Abstract
Key points
Magnetic resonance spectroscopy was conducted before and after high‐intensity interval exercise.
Sensorimotor cortex GABA concentration increased by 20%.
The increase was positively correlated with the increase in blood lactate.
There was no change in dorsolateral prefrontal cortex.
There were no changes in the glutamate‐glutamine‐glutathione peak.
Abstract
High‐intensity exercise increases the concentration of circulating lactate. Cortical uptake of blood borne lactate increases during and after exercise; however, the potential relationship with changes in the concentration of neurometabolites remains unclear. Although changes in neurometabolite concentration have previously been demonstrated in primary visual cortex after exercise, it remains unknown whether these changes extend to regions such as the sensorimotor cortex (SM) or executive regions such as the dorsolateral prefrontal cortex (DLPFC). In the present study, we explored the acute after‐effects of high‐intensity interval training (HIIT) on the concentration of gamma‐Aminobutyric acid (GABA) and the combined glutamate–glutamine–glutathione (Glx) spectral peak in the SM and DLPFC, as well as the relationship with blood lactate levels. Following HIIT, there was a robust increase in GABA concentration in the SM, as evident across the majority of participants. This change was not observed in the DLPFC. Furthermore, the increase in SM GABA was positively correlated with an increase in blood lactate. There were no changes in Glx concentration in either region. The observed increase in SM GABA concentration implies functional relevance, whereas the correlation with lactate levels may relate to the metabolic fate of exercise‐derived lactate that crosses the blood–brain barrier.
Keywords: magnetic resonance spectroscopy, gamma‐aminobutyric acid, high‐intensity exercise
Key points
Magnetic resonance spectroscopy was conducted before and after high‐intensity interval exercise.
Sensorimotor cortex GABA concentration increased by 20%.
The increase was positively correlated with the increase in blood lactate.
There was no change in dorsolateral prefrontal cortex.
There were no changes in the glutamate‐glutamine‐glutathione peak.
Introduction
Cardiovascular exercise has been shown to benefit the brain, protect against cognitive decline and have efficacy as an adjunct treatment for neuropsychiatric conditions (Pedersen & Saltin, 2015; Prakash et al. 2015; Rector et al. 2015; Brellenthin & Koltyn, 2016). How this occurs remains unclear, although is probably related to the effects of exercise on brain plasticity and metabolism (Johansen‐Berg & Duzel, 2016).
One candidate mechanism involves exercise‐induced increases in lactate. When high power output is required during exercise, there is an accumulation of systemic lactate due to the glycolytic rate increasing in excess of lactate oxidation (Brooks et al. 2011). Substantial lactate accumulation occurs during high‐intensity interval exercise (HIIT), which involves alternating epochs of high and low intensity (a feature of sports played worldwide, e.g. football). It is now known that lactate crosses the blood–brain barrier (Bergersen, 2015; Machler et al. 2016), that cortical concentrations of lactate increase during and after exercise (Kemppainen et al. 2005; van Hall et al. 2009; Rasmussen et al. 2011), and that lactate may subsequently enter metabolic pathways involved in the synthesis of neurotransmitters such as glutamate and GABA (Maddock et al. 2016). Nonetheless, the functional relevance of this increase in brain lactate is unclear. Thus, lactate is a focus of research to potentially explain the benefits of HIIT exercise relative to low intensity exercise (Thomas et al. 2016).
There are only a handful of studies that have used 1H proton magnetic resonance spectroscopy (MRS) to investigate neurometabolites during the acute post‐exercise state (Maddock et al. 2011, 2016; Dennis et al. 2015). Typically, MRS spectra are acquired from a small region of the brain (i.e. voxel) both before and after exercise. Two studies have shown that lactate concentration in the visual cortex increased after exercise in direct correlation with the peripheral increases in blood lactate (Maddock et al. 2011; Dennis et al. 2015). Additionally, increases in GABA and the composite signal Glx have been reported in the visual cortex and anterior cingulate cortex, in some (Maddock et al. 2011, 2016) but not all (Dennis et al. 2015) studies. Thus, some of the lactate produced during exercise may be channeled into de novo synthesis of neurotransmitters via tricarboxylic cycle intermediates such as alpha ketoglutarate (Maddock et al. 2011).
It has been suggested that this influence of post‐exercise lactate on neurometabolite concentrations might be global, reflecting a widespread effect across the brain (Maddock et al. 2011, 2016). This remains largely untested because most measurements have been obtained from primary visual cortex. Also, the observed increase in GABA concentration in the visual cortex (Maddock et al. 2016) appears to be potentially at odds with findings from the sensorimotor cortex (SM) that has been examined using transcranial magnetic stimulation (TMS). Several TMS studies have reported reduced synaptic GABA inhibition in the motor cortex after exercise (Singh et al. 2014; Smith et al. 2014; Mooney et al. 2016), including one study where HIIT exercise was utilized (Stavrinos & Coxon, 2017). These TMS studies propose that the reduction in GABA is a permissive factor for plasticity processes in the motor system. Such findings potentially challenge the notion that all cortical regions are influenced in the same way by high‐intensity exercise. Alternatively, it could be that TMS and MRS are providing measures of different aspects of inhibitory function (Stagg et al. 2011c; Tremblay et al. 2013; Dyke et al. 2017). Interestingly, acute high‐intensity exercise is also known to have benefits for executive function (Chang et al. 2012), although the influence of HIIT on MRS measures of GABA and Glx in prefrontal regions such as the dorsolateral prefrontal cortex also remains unknown.
The present study aimed to explore the effects of HIIT exercise on MRS‐derived GABA and Glx neurometabolite concentrations in the SM cortex and dorsolateral prefrontal cortex (DLPFC), as well as their correlation with exercise‐induced changes in lactate. The results provide fundamental new information regarding the effects of exercise on the concentration of excitatory and inhibitory neurometabolites in the brain, including an initial exploration into the possible regional specificity of the effects.
Methods
Ethical approval
The Monash University Human Research Ethics Committee approved the study and all participants provided their written informed consent. The study conformed to the standards set by the Declaration of Helsinki, except for registration in a database.
Participants
The sample size was determined from the only previous study investigating the effects of exercise on GABA using MRS, for which an effect size of d = 1.35 was reported (Maddock et al. 2016). For this effect size and a desired power of 90%, a sample size of nine was required. Thus, we conservatively performed the experiment on ten right‐handed individuals (eight male, two female). Participants were screened for contraindications to exercise (Physical Activity Readiness Questionnaire) and magnetic resonance imaging (MRI), and were asked to refrain from strenuous physical activity for 24 h prior to the study.
