested meristematic region of Arabidopsis (Arabidopsis thaliana) experiences a local energy deprivation state and confirm previous findings that the PIN1 auxin transporter is diffusely localized in the dark. Light triggers a rapid removal of the starvation state and the establishment of PIN1 polar membrane localization consistent with auxin export, both preceding the induction of cell cycle- and cytoplasmic growth-associated genes. We demonstrate that shoot meristematic activity can occur in the dark through the manipulation of auxin and cytokinin activity as well as through the activation of energy signaling, both targets of photomorphogenesis action, but the organ developmental outcomes differ: while TOR-dependent energy signals alone stimulate cell proliferation, the development of a normal leaf lamina requires photomorphogenesis-like hormonal responses. We further show that energy signaling adjusts the extent of cell cycle activity and growth of young leaves non-cellautonomously to available photosynthates and leads to organs constituted of a greater number of cells developing under higher irradiance. This makes energy signaling perhaps the most important biomass growth determinant under natural, unstressed conditions.
Leaves are biological solar panels, the development of which begins as primordia at the flanks of the shoot apical meristem (Tsukaya, 2005; Kalve et al., 2014a). This meristem consists of a pool of stem cells and their close descendants, is organized during embryogenesis, and arrests as the embryo enters dormancy, becoming protected within the seed. Following germination, which frequently occurs underground, the development of leaf primordia is arrested in darkness (Chory, 2010). This constitutes part of the skotomorphogenesis developmental program, which helps young seedlings to emerge through the ground, before the photomorphogenesis program commences aboveground. Emergence into light reinitiates leaf development, including that of leaf mesophyll cells filled with chloroplasts (Nemhauser and Chory, 2002). In most gymnosperm plants, however, leaves can develop and cells with chloroplasts can differentiate in the dark, suggesting that the skotomorphogenesis program is an evolutionary innovation to assist seedling establishment (Hills et al., 2015). As a consequence, upon first exposure to light, photosynthesis cannot immediately commence; instead, photomorphogenesis is activated by informational photoreceptors, most notably the phytochrome and cryptochrome families (Chory, 2010) that detect the presence, quality, and quantity of light. Accordingly, the combined deficiency of phytochromes and cryptochromes prevents leaf initiation in the light (López-Juez et al., 2008). Repressors of photomorphogenesis, including DET1 and COP1, target light signaling proteins for degradation in the dark, as revealed by the fact that their loss of function leads to constitutive photomorphogenic development (Chory et al., 1994; Lau and Deng, 2012).
The response of seedlings to the first light exposure postgermination is so dramatic that it constituted the very first target for large-scale gene expression profiling, followed by many subsequent genome-wide studies (Jiao et al., 2007). However, these studies were of limited use in understanding the initiation of leaves at the meristem in response to light, since the various organs show distinct responses to light (e.g. the cotyledons undergo expansion, while hypocotyls cease to elongate). A developmental and transcriptome-wide analysis of dissected, etiolated shoot apices when the growth of leaf primordia is initiated upon the first exposure to light addressed this question (López-Juez et al., 2008). This analysis revealed a dramatic stimulation of cell proliferation, peaking between 6 and 24 h after light exposure. Gene expression signatures associated with cell proliferation and cytoplasmic growth (protein translation) peaked at 6 h and were followed by expansion growth-associated signatures, including cell wall remodeling and water influx. A direct regulation of cell cycle-associated E2F transcription factors by photoreceptors, under DET1 and COP1 control, provided a possible mechanism for meristem activation by light (López-Juez et al., 2008; Berckmans et al., 2011). Furthermore, based on diagnostic gene expression signatures, a transient down-regulation of auxin and ethylene signaling at the apex was postulated, one that preceded an up-regulation of cytokinin responses. The latter coincided with the peak in the cell cycle and ribosome-related gene expression activity.
Hormonal responses are central to leaf initiation. Consecutive leaves develop at the flanks of the shoot meristem in striking geometric arrangement, known as phyllotaxy, which can be explained by inhibitory fields generated by emerging leaves. Elegant experiments have revealed those fields to be based on the self-regulating dynamics of auxin transport (Braybrook and Kuhlemeier, 2010). Positions for leaf primordia on the epidermis at the peripheral zone of the meristem are selected where the auxin concentration is high, and these points become sinks for further auxin transported from nearby epidermal cells, due to the polar relocalization of the PIN1 auxin transport protein. Cells at this position then enter rapid cell proliferation, but leaf emergence requires a second relocalization of PIN1 proteins to export auxin away from the primordium into the rib meristem (Reinhardt et al., 2003). These events constitute the first steps in leaf development. Auxin is further involved in the proliferation of leaf cells and in the differentiation of vasculature (Scarpella et al., 2006, 2010). Thus, auxin plays fundamental and separate roles in the positioning and early development of leaves (Capua and Eshed, 2017).
An elegant study carried out in tomato (Solanum lycopersicum) shoot meristems showed that the auxin efflux transporter, PIN1, became internalized when light-grown shoot apices were transferred to the dark, while in the light, the auxin maxima established by plasma membrane-localized PIN1 determined the positions for cytokinin action to drive leaf initiation (Yoshida et al., 2011). A subsequent study (Pfeiffer et al., 2016) demonstrated that sugars acting through the Target of Rapamycin (TOR) kinase pathway, together with cytokinin activity, lead to the induction of WUS expression and subsequent meristem activation in the light.
Growth is the most resource-consuming process living organisms undertake, and it is not surprising that mechanisms have evolved to sense and interpret the availability of energy and nutrients. Besides their roles as reduced carbon sources for oxidative phosphorylation, both Glc and Suc can trigger direct responses in plants. Exhaustion of reduced carbon has been shown to trigger a common set of genes, named starvation genes, regardless of the means by which the exhaustion takes place (e.g. change of medium composition or loss of available starch in leaves after an unexpectedly long night). Starvation genes are turned off when reduced carbon becomes available (Usadel et al., 2008; Sulpice et al., 2009). The starvation state is perceived as a deficiency in the metabolite trehalose-6-phosphate and acts through the SNF-related protein kinase, SNRK (Robaglia et al., 2012; Tsai and Gazzarrini, 2014), which negatively regulates TOR, a central kinase that universally mediates resource signals in eukaryotes (Laplante and Sabatini, 2012; Nukarinen et al., 2016). TOR is a master regulator, controlling a number of growth-signaling cascades, which responds to sugar and amino acid availability. One of the fundamental outputs of TOR activity is an enhanced ability to manufacture cellular components through an increase in the cellular translation capacity. TOR also promotes cell proliferation (Xiong et al., 2013). In plants, TOR responds both to sugar signals (Baena-González et al., 2007; Deprost et al., 2007; Xiong et al., 2013; Dobrenel et al., 2016a) and to auxin (Schepetilnikov et al., 2013, 2017). It was shown recently that, in shoot meristems, light stimulates the TOR activity via two parallel pathways, through photosynthates and through light signaling linked to auxin biosynthesis (Li et al., 2017).
In this study, we demonstrate that cell proliferation can be arrested in young primordia by dark exposure, or reduced at low light, through a local starvation state in the meristem, and reinitiated by transfer to light, which rapidly overcomes such a state. We show that, upon light exposure of dark-arrested leaf primordia, PIN becomes rapidly polarized and that this precedes cell proliferation and growth gene responses. We also show that shoot meristematic activity can be induced in the dark by exposure to cytokinin, and more efficiently so under reduced auxin sensitivity. It also can occur in the dark by direct access of the meristem to sugar, in a TOR-dependent manner (i.e. through the activation of energy signaling, the second target of photomorphogenesis action), but with differing results: energy signals stimulate cell proliferation, but the development of a normal leaf lamina requires photomorphogenesis-like hormonal responses. Lastly, we show that available photosynthates impact energy signaling and adjust the extent of cell cycle activity in meristematic cells in a non-cellautonomous manner, which, under higher irradiance, leads to organs constituted of a greater number of cells.
RESULTS
The Shoot Meristem and Arrested Primordia of Dark-Grown Seedlings Experience Local Energy Starvation
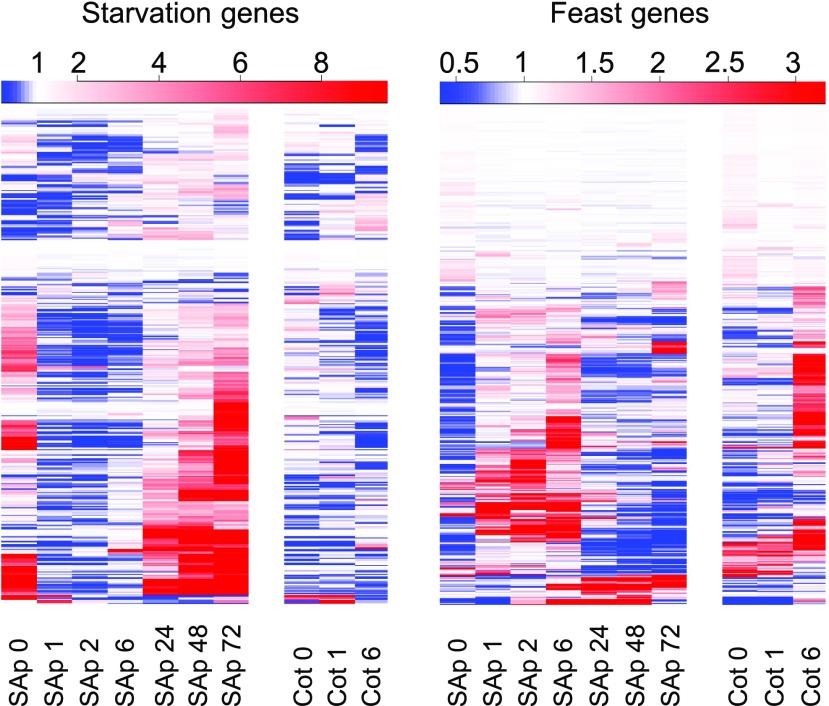
In our earlier analysis of light responses at the shoot apex upon first exposure of dark-grown seedlings, we identified nearly 6,000 differentially expressed genes (López-Juez et al., 2008). Among these genes, we identified one cluster that was composed of hundreds that responded rapidly, within 1 h, and negatively to light, exclusively in the shoot apex, not in the cotyledons. Subsequent analysis revealed that this cluster was highly enriched in common carbon-repressed starvation genes, as classified by a previous study (Usadel et al., 2008). We reexamined the expression of all such genes in our transcriptome data. The resulting expression plot of the complete set of starvation-defined genes shows a generalized, rapid down-regulation of transcript levels (Fig. 1). More than 50% of starvation genes were expressed 2-fold or higher in the dark than after 1 h in the light in the shoot apex, with more than 20% being 10-fold or higher. Because the etiolated cotyledons are unlikely to become photosynthetically competent in the short time interval of 1 h, we postulate that the rapid repression of starvation genes in the shoot apex is a consequence of the rapid mobilization of reserves stored in the cotyledons upon light exposure. This is in contrast to the growth of the hypocotyl, which occurs rapidly at the etiolated stage, demonstrating that resources do not limit the growth of another organ in the dark. Interestingly, the down-regulation of starvation genes in the shoot apex was transient in most cases, expression becoming high again 24 h later. The reason for this is not clear, but it might represent the fact that the carbon supply could not keep up with the rapid growth taking place within the shoot apex. In contrast, carbon-induced genes, which we refer to as feast genes, exhibited the opposite expression pattern, a strong expression between 1 and 6 h after light exposure (Fig. 1). We conclude that skotomorphogenesis in the dark imposes a starvation state specifically on cells within the shoot apex and that this state is released rapidly upon light exposure.
Figure 1.
Shoot apices of 3-d-old seedlings exhibit in the dark a starvation response, which disappears within 1 h of light exposure. The expression levels of genes defined as carbon-repressed starvation and carbon-induced feast (Usadel et al., 2008) were plotted using data from a previous microarray experiment (López-Juez et al., 2008). Heat maps represent levels at each time point relative to the average level for the same gene across all time points (red, above; blue, below). SAp, Shoot apex; Cot, cotyledons. The number after each sample type indicates hours after light exposure. Color scales are shown above each plot.
