Abstract
Key points
Disuse in older adults can critically decrease lower limb muscle power, leading to compromised mobility and overall quality of life.
We studied how muscle power and its determinants (muscle mass, single muscle fibre properties and motor control) adapted to 2 weeks of disuse and subsequent 2 weeks of physical training in young and older people.
Disuse decreased lower limb muscle power in both groups; however, different adaptations in single muscle fibre properties and co‐contraction of leg muscles were observed between young and older individuals.
Six physical training sessions performed after disuse promoted the recovery of muscle mass and power. However, they were not sufficient to restore muscle power to pre‐disuse values in older individuals, suggesting that further countermeasures are required to counteract the disuse‐induced loss of muscle power in older adults.
Abstract
Disuse‐induced loss of muscle power can be detrimental in older individuals, seriously impairing functional capacity. In this study, we examined the changes in maximal explosive power (MEP) of lower limbs induced by a 14‐day disuse (bed‐rest, BR) and a subsequent 14‐day retraining, to assess whether the impact of disuse was greater in older than in young men, and to analyse the causes of such adaptations. Sixteen older adults (Old: 55–65 years) and seven Young (18–30 years) individuals participated in this study. In a subgroup of eight Old subjects, countermeasures based on cognitive training and protein supplementation were applied. MEP was measured with an explosive ergometer, muscle mass was determined by magnetic resonance, motor control was studied by EMG, and single muscle fibres were analysed in vastus lateralis biopsy samples. MEP was ∼33% lower in Old than in Young individuals, and remained significantly lower (−19%) when normalized by muscle volume. BR significantly affected MEP in Old (−15%) but not in Young. Retraining tended to increase MEP; however, this intervention was not sufficient to restore pre‐BR values in Old. Ankle co‐contraction increased after BR in Old only, and remained elevated after retraining (+30%). Significant atrophy occurred in slow fibres in Old, and in fast fibres in Young. After retraining, the recovery of muscle fibre thickness was partial. The proposed countermeasures were not sufficient to affect muscle mass and power. The greater impact of disuse and smaller retraining‐induced recovery observed in Old highlight the importance of designing suitable rehabilitation protocols for older individuals.
Keywords: ageing, explosive muscle power, disuse, single muscle fiber, myosin isoforms
Key points
Disuse in older adults can critically decrease lower limb muscle power, leading to compromised mobility and overall quality of life.
We studied how muscle power and its determinants (muscle mass, single muscle fibre properties and motor control) adapted to 2 weeks of disuse and subsequent 2 weeks of physical training in young and older people.
Disuse decreased lower limb muscle power in both groups; however, different adaptations in single muscle fibre properties and co‐contraction of leg muscles were observed between young and older individuals.
Six physical training sessions performed after disuse promoted the recovery of muscle mass and power. However, they were not sufficient to restore muscle power to pre‐disuse values in older individuals, suggesting that further countermeasures are required to counteract the disuse‐induced loss of muscle power in older adults.
Introduction
People above the age of 60 years represent the fastest growing segment of the population in developed countries, and their quality of life can be dramatically compromised by reduced mobility (McPhee et al. 2013). Epidemiological research has associated reduced mobility with loss of muscle mass and muscular weakness (Janssen, 2006; Hairi et al. 2010). In particular, sarcopenia is a condition that can result from different factors, and can lead to outcomes characterized by progressive and generalized loss of skeletal muscle mass and function (strength and physical performance) (see Cruz‐Jentoft et al. 2010). The loss of muscle mass leads to a decrease in muscle strength; additionally, older people also experience a loss of strength per unit of muscle mass (Rutherford & Jones, 1992; Goodpaster et al. 2006), which may explain why muscle weakness in the elderly is a better predictor of mortality than muscle mass alone (Newman et al. 2006). Interestingly, skeletal muscle power has been proposed as a more critical determinant of physical functioning in the elderly population than muscle strength (Reid & Fielding, 2012) or size (Runge et al. 2004). Muscle power exerted by knee and lower limb extensors has been shown to decline earlier and more rapidly than muscle strength with advancing age (Aagaard et al. 2010), and it is considered a better performance predictor of various motor tasks (i.e. rising from a chair, climbing stairs) as compared to muscle strength in mobility‐limited elderly people (Bassey et al. 1992; Foldvari et al. 2000; Reid & Fielding, 2012).
Besides ageing, disuse is an important cause of muscle deterioration. Periods of skeletal muscle disuse or unloading can occur in healthy people as a consequence of injury or illness. The physiological effects of disuse and unloading have been widely studied by bed rest (BR), which is a recognized experimental model to induce substantial muscle dysfunction within a few weeks, remarkably compromising the generation of muscle power. Young healthy males decrease their ability to generate maximal muscular power of the lower limbs by about 24–30% after 35–90 days of BR without countermeasures (Ferretti, 1997; Rittweger et al. 2007; Rejc et al. 2015). The loss of muscle power after disuse is closely correlated to muscle atrophy. BR studies on young healthy males showed that disuse‐induced muscle atrophy is unevenly distributed, being larger in postural than non‐postural muscles, larger in the extensors than in the other thigh muscle groups, and greater in the calf muscles than in the other leg muscle groups (Ferretti et al. 2001; Alkner & Tesch, 2004; de Boer et al. 2008; Belavy et al. 2009). Atrophic response to BR can be rapid; for example, 7 days of BR induced a 3% decrease of thigh muscle mass (Ferrando et al. 1995). Longer BR induced greater atrophic effects, with a 12% and 8% decrement in gastrocnemius medialis and vastus lateralis muscle thickness after a 35‐day BR, respectively (de Boer et al. 2008), and a reduction of quadriceps and calf muscle mass by about 30% after 90–120 days of BR (Alkner & Tesch, 2004; Shackelford et al. 2004). Other important components of the loss of muscle power in relation to disuse are the decreased ability in motor unit recruitment (Lambertz et al. 2001; Clark et al. 2006) and the reduction of muscle intrinsic force (force/cross sectional area) (Pavy‐Le Traon et al. 2007; Narici & de Boer, 2011).
In spite of the vast amount of literature focused on the separate effects of either ageing or disuse, there are rather few studies that have investigated the combined effects of these two factors (ageing and disuse) on neuromuscular function. However, the occurrence of disuse periods in the elderly population is frequent (Suetta et al. 2007) and can lead to serious consequences such as further reduction of daily physical activity (Kortebein et al. 2008), functional decline (Hoenig & Rubenstein, 1991; Creditor, 1993), greater risks for falls and consequent hip fractures, which have long‐lasting negative effects on quality of life and a strong association with mortality (Wall et al. 2013). In healthy elderly individuals, neuromuscular function seems differently affected by short‐term disuse than in the young population. In particular, 2 weeks of immobilization by unilateral, whole leg casting reduced quadriceps femoris activation in old but not young men, while the decline in quadriceps volume was smaller in old compared to young (Suetta et al. 2009). Under the same experimental design, elderly individuals also showed a greater decrease in rapid force capacity (Hvid et al. 2010). Similarly, Deschenes et al. (2008) observed that muscle performance during faster contractions of the quadriceps femoris was more impaired in elderly than young individuals after 1 week of lower limb suspension. Shorter periods of disuse (i.e. 4 days of one‐leg immobilization) also have a greater impact on the neuromuscular system in old than in young subjects (Hvid et al. 2013, 2014). Interestingly, elderly individuals also showed reduced recovery of neuromuscular function as compared to young subjects when physical retraining was proposed after immobilization. For example, 4 weeks of physical training subsequent to 2 weeks of immobilization induced increments in quadriceps femoris volume that were smaller in elderly than in young males (Suetta et al. 2009), and promoted full recovery of muscle fibre area and rapid force capacity in young but not in elderly subjects (Hvid et al. 2010). Age‐related differences in the recovery of neuromuscular function seem to increase with shorter immobilization and retraining periods, as 7 days of recovery subsequent to 4 days of immobilization were not sufficient to restore isometric and dynamic muscle strength as well as rapid muscle force capacity in elderly, while these parameters were fully recovered in young subjects (Hvid et al. 2013, 2014).
The differential impact of a period of disuse on bone metabolism (Buehlmeier et al. 2017), protein synthesis (Biolo et al. 2017), plasma brain‐derived neurotrophic factor (BDNF) levels (Soavi et al. 2016) and adipokine values (Jurdana et al. 2015) in older adults compared to young people has been investigated by our group in a recent BR study. Also, we have examined the combined effects of ageing and disuse, followed by physical retraining, on muscle mass and performance (Pisot et al. 2016). Because ageing can exacerbate the negative effects of disuse on different systems, specific countermeasures (cognitive training during BR and protein supplementation during physical retraining) were proposed to an experimental subgroup of older adult individuals. Computerized cognitive training during BR improved executive/attention ability as well as processing speed (Marusic et al. 2018), and significantly modulated plasma BDNF levels (Passaro et al. 2017). On the other hand, Pisot et al. (2016) noted that these two countermeasures did not promote significant effects on muscle mass and performance in the experimental subgroup of older adults as compared to control older adults who did not receive any countermeasure. Among the combined effects of age and disuse, the loss of lower limb extensors power has particular relevance with regard to everyday life movements such as rising from a chair or climbing stairs (Bassey et al. 1992). Importantly, Pisot et al. (2016) observed that in older adults, the reduction of maximal explosive power (MEP) of lower limb extensors induced by the 14‐day BR was higher (−15.2%) than expected from the reduction of muscle mass (−8.3%). This prompted us to further examine different factors that may contribute to such MEP decrease. In this study, we re‐examined the issue by investigating how BR affected muscle mass, single muscle fibre properties and motor control, and whether these factors played a similar role on the BR‐induced loss of MEP in young and older subjects. In addition, these parameters were also analysed in both young and older individuals after a period of physical retraining to gain insight into how ageing affects muscle function recovery. Finally, the results were also analysed separately for the two subgroups of older adults (with and without countermeasures) to corroborate the previous statements related to the lack of positive effects of cognitive training and protein supplementation on these parameters.
Methods
Ethical approval
The present study was conducted according to the standards set by the latest revision of the Declaration of Helsinki except for registration in a database, and was approved by the National Ethical Committee of the Slovenian Ministry of Health on 17 April 2012, under the acronym IR‐aging 1200. The purposes and objectives of this study were carefully explained to the subjects and written informed consent was obtained from all of them.
Subjects
Sixteen healthy older adult males (Old; age: 59.6 ± 3.4 years) and seven healthy young males (Young; age: 23.1 ± 2.9 years) participated in this study. Before the start of the study, all subjects filled out a physical activity‐related questionnaire (Craig et al. 2003), had a full medical history and physical examination that also included routine haematology and biochemistry screens, and underwent a fitness battery test. Exclusion criteria were: smoking; regular alcohol consumption; ferromagnetic implants; history of deep vein thrombosis with D‐dimer > 500 μg L−1; acute or chronic skeletal, neuromuscular, metabolic and cardiovascular disease conditions; pulmonary embolism; or a short physical performance battery score <9 (Guralnik et al. 1994). None of the subjects experienced any significant disease and none was taking medications regularly or made use of any medication known to influence physical performance.
