Abstract
Key points
We recently found that feeding healthy mice a diet with reduced levels of branched-chain amino acids (BCAAs), which are associated with insulin resistance in both humans and rodents, modestly improves glucose tolerance and slows fat mass gain.
In the present study, we show that a reduced BCAA diet promotes rapid fat mass loss without calorie restriction in obese mice.
Selective reduction of dietary BCAAs also restores glucose tolerance and insulin sensitivity to obese mice, even as they continue to consume a high‐fat, high‐sugar diet.
A low BCAA diet transiently induces FGF21 (fibroblast growth factor 21) and increases energy expenditure.
We suggest that dietary protein quality (i.e. the precise macronutrient composition of dietary protein) may impact the effectiveness of weight loss diets.
Abstract
Obesity and diabetes are increasing problems around the world, and although even moderate weight loss can improve metabolic health, reduced calorie diets are notoriously difficult to sustain. Branched‐chain amino acids (BCAAs; leucine, isoleucine and valine) are elevated in the blood of obese, insulin‐resistant humans and rodents. We recently demonstrated that specifically reducing dietary levels of BCAAs has beneficial effects on the metabolic health of young, growing mice, improving glucose tolerance and modestly slowing fat mass gain. In the present study, we examine the hypothesis that reducing dietary BCAAs will promote weight loss, reduce adiposity, and improve blood glucose control in diet‐induced obese mice with pre‐existing metabolic syndrome. We find that specifically reducing dietary BCAAs rapidly reverses diet‐induced obesity and improves glucoregulatory control in diet‐induced obese mice. Most dramatically, mice eating an otherwise unhealthy high‐calorie, high‐sugar Western diet with reduced levels of BCAAs lost weight and fat mass rapidly until regaining a normal weight. Importantly, this normalization of weight was mediated not by caloric restriction or increased activity, but by increased energy expenditure, and was accompanied by a transient induction of the energy balance regulating hormone FGF21 (fibroblast growth factor 21). Consumption of a Western diet reduced in BCAAs was also accompanied by a dramatic improvement in glucose tolerance and insulin resistance. Our results link dietary BCAAs with the regulation of metabolic health and energy balance in obese animals, and suggest that specifically reducing dietary BCAAs may represent a highly translatable option for the treatment of obesity and insulin resistance.
Keywords: branched‐chain amino acids, protein restriction, obesity, diabetes
Key points
We recently found that feeding healthy mice a diet with reduced levels of branched-chain amino acids (BCAAs), which are associated with insulin resistance in both humans and rodents, modestly improves glucose tolerance and slows fat mass gain.
In the present study, we show that a reduced BCAA diet promotes rapid fat mass loss without calorie restriction in obese mice.
Selective reduction of dietary BCAAs also restores glucose tolerance and insulin sensitivity to obese mice, even as they continue to consume a high‐fat, high‐sugar diet.
A low BCAA diet transiently induces FGF21 (fibroblast growth factor 21) and increases energy expenditure.
We suggest that dietary protein quality (i.e. the precise macronutrient composition of dietary protein) may impact the effectiveness of weight loss diets.
Introduction
Over the last four decades, the prevalence of obesity has increased dramatically. In the USA, more than two in three adults are now considered to be overweight or obese (Flegal et al. 2012). A similar proportion of males in the European Union are likewise afflicted (Janda et al. 2013). Obesity is associated with an increased risk of many diseases, most notably type 2 diabetes, which is also increasing around the world. Although weight loss is a highly effective means of improving metabolic health, reduced calorie diets are notoriously difficult to sustain. Altering the macronutrient composition of the diet while keeping the total number of calories constant is an intriguing alternative that may be more sustainable (Fontana & Partridge, 2015).
Several recent studies have found that high protein consumption is correlated with insulin resistance, diabetes, and increased mortality in both mice and humans (Lagiou et al. 2007; Sluijs et al. 2010; Solon‐Biet et al. 2014). Conversely, low protein (LP) diets are associated with metabolic health and increased survival (Levine et al. 2014; Solon‐Biet et al. 2014; Simpson et al. 2017), and a recent randomized controlled trial found that a LP diet promotes leanness and decreases fasting blood glucose in humans (Fontana et al. 2016). An LP diet also promotes metabolic health in rodents, reducing the accumulation of white adipose tissue (WAT) and increasing glucose tolerance and insulin sensitivity in animals fed a normal diet, as well as improving glucose homeostasis in mice fed a high‐fat diet (Laeger et al. 2014; Solon‐Biet et al. 2015; Fontana et al. 2016; Maida et al. 2016).
We hypothesized that the beneficial effects of a LP diet might be driven by reduced consumption of specific essential amino acids. We focused on the branched‐chain amino acids (BCAAs; leucine, isoleucine and valine) as blood levels of BCAAs correlate with insulin‐resistant obesity and diabetes in humans and rodents (Felig et al. 1969; Newgard et al. 2009; Batch et al. 2013; Lynch & Adams, 2014; Connelly et al. 2017). BCAA levels also correlate well with outcomes in weight loss regimens (Shah et al. 2012), and are reduced in both mice and humans consuming LP diets (Solon‐Biet et al. 2014; Fontana et al. 2016). We determined that specifically reducing dietary BCAAs by two‐thirds recapitulates many beneficial effects of a LP diet, promoting leanness and glucose tolerance in metabolically normal C57BL/6J mice (Fontana et al. 2016). Reducing dietary BCAAs from a young age also slows the accumulation of visceral adipose tissue in Zucker‐fatty rats and preserves insulin sensitivity (White et al. 2016).
These results led us to hypothesize that specifically reducing dietary BCAAs might not only preserve the metabolic health of young animals, but also might be an effective strategy to restore metabolic health to animals with pre‐existing diet‐induced metabolic dysfunction. Herein, we test if specifically reducing dietary BCAAs can restore metabolic health to C57BL/6J mice preconditioned with a high‐calorie, high‐fat, high‐sugar Western diet (WD), a well characterized model of diet‐induced obesity (DIO) and early type 2 diabetes (Winzell & Ahren, 2004; Newberry et al. 2006; Peterson et al. 2011; Williams et al. 2014). We find that specifically reducing dietary BCAAs is sufficient to promote weight normalization without caloric restriction in less than 4 weeks, primarily as a result of a dramatic reduction in fat mass, and improves metabolic health. Reduction of dietary BCAAs in the context of an otherwise WD transiently induces the energy balance regulating hormone FGF21 (fibroblast growth factor 21) and induces a sustained increase in energy expenditure. Our results suggest that specifically reducing dietary BCAAs, or treatment with pharmaceuticals that mimic this effect, could be an effective and translatable intervention for promoting weight normalization, control of blood glucose, and overall metabolic health.
Methods
Ethical approval and animals
All procedures conformed with institutional guidelines and were approved by the Institutional Animal Care and Use Committee of the William S. Middleton Memorial Veterans Hospital (Madison, WI, USA). Animals were killed using methods consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. The research complies with the policies of The Journal of Physiology (Grundy, 2015).
Animals and diets
Male C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) at 5 weeks of age, and preconditioned with WD (TD.88137; Envigo, Madison, WI, USA) starting at 6 weeks of age for 12 weeks; chow control mice were fed Purina 5001 (Purina Mills, Richmond, IN, USA). Mice were then switched to amino acid defined diets or a WD supplemented with additional BCAAs; all diets were obtained from Envigo and diet descriptions, compositions and item numbers are provided in Tables 1, 2, 3. Mice were housed in a SPF mouse facility under a 12:12 h light/dark cycle with free access to food and water, except where noted in the procedures below. Animals were group housed in static microisolator cages, except when temporarily housed in a Oxymax/CLAMS metabolic chamber system (Columbus Instruments, Columbus, OH, USA). Group sizes are provided as appropriate. The randomization of obese, WD fed mice was performed at the cage level to ensure that all groups had approximately the same initial starting weight.
Table 1.
Diet composition of diets used to investigate the effect of reduced levels of dietary BCAAs in the context of a normal calorie diet
| Amino acid defined diets | Control AA | ExLow AA | ExLow BCAA | Western (WD) | WD + BCAA |
|---|---|---|---|---|---|
| Teklad diet number | TD.140711 | TD.140918 | TD.150387 | TD.88137 | TD.150386 |
| Colour | Red | Orange | Blue | Tan | Green |
| Formula (g kg−1) | |||||
| l‐Alanine | 9.38 | 2.18 | 12.1566 | – | – |
| l‐Arginine | 6.3 | 1.46 | 6.3 | – | – |
| l‐Asparagine | 20.58 | 4.79 | 22.6388 | – | – |
| l‐Aspartic acid | 20.58 | 4.79 | 24.729 | – | – |
| l‐Cysteine | 7.2 | 1.67 | 7.2 | – | – |
| l‐Glutamic acid | 28.97 | 6.74 | 33.5548 | – | – |
| l‐Glutamine | 33.77 | 7.87 | 36.0672 | – | – |
| Glycine | 2.96 | 0.69 | 5.2991 | – | – |
| l‐Histidine HCl, monohydrate | 4.6 | 1.07 | 4.6 | – | – |
| l‐Isoleucine | 7.8 | 1.81 | 1.81 | – | 8.8725 |
| l‐Leucine | 25.4 | 5.9 | 5.9 | – | 15.6195 |
| l‐Lysine HCl | 20.38 | 4.74 | 20.38 | – | – |
| l‐Methionine | 6.7 | 1.56 | 6.7 | – | – |
| l‐Phenylalanine | 6.6 | 1.54 | 6.6 | – | – |
| l‐Proline | 7.41 | 1.72 | 10.9965 | – | – |
| l‐Serine | 7.41 | 1.72 | 10.6844 | – | – |
| l‐Threonine | 9.7 | 2.26 | 9.7 | – | – |
| l‐Tryptophan | 3.4 | 0.79 | 3.4 | – | – |
| l‐Tyrosine | 6.9 | 1.61 | 6.9 | – | – |
| l‐Valine | 8.4 | 1.95 | 1.95 | – | 10.725 |
| dl‐Methionine | – | – | – | 3.0 | 3.0 |
| Casein | – | – | – | 195 | 195 |
| Sucrose | 291.248 | 291.248 | 291.248 | 341.46 | 341.46 |
| Corn starch | 150.0 | 243.79 | 153.4368 | 150 | 114.683 |
| Maltodextrin | 150.0 | 243.79 | 153.4368 | – | – |
| Anhydrous milkfat | – | – | – | 210 | 210 |
| Cholesterol | – | – | – | 1.5 | 1.5 |
| Corn oil | 52.0 | 52.0 | 52.0 | – | – |
| Olive oil | 29.0 | 29.0 | 29.0 | – | – |
| Cellulose | 30.0 | 30 | 30 | 50 | 50 |
| Mineral mix, AIN‐93M‐MX (94049) | 35.0 | 35 | 35 | – | – |
| Mineral mix, AIN‐76 (170915) | – | – | – | 35 | 35 |
| Calcium carbonate | – | – | – | 4 | 4 |
| Calcium phosphate Ca(H2PO4)2·H2O | 8.2 | 8.2 | 8.2 | – | – |
| Vitamin mix, Teklad (40060) | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| TBHQ, anti‐oxidant | 0.012 | 0.012 | 0.012 | – | – |
| Ethoxyquin, anti‐oxidant | – | – | – | 0.04 | 0.04 |
| Food colouring | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| % kcal from: | |||||
| Protein (based on N × 6.25) | 22 | 5.1 | 21.9 | 15.2 | 18.3 |
| Carbohydrates | 59.4 | 76.4 | 59.6 | 42.7 | 39.8 |
| vFat | 18.6 | 18.5 | 18.5 | 42 | 41.9 |
| kcal g−1 | 3.9 | 3.9 | 3.9 | 4.5 | 4.6 |
Table 2.