Light Triggers the Polar Localization of PIN1 to the Plasma Membrane, Allowing Auxin Export That Precedes Primordia Growth
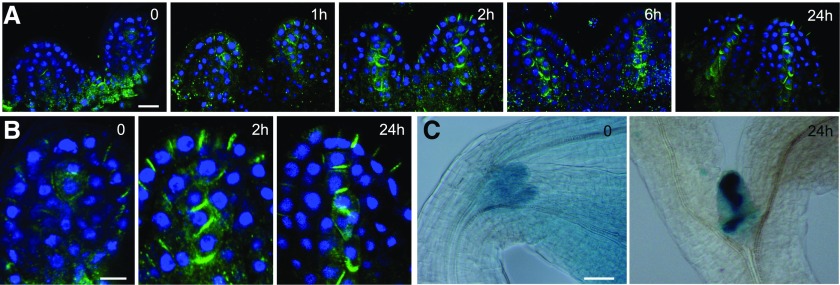
Auxin-responsive genes were shown to be highly expressed in the dark-arrested shoot apex, and upon light exposure, the expression of these genes was rapidly and transiently reduced (López-Juez et al., 2008). This could be explained if light activates auxin export from emerging leaf primordia. To test this hypothesis, we examined the localization of the PIN1 protein in the arrested meristems of dark-germinated seedlings, before and after their first exposure to light, using immunofluorescence labeling. Confirming a previous report (Yoshida et al., 2011), the PIN1 signal was weak, with limited membrane localization, largely diffused inside the cells, and difficult to distinguish from background in the dark-arrested shoot apex. We found that, upon light exposure of dark-arrested meristems, the PIN1 localization became polar on the plasma membrane within 2 h, in a pattern pointing toward the tips of emerging leaf primordia at the epidermal cell layer and away from the tips of primordia in a cell file at the center of the leaf lamina. This pattern was particularly evident 24 h after exposure to light, the position of the PIN1 signal indicating auxin transport toward the primordia tips and export toward the rib meristem (Fig. 2A).
Figure 2.
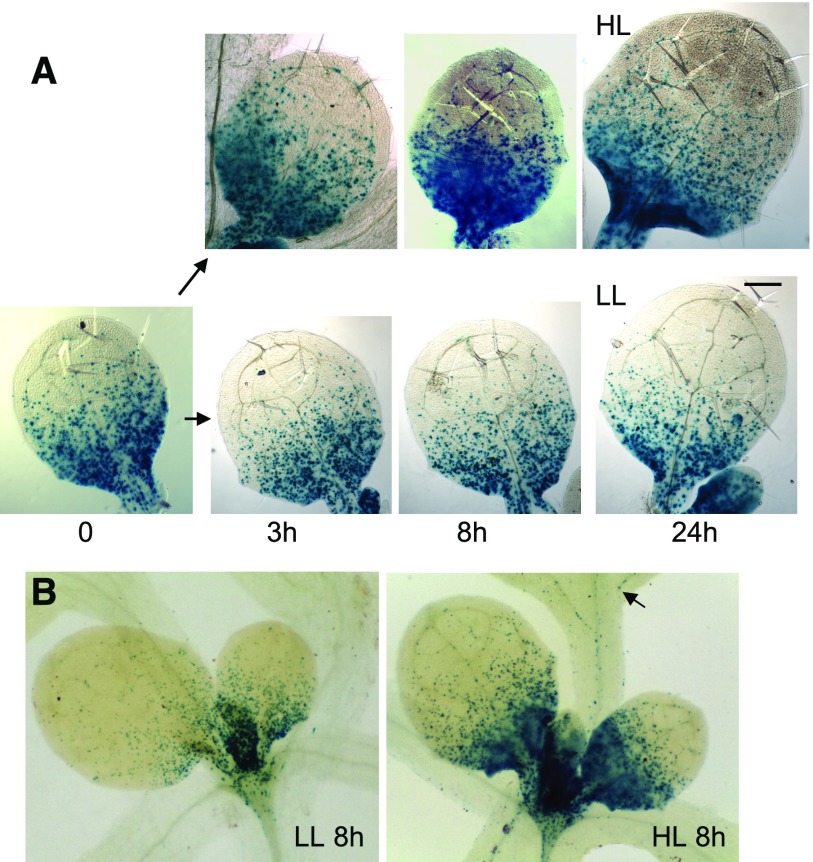
Light exposure of dark-grown seedlings triggers a rapid PIN1 polarization at the leaf primordia and the establishment of localized auxin maxima. A, PIN1 immunofluorescence localization in the first two leaf primordia of wild-type seedlings: PIN1 (green) and 4′,6-diamino-2-phenylindole (DAPI; blue). Seedlings were germinated in the dark for 3 d, then examined immediately or after exposure to continuous white light for the times indicated (in hours). B, Enlargements of a primordium tip from the PIN1 localization images in A after 0, 2, and 24 h. C, DR5:GUS reporter activity of seedlings in the dark and exposed to white light for 24 h. Bars = 10 µm (A), 5 µm (B), and 50 µm (C).
Consistent with the diffuse PIN1 localization pattern in the dark, the DR5:GUS auxin activity reporter (Ulmasov et al., 1997) revealed a relatively high but delocalized auxin response in dark-grown shoot apices, including the meristem and the arrested primordia. Given that the GUS protein is stable, we could not monitor the changes of GUS signal in a similar time scale to that used for PIN1 localization. However, 24 h after transfer to light, the DR5:GUS activity was no longer diffuse and coincided with known, strong auxin maxima at the primordia tips, and a distinct signal accompanying the differentiation of provascular cells emerged in the future mid vein (Fig. 2B).
Reduction of Auxin Sensitivity Enhances the Ability of Cytokinin to Induce Leaf Initiation in the Dark
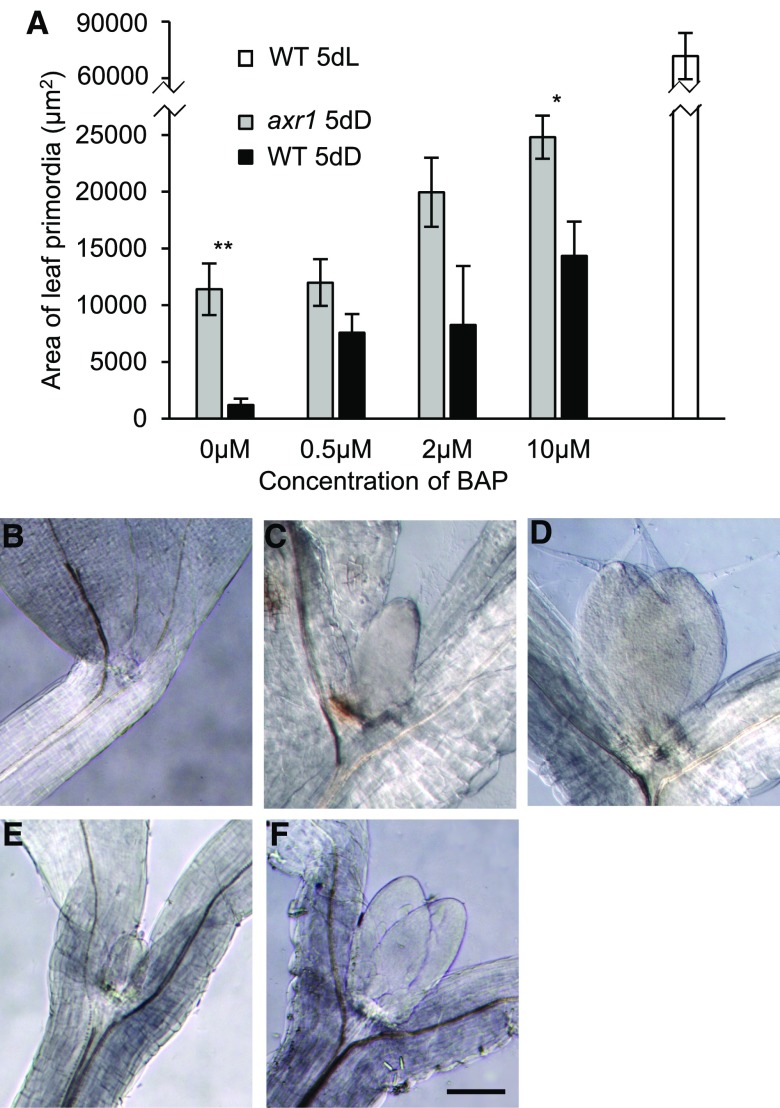
The expression of auxin and cytokinin signature genes when the dark-arrested meristem was exposed to light suggested that, in the dark, auxin might prevent leaf primordia growth, and this auxin action is rapidly removed upon light exposure, to be replaced by cytokinin to drive growth (López-Juez et al., 2008; Yoshida et al., 2011; Pfeiffer et al., 2016). In agreement, it has been shown that an auxin partially insensitive mutant (axr1-12; Leyser et al., 1993) and a cytokinin-overproducing one (amp1; Chaudhury et al., 1993) exhibit a deetiolated state in the dark, manifest as short hypocotyl and open cotyledons. It also has been shown that exposure of wild-type Arabidopsis (Arabidopsis thaliana) to the synthetic cytokinin 6-benzylaminopurine (BAP) causes leaf initiation in the dark (Chory et al., 1994). We attempted to experimentally transform the hormonal balance characteristic of dark-arrested meristems (high auxin and low cytokinin activity) into the one normally found after light exposure (low auxin and high cytokinin activity) and asked whether such manipulation would allow leaf initiation in the dark. To this end, we exposed the axr1-12 mutant to BAP on Suc-containing plates in the dark. Without BAP, the leaf primordia remained arrested in the dark in the wild type, while a substantial increase in leaf primordia size was observed in the axr1-12 mutant grown in the same conditions (Fig. 3). As expected, cytokinin could stimulate wild-type leaf primordia growth in the dark, but the size of primordia observed after the addition of BAP was increased further in the axr1 mutant. After 5 d in the dark, the leaf primordium size of BAP-treated axr1 seedlings reached about one-third that of the wild type in the light in the absence of exogenous hormones. Data obtained from these experiments are consistent with the idea that the removal of auxin and the activation of the cytokinin response are required for leaf primordia growth.
Figure 3.
In the axr1-12 mutant, leaves initiate in the dark, this being enhanced by the addition of cytokinin. A, Seedlings of the Columbia wild type (WT) and axr1-12 were germinated and grown in the dark for 5 d, on 1% Suc-containing medium with or without BAP at the concentrations indicated, or for the wild type in the light for 5 d on medium without BAP. The area of one of the first two leaf primordia is indicated. Error bars represent se. Asterisks reflect the significance of differences between axr1 and the wild type. B to F, Images of leaf primordia of representative shoot apical regions of seedlings as in A. B to D, The wild type. E and F, The axr1-12 mutant. B and E, Dark, no BAP. C and F, Dark, 10 µm BAP. D, Light. Bar = 200 µm.
