Abstract
T cell mediated hypersensitivity to nickel (Ni2+) is one of the most common causes of allergic contact dermatitis. Ni2+ sensitization may also contribute to the failure of Ni2+ containing joint implants, and revision to non-Ni2+ containing hardware can be costly and debilitating. Previously, we identified Ni2+ mimotope peptides, which are reactive to a CD4+ T cell clone, ANi2.3 (Vα1, Vβ17), isolated from a Ni2+ hypersensitive patient with contact dermatitis. This T cell is restricted to major histocompatibility complex class II (MHCII) molecule, Human Leukocyte Antigen (HLA)-DR52c (DRA, DRB3*0301). However, it is not known if Ni2+ induced T cell responses in sensitized joint replacement failure patients are similar to subjects with Ni2+ induced contact dermatitis. Here, we generated DR52c/Ni2+ mimotope tetramers, and used them to test if the same Ni2+ T cell activation mechanism could be generalized to Ni2+ sensitized patients with associated joint implant failure. We confirmed the specificity of these tetramers by staining of ANi2.3 T cell transfectomas. The DR52c/Ni2+ mimotope tetramer detected Ni2+ reactive CD4+ T cells in the peripheral blood mononuclear cells (PBMC) of patients identified as Ni2+ sensitized by patch testing and/or a positive Ni2+ LPT. When HLA-typed by a DR52 specific antibody, three out of four patients were DR52 positive. In one patient, Ni2+ stimulation induced the expansion of Vβ17 positive CD4+ T cells from 0.8% to 13.3%. We found that the percentage of DR52 positivity and Vβ17 usage in Ni2+ sensitized joint failure patients are similar to Ni sensitized skin allergy patients. Ni2+ independent mimotope tetramers may be a useful tool to identify the Ni2+ reactive CD4+ T cells.
Keywords: metal allergy, HLA, tetramer, joint implants, metal toxicology, CD4+ T cells
Graphical abstract
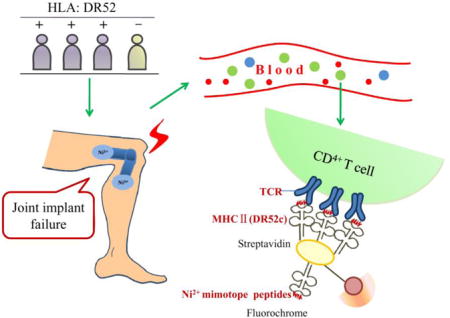
Introduction
Nickel (Ni2+) is one of the most common sensitizers according to the American Academy of Dermatology, and was voted ‘Allergen of the Year’ in 2008. The prevalence of Ni2+ sensitization is estimated to be rising from 15.5% in 2009–10, to 18.5% in 2011–12, and the ATSDR estimates that 10–20% of the US population is sensitized to Ni2+. Hypersensitivity to Ni2+ is an important factor that can cause joint replacements failure; after such failed implants are replaced with non-Ni2+ containing hardware, many of the symptoms are relieved (Pacheco, 2015).
Despite the high prevalence of Ni2+ allergy, the specific immune components of the sensitization process are poorly understood. There is strong evidence for specific T cell involvement in the pathogenesis of the disease, with an expansion of αβ T cells specific for the metal (Sinigaglia et al., 1985; Kapsenberg et al., 1987; Emtestam et al., 1989; Silvennoinen-Kassinen et al., 1991). Vβ17 elements in Ni2+ specific human T cell receptors dominate in contact dermatitis, and greater frequency of these T cells correlates with the severity of the dermatitis (Vollmer et al., 1997). In a Ni2+ reactive CD4+ T cell transfectoma-ANi2.3, derived from a subject with Ni2+ induced contact dermatitis, MHCII molecule DR52c (DRA*0101, DRB3*0301) interacts with the T cell receptor (TCR) (Vα1, Vβ17) in complex with an unknown peptide (Lu et al., 2003). We found several peptide mimotopes that, when bound to DR52c, engaged the TCR and activated ANi2.3 T cells in the absence of Ni2+ (Yin et al., 2012). The mimotope p7 lysine was suggested to mimic Ni2+ in the natural TCR ligand, and MHCII β chain flexibility in the area around the peptide p7 position forms a common site for cation binding in metal allergies.
Fluorescently labeled MHC-peptide tetramers are powerful tools in the analysis of antigen-specific T cell immune responses (Nepom, 2012). The goal of our research was to use the DR52c/Ni2+ mimotope tetramers to study Ni2+ sensitized patients with joint implant failure, and thus provide insight into the Ni2+ sensitization process. The subjects in this study were referred by their orthopedic surgeon for evaluation as to whether sensitization to an implant component had contributed to their joint replacement failure. They were determined to be Ni2+ sensitized by both patch testing and blood lymphocyte proliferation test (LPT), and to have a Ni2+ containing implant (Pacheco et al., 2013). To date, it has been difficult to study how Ni2+ haptens are incorporated into self-peptide and became T cell epitopes for several reasons. First, the binding affinity between TCRs and the peptide-Ni2+ ligands are low (Yin et al., 2012). Second, the metal binding sites are commonly solvent exposed and heavily influenced by the media or buffers. In previous baculovirus DR52c – peptide library screening experiments, we showed that the εNH2 group of lysine mimicked the Ni2+ ions in the engagement of TCRs with stronger and stable binding (Yin et al., 2012). We speculated that Ni2+ mimotopes would circumvent both problems, and could be a valuable tool to study T cells. We expanded Ni2+ specific CD4+ T cells by stimulating PBMCs with Ni2+, and measured the ability of one of our Ni2+ specific mimotopes, complexed with the soluble DR52c conjugated with fluorescein, to bind to these Ni2+ expanded T cells. At the same time, we assessed the change in percentage of V β17+ CD4+ T cells. We found that the DR52c-mimotope tetramer identified in skin Ni2+ allergy is also capable of binding T cells in Ni2+ sensitized patients with joint implant failure, and the percentage of Vβ17+ CD4+ T cells paralleled tetramer staining.