General procedure
Repeated measures of GABA were obtained from MRS before and after 20‐min of HIIT exercise (Fig. 1 A). The exercise protocol was designed to ensure high levels of blood lactate concentration (>10 mmol L−1) at the same time as limiting fatigue.
Figure 1. Overview of experiment.
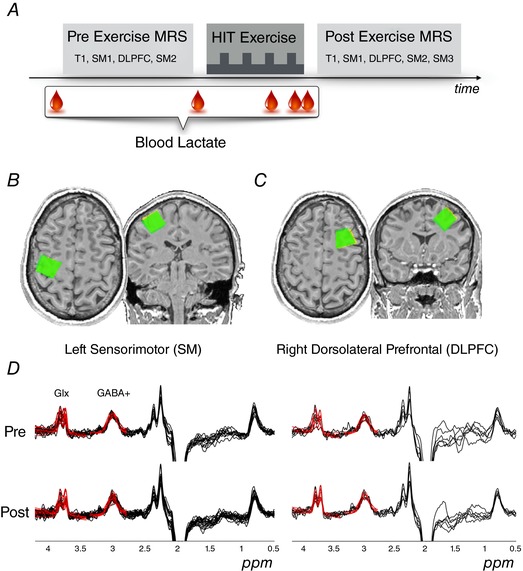
MRS measures were obtained before and after HIIT exercise using MEGA‐PRESS tuned to detect GABA. A, following acquisition of an anatomical image (T1), MRS sequences were repeated for the left SM hand knob region and right DLPFC voxels. Lactate was measured from capillary blood at rest (× 2 baseline measures), during HIIT, as well as at the cessation of exercise. B and C, positioning of the SM and DLPFC voxels, respectively. The post‐exercise voxel (green) is overlaid upon the pre exercise voxel (yellow). D, difference spectra for all participants and timepoints are shown for each voxel (black lines). Model fit for each acquired difference spectrum is superimposed (red lines) for the GABA peak at 3.0 ppm and the Glx peak at 3.75 ppm. [Color figure can be viewed at wileyonlinelibrary.com]
MRI
GABA‐edited MRS data were acquired from two regions of cortex with a Skyra 3T MRI scanner (32‐channel head coil for receive, body coil for transmit) (Siemens AG, Munich, Germany). Spectra were acquired from 2 × 2 × 2 cm voxels placed over the SM spanning the hand knob region of the central sulcus in the dominant (left) hemisphere (Fig. 1 B) and the right DLPFC (Fig. 1 C). GABA concentrations do not typically show hemispheric differences in healthy individuals (Grewal et al. 2016). The SM hand knob region was chosen, as opposed to the leg motor cortex, because we were interested in the general effects of exercise on the brain beyond the region driving the exercise. For both the pre‐ and post‐exercise scans, a 1 × 1 × 1 mm isotropic T1‐weighted image was first acquired (TR = 1.54 s, TE = 2.55 ms, flip angle = 9°) for voxel localization. During voxel positioning, a pulsed arterial spin labelling sequence with a 3‐D gradient and spin echo readout was obtained (data not shown). GABA‐edited Meshcher‐Garwood Point Resolved Spectroscopy (MEGA‐PRESS) data were then acquired with the parameters: TE = 68 ms, editing pulses at 1.9 ppm and 7.5 ppm, TR = 1.5 s, edit pulse bandwidth = 45 Hz, 96 ON‐OFF averages and 4 min 54 s per acquisition. Unsuppressed water sequences (8 ON‐OFF averages) with the same parameters and location were also acquired following each MRS acquisition.
Before exercise, two spectra were obtained from the SM voxel and one spectrum from the DLPFC voxel. Two SM spectra were obtained to assess the stability of MRS measures prior to exercise (sequence order: T1, pASL, SM1, DLPFC, SM2). After exercise, the participant was immediately repositioned in the scanner and three spectra were obtained from the SM voxel, along with one spectrum from the DLPFC voxel. The sequence order and time post‐exercise cessation comprised: T1 at 10 ± 2:46 min, pASL at 15 ± 3:10 min, SM1 at 24 ± 3:28 min, DLPFC at 31 ± 3:35 min, SM2 at 38 ± 3:48 min and SM3 at 45 ± 3:31 min). Participants were instructed to lay at rest with their eyes open viewing a fixation cross during the MRI scans.
HIIT exercise protocol
The HIIT exercise session was performed on a stationary cycle ergometer (Wattbike, Geelong, Australia) with the participant wearing a Polar H7 heart rate monitor (Polar Electro, Kempele, Finland). Resting heart rate (RHR) was obtained whilst sitting and exercise intensity was tailored to each individual based on heart rate reserve (HRR):
where HRage predicted max = 220 – age.
Participants exercised for 20 min, alternating between periods of low‐intensity cycling for 3 min at ∼50% HRR (HRR × 0.5 + RHR) and high‐intensity cycling for 2 min with a target heart rate of 90% HRR (HRR × 0.9 + RHR) by the end of the protocol. The periods of lower intensity active recovery are required because, by definition, high‐intensity exercise cannot be sustained for prolonged periods of time. This exercise protocol is known to increase lactate levels above 10 mmol L−1 (Roig et al. 2012; Mang et al. 2014; Stavrinos & Coxon, 2017). Fluid loss was minimized by ensuring the exercise occurred in a temperature controlled environment, requesting that participants arrived well hydrated, and with the provision of water ad libitum both during and immediately after exercise. At the end of the protocol, participants then continued to cycle at low intensity for 2–3 min as the heart rate recovered. During exercise, the measures recorded were: heart rate (beats min–1), Cadence (rpm), power output (W) and Borg's 6–20 scale for rating of perceived exertion. For each measure, the average was determined over the last minute of each low‐ and high‐intensity epoch.
Blood lactate was quantified using an automated portable lactate analyser and test strips (Lactate Pro2; Arkray, Kyoto, Japan). In accordance with standard procedures, a spring‐loaded lancet was used to obtain capillary blood samples (∼0.3 μL) from the tip of the left index finger. Baseline lactate was measured twice at rest, along with measurements immediately following the third and fourth high‐intensity exercise epochs (i.e. at 15 min and twice at 20 min) (Fig. 1 A).