Active Cell Proliferation in Young Leaf Primordia Can Be Reversibly Arrested in the Dark
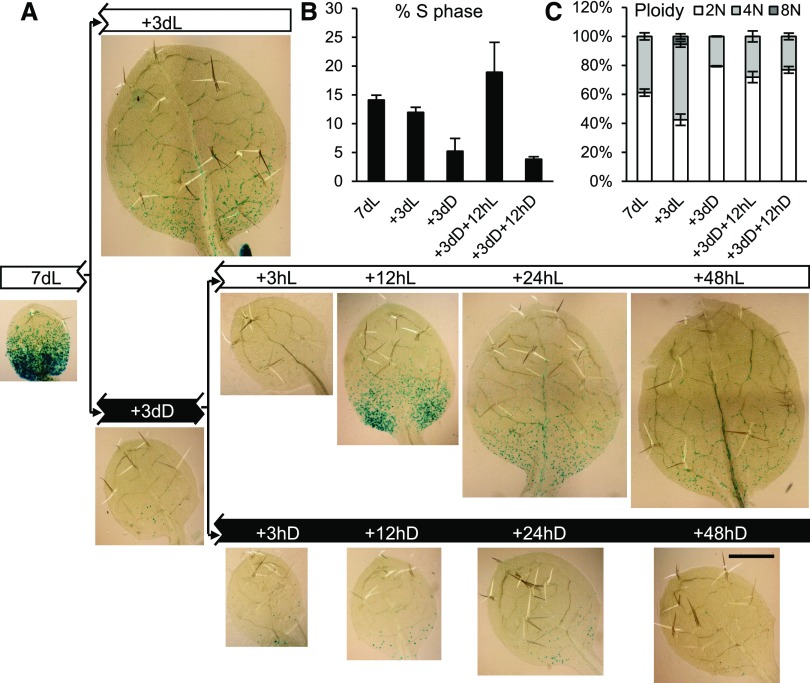
Skotomorphogenesis facilitates seedling establishment upon germination in soil, but photoreceptors remain active throughout the life of the plant. We asked whether the control of leaf development by photomorphogenic pathways remained active after the establishment of leaf primordia, using the well-established CYCLINB1;1:DB-GUS mitotic reporter (Colón-Carmona et al., 1999; Donnelly et al., 1999). Seven-day-old, light-grown seedlings displayed leaves 1 and 2, which were about 0.5 mm in length and which exhibited abundant mitotic activity in the proximal region (Fig. 4A; Supplemental Fig. S1). Flow cytometric ploidy analysis of these leaf primordia showed that around 60% of cells had 2N and 40% had 4N nuclear DNA content (Fig. 4C). Cell cycle analysis of the flow cytometry data revealed that a high proportion of nuclei were undergoing DNA synthesis (Fig. 4B; for extended data, see Supplemental Fig. S2A), indicating that these cells are very actively proliferating. A further 3 d in the light led to a pronounced increase in organ size as cells exited proliferation and entered cellular expansion. Flow cytometry confirmed an increase in the number of cells with higher ploidy levels, including cells that entered endoreduplication (with 8N nuclei; Fig. 4C; Supplemental Fig. S2B). In contrast, transfer to dark for 3 d led to the almost complete losses of mitotic activity, organ expansion, and endoreduplication; instead, an increase in the proportion of 2N nuclei occurred, indicating a widespread G1 arrest in the dark (Fig. 4; Supplemental Fig. S2). Reexposure to light triggered a reinitiation of cell proliferation, as indicated at 12 h by the increased mitotic activity (note the GUS mitotic signal in Fig. 4A), increased number of cells undergoing DNA synthesis as measured by flow cytometry (note the increase of S-phase nuclei in Fig. 4B; Supplemental Fig. S2), and an increased percentage of 4N nuclei, indicating cells that had passed through DNA synthesis (Fig. 4C). At the later time point of 48 h, cells with 8N nuclei also appeared, indicating the start of endoreduplication-associated cell expansion (Supplemental Fig. S2B).
Figure 4.
Proliferation activity arrest following transfer to dark, and reinitiation of mitotic activity in the light in proliferation-competent cells at the leaf base. A, CYCB1;1::DB-GUS-expressing seedlings were grown for 7 d in continuous light (7dL), harvested immediately or transferred to 3 d of continuous light (+3dL) or continuous dark (+3dD), and the latter were transferred back to light, after which they were harvested at the times indicated in hours. A leaf of the first leaf pair, after visualizing the GUS reporter, is shown. Blue GUS stain indicates cells undergoing mitosis in an acropetal gradient. Bar = 500 µm. B, S-phase percentage of total nuclei determined by flow cytometry and cell cycle analysis of nuclei from leaf primordia under the conditions indicated. C, Percentage of nuclei with different ploidy levels under the conditions indicated. Error bars represent sd (n = 3, with each sample containing a pool of at least five leaves).
The above observations were made on seedlings grown on Suc-containing plates, but similar phenomena took place in the absence of exogenous Suc as well. While some aspects of the response, like the increase in the proportion of nuclei in S phase in the light, were not as pronounced (Supplemental Fig. S2), others, like the reinitiation of mitotic events, were even more so (Supplemental Fig. S3). These experiments suggest that prolonged dark exposure of young, developing leaves leads to G1 arrest and block of endoreduplication irrespective of whether the seedlings are grown on Suc-free or Suc-containing plates. Upon light exposure, the arrest in G1 cell cycle phase is reversed and cells rapidly enter into S phase and mitosis.
Dark-Arrested and Light-Reactivated Leaf Primordia Exhibit an Arrest/Growth Gene Expression Program
We previously observed a program of rapid up-regulation of the expression of growth-related genes at the shoot apex, as leaves initiated development in the light (López-Juez et al., 2008). Having established a system of dark arrest, light reactivation of leaf growth, we made use of it to monitor the expression of genes selected to represent DNA synthesis and mitosis and translation capacity/ribosome buildup (Table I). We assessed whether a comparable gene expression program to that seen during deetiolation took place during dark arrest and light reactivation of growth in the dissected first leaf pair. We performed these experiments on seedlings grown on Suc-containing medium.
Table I. Genes monitored as representatives of biological growth processes, and products they encode.
| Process | Gene | Arabidopsis Genome Initiative Code | Encoded Product |
|---|---|---|---|
| Cell cycle entry block | KRP4 | At2g32710 | Kip-related protein4 |
| DNA synthesis (S phase) | RNR2A | At3g23580 | Ribonucleoside-diphosphate reductase small chain A |
| H2A | At1g51060 | Histone 2A | |
| Mitosis (M phase) | CYCB1;1 | At4g37490 | Cyclin B1;1 |
| Ribosome biosynthesis | RPS6 | At4g31700 | 40S ribosomal protein S6-1 |
| EBP1 | At3g51800 | ERBB-3 binding protein 1 | |
| Auxin response | AUX1 | At2g38120 | Auxin resistant1 |
| IAA1 | At4g14560 | indole-3-acetic acid inducible1 | |
| HAT2 | At5g47370 | Homeobox-Leu zipper protein2 | |
| Auxin synthesis | TAA1 | At1g70560 | Trp aminotransferase1 |
| TAR2 | At4g24670 | Trp aminotransferase-related protein2 | |
| Cytokinin response | ARR5 | At3g48100 | Arabidopsis two-component response regulator5 |
| Ethylene response | EIN3 | At3g20770 | Ethylene insensitive3 |
| EBP | At3g16770 | Ethylene-responsive element binding protein | |
| Starvation of reduced carbon | bZIP1 | At5g49450 | Basic Leu zipper1 |
| TPS9 | At1g23870 | Trehalose-6-phosphatase/synthase9 | |
| Mesophyll cell (chloroplast) development | GC1 | At2g21280 | Giant chloroplast1 |
| ARC5 | At3g19720 | Accumulation and replication of chloroplasts5 | |
| Vascular/vein development | VND6 | At5g62380 | Vein deficient6 |
| ATHB8 | At4g32880 | Homeobox-Leu zipper protein8 |
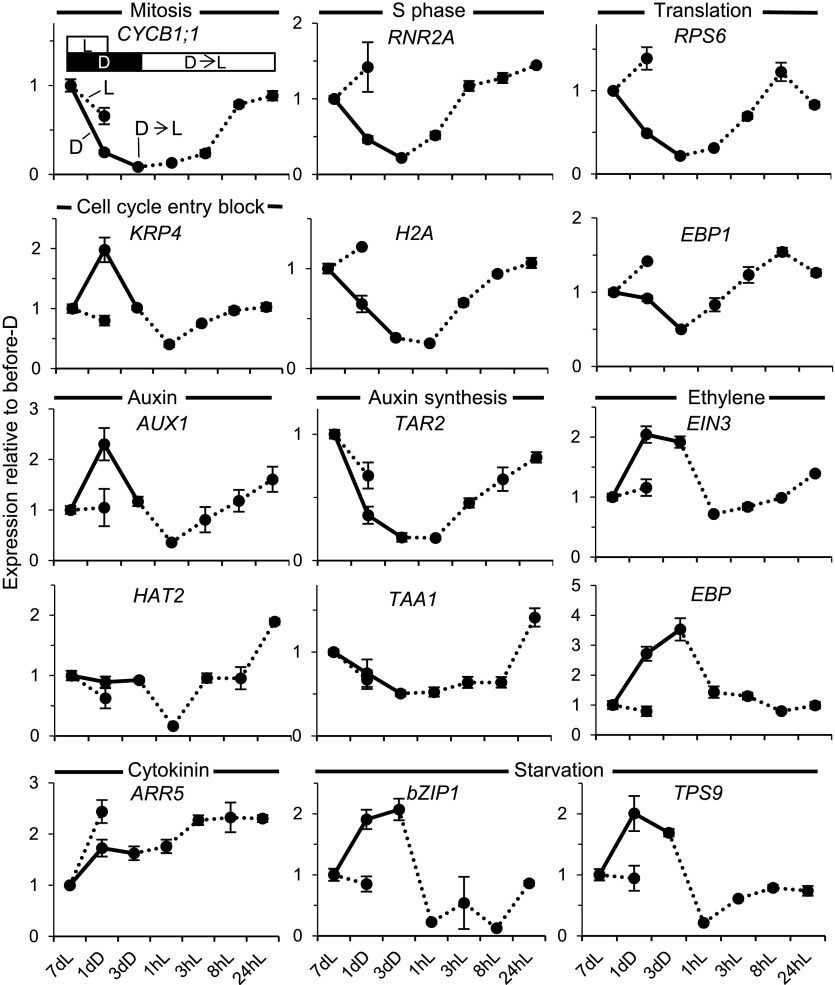
Genes associated with mitosis (CYCB1;1), DNA synthesis (RNR2A and H2A), and translation (RPS6 and EBP1) were all repressed during the 3-d dark period and were up-regulated in the first leaf pair within 8 h following reexposure to light; in several cases, up-regulation could be detected already at 3 h after reexposure (Fig. 5).
Figure 5.
Expression of signature genes during dark arrest and subsequent light exposure in young leaf primordia. The dark arrest blocks the cell proliferation and growth genetic program and activates starvation genetic responses. Light reverses these and brings about hormonal resetting. Wild-type seedlings were gown in light on Suc-containing plates, transferred to dark, and returned to light under conditions and times identical to those for Figure 4 or after 8 d in continuous light. Seedlings harvested at the corresponding times had the primordia of leaves 1 and 2 dissected, and the expression of the genes shown, representing the biological process indicated above each graph and in Table I, was monitored by quantitative real-time PCR. Error bars indicate se (between biological replicates).
The originally observed rapid changes in hormonal responses in the shoot apex also took place in the developing leaves: transfer to dark caused a mild elevation of auxin responses, as indicated by the auxin-responsive AUX1 gene, while light exposure brought about within 1 h a transient, substantial drop, which preceded a mild up-regulation of cytokinin-responsive gene expression (ARR5). At later time points during light-reinitiated leaf growth, between 3 and 24 h, both the expression of auxin biosynthesis genes (TAR2 and TAA1) and that of auxin-responsive AUX1 and HAT2 increased. In contrast, the expression of two genes representing ethylene response (EIN3 and EBP) was elevated consistently in the dark and reduced in the light.
As expected, the expression of starvation genes became up-regulated in the dark, reflecting the establishment of a starvation state, and rapidly dropped upon transfer to light, within 1 h (Fig. 5). Since this happened in spite of the fact that the seedlings were grown on Suc-containing plates, the dark-induced elevation of transcript levels of starvation genes and their rapid decrease upon light exposure might be under photomorphogenic control in young developing leaves. The gene expression changes upon dark arrest and light reexposure on plates with Suc also were largely replicated when seedlings were grown in the absence of exogenous Suc (Supplemental Fig. S4). A notable difference between the experiments on Suc-containing and Suc-free plates was that, in the latter, cell cycle- and growth-associated genes declined both in the dark and when seedlings remained in the light. This might relate to differences in the leaf growth kinetics under these two conditions. However, a clear, further suppression during dark acclimation occurs in both conditions. These gene expression changes are unlikely to be circadian regulated. Although eight out of the 20 selected genes monitored in this study were reported to exhibit circadian expression, the circadian pattern of expression of only one (ARR5) coincided with the observed pattern in our experiment, an elevation at the start of light exposure (dawn [Zeitgeber 0 h]; Supplemental Table S1). The extended, slightly finer time course examined for seedlings in the absence of Suc also showed that the changes occurring did not fit an underlying endogenous, circadian control and were most likely a direct consequence of the light exposure.
