SUMMARY
Aim
Telemonitoring (TM) is a safe and efficient monitoring system for internal cardioverter defibrillator device (ICD) recipients. TM has been used to track info on the clinical status of heart failure patients treated by ICD and/or cardiac resynchronisation therapy defibrillator (CRT-D). The aim of this study was to investigate the impact of TM on clinical outcomes in a population of CRT-D patients with heart failure.
Methods
In a multicentre, randomised study, patients with chronic heart failure, New York Heart Association (NYHA) functional class II or III, left bundle branch block, severe left ventricle ejection fraction reduction (LVEF < 35%) have been identified and screened.
Results
One hundred and ninety-one patients have been randomised to receive either a CRT-D with TM or a CRT-D with traditional ambulatory monitoring (control group) and completed the 12-month study follow-up. Primary endpoints were all cause death, cardiac death and hospital admission for heart failure. Secondary endpoints were atrial fibrillation, sustained episodes, non-sustained and self terminated ventricular tachyarrhythmia, sustained ventricular tachycardia, and ventricular fibrillation, ICD shocks and percentage of CRT-D responder patients. Univariate analysis identified the following factors predicting hospitalisation: TM, age, chronic kidney disease, hypercholesterolaemia, LVEF and NYHA class. At multivariate analysis, TM was the only factor predicting heart failure hospitalisation (hazard ratio 0.6, 0.42–0.79, 95% CI, p = 0.002), without affecting overall mortality and cardiac deaths events.
Conclusions
Taken together, our data indicate the importance of TM in predicting heart failure hospitalisation in patients treated with CRT-D.
Introduction
Since its introduction in the clinical scenario, telemonitoring (TM) has rapidly become a diffused system to monitor patient’s clinical course and device function (1–6). Large clinical trials have studied the TM impact on clinical or device-related events, medical care and resource consumption and follow-up visits costs (1,7–12). Previous trials have investigated the TM impact in a population of heart failure patients treated by ICD, and/or cardiac resynchronisation therapy defibrillator (CRT-D). In heart failure patients, CRT-D is the choice treatment to improve symptoms, quality of life, NYHA class and clinical outcomes, and to prevent heart failure events and progression (13–15). CRT-D induces a reverse cardiac remodelling in a percentage of 65% of treated patients, the so-called ‘CRT-D responders’ (13,16,17). On the other hand, CRT-D non-responders display poor outcomes, mainly related to a progressive ventricular dysfunction with an increased risk of worse clinical outcomes (16,17).
In this scenario, TM, which has just been successfully utilised in heart failure patients treated by ICD and/or CRT-D, may represent a helpful tool to improve clinical outcomes and CRT-D response. To our knowledge there are no clinical studies focused on TM impact in a homogenous population of heart failure patients (chronic heart failure patients with left bundle branch block, and left ventricle ejection fraction reduction (LVEF) < 35%, in NYHA class II/III) treated by CRT-D. Herein, we have investigated in a randomised, multicentre, prospective study conducted on a population of heart failure patients treated by CRT-D, the impact of CRT on primary and secondary clinical study endpoints.
Methods
In a randomised, multicentre study conducted in different Italian centres (Catholic University of Sacred Heart, Campobasso; ‘John Paul II’ Research and Care Foundation, Campobasso; Second University of Naples, Naples) between September 2010 and September 2014 (follow-up has been closed in June 2015), we have enrolled patients with standard indications (18) for a CRT-D implant, enabled with [Biotronik (Berlin, Germany) CRT-D models, Lumax 640HF, Iforia 3HF, Iforia 5HF] or without TM technology [Inogen CRT-D, Incepta CRT-D; Boston Scientific (Marlborough, MA, USA), Unify Assura CRT-D; St Jude Medical (St. Paul, MN, USA), Brava CRT-D; Medtronic (Minneapolis, MN, USA)]. Informed consent was obtained from all participants included in the study. All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration. Enrolled patients had chronic heart failure lasting for at least 3 months, New York Heart Association (NYHA) functional class II or III, left bundle branch block, severe left ventricle ejection fraction reduction (LVEF < 35%) and an indication for CRT-D treatment according to the American College of Cardiology/American Heart Association guidelines (18). Exclusion criteria were as follows: age < 18 or > 75 years, ejection fraction > 35%, previous internal ICD, CRT-D and/or pacemaker implant, prior cardiac surgery, absence of informed written consent and any condition that would make survival for 1 year unlikely. All patients were informed of the nature of the study and provided written consent. Screened patients to receive a CRT-D were defined according to the American College of Cardiology/American Heart Association guidelines for the management of patients with heart failure refractory to a maximal medical therapy (18). After screening phase, 196 patients have been enrolled in the study (these patients met criteria reported above). This population of patients receiving CRT-D has been randomly divided in TM group and traditional monitoring (control) group. Baseline parameters have been determined before interventions and the follow-up has been concluded 12 months after CRT-D implant. Responders to CRT-D treatment have been defined as previously described (16).