Material and methods
Subject Selection
Subjects were enrolled in an IRB approved metal allergy study after providing written informed consent. We randomly selected the initial 4 subjects in our study on the basis of a positive Ni2+ patch test and/or positive Ni2+ LPT, and a poorly functioning Ni2+ containing implant from a cohort of 1054 enrolled patients referred for evaluation of possible metal allergy. The mean age was 56.75 years (range 37 – 74), and 3/4 subjects were female. Most (67%) had a positive Ni2+ patch test, and all had a positive Ni2+ LPT, with a mean peak stimulation index of 61 (positive threshold=5.7), and a range from 12.2 to 138.3. Patients KR, BS, RB and LJ were post-operative patients with the following Ni2+ containing implants: two right total knee arthroplasties (TKA), one left total hip arthroplasty, and one Essure implant. Patient MH is a pre-operative patient with positive Ni2+ patch test and Ni2+ LPT. Patient KG has both negative Ni2+ patch test and Ni2+ LPT. To compare with previous study on the European allergic contact dermatitis patients (Moulon et al., 1995), only Caucasian subjects were selected.
Cell Lines
The Ni2+ reactive CD4+ T cell transfectoma bearing Vα and Vβ TCR segments of ANi2.3 T cell, and Cα and Cβ segments of a mouse TCR, has been described previously (Vollmer et al., 1999).The EBV transformed B cell line, HO301, expresses DRB3*0301 (DR52c), DRA*01012, DRB1*1302, DQA*101021, DQB1*0604, DPA1*01, and DPB1*1601 (Gorski et al., 1989; Lu et al., 2003). The two cell lines are gifts of Dr. John Kappler (National Jewish Health, Denver, CO).
Monoclonal Antibodies
FK7.3.19.1 is a monoclonal antibody (mAb) specific for DR52 (also cross-reactive to DRB1*0301 and DRB1*0302) (Bontrop et al., 1990). Biotin mouse IgG2b κ was from PharMingen (03042C). Tetramer staining used the following MAbs: Streptavidin-R-Phycoerythrin (PE)/Cy7anti-human CD4 antibody (Biolegend, 300511); Streptavidin-Allophycocyanin (APC)/Cy7 anti-human CD8 antibody (Biolegend, 344713); PE anti-human CD8 antibody (for single staining for PE tetramer, Becton Dickinson, 7317); APC anti-human CD8a antibody (for single staining for APC tetramer, eBioscience, 17-0088-41); PB anti-human CD14 antibody (Caltaglab, MHCD1428); BV421 anti-human CD19 antibody (BD, 562441); PerCP/Cy5.5anti-human/mouse CD44 antibody (eBioscience, 45-0441); BV605 anti-human CD3 antibody (Biolegend, 327321); HLA typing antibody: BV605 anti-human CD3 antibody (Biolegend, 327321); PerCP anti-human CD14 antibody (BD, 340585); BV421 anti-human CD19 antibody (BD, 562441); Others: FITC anti-human TCR Vβ17 antibody (Beckman Coulter, IM1234).
Reagents
Purified human Fc receptor binding inhibitor (eBioscience, 45-0441).PE-SA for detecting biotin-Ab in flow cytometry (Molecular Probes, S-866). Phytohemagglutinin-L (PHA-L) solution (500 χ, eBioscience, 00-4977). Ficoll (Ficoll-paque™ PLUS, GE Healthcare, 17-1440-03). PE (ProZyme, PJRS25) and APC (ProZyme, PJ25S) for making tetramers.
Preparation of soluble DR52c with Ni2+ mimotope peptide and Ni2+ mimotope peptide-DR52c tetramers
The DR52c α and β chains were cloned into a two-promoter baculovirus transfer vector described previously (Dai et al., 2008). Briefly, the DNA encoding a DR52c-binding Ni2+ mimotope peptide followed by a linker (GPSKVATLVPRGSGGGGS) was inserted into the frame between the signal peptide and the N terminus of the DR52c β chain, such that after expression and signal peptide cleavage, the peptide would occupy the peptide-binding groove of DR52c (Kozono et al., 1994). Three mimotope peptides were chosen to make tetramers: QHIRCNIPKRI (pHIR), QWIRVNIPKRI (pWIR) and QHISINLPKRI (pHIS). The final construction was sequenced and then transfected SF9 insect cells using standard homologous recombination and Baculogold (PharMingen) as the recipient baculovirus DNA. Then the virus was expanded to provide a high-titer stock for large-scale production of DR52c-mimotope peptides.
