SUMMARY
Sonic hedgehog (Shh) determines cerebellar granule cell (GC) progenitor proliferation and medulloblastoma pathogenesis. However, the pathways regulating GC progenitors during embryogenesis before Shh production by Purkinje neurons and their roles in tumorigenesis remain unclear. The cilium-localized G-protein-coupled receptor Gpr161 suppresses Shh-mediated signaling in the neural tube. Here, by deleting Gpr161 in mouse neural stem cells or GC progenitors, we establish Gpr161 as a tumor suppressor in Shh subtype medulloblastoma. Irrespective of Shh production in the cerebellum, Gpr161 deletion increased downstream activity of the Shh pathway by restricting Gli3-mediated repression, causing more extensive generation and proliferation of GC progenitors. Moreover, earlier deletion of Gpr161 during embryogenesis increased tumor incidence and severity. GC progenitor overproduction during embryogenesis from Gpr161 deletion was cilium dependent, unlike normal development. Low GPR161 expression correlated with poor survival of SHH subtype medulloblastoma patients. Gpr161 restricts GC progenitor production by preventing premature and Shh-dependent pathway activity, highlighting the importance of basal pathway suppression in tumorigenesis.
In Brief
Shimada et al. identify the ciliary G-protein-coupled receptor Gpr161 as a tumor suppressor in Shh subtype medulloblastoma. The authors suggest that Gpr161 restricts premature Shh pathway activity during granule cell progenitor development, implying that cilium-mediated pathway suppression preceding Shh signaling prevents tumorigenesis.
INTRODUCTION
A fundamental question in biology is the mechanism by which morphogen signaling determines the development of neural stem cells (NSCs) and neuroprogenitors. A less appreciated aspect of this problem is the role cell-intrinsic factors play in basally suppressing morphogenetic pathways even before morphogen gradients are established. Granule cells (GCs) in the cerebellum are the most abundant neuronal type in the brain, constituting ~80% of human brain neurons (Herculano-Houzel, 2009). GC progenitors arise from atonal homolog 1 (Atoh1)-expressing cells in the upper rhombic lip (uRL) of the embryonic cerebellum (cerebellar anlage) starting from embryonic day 13 (E13) (Machold and Fishell, 2005; Wang et al., 2005). Sonic hedgehog (Shh) is a critical mitogen regulating GC progenitor proliferation (Dahmane and Ruiz i Altaba, 1999; Wallace, 1999; Wechsler-Reya and Scott, 1999). However, Shh is secreted by Purkinje neurons, starting only from E18.5, resulting in a postnatal growth spurt of GC progenitors (Corrales et al., 2004; Fuccillo et al., 2006; Huang et al., 2010; Kim et al., 2011; Lewis et al., 2004). The factors that regulate the generation and maintenance of GC progenitor pools during embryogenesis in the low Shh environment, and whether basal suppression of Shh signaling plays any role during these critical developmental stages, are not known.
Medulloblastoma is the most common malignant pediatric brain tumor that originates in the cerebellum. Recent molecular profiling of these tumors has resulted in its classification into four subgroups: Wnt, SHH, group 3, and group 4 (Northcott et al., 2011). Shh subtype medulloblastoma (SHH-MB) represents about 30% of the total number of medulloblastomas. It also constitutes the most common subgroup of medulloblastomas affecting infants (< 3 years) and adults (> 16 years) alike. Shh-MBs result from abnormal expansion of GC progenitors (Schüller et al., 2008; Yang et al., 2008). Following generation in the uRL, the GC progenitors migrate tangentially into the formative external granule layer (EGL), where they initiate proliferation in the outer EGL. Around E17, and before the spurt in postnatal proliferation, cerebellar folia start appearing. The GC progenitors proliferate multiple times postnatally in a Shh-dependent manner before exiting the cell cycle. The post-mitotic GCs extend axons chronologically forming the molecular layer (ML) and migrate radially along the Bergmann glia into their final location for maturation in the inner granule layer (IGL). Thus, the pathogenesis of Shh-MB can be best understood in the context of normal development of GCs (Leto et al., 2016).
GC progenitors in the postnatal EGL are known to be ciliated (Spassky et al., 2008), and Shh-mediated proliferation at this stage is cilium dependent (Chizhikov et al., 2007; Spassky et al., 2008). The primary cilium is a microtubule-based dynamic cellular appendage that is templated from the mother centriole. Both activation and repression of the Shh pathway are cilium dependent (Goetz and Anderson, 2010). Binding of Shh to Patched (Ptch1) triggers the removal of Ptch1 from cilia and promotes Smo enrichment in cilia, which mediates Gli transcriptional activator (GliA) formation. Germline heterozygous or conditional knockout of Ptch1 or expression of the constitutively active Smo mutant SmoM2 in NSCs or GC progenitors results in Shh-MBs (Goodrich et al., 1997; Schüller et al., 2008; Yang et al., 2008), whereas lack of cilia prevents SmoM2-induced medulloblastoma formation (Han et al., 2009).
In contrast, basal repression of Shh signaling involves protein kinase A (PKA)-mediated Gli repressor (GliR) formation in a cilium-dependent manner (Mukhopadhyay and Rohatgi, 2014). Lack of cilia promotes medulloblastoma formation upon expression of an active non-repressible form of Gli2, suggesting that the cilium-generated Gli3 repressor (Gli3R) restricts tumor progression (Han et al., 2009). Mutations in Suppressor of fused (SUFU), a negative regulator that functions by restraining cytoplasmic Gli3 and promoting Gli3R processing, have been reported in SHH-MBs (Taylor et al., 2002). However, early embryonic deletion of Sufu in the cerebellum did not exhibit tumorigenesis (Kim et al., 2011), whereas heterozygotes developed Shh-MBs only in a p53-null background (Lee et al., 2007). We recently identified the cilium-localized orphan G-protein-coupled receptor (GPCR) Gpr161 to be a negative regulator of Shh signaling during neural tube development (Mukhopadhyay et al., 2013). Gpr161 determines PKA-mediated Gli3R formation by constitutive cAMP signaling (Mukhopadhyay et al., 2013). Furthermore, the intraflagellar transport A (IFT-A) core complex and the tubby family protein Tulp3 coordinate Gpr161 trafficking to cilia (Badgandi et al., 2017), with either IFT-A or Tulp3 mutants exhibiting high Shh signaling, unlike other mutants affecting cilia (Goetz and Anderson, 2010). Here we describe the role of Gpr161 in development of Shh-MBs by restricting premature and postnatal Shh signaling, ultimately limiting GC progenitor generation and proliferation.
RESULTS
Gpr161 Is Expressed in the Embryonic and Postnatal Cerebellum
Gpr161 was broadly expressed in the mouse cerebellum during late embryonic and postnatal development, as determined by radioisotopic in situ hybridization (Figure S1A) and qRT-PCR, respectively (Figure S1B). In particular, Gpr161 was highly expressed in the E15.5 cerebellar anlage, including the ventricular zone (VZ), uRL, and EGL (Figure S1A). Gpr161 also localized to the cilia of primary cultured GC progenitors (Figure S1C).