Study protocol
The subjects spent 19 consecutive days at the Orthopedic Hospital of Valdoltra (Ankaran, Slovenia), including 3 days of familiarization to study environment and diet, baseline data collection, and 14 days of BR. Immediately after BR, subjects underwent supervised physical training, which was conducted at the same facility and at a nearby gym. To explore possible interventions aimed at mitigating the negative disuse‐induced adaptations and enhancing physical retraining effects, a subgroup of eight randomly selected older adults (Old_Int) underwent daily 45 min of computerized cognitive training by navigating through virtual mazes with the use of a joystick and computer during the 14‐day BR. The same eight subjects also received a nutritional support based on 0.4 g whey protein kg body weight−1 day−1 at breakfast during the initial 14 days of physical training. By contrast, the eight older adults included in the control subgroup (Old_Ctrl) did not receive any additional countermeasure throughout the study.
Anthropometric characteristics, body composition and MEP of the lower limbs were measured 1 day before the BR (Pre‐BR), the day after the 14‐day BR (Post‐BR) and after 2 weeks of physical training (R + 14). Quadriceps muscle volume was assessed after 12 h of BR initiation (Pre‐BR), on the evening of the last day of BR (Post‐BR) and following 12 h of horizontal position after 2 weeks of physical training (R + 14).
Bed rest
The participants were housed in standard air‐conditioned hospital rooms and were under constant visual surveillance with 24‐h medical care. During BR, the subjects performed all daily activities in bed with no deviations from the lying position permitted, and both exercise and muscle contraction tests were not allowed. All participants received hospital meals three times a day and followed an individually controlled eucaloric diet during the BR period. Dietary energy requirements were designed for each subject multiplying resting energy expenditure by factors 1.2 and 1.4 in the bed rest and ambulatory periods, respectively (Biolo et al. 2008). The macronutrient food content was set at 60% carbohydrates, 25% fat and 15% proteins. Energy balance was checked weekly by fat mass assessment.
During BR, all subjects received passive physiotherapy treatments (i.e. joint mobility and stretching, relief massage in the presence of acute back pain) three times per week. To prevent thrombosis, a D‐dimer test was repeated on Day 7 of bed rest where participants reached elevated values but scored <500 μg L−1; however, additional Doppler ultrasound check‐up was conducted in all of them and all wore compression socks.
Physical training programme
Subjects began a 28‐day physical training programme on the second day after the end of BR; however, only the initial 14 days of physical training were considered in this study, i.e. the same duration of the disuse period. Physical training consisted of six sessions in total (three per week); each session, which lasted about 65 min, was followed by 1 or 2 days of routine daily activity. Physical training was aimed at reconditioning both the neuromuscular and the aerobic systems, proposing a series of exercises that did not require specific training equipment so that they could be translated to any home and community environment. The first 12 min of each training session was devoted to warm‐up; subjects performed 6 min of Nordic walking, its speed being determined from a 2‐km walking test performed before BR, and 6 min of active stretching (10 exercises). Subjects then performed 20 min of strength and balance exercises. This section started with half squat (one set; 10 repetitions; overload: from no overload to a 6 kg ball held with both hands), and continued with a circuit training (30 s of exercise followed by 30 s of rest) consisting of eight motor tasks. In particular, the following strength exercises focused on lower limbs were proposed: frontal and sagittal plane lunge, double leg heel raise with elastic resistance; hip extension with elastic resistance. Also, balance exercises mainly consisted of dynamic standing balance activities (i.e. standing on toes; standing on balance foams) and functional movements that involved reaching and passing objects. Strength and balance exercises were followed by 30 min of aerobic exercise (e.g. Nordic walking, brisk walking, running). The last 3 min were devoted to cool down (relaxation and breathing exercises). Subjects’ heart rate was preventively monitored during each training session. Training was conducted at the hospital and in the gym near the hospital and supervised by six physical trainers who instructed the subjects to properly perform the different exercises. All subjects performed all planned training sessions.
Measurements
Anthropometric characteristics and body composition
Body mass (BM) was measured to the nearest 0.1 kg with a manual weighing scale (Seca 709, Hamburg, Germany) with the subject dressed only in light underwear and no shoes. Stature was measured to the nearest 0.5 cm on a standardized wall‐mounted height board.
Body composition was measured by using bioelectrical impedance analysis with a tetra‐polar impedance‐meter (BIA101, Akern, Florence, Italy), according to an accepted method (Lukaski et al. 1986). Body composition [fat‐free mass (FFM) and fat mass (FM)] was obtained from the software provided by the manufacturer. This method has already been utilized and validated to investigate changes in body composition during BR and in clinical settings (Birch & Fisher, 1998; Kyle et al. 2004).
Magnetic resonance imaging
Quadriceps femoris muscle volume of the right leg was measured from turbo spin‐echo, T1‐weighted, magnetic resonance images (MRI) obtained with a 1.5 T Magnetom Avanto device (Siemens Medical Solution, Erlangen, Germany). On each MRI slice, contours corresponding to the quadriceps muscles were delineated by an MRI expert, using the image processing tools OsiriX (Pixmeo Sarl, v.4.1.2). Quadriceps muscle volume was then derived by summation of a series of evenly spaced truncated cones between each of two axial images, a process that included an average of 25 images (range 23–28) and covered the entire length of the quadriceps.
Maximal explosive power of the lower limbs
The biomechanical parameters of the explosive efforts were studied by means of an Explosive Ergometer (EXER), described previously in detail (Lazzer et al. 2009). Briefly, the EXER consists of a metal frame supporting one rail, which was inclined by 20 deg. A seat, fixed on a carriage, was free to move on the rail, its velocity along the direction of motion being continuously recorded by a wire tachometer (LIKA SGI, Vicenza, Italy). The subject was able to accelerate him/herself and the carriage seat backward by pushing on two force platforms (LAUMAS PA 300, Parma, Italy) positioned perpendicular to the rail. The total moving mass of the EXER (seat and carriage together) was equal to 31.6 kg. Force and velocity analog outputs were sampled at 2000 Hz using a data acquisition system (MP100; BIOPAC Systems, Inc., Goleta, CA, USA). The instantaneous power was calculated from the product of instantaneous force and velocity values.
The subject was seated on the carriage seat, secured by a safety harness tightened around the shoulders and abdomen, with their arms on the handlebars. Two mechanical blocks were used to set the distance between the seat and the force platforms, so that the knee angle at rest was 110 deg. The blocks also prevented any countermovement and, consequently, any recovery of elastic energy during the pushing phase. After a brief familiarization session with the laboratory equipment, the subjects performed four maximal explosive efforts, the duration of which was about 400 ms. After each push, subjects rested for 2 min with their feet placed on a support. The attempt with the greatest peak power (MEP) was taken into account for further analysis.
Surface electromyography recordings
Surface electromyography (EMG) data were collected from four muscles of the right lower limb: vastus lateralis (VL), biceps femoris (BF), gastrocnemius medialis (GM) and tibialis anterior (TA). Pre‐gelled surface EMG electrodes (circular contact area of 1 cm diameter, BIOPAC Systems) were placed (inter‐electrode distance 20 mm) at the following locations (Hermens et al. 2000): (a) for VL at two‐thirds on the line from the anterior superior iliac spine to the lateral side of the patella; (b) for BF midway between the ischial tuberosity and the lateral epicondyle of the tibia; (c) for GM on the most prominent bulge of the muscle; (d) for TA at one‐third on the line between the tip of the fibula and the tip of the medial malleolus. To ensure a good electrode–skin interface, prior to the application of the electrodes, the subject's skin was shaved, rubbed with an abrasive paste, cleaned with an alcohol solution and dry‐cleaned with gauze.
EMG data were sampled at a frequency of 2 kHz, and recorded by a four‐channel EMG system (EMG100C, BIOPAC Systems; Band‐pass Filter: 10–500 Hz; RMS noise voltage: 0.2 μV; input impedance: 2 MΩ; common mode rejection ratio: 110 dB). To place electrodes in the same anatomical location during the three different experimental sessions, the position of electrodes was marked on acetate paper using moles and small angiomas (which may be assumed to maintain a fixed position) as reference points. The EMG electrodes were fixed at the beginning of each experimental session and were not removed between explosive efforts and isometric contractions (see below) of the lower limbs.
Maximal voluntary contractions
To normalize EMG signal recorded from the four analysed muscles (VL, BF, GM and TA) during explosive efforts, maximal voluntary isometric contractions (MVCs) of the right lower limb were performed either on a special chair (a) or on an adapted examination bed (b):
-
a)
The subject was seated with his legs hanging vertically down. A strap, connected in series to a force sensor (TSD121C, BIOPAC Systems), was tightened around the subject's right ankle. The force sensor was fixed in series to a steel frame. The position of this frame was set prior the execution of isometric knee extension and knee flexion to obtain a knee angle of 110 deg.
-
b)
The subject lay prone on an examination bed. His right foot was tightened around a custom made attachment connected to an isometric dynamometer. The anterior part of the foot sole was placed against the attachment in a flat standardized position, to obtain an ankle angle of 90 deg.
Force and EMG exerted during MVCs were recorded at a frequency of 2 kHz using a data acquisition system (MP100, BIOPAC Systems). Subjects were asked to perform three MVCs of 4–5 s for each isometric effort. To prevent fatigue, after each contraction subjects rested for 2 min.
EMG data analysis
The EMG activity defined in a 500‐ms window centred on maximal force exerted during MVC was analysed: EMG raw signal was processed using a 5‐ms running‐window root mean square, and its mean value was considered as 100% MVC.
EMG raw signal recorded during the push (i.e. throughout the period of force development) of the explosive efforts was processed using a 5‐ms running‐window root mean square to obtain its mean value throughout each push. This was then expressed as percentage of the value obtained during MVC. To investigate a co‐contraction feature during the push phase of the explosive efforts at the knee and ankle joints, the ratio between EMG amplitude of BF (%MVC) and VL (%MVC) (knee co‐contraction) and the ratio between EMG amplitude of TA (%MVC) and GM (%MVC) (ankle co‐contraction) were calculated. The greater the value of these indexes the greater the level of co‐contraction.