To prepare protein for the tetramers, 4 liters of Hi-5 insect cells (≈5×105/ml) were infected at a multiplicity of infection of ≈5. After 5 days, the culture supernatant was collected by centrifugation and passed through a 0.2-μm filter. The soluble DR52c-mimotope peptides were isolated from the supernatant by anti-DRα-specific mAb LB3.1 affinity column (American Type Culture Collection). The eluate from the column was concentrated and further purified in a Superdex-200 size-exclusion chromatograph. The homogeneous peak corresponding to a molecular mass of ≈60KD was collected. The proteins were biotinylated with BirA (Avidity, Denver) and incorporated into saturated complexes with PE or APC as described previously (Crawford et al., 1998).
PBMC preparation and cryopreservation
Blood was separated on a Ficoll Hypaque gradient, according to standard methods. In brief, the blood was diluted 1: 1 with PBS (no more than 40 ml) and slowly added onto 10 ml of Ficoll in 50 ml conical tube. The tube was spun at 740 g for 20 minutes. The buffy coat layer (PBMC) was carefully transferred into a new tube by pipette, and an equal volume of PBS was added. The tube was centrifuged at 350 g for 10 minutes, and the supernatant was discarded. Ten ml of fresh PBS was added, and 10 μl of sample was used for cell quantification. The tube was again centrifuged at 350 g for 10 minutes. PBMCs weresuspended in 10% DMSO and 90% FBS at a concentration of 5–10 million cells per ml in 1 ml aliquots. The cells were frozen at −80°C for 2–3 days, and then were transferred into liquid nitrogen until used in experiments.
DR52 typing
The B cells from patients’ PBMCs were stained with bio-FK7.3.19.1, followed by binding to PE conjugated Streptavidin. In the flow cytometry experiments, 5 million PBMCs were used for each sample. For single staining samples, 10 thousand PBMCs were used. After PBMCs were thawed, they were washed once with balanced salt solution (BSS). PBMCs were stained with BV605 anti-human CD3 antibody, PerCP anti-human CD14 antibody, BV421 anti-human CD19 antibody, and bio-FK7.3.19.1 or bio-mouse IgG2b at 4°C for 20–30 minutes. Cells were then washed three times with BSS. The cells were stained with PE-SA at 4°C for 20–30 minutes, and washed with BSS three times. Cells were fixed by 1% parafomaldehyde for 20 minutes at room temperature, then washed twice and analyzed by flow cytometry (CyAn ADP, Beckman Coulter). The data were analyzed by FlowJo v8.8.7.
PBMC T cell stimulation
10 million thawed PBMCs were washed once with BSS, and then cultured in CTM (complete tumor medium, MEM enriched with essential and nonessential amino acids, glutamine, sodium bicarbonate, 10% fetal bovine serum, and 20 μM 2-mercaptoethanol). For Ni2+ activation, 10−4 M NiSO4 was added to cells and cultured for 7–8 days before staining and analyzed by flow cytometry. During the 7–8 days, the culture medium with Ni2+ was exchanged once. For PHA-L activation, cells were activated by 1xPHA-L for 3 days, followed by another 4–5 days in culture media without PHA-L.
Immunofluorescence analysis for DR52c/Ni2+ mimotope tetramer binding
In the tetramer staining of T transfectoma cells, 1 million cells were stained with tetramers (20 μg/ml) at 37°C for 2 hours. To enhance peptide-MHC tetramer binding, separate samples also included mAb H597 specific to murine Cβ (Kubo et al., 1989) at 2 μg/ml during the incubation period. Then cells were washed three times and re-suspended in FACs buffer (2% FBS/2 mM EDTA/PBS). The cells were analyzed by CyAn ADP analyzer (Beckman Coulter). In the Ni2+ and PHA-L stimulated PBMC groups, the cells were washed with FACs buffer twice. For the control (no activation) group, 10 million cells were thawed and washed once with FACs buffer. Then cells were blocked by PBS with 10% FBS and human Fc block (1:10) at room temperature for 30 minutes. Cells were stained with tetramers (20 μg/ml) at 37°C for 2 hours. Antibodies cocktail were directly added and incubated at 4°C for another 20–30 minutes. Fluorescent stain 4′,6-diamidino-2-phenylindole (DAPI) was added for 5 minutes at room temperature. Cells were washed three times and re-suspended in FACS buffer. The cells were analyzed by Aria Fusionsorter (BD Biosciences). The data were analyzed by FlowJo v8.8.7.
Results
DR52c/Ni2+ mimotope tetramers specifically stained ANi2.3 T cell transfectomas
The Ni2+ reactive DR52c restricted T cell ANi2.3 is a tool for studying Ni2+ hypersensitivity in patients. Previously a series of Ni2+ -independent mimotopes were found in baculovirus DR52c-peptide libraries (Yin et al., 2012). We chose the mimotopes that best stimulated ANi2.3 T cell (pHIR, pWIRand pHIS) to make tetramers. The soluble mimotope-DR52c molecules were expressed and purified. A BirA tag was present in the C terminal of the β chain of DR52c. The soluble mimotope-DR52c molecules could be made into biotinylated version of complexes. Tetramers were prepared by ligating PE-streptavidin or APC-streptavidin to the biotinylated peptide-DR52c molecules.