Gpr161 Deletion in Neural Stem Cells Induces Cerebellar Tumorigenesis
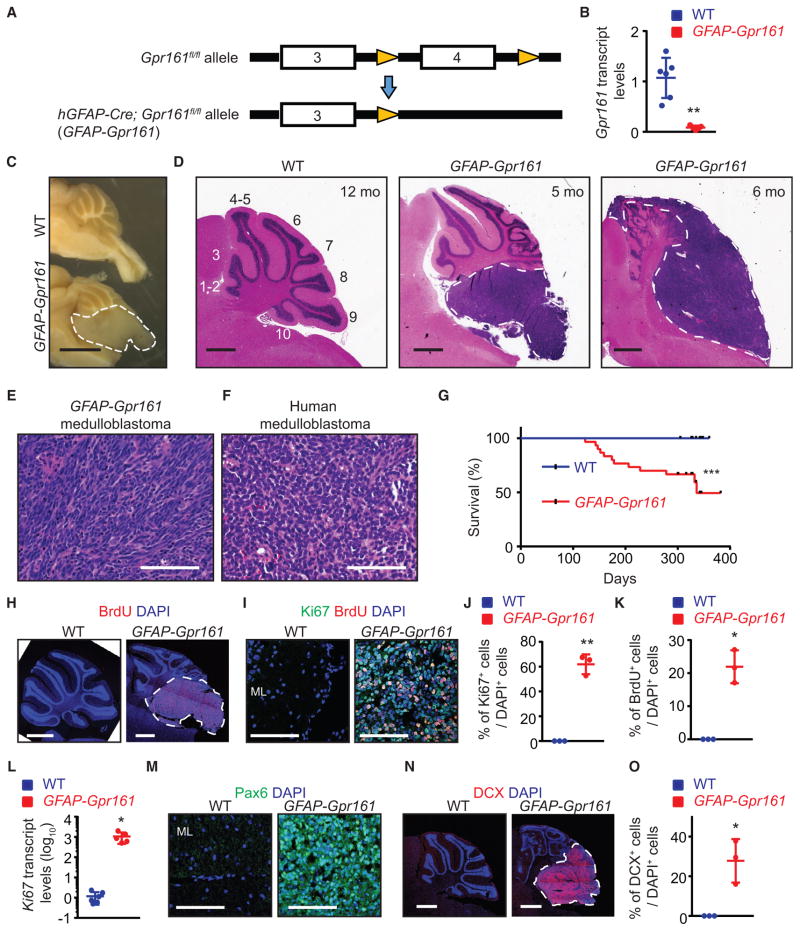
Gpr161 knockout mice are embryonic lethal by E10.5 (Mukhopadhyay et al., 2013). To conditionally delete Gpr161, we used a newly generated allele with loxP sites on either side of exon 4, which, upon recombination, results in a null allele (Hwang et al., 2017). We conditionally deleted Gpr161 in mouse NSCs using hGFAP-Cre (Zhuo et al., 2001) (hereafter referred to as GFAP-Gpr161 conditional knockout [cko]) (Figure 1A). Particularly in the cerebellar anlage, hGFAP-Cre can efficiently recombine genes in proliferating progenitors in the VZ and uRL that ultimately give rise to GC progenitors (Spassky et al., 2008). We confirmed that Gpr161 was efficiently deleted from the cerebellum of GFAP-Gpr161 cko mice using qRT-PCR (Figure 1B). Normally, GCs completely migrate out of the EGL into the IGL by postnatal day 16 (P16) (Corrales et al., 2004). However, even at P50, in GFAP-Gpr161 cko mice, we observed ectopic foci in the posterior cerebellum, constituting proliferating GC progenitors (based on Ki67 levels and bromodeoxyuridine [BrdU] incorporation in Pax6-positive GCs) (Figures S1D and S1F; Lin et al., 2001) and immature GC neurons (based on doublecortin immunostaining in Pax6-positive GCs) (Figures S1E and S1F). Strikingly, by 3–10 months, GFAP-Gpr161 cko mice developed tumors in the cerebellum (Figures 1C and 1D). The tumors resembled histological features of human classic medulloblastoma, with solid sheets of tumor cells having uniform large nuclei and scant cytoplasm (Figures 1E and 1F). By 12 months, more than 50% of GFAP-Gpr161 cko mice failed to survive (Figure 1G). As noted before for the ectopic foci, the tumors were positive for proliferating GC progenitors and immature GC neurons (Figures 1H–1O). Thus, Gpr161 deletion in NSCs induces formation of cerebellar tumors.
Figure 1. Gpr161 Deletion in Neural Stem Cells Induces Cerebellar Tumorigenesis.
(A) Schematic showing Gpr161 exon 4 deletion with the hGFAP-Cre allele.
(B) Gpr161 transcript levels in tumors of GFAP-Gpr161 cko (hGFAP-Cre; Gpr161fl/fl) mice (n = 5) are significantly decreased compared with the cerebellums of littermate controls designated as wild-type (WT) (Gpr161fl/fl or Gpr161fl/+; n = 6) collected from 3- to 5-month-old mice.
(C) Representative picture showing brains of GFAP-Gpr161 cko and WT mice (both 10 months old) after sectioning at the midline. Note the tumor (white dotted line) in the posterior cerebellum.
(D) H&E-stained sagittal sections showing tumors (white dotted lines) in the posterior cerebellum of GFAP-Gpr161 cko mice (5–6 months old).
(E and F) H&E-stained section of tumor from GFAP-Gpr161 cko mice (E) resembling that of human classic medulloblastoma (F).
(G) Kaplan-Meier survival curves of control (n = 19) and GFAP-Gpr161 cko mice (n = 27). Data were collected when mice were found dead or mice were sick (hunched back and reduced mobility).
(H and I) Representative images of immunostaining for BrdU (upon 1-h pulse) (H) and Ki67/BrdU (I) in the cerebellum of 3- to 5-month-old mice.
(J and K) Quantification of Ki67+ (J) or BrdU+ (K) cells co-stained for DAPI per field of view. n = 3 fields/mice, 3 mice/genotype.
(L) qRT-PCR analysis of Ki67 transcript levels in the GFAP-Gpr161 cko tumors (n = 5) with respect to normal cerebellum from the WT (n = 6).
(M) Pax6+ cells were present in the GFAP-Gpr161 cko tumor but not in the WT molecular layer (ML).
(N and O) Representative images of doublecortin (DCX) immunostaining (N) and percentage of DCX+ cells co-stained for DAPI per field of view (O) in the tumors of GFAP-Gpr161 cko mice and cerebellum of the WT. n = 3 fields/mice, 3 mice/genotype, 3–5 months old.
Data represent mean ± SD. *p < 0.05, **p < 0.01 by log rank test (G) and Student’s t test (B and J–L, and O). Scale bars indicate 2mm(C), 1mm (D, H, and N), and 100 μm (E, F, I, and M). (H) and (N) are tiled images. See also Figure S1.
Gpr161 Deletion Induces Shh-MB Formation
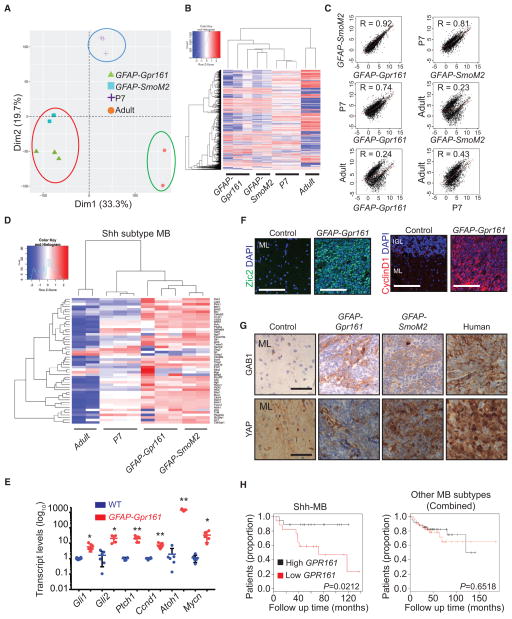
Because Gpr161 is a basal suppressor of Shh signaling (Mukhopadhyay et al., 2013), we compared gene expression in GFAP-Gpr161 cko tumors with a known model of Shh-MB generated by co-expressing a constitutively active Smo allele (SmoM2(W539L)EYFP/+) (Jeong et al., 2004) with hGFAP-Cre (GFAP-SmoM2) and cerebellum from control P7 and adult mice. Principle-component analysis and hierarchical clustering of all transcripts demonstrated that GFAP-Gpr161 cko tumors clustered with hGFAP-SmoM2 tumors (Figures 2A and 2B), whereas regression analysis confirmed a direct correlation only between their transcription profiles (Figure 2C). Furthermore, the clustering analysis showed similarities between known Shh subtype-specific transcripts from these tumors (Figure 2D) but not with transcripts from other medulloblastoma subtypes (Figure S2A; Northcott et al., 2011). Expression of Shh signaling-specific transcripts such as Gli1, Ptch1, CyclinD1 (Ccnd1), and N-myc (Mycn) and of the transcription factor Gli2 in GFAP-Gpr161 cko tumors was high with respect to control littermates by qRT-PCR (Figure 2E). Immunostaining showed increased CyclinD1 levels (Figure 2F) and high expression of other GC progenitor-specific markers, such as Atoh1 (Figure 2E) and Zic2 (Aruga et al., 2002; Figure 2F), in the tumors. In addition, histological markers used in human SHH-MB diagnosis, such as GAB1 and YAP (Ellison et al., 2011), were highly expressed in GFAP-Gpr161 cko and GFAP-SmoM2 tumors, similar to human samples (Figure 2G). However, WNT subtype medulloblastoma-specific nuclear localization of β-catenin was absent (Figure S2B). In addition, GFAP-Gpr161 cko medulloblastomas showed scattered neoplastic cells positive for the glial marker GFAP and neuronal differentiation by synaptophysin immunostaining resembling that of GFAP-SmoM2 tumors and human Shh-MB samples (Figure S2B). Thus, Gpr161 deletion in NSCs induces Shh-MB in mice, resembling the human SHH-MB subtype.