Composition of amino acid defined WDs
| Diet name | Description |
|---|---|
| WD Control AA | AA‐defined WD – 41% of calories from fat, 38% of calories from carbohydrates (high sucrose), with cholesterol |
| WD High BCAA | Similar to WD Control AA; with 2× BCAAs |
| WD Low BCAA | Similar to WD Control AA; with 67% reduction in the BCAAs |
| WD Low AA | Similar to WD Control AA; with 67% reduction in all AAs |
| Control AA | AA defined normal calorie diet |
The exact formulation of these diets is provided in Table 3.
Table 3.
Diet composition of diets used to investigate the effect of specifically altering dietary BCAAs in the context of a WD
| Amino acid defined diets | Control AA | WD Control AA | WD High BCAA | WD Low BCAA | WD Low AA |
|---|---|---|---|---|---|
| Teklad diet number | TD.140711 | TD.160186 | TD.160189 | TD.160188 | TD.160187 |
| Colour | Red | Aqua | Green | Black | Orange |
| Formula (g kg−1) | |||||
| l‐Alanine | 9.38 | 9.38 | 5.5861 | 11.8183 | 3.05 |
| l‐Arginine | 6.3 | 6.3 | 6.3 | 6.3 | 2.05 |
| l‐Asparagine | 20.58 | 20.58 | 17.7668 | 22.388 | 6.7 |
| l‐Aspartic acid | 20.58 | 20.58 | 14.9108 | 24.2237 | 6.7 |
| l‐Cysteine | 7.2 | 7.2 | 7.2 | 7.2 | 2.34 |
| l‐Glutamic acid | 28.97 | 28.97 | 22.7053 | 32.9963 | 9.43 |
| l‐Glutamine | 33.77 | 33.77 | 30.6311 | 35.7873 | 11.0 |
| Glycine | 2.96 | 2.96 | 0.96 | 5.0141 | 0.96 |
| l‐Histidine HCl, monohydrate | 4.6 | 4.6 | 4.6 | 4.6 | 1.5 |
| l‐Isoleucine | 7.8 | 7.8 | 15.6 | 2.54 | 2.54 |
| l‐Leucine | 25.4 | 25.4 | 50.8 | 8.27 | 8.27 |
| l‐Lysine HCl | 20.38 | 20.38 | 20.38 | 20.38 | 6.64 |
| l‐Methionine | 6.7 | 6.7 | 6.7 | 6.7 | 2.18 |
| l‐Phenylalanine | 6.6 | 6.6 | 6.6 | 6.6 | 2.15 |
| l‐Proline | 7.41 | 7.41 | 2.5094 | 10.5596 | 2.41 |
| l‐Serine | 7.41 | 7.41 | 2.9359 | 10.2855 | 2.41 |
| l‐Threonine | 9.7 | 9.7 | 9.7 | 9.7 | 3.16 |
| l‐Tryptophan | 3.4 | 3.4 | 3.4 | 3.4 | 1.1 |
| l‐Tyrosine | 6.9 | 6.9 | 6.9 | 6.9 | 2.25 |
| l‐Valine | 8.4 | 8.4 | 16.8 | 2.735 | 2.735 |
| Sucrose | 291.248 | 341.46 | 341.46 | 341.46 | 341.46 |
| Corn starch | 150.0 | 49.63 | 45.3573 | 52.6511 | 132.0625 |
| Maltodextrin | 150.0 | 49.63 | 45.3573 | 52.6511 | 132.0625 |
| Anhydrous milkfat | – | 210 | 210 | 210 | 210 |
| Cholesterol | – | 1.5 | 1.5 | 1.5 | 1.5 |
| Corn oil | 52.0 | – | – | – | – |
| Olive oil | 29.0 | – | – | – | – |
| Cellulose | 30.0 | 50 | 50 | 50 | 50 |
| Mineral mix, AIN‐93M‐MX (94049) | 35.0 | 35 | 35 | 35 | 35 |
| Calcium phosphate Ca(H2PO4)2·H2O | 8.2 | 8.2 | 8.2 | 8.2 | 8.2 |
| Vitamin mix, Teklad (40060) | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| TBHQ, anti‐oxidant | 0.012 | 0.04 | 0.04 | 0.04 | 0.04 |
| Food colouring | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| % kcal from: | |||||
| Protein (based on N × 6.25) | 22 | 20.7 | 21.4 | 20.2 | 6.8 |
| Carbohydrates | 59.4 | 38.5 | 37.8 | 39.0 | 52 |
| Fat | 18.6 | 40.9 | 40.8 | 40.9 | 41.2 |
| kcal g−1 | 3.9 | 4.6 | 4.6 | 4.6 | 4.6 |
Procedures
Glucose tolerance tests were performed by fasting the mice overnight for 16 h and then injecting glucose (1 g kg−1) i.p. (Arriola Apelo et al. 2016; Fontana et al. 2016). Insulin tolerance tests were performed by fasting mice for 4 h starting at lights on and then injecting insulin (0.75 U kg−1) i.p. Glucose measurements were taken using a Bayer Contour blood glucose meter (Bayer, Leverkusen, Germany) and test strips. Blood for fasting insulin and FGF21 was obtained following an overnight fast; insulin (Crystal Chem, Elk Grove Village, IL, USA) and FGF21 (R&D Systems, Minneapolis, MN, USA) levels were determined by an enzyme‐linked immunosorbent assay. Mouse body composition was determined using an EchoMRI 3‐in‐1 Body Composition Analyser (EchoMRI, Houston, TX, USA). For assay of multiple metabolic parameters [O2, CO2, food consumption, respiratory exchange ratio (RER), energy expenditure] and activity tracking, mice were acclimated to housing in a Oxymax/CLAMS metabolic chamber system (Columbus Instruments) for ∼24 h and data from a continuous 24 h period was then recorded and analysed. Triglycerides were measured by Triglyceride Colorimetric Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA) in liver collected after fasting for ∼16 h. Other tissues for molecular analysis were flash‐frozen in liquid nitrogen or fixed and prepared as described below.
Histology
Samples of brown adipose tissue (BAT), WAT, liver and skin were isolated following death. Adipose was fixed in 4% paraformaldehyde overnight, and then sectioned and haematoxylin and eosin (H&E) stained by the University of Wisconsin Carbone Cancer Center (UWCCC) Experimental Pathology Laboratory. Liver was embedded in OCT, and then cryosectioned and Oil‐Red‐O stained by the UWCCC Experimental Pathology Laboratory. Liver, BAT and WAT sections were imaged using an EVOS microscope (Thermo Fisher Scientific Inc., Waltham, MA, USA) as described previously (Linnemann et al. 2015). Skin was isolated from the belly and back of mice, paraformaldehyde‐fixed (4%) overnight, and then paraffin‐embedded for evaluation (Kasza et al. 2014). Scale bars were inserted automatically or manually by the investigator. For quantification of lipid droplet size, six independent fields were obtained for each tissue from each mouse and quantified using ImageJ (NIH, Bethesda, MD, USA).
Quantitative PCR
Liver or adipose RNA was extracted with Trireagent (Sigma, St Louis, MO, USA). Then, 1 μg of RNA was used to generate cDNA (Superscript III; Invitrogen, Carlsbad, CA, USA). Oligo dT primers and primers for real‐time PCR were obtained from Integrated DNA Technologies (IDT, Coralville, IA, USA). Reactions were run on an StepOne Plus machine (Applied Biosystems, Foster City, CA, USA) with Sybr Green PCR Master Mix (Invitrogen). Actin was used to normalize the results from gene‐specific reactions. The primer sequences used for quantitative PCR are as described previously (Hagiwara et al. 2012; Fontana et al. 2016) or were designed using the IDT quantitative PCR design tool and were: Acc1: F: AAGGCTATGTGAAGGATG, R: CTGTCTGAAGAGGTTAGG; Acl: F: GCCAGCGGGAGCACATC, R: CTTTGCAGGTGCCACTTCATC; Actb: F: ACCTTCTACAATGAGCTGCG, R: CTGGATGGCTACGTACATGG; Bmp8: F: TCAACACAACCCTCCACATCA, R: AGATCGGAGCGTCTGAAGATC; Dgat1: F: TGGTGTGTGGTGATGCTGATC, R: GCCAGGCGCTTCTCAA; Dgat2: F: AGTGGCAATGCTATCATCATCAT, R: TCTTCTGGACCCATCGGCCCCAGGA; Fasn: F: CCCCTCTGTTAATTGGCTCC, R: TTGTGGAAGTGCAGGTTAGG; Fgf21: F: CAAATCCTGGGTGTCAAAGC, R: CATGGGCTTCAGACTGGTAC; Gpat: F: CAACACCATCCCCGACATC, R: GTGACCTTCGATTATGCGATCA; Pparg: F: GTACTGCCGTTTTCACAAGTG, R: TCTTTCAGGTCGTGTTCACAG; Scd1: F: CTGACCTGAAAGCCGAGAAG, R: AGAAGGTGCTAACGAACAGG; Srebp1c: F: GGAGCCATGGATTGCACATT, R: GGCCCGGGAAGTCACTGT.
Immunoblotting
Tissue samples were lysed in cold RIPA buffer supplemented with phosphatase and protease inhibitor cocktail tablets. Tissues were lysed as described previously (Baar et al. 2016) using a FastPrep 24 (MP Biomedicals, Santa Ana, CA, USA) with bead‐beating tubes (13119‐500) and ceramic beads (13113‐325) from Mo‐Bio Laboratories (San Diego, CA, USA) and then centrifuged. Protein concentration was determined by Bradford (Pierce Biotechnology, Rockford, IL, USA). Next, 20 μg of protein was separated by SDS‐PAGE on 10% resolving gels (Life Technologies, Grand Island, NY, USA). Antibody for HSP90 (#4874) was purchased from Cell Signaling Technology (Beverly, MA, USA). Antibody for UCP1 (ab10983) was purchased from Abcam (Cambridge, MA, USA). Imaging was performed using a GE ImageQuant LAS 4000 imaging station (GE Healthcare Chicago, IL, USA). Quantification was determined by densitometry using ImageJ.
Islet isolation and ex vivo studies
Islets were isolated and an ex vivo glucose stimulated insulin secretion assay was performed as described previously (Neuman et al. 2014; Truchan et al. 2015; Fontana et al. 2016). Briefly, mice were anaesthetized with 240 mg kg−1 of freshly prepared avertin, a form of anaesthesia that does not dilate the vasculature or alter blood glucose levels, and the mouse was then killed by exsanguination while the pancreas was inflated with collagenase to isolate the islets. Mitochondrial membrane potential was measured in islets pre‐loaded with Rhodamine123 (5 μm, 5 min) (Sigma) and perfused with a standard external solution (135 mm NaCl, 4.8 mm KCl, 5 mm CaCl2, 1.2 mm MgCl2 and 20 mm Hepes, pH 7.35) containing 2 or 20 mm glucose, followed by a reference solution containing 20 mm glucose and 5 mm KCN, which was used to normalize the data. Excitation (500/20×) and emission (535/30 m) filters (ET type; Chroma Technology Corporation, Rockingham, VT, USA) were used in combination with an FF444/521/608‐Di01 dichroic (Semrock, Rochester, NY, USA) on a Ti‐Eclipse microscope (Nikon, Tokyo, Japan); the imaging system has been described previously (Gregg et al. 2016). A single region of interest was used to quantify the average response of each islet using Elements (Nikon).