Interventions
All patients identified to be treated with CRT-D (heart failure, NYHA class 2–3, LVEF < 35% and left bundle branch block) were randomly assigned (1 : 1) to receive TM (TM group) in addition to standard care (traditional ambulatory clinical and instrumental assessment), or to standard care without TM (control group) for 12 months. The random allocation sequence with variable and randomised block size (sizes four) was computer-generated and concealed from the sites. A small portable patient device receives the data and relays them automatically over mobile phone links to the Home Monitoring Service Center. Data were processed automatically and posted on a server. In the TM group, transmitted data were reviewed by independent investigators according to their clinical routine. In parallel, transmitted data were reviewed by a central monitoring unit composed of trained study nurses and supporting physicians, located at the Giovanni Paolo II Research and Care Foundation, Campobasso (Italy). The role of this unit was to ensure the awareness of investigational sites to predefined medical events including ventricular and atrial tachyarrhythmia episodes, low percentage of biventricular pacing, increase in the frequency of ventricular extrasystoles, decreased patient activity and abnormal intracardiac electrogram, as described in previous reports (19). On working days, the central monitoring unit redundantly forwarded these events and standard technical safety notifications issued by the TM system to investigational sites. The investigational site had to con-firm receipt of the reports within 48 h. A clinical response to TM observations was done at the discretion of investigators. When contacting patients on the basis of TM data, the investigators did a standard (prespecified in the protocol) telephone interview to establish whether the patient’s overall condition or dyspnoea had worsened, whether the patient was regularly taking prescribed drugs, and whether the patient’s weight had increased by more than 2 kg over the preceding 3 days, followed in any case by a clinical examination. The investigators reported the additional clinical follow-up and whether a visit to the family doctor was recommended. In the control group, no study participant had access to TM data, but followed regularly in outpatient clinic ambulatory follow-up visits. All patients were treated according to international guidelines (18).
Surgical procedure
The left ventricle lead was inserted transvenously via the subclavian route. A coronary sinus venogram was obtained using a balloon catheter, and the left ventricle pacing lead was inserted through the coronary sinus with the help of an 8-F or 9-F guiding catheter and positioned as far as possible in the venous system, preferably in the lateral or posterolateral vein. The atrial and right ventricular leads were placed in the right atrial appendage and the right ventricular apex, respectively. All leads were connected to a dual-chamber biventricular implantable cardiac device, with defibrillator function (CRT-D). The atrioventricular interval was optimised by Ritter’s method with transthoracic echocardiography, as previously described (16).
Patients monitoring
Patients were scheduled for in office follow-up visits 10 days after clinical discharge and after 1, 3, 6 and 12 months by the treating physician (TM and control group), and every patient was under continuous, automatic remote monitoring during the entire study (TM group). The frequency of TM data analysis and the response to TM alerts was left to the investigator’s discretion.
The study has been conducted in accordance with the Declaration of Helsinki. The protocol has been approved by the Ethics Committees of participating Institutions. All the patients gave their written informed consent to participate in the trial.
Data collection and use
All data have been collected at admission visits, follow-up visits and clinical database and during TM (TM group) and clinical examination (control and TM group) follow-up. Clinical evaluation included physical examination, vital signs, review of adverse events, fasting venous blood withdrawal (at least 12 h from last meal) have been performed for glycaemia and lipid profile at every visit. Follow-up visits have been scheduled 10 days after hospital discharge and at 1, 3, 6 and 12 months by the treating physician (the 12th month visit was conducted at the end of follow-up). At each clinical follow-up, NYHA classification was re-assessed and patients graded their overall condition as unchanged or slightly, moderately, or markedly worsened, or improved since randomisation by global self-assessment. All patients have been instructed to regularly assess body weight, occurrence of dyspnoea and any clinical symptom. At each visit, patients have been asked whether medical events or symptoms suggestive of cardiac arrhythmias occurred; moreover, both ECG and ECG Holter-monitoring have been performed to detect the presence of asymptomatic arrhythmias (16).