Figure 2. Gene Expression Profiles of Tumors Induced upon Gpr161 Deletion Resemble Shh Subtype Medulloblastomas.
(A and B) Principal-component analysis (PCA) (A) and hierarchical clustering analysis (B) of RNA-seq expression profiles of tumors from GFAP-Gpr161 cko mice (3–5 months old, n = 3), Shh-MBs from GFAP-SmoM2 mice (1 month old, n = 2), cerebellums of post-natal day 7 (P7, n = 3) mice, and adult mice (2 months old, n = 2). The x and y axes in the PCA plot are first- and second-principal components, and the percentages shown on each axis refer to the percent of variance being explained by the corresponding principal components (Dim1 and Dim2). GFAP-Gpr161 cko mice cluster with each other and are closely related to GFAP-SmoM2 mice. The heatmap shows genes with an average expression level higher than 1 transcript per kilobase million (TPM) and variance of expression of more than 2 across all 10 samples.
(C) Regression analysis of RNA-seq data shows that tumors of GFAP-Gpr161 cko mice and Shh subtype medulloblastoma of GFAP-SmoM2 mice have a higher Pearson correlation coefficient (R) than other comparisons. The x and y axes show the log2-transformed TPM values.
(D) Heatmap and clustering analysis of Shh subtype-specific transcripts show that tumors of GFAP-Gpr161 cko mice are similar to GFAP-SmoM2 tumors.
(E) qRT-PCR analysis of transcript levels of Shh-MB candidate genes in tumors of GFAP-Gpr161 cko mice (n = 6) compared with the WT cerebellums (n = 5).
(F) Tumors of GFAP-Gpr161 cko mice (3–10 months old) expressed the GC marker Pax6 and the Shh pathway target CyclinD1 upon immunostaining and co-staining with DAPI.
(G) Immunohistochemical analysis confirms that tumors from GFAP-Gpr161 cko mice exhibit an SHH subtype-specific staining pattern similar to mouse GFAP-SmoM2 tumors and human SHH-MB (human).
(H) Poor survival specifically in SHH-MB patients with low GPR161 expression in resected tumors (n = 52 in the SHH subtype and 135 in other subtypes combined). The top 50% and bottom 50% are considered high and low gene expression groups, respectively.
All data represent mean ± SD. Scale bars indicate (F) 100 μm and (G) 200 μm. *p < 0.05, **p < 0.01 by Student’s t test or as shown using log rank test (H). IGL, internal granule layer; WT, littermate control (Gpr161fl/fl or Gpr161fl/+). See also Figure S2.
Low Expression of GPR161 Correlates with Poor Prognosis in SHH-MB Patients
Mutations in the Gpr161 locus were not seen in 300 sequenced tumors (Jones et al., 2012; Pugh et al., 2012; Robinson et al., 2012). In addition, we did not detect any homozygous deletions in the Gpr161 locus in 827 patients tested (Northcott et al., 2017), implying that this locus is not a common hotspot for tumor initiation in patients. However, upon reevaluating the expression of GPR161 from previously published expression data (Cho et al., 2011) with patient survival, we noted that the expression levels of GPR161 tightly correlated with decreased patient survival length only in the SHH-MB subtype but not in other subtypes combined (Figures 2H and S2C). Thus, low GPR161 is a prognostic indicator for survival in SHH-MB patients, implying that negative regulation of the SHH pathway might be critical for preventing tumor progression in humans.
Gpr161 Deletion in NSC or GC Progenitor-Specific Lineages Results in Shh-MB
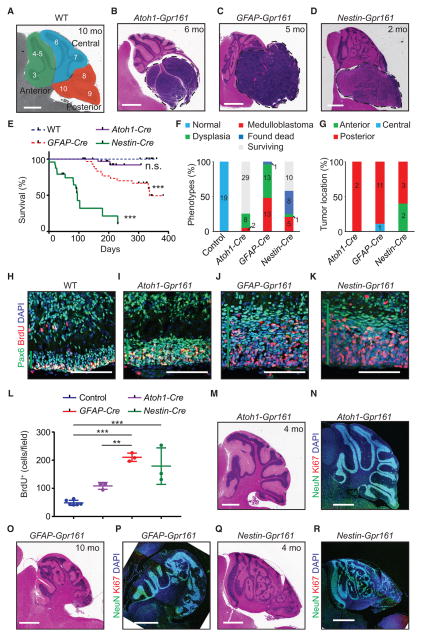
Shh-MBs can be initiated in GC progenitors or NSCs, but tumorigenesis is associated with commitment to the GC lineage (Schüller et al., 2008; Yang et al., 2008). Multipotent NSCs expressing GFAP in the VZ give rise to Purkinje cells, Bergmann glia, astrocytes, and oligodendrocytes and also generate GC progenitors in the uRL (Schüller et al., 2008; Spassky et al., 2008; Yang et al., 2008). hGFAP-Cre starts expression in NSCs in the VZ by E12.5, followed by GC progenitors in the uRL (Spassky et al., 2008; Yang et al., 2008). In contrast, the Nestin-Cre line causes earlier and consistent expression in the entire neural tube by E11.5 (Blaess et al., 2008), whereas Atoh1-expressing GC progenitors appear in the embryonic uRL by E12.5 (Machold and Fishell, 2005; Wang et al., 2005). Nestin-Cre; Gpr161fl/fl and Atoh1-Cre; Gpr161fl/fl are hereafter referred to as Nestin-Gpr161 cko and Atoh1-Gpr161 cko, respectively. All three mouse models developed Shh-MBs (Figures 3A–3G). Although ~50%GFAP-Gpr161 cko mice developed Shh-MBs by 3 months or later (Figures 3C and 3E–3G), Nestin-Gpr161 cko mice developed tumors by 2–8 months, and most were deceased by 8 months (Figures 3D and 3E–3G). Among the dying mice (n = 6), we observed a high tumor incidence upon histological examination (~83%) (Figure 3F). Atoh1-Gpr161 cko mice also developed tumors, but with much less severity and frequency. Only ~20% of analyzed mice (n = 10) developed tumors by 4–6 months, and ~6% died by 10 months of age (Figures 3B and 3E–3G). Because Atoh1-Cre results in GC progenitor-specific recombination, Gpr161 deletion in GC progenitors is sufficient to induce tumorigenesis.
Figure 3. Loss of Gpr161 in Either NSC or GC Progenitor-Specific Lineages Causes Shh-MB with Increased Incidence upon Earlier Depletion.
(A–D) H&E-stained sagittal sections show (A) normal cerebellum in a WT (Gpr161fl/fl or Gpr161fl/+) mouse and medulloblastoma in (B) Atoh1-Gpr161 cko, (C) GFAP-Gpr161 cko, and (D) Nestin-Gpr161 cko mice at the designated ages. In (A), “anterior” indicates lobes 1–5, “central” indicates lobes 6 and 7, and “posterior” indicates lobes 8–10 (Legué et al., 2016).
(E) Kaplan-Meier survival curves of the WT (n = 19), GFAP-Gpr161 cko mice (n = 27), Atoh1-Gpr161 cko mice (n = 31), and Nestin-Gpr161 cko mice (n = 24). Data of WT and GFAP-Gpr161 cko mice (dotted line) from Figure 1G are shown for comparison.
(F) Histological analysis of the cerebellum in WT (10–12 months old, n = 19), Nestin-Gpr161 cko mice (0–8 months old, n = 24; 6 dying mice were histologically analyzed at 2–8 months age; 9 of the 10 surviving mice were less than 93 days old during generation of the survival curve; E), GFAP-Gpr161 cko mice (5–12 months, n = 27), and Atoh1-Gpr161 cko mice (4–10 months old; n = 39; 8 histologically analyzed at 3–5 months age, 2 analyzed from dying animals at 5 and 7 months). Medulloblastoma, medulloblastoma with/without dysplasia; Dysplasia, misplaced granule neurons, both global and focal; Found dead, found dead with a disintegrated brain and excluded from histological analysis.
(G) Regional analysis of tumor location by H&E staining in different strains as follows: Nestin-Gpr161 cko mice (3–8 months old, n = 5), GFAP-Gpr161 cko mice (5–10 months old, n = 10), and Atoh1-Gpr161 cko mice (5–7 months old; n = 2). Lobe designation is as in (A).