Statistical analysis
Statistical analysis was conducted using Prism, version 7 (GraphPad Software Inc., San Diego, CA, USA). Tests involving repeated measurements were analysed with two‐way repeated‐measures ANOVA, followed by a Tukey–Kramer or Dunnett's post hoc test as specified. All other comparisons of three or more means were analysed by one‐way ANOVA followed by a Dunnett's or Tukey–Kramer post hoc test as specified where appropriate. Additional comparisons, if any, were corrected for multiple comparisons using the Bonferroni method.
Results
DIO mice switched to normal calorie diets with reduced BCAAs rapidly lose weight and improve glycaemic control
We induced obesity and metabolic dysfunction by feeding C57BL/6J mice a WD for 12 weeks (DIO mice). DIO mice were then switched to one of several different diets of varying amino acid compositions, with an energy density and macronutrient composition typical of rodent chow (Fig. 1 A). One group of mice was maintained on WD, whereas an additional group of mice was fed WD supplemented with BCAAs. Finally, a parallel group of mice never exposed to a WD was placed on a Control amino acid defined diet. Exact diet formulations are provided in Table 1.
Figure 1. Normal calorie diets with reduced levels of BCAAs promote rapid weight loss.
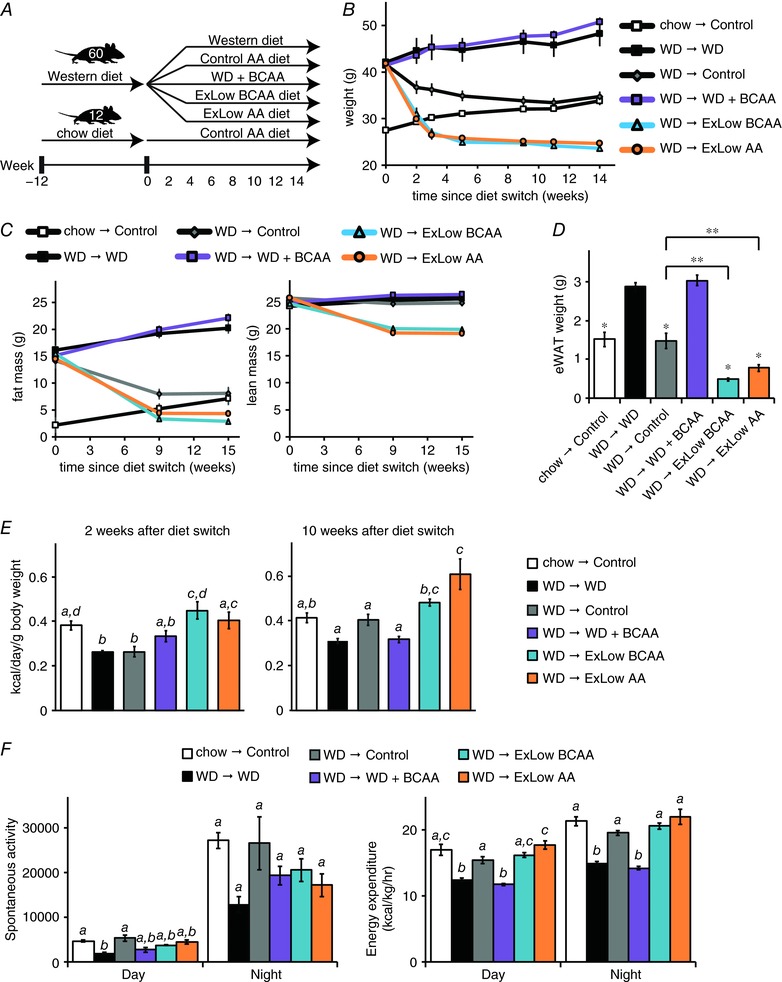
A, schematic representation of the experimental plan; mice were preconditioned with a WD for 12 weeks and then randomized to the five experimental groups shown, whereas chow‐fed Control mice were placed on an amino acid defined Control diet. B, weight as well as (C) adipose and lean mass, of mice in each experimental group, was tracked (n = 12 mice per group). D, epididymal WAT was collected at necropsy and weighed (n = 11–12 mice per group; * P < 0.05, Dunnett's test following ANOVA, ** P < 0.05, Bonferroni's test). E, 2 and 10 weeks following the start of the specified dietary intervention, food intake was assessed over a 4 day period in home cages, calculated as kcal day g−1 body weight (n = 6–7 cages per group; means with the same lowercase letter are not significantly different from each other, Tukey–Kramer test following ANOVA, P < 0.05). F, spontaneous activity and energy expenditure were measured using metabolic chambers ∼7–8 weeks after the start of the dietary intervention (n = 5–8 mice per group; means with the same lowercase letter are not significantly different from each other, Tukey–Kramer test following ANOVA, P < 0.05). Error bars represent the SE.
All DIO mice lost weight when switched to a normal calorie diet, whereas mice consuming a WD (with or without supplemental BCAAs) continued to gain weight (Fig. 1 B). Mice consuming diets in which the BCAAs [extra low (ExLow) BCAA] or all amino acids (ExLow AA) were specifically reduced lost weight very rapidly, shedding ∼25% of their body weight in 2 weeks before eventually stabilizing at a weight lower than mice never exposed to a WD. In contrast, DIO mice switched to a normal calorie Control diet normalized their weight more slowly, over ∼2 additional months. Mice fed the ExLow BCAA or ExLow AA diets lost fat mass, including epididymal WAT, and lean mass, with a net effect of greatly reduced adiposity relative to mice switched to the Control diet as well as to those remaining on a WD (Fig. 1 C and D).
The dramatic weight loss of mice consuming the ExLow BCAA and ExLow AA diets was not the result of decreased food consumption. Indeed, the absolute caloric intake of mice consuming these diets was similar to that of mice switched to the normal calorie Control diet (data not shown) and, relative to their body weight, the caloric intake of mice on either the ExLow BCAA or ExLow AA diets was increased (Fig. 1 E). Low protein and low amino acid diets promote energy expenditure (Laeger et al. 2014; Fontana et al. 2016). We assessed activity and energy expenditure via indirect calorimetry once the weights of all groups had stabilized. Although all mice switched to normal calorie diets had similar levels of activity, we observed increased energy expenditure in mice switched to the ExLow AA diet (Fig. 1 F).
DIO mice switched to any of the normal calorie diets had significantly thinner dermal WAT (dWAT) (Fig. 2 A and B) than mice remaining on a WD. DIO mice fed the ExLow BCAA diet had thinner dWAT than DIO mice switched to the Control diet. DIO mice consuming WD or WD supplemented with BCAAs had evident hepatic steatosis with large fat droplets, whereas DIO mice switched to either the Control or ExLow BCAA diets had decreased liver droplet size and normal liver histology by the conclusion of the experiment (Fig. 2 C and D). DIO mice switched to the ExLow AA diet had a trend towards reduced lipid droplet size (corrected P = 0.12), but hepatic steatosis was still evident. mRNA expression of many lipogenic genes and transcription factors was reduced in the livers of mice switched to Control or ExLow BCAA diets, with similar but less dramatic effects in mice switched to the ExLow AA diet (Fig. 2 E). Surprisingly, mice fed WD supplemented with BCAAs also had decreased hepatic expression of many lipogenic genes and transcription factors.
Figure 2. Consumption of an ExLow BCAA diet reduces adipose tissue and reverses diet‐induced hepatic steatosis.
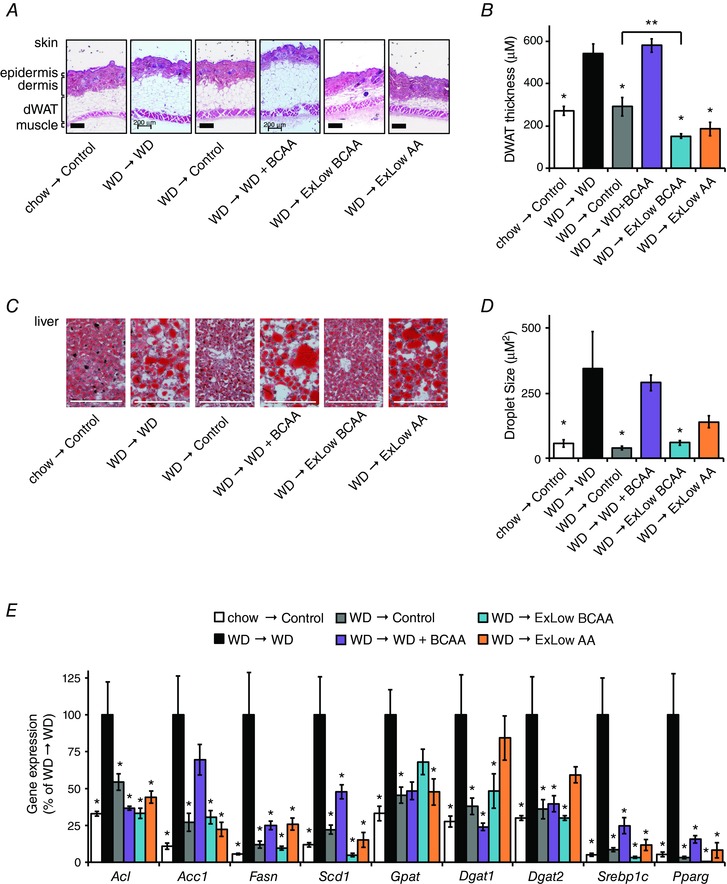
A, paraffin‐embedded skin samples were collected at necropsy ∼15 weeks following the start of the specified dietary intervention, sectioned, and H&E stained, and the thickness of dermal WAT was quantified (B) for non‐anagen stage skin samples, measuring from muscle to dermis; scale bar = 200 μm (n = 5–7 mice per group, * P < 0.05, Dunnett's test following ANOVA, ** P < 0.05, Bonferroni's test). C, liver samples were stained with Oil‐Red‐O and (D) droplet size was quantified; scale bar = 200 μm (n = 3 mice per group, * P < 0.05 vs. WD, Dunnett's test following ANOVA). E, lipogenic gene expression was measured in the livers of fasted mice (n = 5–6 mice per group, * P < 0.05 vs. WD, Dunnett's test following two‐way repeated measures ANOVA). Error bars represent the SE.
Glucose tolerance improved in all mice switched to normal calorie diets; after 3 weeks, mice switched to ExLow BCAA and ExLow AA diets had improved glucose tolerance relative to all other groups, including mice never exposed to a WD (Fig. 3 A), which was an effect that was sustained throughout the experiment. In contrast, supplementing a WD with BCAAs resulted in worse glucose tolerance vs. all other groups after 9 weeks (data not shown). DIO mice that remained on a WD were insulin resistant; all mice placed on normal calorie diets showed improved insulin sensitivity relative to WD mice, with mice switched to an ExLow AA diet showing improved insulin sensitivity relative to all groups (Fig. 3 B). DIO mice that remained on a WD had fasting hyperglycaemia and hyperinsulinaemia, as well as increased HOMA2‐IR (Levy et al. 1998; Mather, 2009), relative to mice never exposed to WD (Fig. 3 C–E). In agreement with our tolerance tests, these deficits were corrected in mice switched to any of the normal calorie diets (Fig. 3 C–E).
Figure 3. Consumption of BCAAs inversely correlates with glucose tolerance and insulin sensitivity.