To test the binding and specificity of the covalent mimotope-DR52c complexes, we used ANi2.3 T cell transfectomas as a positive control. These transfectomas responded to autologous antigen-presenting cells pulsed with Ni2+ (Vollmer et al., 2000; Lu et al., 2003).The ANi2.3 T cells and control cells were incubated with tetramers linked to pHIR, pHIS, pWIR and pTu peptides, respectively. pTu is an irrelevant peptide that served as a negative control (Fig.1). PE-tetramers with pHIR, pHIS and pWIR peptides and APC-tetramer with pHIR peptide could specifically stain the ANi2.3 T cells but not the control cells (Fig.1A,B,C and D). The anti-mouse Cβ mAb, H597, enhanced the tetramer staining (Fig. 1E and F).
Figure 1.
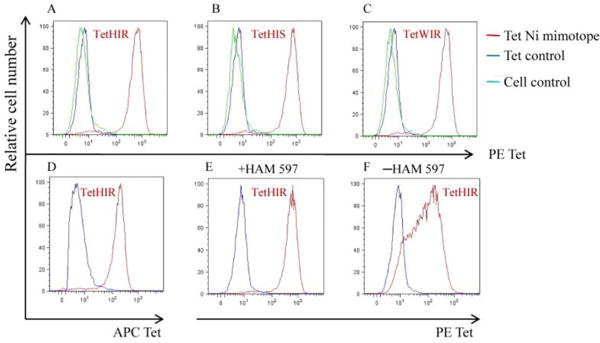
Staining of the Ni specific T transfectoma cell (ANi2.3) with the Ni mimotope tetramers. In A, B and C, ANi2.3 T cells and cell control were separately stained with PE tetramer DR52c-pHIR DR52c-pHIS and DR52c-pWIR; ANi2.3 T cells were also stained with the control tetramer DR52c-pTu. In D, the ANi2.3 T cell and cell control separately were stained with DR52c-pHIR APC tetramer. HAM 597 was also used in A–D. In E and F, ANi2.3 T cells were stained with PE tetramer HIR and control PE tetramer, with or without HAM 597. Tet is tetramer for short.
The DR52 specific mAb FK7.2.19.1 was used to MHC-type the implant failure patients
The ANi2.3 T cell is restricted to DR52c. We wanted to see if DR52c was proportionately represented in our Ni2+ hypersensitive patients. First, we tested the antibody specificity in a DR52c expressed B lymphoblastoid cell line, HO301. We used bio-FK7.3.19.1 to stain the HO301 cells and the control cells, then added PE-streptavidin to detect the biotinylated antibody. The staining was analyzed by flow cytometry. The bio-FK7.3.19.1 could specifically stain the HO301 cells but not the control cells. The isotype control bio-mouse IgG2b κ could not stain the HO301 cells (Fig.2A). This demonstrated that bio-FK7.3.19.1 could specifically stain the DR52 on the surface of HO301 cells.
Figure 2.
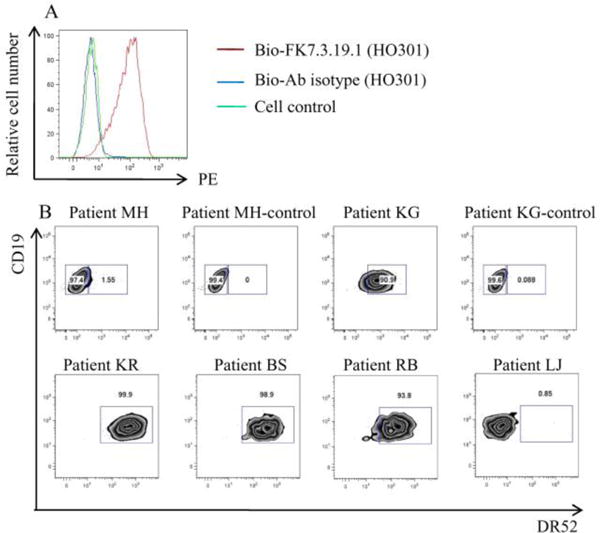
The staining of DR52 specific antibody-FK7.3.19.1 in DR52c positive B lymphoblastoid cells (HO301). In A, HO301 cells were stained with biotinylated FK 7.3.19.1 and biotinylated antibody isotype, cell control was stained with biotinylated FK 7.3.19.1.The detecting reagent is PE-streptavidin. In B, PBMC gated B cells of patient MH and patient KG were stained with biotinylated FK 7.3.19.1 and biotinylated antibody isotype (top). The other patients were stained with biotinylated FK 7.3.19.1 (bottom). Patient KR BS and RB are DR52 positive; Patient LJ is DR52 negative.
We used the same staining strategy to study DR52 alleles in the B cells from 4 subject PBMCs. The PBMCs were first gated on live lymphocytes, then macrophages and CD3+ T cells were excluded. CD19+ B cells were analyzed for DR52 allele staining. Typically, positive DR52 staining was observed in over 90% of B cells. Our isotype control (bio-mouse IgG2b κ) does not stain B cells (Fig. 2B). In the 4 selected patients, patient KR, BS and RB are DR52 positive, and patient LJ is DR52 negative.