Processing of MRS data
Gannet, version 2.0 (http://github.com/cjohnevans/Gannet2.0/archive/master.zip) (Edden et al. 2014; Mullins et al. 2014) was used for analysis of the MRS spectra. Both GABA (3.0 ppm) and Glx (3.75 ppm) concentrations were calculated relative to the unsuppressed water signal from the same region, obtained immediately after each sequence. Automated processing involved phased‐array channel combination of the raw k‐space data (Siemens ‘twix’ files), frequency‐ and phase‐correction in the time domain with spectral registration (Near et al. 2015) and filtering with 3‐Hz exponential line broadening. GABA concentration was estimated with a single Gaussian peak superimposed on a linear baseline. The model for Glx incorporated a double Gaussian. To acknowledge a potential macromolecule contribution to the signal, GABA+ is referred to as appropriate. Model fits are shown in red in Fig. 1 D.
The metabolites of interest (GABA, Glx) were quantified relative to both the unsuppressed water signal (fit with a Gaussian–Lorentzian model), and to the creatine (Cr) peak (integral of a two‐Lorentzian model of Cr and choline in the OFF spectrum). The GABA:H2O and Glx:H2O ratios are expressed in institutional units and the formula used within Gannet takes into account the editing efficiency and approximate macromolecular contributions to the GABA+ peak.
The T1 anatomical image was segmented within each voxel (using FSL's FAST, available at https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL). Metabolite ratios were corrected post hoc for voxel grey matter (GM) and white matter (WM) tissue fractions, according to the equations:
Tissue concentrations therefore reflect cerebrospinal fluid‐corrected individual GABA+ and Glx values.
The full‐width half‐maximum of the creatine linewidth (Cr FWHM), the SD of the water frequency in Hz and the GABA signal fit error (E GABA‐Water) were used as metrics of data quality. E GABA‐Water is a combined measure of the SD of the residuals after fitting models to the water and GABA peaks, expressed as a percentage of peak height. Spectra were accepted if Cr FWHM <10 Hz, SD of water frequency <1 Hz, and EGABA‐Water <15%. For the DLPFC voxel, data from two participants did not meet these criteria and thus were omitted from the DLPFC analysis.
A secondary analysis of the MRS spectra was performed using LCModel software, version 6.3‐H (http://s-provencher.com/lcmodel.shtml) as reported in (Maddock et al. 2016). The difference spectra were fit using a simulated MEGA‐PRESS basis set, which included GABA, Glx and N‐acetylaspartate, N‐acetylaspartyl‐glutamate (NAA). From this, GABA+:NAA and Glx:NAA ratios were calculated for each of the acquired difference spectra. The off‐resonance spectra were fit using a simulated PRESS basis set, which included NAA, Glx, Cr (creatine, phosphocreatine), choline (phosphocholine, glycerophosphocholine), Ins (myo‐inositol), scyllo‐inositol and taurine. From this, NAA:Cr, Glx:Cr, Cho:Cr and Ins:Cr ratios were calculated.
Statistical analysis of MRS data
One‐way repeated measures ANOVA (rmANOVA) was performed for blood lactate, as well as for MRS variables obtained from the SM voxel (factor = Time with five levels). Greenhouse–Geisser adjusted P values are reported for instances where sphericity was violated. Contingent upon a significant rmANOVA, planned contrasts interrogated (i) the stability of the baseline measures prior to exercise (contrast vector [–1 1 0 0 0]) and (ii) whether effects could be ascribed to exercise (contrast vector [–3 –3 2 2 2]). DLPFC MRS variables were subjected to paired t tests. A Pearson correlation tested for a positive relationship between percentage change in GABA concentration after exercise and change in blood lactate. Grubbs’ test was applied to both variables and identified one significant outlier. This data point was omitted from the analysis to satisfy the assumption of normality. This participant demonstrated an abnormally low lactate reading in response to the exercise protocol and may have been the result of a procedural error when obtaining the blood lactate measure (sweat contamination), or their status as a highly trained endurance cyclist, which is known to enhance lactate utilization within muscle (Billat et al. 2003). Data are reported as the mean ± SD within the text and tables and as the mean ± SE in the figures.
Results
On average (mean ± SD), participants were aged 29.40 ± 10.72 years, with a height of 1.76 ± 0.05 m, weighed 74.40 ± 9.37 kg and had a body mass index of 23.91 ± 2.80 kg m−2. Resting heart rate when seated was 63.30 ± 4.32 beats min–1, age‐predicted maximum heart rate was 190.60 ± 10.72 beats min–1 and HRR was 127.30 ± 10.99 beats min–1. A detailed description of the HIIT exercise session is provided in Table 1. By the end of the protocol, participants were exercising at 91 ± 8.2% of the HRR (182 ± 8.2 beats min–1). Importantly, blood lactate measures (Fig. 2) confirmed that the protocol was successful in achieving high‐intensity exercise. The rmANOVA main effect for blood lactate was significant (F 4,36 = 66.08, P < .001, η2 p = 0.88), with planned contrasts confirming that blood lactate was stable at baseline (F 1,9 = 0.61, P = .46, η2 p = 0.06) and elevated during exercise (F 1,9 = 102.7, P < .001, η2 p = 0.92). Peak blood lactate during exercise was 12.3 ± 3.5 mmol L−1.
Table 1.
Exercise parameters
| Time (min) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0–3 | 3–5 | 5–8 | 8–10 | 10–13 | 13–15 | 15–18 | 18–20 | |
| Exercise parameters | L1 | H1 | L2 | H2 | L3 | H3 | L4 | H4 |
| Cadence (rpm) | 78.7 (7.1) | 98.5 (6.2) | 80.9 (7.1) | 102.4 (7.8) | 83.6 (8.1) | 107.1 (6.5) | 83.7 (8.3) | 111.7 (8.3) |
| Power (W) | 90 (13) | 184 (60) | 87 (17) | 211 (92) | 94 (27) | 219 (80) | 85 (19) | 255 (78) |
| Power:Weight (W kg−1) | 1.21 (0.14) | 2.47 (0.71) | 1.17 (0.21) | 2.81 (1.13) | 1.27 (0.34) | 2.91 (0.94) | 1.14 (0.18) | 3.40 (0.89) |
| Heart rate (beats min–1) | 117 (13.0) | 149 (10.9) | 132 (13.3) | 164 (9.6) | 141 (13.0) | 173 (8.6) | 146 (12.3) | 182 (8.3) |
| % HRR | 39 (6.8) | 65 (6.7) | 51 (7.5) | 77 (8.2) | 58 (8.3) | 84 (8.5) | 63 (8.2) | 91 (8.2) |
| Borg's RPE (scale 6–20) | 10.0 (1.56) | 13.4 (1.90) | 10.6 (0.84) | 14.6 (1.35) | 11.2 (1.55) | 16.0 (0.47) | 11.3 (2.26) | 18.1 (1.45) |
L, low‐intensity epoch; H, high‐intensity epoch; RPE rating of perceived exertion; HRR, heart rate reserve. Data are reported as group mean (SD).