Endpoints
Primary endpoints
The primary endpoints were all cause death, cardiac death and hospital admission for heart failure (hospitalisation for objective worsening evidence of change in clinical status, NYHA functional class changing, patient’s symptoms and quality of life, or moderately to markedly worse self-reported overall condition compared with at randomisation). Heart failure worsening has been also reported as unplanned overnight admission to hospital, worse NYHA functional class, or had moderately to markedly worse self-reported overall condition compared with at randomisation, as described in previous studies (7,8,20).
Secondary endpoints
Atrial fibrillation (AF) sustained episodes, non-sustained and self terminated ventricular tachyarrhythmia (VTt), sustained VT and ventricular fibrillation (VF), ICD shocks, percentage of CRT-D responder patients. The determination of endpoints was adjudicated by an independent clinical committee, according to criteria prespecified in the protocol.
Statistical analyses
Continuous data, non-normally distributed, has been compared with the Mann–Whitney–Wilcoxon rank sum test. We compared categorical data, including the primary endpoint with the exact Pearson’s χ2 test. Cox regression models were used to calculate hazard ratios. We considered a two-sided p value of less than 0.05 as statistically significant. Sample size was calculated using a power of 80% and confidence of 95%. The analysis was performed by using SPSS version 21 (Chicago, IL, USA).
Results
We included in the study 196 eligible patients; 191 received a CRT – subdivided in TM treatment and traditional CRT-D ambulatory monitoring (Figure 1); 183 patients terminated the study follow-up (94 patients in control group, no TM, and 89 in TM group).
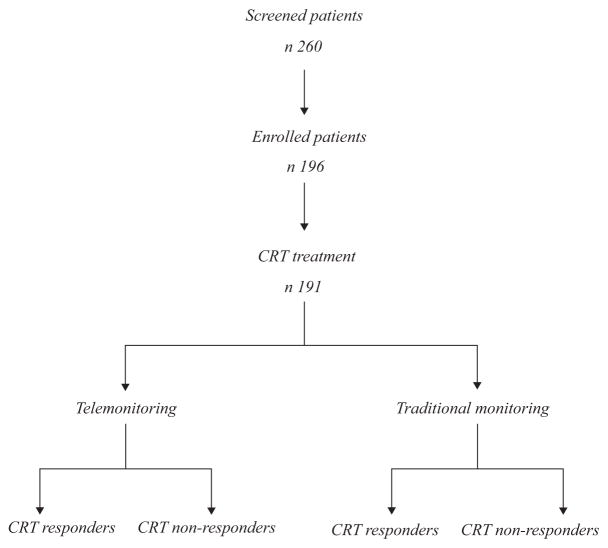
Figure 1.
Schematic flowchart of the study. Two hundred and sixty patients have been screened because they presented chronic heart failure in New York Heart Association (NYHA) class 2,3, left bundle branch block, left ventricle ejection fraction (LVEF) < 35%. After this screening phase, 196 patients have been enrolled in the study (these patients met criteria reported in Methods). Of these patients four have refused to participate in the study and one has refused to be treated by cardiac resynchronisation therapy (CRT). After this phase, 191 patients have received a CRT, randomly divided to receive telemonitoring (TM) and traditional monitoring. 183 patients completed the follow-up, 89 in TM group and 94 in control group (four patients lost at follow-up in no TM group, two discontinued the study in TM group, two patients referred to other centres for follow-up visits in control group)
Mean population age was 72.2 ± 7.2. The clinical characteristics at enrolment were similar and balanced between two groups, as shown in detail in Table 1. The study population was represented by chronic heart failure patients, in maximal pharmacological treatment, receiving CRT-D. No significant difference was observed when comparing pharmacological treatment between the two groups (Table 2). At 1-year follow-up primary and secondary study endpoints have been examined, comparing TM to control group (Table 3). We evaluated all different parameters revealed by TM or by traditional