The DR52c/Ni2+ mimotope tetramers detected Ni2+ reactive T cells in Ni2+ sensitized implant failure subjects
We used the DR52c-pHIR tetramer to detect Ni2+ reactive T cells in the PBMCs of Ni2+ sensitized patients with implant failure. We stained non-stimulated, and Ni2+ or phytohemagglutinin-L (PHA-L) stimulated PBMCs with tetramers, cell antibody markers, and the live cell marker, DAPI. Incubation with PHA-L served as a nonspecific T cell stimulation control, compared to Ni2+ stimulation. We expected that T cells stained with DR52c-pHIR tetramer should be Vβ17 positive, and we measured TCR Vβ17 usage at the same time. To exclude nonspecific binding in one color staining, we used two colors of the tetramer, PE-tetramer and APC-tetramer, in staining. For specific staining, there should be a diagonal in the double positive region. We found that both staining levels were similar, independent of the conjugated fluoresceins.
During the stimulation process, examination by microscope demonstrated that the cells proliferated well in both Ni2+ and PHA-L stimulation groups. When analyzed by flow cytometry, the percentage of DR52c-pHIR tetramer positive CD4+ T cells increased dramatically after Ni2+ stimulation, compared to control non-stimulated cells (Fig.3A and B). In contrast, the control DR52c-pTu tetramer staining of the CD4+ T cell population was almost undetectable. In the PHA-L stimulated positive controls, the DR52c-pHIR tetramer staining is much lower than that seen with Ni2+ stimulation. PHA-L can induce non-specific proliferation of T cells. CD44 expression is an indicative marker for effector-memory T-cells. As shown in figure 3, in patient KR, the tetramer-positive cells after Ni2+ stimulation were CD44 high CD4+ and CD44 low CD4+ T cells, while the cells after PHA-L stimulation were CD44 low CD4+ T cells (Fig.3B and C). It implies that the DR52c-pHIR positive CD4+ T cells were Ni2+ re-stimulated cells. These results demonstrate that the DR52c-pHIR tetramer can specifically detect Ni2+ reactive T cells (Fig.3A, B and C). The percentage of Vβ17 T cells increased in tandem with tetramer staining after Ni2+ stimulation, which was not observed in the PHA-L stimulated controls (Fig. 3D, E and F).
Figure 3.
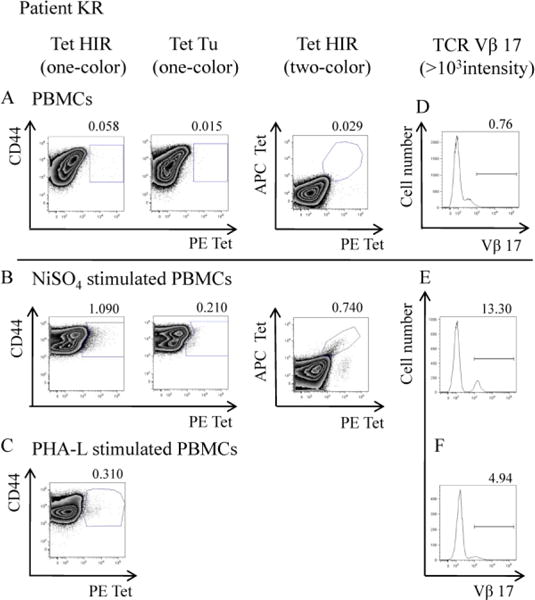
The tetramer DR52c-pHIR staining and TCR Vβ17 staining in CD4+ T cells in PBMC of patient KR. In panel A, B and C, the PBMCs were stained with tetramers and cell markers. The CD4+ T cells were gated; the tetramer staining was analyzed. In panel A, B and C, the analysis of CD4+ T cells stained with PE-tetramer DR52c-pHIR, PE-tetramer DR52c-pTu and two colors of tetramer-DR52c-pHIR (PE-tetramer DR52c-pHIR and APC-tetramer DR52c-pHIR). In panel A, the PBMCs were not stimulated. In panel B, after stimulation of NiSO4 of PBMCs, the analysis of CD4+ T cells. In panel C, after stimulation of PHA-L of PBMCs, the analysis of CD4+ T cells. In panel D, E and F, the PBMCs were stained with cell markers and TCR Vβ17, the percentages of TCR Vβ17+ CD4+ T cells in different samples were analyzed. In panel D, E and F, it is the staining of no stimulation of PBMCs, stimulation of NiSO4 and stimulation of PHA-L of that separately. In each staining, about 1~1.5× 105 PBMCs were analyzed on flow cytometry. Tet is tetramer for short.
We then examined DR52c-pHIR tetramer binding of PBMCs stimulated by Ni2+ from 4 Ni2+ sensitized patients with implant failure (Fig. 4A).The two-color tetramer staining was similar to one-color staining. Patient KR is HLA-DR52 positive, and demonstrated the greatest DR52c-pHIR tetramer staining, accounting for 1.09% in one color staining (Fig. 3B and Fig. 4A). The percentage of TCR Vβ17 staining also increased from <1% up to 13.3% after Ni2+ stimulation for this patient, demonstrating a significantly represented proportion of T cells specific to Ni2+ expressing this beta chain (Fig. 4B). For the other three patients, tetramer staining and Vβ17 percentages remained low (Fig. 4A).