Single muscle fibre experiments
Single muscle fibre analysis was performed on samples obtained from the mid‐region of the left vastus lateralis muscle. Biopsy was done after anaesthesia of the skin, subcutaneous fat tissue, and muscle fascia with 2 mL of lidocaine (2%). A small incision was then made to penetrate skin and fascia, and the tissue sample was harvested with a purpose‐built rongeur (Zepf Instruments, Tuttlingen, Germany). A fragment of the sample, used for single fibre analysis, was quickly stored in skinning solution with 50% glycerol at −20°C, while another fragment was frozen in isopentane cooled with liquid nitrogen. The solutions used had the following composition (mm): skinning solution: potassium propionate 150, magnesium acetate 5, Na‐ATP 5, EGTA 5 and KH2PO4 5; relaxing solution: KCl 100, imidazole 20, MgCl2 5, Na‐ATP 5 and EGTA 5; preactivating solution had a similar composition except that EGTA concentration was reduced to 0.5 mm, and 25 mm creatine phosphate and 300 U mL−1 creatine phosphokinase were added; activating solution (pCa 4.6) was also similar to relaxing solution with the addition of 5 mm CaCl2, 25 mm creatine phosphate and 300 U mL−1 creatine phosphokinase. The pH of all solutions was adjusted to 7.0 at the temperature at which solutions were used (12°C). Protease inhibitors (E64 10 μm and leupeptine 40 μm) were present in all solutions.
On the day of the experiment, single muscle fibre segments were dissected from the samples stored and mounted in a drop of relaxing solution between the force transducer (AME‐801 SensorOne, Sausalito, CA, USA) and the electromagnetic puller (Scientific Instruments, Heidelberg, Germany) equipped with a displacement transducer. The signals from the force and displacement transducers were fed and stored in a computer after A/D conversion (interface CED 1401 plus, CED, Cambridge, UK). For data storage, recall and analysis the software Spike 2 (CED) was used. The experimental setup consisted of an inverted microscope (Axiovert 25, Zeiss, Oberkochen, Germany) with a movable and thermo‐regulated aluminium plate on the stage. On the aluminium plate, three drops (70 μl volume) of relaxing, pre‐activating and activating solution, respectively, were kept under a coverslip connected with a movable arm. Under each solution drop, an opening through the aluminium plate made the fibre segment visible via the objective piece of the inverted microscope. A stereomicroscope (Konus Diamond, Konus, Italy) placed above the inverted microscope was used for manipulation of the fibre segment. A digital camera (Optikam B5, Optika, Bergamo, Italy) allowed measurements of sarcomere length with a custom‐designed software in at least two regions of interest covering approximately 20 sarcomeres each with resolution of 19.5 pixels μm–1. Further details of the setup are reported elsewhere (Doria et al. 2011). Once mounted in the setup, fibre segments were gently elongated in the relaxing solution to 120% of the slack length, which corresponded to a sarcomere length of 2.685 ± 0.011 μm (mean ± SE, n = 710). The segments then transferred to the pre‐activating solution for at least 1 min and finally maximally activated by immersion in the activating solution. During maximal activation, isometric force (F o) was measured in several consecutive contractions and unloaded shortening velocity (V o) was determined according to the slack test procedure. To this end, five instantaneous length changes (<1 ms) were imposed with amplitudes ranging from 5 to 15% of the resting length. Unloaded shortening velocity was obtained from the slope of the linear regression between the time required to take up the slack and the amount of shortening imposed and was expressed in fibre length per second. Cross‐sectional area (CSA) was calculated from the measurements of three fibre diameters, assuming a circular shape of the fibre section, while the fibre was immersed in relaxing solution. Furthermore, specific force P o = F o/CSA was also calculated.
The composition in Myosin Heavy Chains (MyHC) isoforms of each fibre segment was determined on 8% polyacrylamide slab gels after denaturation in SDS (SDS‐PAGE) as described by Doria et al. (2011). Gels were silver stained and three bands were separated in the region of 200 kDa, corresponding (in order of migration from the fastest to the slowest) to MyHC‐1, MyHC‐2A and MyHC‐2X. The same protocol was followed to separate MyHC isoforms in the frozen fragment of the biopsy, with Coomassie Blue staining of the gels. The relative proportions of MyHC isoforms were obtained from the measurements of the brightness area product (BAP, i.e. the product of the area of the band by the average brightness subtracted local background after black–white inversion) after scanning the gels to an accuracy of 600 d.p.i. For each sample the electrophoretic separation and the densitometry measurements were repeated three times.
Statistical analyses
Statistical analyses were performed using SPSS 19.0 software (IBM, Chicago, MI, USA), with significance set at P < 0.05. Normal distribution of the data was tested using the Shapiro–Wilk test. Sphericity was verified by Mauchly's test. When the assumption of sphericity was not met, the significance of the F‐ratios was adjusted according to the Greenhouse–Geisser procedure. Differences in baseline data of body mass, stature and body mass index among groups were analysed by a general linear model of the main effect of group (Old_Int vs. Old_Ctrl vs. Young). Changes of anthropometric characteristics, body composition, quadriceps femoris volume (QF), MEP, MVC, EMG data and per cent distribution of MyHC isoforms were analysed with general linear model repeated measures with two factors considering three groups (Old_Int vs. Old_Ctrl vs. Young) and time (Pre‐BR vs. Post‐BR vs. R + 14). When significant differences were found, a Bonferroni post hoc test was used to determine the exact location of the difference. Changes of muscle fibres characteristics (CSA, F o, P o and V o) were analysed with a generalized linear mixed model, which accounts for fixed effects due to group (Old_Int vs. Old_Ctrl vs. Young) and time (Pre‐BR vs. Post‐BR vs. R + 14) taking into account the correlation of the data. The same analyses were also performed pooling together the two subgroups of older adults (Old group) to strengthen the focus of the present study on ageing, disuse and physical training.
Results
Physical characteristics of subjects
All participants were able to comply with the study protocol. There was no dropout, and no medical complications occurred beside transient hypotension during the 10 min orthostatic tolerance testing at the end of BR in seven participants (three in Old and four in Young). Baseline values of body mass, stature, body mass index, body composition and lifestyle were not different among the two groups of older adults and the Young group (Table 1). However, quadriceps femoris muscle volume was significantly greater in Young compared to Old (+15.3%, P = 0.038, Table 2), while it was similar between Old_Int and Old_Ctrl (P = 0.757).
Table 1.
Baseline characteristics of the Control (Old_Ctrl) and Interventions (Old_Int) groups of older adults and Young subjects
| Old_Ctrl (n = 8) | Old_Int (n = 8) | Young (n = 7) | P | |
|---|---|---|---|---|
| Age (years) | 59.9 ± 3.3* | 59.4± 3.6* | 23.1± 2.9 | <0.001 |
| Stature (m) | 1.73 ± 0.04 | 1.74 ± 0.06 | 1.77 ± 0.07 | 0.358 |
| Body mass (kg) | 79.6 ± 10.5 | 80.3 ± 14.7 | 74.8 ± 8.8 | 0.633 |
| BMI (kg m−2) | 26.8 ± 4.2 | 26.4 ± 4.8 | 24.0 ± 2.4 | 0.342 |
| Physical activity (min week−1) | 874 ± 549 | 922 ± 587 | 597 ± 238 | 0.354 |
| SPPB score | 11.6 ± 0.2 | 12.2 ± 0.3 | 12.0 ±0.0 | 0.625 |
| Self‐assessed general health | 3.9 ± 0.9 | 3.5 ± 0.9 | 4.3 ± 0.8 | 0.215 |
| Self‐assessed quality of life | 3.2 ± 1.0 | 3.1 ± 1.0 | 3.7 ± 1.1 | 0.312 |
All values are means ± SD.
P: significance by general linear model of the main effects of Group (Old_Ctrl vs. Old_Int vs. Young).
*Significantly different from Young.
BMI: body mass index; SPPB: short physical performance battery score (Guralnik et al. 1994).
Table 2.
Effects of 2 weeks of bed rest and 2 weeks of physical retraining on physical characteristics of the Control group (Old_Ctrl) and Interventions group (Old_Int) of older adults and Young subjects
| Old_Ctrl (n = 8) | Old_Int (n = 8) | Young (n = 7) | Significance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐BR | Post‐BR | R + 14 | Pre‐BR | Post‐BR | R + 14 | Pre‐BR | Post‐BR | R + 14 | Group | Time | G × T | |
| BM (kg) | 79.6 ± 10.5 | 77.6 ± 10.4 | 79.5 ± 10.1 | 80.3 ± 14.7 | 77.35 ± 13.6 | 79.2 ± 13.7 | 74.8 ± 8.8 | 71.6 ± 8.3 | 74.4 ± 8.1 | 0.590 | <0.001 | 0.243 |
| FFM (kg) | 59.8 ± 6.9 | 56.7 ± 6.8 | 60.4 ± 6.9 | 63.8 ± 11.1 | 59.3 ± 7.9 | 60.6 ± 10.4 | 60.9 ± 3.9 | 56.3 ± 3.8 | 57.8 ± 4.4 | 0.709 | <0.001 | 0.131 |
| FM (kg) | 19.9 ± 4.9 | 20.9 ± 4.8 | 19.1 ± 4.6 | 16.4 ± 6.9 | 18.1 ± 8.8 | 18.6 ± 7.2 | 14.0 ± 6.2 | 15.3 ± 7.0 | 16.6 ± 6.6 | 0.370 | 0.066 | 0.173 |
| QF (cm3) | 1663 ± 173 | 1524 ± 197 | 1613 ± 157 | 1702 ± 298 | 1561 ± 246 | 1648 ± 233 | 1988 ± 270 | 1867 ± 204 | 1954 ± 211 | 0.016 | <0.001 | 0.981 |
All values are means ± SD.
Pre‐BR: before bed rest; Post‐BR: after bed rest; R + 14: after physical retraining; BM: body mass; FFM: fat‐free mass; FM: fat mass; QF: quadriceps femoris muscle volume.
Significance by general linear model of the main effects of Group (Old_Ctrl vs. Old_Int vs. Young), Time (Pre‐BR vs. Post‐BR vs. R + 14) and Group × Time interaction (G × T).
Body mass, total body fat‐free mass and quadriceps femoris muscle volume were similar between Old_Ctrl and Old_Int both after BR and after physical training (P values ranging from 0.394 to 0.971; Table 2).
BR induced significant decreases in body mass (−3.1 and −4.4%, P < 0.001, in Old and Young, respectively) and in total body fat‐free mass (−6.2 and −7.6%, P < 0.005, in Old and Young, respectively) in both Old and Young (Table 2). Similarly, a significant decrease in muscle volume of quadriceps femoris occurred in Old (−8.3%, P < 0.001), and the same trend was observed in Young (−6.1%, P = 0.052). Physical training performed after BR increased body mass in both groups (+2.4 and +3.9%, P < 0.001, in Old and Young, respectively); however, the increment in total body fat‐free mass and quadriceps femoris muscle volume was significant in Old only (+4.4%, P < 0.006, and +5.7%, P < 0.001, respectively). It is important to note that physical training tended to restore quadriceps femoris muscle volume to Pre‐BR values in Old (−3.1%, P = 0.048), and to fully restore it in Young (−1.7%, P = 0.428) (Table 2).
MEP and MVCs of the lower limbs
MEP, peak force, peak velocity and specific MEP values were similar between the two groups of older adults (Old_Ctrl and Old_Int) at all three investigated time points (P values ranging from 0.561 to 0.982; Fig. 1 and Table 3).