visits, to differentiate CRT-D responders from non-responders and to study the primary and the secondary study endpoints. The patients have been then divided in CRT-D responders and CRT-D non-responders, as indicated by clinical characteristics and response during follow-up to the CRT-D using criteria previously described (16). At 1-year follow-up 26 patients were in persistent AF (7 patients in TM group vs. 19 patients in control group, p = 0.048). There was a significant difference in hospitalisation events (15.7 vs. 28.7, p = 0.02) comparing TM patients to control group. There was no significant difference when considering all cause mortality (7.9 vs. 8.5, p = 0.54) or cardiac death events (3.4 vs. 5.3, p = 0.39), comparing TM to non-TM patients. We also detected no significant differences when examining responder percentage, stroke events and number of sustained VT/VF episodes or ICD shocks events. Notably, at 1-year follow-up seven patients in TM group vs. 17 patients in control group reported ventricular non-sustained tachiarrhytmias events (VTt) (p = 0.04). When considering secondary endpoints, we did not observe a significant difference in ICD shock events (event numbers 10 vs. 16, p = 0.208), CRT-D responder patients percentage [n = 60 (67.4%) vs. 59 (62.8%), p = 0.31], stroke events (n = 3 vs. 4, p = 0.549) and VT/VF events (n = 18 vs. 23, p = 0.35) comparing TM to non-TM patients. A significant difference has been found when examining sustained AF episodes (events 7 vs. 19, p = 0.048) and non-sustained VT episodes (VTt episodes 7 vs. 17, p value 0.04), comparing TM to non-TM patients.
Table 1.
Baseline parameters of the study population
| Total | TM | non-TM | p | |
|---|---|---|---|---|
| Number of patients | 183 | 89 | 94 | |
| Age | 72.2 ± 7.2 | 71.8 ± 8.5 | 72.6 ± 5.7 | 0.43 |
| Male gender, n (%) | 139 (75.9) | 64 (71.9) | 75 (79.8) | 0.23 |
| Hypertension, n (%) | 109 (59.5) | 52 (58.4) | 57 (60.6) | 0.46 |
| Hypercholesterolaemia, n (%) | 106 (57.9) | 61 (68.5) | 45 (47.9) | 0.07 |
| Diabetes, n (%) | 110 (60.1) | 53 (59.6) | 57 (60.6) | 0.51 |
| Glucose (mg/dl) | 136.4 ± 44.8 | 141.1 ± 48 | 132.6 ± 41 | 0.17 |
| Creatinine | 1.23 ± 0.48 | 1.23 ± 0.48 | 1.22 ± 0.36 | 0.46 |
| NYHA (II/III) | 83/100 | 37/52 | 46/48 | 0.19 |
Data are expressed as mean ± standard deviation. HF, heart failure; NYHA, New York Heart Association; TM, telemonitoring.
Table 2.
Pharmacological treatment of the study population
| Drugs | Total | TM | non-TM | p |
|---|---|---|---|---|
| Ivabradin | 51 (27.9) | 27 (30.3) | 24 (25.5) | 0.28 |
| Carvedilol | 71 (38.8) | 32 (36.0) | 39 (41.5) | 0.27 |
| Bisoprolol | 102 (55.7) | 54 (52.9) | 48 (47.1) | 0.057 |
| Furosemide | 162 (88.50 | 82 (92.1) | 80 (85.1) | 0.10 |
| Ace Inhibitors | 73 (39.9) | 32 (36.0) | 41 (43.6) | 0.18 |
| Sartans | 73 (39.9) | 39 (43.8) | 34 (36.2) | 0.18 |
| Digitalis | 51 (27.9) | 26 (29.2) | 25 (26.6) | 0.41 |
| Statins | 101 (55.2) | 58 (57.4) | 43 (42.57) | 0.05 |
| Fibrates | 22 (12.0) | 14 (15.7) | 8 (8.5) | 0.10 |
| Oral hypocglycaemic drugs | 61 (33.3) | 34 (38.2) | 27 (28.7) | 0.11 |
| Insulin | 51 (27.9) | 30 (31.9) | 21 (23.6) | 0.13 |
| Amiodarone | 37 (20.9) | 22 (24.2) | 15 (17.4) | 0.18 |
Data are expressed as mean ± standard deviation. TM, telemonitoring.
Table 3.