Figure 4.
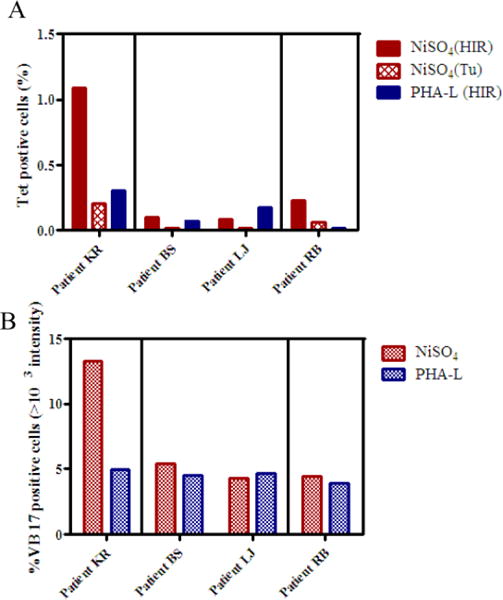
The tetramer staining and TCR Vβ17 staining in CD4+ T cells in four patients. A, Analysis of one color tetramer staining. Tetramer DR52c-pHIR and tetramer DR52c-pTu staining in CD4+ T cells of NiSO4 stimulation of PBMCs and tetramer DR52c-pHIR staining in PHA-L stimulation cultures. B, TCR Vβ17 staining in CD4+ T cells of NiSO4 and PHA-L stimulated PBMCs. There were three groups of staining and analyzing: patient KR; patient BS and LJ; patient RB.
Table 1 summarizes the clinical and research data of the four Ni2+ sensitized patients with implant failure that were evaluated with tetramer staining. WW found there is a tendency of correlation between the Vβ17 ratio (the ratio of the Vβ17+ cell percentages in Ni2+-specific versus PHA-L-activated cultures) and the size of the patch test reaction and the Ni2+ LPT PSI (peak stimulation index) for all patients. Interestingly, the patient (KR) with the highest percent ratio of Vβ17 T cells, highest staining of tetramer also had the largest skin test reaction. In contrast, the one HLA DR52 negative patient also had the lowest Ni2+ LPT PSI, the smallest Ni2+ patch test reaction, the lowest percentage ratio of Vβ17 T cells, and the lowest tetramer staining, suggesting a different pathway of Ni2+ sensitization.
Table 1.
Summary of clinical data and research data
| Patient KR | Patient BS | Patient RB | Patient LJ | |
|---|---|---|---|---|
| Size of patch test | 3+ | 2+ | 2+ | 1+ |
| Ni2+ LPT – PSI | 51.2 | 138.3 | 127.6 | 12.2 |
| Vβ17 ratio in CD4 T cellsa | 2.69 | 1.20 | 1.12 | 0.93 |
| DR52 | + | + | + | − |
| one-color Tet-HIR(%)b | 1.09 | 0.11 | 0.23 | 0.09 |
The ratio of the percentages of Vβ17+ cells in Ni-specific versus PHA-L-activated cultures.
Percentages of DR52c-pHIRtetramer staining positive cells in CD4+ T cells.
Discussion
Our initial studies of the process of Ni2+ sensitization were based on a specific Ni2+ reactive human CD4+ T cell, ANi2.3, which was isolated from a Ni2+ allergic contact dermatitis patient. ANi2.3 T cells are restricted to HLA DR52c, and can be activated by Ni2+ mimotope peptides. The structural data showed that ANi2.3 T cells recognize an invariant lysine at the p7 position of mimotopes in the absence of Ni2+ (Yin et al., 2012), though the exact Ni2+ associated self-peptides remain undefined. Little is known about how Ni2+ ions are presented to pathologic T cells present in joint failure due to Ni2+ sensitization. One of our main questions is whether Ni2+ reactive T cells share the same Ni2+ presenting self-peptides in both skin and implant hypersensitivity, indicating that Ni2+ hypersensitivity is either an organ specific or systemic disease. Here, we used tetramers of identified Ni2+ mimotope peptides linked to DR52c to study T cell responses in subjects with implant failure due to Ni2+ hypersensitivity, and compared their results to a Ni2+ specific clone from a patient with contact dermatitis due to Ni2+ hypersensitivity.
We were able to detect similarities between Ni2+ induced contact dermatitis and Ni2+ induced joint failure. Patient KR, who had the strongest Ni2+ patch test reaction, also had the highest percentage of T cell Vβ17 usage. In patient with Ni2+ induced contact dermatitis, the dominance of TCR Vβ17 usage is related to the severity of disease (Vollmer et al., 1997). The other three patients with a small Ni2+ patch test reaction also had a much lower percentage of TCR Vβ17 usage. This may be not surprising, as Ni2+ reactive CD4+ T cells from peripheral blood could have a very broad range of frequency, depending on prior antigen exposure, exposure history, and timing of sensitization.