(H–K) Proliferating GC progenitors in the posterior (lobes 9 and 10) external granule layer (EGL) of cerebellums from (H) WT, (I) Atoh1-Gpr161, (J) GFAP-Gpr161, and (K) Nestin-Gpr161 cko mice at P7. Vertical green lines mark the extent of the EGL. (L) The number of BrdU+ cells (upon 1-h pulse) was measured upon co-staining for DAPI cells per field of view. Three fields per mouse were analyzed. n = 3–6 mice/genotype.
(M–R) H&E-stained and NeuN+-immunostained sagittal sections show global cerebellar dysplasia and/or focal ectopic granule neurons of (M and N) Atoh1-Gpr161, (O and P) GFAP-Gpr161, and (Q and R) Nestin-Gpr161 cko mice at the designated ages. In (N), (P), and (R), NeuN+ neurons are shown. Note that Ki67+ cells were not observed.
Scale bars are 1mm(A–D and M–R) and 100 μm(H–K). ***p < 0.001 by log rank test compared with the WT (E). n.s., not significant. **p < 0.01 by one-way ANOVA with Tukey’s multiple comparisons test (L). Nuclei are stained by DAPI. (N), (P), and (R) are tiled images. See also Figure S3.
Similar to GFAP-Gpr161 cko tumors, both Nestin-Gpr161 and Atoh1-Gpr161 cko tumors were GC-specific (Pax6-positive) (Figure S3A) and resembled human classic medulloblastomas in histology (Figures 1F and S3B) and Shh-MBs by immunohistochemistry against GAB1 and YAP (Figures S3C–S3E). Interestingly, although tumors arising in Atoh1-Gpr161 cko and GFAP-Gpr161 cko predominantly affected posterior lobes, tumors in Nestin-Gpr161 cko were also distributed anteriorly, possibly corresponding to the earlier depletion in NSCs (Figure 3G). All models were associated with an increase in thickness and proliferation of GC progenitors in the posterior EGL at P7 (Figures 3H–3L), suggesting increased proliferation postnatally. Overall, NSC-specific deletion causes more occurrences and a broader distribution of tumors and high mortality compared with GC progenitor-specific deletion.
Cerebellar Dysplasia and a Limited Number of Folia upon Loss of Gpr161
Irrespective of the development of tumors, we noticed abnormal cerebellar development (cerebellar dysplasia) resulting from mislocalized GCs during postnatal development (Figure 3F). The dysplastic regions were positive for the pan-neuronal marker NeuN and generally lacked proliferation (Figures 3M–3R), suggesting gradual regression into delayed neurogenesis starting from earlier proliferation (Figures 3H–3K). The dysplasia was strongest, earliest, and global in appearance in Nestin-Gpr161 cko mice, followed by reduced severity in GFAP-Gpr161 cko mice and only focal lesions in Atoh1-Gpr161 cko mice (Figures 3M–3R). In addition, Nestin-Gpr161 cko mice had a limited number of folia irrespective of developing tumors and/or dysplasia (Figures 3D and 3Q). Thus, Gpr161 is critical for normal cerebellar development in addition to regulating tumorigenesis.
Temporal Progression of GC Progenitor Proliferation in Nestin-Gpr161 cko Mice
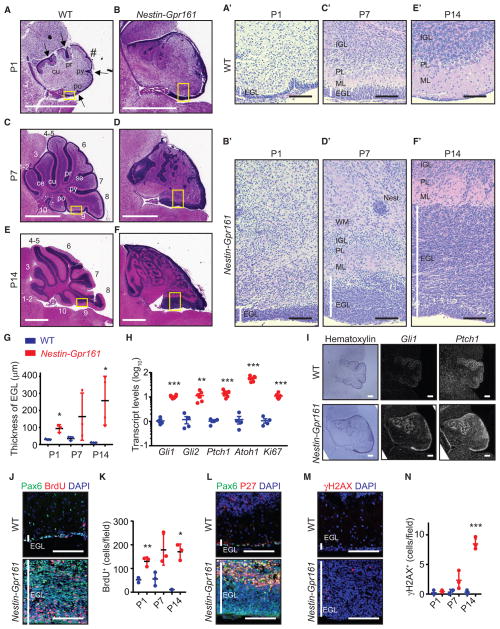
To investigate tumorigenesis following Gpr161 deletion, we further characterized the temporal progression of proliferative changes in GC progenitors. Deletion of Gpr161 was confirmed by qRT-PCR in Nestin-Gpr161 cko mice at P14 (Figure S4A). The thickness of the EGL was increased in Nestin-Gpr161 cko mice compared with the littermate controls during postnatal development (Figures 4A–4G). The histological appearance of the proliferative cells in the outer EGL of Nestin-Gpr161 cko mice showed gradual transformative changes with enlarged nuclei and a spindle-like appearance compared with the inner EGL (Figures 4A′–4F′). Shh pathway transcripts were highly upregulated in the cerebellum of Nestin-Gpr161 cko mice compared with littermate controls at P14 by qRT-PCR (Figure 4H). Furthermore, radioisotopic RNA in situ hybridization demonstrated increased Gli1 and Ptch1 transcripts in the EGL of Nestin-Gpr161 cko mice compared with littermate controls by P1 (Figure 4I). Proliferation of GC progenitors in the EGL was increased in Nestin-Gpr161 cko mice compared with controls during postnatal development (Figures 4J and 4K and S4B). The inner EGL with P27-positive cells was also thickened, demonstrating that differentiation of GC progenitors was occurring in the EGL of Nestin-Gpr161 cko mice. (Figures 4L and S4C). Increased proliferation of GC progenitors is associated with replication stress, accumulating DNA damage, and tumorigenesis (Mille et al., 2014), Interestingly, we observed increased DNA damage by γH2AX immunostaining in the posterior EGL of Nestin-Gpr161 cko mice at P14 (Figures 4M and 4N and S4D) and in medulloblastomas of GFAP-Gpr161 cko mice, indicating genomic instability (Figure S4E). Sox2 marks a rare quiescent stem cell population driving Shh-MB growth and relapse (Vanner et al., 2014). GFAP-Gpr161 cko mouse tumors were also positive for Sox2, indicating accumulating cells with tumorigenic potential (Figure S4F). Thus, overproliferation of GC progenitors, accumulating DNA damage, and gradual transformative changes culminate into developing Shh-MB.
Figure 4. GC Progenitor Proliferation and Dysplasia during Postnatal Development following Gpr161 Deletion.
(A–F) H&E-stained sagittal sections showing the thickened EGL, decreased foliation, and cerebellar dysplasia of Nestin-Gpr161 cko mice (B, D, and F) compared with the WT (Gpr161fl/fl or Gpr161fl/+) (A, C, and E) for P1 (A and B), P7 (C and D), and P14
(E and F). Arrows indicate fissures, and # indicates an expected fissure. Abbreviations for fissures: ce, precentral; cu, preculminate; pr, primary; se, secondary; py, pyramidal; po, posteriolateral. (A′–F′) High-magnification pictures of the yellow boxes in (A)–(F). White vertical lines indicate the EGL layer. PL, Purkinje/Bergmann glia layer; Nest, small proliferative nest (confirmed by BrdU staining); WM, white matter.
(G) The thickness of the EGL is increased in Nestin-Gpr161 cko mice compared with littermate controls. n = 3–4 mice/genotype.
(H) qRT-PCR analysis shows increased Shh pathway transcripts in the cerebellums of Nestin-Gpr161 cko mice compared with that of the WT at P14. n = 4–6 per genotype.
(I) Radioisotopic in situ hybridization shows increased Gli1 and Ptch1 transcript levels in the EGL of Nestin-Gpr161 cko mice compared with littermate control mice at P1. Hematoxylin staining shows the morphology of the cerebellum.
(J–N) Temporal changes of (J and K) BrdU+ (1-h pulse) GC progenitors, (L) P27+ GC progenitors, and (M and N) γH2AX+ cells in the EGL of cerebellums of Nestin-Gpr161 cko mice at P14. Note that data from Figure 3L at P7 are included in (K) for comparison. n = 3 mice/genotype.
All data represent mean ± SD. Scale bars indicate 1mm(A–F), 100 μm(A′–F′, J, L, and M), and 200 μm(I). *p < 0.05, **p < 0.01, and ***p < 0.01 by Student’s t test. Nuclei are stained by DAPI. See also Figure S4.
Dysplastic changes in Nestin-Gpr161 cko mice were apparent from a gradual regression of proliferation into neurogenesis in non-tumor areas, except peripherally and in a few remaining pockets of small proliferative nests (Figures S4G and S4H). Similarly, there was a gradual regression of thickened EGL (P7) and cellular proliferation in the posterior lobe (1 month) (Figure S4I) into focal dysplasia, irrespective of Shh-MB formation in Atoh1-Gpr161 cko mice. A limited number of folia in Nestin-Gpr161 cko was apparent as early as P0, with reduction of the principal fissures (Figures 4A–4F). Thus, Gpr161 lacks results in a continuum of phenotypes, including tumorigenesis, dysplasia, and limited foliation.