Relevant clinical events in telemonitoring (TM) and non-telemonitoring (non-TM) groups
| TM | non-TM | p | |
|---|---|---|---|
| AF | 7 | 19 | 0.048* |
| All cause mortality | 7 (7.9) | 8 (8.5) | 0.54 |
| Cardiac death | 3 (3.4) | 5 (5.3) | 0.39 |
| Heart failure Hospitalisation | 14 (15.7) | 27 (28.7) | 0.02* |
| ICD shocks | 10 | 16 | 0.208 |
| Responders | 60 (67.4) | 59 (62.8) | 0.31 |
| Stroke | 3 | 4 | 0.549 |
| VTt | 7 | 17 | 0.04* |
| VT/VF | 6 | 11 | 0.35 |
Data are expressed as mean ± standard deviation. AF, atrial fibrillation; VTt, total ventricular tachycardia events; VT/VF, sustained ventricular tachycardia and ventricular fibrillation episodes; ICD, internal cardioverter defibrillator.
p < 0.05.
Then, we evaluated the relative benefits of TM in CRT-D responders and non-responders by univariate analysis of factors predicting heart failure hospitalisation (Table 4). Strikingly, at multivariate analysis of factors predicting heart failure hospitalisation (Table 5), TM is the only factor predicting heart failure hospitalisation [hazard ratio (HR) 0.6, 0.42–0.79, 95% CI, p value 0.002].
Table 4.
Univariate analysis of factors predicting hospitalisation
| HR | p | |
|---|---|---|
| Age (year) | 1.11 | 0.007* |
| Chronic kidney disease (+ vs −) | 1.24 | 0.011* |
| Diabetes (+ vs −) | 1.39 | 0.312 |
| Hypercholesterolaemia (+ vs −) | 0.76 | 0.014* |
| Hypertension (+ vs −) | 1.21 | 0.106 |
| LV ejection fraction (%) | 0.96 | 0.048* |
| Male vs female | 1.11 | 0.113 |
| NYHA class (class) | 0.79 | 0.016* |
| TM vs non-TM | 0.59 | 0.002* |
HR was calculated via Coz regression models (fixed covariates: baseline predictors). Data are expressed as mean ± standard deviation. NYHA, New York Heart Association; LV, left ventricle; TM, telemonitoring. HR, hazard ratio.
p < 0.05.
Table 5.
Multivariate analysis of factors predicting hospitalisation
| HR | 95% CI | p | |
|---|---|---|---|
| TM vs non-TM | 0.60 | 0.42–0.79 | 0.002* |
| Age (year) | 1.16 | 0.81–1.13 | 0.052 |
| Hypercholesterolaemia (+ vs −) | 0.78 | 0.57–1.04 | 0.258 |
| Chronic kidney disease (+ vs −) | 1.13 | 0.85–1.37 | 0.066 |
| NYHA class (class) | 0.74 | 0.49–1.12 | 0.058 |
| LV ejection fraction (%) | 0.97 | 0.89–0.98 | 0.083 |
After testing for collinearity, variables with p < 0.10 on univariate analysis were included in the multivariate analysis. Data are expressed as mean ± standard deviation. NYHA, New York Heart Association; LV, left ventricle; TM, telemonitoring; HR, hazard ratio. CI, confidence interval.
p < 0.05.
Discussion
To our knowledge, this is the first study reporting TM as a powerful diagnostic tool that can independently predict heart failure hospitalisation in patients treated with CRT-D. In the MADIT-CRT trial, randomisation to CRT-D was associated with a significant reduction in heart failure or death among patients treated with CRT-D as compared with patients treated with ICD (13). CRT-D has been also shown to improve symptoms, quality of life and NYHA class in responders (13,16,18). Notably, we did not observe a significant difference in all cause mortality comparing TM patients to control group, in contrast with the IN-TIME study, where a lower mortality in the TM group than in the control group had been reported. Such a finding may be related to the study population characteristics. A possible explanation is that in the IN-TIME study there was a mixed population including patients treated by ICD and/or CRT-D: specifically, CRT-D recipients were 58.7% and there were different percentages of CRT-D responses between TM and non-TM groups (8). We studied a homogenous population (heart failure patients, NYHA class 2–3, LVEF < 35% and left bundle branch block) of CRT-D recipients with overall similar clinical characteristics. Heart failure disease progression and ventricular dyssynchrony might differently affect the prognosis as compared with overall population and ICD recipients without left ventricle dyssynchrony. These data have been confirmed by major trials, including the Mode Selection Trial (MOST) and the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial (21,22). These studies have determined that right ventricular pacing is detrimental in terms of heart failure symptoms, and the current practice is to avoid unnecessary ventricular pacing in ICD recipients (21,22). Disease progression, comorbidities and loss of response to CRT-D may all affect prognosis (21–25), and such parameters may be not influenced by TM. In our study population, having similar percentages of CRT-D response (TM vs. non-TM), we found comparable mortality events rates. Moreover, we did not observe a significant difference in cardiac death (3.4% vs. 5.3%, p: 0.39) comparing TM to non-TM patients. Thus, from our analysis, TM does not seem to affect cardiac mortality in heart failure patients treated by CRT-D.