We used a DR52 specific mAbFK-7.3.19.1 to type the patients. HLADR52 has three sub-alleles: DR52a, DR52b, and DR52c (Dai et al., 2008). In fact, the three DR52 alleles are closely related, as they derive from a common precursor gene through a gene conversion or other type of recombination event (Gorski and Mach, 1986; Gorski et al., 1989). Their sequence data suggest that DR52c was derived from a recombination between DR52a and DR52b (Gorski et al., 1989). It is possible that our DR52c-pHIR tetramer could also cross-react with Ni2+ specific T cells from DR52a or DR52b positive patients. The similarity of certain peptide-binding pockets between DR52c, DR52b, and DR52a, may explain why certain autoimmune diseases are associated with two of the three alleles; depending on the relative use of particular binding sites, a given peptide might bind to more than one of these DR52 allelic forms (Dai et al., 2008).
In our small sample of four patients with implant failure due to Ni2+ sensitization, the combined DR52 frequency is 75% (Fig 2B). This is similar to the frequency of HLA DR52 in a study of Ni2+ allergy in contact dermatitis, where the combined DR52 frequency was 66.7% (Vollmer et al., 1997). Interestingly, it is much higher than the frequency of HLA DR52 in the general Caucasian population, where it is 38.7% (www.allelefrequencies.net)
In another example of the immune specificity of metal sensitization, Beryllium (Be2+) hypersensitivity are linked to a Glu 69 present inDP2 beta chain (Dai et al., 2010). We showed that the Be2+ ion was buried in the acidic pocket between MHC and self-peptides (Clayton et al., 2014), which limits the alleles of Be2+ presenting MHC molecules. In contrast, Ni2+ presentation is far more promiscuous, since Ni2+ ions are coordinated on top of MHC molecules and self-peptides (Yin et al., 2012). As long as the amino acids on the face of MHC and self-peptides satisfy the Ni2+ coordination chemistry, quite diverse MHCs and peptides could present Ni2+ to T cells. Vollmeret al. showed that DR52c was not the only MHC restriction element for Ni2+ reactive CD4+ T cells (Vollmer et al., 1999).
In summary, we have demonstrated that the DR52c-pHIR tetramer specific to one Ni2+ clone was able to also detect Ni2+ specific CD4+ T cells in other sensitized patients. These tetramers can provide a powerful tool to study T cells from Ni2+ allergic patients, and could potentially develop into a clinically useful biomarker. Using this patient population, we plan to study the exact peptides associated with Ni2+ reactive TCRs by isolating tetramer-positive CD4+ T cells, and determining TCR usage using single cell sequencing.
Highlight.
Nickel hypersensitive patients with implant failure are dominantly DR52 positive.
The nickel hypersensitive patients with implant failure may have similar TCR beta chain usage of CD4+ T cells to that of contact dermatitis.
The nickel independent mimotope tetramers are a useful tool to identify the nickel reactive CD4+ T cells.
Acknowledgments
We thank the Kappler/Marrack lab for their generous help and sharing of reagents, and Shirley Sobus of the Flow Cytometry Facility at National Jewish Health. This work was supported by a grant from NIEHS R01-ES025797. Y.W. was supported by NIH grant F32-AI074491. K.A. was supported by NIH grant T32-AR007534.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare there is no conflict of interest.
References
- Bontrop RE, Elferink DG, Otting N, Jonker M, de Vries RR. Major histocompatibility complex class II-restricted antigen presentation across a species barrier: conservation of restriction determinants in evolution. J Exp Med. 1990;172:53–59. doi: 10.1084/jem.172.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowerman NA, Falta MT, Mack DG, Wehrmann F, Crawford F, Mroz MM, Maier LA, Kappler JW, Fontenot AP. Identification of multiple public TCR repertoires in chronic beryllium disease. J Immunol. 2014;192:4571–4580. doi: 10.4049/jimmunol.1400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton GM, Wang Y, Crawford F, Novikov A, Wimberly BT, Kieft JS, Falta MT, Bowerman NA, Marrack P, Fontenot AP, Dai S, Kappler JW. Structural basis of chronic beryllium disease: linking allergic hypersensitivity and autoimmunity. Cell. 2014;158:132–142. doi: 10.1016/j.cell.2014.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- Dai S, Crawford F, Marrack P, Kappler JW. The structure of HLA-DR52c: comparison to other HLA-DRB3 alleles. Proc Natl Acad Sci U S A. 2008;105:11893–11897. doi: 10.1073/pnas.0805810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Murphy GA, Crawford F, Mack DG, Falta MT, Marrack P, Kappler JW, Fontenot AP. Crystal structure of HLA-DP2 and implications for chronic beryllium disease. Proc Natl Acad Sci U S A. 2010;107:7425–7430. doi: 10.1073/pnas.1001772107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emtestam L, Carlsson B, Marcusson JA, Wallin J, Moller E. Specificity of HLA restricting elements for human nickel reactive T cell clones. Tissue Antigens. 1989;33:531–541. doi: 10.1111/j.1399-0039.1989.tb01704.x. [DOI] [PubMed] [Google Scholar]