The reinitiation of leaf development necessitates the differentiation of all cell types that, in essence, consist of an epidermis enclosing a combination of photosynthetic mesophyll and vascular cells. We could indeed observe that the dark arrest was accompanied by a reduction of the expression of marker genes for early chloroplast biogenesis (GC1 and ARC5) and for the initiation of vascular development (VND6 and ATHB8) and that both types of cellular differentiation were promoted by reexposure to light (Supplemental Fig. S5A).
The Starvation/Growth Arrest Gene Expression Program Is Largely under the Control of the COP1-Dependent Photomorphogenic Pathway
To address whether the gene expression program upon dark arrest and light reexposure of young developing leaves is imposed by photosynthetic activity status or light signaling, we performed these experiments using the cop1-1 mutant. In the dark, this mutant maintains active photomorphogenic signaling pathways, even though photosynthesis is completely absent. Transfer of cop1-1 seedlings to dark did not cause a leaf growth arrest, as revealed by the additional area of white tissue produced in the young leaves during the dark exposure, proximal to the green tip developed prior to the dark transfer (Fig. 6, inset). We then monitored gene expression signatures associated with growth, hormones (auxin, ethylene, and cytokinin), and starvation in cop1-1 mutant seedlings compared with the wild type upon 3 d in dark (Fig. 6A). Compared with the wild type, cell proliferation and growth gene expression signatures were less impacted by the dark adaptation in cop1-1 (Fig. 6A). The reduced expression of a gene involved in auxin synthesis, as well as the up-regulation of ethylene action and of the starvation response in the dark, were all attenuated in cop1 (Fig. 6A). The same difference in expression was observed for the cell type-specific signature genes (Supplemental Fig. S5B). We then examined the kinetics of the auxin response by monitoring AUX1 gene expression both during dark arrest and light reactivation. The rapid, transient down-regulation of auxin response following reexposure to light was present in the cop1-1 mutant (Fig. 6B). This implies that a COP1-dependent photomorphogenic pathway is responsible for the bulk of the gene expression program in the dark. However, the transient down-regulation of auxin signaling during the dark-to-light transition appears to be independent of COP1 action.
Figure 6.
The gene expression program change in the light is brought about to a large extent by COP1-dependent photomorphogenesis pathways. A, Expression in leaf primordia of the genes indicated after 7dL + 3dD is shown, plotted on a log2 scale relative to the levels after 7dL, in the cop1 mutant and its wild type (WT) grown on Suc-containing plates. The inset shows leaf primordia of a 3dD-adapted cop1 seedling. B, Expression of AUX1 after 7dL, + 3dD, and following transfer back to light (times indicated). Error bars indicate se.
Direct Suc Access to the Shoot Apex Activates Cell Proliferation and the Growth Gene Expression Program in the Dark
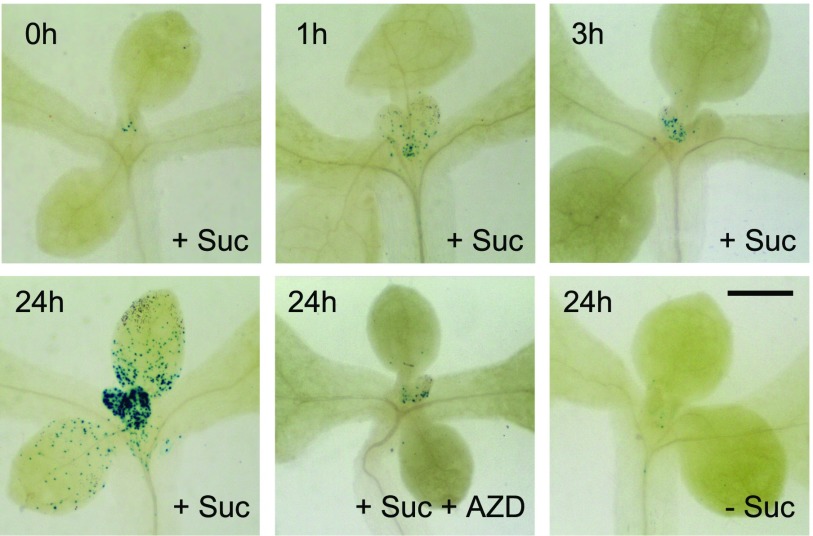
We have shown that the shoot apex in the dark locally experiences a starvation state, which is terminated rapidly by light in a way that cannot be explained by photosynthetic activity. Intriguing observations have shown that exposure of the meristem to Suc or Glc can trigger the further growth of organs in the dark (Roldán et al., 1999; Li et al., 2017). We made Suc available to the shoot apices of seedlings in the dark using the following strategy: 7-d light-grown seedlings, exhibiting active meristematic activity, were arrested by transferring to dark in Suc-free liquid culture, and after 3 d, the culture medium was replaced, under very dim green safelight, with Suc-containing medium, in which the seedlings continued to grow. Monitoring of the CYCB1;1:DB-GUS reporter demonstrated that the mitotic activity of young developing leaves in light (Fig. 4) all but disappeared during dark adaptation in the absence of Suc, while exposure to Suc resulted in a reemergence of mitotic activity, which was most pronounced after 24 h (Fig. 7; Supplemental Fig. S6A). The most frequent localization of such events was the proximal region of leaf primordia (Fig. 7).
Figure 7.
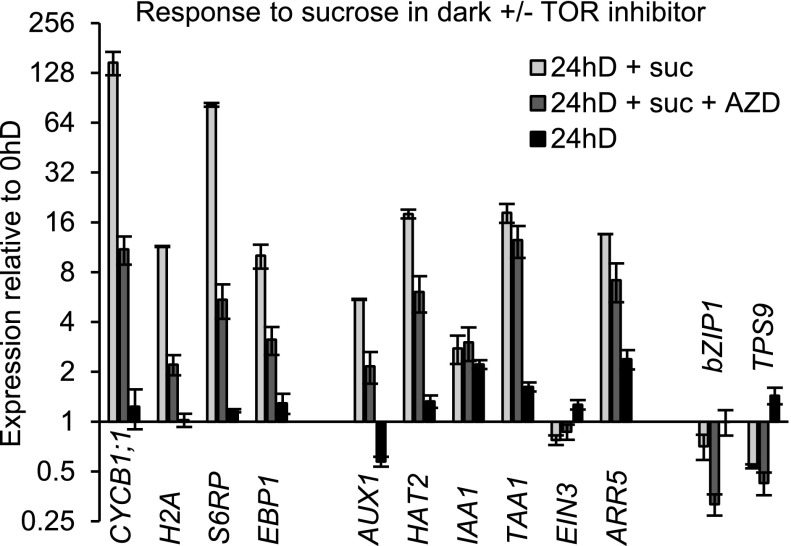
Direct Suc access to the meristem reactivates cell proliferation in the absence of light in a TOR-dependent manner. CYCB1;1::DB-GUS-expressing seedlings were grown on solid medium plates in light for 7 d, transferred to Suc-free liquid medium in the dark for 3 d, and visualized for GUS expression as follows: after subsequent transfer to medium containing Suc, or to medium containing Suc plus AZD-8055, or to Suc-free medium, for the times indicated. Bar = 500 µm.
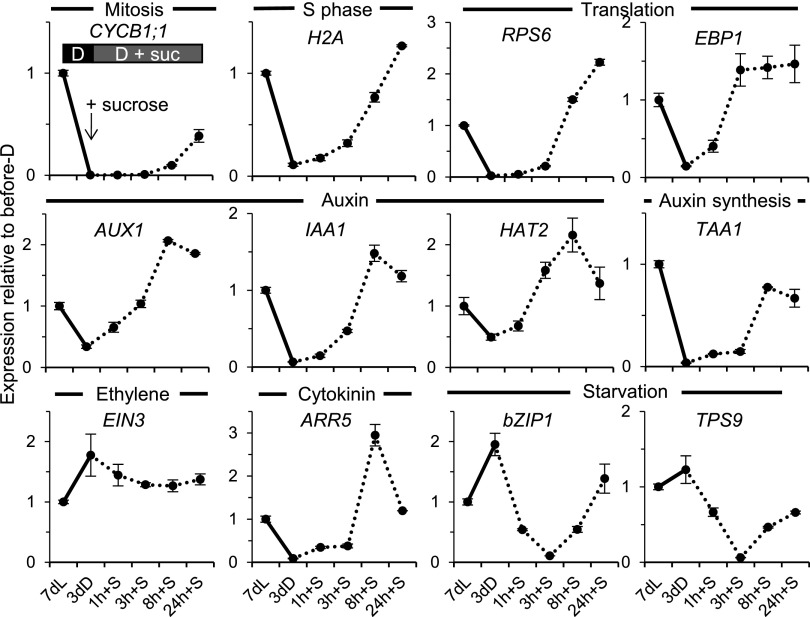
We monitored the gene expression program initiated by direct exposure of the meristem to Suc in the dark (Fig. 8). As expected from the observation of reactivation of mitotic activity visualized by the CYCB1;1:DB-GUS reporter, the cell proliferation- and growth-associated gene expression also was strongly stimulated by direct Suc access, with a simultaneous rapid down-regulation of starvation signature genes (Fig. 8). The induction of genes associated with plastid biogenesis and vasculature development also exhibited light-like responses (Supplemental Fig. S5C). Three notable differences, however, could be observed in comparison with the response to light. First, the response of growth-related genes to direct Suc supply was somewhat slower than that to light, generally clear after 8 h rather than 3 h. Second, the rapid, transient down-regulation of auxin responses upon the dark-to-light transition was not seen when dark-adapted seedlings were exposed to Suc; only a strong increase of such responses was observed, as confirmed by three separate signature genes, suggesting that a rapid activation of auxin export had not taken place under Suc influence, only the up-regulation of auxin synthesis had. Third, ethylene responses, which were rapidly down-regulated by light, were reduced only mildly after Suc exposure in the dark (Fig. 8). We conclude that, during leaf development, cell proliferation, cytoplasmic growth, aspects of plastid biogenesis, and vasculature differentiation all are under Suc control and can occur in the dark.
Figure 8.
Direct Suc access activates a proliferation and growth gene expression program. Wild-type seedlings grown for 7 d in continuous light on solid medium were transferred to Suc-free liquid medium in darkness for 3 d, then transferred to Suc-containing medium for the times indicated. Seedling shoot apices were dissected, and gene expression was quantified and displayed as in Figure 5.