Figure 2. Blood lactate measures are displayed relative to exercise onset.
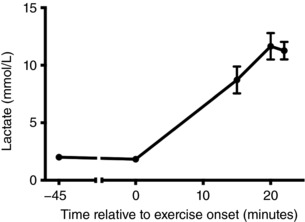
The second baseline measure was obtained within the 5 min prior to exercise commencing. During exercise, measures were obtained at the end of the third and fourth high‐intensity epochs.
MRS voxel localization and tissue composition
The overlap in voxel position for pre‐ and post‐scans was 86.3 ± 12.33% for the SM voxel and 81.2 ± 16.03% for the DLPFC voxel. Within each MRS voxel, paired t tests established that there were no significant differences across pre‐ and post‐exercise scans in the proportion of grey matter (SM: pre 0.33 ± 0.06, post 0.33 ± 0.06; DLPFC: pre 0.36 ± 0.03, post 0.37 ± 0.03), white matter (SM: pre 0.56 ± 0.06, post 0.55 ± 0.07; DLPFC: pre 0.55 ± 0.04, post 0.54 ± 0.03) or cerebrospinal fluid (SM: pre 0.11 ± 0.04, post 0.11 ± 0.05; DLPFC: pre 0.08 ± 0.02, post 0.08 ± 0.01) (all P > 0.17).
SM
There was a significant main effect of Time for GABA in the SM voxel, expressed relative to both water and creatine (Table 2). Planned contrasts revealed that GABA ratios were stable at baseline but significantly elevated post‐exercise. Averaging across pre‐ and post‐acquisitions, HIIT exercise was associated with a 29% increase in GABA:H2O (Fig. 3 A) and a 24% increase in GABA:Cr (Fig. 3 C). By contrast, Glx ratios did not show significant modulation as a function of Time (Fig. 3 B and D and Table 2). The rmANOVA model applied to data quality metrics indicated that measures were stable over Time (Table 2). The increase in GABA may be related to the accumulation of lactate during exercise because there was a significant positive correlation (r = 0.63, P = 0.034) between the increase in blood lactate as a result of exercise and the percentage increase in GABA in the SM voxel (Fig. 4).
Table 2.
GABA+ and Glx ratios obtained from the primary analysis using GANNET for the SM voxel, along with associated data quality metrics
| Neurometabolite | Pre 1 | Pre 2 | Post Ex 1 (24 min) | Post Ex 2 (38 min) | Post Ex 3 (46 min) | rmANOVA | Contrast 1 (Pre 1 vs. Pre 2) | Contrast 2 (Post vs. Pre) |
|---|---|---|---|---|---|---|---|---|
| GABA+:H2O | 2.00 (0.33) | 1.81 (0.29) | 2.45 (0.53) | 2.50 (0.54) | 2.35 (0.37) |
F4,36 = 5.24,
P = 0.002 η2p = 0.37 |
F
1,9 = 2.56, P = 0.14 η2 p = 0.22 |
F1,9 = 25.18,
P = 0.001 η2p = 0.74 |
| GABA+:Cr | 0.121 (0.0160) | 0.111 (0.0197) | 0.144 (0.0353) | 0.147 (0.0356) | 0.138 (0.0168) |
F4,36 = 3.93,
P = 0.01 η2p = 0.30 |
F
1,9 = 1.52, P = 0.25 η2 p = 0.14 |
F1,9 = 16.72,
P = 0.003 η2p = 0.65 |
| Glx:H2O | 1.57 (0.25) | 1.62 (0.38) | 1.53 (0.40) | 1.50 (0.43) | 1.66 (0.36) |
F
4,36 = 0.50, P = 0.74 η2 p = 0.05 |
– | – |
| Glx:Cr | 0.094 (0.0180) | 0.098 (0.0223) | 0.088 (0.0214) | 0.085 (0.0200) | 0.095 (0.0179) |
F
4,36 = 0.90, P = 0.47 η2 p = 0.09 |
– | – |
| Cr FWHM (Hz) | 7.98 (0.60) | 7.93 (0.65) | 8.06 (0.56) | 7.85 (0.46) | 7.92 (0.40) |
F
4,36 = 0.44, P = 0.78 η2 p = 0.05 GGε = 0.28 |
– | – |
| Water frequency SD (Hz) | 0.62 (0.23) | 0.55 (0.19) | 0.49 (0.10) | 0.59 (0.13) | 0.60 (0.14) |
F
4,36 = 1.40, P = 0.25 η2 p = 0.14 |
– | – |
| Normalized fitting error E GABA,Water | 9.56 (2.57) | 9.67 (2.01) | 8.74 (2.16) | 10.03 (2.71) | 9.37 (1.99) |
F
4,36 = 0.53, P = 0.71 η2 p = 0.06 |
– | – |
Significant results are highlighted in bold. Planned comparisons were only carried out if a main effect was present in the rmANOVA. Data are reported as group mean (SD).
Figure 3. SM voxel GABA and Glx ratios.
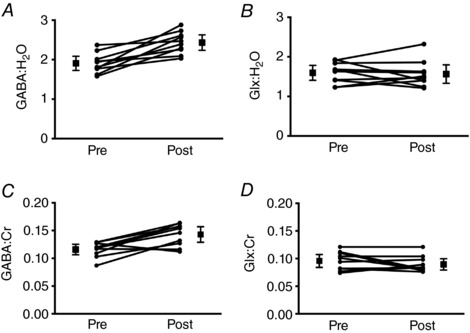
Concentrations are shown for pre‐ and post‐HIIT exercise, depicting the main contrast of interest. Metabolite concentrations are shown relative to water (A and B) and creatine (C and D). Squares depict the mean and 95% confidence interval. Circles show individual subject data.
Figure 4. Exercise‐induced change in Lactate and GABA.
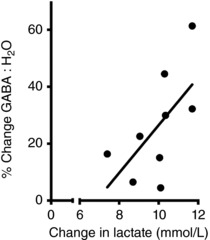
Correlation between increase in blood lactate and the percent increase in GABA concentration in the SM voxel following HIIT (r = 0.63, P = 0.034).