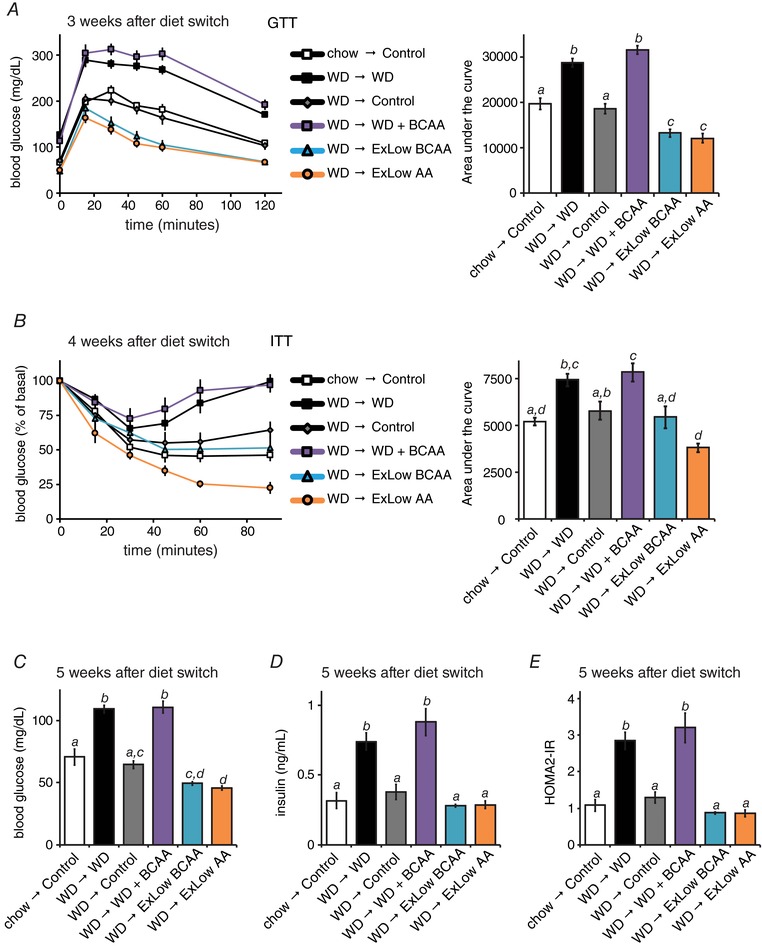
Glucose (A) and (B) insulin tolerance tests were conducted at the specified times after the start of the dietary interventions (n = 10–12 per group; for the area under the curve, means with the same lowercase letter are not significantly different from each other, Tukey–Kramer test following ANOVA, P < 0.05). C–E, mice were fasted overnight and (C) blood glucose and (D) insulin were measured and (E) the HOMA2‐IR was calculated after 5 weeks on the specified diets (n = 5–7 mice per group; means with the same lowercase letter are not significantly different from each other, Tukey–Kramer test following ANOVA, P < 0.05). Error bars represent the SE.
Specifically reducing dietary BCAAs restores metabolic health to DIO mice continuing to consume a WD
While these results supported our hypothesis that reducing dietary BCAAs would restore metabolic health, the simultaneous alterations in energy density and macronutrient ratios made it difficult to elucidate the precise contribution of the BCAAs. To specifically address this question, we designed a new series of diets based on a novel amino acid‐defined WD (WD Control AA) closely matching the macronutrient profile of the naturally sourced diet WD TD.88137. Using this WD Control AA diet as our base, we developed several additional isocaloric WDs with increased or decreased dietary levels of BCAAs (Table 2).
As shown in Fig. 4 A, we induced obesity in 48 C57BL/6J mice by feeding them a WD for 12 weeks; these mice were then randomized into four groups of 12 mice each, and each group was placed on either a WD Control AA, WD High BCAA, WD Low BCAA or WD Low AA diet. In parallel, a group of 12 mice never exposed to a WD were switched to the Control amino acid defined diet. Exact diet formulations are provided in Table 3.
Figure 4. Specifically reducing dietary BCAAs in the context of a WD promotes rapid weight loss and reduced adiposity.
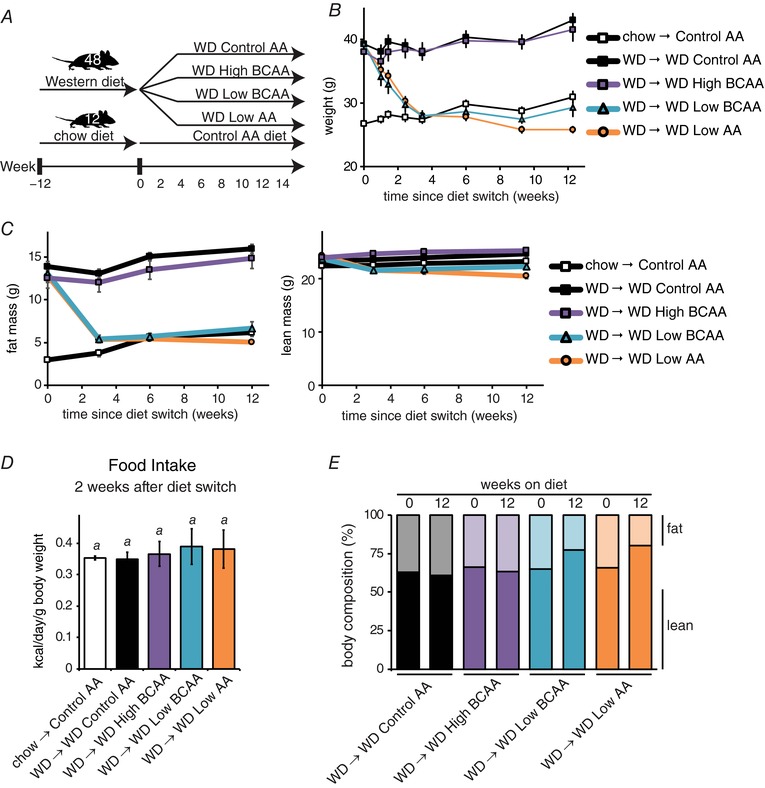
A, schematic representation of experimental plan; mice were preconditioned with a WD for 12 weeks and then randomized to the four experimental groups shown, whereas chow‐fed Control mice were placed on an amino acid defined Control diet. B, weight, as well as (C) adipose and lean mass of mice in each experimental group was tracked (n = 12 mice per group). D, food intake was measured 2 weeks after special diet feeding began (n = 8 cages per group; means with the same lowercase letter are not significantly different from each other, Tukey–Kramer test following ANOVA, P < 0.05). E, body composition at diet intervention start and 12 weeks later. Error bars represent the SE.
DIO mice fed either the WD Control AA or WD High BCAA diets maintained or gained weight, whereas DIO mice switched to the WD Low BCAA or the WD Low AA diets progressively lost weight for 3 weeks (Fig. 4 B). The weight of mice fed a WD Low BCAA diet then stabilized, matching the weight of Control AA‐fed mice never exposed to a WD; mice fed the WD Low AA diet continued to lose weight at a greatly reduced rate. The weight loss of these mice was primarily due to a dramatic decrease in fat mass, whereas lean mass was preserved (Fig. 4 C), and was not associated with decreased food consumption (Fig. 4 D). Overall, the mice fed the WD Low BCAA or WD Low AA diets had improved body composition, with decreased adiposity and a resulting increase in the lean fraction (Fig. 4 E). The dWAT thickness of mice fed the WD Low BCAA or WD Low AA diets was significantly decreased; curiously, mice fed the WD High BCAA diet also had thinner dWAT (Fig. 5 A).
Figure 5. Specifically reducing dietary BCAAs in the context of a WD reduces adipose tissue and reverses diet‐induced hepatic steatosis.
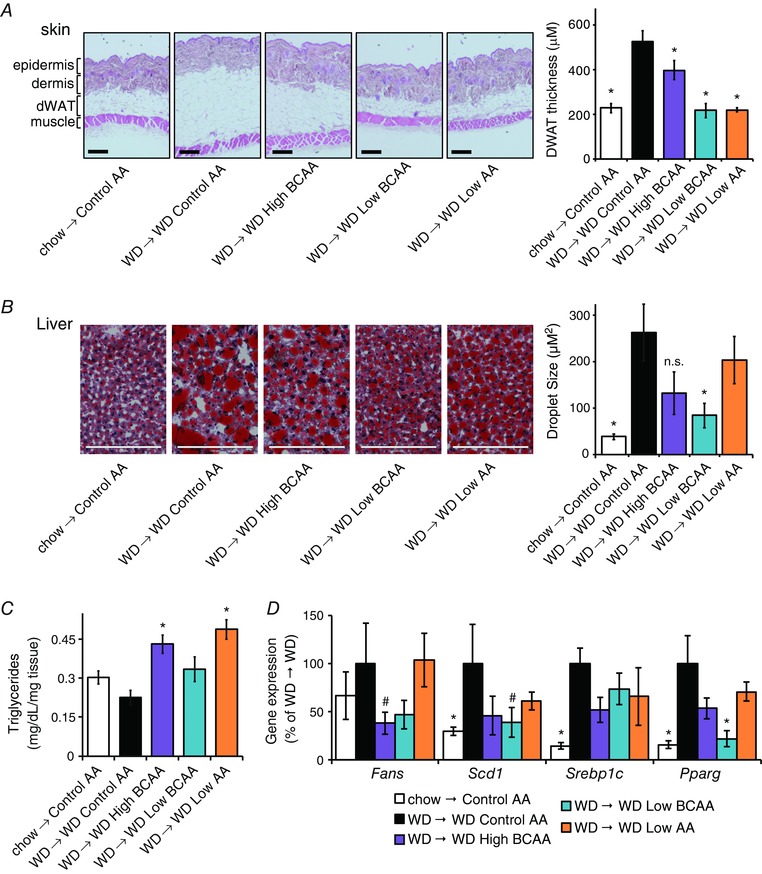
A, skin samples were collected after feeding mice the indicated diets for ∼14 weeks, sectioned, H&E stained and the thickness of dermal WAT was quantified for non‐anagen stage skin samples, measuring from muscle to dermis; scale bar = 200 μm (n = 6 mice per group, * P < 0.05 vs. WD Control AA, Dunnett's test following ANOVA). B, OCT‐embedded liver samples were cryosectioned and stained with Oil‐Red‐O, and droplet size was quantified; scale bar = 200 μm (n = 3 mice per group, * P < 0.05 vs. WD Control AA, Dunnett's test following ANOVA). C, triglyceride levels in liver as mg dL–1 mg–1 of tissue assayed (n = 6 mice per group, * P < 0.05 vs. WD Control AA, Dunnett's test following ANOVA). D, lipogenic gene expression in the livers of mice on the specified diets was measured by quantitative PCR after an overnight fast (n = 8–9 mice per group, * P < 0.05, #P < 0.1 vs. WD Control AA, Dunnett's test following ANOVA). Error bars represent the SE.
Mice fed the WD Low BCAA diet had smaller hepatic lipid droplets than mice fed the WD Control AA diet, and cleared much of the hepatic fat deposited by WD feeding (Fig. 5 B). Intriguingly, there was also qualitative histological improvement and a non‐significant trend towards reduced lipid droplet size in mice consuming extra BCAAs (WD High BCAA). The livers of mice fed the WD Low AA diet did not have smaller lipid droplets and retained large lipid droplets (Fig. 5 B). Mice fed the WD Low AA or WD High BCAA diets had increased hepatic triglyceride levels (Fig. 5 C). We observed numerical decreases in the mRNA expression of two lipogenic genes (Fasn, Scd1) in the livers of mice fed either the WD Low BCAA diet or the WD High BCAA diet, as well as a statistically significant decrease in the transcription factor Pparg in the livers of mice switched to a WD Low BCAA diet (Fig. 5 D).
We examined glycaemic control by conducting glucose and insulin tolerance tests, as well as by determining fasting glucose and insulin levels. Glucose tolerance was improved in mice eating the WD Low BCAA and WD Low AA diets 3 weeks after the diet switch, a time when these mice still weighed more than Control AA‐fed mice never exposed to a WD (Fig. 6 A). Insulin sensitivity was similarly improved in both groups (Fig. 6 B), even as the mice continued to consume a WD. These improvements were maintained over the course of the study (data not shown). Mice on WD Low BCAA and WD Low AA diets had decreased fasting blood glucose and insulin levels (Fig. 6 C and D), whereas increasing dietary BCAAs resulted in fasting hyperglycaemia and hyperinsulinaemia. HOMA2‐IR calculated from these values indicate that insulin sensitivity is inversely correlated with dietary BCAAs (Fig. 6 E).