The Organs Developed by Meristem Activation through Direct Access to Sugar Differ in the Dark
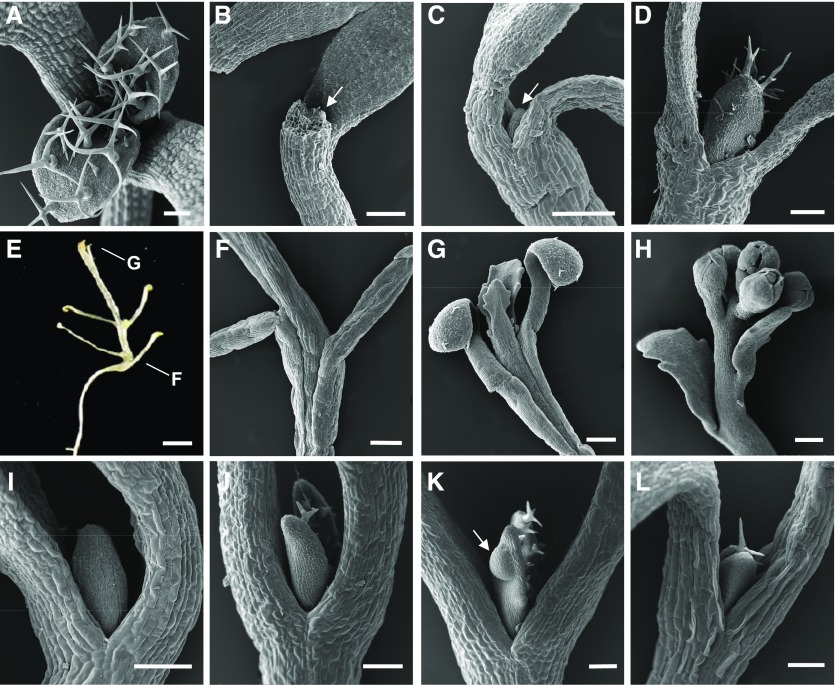
Following an extended 6-d incubation in Suc-containing liquid medium in the dark, we observed the appearance of an internode between the youngest leaf primordia and the point of cotyledon emergence (Supplemental Fig. S7). To examine this further, we administered a prolonged exposure of the meristem to Suc in the dark while avoiding the hypoxia that characteristically occurs in liquid culture, by growing seedlings on vertical Suc-containing solid medium, where apices of seedlings contact the medium’s surface, as carried out by Roldán et al. (1999). The shoot apex of seedlings grown on horizontal, Suc-containing medium developed leaves only in the light but was completely arrested in the dark (Fig. 9, A and B). The meristem of seedlings grown in the dark in liquid medium without Suc also was arrested, while if the medium contained Suc, the leaf primordia developed (Fig. 9, C and D). This indicated that direct sugar access is required for leaf initiation. Prolonged growth of shoot apices in contact with Suc led to extraordinarily elongated seedlings (Fig. 9E), with unusually long petioles of cotyledons and new leaves as well as internodes (Fig. 9, E–H). Elongation of the internodes reflects premature activation of the rib meristem. Leaf lamina barely developed (Fig. 9G); however, the transition to flowering occurred (Fig. 9H). Addition of BAP to the medium of seedlings whose shoot meristems were not in contact with Suc also initiated leaf development, both in the wild type and in the axr1 mutant background (Fig. 9, I–J). In addition, we noted in the axr1 mutant occasional tumor-like growths on some leaf primordia when exposed to cytokinin (Fig. 9K). The cop1 mutant also developed leaf primordia in the dark without direct contact with Suc-containing medium (Fig. 9L). We conclude that sugar can promote leaf initiation in the dark only through direct access to the shoot apex and that the dark arrest also can be overcome by a light-like shift in hormonal activity or by the removal of COP1, thus activating photomorphogenic signaling.
Figure 9.
The dark arrest of leaf initiation can be overcome by direct access to Suc, change in hormonal response, or by the loss of COP1. Scanning electron micrographs show shoot apices of seedlings of the wild type (A–I), axr1-12 mutant (J and K), and cop1 mutant (L) genotypes. All seedlings except that in A were grown in continuous dark. A, Wild type, continuous light, 7 d, horizontal Suc-containing plate. B, As in A but in continuous dark. C, Wild type, 17 d, Suc-free liquid medium. D, Wild type, 7 d, Suc-containing liquid medium. E, Wild type, 28 d, vertical Suc-containing plate. F, Detail of a seedling equivalent to that in E. G, Detail of a seedling equivalent to that in E. H, Detail of a seedling equivalent to that in E but grown for 42 d. I, Wild type, 7 d, horizontal Suc-containing plates with 10 µm BAP. J, axr1 mutant, 7 d, horizontal Suc-containing plates with 2 µm BAP. K, As in J. L, cop1 mutant, 7 d, horizontal Suc-containing plates. Arrows in B and C indicate leaf primordia; the arrow in K indicates a tumor-like growth. Bars = 100 µm (A–D and I–L), 200 µm (F–H), and 2 mm (E).
The strategy of enhancing Suc access through the growth of seedlings on Suc-containing vertical plates maintains full exposure of the seedlings to ambient air. This allowed us to also test whether the growth response of the meristem and young leaf primordia relies on photosynthesis-generated Suc in the light. To this end, we designed an experimental setup that depletes CO2 in air (see “Materials and Methods”; Supplemental Fig. S8). The transfer of seedlings for 3 d into darkness on vertical plates without Suc led to the almost complete cessation of cell proliferation activity, while on Suc-containing vertical plates, with apices being in contact with the plate, cell proliferation remained active. An 8-h light exposure reactivated cell proliferation in the shoot meristem of seedlings grown in Suc-free medium, but this was prevented in CO2-free air, where photosynthesis cannot take place (Supplemental Fig. S8; for quantitation, see Supplemental Fig. S6B). We conclude that the photomorphogenic response of the meristem and leaf primordia to light requires photosynthesis-generated reduced carbon.
The Growth Response to Suc Is Mediated by the TOR Pathway
Having observed key aspects of the genetic program that are initiated by exposure to Suc, we used a pharmacological approach to determine which of those aspects were TOR dependent. TOR is a structurally and functionally conserved protein kinase belonging to the PI3K-like protein kinase family (Dobrenel et al., 2016a). Because of this conservation, highly specific ATP-competitive TOR inhibitors developed in animal or yeast cells (Liu et al., 2012), including AZD-8055, have been shown to be effective in plants (Montané and Menand, 2013; Dong et al., 2015; Kravchenko et al., 2015; Schepetilnikov et al., 2017). The fact that AtTOR heterozygous knockout plants are hypersensitive to AZD-8055 in terms of root growth (the TOR gene becomes haploinsufficient; Montané and Menand, 2013) is a strong indication that TOR is the genuine target. This was experimentally proven by measuring the activity of direct downstream TOR targets, S6 kinase and S6 phosphorylation, both of which were strongly inhibited by AZD-8055 (Dobrenel et al., 2016b; Schepetilnikov et al., 2017). We carried out treatment with this selective TOR inhibitor, at previously used concentrations, and observed that it dramatically reduced the mitotic activity in young leaf primordia (Fig. 7; Supplemental Fig. S6C). It also reduced the Suc-induced expression of cell cycle and cell growth signature genes, confirming that these processes are, to a large extent, mediated by the TOR pathway (Fig. 10; note the log scale). Remarkably, the up-regulation of two out of three auxin-response genes also was found to be partially TOR dependent. Interestingly, we found genes involved in plastid biogenesis to be particularly sensitive to TOR inhibition (Supplemental Fig. S5D). In the dark, the addition of Suc repressed the expression of starvation genes in leaf primordia, but only to some extent after 24 h (Fig. 10). Unexpectedly, the addition of AZD-8055 further reduced their expression, indicating that the sugar repression of starvation genes is modulated, but not dependent on TOR signaling. We conclude that Suc access acts on the meristem in a TOR pathway-dependent manner, which leads to the bulk of responses impacting on cell and organ growth.
Figure 10.
The gene expression program induced by Suc in the dark is largely TOR dependent. Expression in the shoot apex and leaf primordia of the genes indicated is shown following the growth treatment described for Figure 7 (7dL in solid medium followed by 3dD in Suc-free liquid medium), after transfer for a further 24 h to medium containing Suc with or without AZD-8055, or without Suc. Expression quantitation by quantitative real-time PCR is displayed as in Figure 6.
Light Fluence Rate Increases Lead to an Accelerated Development of Leaves with More Cells
One obvious advantage for plants to utilize energy signaling to determine meristematic activity would be that it would allow them to adjust organ growth to the constantly changing level of available resources, the products of photosynthetic activity. It is known that, under high irradiance, leaves develop with a multilayer palisade mesophyll to support photosynthetic performance (López-Juez et al., 2007; Kalve et al., 2014b). Here, we tested how the mitotic activity becomes modulated in response to changing light intensity throughout the leaf by analyzing the CYCB1;1:DB-GUS reporter in the palisade layer. We found a rapid increase of mitotic activity soon after the transfer from low light (LL) to high light (HL; Fig. 11A) as well as an increased S-phase proportion measured by flow cytometry (Supplemental Fig. S9). The mitotic events occurred in the competent, proximal region of young leaf primordia (leaf 3 onward), but a few were visible even in leaves 1 and 2 only under HL (Fig. 11B). Cells of leaves 3 and 4 also entered endoreduplication at an accelerated rate in HL (Supplemental Fig. S9), as could be expected given the greater extent of cell expansion under those conditions.
Figure 11.
During growth in the light, exposure to HL for 8 or 24 h increases cell proliferation. A, CYCB1;1::DB-GUS-expressing seedlings were grown for 7dL, transferred to soil, adapted to LL (40 μmol m−2 s−1) until day 11 (see “Materials and Methods”), then harvested immediately or after transfer to HL (300 μmol m−2 s−1; top row) or maintained at LL (bottom row) for the times indicated, and visualized for GUS reporter activity. Leaf 3 is shown. Bar = 200 µm. B, Apical region, displaying primordia of leaves 3 and 4, 8 h after the light transfer, visualized for the GUS reporter. The arrows indicates mitotic events in the young leaf 2.
As a result of an increased mitotic activity, the cell number across the leaf, as calculated by dividing leaf area by weighted, average palisade cell areas at proximal, middle, and distal regions, over a longer time course, also increased (Supplemental Fig. S10A). The average size of mesophyll cells was much smaller in the proximal than in the middle and distal regions, whether grown under LL or HL, with size increasing as cell expansion took place. Here, we detected a higher mitotic activity in leaves at LL compared with those of the same age at HL, indicating that the entire developmental program is slowed down and that there is a delayed exit from proliferation to differentiation under LL (Supplemental Fig. S10A). In agreement, while after 4 d some mitotic activity remains at the distal region of the leaf in LL, mitotic activity had already ceased in this area in leaves developing in HL (Supplemental Fig. S10B).
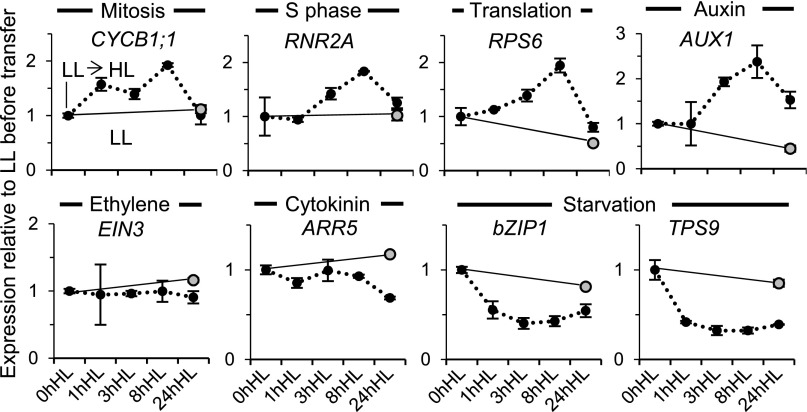
Correspondingly with the immediate increase in cell proliferation activity upon transfer of seedlings from LL to HL, the expression of cell cycle and cell growth signature genes in young developing leaves of the seedling apex also showed up-regulation (Fig. 12). Notably, the auxin-responsive AUX1 expression also increased in HL, while starvation gene transcript levels decreased, showing that light quantity sensitively modulates hormone and energy signaling in developing leaves (Fig. 12). Genes for chloroplast biogenesis and vascular differentiation, ARC5 and VND6, respectively, showed a transient decrease followed by an increase upon HL transfer, indicating that the transient burst in cell proliferation is accompanied by an early but transient arrest in cellular differentiation (Supplemental Fig. S5E). We conclude that, like the dark-to-light transition, a change in light intensity rapidly alters the energy, hormonal, cell proliferation, and differentiation programs.
Figure 12.
Gene expression changes after transfer to HL. The expression of signature genes in the shoot apex and leaf primordia, following the transfer to HL as described for Figure 11, is shown. Expression quantitation by quantitative real-time PCR is displayed as for Figure 5.