DLPFC
Paired t tests revealed no significant differences pre‐ to post‐exercise in the DLPFC voxel for either GABA ratio (GABA:H2O: pre 2.03 ± 0.67, post 2.07 ± 0.32, t 7 = 0.12, P = .91; GABA:Cr: pre 0.123 ± 0.033, post 0.124 ± 0.026, t 7 = 0.12, P = .91), or Glx ratio (Glx:H2O: pre 1.54 ± 0.50, post 1.65 ± 0.07, t 7 = 0.62, P = .56; Glx:Cr: pre 0.093 ± 0.027, post 0.097 ± 0.010, t 7 = 0.46, P = .66), nor for any of the data quality metrics (all P > .13).
Secondary analysis using the LCModel
As reported above for the SM voxel (primary GANNET analysis), the significant main effect of Time for GABA (but not Glx) was confirmed using the LCModel software package, with NAA as the denominator (Table 3). A t test indicated that GABA:NAA was significantly elevated at the first post‐timepoint compared to the average of Pre (t 9 = 3.75, P = 0.007). The percentage increase in GABA:NAA was also positively correlated with change in blood lactate (r = 0.57, P = 0.042). The signal‐to‐noise ratio (SNR) and line width (FWHM) values were stable (Table 3) and similar to those reported by (Maddock et al. 2016). The mean Cramer–Rao lower bound (CRLB) for GABA was 5.5 (range 4–8) indicating the reliability of the values obtained.
Table 3.
Secondary analysis using LCModel for the SM voxel, along with associated data quality metrics
| Neurometabolite | Pre 1 | Pre 2 | Post Ex 1 (24 min) | Post Ex 2 (38 min) | Post Ex 3 (46 min) | rmANOVA | Contrast 1 (Pre 1 vs. Pre 2) | Contrast 2 (Post vs. Pre) |
|---|---|---|---|---|---|---|---|---|
| LC‐Model: MEGA‐PRESS difference spectrum | ||||||||
| GABA+:NAA | 0.34 (0.05) | 0.34 (0.06) | 0.38 (0.05) | 0.35 (0.06) | 0.36 (0.05) |
|
F
1,9 = 0.06, P = 0.81 η2 p = 0.01 |
F
1,9 = 3.33, P = 0.10 η2 p = 0.27 |
| Glx:NAA | 0.99 (0.10) | 1.01 (0.09) | 1.02 (0.11) | 1.01 (0.12) | 0.99 (0.13) |
|
– | – |
| Line width (ppm) | 0.037 (0.016) | 0.037 (0.013) | 0.034 (0.004) | 0.034 (0.004) | 0.033 (0.00) |
|
– | – |
| SNR | 26.7 (5.98) | 26.8 (6.29) | 25.9 (3.41) | 26.0 (3.40) | 25.9 (3.75) |
F
4,36 = 0.21, P = 0.76 η2 p = 0.02 |
– | – |
| LC‐Model: ‘Off’ resonance PRESS spectrum | ||||||||
| NAA:Cr | 1.90 (0.12) | 1.90 (0.13) | 1.86 (0.14) | 1.93 (0.28) | 1.90 (0.16) |
F
4,36 = 0.73, P = 0.046 η2 p = 0.08 GGε = 0.35 |
– | – |
| Glx:Cr | 0.74 (0.11) | 0.74 (0.11) | 0.76 (0.10) | 0.75 (0.11) | 0.76 (0.13) |
|
– | – |
| Ins:Cr | 0.91 (0.13) | 0.91 (0.11) | 0.89 (0.14) | 0.92 (0.12) | 0.91 (0.15) |
|
– | – |
| Cho:Cr | 0.25 (0.04) | 0.25 (0.05) | 0.24 (0.04) | 0.24 (0.04) | 0.25 (0.41) |
|
– | – |
| Line width (ppm) | 0.037 (0.012) | 0.036 (0.011) | 0.033 (0.003) | 0.034 (0.003) | 0.035 (0.004) |
|
– | – |
| SNR | 34.4 (5.42) | 35.3 (5.40) | 34.2 (2.49) | 33.2 (5.27) | 34.5 (2.95) |
|
– | – |
Significant results are highlighted in bold. Planned comparisons were only carried out if a main effect was present in the rmANOVA. Data are reported as group mean (SD).
Analysis of the off‐resonance spectra revealed no significant main effects for NAA:Cr, Glx:Cr, Ins:Cr or Cho:Cr (Table 3), again with similar data quality as reported by (Maddock et al. 2016). The mean CRLB was 7.06 (range 6–10) for Glx and ≤8 for the other metabolite resonances reported in Table 3.
For DLPFC, paired t tests revealed no significant differences for any of the metabolite ratios or data quality metrics (all t < 1.83, P > 0.11).
Discussion
The present study investigated the effects of HIIT‐exercise on systemic lactate accumulation and on the concentration of the neurometabolite GABA, which is the primary inhibitory neurotransmitter in the brain. We report novel evidence of a large 20% increase in GABA concentration in the upper‐limb SM in the first hour after lower‐limb cycling exercise. This result complements a previous study (Maddock et al. 2016) that reported a 7% GABA increase in the visual cortex. In the present study, we show that the increase in GABA is of a greater magnitude and persists for a longer duration in the SM compared to that reported previously in the visual cortex. We did not observe any change in GABA in the DLPFC, providing some preliminary evidence that increased GABA after exercise may be regionally specific as opposed to a global phenomenon. We also report novel evidence of a positive correlation between greater increases in systemic lactate and the increase in SM GABA.
Cortical changes in neurometabolite concentrations evoked by HIIT
To our knowledge, this is the first study to investigate the effects of high‐intensity exercise on GABA and Glx concentrations in the SM and DLPFC. Previous studies demonstrated the modulation of neurometabolite concentrations after exercise in visual and anterior cingulate cortex (ACC) in samples of eight to 11 participants (Maddock et al. 2011, 2016; Dennis et al. 2015). More specifically, in agreement with the increase in the SM in the present study, GABA levels have been reported to be increased in the visual cortex (Maddock et al. 2016). By contrast, markers of glutamatergic neurometabolite concentration have been reported to be increased in the visual cortex and ACC (Maddock et al. 2016) or remain unchanged in the visual cortex (Dennis et al. 2015). It remains unclear to what extent such regions might be functionally involved in high‐intensity exercise or represent more generalized and global influences of exercise on neurometabolite levels. Although it has been noted that the ACC plays a functional role in executive function (Maddock et al. 2016), it is also involved in a variety of autonomic functions (e.g. regulation of blood pressure and heart rate) and tasks requiring focused mental effort (Allman et al. 2001). In the present study, we sampled the DLPFC, which is consistently implicated in executive function, and saw no changes in GABA and Glx levels. This is perhaps to be expected given the relatively automatic nature of exercise on a stationary cycle ergometer with relatively low cognitive demands. Nonetheless, these results indicate a complex picture in which neurometabolite changes are neither global, nor fully explained by the functional relevance of a cortical region.