Figure 1. Effects of bed rest and physical retraining on maximal explosive power of the lower limbs in older adults enrolled in the Control group, Interventions group and Young subjects.
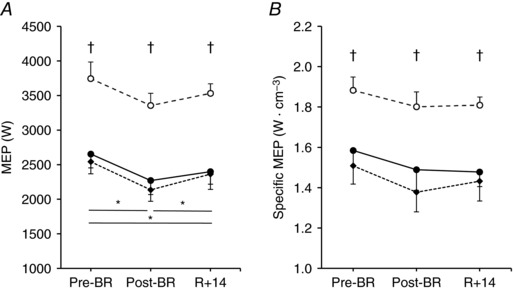
Values are mean ± SE. Maximal explosive power (MEP, A) and MEP normalized per unit of quadriceps femoris muscle volume (Specific MEP, B) exerted before bed rest (Pre‐BR), after bed rest (Post‐BR) and after physical retraining (R + 14). Control group (Old_Ctrl, ●), Interventions group (Old_Int, ♦) and Young subjects (○). Differences in MEP and specific MEP were tested using general linear model and following post hoc analysis with Bonferroni corrections. *Difference between periods in the two Old groups; †difference between the two Old groups and Young.
Table 3.
Effects of 2 weeks of bed rest and 2 weeks of physical retraining on peak force and velocity during maximal explosive efforts of the Control (Old_Ctrl) and Interventions (Old_CT) groups of older adults and Young subjects
| Old_Ctrl (n = 8) | Old_Int (n = 8) | Young (n = 7) | Significance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐BR | Post‐BR | R + 14 | Pre‐BR | Post‐BR | R + 14 | Pre‐BR | Post‐BR | R + 14 | Group | Time | G × T | |
| F (N) | 1457 ± 185 | 1297 ± 191 | 1319 ± 176 | 1466 ± 185 | 1299 ± 160 | 1367 ± 221 | 1693 ± 210 | 1588 ± 190 | 1649 ± 112 | 0.009 | < 0.001 | 0.364 |
| v (m·s−1) | 2.02 ± 0.22 | 1.94 ± 0.29 | 1.99 ± 0.24 | 2.03 ± 0.19 | 1.87 ± 0.21 | 1.98 ± 0.19 | 2.44 ± 0.16 | 2.31 ± 0.15 | 2.32 ± 0.11 | 0.001 | 0.002 | 0.645 |
All values are means ± SD.
Pre‐BR: before bed rest; Post‐BR: after bed rest; R + 14: after physical retraining; F: peak force; v: peak velocity.
Significance by general linear model of the main effects of Group (Old_Ctrl vs. Old_Int vs. Young), Time (Pre‐BR vs. Post‐BR vs. R + 14) and Group × Time interaction (G × T).
Young subjects consistently exerted higher MEP than Old (∼ +33%, P < 0.001) across the three investigated time points (Fig. 1 A). Accordingly, greater peak force and velocity (∼ +16% for both measurements, P < 0.005) were found in Young compared to Old (Table 3). Importantly, MEP normalized to quadriceps femoris muscle volume (specific MEP) was also consistently higher in Young than in Old (∼ +19%, P < 0.005) at Pre‐BR, Post‐BR and R + 14 (Fig. 1 B).
BR caused a decrease in MEP (−15.2%, P < 0.001, Fig. 1 A) as well as peak force and velocity (Table 3) in Old; a similar trend was observed in Young, as MEP decreased by 10.4% (P = 0.067) after BR. The subsequent physical retraining significantly increased MEP only in Old (+8.1%, P = 0.018; Fig. 1 A). However, MEP developed at R + 14 was still significantly lower than before BR for Old (−8.3%, P = 0.011) but not for Young (−5.7%, P = 0.370). In Old, this lack of complete MEP recovery at R + 14 was accompanied by a significantly lower peak force (−8.1%, P < 0.001) compared to Pre‐BR (Table 3). Specific MEP did not change significantly across the three experimental time points in both Old and Young groups (Fig. 1 B). However, it might be of note that in Old specific MEP tended to decrease after BR (−7.7%, P = 0.133) and to remain lower than at Pre‐BR (−7.0%, P = 0.148) also after the completion of physical training.
To examine the changes in contractile force related to disuse and retraining, the force developed during MVCs of quadriceps femoris muscle was analysed. As seen in Fig. 2 A, MVC values were lower in Old compared to Young (by approximately −20%) at any time point, and showed a significant decrease at the end of BR only in older adults (Old: −13%, P < 0.001; Old_Ctrl: −15%, P = 0.012; Old_Int: −11%, P = 0.006). MVC values recorded at R + 14 were comparable to those collected before BR for both Old and Young. No significant differences were observed between MVC values exerted by Old_Ctrl and Old_Int at any time point (difference ranging from 6 to 11%; P values ranging from 0.204 to 0.521). MVC normalized by quadriceps femoris muscle volume (specific MVC; Fig. 2 B) was not significantly different among Old_Ctrl, Old_Int and Young, and did not change significantly across the three experimental time points; however, it tended to follow the trend observed for MVC.
Figure 2. Effects of bed rest and physical retraining on maximal voluntary isometric contractions of the right quadriceps femoris muscle in older adults enrolled in the Control group, Interventions group and Young subjects.
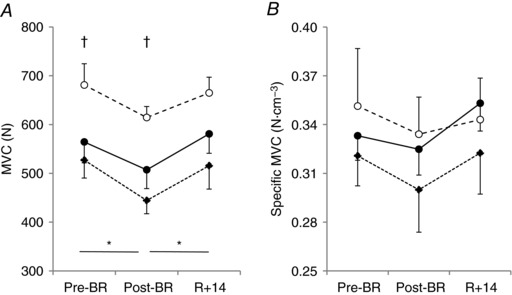
Values are mean ± SE. Force exerted during maximal voluntary isometric contractions (MVC, A), and MVC normalized per unit of quadriceps femoris muscle volume (Specific MVC, B). Control group (Old_Ctrl, ●), Interventions group (Old_Int, ♦) and Young subjects (○). Pre‐BR: before bed rest; Post‐BR: after bed rest; R + 14: after physical retraining. Differences in force were tested using general linear model and following post hoc analysis with Bonferroni corrections. *Difference between periods in the two Old groups; †difference between the two Old groups and Young.
Single muscle fibre variations with age and bed rest
To assess whether the structural and functional characteristics of single muscle fibres could contribute to the difference between older adults and young individuals and to the changes of lower limb muscle force and power following BR and physical retraining, large populations of fibres were dissected from biopsy samples taken from vastus lateralis and analysed as described in the Methods.
The total populations analysed with single fibre experiments comprised 233 fibres (84 in Old_Ctrl; 77 in Old_Int; 72 in Young) in Pre‐BR biopsy sampling, 243 fibres (84 in Old_Ctrl; 86 in Old_Int; 73 in Young) in Post‐BR and 241 fibres (84 in Old_Ctrl; 87 in Old_Int; 70 in Young) in post physical retraining sampling. When grouped according to their myosin isoform composition, which was adopted as a molecular marker of fibre type, slow fibres represented 27–28% in the biopsies of Young subjects and 33–39% in the biopsies of Old, suggesting an age‐related fibre type transition. Fast 2A fibres represented 35–44% in the biopsy samples of Young and 24–31% in the biopsy samples of Old. The remaining fraction comprised hybrid fibres (slow‐fast 2A and fast 2A‐2X) and in Young subjects a minor group of pure 2X fibres (see also Table 4). No difference was detectable in electrophoretic separation of MyHC isoforms on muscle homogenates (Table 5).
Table 4.
Baseline data of single fibres analysed from biopsy samples collected prior to the beginning of bed rest; in addition to the three groups of fibres reported in the table, hybrid slow‐2A (n = 16 in Old_Ctrl; n = 14 in Old_Int; n = 7 in Young) and few pure fast 2X (n = 2 in Old_Ctrl; n = 0 in Old_Int; n = 3 in Young) fibres were also found and characterized
| Slow/1 | Fast 2A | 2A‐2X | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Old_Ctrl | Old_Int | Young | P | Old_Ctrl | Old_Int | Young | P | Old_Ctrl | Old_Int | Young | P | |
| Fibre (number) | 29 | 36 | 19 | 24 | 17 | 32 | 13 | 10 | 11 | |||
| CSA (μm2) | 6426 ± 2768 | 6335 ± 3233 | 6256 ± 3918 | 0.984 | 5607 ± 1820* | 5601 ± 2363* | 7662 ± 3388 | 0.009 | 5338 ± 2731 | 4561 ± 1289 | 7282 ± 3131 | 0.053 |
| F o (mN) | 0.683 ± 0.389 | 0.732 ± 0.320 | 0.864 ± 0.583 | 0.333 | 0.565 ± 0.338* | 0.650 ± 0.239* | 0.962 ± 0.611 | 0.005 | 0.897 ± 0.459* | 0.555 ± 0.252* | 1.447 ± 1.060 | 0.017 |
| P o (mN mm−2) | 116 ± 69 | 133 ± 76 | 144 ± 78 | 0.445 | 103 ± 54 | 135 ± 86 | 132 ± 54 | 0.179 | 177 ± 89 | 130 ± 66 | 188 ± 96 | 0.265 |
| V o (L s−1) | 0.543 ± 0.681 | 0.854 ± 0.820 | 0.911 ± 0.798 | 0.268 | 2.873 ± 2.970 | 1.795 ± 1.277 | 1.771 ± 1.715 | 0.168 | 2.924 ± 2.440 | 2.939 ± 2.132 | 3.417 ± 2.658 | 0.907 |
All values are means ± SD.
CSA: cross sectional area; F o: isometric force; P o: specific force; V o: unloaded shortening velocity.
P: significance by general linear mixed model of the main effect of group (Old_Ctrl vs. Old_Int vs. Young).
*Significantly different from Young.
Table 5.
Per cent distribution of MyHC isoforms in the Control (Old_Ctrl) and Interventions (Old_Int) groups of older adults and Young subjects before bed rest, after bed rest and after physical retraining
| Old_Ctrl (n = 8) | Old_Int (n = 8) | Young (n = 7) | Significance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre‐BR | Post‐BR | R + 14 | Pre‐BR | Post‐BR | R + 14 | Pre‐BR | Post‐BR | R + 14 | Group | Time | G × T | |
| MyHC 1 | 47.4 ± 13.0 | 43.8 ± 10.8 | 44.0 ± 8.7 | 41.6 ± 7.7 | 41.8 ± 8.1 | 41.0 ± 9.7 | 35.7 ± 7.5 | 38.0 ± 8.4 | 35.9 ± 13.4 | 0.095 | 0.623 | 0.871 |
| MyHC 2A | 36.3 ± 10.2 | 35.0 ± 7.5 | 38.2 ± 16.5 | 37.1 ± 8.6 | 38.6 ± 9.1 | 40.3 ± 13.6 | 45.2 ± 13.5 | 48.3 ± 6.6 | 47.7 ± 12.3 | 0.084 | 0.536 | 0.921 |
| MyHC 2X | 15.8 ± 11.8 | 21.2 ± 9.3 | 17.1 ± 14.8 | 18.1 ± 11.2 | 19.6 ± 9.4 | 18.3 ± 15.9 | 19.1 ± 12.6 | 15.2 ± 8.8 | 16.4 ± 12.7 | 0.889 | 0.963 | 0.679 |
All values are means ± SD.