The Effect of HL on Cell Proliferation in Young Leaves Is Non-Cellautonomous
If available photosynthates, produced by photosynthetically-competent leaves, are indeed the proliferative signal in young leaf primordia, one would predict that the exposure of mature leaves to HL would be sufficient to stimulate cell proliferation in primordia emerging from the meristem. To test this hypothesis, we allowed Arabidopsis rosettes to develop to a larger size and acclimated them to LL. We then used local shading of only the meristematic region, including young primordia, or of the entire seedling except that region, during a shift from LL to HL for 8 h (Supplemental Fig. S11) and monitored the expression of the mitotic reporter. We found an increase in the mitotic activity in the young leaf 8 even when it was itself shaded (remained under LL) and only the mature leaves became exposed to HL. This increase was similar to that when the whole plant was uniformly exposed to HL, indicating a systemic action of the HL effect from mature leaves to very young ones (Supplemental Fig. S11).
We then asked whether the ability to respond to the HL signal was restricted to a developmental window. We showed earlier that the first leaf primordia pair of light-grown seedlings on Suc-containing medium exhibited extensive numbers of cells undergoing mitotic activity at day 7, a much reduced number if development continued until day 10 in the light, and almost none if development was arrested for 3 d in the dark (Fig. 4). We exposed identically grown, 10-d, constant light-grown seedlings to HL for 48 h. This led to a few extra events of mitotic activity (Supplemental Fig. S12), but their number was minimal compared with that caused by the light exposure of dark-arrested primordia of 7-d-old seedlings (Fig. 4). Interestingly, such events at this later stage tended to be associated with provascular or vascular cells throughout the leaf lamina, not just the proximal region. We conclude that most leaf primordia cells are competent to respond to light signals with increased mitotic activity only during a very early developmental window and that, at a later stage, when most cells have already exited the cell cycle during normal development, only vascular cells are competent to respond to HL exposure through cell division.
DISCUSSION
How leaves form at the shoot meristem is a central developmental question. Understanding how light, as a natural trigger, brings about the transition from meristem arrest to activity, or how light intensity changes modulate leaf emergence, can provide fundamental clues to this basic biological phenomenon. Taking together the results of this and previous studies (López-Juez et al., 2008; Yoshida et al., 2011; Pfeiffer et al., 2016; Li et al., 2017), a picture of how light, hormonal, and energy signaling mechanisms regulate leaf development emerges.
The hormonal switch centers on the biology of auxin. Auxin has a complex role in leaf initiation (Braybrook and Kuhlemeier, 2010; Capua and Eshed, 2017), both growth promoting and growth inhibiting, but it appears from our data that, in the dark, auxin becomes diffusely localized in the meristem and that this inhibits the emergence of primordia. One outcome of such activity is to prevent the occurrence of auxin maxima, while another may be to prevent cytokinin action. Such an antagonistic action would be consistent with the unexpected observation of occasional, tumor-like growths in primordia of the auxin-resistant mutant exposed to cytokinin. At least two mechanisms are known by which this auxin/cytokinin antagonism could take place: the auxin response factor Monopteros inhibits cytokinin signaling (Pacifici et al., 2015; Pfeiffer et al., 2016), and auxin also promotes the expression of CKX6, a gene for cytokinin inactivation, in young leaves under simulated shade (Carabelli et al., 2007). A close homolog of this gene, CKX5, also is repressed in the shoot apex by the first light exposure (López-Juez et al., 2008), and simultaneous inactivation of CKX5 and CKX6 enhances the expression of the meristem-organizing WUSCHEL gene (Pfeiffer et al., 2016), helping to explain, at least in part, the initial meristem-repressive auxin role. Meanwhile, the absence of auxin maxima prevents the initiation of auxin export, necessary for leaf initiation (Reinhardt et al., 2003). Indeed, we observed the simultaneous establishment of polar localization of PIN1 toward primordia tip maxima in the epidermis and away in the developing mid vein toward the rib meristem. Once maxima are established, auxin clearly plays a positive role, needed to direct the expansion of primordia and the differentiation of vasculature (Scarpella et al., 2006, 2010). As part of the complex action of auxin, we confirmed that a strong, localized auxin activity occurs at the tips of emerging primordia in the light and that light promotes the expression of at least some auxin biosynthesis genes. Meanwhile, cytokinin plays an unambiguously positive role, as demonstrated previously (Chory et al., 1994; Yoshida et al., 2011; Pfeiffer et al., 2016), and our data show that reduced auxin and enhanced cytokinin activity not only phenocopy a photomorphogenic state but form an intrinsic part of the endogenous, early photomorphogenic program under direct light regulation. Our results further show that their effects interact, confirming their shared underlying growth output.
A finding, surprising at first, in our experiments was the fact that energy signaling through direct exposure of the meristem to Suc is itself capable of promoting at least some auxin responses, as evidenced by the regulation of signature genes (Fig. 8). This action was, for two out of three genes tested, TOR dependent (Fig. 10). It has been demonstrated that the TOR kinase, in addition to mediating cell proliferation and protein synthesis in response to sugar, also mediates the translational control of expression of several auxin response factors in response to auxin (Schepetilnikov et al., 2013). The activation of TOR by auxin occurs through a family of small GTPases (Schepetilnikov et al., 2017). Therefore, this central growth kinase may occupy a crucible of growth actions underpinning energy and auxin signaling and explain some of their partly shared responses.
Energy signaling plays a central role in the control of both cellular growth (Dobrenel et al., 2016a, 2016b) and cell proliferation (Xiong et al., 2013). It can boost meristematic activity (Pfeiffer et al., 2016; Li et al., 2017) and, indeed, through direct sugar access to the meristem, override the dark repression completely. However, on its own, it cannot lead to photomorphogenic-like rosette leaves. Instead, the meristem overwhelmingly produces petioles and internodes (Fig. 9; Supplemental Fig. S7). While such developmental behavior resembles the phenotype of auxin-overproducing seedlings (Chen et al., 2014), a central key factor may be ethylene, responses to which are strong in the dark and are barely affected by Suc exposure. Ethylene signaling is necessary for hypocotyl hook formation, a component of the skotomorphogenic program (Marín-de la Rosa et al., 2014), and pea (Pisum sativum) phytochrome mutants have been shown to exhibit strong ethylene responses (Foo et al., 2006). Auxin synthesis genes were identified in genetic screens for weak ethylene insensitivity (Stepanova et al., 2008), because the ethylene actions under observation were mediated by newly synthesized auxin. Tellingly, pea phytochrome mutants produced leaves with limited laminae (Weller et al., 2015), as do phytochrome mutants of Arabidopsis (Tsukaya, 2005), and loss of an ethylene-dependent transcription factor gene restored in those pea mutants the wild-type leaf phenotype (Weller et al., 2015). Our observations not only confirm a fundamental role for auxin in leaf organ differentiation but also support a role for ethylene in directing the meristematic cellular activity toward elongating organs, like internodes and petioles in the dark, when ethylene response is high, or toward leaf laminae, with their distinct epidermal and mesophyll cellular makeup in the light, when ethylene responses are repressed. Whether this possible ethylene switch of the proliferative potential acts solely through auxin activities is unknown at present. An elegant genetic screen recently identified the LEAFLESS tomato gene, deficiency in which results in meristem cells producing only elongating internodes under auxin action (Capua and Eshed, 2017). The role of such genes in photomorphogenic leaf initiation also awaits further study. We should note, nevertheless, that following a substantially extended period of dark growth on Suc, after the transition to flowering, one could observe comparatively normal cauline leaves as well as floral buds (Fig. 9H). This could reflect environmental plasticity early in development, fully subjected to skotomorphogenic or photomorphogenic regulation, yet enhanced homeostasis of development following the transition to flowering. Whether this in any way relates to ethylene signaling, or competence to respond to it, is only a matter of conjecture at present.
Photomorphogenesis acts through a COP1-dependent pathway. Transcription factors that positively regulate light responses, including hypocotyl repression, cotyledon unfolding, and the initiation of chloroplast biogenesis, are marked by COP1 for proteolysis and are degraded through a proteasome-dependent activity in the dark (Lau and Deng, 2012). Although we could observe some degree of response to dark adaptation by the cop1 mutant, overall, those responses were clearly attenuated. It is a particularly intriguing aspect of the response to light that it can be overridden in terms of meristem activation, but not of developmental fate, by energy signaling. Light appears to play what could be described as a gating, or permissive, role toward energy signaling in that the extent of meristem activity is dependent on seed reserves or, later, photosynthates, but only when light is present does this reduced carbon become accessible to the meristem. This light role is dependent on photomorphogenic pathways, as it depends on photoreceptors (López-Juez et al., 2008) and COP1 (this study). One attractive hypothesis for the mechanism underlying the light-gating phenomenon is that, in a manner analogous to auxin export, sugar import into the meristem is under photoreceptor control in a COP1-dependent manner. This would explain the dramatic observations that direct sugar access to the meristem is capable of fully activating the meristem in the dark, which the growth of seedlings on Suc-containing solid medium alone cannot.
One exception to the involvement of COP1 is the transient drop of auxin responses in the light. COP1 in the dark is localized in the nucleus, where its targets are light-associated transcription factors. Thus, the transient auxin response drop is most probably the result of a postranslational control of auxin export via PIN1, the control being mediated by PIN1 localization. The nature of this control remains poorly understood, but posttranslational signaling cascades, mediated by protein kinases, control PIN1 localization under other developmental scenarios (Benjamins et al., 2001; Jia et al., 2016; Dory et al., 2017). Transcript levels of PIN1 also are activated in the shoot apical region by light exposure (López-Juez et al., 2008), further contributing to the establishment of fully fledged auxin transport capacity in the light.
The role of energy signaling becomes most apparent in the control of the cellular makeup of leaves under different irradiances. This control is mediated by the regulation of cell proliferation and cell growth pathways, crucially dependent on the central, TOR pathway. It is well established that HL-grown leaves develop a multilayer palisade (Weston et al., 2000; Tsukaya, 2005), and it would be tempting to assume that further cell proliferation events occur in the mesophyll to generate such cellular anatomy. However, the multilayer palisade is present in the youngest leaf primordium which is physically possible to examine, composed of just a few tens of cells (Kalve et al., 2014b), suggesting that it may actually arise from the recruitment of a larger number of meristematic cells into the primordium. Given that a previous study demonstrated that the cellular anatomy of very young leaves is determined by the light exposure of mature ones (Yano and Terashima, 2001), one can conclude that the recruitment of meristematic cells to primordia is under non-cellautonomous, systemic control. Our observations complement those by showing that proliferation events in division-competent cells of the young leaves increase the number of cells observed and are followed by accelerated endoreduplication and cellular expansion, which thus increases the surface area of the solar panel. This contrasts with observations of high- and low-irradiance leaves of a different species, in which no change in the total number of palisade cells was observed (Yano and Terashima, 2004). Those authors suggested that, in their experimental system, light irradiance only controlled the angle of cell division: anticlinal to form extra palisade in sun (HL), periclinal to extend the lamina in shade (LL). This is clearly not the case in our observations (Fig. 11; Supplemental Figs. S9 and S10), where high irradiance promoted extra cell proliferation in our leaf primordia of HL-exposed plants. Our data show that this also is a systemic response, dependent on the irradiance received by mature leaves, adding a further dimension to the impact of photosynthate signaling on meristematic activity. It was shown recently (Van Dingenen et al., 2016) that the larger organs under HL are explained to an extent by an increased import capacity of Glc into chloroplasts of young, meristematic, proximal leaf cells. This causes a down-regulation of the overall transcriptional activity in chloroplasts, which, in turn, delays the exit of those cells from proliferation. The extended proliferative phase contributes to increasing the final organ size. Such a mechanism would be expected to be cell autonomous, while the response we observed is not. How these interorganellar and energy-signaling regulatory mechanisms interact and delay or accelerate the exit into endoreduplication/differentiation remains to be answered.
CONCLUSION
Two stages in which the action of light determines meristematic activity become apparent in this study (for model, see Supplemental Fig. S13). First, the presence of light plays a permissive role (i.e. no cell cycle and growth activities can occur in prolonged dark). This action utilizes photomorphogenic pathways and is photoreceptor and, largely, COP1 dependent and makes use of auxin-, cytokinin-, and ethylene-dependent mechanisms of meristem organization, leaf initiation, and cell fate decision making, together with a photomorphogenic gating control of energy signaling. The latter may be due to the control of access to reduced carbon, activates the TOR signaling pathway, and has cell proliferation and growth as its output. Second, light irradiance determines the extent of cellular growth activities, adjusting the number of cells supplied and the extent of organ growth through the availability of photosynthates, and its action is mediated by the TOR pathway.