Interestingly, the increase in GABA concentration in the SM was strongly correlated with blood lactate levels. One plausible candidate mechanism for the observed increase in GABA is the cortical uptake of lactate and its non‐oxidative metabolism and conversion to GABA via α‐ketoglutarate transamination (Maddock et al. 2016). This result may be partially mediated by the neuronal populations that are active during exercise: visual processing and SM activity both occur during exercise. Active cortical regions receive more blood flow during tasks as evidenced in blood oxygen level‐dependent functional MRI studies (Logothetis, 2003) and may therefore receive more peripheral lactate and then process this metabolically. This may provide a degree of regional specificity in terms of the increase in lactate and neurometabolite concentrations. Future studies could investigate this notion by determining whether functional activation of cortical regions during or following exercise, such as the activation of DLPFC by a cognitive task, would similarly increase local blood flow, lactate and, consequently, neurometabolite levels in the activated regions.
The exercise‐induced increase in the SM GABA concentration was supported by two analyses employing different processing pipelines. The effects were stronger and more enduring for our primary analysis using Gannet than our secondary analysis using the LCModel. The Gannet pipeline was chosen for our primary analysis because this analysis package is specifically designed and optimized for the detection of GABA using the MEGA‐PRESS sequence (Edden et al. 2014; Mullins et al. 2014). Additionally, one advantage of Gannet is that outliers (frames exhibiting excessive frequency shift; e.g. as a result of subject movement) are identified and rejected during preprocessing to maximize the signal:noise of the averaged spectrum, which may improve measurement reliability. The use of a larger voxel size and longer scanning acquisition (e.g. 160 averages) also increases the reliability of GABA concentration measurement (Brix et al. 2017). Our relatively short acquisition duration (96 averages per timepoint) and small voxel (8 cm3) were chosen to maximize the temporal and spatial resolution of our results; however, this was at the expense of a higher coefficient of variation (16% at baseline) compared to that reported for larger voxels and longer acquisition durations (Mullins et al. 2014; Maddock et al. 2016; Brix et al. 2017). Our use of rmANOVA assists in mitigating the reduced signal:noise ratio of each individual measure.
Our data show no evidence for increases in neurometabolites involved in excitatory neurotransmission in the SM or DLPFC. The literature to date is mixed with respect to the effects of exercise on MRS derived measures of excitatory neurometabolites in different cortical regions, with some studies reporting an increase (Maddock et al. 2011; Maddock et al. 2016), whereas others were unable to replicate the effect (Dennis et al. 2015). Lactate is linked to metabolic processes that can upregulate the de novo synthesis of glutamate (Maddock et al. 2011) and so it is surprising that no changes in Glx were observed in the present study with high‐intensity exercise, as well as in a previous study using moderate level exercise (Dennis et al. 2015). This may relate to the fact that we used a sequence optimized for the detection of GABA, although, in addition, we did not observe any changes in Glx in a separate analysis of the ‘off‐resonance’ PRESS sequence. Alternatively, it could also be a result of only being able to resolve the composite glutamatergic signal Glx on our 3T scanner. It is possible that this composite signal could miss some effects that are specific to glutamate or glutamine. Interestingly, a 7T MRS study in which rats were exercised to exhaustion revealed greater increases in glutamine relative to glutamate (Swiatkiewicz et al. 2017). Nonetheless, we note that Dennis et al. (2015), who were able to resolve separate peaks for glutamate and glutamine at 7T in humans, reported no increase after exercise. Yet another possibility is that this may reflect regional specificity of HIIT effects. Further studies are required to better understand this discrepancy.
What might an exercise‐induced change in GABA concentration represent?
One question that arises is whether the increase in GABA concentration, as observed in the present study and previously, reflects synaptic neurotransmitter concentrations or, alternatively, represents other pools of GABA. GABA is utilized in synaptic neurotransmission, extrasynaptic neurotransmission and in cell metabolism via the ‘GABA shunt’ (Martin & Rimvall, 1993). Although GABA concentrations have been associated with functions such as motor learning (Stagg et al. 2011a; Kim et al. 2014), it is assumed that the majority of the GABA‐MRS signal primarily reflects extrasynaptic and metabolic pools of GABA (Stagg et al. 2011b). Indeed, most common TMS measures of inhibition are uncorrelated with GABA concentrations as derived by MRS (Stagg et al. 2011c; Tremblay et al. 2013; Dyke et al. 2017). Therefore, the changes observed in the present study, especially because they are being measured at rest during scanning, probably do not reflect an increase in synaptic neurotransmission but rather changes at the metabolic or extrasynaptic level. Increases in GABA in the extrasynaptic space typically occur following large amounts of GABA release and subsequent pooling in the synaptic and extrasynaptic space (Brickley & Mody, 2012). However, TMS studies, which typically measure the strength of synaptic GABAergic neurotransmission (Kujirai et al. 1993; Ziemann et al. 2015), indicate that synaptic GABA function is decreased after exercise (Singh et al. 2014; Smith et al. 2014; Mooney et al. 2016; Stavrinos & Coxon, 2017). Although GABA pooling can result in synaptic disinhibition via extrasynaptic GABAB autoreceptors, extrasynaptic GABAB is typically cleared within hundreds of milliseconds (Cash et al. 2010). GABA may also be found in the extracellular fluid, where it may impact on non‐synaptic GABAergic tone; however, the impact of this on synaptic transmission remains unclear (Stagg et al. 2011c). Overall, these arguments suggest that the increase in the GABA‐MRS signal probably does not reflect synaptic or extrasynaptic GABA content (Stagg et al. 2011c; Tremblay et al. 2013; Dyke et al. 2017). Instead, the increase in MRS GABA observed in the present study may reflect an increase in intracellular GABA content following HIIT, whereby GABA might be preferentially utilized to support the energetic demands of mitochondria. The underlying processes, as well as implications of this for brain plasticity, require further investigation.
It is conceivable that brain‐derived neurotrophic factor, plays a role in this process alongside lactate, given that this neurotrophic factor is released during high intensity exercise (Saucedo Marquez et al. 2015), plays a regulatory role in mediating the balance between excitation and inhibition (Cash et al. 2017) and enhances clearance of GABA from the synapse (Vaz et al. 2011). Future studies could explore this interplay and how the balance between excitation and inhibition is restored over time following HIIT by obtaining, in the same participants, MRS and TMS measures, as well as lactate and brain‐derived neurotrophic factor concentrations.