Heart failure patients treated with CRT-D (and particularly CRT-D non-responders) may have a worse outcome that is more related to disease progression and ventricular arrhythmias than to the monitoring technique used. These data are confirmed in large clinical trials as the COMPANION trial, where CRT-D recipients have a 1-year survival of ~88% (25), and the MADIT-CRT trial, reporting 1-year survival at 80% (13). We can speculate that heart failure disease progression is related to numerous different factors that may not be influenced by TM. In fact, one relevant aspect of cardiac death in CRT-D recipients may be related to VT and/or VF episodes (26). In this sense, disease progression, occurrence of VT/VF episodes and ICD shock therapy are not attributable to the monitoring system utilised, but more to patient risk factors, clinical characteristics and disease stage. Since in our study we did not detect significant differences when considering VT/FV and shock episodes, we expect to have similar cardiac deaths rates comparing the two groups. Again in contrast with our current results, in the IN-TIME study (19), with a 1-year cardiovascular mortality of ~2.7% in the TM group vs. 6.8% in the control group (log-rank p = 0.012; HR 0.37, 95% CI 0.16–0.83), the Authors concluded that TM may reduce the percentage of cardiovascular mortality as compared with non-TM patients, treated by ICD/CRT-D (19). This discrepancy might be because of the differences in CRT-D non-responders percentage between TM and non-TM groups, which may impact heart failure progression (26). In our population we have similar CRT-D response percentages comparing TM vs. non-TM, and TM does not ameliorate the CRT-D response percentage.
A significant difference between TM and non-TM patients (15.7% vs. 28.7%) has been observed when examining the hospital admission for heart failure disease progression (objective worsening evidence of change in clinical status, NYHA functional class changing, patient’s symptoms and quality of life, or moderately to markedly worse self-reported overall condition) (16). Thus, TM does not reduce overall cause mortality and cardiac death, in CRT-D recipients but may change the clinical course of disease progression. Continuous monitoring and data collection, interpretation and alarm settings may help the clinicians in immediate therapy management and adjustments to have the better CRT-D response. We could consider to use TM to achieve a better clinical control of CRT-D recipients exploiting common monitoring mechanisms, including low percentage of biventricular pacing, early detection of the onset or progression of arrhythmias, number of device interventions, early recognition of leads and device dysfunction. Continuous monitoring may be reflected in a better patient therapeutic management and lower hospital admission, as compared with periodic out-patient follow-up.
We did not detect significant differences when examining stroke events, number of sustained VT/VF episodes or ICD shocks events. A significant difference comparing TM to non-TM patients has been found when examining sustained AF and non-sustained VT episodes. This effect may be in part attributable to a higher prevalence of immediate anti arrhythmic treatment to restore sinus rhythm in TM patients as compared with non-TM (27).
In our study, LVEF and NYHA functional class are predictive factors of hospitalisation. In line with this observation, LVEF has been classified as an independent factor for heart failure worse prognosis in the general population and in CRT-D recipients (26). Indeed, LVEF improvement is an index to define CRT-D-positive response, and NYHA functional class is an indicator of clinical status and outcomes (28). Hence, NYHA class worsening is linked to increasing fatigue, dyspnoea and other symptoms of failing heart, and may be regulated in positive manner by pharmacological and electrical therapy in responders (26).
A clinically relevant result emerging from our data analysis is that TM is predictive of heart failure hospitalisation rate. CRT-D recipients are hospitalised for dyspnoea worsening, for an increasing weight and for all symptoms related to heart failure disease worsening. TM may represent a useful monitoring system to follow heart failure CRT-D recipients, who may receive an appropriate, safe to use for physicians and patients (8), continuous follow-up monitoring and consequently the care that they need.