- Gorski J, Irle C, Mickelson EM, Sheehy MJ, Termijtelen A, Ucla C, Mach B. Correlation of structure with T cell responses of the three members of the HLA-DRw52 allelic series. J Exp Med. 1989;170:1027–1032. doi: 10.1084/jem.170.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J, Mach B. Polymorphism of human Ia antigens: gene conversion between two DR beta loci results in a new HLA-D/DR specificity. Nature. 1986;322:67–70. doi: 10.1038/322067a0. [DOI] [PubMed] [Google Scholar]
- Kapsenberg ML, Res P, Bos JD, Schootemijer A, Teunissen MB, Van Schooten W. Nickel-specific T lymphocyte clones derived from allergic nickel-contact dermatitis lesions in man: heterogeneity based on requirement of dendritic antigen-presenting cell subsets. Eur J Immunol. 1987;17:861–865. doi: 10.1002/eji.1830170620. [DOI] [PubMed] [Google Scholar]
- Kozono H, White J, Clements J, Marrack P, Kappler J. Production of soluble MHC class II proteins with covalently bound single peptides. Nature. 1994;369:151–154. doi: 10.1038/369151a0. [DOI] [PubMed] [Google Scholar]
- Kubo RT, Born W, Kappler JW, Marrack P, Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989;142:2736–2742. [PubMed] [Google Scholar]
- Lu L, Vollmer J, Moulon C, Weltzien HU, Marrack P, Kappler J. Components of the ligand for a Ni++ reactive human T cell clone. J Exp Med. 2003;197:567–574. doi: 10.1084/jem.20021762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molins H, Michelet L, Lanquar V, Agorio A, Giraudat J, Roach T, Krieger-Liszkay A, Thomine S. Mutants impaired in vacuolar metal mobilization identify chloroplasts as a target for cadmium hypersensitivity in Arabidopsis thaliana. Plant, Cell & Environment. 2013;36:804–817. doi: 10.1111/pce.12016. [DOI] [PubMed] [Google Scholar]
- Moulon C, Vollmer J, Weltzien HU. Characterization of processing requirements and metal cross-reactivities in T cell clones from patients with allergic contact dermatitis to nickel. Eur J Immunol. 1995;25:3308–3315. doi: 10.1002/eji.1830251216. [DOI] [PubMed] [Google Scholar]
- Nepom GT. MHC class II tetramers. J Immunol. 2012;188:2477–2482. doi: 10.4049/jimmunol.1102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco K, Barker L, Maier L, Erb S, Sills M, Knight V. Development of a validated blood test for nickel sensitization. J Allergy Clin Immunol. 2013;132:767–769. doi: 10.1016/j.jaci.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Pacheco K, Mayer A, Erb S, Shirname-More L, Maier LA. High Rates Of Sensitization To Selected Metals and Bone Cement In Joint Replacement Failure Patients and Preoperative Evaluations. J All Clin Immunol. 2014;133:AB150. [Google Scholar]
- Pacheco KA. Allergy to Surgical Implants. J Allergy Clin Immunol Pract. 2015;3:683–695. doi: 10.1016/j.jaip.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Silvennoinen-Kassinen S, Poikonen K, Ikaheimo I. Characterization of nickel-specific T cell clones. Scand J Immunol. 1991;33:429–434. doi: 10.1111/j.1365-3083.1991.tb01791.x. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F, Scheidegger D, Garotta G, Scheper R, Pletscher M, Lanzavecchia A. Isolation and characterization of Ni-specific T cell clones from patients with Ni-contact dermatitis. J Immunol. 1985;135:3929–3932. [PubMed] [Google Scholar]
- Uchtenhagen H, Rims C, Blahnik G, Chow IT, Kwok WW, Buckner JH, James EA. Efficient ex vivo analysis of CD4+ T-cell responses using combinatorial HLA class II tetramer staining. Nature Communications. 2016;7:12614. doi: 10.1038/ncomms12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer J, Fritz M, Dormoy A, Weltzien HU, Moulon C. Dominance of the BV17 element in nickel-specific human T cell receptors relates to severity of contact sensitivity. Eur J Immunol. 1997;27:1865–1874. doi: 10.1002/eji.1830270808. [DOI] [PubMed] [Google Scholar]
- Vollmer J, Weltzien HU, Dormoy A, Pistoor F, Moulon C. Functional expression and analysis of a human HLA-DQ restricted, nickel-reactive T cell receptor in mouse hybridoma cells. J Invest Dermatol. 1999;113:175–181. doi: 10.1046/j.1523-1747.1999.00646.x. [DOI] [PubMed] [Google Scholar]
- Vollmer J, Weltzien HU, Gamerdinger K, Lang S, Choleva Y, Moulon C. Antigen contacts by Ni-reactive TCR: typical alphass chain cooperation versus alpha chain-dominated specificity. Int Immunol. 2000;12:1723–1731. doi: 10.1093/intimm/12.12.1723. [DOI] [PubMed] [Google Scholar]
- Yin L, Crawford F, Marrack P, Kappler JW, Dai S. T-cell receptor (TCR) interaction with peptides that mimic nickel offers insight into nickel contact allergy. Proc Natl Acad Sci U S A. 2012;109:18517–18522. doi: 10.1073/pnas.1215928109. [DOI] [PMC free article] [PubMed] [Google Scholar]