Pre‐BR: before bed rest; Post‐BR: after bed rest; R + 14: after physical retraining; F: peak force; v: peak velocity.
Significance by general linear model of the main effects of Group (Old_Ctrl vs. Old_Int vs. Young), Time (Pre‐BR vs. Post‐BR vs. R + 14) and Group × Time interaction (G × T).
The variations of CSA and of isometric force developed during maximal activation (F o) are reported in Fig. 3 together with their ratio, P o = F o/CSA, and the value of unloaded shortening velocity (V o). Only the most abundant fibre types, slow, fast 2A and fast 2A‐2X, are considered, because for the other types (hybrid slow‐fast 2A and pure 2X) there were too few fibres to produce reliable measurements and statistical comparisons. Interestingly, the response to disuse was different in slow and fast 2A between Young and Old subjects, while the two subgroups of older adults (Old_Ctrl and Old_Int) showed very similar results. In baseline sampling, slow fibres showed similar CSA in Young and Old, while fast 2A fibres showed significantly lower CSA values in Old (−27%, P < 0.05) compared to Young subjects (Table 4 and Fig. 3 A and E). BR caused a significant reduction of CSA, i.e. atrophy at the single fibre level, in fast 2A fibres of Young subjects (−28%, Fig. 3 E) and in slow fibres of Old subjects (−19%, Fig. 3 A), clearly pointing to a differential sensitivity to disuse. By contrast, CSA values after BR were similar between Old_Ctrl and Old_Int both in slow, in fast 2A and in fast 2A2X fibres (P values ranging from 0.512 to 0.881, Fig. 3 A, E and I). The determination of isometric force (F o, see Fig. 3 B) revealed a significant decrease in slow fibres of both Young (−42%) and older adults (−22% in Old_Ctrl; −29% in Old_Int) at the end of BR and this resulted in a reduction in specific force (P o) in the slow fibres of the Young (−44%, Fig. 3 C) subjects. No significant difference was observed in unloaded shortening velocity comparing Pre‐BR, Post‐BR and R + 14 (Fig. 3 D, H and N). In both Young and Old, fast 2A‐2X fibres showed a trend to a decrease in F o and P o after BR, which remained below statistical significance. Importantly, at R + 14 CSA values of slow and fast 2A fibres and F o values of slow fibres were lower than before BR in the older adult subjects, indicating an incomplete structural and functional recovery (Fig. 3 A, B and E). Moreover, fast 2A‐2X fibres of Young showed lower values of F o and P o at R + 14 than before BR (Fig. 3 L and M).
Figure 3. Effects of bed rest and physical retraining on single fibre parameters.
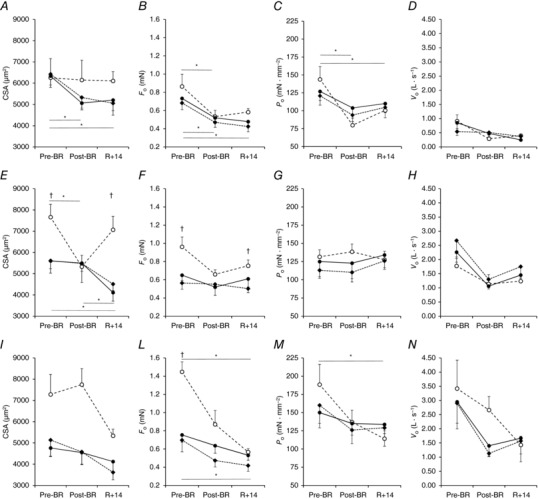
Cross sectional area (CSA: A, E, I), isometric force during maximal activation (F o: B, F, L), specific force or tension (P o: C, G, M) and single fibre shortening velocity (V o: D, H, N) in slow or type 1 fibres (A–D), fast 2A (E–H) and fast 2A2X (I–N) in the older adult Control group (Old_Ctrl, ●), Interventions group (Old_Int, ♦) and in Young subjects (○). Values are mean ± SE. Differences in CSA, F o, P o and V o were tested using general linear mixed model and are indicated in the lower part of each panel for Old and the upper part for Young. *Difference between periods; †difference between the two Old groups and Young.
Knee and ankle co‐contraction
Finally, to gain insight into the contribution of motor control to the variations in MEP, we recorded the EMG activity of knee and ankle flexor and extensor muscles to calculate an index of co‐contraction between antagonist muscles (see Methods). As shown in Fig. 4, the level of knee and ankle co‐contraction during explosive extensions of the lower limbs was not significantly different among Old_Ctrl, Old_Int and Young at any of the three investigated time points. Also, knee co‐contraction did not change significantly across the investigated time points in all three groups. In Young, ankle co‐contraction was also not affected by BR and physical retraining. Conversely, in both older adult groups, ankle co‐contraction showed an increase after BR (Old: +27.6%, P = 0.035), and remained greater than at Pre‐BR also at R + 14 (Old: +30.4%, P = 0.017) (Fig. 4 ).
Figure 4. Effects of bed rest and following physical retraining on the level of co‐contraction at the knee and ankle joint in older adults enrolled in the Control group, Interventions group and in Young subjects.
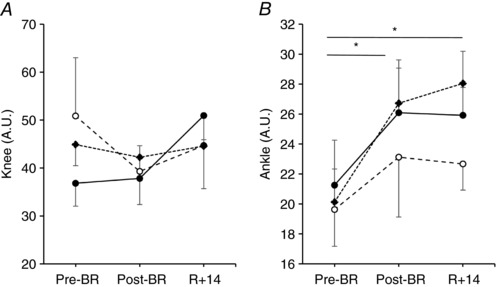
Values are mean ± SE. Control group (Old_Ctrl, ●), Interventions group (Old_Int, ♦) and Young subjects (○). Level of co‐contraction between biceps femoris and vastus lateralis (Knee, A) and between tibialis anterior and gastrocnemius medialis (Ankle, B) recorded during explosive efforts of the lower limbs before bed rest (Pre), after bed rest (Post‐BR) and after physical retraining (R + 14). Differences in the level of Knee and Ankle co‐contraction were tested using general linear model and following post hoc analysis with Bonferroni corrections. *Difference between periods in the two Old groups.
Discussion
In this study, we showed that the MEP of lower limbs in healthy older adult males (mean age: 60 years) was about 30% lower than in young males (mean age: 23 years). MEP normalized by quadriceps femoris muscle volume was also substantially lower (−19%) in older adults. Fourteen days of BR induced a significant decrement of MEP in Old, and a similar trend was observed in Young. A period of physical retraining that had the same duration of BR (2 weeks) tended to increase MEP in both groups; however, this intervention was not sufficient to restore muscle power to Pre‐BR values in older adults. Additional interventions (cognitive training and protein supplementation) tested on one subgroup of older adults (Old_Int) did not mitigate BR‐induced muscle atrophy and MEP decrement and did not enhance physical training‐induced adaptations.
Lower limb muscle function was affected by age
Before any intervention, MEP developed by a group of older adult men was about 30% lower than that exerted by a group of young subjects with similar life style, body weight and stature (Fig. 1 A). Note that the difference in MEP as well as in other parameters was clearly detectable even if the age of the older group was not very advanced (approximately 60 years). The rate of decline of muscle function is relatively slow from 20 to 50 years of age, and becomes marked after 50 years of age (Wilmore, 1991). Previous studies found that muscle power decreases over the age range 65–89 years by about 3.5% every year (Skelton et al. 1994), with longitudinal observations indicating a 6% annual loss of muscle power over 3 years among adults aged 70–85 years (Clark et al. 2013). Muscle mass of the lower limbs, and in particular of the knee extensors (quadriceps femoris), directly affects the level of muscle power that can be exerted by lower limbs (Ferretti et al. 2001). As expected, our results indicated that quadriceps muscle volume was lower (−15.3%; Table 2) in older adults (Rosenberg, 1997; McPhee et al. 2013), contributing to explain their lower MEP. However, when MEP was normalized per unit of quadriceps muscle volume (specific MEP), it was still substantially lower (∼−19%) in Old than in Young (Fig. 1 B). This difference in specific MEP generated during a fast (∼400 ms) and relatively complex movement that involves three joints and the interaction of uni‐ and multi‐articulate muscle–tendon units was conceivably due to different components. One of them can be related to different single muscle fibre characteristics between Young and Old. Single muscle fibre analysis showed a greater abundance of slow fibres in Old (≈ 40%) compared to Young (≈ 28%) (Table 4). These data are in agreement with previous observations (Lexell et al. 1988; Klitgaard et al. 1990b), but are not supported by electrophoresis and densitometry of MyHC, which accounts not only for fibre abundance but also for fibre size. Slow fibres develop less force and shorten at lower speed (see Table 4) and this implies a lower peak power output. In addition, fast 2A fibres were not only less abundant but also thinner (see Table 4) in Old compared to Young. An age‐related selective atrophy of fast fibres in knee extensor muscles was observed for the first time by Klitgaard et al. (1990a) and confirmed by more recent studies (e.g. Brunner et al. 2007; Murgia et al. 2017). Thus, quantitative variations in muscle fibre type distribution and size, together with different levels of intramuscular fat and fibrosis, would be expected to play a role in age‐related loss of specific power (Marcus et al. 2012; McGregor et al. 2014).
In addition, differences in muscle architecture and tendon properties between young and older adults have been previously found, making these factors potential contributors to the age‐related decline in muscle power and mobility (Stenroth et al. 2015). In particular, muscle fascicle length affects muscle power production capacity according to force–length and force–velocity relationships (Narici & Maganaris, 2007). Also, the lower tendon stiffness shown by elderly subjects (Stenroth et al. 2012) conceivably impairs the tendon roles of “energy re‐distributor” and “power amplifier” during explosive movements (Hof et al. 1983; Fukashiro et al. 2006; Cormie et al. 2011).
Finally, motor control is an important component for the multi‐articular development of muscle power, and its deterioration has been recently indicated as one of the relevant mechanisms responsible for age‐related loss of muscle function (Venturelli et al. 2015). In particular, ageing leads to the loss of spinal motor neurons and changes in maximal motor neuron firing frequency, activation capacity, co‐contraction of antagonist muscles and spinal inhibitory circuitry (Klass et al. 2008; Aagaard et al. 2010). Our results indicated that the level of co‐contraction at the knee and ankle joints was similar between Old and Young (Fig. 4); hence, it can be assumed that this neuromuscular feature was not responsible for the MEP difference between groups. However, activation capacity was not examined in the present study, and its age‐related decrement (Onambele et al. 2007) could have played a role in the lower absolute and specific MEP exerted by Old individuals.