The main limitation of this study is the small size of our population of patients treated by CRT-D, monitored or not by TM, also attributable to loss of patients during follow-up, and to the low adherence of patients to the study protocol, as mentioned in the results section. Unfortunately, we do not have sufficient data on sympathetic nervous activity, which plays a crucial role in the pathophysiology of heart failure (29–34) and can be modulated by CRT (35). Besides, our conclusions remain linked to the relatively short follow-up duration and should not be extrapolated to long-term clinical outcomes.
What’s known
Telemonitoring is an efficient monitoring system for internal cardioverter defibrillator device (ICD) recipients. It has been used to track info on the clinical status of heart failure patients treated by ICD and/or cardiac resynchronisation therapy.
What’s new
This study demonstrates that telemonitoring is an independent prognostic factor predicting heart failure hospitalisation in patients treated with cardiac resynchronisation therapy defibrillator (CRT-D).
Acknowledgments
Dr Gaetano Santulli, MD, PhD is supported by the NIH (K99DK107895).
Footnotes
Disclosures
All Authors declare that they have no conflict of interest.
References
- 1.Inglis SC, Clark RA, Dierckx R, et al. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev. 2015;10:CD007228. doi: 10.1002/14651858.CD007228.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotooka N, Asaka M, Sato Y, et al. Home telemonitoring study for Japanese patients with heart failure (HOMES-HF): protocol for a multicentre randomised controlled trial. BMJ Open. 2013;3:e002972. doi: 10.1136/bmjopen-2013-002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasan A, Paul V. Telemonitoring in chronic heart failure. Eur Heart J. 2011;32:1457–64. doi: 10.1093/eurheartj/ehr005. [DOI] [PubMed] [Google Scholar]
- 4.Halimi F, Clementy J, Attuel P, et al. Optimized post-operative surveillance of permanent pacemakers by home monitoring: the OEDIPE trial. Europace. 2008;10:1392–9. doi: 10.1093/europace/eun250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houston BA, Kalathiya RJ, Kim DA, Zakaria S. Volume overload in heart failure: an evidence-based review of strategies for treatment and prevention. Mayo Clin Proc. 2015;90:1247–61. doi: 10.1016/j.mayocp.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Hutchinson K, Pellicori P, Dierckx R, et al. Remote telemonitoring for patients with heart failure: might monitoring pulmonary artery pressure become routine? Expert Rev Cardiovasc Ther. 2014;12:1025–33. doi: 10.1586/14779072.2014.935340. [DOI] [PubMed] [Google Scholar]
- 7.Heidbuchel H, Hindricks G, Broadhurst P, et al. EuroEco (European Health Economic Trial on Home Monitoring in ICD Patients): a provider perspective in five European countries on costs and net financial impact of follow-up with or without remote monitoring. Eur Heart J. 2015;36:158–69. doi: 10.1093/eurheartj/ehu339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hindricks G, Taborsky M, Glikson M, et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet. 2014;384:583–90. doi: 10.1016/S0140-6736(14)61176-4. [DOI] [PubMed] [Google Scholar]
- 9.Dubner S, Auricchio A, Steinberg JS, et al. ISHNE/EHRA expert consensus on remote monitoring of cardiovascular implantable electronic devices (CIEDs) Ann Noninvasive Electrocardiol. 2012;17:36–56. doi: 10.1111/j.1542-474X.2011.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition-heart failure (BEAT-HF) randomized clinical trial. JAMA Intern Med. 2016;176:310–8. doi: 10.1001/jamainternmed.2015.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–9. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myrvang H. Automatic remote home monitoring is a safe option for ICD follow-up. Nat Rev Cardiol. 2010;7:541. doi: 10.1038/nrcardio.2010.131. [DOI] [PubMed] [Google Scholar]
- 13.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 14.Sardu C, Marfella R, Santulli G, Paolisso G. Functional role of miRNA in cardiac resynchronization therapy. Pharmacogenomics. 2014;15:1159–68. doi: 10.2217/pgs.14.76. [DOI] [PubMed] [Google Scholar]
- 15.Santulli G, D’Ascia C. Atrial remodelling in echocardiographic super-responders to cardiac resynchronization therapy. Heart. 2012;98:517. doi: 10.1136/heartjnl-2012-301731. [DOI] [PubMed] [Google Scholar]