Limitations
There are some limitations to the present study. First, the lactate concentration was measured peripherally from the blood, rather than centrally via MRS. However, previous studies have shown that cerebral increases in lactate after exercise co‐occur with peripheral blood changes in lactate (Maddock et al. 2011) and that these measures are correlated with one another (Dennis et al. 2015). Brain lactate levels increase during and following exercise, albeit to a lesser extent than the increases in peripheral lactate, presumably because this incoming lactate is rapidly metabolized by the brain (Dennis et al. 2015; Ide et al. 2000; Dalsgaard et al. 2004; van Hall et al. 2009; Maddock et al. 2011). We anticipate that the strength of relationship between GABA and central lactate levels may, if anything, be potentially underestimated by measuring peripheral blood lactate levels. Second, it was not feasible to include a control study at rest without HIIT. However, Glx, NAA, choline and Myo‐Inositol levels remained stable across all time points (Tables 2 and 3) and GABA levels were stable at baseline, before increasing after exercise in the SM but not the DLPFC voxel (although this latter result may be driven by inter‐subject variability in DLPFC concentrations observed at baseline). The relationship between GABA levels and lactate levels supports a functional relationship to exercise. Third, it could be argued that GABA:H20 and Glx:H20 metrics would be influenced by dehydration during exercise. However, we took various steps to avoid dehydration (see Methods) and also examined ratios using creatine as the denominator, which confirmed our results (Fig. 3; see also secondary LCModel analysis reported in Table 3). Dehydration cannot explain why the effects were observed in the SM but not DLPFC. Furthermore, if the results for GABA:H20 were the result of a change in water levels, then Glx:H20 would not have remained stable, as was the case in the present study. Lastly, the present findings demonstrate a functional but correlative, relationship between lactate levels and GABA neurometabolite concentration. Future studies should aim to explore this relationship by testing whether neurometabolite concentrations change following injection of lactate peripherally, in the absence of HIIT.
Conclusions
The present study provides the first evidence of changes in GABA (but not Glx) neurometabolite concentrations following HIIT in human SM using MRS. Our data indicate, for the first time, a potential regional specificity of these changes, which has not been recognized previously. The correlation between GABA and lactate concentration warrants investigation in future studies.
Additional information
Competing interests
The authors declare that they have no competing financial interests.
Author contributions
This work was performed in the Movement and Exercise Neuroscience Laboratory, Monash Institute of Cognitive and Clinical Neurosciences. JC, NR and MY were responsible for the conception or design of the study. JC, CS, RC, JH and ES were responsible for the acquisition, analysis or interpretation of data. JC and RC were responsible for drafting the paper. JC, RC, JH, CS, NR and MY were responsible for revising the paper critically for important intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by a Monash University Platform Access Grant. Murat Yücel is supported by a National Health and Medical Research Fellowship Principal Research Fellowship, as well as by the David Winston Turner Endowment Fund.
Acknowledgements
We are grateful to the staff at Monash Biomedical Imaging and to Dr Mark Mikkelsen for technical support implementing Glx fitting within Gannet.
Edited by: Ole Paulsen & Richard Carson
References
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E & Hof P (2001). The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann NY Acad Sci 935, 107–117. [PubMed] [Google Scholar]
- Bergersen LH ( 2015). Lactate transport and signaling in the brain: potential therapeutic targets and roles in body‐brain interaction. J Cereb Blood Flow Metab 35, 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billat VL, Sirvent P, Py G, Koralsztein JP & Mercier J (2003). The concept of maximal lactate steady state – a bridge between biochemistry, physiology and sport science. Sports Med 33, 407–426. [DOI] [PubMed] [Google Scholar]
- Brellenthin AG & Koltyn KF (2016). Exercise as an adjunctive treatment for cannabis use disorder. Am J Drug Alcohol Ab 42, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG & Mody I (2012). Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron 73, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix MK, Ersland L, Hugdahl K, Dwyer GE, Gruner R, Noeske R, Beyer MK & Craven AR (2017). Within‐ and between‐session reproducibility of GABA measurements with MR spectroscopy. J Magn Reson Imaging 46, 421–430. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Fahey TD & Baldwin KM (2011). Exercise Physiology: Human Bioenergetics and Its Applications. McGraw Hill, Boston, MA. [Google Scholar]
- Cash R, Udupa K, Gunraj C, Mazzella F, Daskalakis ZJ, Wong AH, Kennedy JL, Fitzgerald PB & Chen R (2017). Influence of the BDNF Val66Met polymorphism on the balance of excitatory and inhibitory neurotransmission and relationship to plasticity in human cortex. Brain Stimulation 10, 502. [Google Scholar]
- Cash RF, Ziemann U, Murray K & Thickbroom GW (2010). Late cortical disinhibition in human motor cortex: a triple‐pulse transcranial magnetic stimulation study. J Neurophysiol 103, 511–518. [DOI] [PubMed] [Google Scholar]
- Chang YK, Labban JD, Gapin JI & Etnier JL (2012). The effects of acute exercise on cognitive performance: a meta‐analysis. Brain Res 1453, 87–101. [DOI] [PubMed] [Google Scholar]
- Dalsgaard MK, Quistorff B, Danielsen ER, Selmer C, Vogelsang T & Secher NH (2004). A reduced cerebral metabolic ratio in exercise reflects metabolism and not accumulation of lactate within the human brain. J Physiol 554, 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis A, Thomas AG, Rawlings NB, Near J, Nichols TE, Clare S, Johansen‐Berg H & Stagg CJ (2015). An ultra‐high field magnetic resonance spectroscopy study of post exercise lactate, glutamate and glutamine change in the human brain. Front Physiol 6, 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke K, Pepes SE, Chen C, Kim S, Sigurdsson HP, Draper A, Husain M, Nachev P, Gowland PA, Morris PG & Jackson SR (2017). Comparing GABA‐dependent physiological measures of inhibition with proton magnetic resonance spectroscopy measurement of GABA using ultra‐high‐field MRI. Neuroimage 152, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RA, Puts NA, Harris AD, Barker PB & Evans CJ (2014). Gannet: A batch‐processing tool for the quantitative analysis of gamma‐aminobutyric acid‐edited MR spectroscopy spectra. J Magn Reson Imaging 40, 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal M, Dabas A, Saharan S, Barker PB, Edden RA & Mandal PK (2016). GABA quantitation using MEGA‐PRESS: Regional and hemispheric differences. J Magn Reson Imaging 44, 1619–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide K, Schmalbruch IK, Quistorff B, Horn A & Secher NH (2000). Lactate, glucose and O2 uptake in human brain during recovery from maximal exercise. J Physiol 522 Pt 1, 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen‐Berg H & Duzel E (2016). Neuroplasticity: effects of physical and cognitive activity on brain structure and function. Neuroimage 131, 1–3. [DOI] [PubMed] [Google Scholar]