Figure 6. Reduction of dietary BCAAs improves glycaemic control even in mice continuing to consume a high‐calorie, high‐fat, high‐sugar WD.
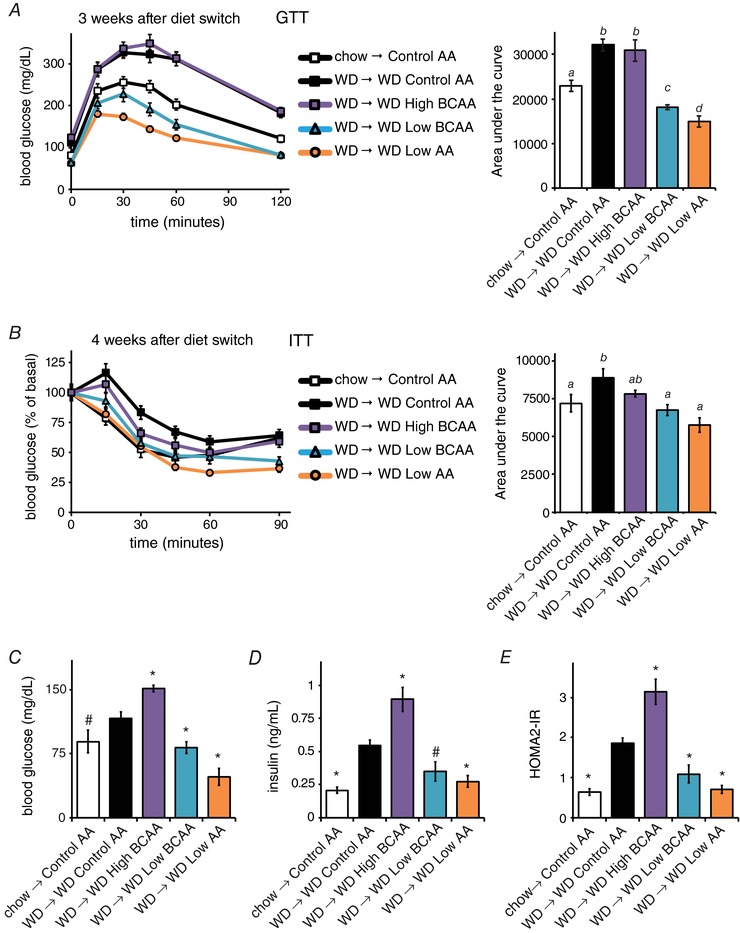
Glucose (A) and (B) insulin tolerance tests were conducted 3 and 4 weeks, respectively, after the start of the dietary interventions (n = 12–16 per group; for the area under the curve, means with the same lowercase letter are not significantly different from each other, Tukey–Kramer test following ANOVA, P < 0.05). C–E, fasting (C) blood glucose and (D) insulin were measured and (E) the HOMA2‐IR was calculated after 5 weeks on the specified diets (n = 3–7 mice per group; * P < 0.05, #P < 0.12 vs. WD Control AA, Dunnett's test following ANOVA).
We examined pancreatic islet function by performing an ex vivo glucose‐stimulated insulin secretion assay (Fig. 7 A). Insulin secretion was decreased in mice fed a WD Low AA diet (Fig. 7 A); however, total islet insulin content was not affected (Fig. 7 A). To precisely examine β cell metabolic stress, we quantified the mitochondrial membrane potential. This was increased in mice eating a WD Control AA diet, and was reduced and essentially normalized in mice consuming WD Low BCAA and WD Low AA diets (Fig. 7 B). In every case except WD High BCAA, β cell function was matched with insulin sensitivity, implying that increased BCAA consumption negatively impacts mitochondrial function and insulin secretion.
Figure 7. Ex vivo analysis of the effect of altering dietary BCAAs on pancreatic beta cells.
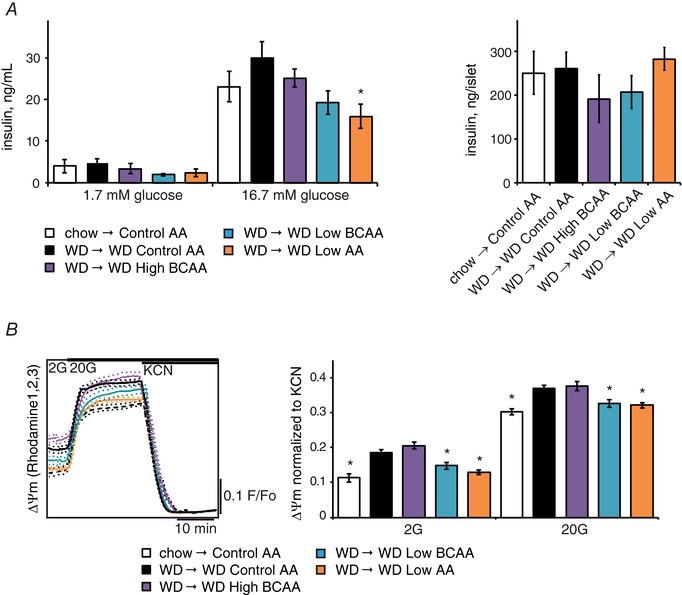
A, an ex vivo insulin secretion assay was performed to assess (left) insulin secretion per islet and (right) islet insulin content in response to low (1.7 mm) and high (16.7 mm) glucose in mice kept on the indicated diets for ∼14 weeks (n = 6 mice per group, * P < 0.05 vs. WD Control AA, Dunnett's test following ANOVA). B, the mitochondrial membrane potential was measured in ex vivo isolated pancreatic islets stimulated with low (2 mm) and high (20 mm) glucose levels (n = 44–74 islets per group; * P < 0.05 vs. WD Control AA, Dunnett's test following ANOVA). Error bars represent the SE.
Chronic consumption of a reduced BCAA WD increases energy expenditure independently of FGF21
To understand how reducing dietary BCAAs promotes leanness without reducing calorie intake, we utilized metabolic chambers to examine food consumption, respiration, activity, and energy expenditure after mice had been on the diets for ∼12 weeks. Mice fed diets with reduced levels of BCAAs or reduced levels of all AAs consumed around twice as many calories than mice fed a WD Control AA diet (data not shown); the increase is even greater when calculated relative to body weight (Fig. 8 A). As expected, the RER was decreased by WD feeding and increased in mice consuming the high carbohydrate WD Low AA diet (Fig. 8 B). Intriguingly, the RER of mice fed the WD Low BCAA diet, containing the same level of carbohydrates as the WD Control AA diet, was also increased, and was indistinguishable from the RER of mice consuming a WD Low AA diet.
Figure 8. Metabolic impact of sustained consumption of diets with altered dietary BCAAs.
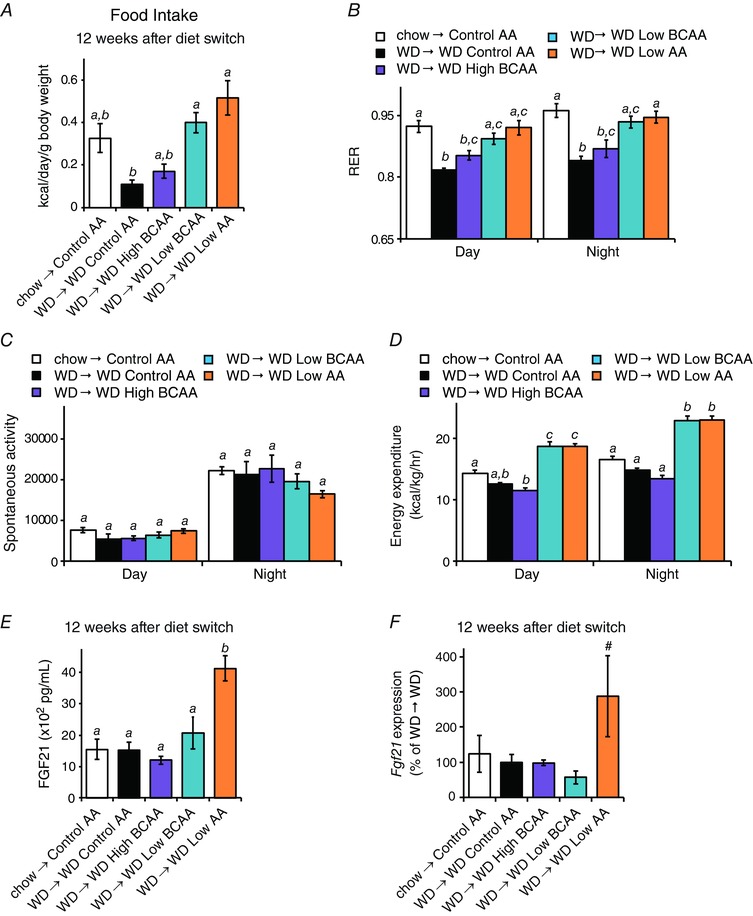
A, food intake over a 24 h period (n = 2–5 mice per group; means with the same lowercase letter are not significantly different from each other, Bonferroni test, P < 0.05). B, RER, (C) spontaneous activity and (D) energy expenditure were measured ∼12 weeks after the start of the dietary intervention (n = 4–5 mice per group; means with the same lowercase letter are not significantly different from each other, Tukey–Kramer test following ANOVA, P < 0.05). E, FGF21 was measured in the plasma of mice following an overnight fast (n = 4 mice per group; means with the same lowercase letter are not significantly different from each other, Tukey–Kramer test following ANOVA, P < 0.05). F, Fgf21 gene expression in the liver of mice following an overnight fast was assessed by quantitative PCR (n = 8–9 mice per group; #P < 0.1 vs. WD Control AA, Dunnett's test following ANOVA). Error bars represent the SE.
There was no difference in spontaneous activity between any of the groups (Fig. 8 C). Mice consuming the WD Low BCAA and WD Low AA diets had greater energy expenditure during both daytime and night‐time (Fig. 8 D). Mice consuming the WD Low AA diet had high levels of the energy balance regulating hormone FGF21 (Fig. 8 E); however, there was no increase in FGF21 in the blood of mice consuming the WD Low BCAA diet and no increase in liver Fgf21 gene expression (Fig. 8 E and F).
Reduction in dietary BCAAs in WD‐fed mice is accompanied by a transient increase in FGF21 levels
From the perspective of weight and body composition, the 3 weeks following the diet switch are distinctly different from the time period during which we analysed energy expenditure above. In particular, rapid weight normalization occurs during the first 3 weeks, whereas weights are relatively stable thereafter. We therefore utilized an additional cohort of mice to intensively analyse weight, body composition, activity, and energy expenditure during the 12 days immediately following the diet switch, during which mice fed the WD Low BCAA and WD Low AA diets progressively lost weight and fat mass (Fig. 9 A and B).
Figure 9. A Western reduced BCAA diet transiently induces FGF21 and increases energy expenditure.