Gpr161 Restricts Generation/Proliferation of GC Progenitors during Embryogenesis
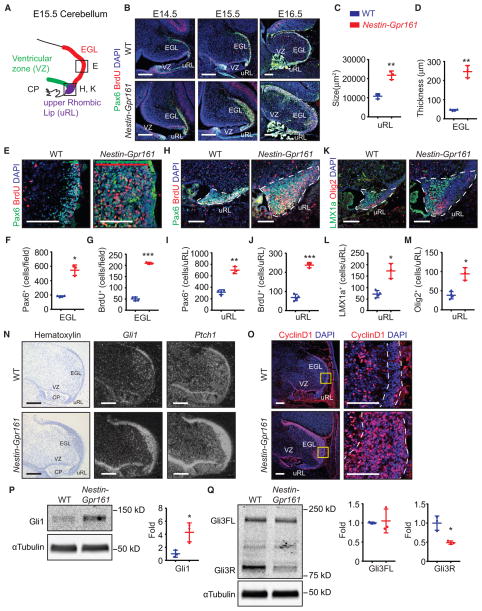
Because deletion of Gpr161 in earlier NSC-specific lineages caused more severe phenotypes than in GC progenitor-specific lineages, we systematically probed for developmental changes that accompany the generation of granule progenitors in the cerebellar anlage. In Nestin-Gpr161 cko mice, we noted an increased size of the uRL and increased thickening of the formative EGL compared with littermate controls, starting as early as E14.5 and clearly evident by E16.5, suggesting that the areas where NSCs and GC progenitors locate are expanded in Nestin-Gpr161 cko mice (Figures 5A–5D).
Figure 5. Gpr161 Limits Premature Shh Signaling in GC Progenitors during Embryogenesis.
(A) A cartoon of the cerebellar anlage at E15.5. EGL, formative external granule layer; uRL, upper rhombic lip; VZ, ventricular zone; CP, hindbrain choroid plexus.
(B) Pax6+- and BrdU+-stained sagittal sections show gradual thickening of the formative EGL at different embryonic stages in Nestin-Gpr161 cko mice.
(C and D) Increased area of the uRL (C) and thickened formative EGL (D) are shown in Nestin-Gpr161 cko mice (n = 3) compared with WT mice (n = 4) at E16.5.
(E–G) Representative images of proliferating GC progenitors in the formative EGL at E16.5 of Nestin-Gpr161 cko mice (n = 3) and WT mice (n = 4) (E). The location of the EGL region depicted is indicated in the black box in (A). The extent of the EGL (Pax6+) and BrdU+ proliferating cells is shown using a green and red bar, respectively. The numbers of (F) Pax6+ GC progenitors and (G) BrdU+ (1 h incorporation) proliferating cells are increased in the EGL regions of Nestin-Gpr161 cko mice compared with that of the WT at E16.5.
(H–J) Representative pictures of Pax6+ GC progenitors and BrdU+ proliferating cells in the uRL of E16.5 Nestin-Gpr161 cko mice and WT mice (H). The location is indicated in the black box in (A). The numbers of (I) Pax6+ GC progenitor cells and (J) BrdU+ proliferating cells are increased in the uRL of Nestin-Gpr161 mice (n = 3) compared with the WT (n = 4) at E16.5.
(K–M) Representative pictures of Lmx1a+ and Olig2+ cells in the uRL of E16.5 Nestin-Gpr161 cko mice (n = 3) and WT mice (n = 4) (K). The location is indicated in the black box in (A). The numbers of (L) Lmx1a+ and (M) Olig2+ cells are increased in the uRL of Nestin-Gpr161 cko mice compared with that of the WT at E16.5.
(N) Radioisotopic in situ hybridization showed increased Gli1 and Ptch1 transcript levels in the formative EGL, outer uRL, and VZ of Nestin-Gpr161 cko mice compared with WT mice at E15.5. Hematoxylin staining shows the morphology of the cerebellum.
(O) Representative images of CyclinD1+ cells in the cerebellum of E15.5 Nestin-Gpr161 cko mice and littermate control mice. The EGL is marked by white dotted lines.
(P and Q) Immunoblotting for Gli1 (P) and Gli3 (Q) and quantification (after normalization with α-tubulin levels) in E15.5 cerebellar anlagen of control littermates and Nestin-Gpr161 cko mice (n = 3 each). High Gli1 levels and low Gli3R levels are seen in Nestin-Gpr161 cko mice. Gli3FL, Gli3 full-length; Gli3R, Gli3 repressor.
All data represent mean ± SD. Scale bars indicate 200 μm (B, N, and O, left) and 100 μm (E, H, K, and O, insets). Nuclei are stained by DAPI. *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s t test. WT, littermate control (Nestin-Cre; Gpr161fl/+ or Gpr161fl/fl or Gpr161fl/+). (B) is a tiled image. See also Figure S5.
Most GC progenitors, including those derived from non-overlapping Atoh1- or Lmx1a-positive lineages, are Pax6-positive (Chizhikov et al., 2006; Lin et al., 2001; Millonig et al., 2000). Sox2 also marks a population of multipotent NSCs in the uRL and of GC progenitors in the formative EGL around E13.5 (Ahlfeld et al., 2013). In addition, a subpopulation of Pax6-positive progenitors in the uRL is also Olig2-positive. In agreement with increased thickness of the formative EGL, proliferating cells, Pax6-positive GC progenitors, as well as Sox2-positive NSCs were increased in the EGL of Nestin-Gpr161 cko mice compared with littermate controls (Figures 5E–5G and S5A and S5B). Pax6-, Lmx1a-, and Olig2-positive GC progenitors along with Sox2-positive NSCs and proliferation in the uRL also increased in Nestin-Gpr161 cko mice (Figures 5H–5M and S5C and S5D). Thus, Gpr161 restricts the generation and proliferation of GC progenitor and NSC pools in the EGL and uRL of the cerebellar anlage.
Premature Shh Signaling in GC Progenitors upon Gpr161 Deletion
Next we determined mechanisms underlying Gpr161–mediated regulation of NSCs and GC progenitors in the cerebellar anlage. During embryogenesis, Shh is released from the hindbrain choroid plexus outside of the cerebellar anlage that promotes proliferation in the adjacent cerebellar VZ (Huang et al., 2010). BrdU incorporation (Figures S5E and S5F), Sox2-expressing radial glial progenitors (Figures S5G and S5H), Pax-2 expressing GABAergic interneuron progenitors (Figures S5I–S5J), and Shh pathway targets such as Gli1 and Ptch1 transcripts (Figure 5N) were increased in the cerebellar VZ of Nestin-Gpr161 cko mice compared with littermate controls. Thus, Gpr161 restricts NSC pools in the VZ of the cerebellar anlage by inhibiting Shh signaling.
In contrast, inside the cerebellar anlage, Shh secretion by Purkinje neurons is not initiated until E18.5 (Corrales et al., 2004; Fuccillo et al., 2006; Huang et al., 2010; Kim et al., 2011; Lewis et al., 2004). Unexpectedly, we detected increased levels of Shh signaling targets (Gli1 and Ptch1 transcripts) and CyclinD1 in the EGL and outer uRL of Nestin-Gpr161 cko mice compared with the littermate controls at E15.5 by in situ hybridization and immunofluorescence analysis (Figures 5N and 5O). Gli1 protein levels were also increased (Figure 5P). Importantly, Gli3R levels were significantly reduced in the cerebellar anlage of Nestin-Gpr161 cko mice (Figure 5Q), concurrent with the role of Gpr161 in basal suppression of the Shh pathway by Gli3R processing prior to initiation of Shh-mediated morphogenetic signaling. Thus, Gpr161-mediated restriction of premature activation of the Shh pathway limits the GC progenitor pool during embryogenesis.