Disuse‐induced loss of lower limb muscle power and volume
Our results showed that 14 days of BR impaired MEP development in Old (−15.2%), and that the same trend was observed in Young (−10.4%) (Fig. 1 A). This was accompanied by lower values of peak force and velocity in Old (Table 3). The amount of MEP lost in the present study was similar to that observed after a 10‐day BR in healthy elderly individuals (−14%) (Kortebein et al. 2008), and is lower than that observed after longer periods of disuse without countermeasures in young participants [about 24–30% after 35–90 days of BR (Ferretti, 1997; Rittweger et al. 2007; Rejc et al. 2015)]. It is worth noting that, in the present study, explosive lower limb extensions were performed under simulated microgravity (i.e. against an after‐load equal to about 48% body mass), allowing fast lower limb extension movements. Interestingly, Deschenes et al. (2008) found that, at slower isokinetic knee extensions, muscle power was similarly affected in elderly and young individuals (∼ −11%) after 1 week of unilateral lower limb suspension. However, at higher contraction speeds, disuse significantly affected muscle power only in elderly. In addition, other studies showed that, after a period of short‐term immobilization, rapid muscle force capacity was more affected in elderly than young men (Hvid et al. 2010, 2014). These findings might suggest that disuse degrades the functional capacity of fast‐twitch muscle tissue in older adults more than in young. However, the present study does not support the hypothesis that BR‐induced muscle atrophy targeted primarily fast fibres in older adults; in fact, atrophy was more pronounced in slow fibres of Old and in fast 2A fibres in Young (see below). Hence, it seems plausible that BR accelerated ageing‐related neural impairments that preferentially affect fast motor neurons (i.e. firing frequency and activation capacity) (Deschenes et al. 2008; Aagaard et al. 2010), thus inhibiting the capacity of older adults to activate fast‐twitch muscle tissue during fast muscle contractions. In addition, we found that co‐contraction between representative plantar flexors and dorsi flexors increased only in older adults after BR. This can be considered an additional disuse‐induced negative neural adaptation that was amplified by ageing (Aagaard et al. 2010), as greater levels of co‐contraction generally impair power exertion (Reeves et al. 2006a). The view that BR may have accelerated ageing‐related neural impairments is also consistent with the fact that specific MEP tended to be more affected in older adults (−7.8%) than in young (−4.3%) (Fig. 1 B). In fact, neural adaptations are one of the factors that can affect muscle‐specific power (see discussion above and Narici & de Boer, 2011). From a functional perspective, the substantial disuse‐induced loss of muscle power in older adults and elderly individuals can be deleterious, as their lower limb muscle power is already closer to the critical threshold required for independent mobility, physical functioning and lower risk of falling (Reid & Fielding, 2012).
Muscle atrophy is another factor that contributed to the loss of lower limb muscle power after disuse. BR‐induced muscle atrophy is larger in postural than non‐postural muscles, larger in the extensors than in the other thigh muscle groups, and larger in the calf muscles than in the other leg muscle groups (Ferretti et al. 2001; Alkner & Tesch, 2004; de Boer et al. 2008; Belavy et al. 2009). In particular, Ferretti et al. (2001) indicated that the loss of lower limb muscle power after 42 days of BR was primarily explained by the reduction of quadriceps femoris CSA, and that other factors such as impaired neural activation or fibre‐specific tension might have accounted for only 5%. In the present study, total‐body fat free mass was not affected by BR in the two groups. Conversely, quadriceps femoris muscle volume decreased by a similar extent in Old (−8.3%) and Young (−6.1%); in both cases, the muscle volume decrement was smaller than the decrease of MEP (−15.2% in Old and −10.4% in Young). A comparable overall amount of muscle atrophy was reported by Suetta et al. (2009) after 2 weeks of immobilization by unilateral, whole leg casting; however, these authors reported that quadriceps muscle volume decreased more in young (−8.9%) than in elderly males (−5.2%). This last finding is not in agreement with the similar muscle atrophy between young and older adults that was found in the present study. Data on the combined effect of ageing and disuse on muscle atrophy seem to be not consistent (Suetta et al. 2009), as also shown by the greater adductor pollicis muscle atrophy found in elderly compared to young individuals after 2 weeks of immobilization (Urso et al. 2006). The differences observed between the present study and the two above mentioned studies could be at least partially explained by the different experimental conditions (i.e. BR vs. immobilization; larger vs. smaller muscle group; age: older adults vs. elderly individuals).
It is important to note that, when analysed at single fibre level, the impact of 2 weeks of disuse is more pronounced in slow fibres of Old and in fast 2A fibres in Young. Fast 2A‐2X fibres are also responsive to BR in Young. This differential response suggests a different functional role of the two major fibre types in the habitual postural and locomotor activities which are suppressed during BR. A greater sensitivity to disuse in fast fibres has been reported in previous studies (e.g. Hvid et al. 2011). In that study, however, the decrease in force and specific force was not different between young and elderly subjects. A possible explanation can be found in the different protocols, as Hvid and co‐workers induced not only disuse (as in BR), but also immobilization by applying a cast on the legs.
Six sessions of physical training performed after disuse were not sufficient to restore lower limb muscle power in older adults
In the present study, a period of physical retraining after BR of the same duration as the BR promoted incomplete MEP recovery in older adults, while it restored MEP to pre‐BR values in young individuals (Fig. 1 A). Similarly, specific MEP tended to remain lower than at Pre‐BR in older adults (−6.5%). These findings are in agreement with previous studies that investigated similar periods of disuse and retraining, showing incomplete recovery of rapid force capacity in elderly but not young individuals (Hvid et al. 2010, 2014). It is plausible that negative adaptations induced by disuse and ageing on the neural system were not fully counteracted by physical retraining in the elderly, playing a role in the incomplete recovery of muscle function during fast muscle contraction. In particular, after physical retraining, co‐contraction of ankle muscles remained similar to that at Post‐BR and greater than at Pre‐BR in older adults (Fig. 4), conceivably contributing to the impaired muscle power exertion (Reeves et al. 2006a). In addition, other potential ageing‐ and disuse‐induced neural adaptations not tested in the present study [i.e. loss of spinal motor neurons; impaired maximal motor neuron firing frequency and activation capacity; altered motor unit recruitment pattern (Klass et al. 2008; Aagaard et al. 2010; Narici & de Boer, 2011)] may not have been restored by physical retraining, contributing as well to the impaired muscle power output observed in the elderly during fast muscle contractions. Interestingly, quadriceps muscle volume did not completely recover Pre‐BR values in older adults (−3.1%), and an incomplete recovery of contractile function was also observed in single muscle fibres of the older participants in partial agreement with previous observations (Suetta et al. 2009; Hvid et al. 2014). Also, the present study further supports the view that recovery of muscle function in older adults is particularly impaired during fast muscle contractions, because muscle strength exerted during isometric MVC of the knee extensors was fully restored after physical retraining in both older adults and young subjects (Fig. 2 A and B). Complete recovery of muscle strength during isometric MVC in the elderly was also found after 4 weeks of retraining, which were subsequent to 2 weeks of immobilization (Suetta et al. 2009). By contrast, shorter retraining (1 week) did not lead to the same positive outcome, even if the immobilization period was shorter (4 days) (Hvid et al. 2014).
These findings seem to highlight the role of training volume as an important determinant for the recovery of muscle function in the elderly. Along this line, most of the studies that reported positive neuromuscular adaptations in the elderly involved a greater number of training sessions (i.e. ∼20–45 sessions) than the above mentioned studies (Reeves et al. 2006a; Reid & Fielding, 2012).
While discussing the lower limb muscle function recovery observed in the present study, it is also important to note that the proposed physical training was not primarily focused on the increment of lower limb muscle power, but rather to a more global physical improvement. In fact, more than half of each training session was devoted to aerobic exercise, and no explosive or high‐speed movements were included during strength exercises.
Interestingly, training interventions that emphasized explosive power focusing on higher movement speed, even without requiring specific resistance training equipment, were safe and effective for improving lower limb muscle power and physical functioning (i.e. stair climbing) in the elderly population (Bean et al. 2002, 2004; Reid & Fielding, 2012). Similarly, physical training performed with isotonic weight‐resistance machines or inertial load has the potential to increase lower limb muscle strength and power in elderly individuals (Reeves et al. 2006a,b; Onambele et al. 2008). Hence, further studies focused on the retraining of muscle power after a period of disuse should include higher‐speed, power‐orientated exercises. The improvement and recovery of muscle power after disuse in older adults and elderly individuals is of particular interest in view of its relevance for independent mobility and quality of life in the elderly population (Reid & Fielding, 2012). In fact, several studies identified lower limb muscle power as a significant predictor of functional performance in older adults (reviewed by Reid & Fielding, 2012).
Cognitive training and protein supplementation did not significantly affect muscle mass and power in older adults
Two distinct countermeasures, cognitive training and protein supplementation, were adopted to attenuate the impact of bed rest and improve recovery, respectively, in a subgroup of older adult participants. Cognitive training by navigating through virtual mazes might activate the same neural systems involved in mobility and this might produce, in turn, subliminal muscle contractions. An improved executive/attention ability and processing speed (Marusic et al. 2018) as well as suppression of the increase in plasma BDNF concentration (Passaro et al. 2017) have been observed after bed rest in the older individuals enrolled in the present study who were exposed to cognitive training. However, cognitive training did not lead to any mitigation of BR‐induced loss of muscle mass and power, as also reported by Passaro et al. (2017), who additionally analysed the correlation between variations of explosive power and BDNF plasma levels.
The usefulness of protein supplementation in BR experiments has been considered in many studies with mixed results (see Stein & Blanc, 2011), possibly in relation to the amount and the timing of administration. In the present BR study, development of insulin resistance (Pisot et al. 2016) and anabolic resistance (Biolo et al. 2017) were detected. The protein supplementation was restricted to the physical retraining phase, thus starting from a condition in which anabolic resistance was present. No significant effect of protein supplementation on the recovery of muscle mass and power was found; however, we cannot rule out the possibility that the lack of effect was just due to the insufficient amount of protein.
Limitations of the study
Two main outcomes of this study are that (1) the loss of lower limb muscle power due to disuse was more pronounced in older adults than in young individuals; and (2) physical retraining that had the same duration as disuse was not sufficient to fully recover muscle power and volume in older adults. However, from a statistical standpoint, these messages are weakened by the small number of participants enrolled in this study; this is a consequence of logistical limitations that are intrinsic to this type of studies.
Similar limitations are also related to the uneven sample size of the groups. Also, the fact that only male subjects were enrolled limits the applicability of the findings reported in the present study. It should be noted that women, and particularly post‐menopausal women, can count on lower muscle mass than matched male subjects, and thus may be more responsive to disuse than men. Finally, bioelectrical impedance analysis has some intrinsic limitation for body composition analysis; however, this methodology was used in the present study only to describe anthropometric characteristics of the population, while a more sensitive technique (MRI) was used for assessing the changes in muscle volume of the representative lower limb extensor examined (quadriceps femoris).