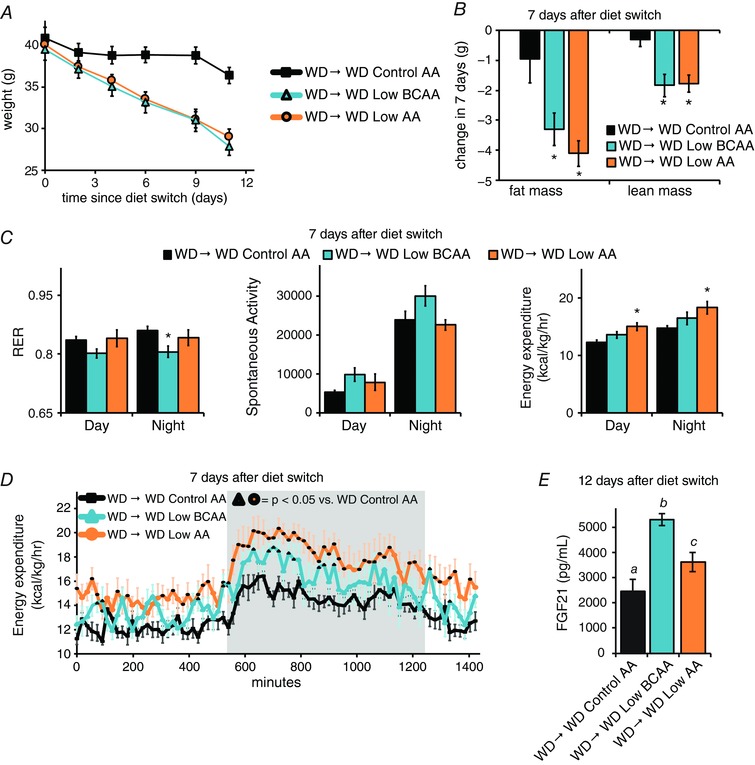
A, weight of DIO mice switched to the indicated diet at time 0 (n = 8 per group). B, change in fat and lean mass of mice placed on each diet for 7 days (n = 8 mice per group, * P < 0.05 vs. WD Control AA, Dunnett's test following ANOVA). C, RER, spontaneous activity and energy expenditure were measured 1 week after the diet switch (n = 6–8 mice per group, * P < 0.05 vs. WD Control AA, Dunnett's test following ANOVA). D, energy expenditure over the course of a 24 h cycle starting at ∼10.00 h (n = 8 mice per group; dark outline of symbol indicates P < 0.05 vs. WD Control AA (Dunnett's test following two‐way repeated measures ANOVA). E, FGF21 was measured in the plasma of mice following an overnight fast (n = 6 mice per group; means with the same lowercase letter are not significantly different from each other, Tukey–Kramer test following ANOVA, P < 0.05). Error bars represent the SE.
In contrast to the increased RER seen at later time points, neither WD Low BCAA or WD Low AA diet fed mice had increased RER 1 week after the diet switch; indeed, WD Low BCAA diet fed mice had a lower RER (Fig. 9 C). Although there were no significant changes in spontaneous activity between groups, we observed a statistically significant increase in energy expenditure during the daytime and night‐time in WD Low AA diet fed mice but, surprisingly, not in WD Low BCAA fed mice (Fig. 9 C). As weight loss in the absence of a change in energy expenditure or activity was puzzling, we examined energy expenditure more closely over a 24 h period. We determined that mice fed the WD Low BCAA diet have a significant increase in energy expenditure for at least 20% of a 24 h period, with the most pronounced difference at night (Fig. 9 D). Following completion of this analysis, we determined that FGF21 levels were significantly increased in the blood of both WD Low BCAA and WD Low AA diet fed mice (Fig. 9 E).
FGF21 promotes browning of WAT and increased activation of BAT (Fisher et al. 2012; Owen et al. 2014; Douris et al. 2015; Hill et al. 2017; Wanders et al. 2017). We observed decreased adipocyte size in inguinal and gonadal WAT, consistent with the observed loss of adipose mass; however, we did not observe a morphology consistent with increased beiging (Fig. 10 A) and the expression of UCP1 was not increased in either gonadal or inguinal WAT of WD Low BCAA or WD Low AA diet fed mice (Fig. 10 B and C). We also did not observe increased expression of Ucp1 in the BAT of mice fed WD Low BCAA or WD Low AA diets (Fig. 10 D); however, we did observe a significant decrease in lipid droplet size (Fig. 10 E) and increased expression of Bmp8 (Fig. 10 F), which are changes consistent with FGF21‐mediated activation of BAT (Whittle et al. 2012; Bendayan & Cammisotto, 2016; Quesada‐Lopez et al. 2016; Wanders et al. 2017).
Figure 10. A Western reduced BCAA diet does not beige WAT but activates BAT.
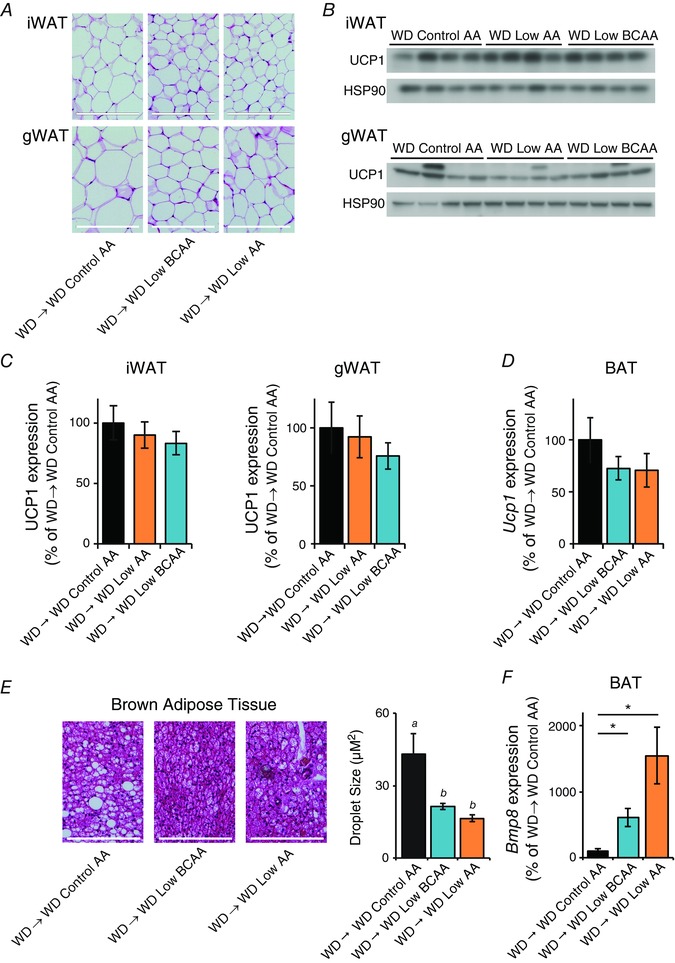
Following an overnight fast, tissues were collected from DIO mice switched to the indicated diets for 12 days. A, inguinal and gonadal WAT (iWAT and gWAT, respectively) depots were collected, sectioned, and H&E stained; scale bar = 200 μm. B, the expression of UCP1 in iWAT and gWAT was determined by Western blotting. C, UCP1 expression in iWAT and gWAT was quantified relative to HSP90 (n = 8 mice per group, * P < 0.05 vs. WD Control AA, Dunnett's test following ANOVA). D, Ucp1 gene expression in BAT was assessed by quantitative PCR (n = 6 mice per group, * P < 0.05 vs. WD Control AA, Dunnett's test following ANOVA). E, BAT was collected, sectioned, and H&E stained, and lipid droplet size was quantified; scale bar = 200 μm (n = 6 mice per group; means with the same lowercase letter are not significantly different from each other, Tukey–Kramer test following ANOVA, P < 0.05). F, Bmp8 gene expression in BAT was assessed by quantitative PCR (n = 6 mice per group; * P < 0.05 vs. WD Control AA, Dunnett's test following ANOVA). Error bars represent the SE.
Discussion
Building on recent studies conducted by our laboratory and others showing that reducing dietary BCAAs can promote or preserve metabolic health in young mice and rats (Xiao et al. 2014; Fontana et al. 2016; White et al. 2016), we tested the hypothesis that reducing dietary BCAAs would be a uniquely potent way to intervene in metabolic syndrome. In the present study, we find that specifically reducing dietary BCAAs, without altering energy density, the caloric contribution of amino acids to the diet, or the protein:carbohydrate ratio (Solon‐Biet et al. 2014, 2015; Simpson et al. 2017), is sufficient to robustly restore metabolic health.
These studies represent the first examination of a reduced BCAA diet in a mouse model of pre‐existing diet‐induced obesity and type 2 diabetes, which are not hyperphagic and have a gradual and reversible onset of metabolic disease (Williams et al. 2014). Specifically reducing BCAAs rapidly normalizes the weight of DIO mice without calorie restriction, even in mice continuing to consume a WD, promoting fat mass loss as well as rapid and dramatic improvements in glucose tolerance and insulin sensitivity. While there are likely multiple mechanisms underlying the metabolic benefits of a reduced BCAA diet, the improvements in glucose homeostasis we observe here are likely due in part to weight loss and decreased adiposity resulting from increased energy expenditure.
As summarized in Fig. 11, reducing either all dietary AAs or specifically reducing the BCAAs improves the metabolic health of DIO mice; however, the specific effects of the diets on energy balance differ. During the acute phase of rapid weight loss, mice eating a WD Low AA diet have increased food intake and a substantial increase in energy expenditure, whereas mice eating a WD Low BCAA diet have no change in food intake and a more modest increase in energy expenditure. In contrast, during the chronic phase of relatively stable weight, energy expenditure and caloric intake are identically increased in mice consuming either the WD Low AA diet or WD Low BCAA diet.
Figure 11. Specifically reducing dietary BCAAs or all AAs restores metabolic health to DIO mice.
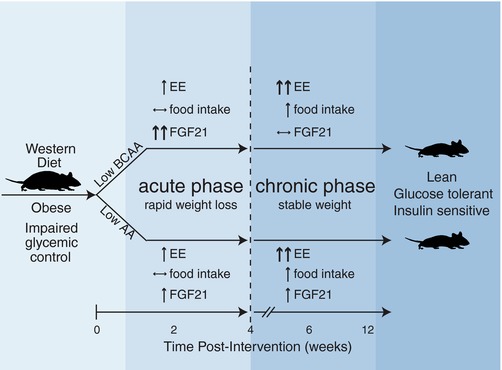
Altering dietary levels of either the BCAAs or all AAs promotes leanness and restores blood glucose control to DIO mice, but these two dietary interventions have distinct effects on energy expenditure (EE), food intake and blood levels of FGF21. Further, these metabolic effects vary between an acute phase (an ∼4 week long period following the diet switch characterized by rapid weight loss), and a chronic phase that persists once mice have reached a stable weight.
A more dramatic difference is observed between mice fed these two diets with regard to the hormone FGF21, which has pleiotropic effects on glucose metabolism and energy expenditure (Berglund et al. 2009; Fisher et al. 2012; Emanuelli et al. 2014; Laeger et al. 2014, 2016; Markan et al. 2014; Owen et al. 2014; Stone et al. 2014). FGF21 is induced in both humans and rodents in response to low protein diets, and is proposed to mediate many of the beneficial metabolic effects of these diets (Laeger et al. 2014, 2016; Fontana et al. 2016; Maida et al. 2016). Although our previous work, conducted in the context of a normal calorie diet, revealed no effect of BCAA reduction on FGF21, in the present study we find that specifically reducing dietary BCAAs in the context of a WD transiently induces FGF21.
This is intriguing, particularly in light of a recent study which suggests that the increase in FGF21 in mice fed a low protein diet is mediated by the protein:carbohydrate ratio (Solon‐Biet et al. 2016). Although other groups have previously determined that restricting or eliminating specific essential dietary amino acids, including methionine or leucine (De Sousa‐Coelho et al. 2012; Lees et al. 2014,2017; Wanders et al. 2015, 2017), is sufficient to induce FGF21, these studies have utilized diets in which either methionine or leucine levels are restricted by 80% or more. Our finding that FGF21 is responsive to a more physiologically relevant 67% reduction in dietary levels of BCAAs, at least in the context of a WD, suggests that the precise amino acid composition of the dietary protein may also play a role. Further research will be required to define the mechanism by which FGF21 expression is regulated by dietary BCAAs and, indeed, by other AAs such as asparagine (Wilson et al. 2015).