Overproliferation of GC Progenitors upon Gpr161 Deletion Is Cilium Dependent
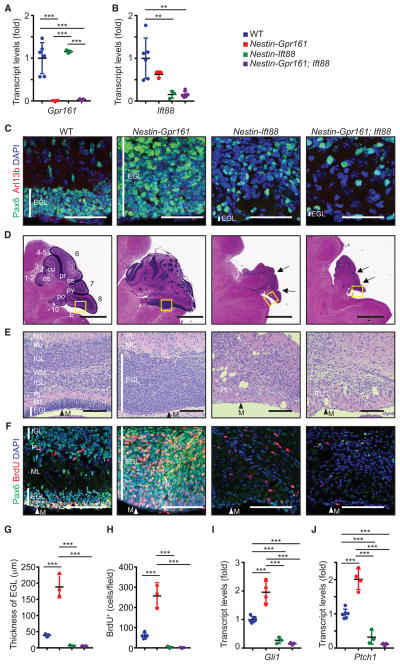
We next tested the role of cilia in phenotypes resulting from Gpr161 deletion by combining it with deficiencies in the intraflagellar transport-B (IFT-B) complex protein lft88 that disrupts cilia (Haycraft et al., 2007). Thus, we generated single and double conditional knockouts of Nestin-Cre; Ift88fl/fl (Nestin-Ift88 cko) and Nestin-Cre; Gpr161fl/fl; Ift88fl/fl (Nestin-Gpr161; Ift88 double conditional knockout [dko]), respectively. We first confirmed that Gpr161 transcripts were reduced in Nestin-Gpr161 cko and Nestin-Gpr161; Ift88 dko mice (Figure 6A), whereas Ift88 transcripts and Arl13b-positive primary cilia in P7 EGL were absent in Nestin-Ift88 cko and Nestin-Gpr161; Ift88 dko mice (Figures 6B and 6C). Nestin-Ift88 cko mice had a smaller cerebellum with rudimentary folia compared with the littermate controls (Figure 6D). Strikingly, the cerebellums of Nestin-Gpr161; Ift88 dko mice were smaller than those of Nestin-Gpr161 cko mice and littermate controls at P7 but were almost indistinguishable from those of Nestin-Ift88 cko mice (Figure 6D). The almost complete lack of EGL in Nestin-Gpr161; Ift88 dko mice was similar to Nestin-Ift88 cko mice (Figures 6E and 6G). In line with a lack of EGL, Pax6-positive GC progenitors, BrdU-positive proliferation, and the Shh pathway targets Gli1 and Ptch1 were drastically decreased in Nestin-Gpr161; Ift88 dko and Nestin-Ift88 cko mice compared with either WT or Nestin-Gpr161 cko mice (Figures 6F and 6H–6J). Thus, primary cilia are required for normal proliferation and overproliferation of GC progenitors caused by Gpr161 deletion in the postnatal cerebellum.
Figure 6. Gpr161 Prevents GC Progenitor Proliferation in a Cilium-Dependent Manner.
(A and B) Gpr161 (A) and Ift88 (B) transcript levels in P7 cerebellums of WT, Nestin-Gpr161 cko, Nestin-Ift88 cko, and Nestin-Gpr161; Ift88 dko mice. n = 3–6 mice per genotype.
(C) Representative pictures of Arl13b+ primary cilia in Pax6+ GC progenitors in the EGL in the cerebellum of WT, Nestin-Gpr161 cko, Nestin-Ift88 cko, and Nestin-Gpr161; Ift88 dko mice at P7. Note that Arl13b+ primary cilia are not observed in the rudimentary EGL of Nestin-Ift88 cko and Nestin-Gpr161; Ift88 dko mice.
(D and E) H&E-stained sagittal sections of the corresponding insets in (D) as depicted in (E) shows increased thickness of the EGL in Nestin-Gpr161 cko mice and almost nonexistent EGL and partial foliation in Nestin-Ift88 cko mice and Nestin-Gpr161; Ift88 dko mice at P7. Arrows point to rudimentary fissures.
(F) Representative pictures of Pax6+ GC progenitors and BrdU+ proliferating cells in the EGL at P7. Vertical white lines denote extent of EGL (C, E, and F). M, meninges (E and F).
(G) Thickness of the EGL of P7 cerebellum. n = 3–6 mice per genotype.
(H) Number of BrdU+ cells (upon 1-h pulse) measured in the EGL of P7 cerebellums of the designated genotypes. n = 3–6 mice/genotype.
(I and J) qRT-PCR analysis shows increased Shh pathway transcripts, Gli1 (I) and Ptch1 (J), in the cerebellums of Nestin-Gpr161 cko mice compared with that of WT and Nestin-Gpr161; Ift88 dko mice at P7. n = 3–6 mice/genotype.
Scale bars indicate (C) 50 μm, (D) 1 mm, and (E and F) 100 μm. Nuclei are stained by DAPI. **p < 0.01, ***p < 0.001 by one-way ANOVA with Tukey’s multiple comparisons test. Nestin-Gpr161 cko is Nestin-Cre; Gpr161fl/fl; Ift88fl/+; Nestin-Ift88 cko is Nestin-Cre; Ift88fl/fl; Gpr161fl/+; Nestin-Gpr161; Ift88 dko is Nestin-Cre; Gpr161fl/fl; Ift88fl/fl; WT is littermate control (Nestin-Cre; Gpr161fl/+; Ift88fl/+ or Gpr161fl/fl; Ift88fl/fl or Gpr161fl/+; Ift88fl/fl or Gpr161fl/fl; Ift88fl/+). The other abbreviations are as in Figures 4 and 5.
Lack of Cilia Prevents Premature Shh Signaling and Excessive Generation of GC Progenitors upon Gpr161 Deletion during Embryogenesis
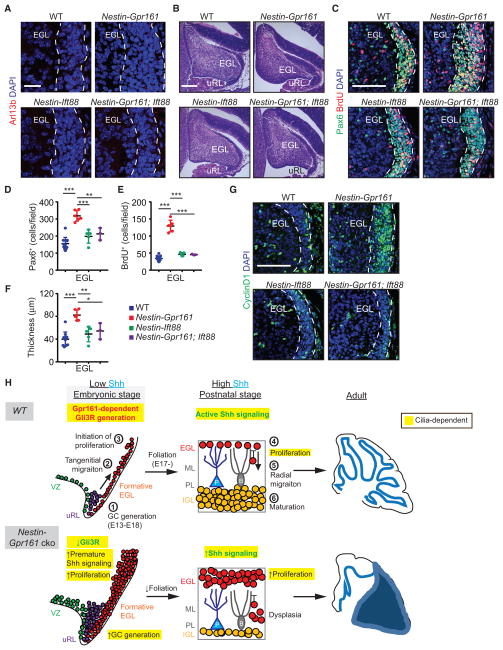
We next examined whether excessive generation and proliferation of GC progenitors during embryogenesis upon lack of Gpr161 are also dependent on cilia at E15.5. Pax6-expressing GC progenitors were ciliated at this stage in the cerebellar anlage (Figure S6A), and cilia were absent upon Nestin-Cre-mediated conditional deletion of Ift88 (Figure 7A). The number and proliferation of GC progenitors in the formative EGL and uRL of Nestin-Ift88 cko mice that lack cilia were similar to littermate controls (Figures 7B–7F and S6B–S6D). In contrast, the thickness of the EGL and the number and proliferation of GC progenitors in the EGL and uRL in Nestin-Gpr161 cko mice were increased compared with the littermate controls (Figures 7B–7F; Figure S6B–S6D). The thickness of the EGL and the number and proliferation of GC progenitors in Nestin-Gpr161; Ift88 dko mice were decreased compared with Nestin-Gpr161 cko mice and similar to littermate controls or Nestin-Ift88 cko mice (Figures 7B–7F and S6B–S6D). The Shh pathway target CyclinD1 was significantly high in Nestin-Gpr161 cko mice compared with Nestin-Gpr161; Ift88 dko mice, which were similar to littermate controls or Nestin-Ift88 cko mice (Figure 7G and S6E). Thus, the presence of cilia prevents excessive generation and proliferation of GC progenitors during embryogenesis by Gpr161-mediated repression of the Shh pathway but has no effect on normal progenitor development during embryogenesis.
Figure 7. Gpr161 Prevents Premature Shh Signaling during Embryogenesis in a Cilium-Dependent Manner.
(A) Arl13b+ primary cilia in the formative EGL (white dashed line) at E15.5.
(B) H&E-stained sagittal sections of the embryonic cerebellum at E15.5.
(C–F) Representative images of proliferating GC progenitors in the formative EGL at E15.5 (C) and quantification (D and E). Also shown is the thicknesses of the EGL at E15.5 (F). The numbers of mice were as follows: WT (n = 11), Nestin-Gpr161 cko (n = 6), Nestin-Ift88 cko (n = 5), and Nestin-Gpr161;Ift88 dko (n = 3).
(G) Increased number of Cyclin D1+ cells in the formative EGL of Nestin-Gpr161 cko mice at E15.5.