Conclusions
Two weeks of disuse decreased the lower limb muscle power in both young and older individuals. Six physical training sessions performed in the 2 weeks subsequent to disuse promoted the recovery of muscle mass and power; however, they were not sufficient to restore muscle function to pre‐disuse values in older individuals. Also, countermeasures based on cognitive training and protein supplementation were not effective for reducing the impact of disuse and improving physical retraining in older adults. Taken together, these findings indicate that susceptibility to the impact of disuse increases with ageing, and suggest that a greater number of training sessions, the inclusion of power‐orientated exercises and more effective countermeasures are required to restore muscle mass and power after 2 weeks of disuse in older individuals.
Additional information
Competing interests
None declared
Author contributions
E.R., P.T., M.N., B.S., R.P., G.B., A.P., J.R., C.R. and S.L. were responsible for study conception and experimental design; E.R., M.F., P.T., A.B., L.T., L.C., J. R. and S.L. performed the experiments; E.R., M.F., P.T., B.S., R.P., C.R. and S.L. analysed and interpreted the data; E.R., M.F., P.T., C.R. and S.L. drafted the article; and E.R., M.F., P.T., M.N., B.S., R.P., G.B., A.P., J.R., C.R. and S.L. revised the article critically for important intellectual content. All authors have approved the final version of the manuscript and agreed to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The study was conducted in the framework of the project PANGeA: CB147 – Physical Activity and Nutrition for Quality Ageing, supported by the Cross‐border Cooperation Program Slovenia–Italy 2007–2013 and co‐financed by the European Regional Development Fund (grant no. 042‐2/2009‐18/052012), as well as Slovenian national project L5‐5550 – Development of noninvasive marker for muscle atrophy (grant no. 1000‐15‐1988).
Acknowledgements
We would like to thank the participants in the study for their time and effort to ensure the success of the project. We acknowledge the excellent assistance of the entire staff of the Orthopaedic Hospital Valdoltra (Koper, Slovenia). Additionally, we thank the research team and the students of Applied Kinesiology of University of Primorska for their help and logistic support and many other researchers and colleagues from different institutes and different countries who contributed to the smooth undertaking of the study.
Biographies
Enrico Rejc is presently Assistant Professor and Director of the Neuromuscular and Skeletal Research Core at the Kentucky Spinal Cord Injury Research Center, Department of Neurosurgery, University of Louisville, USA. Prior to taking this position, he was also involved in research activities at the University of Udine, University of California, Los Angeles, and Manchester Metropolitan University. He has studied the effects of disuse, ageing, spinal cord injury and physical exercise on the human neuromuscular system for about 10 years. His research is also focused on the recovery of motor function after severe spinal cord injury using spinal cord epidural stimulation and activity‐based training.

Stefano Lazzer is Professor and Director of the School of Sport and Exercise Sciences, Department of Medicine, University of Udine, Italy. He studies the physiology of muscle contraction, bioenergetics and cardio‐respiratory adaptations to exercise on human health and performance. His current research programme is focused on the metabolic responses during exercise and the adaptation of humans disuse and training.
Edited by: Michael Hogan & Karyn Hamilton
References
- Aagaard P, Suetta C, Caserotti P, Magnusson SP & Kjaer M (2010). Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports 20, 49–64. [DOI] [PubMed] [Google Scholar]
- Alkner BA & Tesch PA (2004). Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur J Appl Physiol 93, 294–305. [DOI] [PubMed] [Google Scholar]
- Bassey EJ, Fiatarone MA, O'Neill EF, Kelly M, Evans WJ & Lipsitz LA (1992). Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 82, 321–327. [DOI] [PubMed] [Google Scholar]
- Bean J, Herman S, Kiely DK, Callahan D, Mizer K, Frontera WR & Fielding RA (2002). Weighted stair climbing in mobility‐limited older people: a pilot study. J Am Geriatr Soc 50, 663–670. [DOI] [PubMed] [Google Scholar]
- Bean JF, Herman S, Kiely DK, Frey IC, Leveille SG, Fielding RA & Frontera WR (2004). Increased Velocity Exercise Specific to Task (InVEST) training: a pilot study exploring effects on leg power, balance, and mobility in community‐dwelling older women. J Am Geriatr Soc 52, 799–804. [DOI] [PubMed] [Google Scholar]
- Belavy DL, Miokovic T, Armbrecht G, Richardson CA, Rittweger J & Felsenberg D (2009). Differential atrophy of the lower‐limb musculature during prolonged bed‐rest. Eur J Appl Physiol 107, 489–499. [DOI] [PubMed] [Google Scholar]
- Biolo G, Agostini F, Simunic B, Sturma M, Torelli L, Preiser JC, Deby‐Dupont G, Magni P, Strollo F, di Prampero P, Guarnieri G, Mekjavic IB, Pisot R & Narici MV (2008). Positive energy balance is associated with accelerated muscle atrophy and increased erythrocyte glutathione turnover during 5 wk of bed rest. Am J Clin Nutr 88, 950–958. [DOI] [PubMed] [Google Scholar]
- Biolo G, Pisot R, Mazzucco S, Di Girolamo FG, Situlin R, Lazzer S, Grassi B, Reggiani C, Passaro A, Rittweger J, Gasparini M, Simunic B & Narici M (2017). Anabolic resistance assessed by oral stable isotope ingestion following bed rest in young and older adult volunteers: relationships with changes in muscle mass. Clin Nutr 36, 1420–1426. [DOI] [PubMed] [Google Scholar]
- Birch LL & Fisher JO (1998). Development of eating behaviors among children and adolescents. Pediatrics 101, 539–549. [PubMed] [Google Scholar]
- Brunner F, Schmid A, Sheikhzadeh A, Nordin M, Yoon J & Frankel V (2007). Effects of aging on Type II muscle fibers: a systematic review of the literature. J Aging Phys Act 15, 336–348. [DOI] [PubMed] [Google Scholar]
- Buehlmeier J, Frings‐Meuthen P, Mohorko N, Lau P, Mazzucco S, Ferretti JL, Biolo G, Pisot R, Simunic B & Rittweger J (2017). Markers of bone metabolism during 14 days of bed rest in young and older men. J Musculoskelet Neuronal Interact 17, 399–408. [PMC free article] [PubMed] [Google Scholar]
- Clark BC, Fernhall B & Ploutz‐Snyder LL (2006). Adaptations in human neuromuscular function following prolonged unweighting: I. Skeletal muscle contractile properties and applied ischemia efficacy. J Appl Physiol (1985) 101, 256–263. [DOI] [PubMed] [Google Scholar]
- Clark DJ, Pojednic RM, Reid KF, Patten C, Pasha EP, Phillips EM & Fielding RA (2013). Longitudinal decline of neuromuscular activation and power in healthy older adults. J Gerontol A Biol Sci Med Sci 68, 1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormie P, McGuigan MR & Newton RU (2011). Developing maximal neuromuscular power: Part 1–biological basis of maximal power production. Sports Med 41, 17–38. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF & Oja P (2003). International physical activity questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc 35, 1381–1395. [DOI] [PubMed] [Google Scholar]
- Creditor MC ( 1993). Hazards of hospitalization of the elderly. Ann Intern Med 118, 219–223. [DOI] [PubMed] [Google Scholar]
- Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M & European Working Group on Sarcopenia in Older P (2010). Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer MD, Seynnes OR, di Prampero PE, Pisot R, Mekjavic IB, Biolo G & Narici MV (2008). Effect of 5 weeks horizontal bed rest on human muscle thickness and architecture of weight bearing and non‐weight bearing muscles. Eur J Appl Physiol 104, 401–407. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Holdren AN & McCoy RW (2008). Adaptations to short‐term muscle unloading in young and aged men. Med Sci Sports Exerc 40, 856–863. [DOI] [PubMed] [Google Scholar]
- Doria C, Toniolo L, Verratti V, Cancellara P, Pietrangelo T, Marconi V, Paoli A, Pogliaghi S, Fano G, Reggiani C & Capelli C (2011). Improved VO2 uptake kinetics and shift in muscle fiber type in high‐altitude trekkers. J Appl Physiol (1985) 111, 1597–1605. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Stuart CA, Brunder DG & Hillman GR (1995). Magnetic resonance imaging quantitation of changes in muscle volume during 7 days of strict bed rest. Aviat Space Environ Med 66, 976–981. [PubMed] [Google Scholar]
- Ferretti G (1997). The effect of prolonged bed rest on maximal instantaneous muscle power and its determinants. Int J Sports Med 18(Suppl 4), S287–289. [DOI] [PubMed] [Google Scholar]
- Ferretti G, Berg HE, Minetti AE, Moia C, Rampichini S & Narici MV (2001). Maximal instantaneous muscular power after prolonged bed rest in humans. J Appl Physiol 90, 431–435. [DOI] [PubMed] [Google Scholar]
- Foldvari M, Clark M, Laviolette LC, Bernstein MA, Kaliton D, Castaneda C, Pu CT, Hausdorff JM, Fielding RA & Singh MA (2000). Association of muscle power with functional status in community‐dwelling elderly women. J Gerontol A Biol Sci Med Sci 55, M192–199. [DOI] [PubMed] [Google Scholar]
- Fukashiro S, Hay DC & Nagano A (2006). Biomechanical behavior of muscle–tendon complex during dynamic human movements. J Appl Biomech 22, 131–147. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M & Newman AB (2006). The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61, 1059–1064. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA & Wallace RB (1994). A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol 49, M85–94. [DOI] [PubMed] [Google Scholar]
- Hairi NN, Cumming RG, Naganathan V, Handelsman DJ, Le Couteur DG, Creasey H, Waite LM, Seibel MJ & Sambrook PN (2010). Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc 58, 2055–2062. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Disselhorst‐Klug C & Rau G (2000). Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10, 361–374. [DOI] [PubMed] [Google Scholar]
- Hoenig HM & Rubenstein LZ (1991). Hospital‐associated deconditioning and dysfunction. J Am Geriatr Soc 39, 220–222. [DOI] [PubMed] [Google Scholar]
- Hof AL, Geelen BA & Van den Berg J (1983). Calf muscle moment, work and efficiency in level walking; role of series elasticity. J Biomech 16, 523–537. [DOI] [PubMed] [Google Scholar]
- Hvid L, Aagaard P, Justesen L, Bayer ML, Andersen JL, Ortenblad N, Kjaer M & Suetta C (2010). Effects of aging on muscle mechanical function and muscle fiber morphology during short‐term immobilization and subsequent retraining. J Appl Physiol (1985) 109, 1628–1634. [DOI] [PubMed] [Google Scholar]