FGF21 has been shown to play a key role in the response to cold exposure and dietary interventions such as calorie restriction and methionine restriction, promoting the beiging of WAT (Fisher et al. 2012; Shabalina et al. 2013; Fabbiano et al. 2016; Wanders et al. 2017). Dietary protein restriction also promotes the beiging of WAT, and increases energy expenditure via a FGF21 and UCP1‐dependent mechanism (Laeger et al. 2016; Hill et al. 2017). We therefore hypothesized that the increase in FGF21 following the reduction of dietary BCAAs or all AAs promoted energy expenditure and weight loss through the beiging of WAT. However, while mice fed either the WD Low AA or WD Low BCAA diet for ∼2 weeks had increased levels of FGF21 and increased energy expenditure, we observed no changes in WAT morphology consistent with beiging, and no increase in the expression of UCP1 in either inguinal or gonadal WAT. Ucp1 was also not increased in BAT, despite the presence of other changes suggestive of BAT activation.
These results demonstrate that BCAA and AA reduction in the context of a WD does not promote energy expenditure solely by engaging the FGF21‐UCP1 axis and promoting WAT beiging. However, as our analysis of WAT and BAT is limited to an early time point, we cannot rule out a role for beiged WAT in the greatly increased energy expenditure we observed in mice consuming a WD Low AA or WD Low BCAA diet for longer periods of time. Determining how UCP1 expression changes in mice fed these diets for longer periods of time will be a key point for further study. FGF21 may also still play a role in the response to reduced dietary BCAAs, as recent research suggests that some of the effects of FGF21 are mechanistically independent of UCP1 (Samms et al. 2015). There are also as yet undefined thermogenic mechanisms that act independently of FGF21 and UCP1 (Hill et al. 2017; Keipert et al. 2017), which could conceivably also play a role in the response to reduced dietary BCAAs. Understanding the molecular mechanisms leading to increased energy expenditure and weight loss, including the role of FGF21 and UCP1, will be key points for further study.
The improvements in the regulation of blood glucose we observe in mice consuming diets with reduced levels of BCAAs are not exclusively the result of weight loss. Notably, while both glucose and insulin tolerance are improved in obese mice switched to diets with reduced levels of the BCAAs, obese mice switched to the WD Low BCAA and WD Low AA diets have better glucose tolerance than Control diet‐fed mice never exposed to a WD. Based on our previous work (Fontana et al. 2016), the decrease in fasting blood glucose we observe in mice switched to BCAA‐reduced diets, and the minimal effects of BCAA‐reduced diets on islet metabolism, we hypothesize that the improvement in glucose tolerance is most likely due to improved hepatic insulin sensitivity. This effect could be due to alterations in lipid metabolism; increased levels of FGF21, which promotes hepatic insulin sensitivity (Berglund et al. 2009); decreased BCAA catabolism, as increased hepatic BCAA catabolism is associated with glucose intolerance in mice (She et al. 2007; Ananieva et al. 2017); or a combination of these effects. Identifying the physiological and molecular basis for the improvements in blood glucose control we observed here may suggest novel approaches for the treatment of prediabetes and type 2 diabetes.
Intriguingly, increased consumption of BCAAs, at least under the conditions examined here, has minimal impact on weight and body composition. In agreement with previous studies demonstrating that supplementing either a LP diet (Maida et al. 2017) or a WD (Newgard et al. 2009) with BCAAs impairs glucose homeostasis in wild‐type animals, there is an observable negative impact of BCAA supplementation on blood glucose control. Interestingly, while there is both preclinical and clinical data suggesting that BCAA supplementation may be therapeutic for hepatic steatosis (Garcia‐Caraballo et al. 2013; Barb et al. 2016; Honda et al. 2017), and we observed decreased expression of several lipogenic genes in mice consuming additional BCAAs, we observed no statistically significant effect of BCAA supplementation on hepatic lipid droplet size. Indeed, we find that BCAA supplementation actually increases liver triglyceride levels. Reconciling our results with data from other groups suggesting a beneficial effect of BCAA supplementation on hepatic steatosis will require additional study.
In contrast to these results, we observed that specifically reducing dietary BCAAs suppresses the expression of numerous lipogenic genes, and also decreases hepatic lipid droplet size. Although the relationship between lipid droplet size and liver health is not clearly understood, smaller lipid droplets may be more metabolically available and better‐stabilized by lipid binding proteins (Suzuki et al. 2011). Notably, reducing all dietary amino acids, as in a low protein diet, did not reduce lipid droplet size and increased hepatic triglyceride levels. These results suggest that specifically reducing dietary BCAAs may have uniquely beneficial effects on hepatic lipid metabolism not achievable from the consumption of a low protein diet.
Collectively, these results demonstrate that reducing dietary levels of all AAs, or specifically reducing dietary levels of the three BCAAs, leucine, isoleucine, and valine, can rapidly reverse the obesity and metabolic dysfunction resulting from consumption of a high‐fat, high‐sugar diet without requiring calorie restriction. While many of these metabolic benefits likely result from increased energy expenditure and a resulting decrease in weight, changes in hormones or BCAA catabolism probably also contribute to the improved regulation of blood glucose that we observe in mice fed diets with reduced levels of BCAAs. While the direct applicability of our results to humans remains to be determined, the correlation of blood levels of BCAAs with obesity and insulin resistance in humans is well established (Newgard et al. 2009; Lynch & Adams, 2014). If dietary BCAAs have similar effects on energy balance and metabolism in humans, it is implicit in our findings that protein quality (i.e. the precise amino acid composition of dietary protein) may have a significant impact on the efficacy of weight loss diets. Finally, selective reduction of dietary BCAAs through the use of customized diet plans or BCAA‐free medical food, or pharmaceuticals that mimic this effect (e.g. by altering BCAA absorption or catabolism), may represent a translatable and sustainable approach to promote metabolic health and treat diabetes and obesity without reducing caloric intake.
Additional information
Competing interests
The University of Wisconsin‐Madison has applied for a patent based in part on the findings reported here, for which NEC and DWL are inventors.
Author contributions
Experiments were performed in the Lamming laboratory, except for the analysis of dWAT, which was performed in the Alexander laboratory, and the analysis of pancreatic islets, which was performed in the Kimple and Merrins laboratories. NEC, MEK, CMA, MJM and DWL conceived the experiments and secured funding. NEC, IK, EMW, ENK, MDS, BAS, CP, DY, SIAA, GG, DSS, SEC, MEB, JAW, RJF and DWL performed the experiments. NEC, IK, EMW, MDS, BAS, CP, KAM, MEK, CMA, MJM and DWL analysed the data. NEC and DWL wrote the manuscript.
Funding
The Lamming laboratory is supported by the NIH NIA (R00 AG041765, R21 AG051974 and R56 AG056771) and a New Investigator Program Award from the Wisconsin Partnership Program. This research was conducted when DWL was an AFAR Research Grant recipient from the American Federation for Aging Research, and was also supported by a Glenn Foundation Award for Research in the Biological Mechanisms of Aging and an Early Career Development Award from the Central Society for Clinical and Translational Research (CSCTR) to DWL. The Merrins and Lamming laboratories are jointly supported by a grant from the NIH NIA (R21 AG050135), and both laboratories are supported by startup funds from the University of Wisconsin‐Madison School of Medicine and Public Health and Department of Medicine. The Merrins laboratory is also supported by the ADA (1‐16‐IBS‐212), the NIH/NIDDK (K01 DK101683 and R01 DK113103), an Early Career Development Award from CSCTR, and the Wisconsin Partnership Program. This work was also supported by grants from the ADA (1‐14‐BS‐115) and the NIH/NIDDK (R01 DK102598) to MEK. The Alexander laboratory is supported by NIH/NIGMS (RO1 GM113142). NEC is supported by a training grant from the UW Institute on Aging (NIA T32 AG000213). IK is supported in part by McArdle Departmental Funds. EMW was supported by the Shapiro Summer Research Program and the UW‐Madison Department of Medicine. SIAA was supported in part by a fellowship from the ADA (1‐16‐PMF‐001). SEC was supported by the Rural and Urban Scholars in Community Health Program. The UWCCC Experimental Pathology Laboratory is supported by the University of Wisconsin Carbone Cancer Centre Support Grant P30 CA014520. This work was supported using facilities and resources from the William S. Middleton Memorial Veterans Hospital. This work does not represent the views of the Department of Veterans Affairs or the United States Government.
Acknowledgements
We thank all the members of the Lamming, Kimple, Merrins and Alexander laboratories, as well as Dr Amelia Linnemann, Dr Dawn B. Davis and the Davis laboratory for their assistance and insight. We thank Dr Tina Herfel (Envigo) for assistance with the formulation of the amino acid defined diets.
Linked articles This article is highlighted by a Perspective by Hill & Morrison. To read this Perspective, visit https://doi.org/10.1113/JP275613.