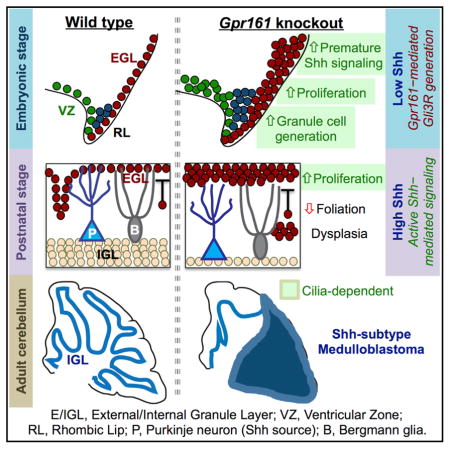
(H) Cartoon summarizing the role of Gpr161 in cerebellar development and Shh-MB pathogenesis. See Discussion for details.
Scale bars indicate (A and B) 200 μmand (C and G) 100 μm. Nuclei are stained by DAPI. *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA with Sidak multiple comparisons test. Abbreviations of genotypes are as in Figure 6. See also Figure S6.
DISCUSSION
Gpr161 Is a Bona Fide Tumor Suppressor in Shh-MB Formation
Here we establish Gpr161 as a bona fide tumor suppressor in Shh-MB. Multiple lines of evidence, including RNA sequencing (RNA-seq) analysis and immunohistochemistry, group the Shh-MB tumors in Gpr161 conditional knockout mice with SmoM2-induced tumors in mice and human SHH-MB. To our knowledge, this is the only rhodopsin family GPCR to be implicated in medulloblastoma pathogenesis. We also note that lower levels of GPR161 transcript correlate with tumor progression in SHH-MB patients but not in other subtypes. Thus, GPR161 levels might also play a role in the progression of human SHH-MB tumors and be useful as a prognostic biomarker. The severity and extent of tumorigenesis is weaker than with respect to Ptch1 conditional knockout mice (Yang et al., 2008) because Shh pathway induction upon Gpr161 depletion is weaker than upon Smo activation (Mukhopadhyay et al., 2013). Nonetheless, the natural progression of tumorigenesis upon Gpr161 deletion instructs on stages of Shh-MB formation, such as increased generation and proliferation of GC progenitors leading gradually to tumorigenesis or dysplasia (Figure 7H). As opposed to Gpr161’s role as a tumor suppressor in Shh-MB, GPR161 levels are elevated in triple-negative breast cancer, with GPR161 over-expression promoting proliferation and invasiveness in mammary epithelial cells by Shh-independent mechanisms (Feigin et al., 2014).
Gpr161 Regulates GC Progenitor Proliferation in a Cilium-Dependent Manner
Lack of Ift88 prevented both basal and Gpr161 deletion-induced excessive postnatal GC proliferation and Shh-mediated signaling. Ift88 is known to play other roles in the cell cycle in addition to IFT in cilia (Delaval et al., 2011). Deletion of the anterograde IFT kinesin II subunit Kif3a using hGFAP-Cre also disrupts cilia in GC progenitors and reduces proliferation in the postnatal EGL (Spassky et al., 2008). Because conditional knockouts of Ift88 phenocopy Kif3a mutants in preventing GC progenitor proliferation postnatally, lack of cilia in Ift88 mutants likely accounts for EGL deficiency in single or double mutants with Gpr161. Overall, Gpr161-mediated negative regulation through cilia functions as a rheostat in fine-tuning Shh-dependent signaling during postnatal GC progenitor proliferation (Figure 7H).
Restriction of Premature Shh Signaling in the Cerebellar Anlage by Gpr161 in a Cilium-Dependent Manner
Gpr161 deletion during embryogenesis promotes prematurely high downstream activity of the Shh pathway from lack of Gli3R formation, causing increased GC progenitor proliferation in the cerebellar anlage in the absence of Shh. Furthermore, we detect a general increase in cellularity in the uRL, along with an increase in Pax6-, Lmx1a-, and Olig2-positive GC progenitors and Sox2-positive NSCs. Interestingly, only excessive generation and overproliferation of GC progenitors during embryogenesis from Gpr161 deletion were cilium dependent, unlike baseline production of GC progenitors. Thus, Gli3R-mediated suppression of premature Shh signaling through cilia critically determines balanced generation of GC progenitors in the uRL and proliferation of GC progenitors in the formative EGL (Figure 7H). The low Shh signaling compartment in the cerebellar anlage might be necessary to restrict generation of GC progenitor pools in the uRL, prevent undue proliferation of GC progenitors during migration into the formative EGL, and limit excessive proliferation in the formative EGL before initiation of foliation. Restricting premature Shh pathway activity in the cerebellar anlage exemplifies a broader role of suppression of downstream signaling in the absence of Shh in developmental (Hwang et al., 2017) and tumor initiation pathways.
EXPERIMENTAL PROCEDURES
Mice
All the animals in the study were handled according to protocols approved by the University of Texas (UT) Southwestern Institutional Animal Care and Use Committee, and the mouse colonies were maintained in a barrier facility at UT Southwestern, in agreement with the State of Texas legal and ethical standards of animal care. The newly generated Gpr161 floxed mouse strain was maintained in a C57BL/6 background (Hwang et al., 2017). Nestin-Cre mice (B6.Cg-Tg(Nes-cre)1Kln/J, stock no. 003771), Atoh1-Cre mice (B6.Cg-Tg(Atoh1-cre)1Bfri/J, stock no. 011104), hGFAP-Cre mice (FVB-Tg(GFAP-cre) 25Mes/J, stock no. 004600), SmoM2 mice (Gt(ROSA)26Sortm1(Smo/EYFP)Amc/J, stock no. 005130), and Ift88fl/fl (B6.129P2-Ift88tm1Bky/J, stock no. 022409) mice were obtained from Jackson Laboratory (Bar Harbor, ME). Both male and female mice were analyzed in all experiments. We did not notice any difference between wild-type or heterozygous animals for both Gpr161 and Ift88 floxed alleles with or without Cre recombinase, and, thus, all were included as littermate controls, as mentioned in the respective figure legends. Noon of the day on which a vaginal plug was found was considered E0.5. Tail DNAs were used for genotyping embryos. Genotyping details are provided in the Supplemental Experimental Procedures.
Patient Survival Analysis
Published patient data were re-analyzed for survival and gene expression levels (Cho et al., 2011), and the top 50% and bottom 50% gene expression groups were considered high and low gene expression groups, respectively. The relationship between mRNA expression and survival time was analyzed according to the Kaplan-Meier method using log rank statistics. Survival plots were generated with R studio. The p values were calculated by log-rank tests.
Cell Quantification and Statistics
For EGL, uRL, and VZ quantification from a “field” (Figures 3, 4, 5, 6, and 7 and S5 and S6), we averaged marker-positive cells from three images of different sections of the designated region from each mouse embryo or pup taken with a 40× objective in an LSM780. For the uRL quantification in Figure 5, we averaged marker-positive cells in the uRL from three images of different sections of the designated region from each embryo taken with 20× objective in an LSM780. Data from 3–11 mice per genotype are shown. The thickness of the EGL layer was measured manually using ImageJ from at least three sections per mouse. No blinding was performed. Sample sizes were based on our experience with these assays. All data in the figures are expressed as mean ± SD. To assess the statistical significance of differences among treatments, we often performed unpaired, two-sided Student’s t tests that assumed unequal variances in treatments, one-way ANOVA with Sidak’s multiple comparisons tests, one-way ANOVA with Turkey multiple comparisons tests, and log rank tests. No mice or samples were excluded from any experiments, except for Figure 3F (because degraded brains were not amenable to analysis). Microsoft Excel and GraphPad Prism (GraphPad, La Jolla, CA) were used for statistical analysis. Values of p < 0.05 were considered significant. Analysis of RNA-seq data is described in the Supplemental Experimental Procedures.
DATA AND SOFTWARE AVAILABILITY
The accession number for the RNA-seq data reported in this paper is SRA: SRP114953.
Supplementary Material
Highlights.
Gpr161 is a tumor suppressor in sonic hedgehog (Shh) subtype medulloblastoma (MB)
Granule cell (GC) progenitor production increased upon Gpr161 deletion and was cilium dependent
Gpr161 restricted premature Shh pathway activity and GC progenitor overproduction in embryos
Reduced GPR161 expression correlated with poor survival of SHH-MB patients
Acknowledgments
This work was supported by a recruitment grant from the Cancer Prevention and Research Institute of Texas (R1220 to S.M.), an A-grant from the Alex’s Lemonade Foundation (to S.M.), and an R01 grant from the NIH (1R01GM113023-01 to S.M.). We thank Dr. Stephen Brown at University of Vermont for the Zic2 antibody. We thank Victor Santana for technical support with histology. We thank Dr. Robert Hammer and the Mouse Transgenic Core, Live Cell Imaging Facility (NIH S10RR029731), Bioinformatic Core (Cancer Prevention and Research Institute of Texas RP150596), and the Molecular Pathology Core at UT Southwestern Medical Center.