Our results here, together with previous studies, contribute to untangling the complex role and interactions of hormonal and energy signaling, through the action of the TOR kinase, to determine the activity of meristematic and early-organ cells in the light. They also have opened many new questions. Understanding the means by which TOR action arises from the combined energy and auxin response, uncovering the mechanism of photomorphogenic energy-signaling gating, the way in which the starvation state is imposed in the absence of photoreceptor action, and unraveling the different cellular and organ fates produced by meristematic activity under light or energy-only signaling should be among the matters addressed by further analyses. It is apparent, nevertheless, that energy signals may constitute the most important determinant of plant growth and, therefore, biomass production in nonstressed conditions.
MATERIALS AND METHODS
Plant Materials, Growth Conditions, and Experimental Treatments
Wild-type Arabidopsis (Arabidopsis thaliana) plants of the Columbia (Col) ecotype and the axr1-12 mutant (Leyser et al., 1993) were obtained from the Nottingham Arabidopsis Stock Centre. The cop1-4 mutant (Deng et al., 1991), the CYCLINB1;1:Dbox-GUS line (Colón-Carmona et al., 1999), and DR5:GUS (Ulmasov et al., 1997) were kind gifts of J. Gray, P. Doerner, and T.J. Guilfoyle respectively. Seedlings were plated on agar-solidified Murashige and Skoog (MS) medium, under continuous fluorescent white light (100 μmol m−2 s−1), in Percival I-30 or I-35 (CLF Plant Climatics) or in a dark incubator at 21°C, on horizontal plates containing 0.8% (w/v) agar-solidified MS medium and 1% (w/v) Suc, unless stated otherwise, and when required in the presence of BAP (Duchefa/Melford Laboratories) at the indicated concentration, as described previously (López-Juez et al., 2008). In liquid culture experiments, unless stated, seedlings were grown for 7 d on horizontal plates in the light as above, then transferred to six-well microtiter plates containing liquid MS medium devoid of Suc, for a further 3 d in the dark, with shaking (80 rpm), at which point the medium was replaced under very dim green safelight with fresh Suc-free or 1% Suc-containing medium, with or without the addition of 2 µm AZD-8055 (Sigma-Aldrich). Liquid-cultured seedlings shown in Figure 9 were grown from germination in the total absence of light. When indicated, seedlings were grown on 1.2% agar-solidified, vertically positioned square plates. For CO2-deprivation experiments, seedlings were grown on Suc-containing vertical plates in the light, and after 7 d, they were transferred to fresh Suc-containing or Suc-free vertical plates and double clear bagged with or without 5 g of indicator-containing soda lime (Fisher Scientific), as described previously (Kircher and Schopfer, 2012). For light quantity experiments, seedlings grown for 7 d on MS horizontal plates were transferred to soil, grown for 2 d at 100 μmol m−2 s−1, and adapted for a further 2 d to 40 μmol m−2 s−1 continuous LL, before transfer if required to 300 μmol m−2 s−1 continuous HL. For the cellular makeup experiment (Supplemental Fig. S10), seedlings were transferred to soil and adapted to LL until day 14, before transfer to HL or being maintained in LL for a further 6 d. Leaf 5 was monitored. To assess local or systemic light effects, seedlings were transferred to soil after plate growth and kept at 150 μmol m−2 s−1 for 6 d to achieve a sufficient rosette size, before adapting to LL for another 3 d, and then subjected to LL, HL, local HL or systemic HL by exposure to HL and the use of custom-sized neutral density celluloid filters. Leaves were collected 8 h later for GUS reporter assay.
Leaf Cellular Analysis, Immunocytochemistry, and Reporter Assay
Histochemical GUS assays took place largely as described (López-Juez et al., 2008) with minor modifications. After fixation (ice-cold 90% acetone), seedlings were infiltrated with GUS staining buffer to a final concentration of 0.3 mg mL−1 5-bromo-4-chloro-3-indolyl-β-glucuronic acid under vacuum with 0.5 atm pressure for 10 min. Seedlings were then kept in the dark at 37°C for 14 h followed by postincubation in 3:1 (v/v) methanol:acetic acid for 2 h and washes in 70% ethanol at 65°C for 10 min. Seedlings were mounted on slides in Hoyer’s solution. Digital images were recorded using a Nikon SMZ1500 equipped with a Nikon DXM1200 camera or Leica EZ4HD (Leica Microsystems) stereomicroscopes.
Primordia of BAP-treated axr1 or Columbia seedlings, or cellular anatomy of varying fluence rate leaves, were observed under Nomarski optics using a Nikon Optiphot 2 microscope equipped with a Nikon DXM1200 or a Micropublisher 5.0 RTV camera. The area of leaf primordia, individual cells, or GUS-stained areas were measured using ImageJ software (https://imagej.nih.gov/ij/). Except where indicated, measurements used 10 seedlings. To quantify cellular anatomy, leaves were divided into basal, mid, and distal thirds, average cell areas were measured in each region, the number of cells for each region was estimated as one-third of the leaf area divided by the corresponding average cell area, and the resulting number of cells was added for the three regions.
For immunocytochemistry, samples were fixed and processed as described previously (Gälweiler et al., 1998). PIN1 was detected in permeabilized seedlings incubated with an affinity-purified mouse anti-PIN1 monoclonal antibody (1:100) and monoclonal secondary antibody (Alexa 488-labeled goat anti-mouse at 1:1,000 dilution). Fluorescence was analyzed with a Zeiss LSM 5 DUO scanning microscope. Fluorescence-labeled anti-PIN antibody and DAPI fluorescence were monitored using multitracking in frame mode. Alexa 488 was excited using the 488-nm laser line in conjunction with a 505- to 530-nm band-pass filter. DAPI was excited with the 405-nm laser line and collected using a 420- to 480-nm band-pass filter.
Flow Cytometry Analysis
To determine cell DNA content, leaf primordia of a minimum of five seedlings per sample were dissected on agar, transferred to a few drops of ice-cold nuclei extraction buffer (CyStain UV Precise P kit; Sysmex Partec), and cells were chopped with a sharp razor blade as described previously (López-Juez et al., 2008). One milliliter of DAPI DNA-staining solution (Partec) was added, the sample was mixed, filtered, and analyzed through a PAS flow cytometer (Partec), and the fluorescence of different ploidy peaks was calibrated using Arabidopsis floral tissue. The proportions of peak areas at different ploidy levels were measured using Flomax software. Where only 2N and 4N peaks were present, cell cycle analysis mode was used to estimate the proportion of nuclei in S phase.
Analysis of Gene Expression
Seedlings were harvested into RNAlater (Sigma-Aldrich) and stored for a maximum of 7 d at 4°C, before dissecting primordia using a stereomicroscope (Nikon SMZ-2T) and flash freezing in liquid nitrogen. Dissected tissue consisted of the primordia of leaves 1 and 2 (dark arrest, liquid culture, AZD-8055, or cop1 experiments) or the shoot apex including the meristem and all leaf primordia (light fluence rate experiments). Arabidopsis total RNA was extracted using the Plant RNA mini spin kit (Macherey-Nagel) following the manufacturer’s instructions and quality checked by agarose gel electrophoresis. Two-microgram aliquots were reverse transcribed using the Maxima first-strand cDNA synthesis kit (Thermo Fisher Scientific). DNA was used for real-time amplification as described previously (Hills et al., 2015). Three independent biological replicates, each containing 150 to 200 dissected apices, were used for each sample type or time point, and all reactions took place in duplicate. Relative quantitation for each target gene used the ∆Ct method against the expression of a constitutive gene, UBQ10. Primers were designed using QuantPrime (http://quantprime.mpimp-golm.mpg.de/). Gene identifiers and corresponding primers are as listed (Supplemental Table S2).
Assessment of the possible circadian behavior of monitored genes (Supplemental Table S1) used the LL_LLHC data series available at the Diurnal tool (http://diurnal.mocklerlab.org/).
Scanning Electron Microscopy
Seedlings were placed in fixative (3% [v/v] glutaraldehyde plus 4% formaldehyde in 0.1 m PIPES, pH 7.2) at room temperature and stored at 4°C for 12 h. The primary fixative was removed, and seedlings were washed 2 × 10 min with 0.1 m PIPES, pH 7.2. Seedlings were dehydrated by immersion in 30%, 50%, 70%, 95%, and 95% ethanol, for 10 min each, followed by 2 × 20 min in 100% absolute ethanol. Fixed specimens were critical point dried in CO2, mounted on an aluminum scanning electron microscope stub with conductive glue, and sputter coated with gold/palladium before observation in an FEI Quanta 200 scanning electron microscope (Biomedical Imaging Unit, Southampton University Hospital).
Accession Numbers
Accession numbers are listed in Table I.
Supplemental Data
The following supplemental data are available.
Supplemental Figure S1. Quantitation of leaf area and CYCB1;1:DB-GUS expression in Figure 4.
Supplemental Figure S2. Flow cytometric cell cycle parameters in cells of leaf primordia equivalent to those in Figure 4.
Supplemental Figure S3. CYCB1;1:DB-GUS expression showing proliferation activity arrest following transfer to dark, and light reinitiation of mitotic activity in proliferation-competent cells, in seedlings grown on Suc-free plates.
Supplemental Figure S4. Gene expression analysis showing that dark arrest blocks the cell proliferation and growth genetic program and activates starvation genetic responses at the shoot apex in seedlings on Suc-free plates.
Supplemental Figure S5. Expression of genes associated with plastid biogenesis (primarily leaf mesophyll) and vascular development at the shoot apex in the dark arrest, cop1, Suc supply, TOR inhibitor, and light fluence rate experiments.
Supplemental Figure S6. Quantitation of the proportion of GUS-positive leaf area in Figures 7 and 11 and Supplemental Figure S8.
Supplemental Figure S7. CYCB1;1:DB-GUS expression showing prolonged access of the meristem to external Suc (in liquid medium) causes cell proliferation that extends petiole and internode organs.
Supplemental Figure S8. CYCB1;1:DB-GUS expression showing that access to external Suc maintains cell proliferation in the dark; light activates cell proliferation in the absence of external Suc, but this requires access to CO2 for photosynthesis.
Supplemental Figure S9. Flow cytometric cell cycle parameters showing that transfer to HL rapidly promotes cell proliferation and subsequently accelerates entry into endoreduplication.
Supplemental Figure S10. Total two-dimensional leaf cell number, percentage of dividing cells, and sample cell images showing that, during growth in the light, exposure to HL produces organs composed of a greater number of cells.
Supplemental Figure S11. Experimental setup and CYCB1;1:DB-GUS expression quantitation showing that HL acts systemically on cell proliferation in young leaf primordia after perception by mature leaves.
Supplemental Figure S12. Additional CYCB1;1:DB-GUS expression of partially developed leaves of 10-d light-grown seedlings transferred to HL occurs almost exclusively in vascular cells.
Supplemental Figure S13. Model of the impact of photoreceptor activation or exposure to HL and the occurrence or extent of leaf organ growth.
Supplemental Table S1. Circadian response, if known, of genes subjected to expression analysis.
Supplemental Table S2. Primers used for gene expression analysis.
Acknowledgments
We are indebted to John Gray, Peter Doerner, Thomas J. Guilfoyle, and the Nottingham Arabidopsis Stock Centre for the supply of materials; Rajat Yadav, James Hall, and Miranda Burke for skillful help with hormone reporter and deetiolation assays; Anton Page and members of the Southampton Bioimaging Unit for excellent support for scanning electron microscopy; and Czaba Papdi and members of the E.L.-J. and L.B. laboratories for constructive criticisms.
Footnotes
Work funded in part by NSF/BBSRC bilateral grant BB/M025047 to L.B.
[CC-BY]: Article free via Creative Commons CC-BY 4.0 license.