Edited by: Kim Barrett & Bettina Mittendorfer
References
- Ananieva EA, Van Horn CG, Jones MR & Hutson SM (2017). Liver BCATm transgenic mouse model reveals the important role of the liver in maintaining BCAA homeostasis. J Nutr Biochem 40, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriola Apelo SI, Neuman JC, Baar EL, Syed FA, Cummings NE, Brar HK, Pumper CP, Kimple ME & Lamming DW (2016). Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell 15, 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar EL, Carbajal KA, Ong IM & Lamming DW (2016). Sex‐ and tissue‐specific changes in mTOR signaling with age in C57BL/6J mice. Aging Cell 15, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barb D, Portillo‐Sanchez P & Cusi K (2016). Pharmacological management of nonalcoholic fatty liver disease. Metabolism 65, 1183–1195. [DOI] [PubMed] [Google Scholar]
- Batch BC, Shah SH, Newgard CB, Turer CB, Haynes C, Bain JR, Muehlbauer M, Patel MJ, Stevens RD, Appel LJ, Newby LK & Svetkey LP (2013). Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism 62, 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendayan M & Cammisotto P (2016). Activation of brown adipose tissue by oral administration of leptin. Austin J Endocrinol Diabetes 3, 1051. [Google Scholar]
- Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB, Kharitonenkov A & Wasserman DH (2009). Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology 150, 4084–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly MA, Wolak‐Dinsmore J & Dullaart RPF (2017). Branched chain amino acids are associated with insulin resistance independent of leptin and adiponectin in subjects with varying degrees of glucose tolerance. Metab Syndr Relat Disord 15, 183–186. [DOI] [PubMed] [Google Scholar]
- De Sousa‐Coelho AL, Marrero PF & Haro D (2012). Activating transcription factor 4‐dependent induction of FGF21 during amino acid deprivation. Biochem J 443, 165–171. [DOI] [PubMed] [Google Scholar]
- Douris N, Stevanovic DM, Fisher FM, Cisu TI, Chee MJ, Nguyen NL, Zarebidaki E, Adams AC, Kharitonenkov A, Flier JS, Bartness TJ & Maratos‐Flier E (2015). Central fibroblast growth factor 21 browns white fat via sympathetic action in male mice. Endocrinology 156, 2470–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelli B, Vienberg SG, Smyth G, Cheng C, Stanford KI, Arumugam M, Michael MD, Adams AC, Kharitonenkov A & Kahn CR (2014). Interplay between FGF21 and insulin action in the liver regulates metabolism. J Clin Invest 124, 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbiano S, Suarez‐Zamorano N, Rigo D, Veyrat‐Durebex C, Stevanovic Dokic A, Colin DJ & Trajkovski M (2016). Caloric restriction leads to browning of white adipose tissue through type 2 immune signaling. Cell Metab 24, 434–446. [DOI] [PubMed] [Google Scholar]
- Felig P, Marliss E & Cahill GF Jr (1969). Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 281, 811–816. [DOI] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos‐Flier E & Spiegelman BM (2012). FGF21 regulates PGC‐1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK & Ogden CL (2012). Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307, 491–497. [DOI] [PubMed] [Google Scholar]
- Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, Cava E, Spelta F, Tosti V, Syed FA, Baar EL, Veronese N, Cottrell SE, Fenske RJ, Bertozzi B, Brar HK, Pietka T, Bullock AD, Figenshau RS, Andriole GL, Merrins MJ, Alexander CM, Kimple ME & Lamming DW (2016). Decreased consumption of branched‐chain amino acids improves metabolic health. Cell Rep 16, 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L & Partridge L (2015). Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Caraballo SC, Comhair TM, Verheyen F, Gaemers I, Schaap FG, Houten SM, Hakvoort TB, Dejong CH, Lamers WH & Koehler SE (2013). Prevention and reversal of hepatic steatosis with a high‐protein diet in mice. Biochim Biophys Acta 1832, 685–695. [DOI] [PubMed] [Google Scholar]
- Gregg T, Poudel C, Schmidt BA, Dhillon RS, Sdao SM, Truchan NA, Baar EL, Fernandez LA, Denu JM, Eliceiri KW, Rogers JD, Kimple ME, Lamming DW & Merrins MJ (2016). Pancreatic beta‐cells from mice offset age‐associated mitochondrial deficiency with reduced KATP channel activity. Diabetes 65, 2700–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara A, Cornu M, Cybulski N, Polak P, Betz C, Trapani F, Terracciano L, Heim MH, Ruegg MA & Hall MN (2012). Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab 15, 725–738. [DOI] [PubMed] [Google Scholar]
- Hill CM, Laeger T, Albarado DC, McDougal DH, Berthoud HR, Munzberg H & Morrison CD (2017). Low protein‐induced increases in FGF21 drive UCP1‐dependent metabolic but not thermoregulatory endpoints. Sci Rep 7, 8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T, Ishigami M, Luo F, Lingyun M, Ishizu Y, Kuzuya T, Hayashi K, Nakano I, Ishikawa T, Feng GG, Katano Y, Kohama T, Kitaura Y, Shimomura Y, Goto H & Hirooka Y (2017). Branched‐chain amino acids alleviate hepatic steatosis and liver injury in choline‐deficient high‐fat diet induced NASH mice. Metabolism 69, 177–187. [DOI] [PubMed] [Google Scholar]
- Janda M, Zeidler D, Bohm G & Schoberberger R (2013). An instrument to measure adherence to weight loss programs: the compliance praxis survey‐diet (COMPASS‐Diet). Nutrients 5, 3828–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza I, Suh Y, Wollny D, Clark RJ, Roopra A, Colman RJ, MacDougald OA, Shedd TA, Nelson DW, Yen MI, Yen CL & Alexander CM (2014). Syndecan‐1 is required to maintain intradermal fat and prevent cold stress. PLoS Genet 10, e1004514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keipert S, Kutschke M, Ost M, Schwarzmayr T, van Schothorst EM, Lamp D, Brachthauser L, Hamp I, Mazibuko SE, Hartwig S, Lehr S, Graf E, Plettenburg O, Neff F, Tschop MH & Jastroch M (2017). Long‐term cold adaptation does not require FGF21 or UCP1. Cell Metab 26, 437–446 e435. [DOI] [PubMed] [Google Scholar]
- Laeger T, Albarado DC, Burke SJ, Trosclair L, Hedgepeth JW, Berthoud HR, Gettys TW, Collier JJ, Munzberg H & Morrison CD (2016). Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Rep 16, 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MW & Morrison CD (2014). FGF21 is an endocrine signal of protein restriction. J Clin Invest 124, 3913–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagiou P, Sandin S, Weiderpass E, Lagiou A, Mucci L, Trichopoulos D & Adami HO (2007). Low carbohydrate‐high protein diet and mortality in a cohort of Swedish women. J Intern Med 261, 366–374. [DOI] [PubMed] [Google Scholar]
- Lees EK, Banks R, Cook C, Hill S, Morrice N, Grant L, Mody N & Delibegovic M (2017). Direct comparison of methionine restriction with leucine restriction on the metabolic health of C57BL/6J mice. Sci Rep 7, 9977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees EK, Krol E, Grant L, Shearer K, Wyse C, Moncur E, Bykowska AS, Mody N, Gettys TW & Delibegovic M (2014). Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell 13, 817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara‐Aguirre J, Wan J, Passarino G, Kennedy BK, Wei M, Cohen P, Crimmins EM & Longo VD (2014). Low protein intake is associated with a major reduction in IGF‐1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 19, 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JC, Matthews DR & Hermans MP (1998). Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 21, 2191–2192. [DOI] [PubMed] [Google Scholar]
- Linnemann AK, Neuman JC, Battiola TJ, Wisinski JA, Kimple ME & Davis DB (2015). Glucagon‐like peptide‐1 regulates cholecystokinin production in beta‐cells to protect from apoptosis. Mol Endocrinol 29, 978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CJ & Adams SH (2014). Branched‐chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 10, 723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maida A, Chan JSK, Sjoberg KA, Zota A, Schmoll D, Kiens B, Herzig S & Rose AJ (2017). Repletion of branched chain amino acids reverses mTORC1 signaling but not improved metabolism during dietary protein dilution. Mol Metab 6, 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maida A, Zota A, Sjoberg KA, Schumacher J, Sijmonsma TP, Pfenninger A, Christensen MM, Gantert T, Fuhrmeister J, Rothermel U, Schmoll D, Heikenwalder M, Iovanna JL, Stemmer K, Kiens B, Herzig S & Rose AJ (2016). A liver stress‐endocrine nexus promotes metabolic integrity during dietary protein dilution. J Clin Invest 126, 3263–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M & Potthoff MJ (2014). Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 63, 4057–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather K ( 2009). Surrogate measures of insulin resistance: of rats, mice, and men. Am J Physiol Endocrinol Metab 296, E398–399. [DOI] [PubMed] [Google Scholar]
- Neuman JC, Truchan NA, Joseph JW & Kimple ME (2014). A method for mouse pancreatic islet isolation and intracellular cAMP determination. JoVE, e50374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry EP, Xie Y, Kennedy SM, Luo J & Davidson NO (2006). Protection against Western diet‐induced obesity and hepatic steatosis in liver fatty acid‐binding protein knockout mice. Hepatology 44, 1191–1205. [DOI] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD & Svetkey LP (2009). A branched‐chain amino acid‐related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9, 311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA & Mangelsdorf DJ (2014). FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab 20, 670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN & Sabatini DM (2011). mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146, 408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada‐Lopez T, Cereijo R, Turatsinze JV, Planavila A, Cairo M, Gavalda‐Navarro A, Peyrou M, Moure R, Iglesias R, Giralt M, Eizirik DL & Villarroya F (2016). The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat Comm 7, 13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samms RJ, Smith DP, Cheng CC, Antonellis PP, Perfield JW 2nd, Kharitonenkov A, Gimeno RE & Adams AC (2015). Discrete aspects of FGF21 in vivo pharmacology do not require UCP1. Cell Rep 11, 991–999. [DOI] [PubMed] [Google Scholar]
- Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B & Nedergaard J (2013). UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep 5, 1196–1203. [DOI] [PubMed] [Google Scholar]
- Shah SH, Crosslin DR, Haynes CS, Nelson S, Turer CB, Stevens RD, Muehlbauer MJ, Wenner BR, Bain JR, Laferrere B, Gorroochurn P, Teixeira J, Brantley PJ, Stevens VJ, Hollis JF, Appel LJ, Lien LF, Batch B, Newgard CB & Svetkey LP (2012). Branched‐chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 55, 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ & Hutson SM (2007). Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab 6, 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SJ, Le Couteur DG, Raubenheimer D, Solon‐Biet SM, Cooney GJ, Cogger VC & Fontana L (2017). Dietary protein, aging and nutritional geometry. Ageing Res Rev 39, 78–86. [DOI] [PubMed] [Google Scholar]
- Sluijs I, Beulens JW, van der AD, Spijkerman AM, Grobbee DE & van der Schouw YT (2010). Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)‐NL study. Diabetes Care 33, 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon‐Biet SM, Cogger VC, Pulpitel T, Heblinski M, Wahl D, McMahon AC, Warren A, Durrant‐Whyte J, Walters KA, Krycer JR, Ponton F, Gokarn R, Wali JA, Ruohonen K, Conigrave AD, James DE, Raubenheimer D, Morrison CD, Le Couteur DG & Simpson SJ (2016). Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metab 24, 555–565. [DOI] [PubMed] [Google Scholar]
- Solon‐Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG & Simpson SJ (2014). The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum‐fed mice. Cell Metab 19, 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon‐Biet SM, Mitchell SJ, Coogan SC, Cogger VC, Gokarn R, McMahon AC, Raubenheimer D, de Cabo R, Simpson SJ & Le Couteur DG (2015). Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Cell Rep 11, 1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone KP, Wanders D, Orgeron M, Cortez CC & Gettys TW (2014). Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes 63, 3721–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Shinohara Y, Ohsaki Y & Fujimoto T (2011). Lipid droplets: size matters. J Electron Microsc (Tokyo) 60(Suppl 1), S101–116. [DOI] [PubMed] [Google Scholar]
- Truchan NA, Brar HK, Gallagher SJ, Neuman JC & Kimple ME (2015). A single‐islet microplate assay to measure mouse and human islet insulin secretion. Islets 7, e1076607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders D, Forney LA, Stone KP, Burk DH, Pierse A & Gettys TW (2017). FGF21 mediates the thermogenic and insulin‐sensitizing effects of dietary methionine restriction but not its effects on hepatic lipid metabolism. Diabetes 66, 858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders D, Stone KP, Dille K, Simon J, Pierse A & Gettys TW (2015). Metabolic responses to dietary leucine restriction involve remodeling of adipose tissue and enhanced hepatic insulin signaling. Biofactors 41, 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, Ilkayeva O, George T, Muehlbauer MJ, Bain JR, Trimmer JK, Brosnan MJ, Rolph TP & Newgard CB (2016). Branched‐chain amino acid restriction in Zucker‐fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl‐glycine export. Mol Metab 5, 538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vazquez MJ, Morgan D, Csikasz RI, Gallego R, Rodriguez‐Cuenca S, Dale M, Virtue S, Villarroya F, Cannon B, Rahmouni K, Lopez M & Vidal‐Puig A (2012). BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149, 871–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Campbell FM, Drew JE, Koch C, Hoggard N, Rees WD, Kamolrat T, Thi Ngo H, Steffensen IL, Gray SR & Tups A (2014). The development of diet‐induced obesity and glucose intolerance in C57BL/6 mice on a high‐fat diet consists of distinct phases. PLoS ONE 9, e106159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GJ, Lennox BA, She P, Mirek ET, Al Baghdadi RJ, Fusakio ME, Dixon JL, Henderson GC, Wek RC & Anthony TG (2015). GCN2 is required to increase fibroblast growth factor 21 and maintain hepatic triglyceride homeostasis during asparaginase treatment. Am J Physiol Endocrinol Metab 308, E283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzell MS & Ahren B (2004). The high‐fat diet‐fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53(Suppl 3), S215–219. [DOI] [PubMed] [Google Scholar]
- Xiao F, Yu J, Guo Y, Deng J, Li K, Du Y, Chen S, Zhu J, Sheng H & Guo F (2014). Effects of individual branched‐chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metabolism 63, 841–850. [DOI] [PubMed] [Google Scholar]