Footnotes
AUTHOR CONTRIBUTIONS
I.S.S. and S.M. conceived the project, designed the experiments, analyzed most of the data, and wrote the paper. I.S.S. performed most of the experiments. S.-H.H. generated Gpr161fl/fl mice and assisted with in situ hybridization experiments. B.N.S. performed the Gpr161 expression analysis and assisted with preliminary characterization of GFAP-Gpr161 cko mice. X.W., P.S., and M.D.T. performed human patient data analysis. J.K., M.K., and Z.X. analyzed RNA-seq data. J.M.S. performed the radiometric in situ hybridization experiments. V.R. provided human tumor samples and served as a consultant pathologist for tumor diagnosis and immunohistochemistry interpretation.
DECLARATION OF INTERESTS
The authors declare no competing interests.
Supplemental information includes Supplemental Experimental Procedures and six figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.01.018.
References
- Ahlfeld J, Favaro R, Pagella P, Kretzschmar HA, Nicolis S, Schüller U. Sox2 requirement in sonic hedgehog-associated medulloblastoma. Cancer Res. 2013;73:3796–3807. doi: 10.1158/0008-5472.CAN-13-0238. [DOI] [PubMed] [Google Scholar]
- Aruga J, Inoue T, Hoshino J, Mikoshiba K. Zic2 controls cerebellar development in cooperation with Zic1. J Neurosci. 2002;22:218–225. doi: 10.1523/JNEUROSCI.22-01-00218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgandi HB, Hwang SH, Shimada IS, Loriot E, Mukhopadhyay S. Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. J Cell Biol. 2017;216:743–760. doi: 10.1083/jcb.201607095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaess S, Stephen D, Joyner AL. Gli3 coordinates three-dimensional patterning and growth of the tectum and cerebellum by integrating Shh and Fgf8 signaling. Development. 2008;135:2093–2103. doi: 10.1242/dev.015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhikov VV, Lindgren AG, Currle DS, Rose MF, Monuki ES, Millen KJ. The roof plate regulates cerebellar cell-type specification and proliferation. Development. 2006;133:2793–2804. doi: 10.1242/dev.02441. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, Yoder BK, Millen KJ. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, Berhoukim R, Amani V, Goumnerova L, Eberhart CG, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29:1424–1430. doi: 10.1200/JCO.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales JD, Rocco GL, Blaess S, Guo Q, Joyner AL. Spatial pattern of sonic hedgehog signaling through Gli genes during cerebellum development. Development. 2004;131:5581–5590. doi: 10.1242/dev.01438. [DOI] [PubMed] [Google Scholar]
- Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- Delaval B, Bright A, Lawson ND, Doxsey S. The cilia protein IFT88 is required for spindle orientation in mitosis. Nat Cell Biol. 2011;13:461–468. doi: 10.1038/ncb2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C, Neale G, Kenney AM, Brat DJ, Perry A, Yong WH, et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121:381–396. doi: 10.1007/s00401-011-0800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin ME, Xue B, Hammell MC, Muthuswamy SK. G-protein-coupled receptor GPR161 is overexpressed in breast cancer and is a promoter of cell proliferation and invasion. Proc Natl Acad Sci USA. 2014;111:4191–4196. doi: 10.1073/pnas.1320239111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Milenković L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009;15:1062–1065. doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134:307–316. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci. 2009;3:31. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Liu J, Ketova T, Fleming JT, Grover VK, Cooper MK, Litingtung Y, Chiang C. Transventricular delivery of Sonic hedgehog is essential to cerebellar ventricular zone development. Proc Natl Acad Sci USA. 2010;107:8422–8427. doi: 10.1073/pnas.0911838107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SH, White KA, Somatilaka BN, Shelton JM, Richardson JA, Mukhopadhyay S. The G-protein-coupled receptor Gpr161 regulates forelimb formation, limb patterning and skeletal morphogenesis in a primary cilium-dependent manner. Development. 2017;145 doi: 10.1242/dev.154054. dev.154054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Jäger N, Kool M, Zichner T, Hutter B, Sultan M, Cho YJ, Pugh TJ, Hovestadt V, Stütz AM, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488:100–105. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Gill PS, Rotin L, van Eede M, Henkelman RM, Hui CC, Rosenblum ND. Suppressor of fused controls mid-hindbrain patterning and cerebellar morphogenesis via GLI3 repressor. J Neurosci. 2011;31:1825–1836. doi: 10.1523/JNEUROSCI.2166-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kawagoe R, Sasai K, Li Y, Russell HR, Curran T, McKinnon PJ. Loss of suppressor-of-fused function promotes tumorigenesis. Oncogene. 2007;26:6442–6447. doi: 10.1038/sj.onc.1210467. [DOI] [PubMed] [Google Scholar]
- Legué E, Gottshall JL, Jaumouillé E, Roselló-Díez A, Shi W, Barraza LH, Washington S, Grant RL, Joyner AL. Differential timing of granule cell production during cerebellum development underlies generation of the foliation pattern. Neural Dev. 2016;11:17. doi: 10.1186/s13064-016-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto K, Arancillo M, Becker EB, Buffo A, Chiang C, Ding B, Dobyns WB, Dusart I, Haldipur P, Hatten ME, et al. Consensus Paper: Cerebellar Development. Cerebellum. 2016;15:789–828. doi: 10.1007/s12311-015-0724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP. Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol. 2004;270:393–410. doi: 10.1016/j.ydbio.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Lin JC, Cai L, Cepko CL. The external granule layer of the developing chick cerebellum generates granule cells and cells of the isthmus and rostral hindbrain. J Neurosci. 2001;21:159–168. doi: 10.1523/JNEUROSCI.21-01-00159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machold R, Fishell G. Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron. 2005;48:17–24. doi: 10.1016/j.neuron.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Mille F, Tamayo-Orrego L, Lévesque M, Remke M, Korshunov A, Cardin J, Bouchard N, Izzi L, Kool M, Northcott PA, et al. The Shh receptor Boc promotes progression of early medulloblastoma to advanced tumors. Dev Cell. 2014;31:34–47. doi: 10.1016/j.devcel.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Millonig JH, Millen KJ, Hatten ME. The mouse Dreher gene Lmx1a controls formation of the roof plate in the vertebrate CNS. Nature. 2000;403:764–769. doi: 10.1038/35001573. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Rohatgi R. G-protein-coupled receptors, Hedgehog signaling and primary cilia. Semin Cell Dev Biol. 2014;33:63–72. doi: 10.1016/j.semcdb.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell. 2013;152:210–223. doi: 10.1016/j.cell.2012.12.026. [DOI] [PubMed] [Google Scholar]
- Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcott PA, Buchhalter I, Morrissy AS, Hovestadt V, Weischenfeldt J, Ehrenberger T, Gröbner S, Segura-Wang M, Zichner T, Rudneva VA, et al. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017;547:311–317. doi: 10.1038/nature22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh TJ, Weeraratne SD, Archer TC, Pomeranz Krummel DA, Auclair D, Bochicchio J, Carneiro MO, Carter SL, Cibulskis K, Erlich RL, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488:106–110. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G, Parker M, Kranenburg TA, Lu C, Chen X, Ding L, Phoenix TN, Hedlund E, Wei L, Zhu X, et al. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488:43–48. doi: 10.1038/nature11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, Huillard E, Sun T, Ligon AH, Qian Y, et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14:123–134. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, Han YG, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo JM, Alvarez-Buylla A. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008;317:246–259. doi: 10.1016/j.ydbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MD, Liu L, Raffel C, Hui CC, Mainprize TG, Zhang X, Agatep R, Chiappa S, Gao L, Lowrance A, et al. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31:306–310. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- Vanner RJ, Remke M, Gallo M, Selvadurai HJ, Coutinho F, Lee L, Kushida M, Head R, Morrissy S, Zhu X, et al. Quiescent sox2(+) cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma. Cancer Cell. 2014;26:33–47. doi: 10.1016/j.ccr.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- Wang VY, Rose MF, Zoghbi HY. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron. 2005;48:31–43. doi: 10.1016/j.neuron.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M, Schüller U, Machold R, Fishell G, Rowitch DH, et al. Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell. 2008;14:135–145. doi: 10.1016/j.ccr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.