- 16.D’Ascia SL, D’Ascia C, Marino V, et al. Cardiac resynchronisation therapy response predicts occurrence of atrial fibrillation in non-ischaemic dilated cardiomyopathy. Int J Clin Pract. 2011;65:1149–55. doi: 10.1111/j.1742-1241.2011.02732.x. [DOI] [PubMed] [Google Scholar]
- 17.Friedman DJ, Upadhyay GA, Rajabali A, et al. Progressive ventricular dysfunction among nonresponders to cardiac resynchronization therapy: baseline predictors and associated clinical outcomes. Heart Rhythm. 2014;11:1991–8. doi: 10.1016/j.hrthm.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Tracy CM, Epstein AE, Darbar D, et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60:1297–313. doi: 10.1016/j.jacc.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Arya A, Block M, Kautzner J, et al. Influence of Home Monitoring on the clinical status of heart failure patients: design and rationale of the IN-TIME study. Eur J Heart Fail. 2008;10:1143–8. doi: 10.1016/j.ejheart.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Varma N, Epstein AE, Irimpen A, et al. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. 2010;122:325–32. doi: 10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 21.Lamas GA, Lee K, Sweeney M, et al. The mode selection trial (MOST) in sinus node dysfunction: design, rationale, and baseline characteristics of the first 1000 patients. Am Heart J. 2000;140:541–51. doi: 10.1067/mhj.2000.109652. [DOI] [PubMed] [Google Scholar]
- 22.Kutalek SP, Sharma AD, McWilliams MJ, et al. Effect of pacing for soft indications on mortality and heart failure in the dual chamber and VVI implantable defibrillator (DAVID) trial. Pacing Clin Electrophysiol. 2008;31:828–37. doi: 10.1111/j.1540-8159.2008.01106.x. [DOI] [PubMed] [Google Scholar]
- 23.Vanderheyden M, Mullens W, Delrue L, et al. Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy responders versus nonresponders. J Am Coll Cardiol. 2008;51:129–36. doi: 10.1016/j.jacc.2007.07.087. [DOI] [PubMed] [Google Scholar]
- 24.Anand IS, Carson P, Galle E, et al. Cardiac resynchronization therapy reduces the risk of hospitalizations in patients with advanced heart failure: results from the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) trial. Circulation. 2009;119:969–77. doi: 10.1161/CIRCULATIONAHA.108.793273. [DOI] [PubMed] [Google Scholar]
- 25.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 26.Moss AJ, Greenberg H, Case RB, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–5. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 27.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sardu C, Marfella R, Santulli G. Impact of diabetes mellitus on the clinical response to cardiac resynchronization therapy in elderly people. J Cardiovasc Transl Res. 2014;7:362–8. doi: 10.1007/s12265-014-9545-9. [DOI] [PubMed] [Google Scholar]
- 29.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res. 2013;113:739–53. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santulli G, Iaccarino G. Adrenergic signaling in heart failure and cardiovascular aging. Maturitas. 2016 doi: 10.1016/j.maturitas.2016.03.022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatton R, Cvjeticanin A, Lymperopoulos A. The adrenergic system of the adrenal glands as a remote control of cardiac function. J Cardiovasc Dis. 2015;5:394–7. [Google Scholar]
- 32.Santulli G. Adrenal signaling in heart failure: something more than a distant ship’s smoke on the horizon. Hypertension. 2014;63:215–6. doi: 10.1161/HYPERTENSIONAHA.113.02382. [DOI] [PubMed] [Google Scholar]
- 33.Lymperopoulos A, Chowdhary S, Sankar K, Simon I. Regulation of Catecholamine Production from the Adrenal Medulla. In: Santulli G, editor. Adrenal Glands: from Pathophysiology to Clinical Evidence. New York: NY Nova Science Publisher; 2015. [Google Scholar]
- 34.Santulli G. beta-Blockers in diabetic patients with heart failure. JAMA Intern Med. 2015;175:657. doi: 10.1001/jamainternmed.2014.8009. [DOI] [PubMed] [Google Scholar]
- 35.Scholtens AM, Braat AJ, Tuinenburg A, et al. Cardiac sympathetic innervation and cardiac resynchronization therapy. Heart Fail Rev. 2014;19:567–73. doi: 10.1007/s10741-013-9400-0. [DOI] [PubMed] [Google Scholar]