References
- Baena-González E, Rolland F, Thevelein JM, Sheen J (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942 [DOI] [PubMed] [Google Scholar]
- Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R (2001) The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128: 4057–4067 [DOI] [PubMed] [Google Scholar]
- Berckmans B, Lammens T, Van Den Daele H, Magyar Z, Bögre L, De Veylder L (2011) Light-dependent regulation of DEL1 is determined by the antagonistic action of E2Fb and E2Fc. Plant Physiol 157: 1440–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braybrook SA, Kuhlemeier C (2010) How a plant builds leaves. Plant Cell 22: 1006–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capua Y, Eshed Y (2017) Coordination of auxin-triggered leaf initiation by tomato LEAFLESS. Proc Natl Acad Sci USA 114: 3246–3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabelli M, Possenti M, Sessa G, Ciolfi A, Sassi M, Morelli G, Ruberti I (2007) Canopy shade causes a rapid and transient arrest in leaf development through auxin-induced cytokinin oxidase activity. Genes Dev 21: 1863–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury AM, Letham S, Craig S, Dennis ES (1993) amp1: a mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant J 4: 907–916 [Google Scholar]
- Chen Q, Dai X, De-Paoli H, Cheng Y, Takebayashi Y, Kasahara H, Kamiya Y, Zhao Y (2014) Auxin overproduction in shoots cannot rescue auxin deficiencies in Arabidopsis roots. Plant Cell Physiol 55: 1072–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J. (2010) Light signal transduction: an infinite spectrum of possibilities. Plant J 61: 982–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J, Reinecke D, Sim S, Washburn T, Brenner M (1994) A role for cytokinins in de-etiolation in Arabidopsis (det mutants have an altered response to cytokinins). Plant Physiol 104: 339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón-Carmona A, You R, Haimovitch-Gal T, Doerner P (1999) Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J 20: 503–508 [DOI] [PubMed] [Google Scholar]
- Deng XW, Caspar T, Quail PH (1991) cop1: a regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev 5: 1172–1182 [DOI] [PubMed] [Google Scholar]
- Deprost D, Yao L, Sormani R, Moreau M, Leterreux G, Nicolaï M, Bedu M, Robaglia C, Meyer C (2007) The Arabidopsis TOR kinase links plant growth, yield, stress resistance and mRNA translation. EMBO Rep 8: 864–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrenel T, Caldana C, Hanson J, Robaglia C, Vincentz M, Veit B, Meyer C (2016a) TOR signaling and nutrient sensing. Annu Rev Plant Biol 67: 261–285 [DOI] [PubMed] [Google Scholar]
- Dobrenel T, Mancera-Martínez E, Forzani C, Azzopardi M, Davanture M, Moreau M, Schepetilnikov M, Chicher J, Langella O, Zivy M, et al. (2016b) The Arabidopsis TOR kinase specifically regulates the expression of nuclear genes coding for plastidic ribosomal proteins and the phosphorylation of the cytosolic ribosomal protein S6. Front Plant Sci 7: 1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong P, Xiong F, Que Y, Wang K, Yu L, Li Z, Ren M (2015) Expression profiling and functional analysis reveals that TOR is a key player in regulating photosynthesis and phytohormone signaling pathways in Arabidopsis. Front Plant Sci 6: 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG (1999) Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol 215: 407–419 [DOI] [PubMed] [Google Scholar]
- Dory M, Hatzimasoura E, Kállai BM, Nagy SK, Jäger K, Darula Z, Nádai TV, Mészáros T, López-Juez E, Barnabás B, et al. (2018) Coevolving MAPK and PID phosphosites indicate an ancient environmental control of PIN auxin transporters in land plants. FEBS Lett 592: 89–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E, Ross JJ, Davies NW, Reid JB, Weller JL (2006) A role for ethylene in the phytochrome-mediated control of vegetative development. Plant J 46: 911–921 [DOI] [PubMed] [Google Scholar]
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Hills AC, Khan S, López-Juez E (2015) Chloroplast biogenesis-associated nuclear genes: control by plastid signals evolved prior to their regulation as part of photomorphogenesis. Front Plant Sci 6: 1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W, Li B, Li S, Liang Y, Wu X, Ma M, Wang J, Gao J, Cai Y, Zhang Y, et al. (2016) Mitogen-activated protein kinase cascade MKK7-MPK6 plays important roles in plant development and regulates shoot branching by phosphorylating PIN1 in Arabidopsis. PLoS Biol 14: e1002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Lau OS, Deng XW (2007) Light-regulated transcriptional networks in higher plants. Nat Rev Genet 8: 217–230 [DOI] [PubMed] [Google Scholar]
- Kalve S, De Vos D, Beemster GTS (2014a) Leaf development: a cellular perspective. Front Plant Sci 5: 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalve S, Fotschki J, Beeckman T, Vissenberg K, Beemster GTS (2014b) Three-dimensional patterns of cell division and expansion throughout the development of Arabidopsis thaliana leaves. J Exp Bot 65: 6385–6397 [DOI] [PubMed] [Google Scholar]
- Kircher S, Schopfer P (2012) Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proc Natl Acad Sci USA 109: 11217–11221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchenko A, Citerne S, Jéhanno I, Bersimbaev RI, Veit B, Meyer C, Leprince AS (2015) Mutations in the Arabidopsis Lst8 and Raptor genes encoding partners of the TOR complex, or inhibition of TOR activity decrease abscisic acid (ABA) synthesis. Biochem Biophys Res Commun 467: 992–997 [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149: 274–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, Deng XW (2012) The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci 17: 584–593 [DOI] [PubMed] [Google Scholar]
- Leyser HMO, Lincoln CA, Timpte C, Lammer D, Turner J, Estelle M (1993) Arabidopsis auxin-resistance gene AXR1 encodes a protein related to ubiquitin-activating enzyme E1. Nature 364: 161–164 [DOI] [PubMed] [Google Scholar]
- Li X, Cai W, Liu Y, Li H, Fu L, Liu Z, Xu L, Liu H, Xu T, Xiong Y (2017) Differential TOR activation and cell proliferation in Arabidopsis root and shoot apexes. Proc Natl Acad Sci USA 114: 2765–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kirubakaran S, Hur W, Niepel M, Westover K, Thoreen CC, Wang J, Ni J, Patricelli MP, Vogel K, et al. (2012) Kinome-wide selectivity profiling of ATP-competitive mammalian target of rapamycin (mTOR) inhibitors and characterization of their binding kinetics. J Biol Chem 287: 9742–9752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Juez E, Bowyer JR, Sakai T (2007) Distinct leaf developmental and gene expression responses to light quantity depend on blue-photoreceptor or plastid-derived signals, and can occur in the absence of phototropins. Planta 227: 113–123 [DOI] [PubMed] [Google Scholar]
- López-Juez E, Dillon E, Magyar Z, Khan S, Hazeldine S, de Jager SM, Murray JAH, Beemster GTS, Bögre L, Shanahan H (2008) Distinct light-initiated gene expression and cell cycle programs in the shoot apex and cotyledons of Arabidopsis. Plant Cell 20: 947–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-de la Rosa N, Sotillo B, Miskolczi P, Gibbs DJ, Vicente J, Carbonero P, Oñate-Sánchez L, Holdsworth MJ, Bhalerao R, Alabadí D, et al. (2014) Large-scale identification of gibberellin-related transcription factors defines group VII ETHYLENE RESPONSE FACTORS as functional DELLA partners. Plant Physiol 166: 1022–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montané MH, Menand B (2013) ATP-competitive mTOR kinase inhibitors delay plant growth by triggering early differentiation of meristematic cells but no developmental patterning change. J Exp Bot 64: 4361–4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser JL, Chory J (2002) Photomorphogenesis. The Arabidopsis Book 1: e0054, 10.1199/tab.0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukarinen E, Nägele T, Pedrotti L, Wurzinger B, Mair A, Landgraf R, Börnke F, Hanson J, Teige M, Baena-González E, et al. (2016) Quantitative phosphoproteomics reveals the role of the AMPK plant ortholog SnRK1 as a metabolic master regulator under energy deprivation. Sci Rep 6: 31697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici E, Polverari L, Sabatini S (2015) Plant hormone cross-talk: the pivot of root growth. J Exp Bot 66: 1113–1121 [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Janocha D, Dong Y, Medzihradszky A, Schöne S, Daum G, Suzaki T, Forner J, Langenecker T, Rempel E, et al. (2016) Integration of light and metabolic signals for stem cell activation at the shoot apical meristem. eLife 5: e17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C (2003) Regulation of phyllotaxis by polar auxin transport. Nature 426: 255–260 [DOI] [PubMed] [Google Scholar]
- Robaglia C, Thomas M, Meyer C (2012) Sensing nutrient and energy status by SnRK1 and TOR kinases. Curr Opin Plant Biol 15: 301–307 [DOI] [PubMed] [Google Scholar]
- Roldán M, Gómez-Mena C, Ruiz-García L, Salinas J, Martínez-Zapater JM (1999) Sucrose availability on the aerial part of the plant promotes morphogenesis and flowering of Arabidopsis in the dark. Plant J 20: 581–590 [DOI] [PubMed] [Google Scholar]
- Scarpella E, Barkoulas M, Tsiantis M (2010) Control of leaf and vein development by auxin. Cold Spring Harb Perspect Biol 2: a001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T (2006) Control of leaf vascular patterning by polar auxin transport. Genes Dev 20: 1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepetilnikov M, Dimitrova M, Mancera-Martínez E, Geldreich A, Keller M, Ryabova LA (2013) TOR and S6K1 promote translation reinitiation of uORF-containing mRNAs via phosphorylation of eIF3h. EMBO J 32: 1087–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepetilnikov M, Makarian J, Srour O, Geldreich A, Yang Z, Chicher J, Hammann P, Ryabova LA (2017) GTPase ROP2 binds and promotes activation of target of rapamycin, TOR, in response to auxin. EMBO J 36: 886–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jürgens G, Alonso JM (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133: 177–191 [DOI] [PubMed] [Google Scholar]
- Sulpice R, Pyl ET, Ishihara H, Trenkamp S, Steinfath M, Witucka-Wall H, Gibon Y, Usadel B, Poree F, Piques MC, et al. (2009) Starch as a major integrator in the regulation of plant growth. Proc Natl Acad Sci USA 106: 10348–10353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AYL, Gazzarrini S (2014) Trehalose-6-phosphate and SnRK1 kinases in plant development and signaling: the emerging picture. Front Plant Sci 5: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukaya H. (2005) Leaf shape: genetic controls and environmental factors. Int J Dev Biol 49: 547–555 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel B, Bläsing OE, Gibon Y, Retzlaff K, Höhne M, Günther M, Stitt M (2008) Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol 146: 1834–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dingenen J, De Milde L, Vermeersch M, Maleux K, De Rycke R, De Bruyne M, Storme V, Gonzalez N, Dhondt S, Inzé D (2016) Chloroplasts are central players in sugar-induced leaf growth. Plant Physiol 171: 590–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JL, Foo EM, Hecht V, Ridge S, Vander Schoor JK, Reid JB (2015) Ethylene signaling influences light-regulated development in pea. Plant Physiol 169: 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston E, Thorogood K, Vinti G, López-Juez E (2000) Light quantity controls leaf-cell and chloroplast development in Arabidopsis thaliana wild type and blue-light-perception mutants. Planta 211: 807–815 [DOI] [PubMed] [Google Scholar]
- Xiong Y, McCormack M, Li L, Hall Q, Xiang C, Sheen J (2013) Glucose-TOR signalling reprograms the transcriptome and activates meristems. Nature 496: 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano S, Terashima I (2001) Separate localization of light signal perception for sun or shade type chloroplast and palisade tissue differentiation in Chenopodium album. Plant Cell Physiol 42: 1303–1310 [DOI] [PubMed] [Google Scholar]
- Yano S, Terashima I (2004) Developmental process of sun and shade leaves in Chenopodium album L. Plant Cell Environ 27: 781–793 [Google Scholar]
- Yoshida S, Mandel T, Kuhlemeier C (2011) Stem cell activation by light guides plant organogenesis. Genes Dev 25: 1439–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]