- Kemppainen J, Aalto S, Fujimoto T, Kalliokoski KK, Langsjo J, Oikonen V, Rinne J, Nuutila P & Knuuti J (2005). High intensity exercise decreases global brain glucose uptake in humans. J Physiol 568, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Stephenson MC, Morris PG & Jackson SR (2014). tDCS‐induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: a 7 T magnetic resonance spectroscopy study. Neuroimage 99, 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P & Marsden CD (1993). Corticocortical inhibition in human motor cortex. J Physiol 471, 501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK (2003). The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci 23, 3963–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machler P, Wyss MT, Elsayed M, Stobart J, Gutierrez R, von Faber‐Castell A, Kaelin V, Zuend M, San Martin A, Romero‐Gomez I, Baeza‐Lehnert F, Lengacher S, Schneider BL, Aebischer P, Magistretti PJ, Barros LF & Weber B (2016). In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab 23, 94–102. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Casazza GA, Buonocore MH & Tanase C (2011). Vigorous exercise increases brain lactate and Glx (glutamate+glutamine): a dynamic 1H‐MRS study. Neuroimage 57, 1324–1330. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Casazza GA, Fernandez DH & Maddock MI (2016). Acute modulation of cortical glutamate and GABA content by physical activity. J Neurosci 36, 2449–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang CS, Snow NJ, Campbell KL, Ross CJ & Boyd LA (2014). A single bout of high‐intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence‐specific implicit motor learning. J Appl Physiol (1985) 117, 1325–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DL & Rimvall K (1993). Regulation of gamma‐aminobutyric acid synthesis in the brain. J Neurochem 60, 395–407. [DOI] [PubMed] [Google Scholar]
- Mooney RA, Coxon JP, Cirillo J, Glenny H, Gant N & Byblow WD (2016). Acute aerobic exercise modulates primary motor cortex inhibition. Experimental Brain Research 234, 3669–3676. [DOI] [PubMed] [Google Scholar]
- Mullins PG, McGonigle DJ, O'Gorman RL, Puts NA, Vidyasagar R, Evans CJ, Cardiff Symposium on MRSoG & Edden RA (2014). Current practice in the use of MEGA‐PRESS spectroscopy for the detection of GABA. Neuroimage 86, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near J, Edden R, Evans CJ, Paquin R, Harris A & Jezzard P (2015). Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med 73, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK & Saltin B (2015). Exercise as medicine ‐ evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Spor 25, 1–72. [DOI] [PubMed] [Google Scholar]
- Prakash RS, Voss MW, Erickson KI & Kramer AF (2015). Physical activity and cognitive vitality. Annu Rev Psychol 66, 769–797. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Wyss MT & Lundby C (2011). Cerebral glucose and lactate consumption during cerebral activation by physical activity in humans. FASEB J 25, 2865–2873. [DOI] [PubMed] [Google Scholar]
- Rector NA, Richter MA, Lerman B & Regev R (2015). A pilot test of the additive benefits of physical exercise to CBT for OCD. Cogn Behav Ther 44, 328–340. [DOI] [PubMed] [Google Scholar]
- Roig M, Skriver K, Lundbye‐Jensen J, Kiens B & Nielsen JB (2012). A single bout of exercise improves motor memory. PLoS ONE 7, e44594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucedo Marquez CM, Vanaudenaerde B, Troosters T & Wenderoth N (2015). High‐intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J Appl Physiol (1985) 119, 1363–1373. [DOI] [PubMed] [Google Scholar]
- Singh AM, Duncan RE, Neva JL & Staines WR (2014). Aerobic exercise modulates intracortical inhibition and facilitation in a nonexercised upper limb muscle. BMC Sports Sci Med Rehabil 6, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AE, Goldsworthy MR, Garside T, Wood FM & Ridding MC (2014). The influence of a single bout of aerobic exercise on short‐interval intracortical excitability. Exp Brain Res 232, 1875–1882. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V & Johansen‐Berg H (2011a). The role of GABA in human motor learning. Curr Biol 21, 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V & Johansen‐Berg H (2011b). What are we measuring with GABA magnetic resonance spectroscopy? Commun Integr Biol 4, 573–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bestmann S, Constantinescu AO, Moreno LM, Allman C, Mekle R, Woolrich M, Near J, Johansen‐Berg H & Rothwell JC (2011c). Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J Physiol 589, 5845–5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrinos EL & Coxon JP (2017). High‐intensity interval exercise promotes motor cortex disinhibition and early motor skill consolidation. J Cogn Neurosci 29, 593–604. [DOI] [PubMed] [Google Scholar]
- Swiatkiewicz M, Fiedorowicz M, Orzel J, Welniak‐Kaminska M, Bogorodzki P, Langfort J & Grieb P (2017). Increases in brain 1H‐MR glutamine and glutamate signals following acute exhaustive endurance exercise in the rat. Front Physiol 8, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R, Johnsen LK, Geertsen SS, Christiansen L, Ritz C, Roig M & Lundbye‐Jensen J (2016). Acute exercise and motor memory consolidation: the role of exercise intensity. PLoS ONE 11, e0159589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, Beaule V, Proulx S, de Beaumont L, Marjanska M, Doyon J, Pascual‐Leone A, Lassonde M & Theoret H (2013). Relationship between transcranial magnetic stimulation measures of intracortical inhibition and spectroscopy measures of GABA and glutamate+glutamine. J Neurophysiol 109, 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G, Stromstad M , Rasmussen P, Jans O, Zaar M, Gam C, Quistorff B, Secher NH & Nielsen HB (2009). Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab 29, 1121–1129. [DOI] [PubMed] [Google Scholar]
- Vaz SH, Jorgensen TN, Cristovao‐Ferreira S, Duflot S, Ribeiro JA, Gether U & Sebastiao AM (2011). Brain‐derived neurotrophic factor (BDNF) enhances GABA transport by modulating the trafficking of GABA transporter‐1 (GAT‐1) from the plasma membrane of rat cortical astrocytes. J Biol Chem 286, 40464–40476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Reis J, Schwenkreis P, Rosanova M, Strafella A, Badawy R & Muller‐Dahlhaus F (2015). TMS and drugs revisited 2014. Clin Neurophysiol 126, 1847–1868. [DOI] [PubMed] [Google Scholar]