- Hvid LG, Ortenblad N, Aagaard P, Kjaer M & Suetta C (2011). Effects of ageing on single muscle fibre contractile function following short‐term immobilisation. J Physiol 589, 4745–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvid LG, Suetta C, Aagaard P, Kjaer M, Frandsen U & Ortenblad N (2013). Four days of muscle disuse impairs single fiber contractile function in young and old healthy men. Exp Gerontol 48, 154–161. [DOI] [PubMed] [Google Scholar]
- Hvid LG, Suetta C, Nielsen JH, Jensen MM, Frandsen U, Ortenblad N, Kjaer M & Aagaard P (2014). Aging impairs the recovery in mechanical muscle function following 4 days of disuse. Exp Gerontol 52, 1–8. [DOI] [PubMed] [Google Scholar]
- Janssen I (2006). Influence of sarcopenia on the development of physical disability: the cardiovascular health study. J Am Geriatr Soc 54, 56–62. [DOI] [PubMed] [Google Scholar]
- Jurdana M, Jenko‐Praznikar Z, Mohorko N, Petelin A, Jakus T, Simunic B & Pisot R (2015). Impact of 14‐day bed rest on serum adipokines and low‐grade inflammation in younger and older adults. Age (Dordr) 37, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass M, Baudry S & Duchateau J (2008). Age‐related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol (1985) 104, 739–746. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Mantoni M, Schiaffino S, Ausoni S, Gorza L, Laurent‐Winter C, Schnohr P & Saltin B (1990a). Function, morphology and protein expression of ageing skeletal muscle: a cross‐sectional study of elderly men with different training backgrounds. Acta Physiol Scand 140, 41–54. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G & Saltin B (1990b). Ageing alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand 140, 55–62. [DOI] [PubMed] [Google Scholar]
- Kortebein P, Symons TB, Ferrando A, Paddon‐Jones D, Ronsen O, Protas E, Conger S, Lombeida J, Wolfe R & Evans WJ (2008). Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci 63, 1076–1081. [DOI] [PubMed] [Google Scholar]
- Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gomez J, Lilienthal Heitmann B, Kent‐Smith L, Melchior JC, Pirlich M, Scharfetter H, Schols MWJA, Pichard C & ESPEN (2004). Bioelectrical impedance analysis‐part II: utilization in clinical practice. Clin Nutr 23, 1430–1453. [DOI] [PubMed] [Google Scholar]
- Lambertz D, Perot C, Kaspranski R & Goubel F (2001). Effects of long‐term spaceflight on mechanical properties of muscles in humans. J Appl Physiol (1985) 90, 179–188. [DOI] [PubMed] [Google Scholar]
- Lazzer S, Pozzo R, Rejc E, Antonutto G & Francescato MP (2009). Maximal explosive muscle power in obese and non‐obese prepubertal children. Clin Physiol Funct Imaging 29, 224–228. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC & Sjostrom M (1988). What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15‐ to 83‐year‐old men. J Neurol Sci 84, 275–294. [DOI] [PubMed] [Google Scholar]
- Lukaski HC, Bolonchuk WW, Hall CB & Siders WA (1986). Validation of tetrapolar bioelectrical impedance method to assess human body composition. J Appl Physiol 60, 1327–1332. [DOI] [PubMed] [Google Scholar]
- Marcus RL, Addison O, Dibble LE, Foreman KB, Morrell G & Lastayo P (2012). Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J Aging Res 2012, 629637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusic U, Giordani B, Moffat SD, Petric M, Dolenc P, Pisot R & Kavcic V (2018). Computerized cognitive training during physical inactivity improves executive functioning in older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 25, 49–69. [DOI] [PubMed] [Google Scholar]
- McGregor RA, Cameron‐Smith D & Poppitt SD (2014). It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhee JS, Hogrel JY, Maier AB, Seppet E, Seynnes OR, Sipila S, Bottinelli R, Barnouin Y, Bijlsma AY, Gapeyeva H, Maden‐Wilkinson TM, Meskers CG, Paasuke M, Sillanpaa E, Stenroth L, Butler‐Browne G, Narici MV & Jones DA (2013). Physiological and functional evaluation of healthy young and older men and women: design of the European MyoAge study. Biogerontology 14, 325–337. [DOI] [PubMed] [Google Scholar]
- Murgia M, Toniolo L, Nagaraj N, Ciciliot S, Vindigni V, Schiaffino S, Reggiani C & Mann M (2017). Single muscle fiber proteomics reveals fiber type‐specific features of human muscle aging. Cell Reports 19, 2396–2409. [DOI] [PubMed] [Google Scholar]
- Narici MV & de Boer MD (2011). Disuse of the musculo‐skeletal system in space and on earth. Eur J Appl Physiol 111, 403–420. [DOI] [PubMed] [Google Scholar]
- Narici MV & Maganaris CN (2007). Plasticity of the muscle–tendon complex with disuse and aging. Exerc Sport Sci Rev 35, 126–134. [DOI] [PubMed] [Google Scholar]
- Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM & Harris TB (2006). Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 61, 72–77. [DOI] [PubMed] [Google Scholar]
- Onambele GL, Maganaris CN, Mian OS, Tam E, Rejc E, McEwan IM & Narici MV (2008). Neuromuscular and balance responses to flywheel inertial versus weight training in older persons. J Biomech 41, 3133–3138. [DOI] [PubMed] [Google Scholar]
- Onambele GL, Narici MV, Rejc E & Maganaris CN (2007). Contribution of calf muscle–tendon properties to single‐leg stance ability in the absence of visual feedback in relation to ageing. Gait Posture 26, 343–348. [DOI] [PubMed] [Google Scholar]
- Passaro A, Soavi C, Marusic U, Rejc E, Sanz JM, Morieri ML, Nora ED, Kavcic V, Narici MV, Reggiani C, Biolo G, Zuliani G, Lazzer S & Pisot R (2017). Computerized cognitive training and brain derived neurotrophic factor during bed rest: mechanisms to protect individual during acute stress. Aging (Albany NY) 9, 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavy‐Le Traon A, Heer M, Narici MV, Rittweger J & Vernikos J (2007). From space to Earth: advances in human physiology from 20 years of bed rest studies (1986–2006). Eur J Appl Physiol 101, 143–194. [DOI] [PubMed] [Google Scholar]
- Pisot R, Marusic U, Biolo G, Mazzucco S, Lazzer S, Grassi B, Reggiani C, Toniolo L, di Prampero PE, Passaro A, Narici M, Mohammed S, Rittweger J, Gasparini M, Gabrijelcic Blenkus M & Simunic B (2016). Greater loss in muscle mass and function but smaller metabolic alterations in older compared with younger men following 2 wk of bed rest and recovery. J Appl Physiol (1985) 120, 922–929. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Narici MV & Maganaris CN (2006a). Musculoskeletal adaptations to resistance training in old age. Man Ther 11, 192–196. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Narici MV & Maganaris CN (2006b). Myotendinous plasticity to ageing and resistance exercise in humans. Exp Physiol 91, 483–498. [DOI] [PubMed] [Google Scholar]
- Reid KF & Fielding RA (2012). Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 40, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejc E, di Prampero PE, Lazzer S, Grassi B, Simunic B, Pisot R, Antonutto G & Narici M (2015). Maximal explosive power of the lower limbs before and after 35 days of bed rest under different diet energy intake. Eur J Appl Physiol 115, 429–436. [DOI] [PubMed] [Google Scholar]
- Rittweger J, Felsenberg D, Maganaris C & Ferretti JL (2007). Vertical jump performance after 90 days bed rest with and without flywheel resistive exercise, including a 180 days follow‐up. Eur J Appl Physiol 100, 427–436. [DOI] [PubMed] [Google Scholar]
- Rosenberg IH (1997). Sarcopenia: origins and clinical relevance. J Nutr 127, 990S–991S. [DOI] [PubMed] [Google Scholar]
- Runge M, Rittweger J, Russo CR, Schiessl H & Felsenberg D (2004). Is muscle power output a key factor in the age‐related decline in physical performance? A comparison of muscle cross section, chair‐rising test and jumping power. Clin Physiol Funct Imaging 24, 335–340. [DOI] [PubMed] [Google Scholar]
- Rutherford OM & Jones DA (1992). The relationship of muscle and bone loss and activity levels with age in women. Age Ageing 21, 286–293. [DOI] [PubMed] [Google Scholar]
- Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL & Lai D (2004). Resistance exercise as a countermeasure to disuse‐induced bone loss. J Appl Physiol (1985) 97, 119–129. [DOI] [PubMed] [Google Scholar]
- Skelton DA, Greig CA, Davies JM & Young A (1994). Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing 23, 371–377. [DOI] [PubMed] [Google Scholar]
- Soavi C, Marusic U, Sanz JM, Morieri ML, Dalla Nora E, Simunic B, Pisot R, Zuliani G & Passaro A (2016). Age‐related differences in plasma BDNF levels after prolonged bed rest. J Appl Physiol (1985) 120, 1118–1123. [DOI] [PubMed] [Google Scholar]
- Stein TP & Blanc S (2011). Does protein supplementation prevent muscle disuse atrophy and loss of strength? Crit Rev Food Sci Nutr 51, 828–834. [DOI] [PubMed] [Google Scholar]
- Stenroth L, Peltonen J, Cronin NJ, Sipila S & Finni T (2012). Age‐related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol (1985) 113, 1537–1544. [DOI] [PubMed] [Google Scholar]
- Stenroth L, Sillanpaa E, McPhee JS, Narici MV, Gapeyeva H, Paasuke M, Barnouin Y, Hogrel JY, Butler‐Browne G, Bijlsma A, Meskers CG, Maier AB, Finni T & Sipila S (2015). Plantarflexor muscle‐tendon properties are associated with mobility in healthy older adults. J Gerontol A Biol Sci Med Sci 70, 996–1002. [DOI] [PubMed] [Google Scholar]
- Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M & Aagaard P (2009). Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol (1985) 107, 1172–1180. [DOI] [PubMed] [Google Scholar]
- Suetta C, Magnusson SP, Beyer N & Kjaer M (2007). Effect of strength training on muscle function in elderly hospitalized patients. Scand J Med Sci Sports 17, 464–472. [DOI] [PubMed] [Google Scholar]
- Urso ML, Clarkson PM & Price TB (2006). Immobilization effects in young and older adults. Eur J Appl Physiol 96, 564–571. [DOI] [PubMed] [Google Scholar]
- Venturelli M, Saggin P, Muti E, Naro F, Cancellara L, Toniolo L, Tarperi C, Calabria E, Richardson RS, Reggiani C & Schena F (2015). In vivo and in vitro evidence that intrinsic upper‐ and lower‐limb skeletal muscle function is unaffected by ageing and disuse in oldest‐old humans. Acta Physiol (Oxf) 215, 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall BT, Dirks ML & van Loon LJ (2013). Skeletal muscle atrophy during short‐term disuse: implications for age‐related sarcopenia. Ageing Res Rev 12, 898–906. [DOI] [PubMed] [Google Scholar]
- Wilmore JH (1991). The aging of bone and muscle. Clin Sports Med 10, 231–244. [PubMed] [Google Scholar]


